Abstract
The gene encoding a novel 5-oxoprolinase without ATP-hydrolyzing activity from Alcaligenes faecalis N-38A was cloned and characterized. The coding region of this gene is 1,299 bp long. The predicted primary protein is composed of 433 amino acid residues, with a 31-amino-acid signal peptide. The mature protein is composed of 402 amino acid residues with a molecular mass of 46,163 Da. The derived amino acid sequence of the enzyme showed no significant sequence similarity to any other proteins reported so far. The 5-oxoprolinase gene was expressed in Escherichia coli by using a regulatory expression system with an isopropyl-β-d-thiogalactopyranoside-inducible tac promoter, and its expression level was approximately 16 mg per liter. The purified enzyme has the same characteristics as the authentic enzyme, except for the amino terminus, which has three additional amino acids. The enzyme was markedly inhibited by p-chloromercuribenzoic acid, EDTA, o-phenanthroline, HgCl2, and CuSO4. The EDTA-inactivated enzyme was completely restored by the addition of Zn2+ or Co2+. In addition, the enzyme was found to contain 1 g-atom of zinc per mol of protein. These results suggest that the 5-oxoprolinase produced by A. faecalis N-38A is a zinc metalloenzyme.
5-Oxoprolinases (EC 3.5.2.9) catalyze a decyclization of l-pyroglutamate (5-oxoproline) to form l-glutamate. Such enzymes have been found in mammalian tissues (21–23), wheat germ (8), Pseudomonas putida (20), and Alcaligenes sp. strain F-137 (3). Among these, rat kidney enzyme catalyzes the decyclization of glutamate and the cleavage of ATP by the same protein. The enzyme is composed of two apparently identical subunits and contains six sulfhydryl groups. Two of the groups are required for catalysis and at least one is involved in ATP cleavage (24). 5-Oxoproline is first phosphorylated with ATP hydrolysis on the amide carbonyl oxygen, and the resulting intermediate is subsequently hydrolyzed to yield γ-glutamyl phosphate, which is hydrolyzed to glutamate and inorganic phosphate (2). In contrast, the Pseudomonas enzyme is composed of two functionally different subunits, A and B (15). Component A, which has sulfhydryl groups, catalyzes the ATP-dependent phosphorylation of 5-oxoproline. Component B cleaves the phosphorylated 5-oxoproline to glutamate and inorganic phosphate (5, 6, 16). Thus, the 5-oxoprolinases reported to date required ATP and metal ions, such as Mg2+ and K+, for their catalytic function. Therefore, these enzymes are classified as “metal-activated” or “sulfhydryl” enzymes. Recently, the gene encoding 5-oxoprolinase has been cloned from a rat kidney cDNA library (26). The predicted amino acid sequence was similar to those of a hypothetical yeast protein, YKL215C, and the bacterial hydantoinases HyuA and HyuB (19). Catalytic residues of the enzyme have not yet been identified.
As reported previously (9, 10), we found a novel type of 5-oxoprolinase without ATP-hydrolyzing activity, named the N-38A enzyme, in a cell extract of Alcaligenes faecalis N-38A. The enzyme catalyzes a decyclization of l-pyroglutamate to l-glutamate without ATP hydrolysis and without requiring metals such as Mg2+ and K+.
We are interested in elucidating the catalytic mechanism and the structure-function relationship. To investigate them, the 5-oxoprolinase gene from A. faecalis N-38A was cloned, sequenced, and expressed in Escherichia coli, and the recombinant enzyme was compared with the natural enzyme. Further, the metal ion participating in the catalytic function of the enzyme was determined.
MATERIALS AND METHODS
Materials.
Restriction endonucleases, DNA polymerase, and DNA ligase were purchased from Nippon Gene Co., Toyama, Japan, or Toyobo Co., Osaka, Japan. AmpliTaq DNA polymerase for sequencing was obtained from the Perkin-Elmer Corp., Norwalk, Conn. l- and dl-pyroglutamate were obtained from Tokyo Chemical Industry Co., Tokyo, Japan, and Butyl-Toyopearl 650M was obtained from Tosoh Co., Tokyo, Japan. DEAE-Sepharose Fast Flow was purchased from Pharmacia Fine Chemical Co., Uppsala, Sweden. Shim-pack SCR-101H was obtained from Shimadzu Co., Kyoto, Japan. Polyvinylidene difluoride membrane was obtained from Bio-Rad Laboratories, Richmond, Calif., and all other materials were purchased from Wako Pure Chemicals Co., Osaka, Japan.
Bacterial strains and plasmids.
E. coli DH5α [deoR endA1 gyrA96 hsd R17(rk−, mk+) recA1 relA1 supE44 thi-1 (lac ZYA-arg F)U169 φ80lacZ M15 F−. 1−] and JM109 [e14-(mcrA) recA1 endA1 gyrA96 thi-1 hsdR17(rk−, mk+) supE44 relA1 (lac-proAB) (F′ traD36 proAB lacIqZ M15)] were used as hosts. Plasmids pUC18, pUC19, and pKK223-3 (1) were used for cloning and sequencing.
Preparation of genomic library and screening.
A. faecalis N-38A was cultured in glucose bouillon (GB) broth (1% polypeptone, 1% meat extract, 1% glucose, and 0.5% NaCl, pH 7.0) at 37°C. GB broth (100 ml) in Sakaguchi flasks was shaken in a reciprocal shaker (120 rpm) for 24 h. Chromosomal DNA was isolated from the harvested cells by the Saito-Miura method (12). Chromosomal DNA was partially digested with Sau3AI, and the resultant 2- to 6-kbp fragments were ligated with the BamHI-cleaved and dephosphorylated plasmid pUC19. The hybrid plasmids obtained were used to transform E. coli DH5α. Ampicillin (final concentration, 50 μg/ml) was added to Luria-Bertani broth (1% tryptone, 1% NaCl, and 0.5% yeast extract, pH 7.0) for selection. Recombinant colonies were transferred into the wells of microtiter plates containing 100 μl of diluted GB broth (0.11% polypeptone, 0.033% meat extract, and 0.055% NaCl, pH 7.0). After overnight incubation at 30°C, 50 μl of 0.1 M Tris-HCl (pH 7.0) and 50 μl of 0.2 M dl-pyroglutamate (pH 7.0) were added to each well. The reaction mixture was incubated at 30°C for 10 min, and generated l-glutamate was measured by adding 76 μl of TEA buffer (3.72% triethanolamine hydrochloride, 1.28% Triton X-100, 120 mM K2HPO4, and 0.1 mM KH2PO4, pH 8.0) and 24 μl of enzyme solution (2.8 mM NAD+, 0.5 mM INT [2-(4-iodophenyl)-3-(4-nitrophenyl)-5-phenyltetrazolium chloride], 0.275 U of diaphorase/ml, and 100 U of glutamate dehydrogenase/ml). Red color development indicated the presence of enzyme activity.
DNA manipulation.
The general procedures for DNA manipulation were based on those described by Sambrook et al. (14). Protocols for PCR and sequencing were as recommended by the respective manufacturers.
Nucleotide sequencing.
Nucleotide sequence was determined by the chain termination method with AmpliTaq DNA polymerase or Stoffel fragment by using an Applied Biosystems model 373S DNA sequencer. The reaction mixture was loaded onto a 5.25% denatured polyacrylamide gel. The nucleotide sequence was analyzed using the DNASIS software programs (Hitachi Co., Tokyo, Japan) for prediction of an amino acid sequence. The similarity search was done using BLAST and FASTA programs (GenomeNet, Institute for Chemical Research, Kyoto University, Kyoto, Japan) with protein and nucleotide databases (SWISSPLOT, SWISSPLOT-upd, pir, pdbstr, prf, nr-aa, and genes).
Enzyme assay and protein concentration.
Enzyme assay and protein concentration were as described previously (10). One unit of decyclization activity was defined as the amount of enzyme required to form 1 μmol of l-glutamate from l-pyroglutamate per min.
Construction of the 5-oxoprolinase expression plasmid, pKK/N38A.
Upstream (5′-TTTGAATTCATGACTTGCCATCGTAT-3′) and downstream (5′-TTTAAGCTTGACAGCAGAATCAAGAA-3′) primers containing the EcoRI or HindIII site, respectively, were designed from both terminal sequences of the proenzyme. Amplification of a DNA fragment mediated by PCR gave a single product approximately 1.5 kbp in length. This PCR product was digested with EcoRI and HindIII, and the resultant fragment was ligated into the same restriction site of pKK223-3. The resultant plasmid, pKK/N38A, was transformed into E. coli JM109.
Expression of the 5-oxoprolinase gene in E. coli.
E. coli JM109 harboring recombinant plasmid was cultured at 25 or 30°C in 50 ml of Terrific broth (TB) (1.2% Bacto tryptone, 2.4% Bacto yeast extract, 0.5% glycerol, 17 mM KH2PO4, and 72 mM K2HPO4, pH 7.2) containing 50 μg of ampicillin/ml until the optical density at 660 nm reached 0.6; then isopropyl-β-d-thiogalactopyranoside (IPTG) was added to a final concentration of 0.1 or 1.0 mM, and the cultivation was continued for an additional 4 h.
Purification of the enzyme.
E. coli JM109 harboring pKK/N38A was aerobically cultured in 15 liters of TB containing ampicillin (50 μg/ml) using a 30-liter jar fermentor (Mitsuwa Rikagaku Co., Osaka, Japan). The culture was grown at 25°C (stirring speed, 150 rpm; aeration, 15 liters per min). When the optical density at 660 nm reached 0.6, IPTG was added to a final concentration of 0.1 mM, and then the cultivation was continued for an additional 4.4 h. Harvested cells were washed with 50 mM Tris-HCl buffer (pH 8.0) and were resuspended in 10 liters of the same buffer. Each liter of the suspension was disrupted with an Ultrasonic Disruptor Sonifier B-12 (Branson Co., Danbury, Conn.) (output 150 W, 30 s at intervals of 30 s, 30 times at 4°C). The resultant suspension was centrifuged at 13,200 × g for 10 min at 4°C. Ammonium sulfate was added to produce 20% saturation in the cell extract. Five-liter portions of the resulting solution (15 liters) were loaded onto a Butyl-Toyopearl 650M column (5.0 [diameter] by 30 cm) equilibrated with 50 mM Tris-HCl buffer (pH 8.0) saturated with 20% ammonium sulfate. The adsorbed enzyme was eluted by 10% ammonium sulfate-saturated buffer. The active fractions were pooled and ammonium sulfate was added to produce 20% saturation. The resulting solution was loaded onto a Butyl-Toyopearl 650M column (5.0 [diameter] by 30 cm) equilibrated with 50 mM Tris-HCl (pH 8.0) saturated with 20% ammonium sulfate. The adsorbed enzyme was eluted by a linear gradient from 20 to 10% ammonium sulfate-saturated buffer. Active fractions were collected and dialyzed against 50 mM Tris-HCl buffer (pH 7.0). The dialyzed sample was loaded onto a DEAE-Sepharose fast-flow column (3.2 [diameter] by 30 cm) equilibrated with the same buffer. The adsorbed enzyme was eluted by a linear gradient in the same buffer from 0 to 0.3 M NaCl. The active fractions were dialyzed against distilled water and lyophilized.
Homogeneity.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was done according to the method of Laemmli (4), using a 7.5% polyacrylamide gel in 0.375 M Tris-HCl buffer (pH 8.8), containing 7.3% acrylamide, 0.2% N,N′-methylenebisacrylamide, and 0.1% SDS. The enzyme preparation (20 μg) was electrophoresed at a constant current of 20 mA at room temperature. The gel was stained with Coomassie brilliant blue R-250 in order to detect protein bands.
Amino-terminal amino acid sequence.
Purified enzyme was separated by SDS-PAGE and then electrophoretically transferred to a polyvinylidene difluoride membrane. Proteins were stained with Coomassie brilliant blue R-250 on the membrane. The stained protein band was directly sequenced with a Shimadzu model PSQ-21 gas-phase sequencer (Shimadzu Co.) according to the Matsudaira method (7).
ICP analysis.
Metal content was assayed by using an inductively coupled plasma (ICP) analyzer, model ICAP-55 (Nippon Jarrell-Ash Co. Ltd., Kyoto, Japan). Purified enzyme (25 mg) was dissolved in 20 ml of 0.1 N HCl solution. Various metals were analyzed by the ICP method in the resulting solution. Wavelengths used for the assay of Zn, Mg, and Co were 231.86, 279.55 or 280.27, and 238.89 nm, respectively.
Effect of various chemical reagents and metal ions on enzyme activity.
A reaction mixture (0.2 ml) containing 1 μmol of each reagent and 50 mg (1.06 pmol) of enzyme in 0.1 M Tris-HCl buffer (pH 8.0) was preincubated at 30°C for 30 min. After that, 0.8 ml of 49 mM l-pyroglutamate in 0.1 M Tris-HCl buffer (pH 8.0) was added and incubated at 30°C for 15 min. The amount of l-glutamate was measured by the o-phthalaldehyde method with the LC-10A amino acid analysis system (Shimadzu Co., Kyoto, Japan). In the control sample, each reagent was replaced by distilled water.
Reactivation of EDTA-inactivated enzyme by several divalent metal ions.
The native enzyme (10 μg/2.0 ml) was dialyzed against 1 liter of 0.1 M Tris-HCl buffer (pH 8.0) containing 1 mM EDTA for 17 h at 5°C. EDTA-inactivated enzyme (50 ng) was mixed with 1 μmol of each metal ion (Co2+, Cu2+, Fe2+, Mg2+, Mn2+, and Zn2+). After incubation at 30°C for 30 min, 0.8 ml of 49 mM l-pyroglutamate in 0.1 M Tris-HCl buffer (pH 8.0) was added to 0.2 ml of reaction mixture. After incubation at 30°C for 15 min, the amount of l-glutamate was measured by the method described above. In the control sample, the dialyzed buffer was replaced by 0.1 M Tris-HCl buffer (pH 8.0).
Nucleotide sequence accession numbers.
The nucleotide sequence data reported in this paper will appear in the DDBJ/EMBL/GenBank nucleotide sequence databases with the accession number AB034726.
RESULTS
Cloning of 5-oxoprolinase gene from Alcaligenes faecalis N-38A.
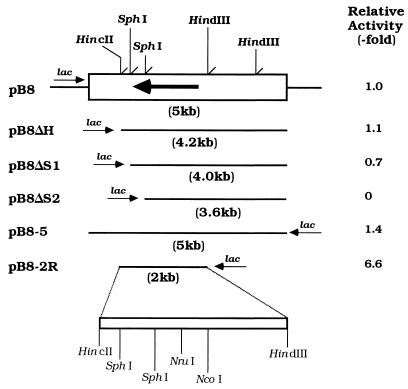
Approximately 9,600 transformants were obtained from the A. faecalis N-38A gene library and screened for 5-oxoprolinase activity. As a result, a clone having 5-oxoprolinase activity was detected. This clone contained the inserted fragment of 5 kbp, which was named pB8 (Fig. 1).
FIG. 1.
Partial restriction map of the plasmid pB8 and deletion analysis. Boxes indicate the insert DNA fragments. 5-Oxoprolinase activities were assayed with sonic extracts prepared from the transformants containing various plasmids. The enzyme activities of each transformant are expressed as values relative to those of E. coli DH5α harboring pB8 at 25°C. The large arrow indicates the position and direction of the enzyme gene. Small arrows show orientations of the lac promoter relative to the insert DNA.
Subcloning of 5-oxoprolinase gene.
To determine the location of the N-38A enzyme gene in pB8, deletion plasmids were constructed. Production of the enzyme in transformants harboring each plasmid was examined. The activity of the transformant cells harboring pB8ΔS1 decreased slightly. With further deletion (pB8ΔS2), the activity totally ceased. Further, the nucleotide sequence predicted by the amino-terminal amino acid sequence of the authentic enzyme was not found in the deletion-containing 0.6-kbp HincII-SphI fragment. These results indicate that the mRNA reading frame and the lac promoter are inserted in opposite directions. The plasmid pB8-5 was digested with HincII and HindIII and ligated into the same site of pUC18. The transformant harboring the resultant plasmid (pB8-2R) showed potent enzyme activity (Fig. 1). These results suggest that the open reading frame of the N-38A enzyme gene is located in the HincII-HindIII fragment.
Structure of 5-oxoprolinase gene.
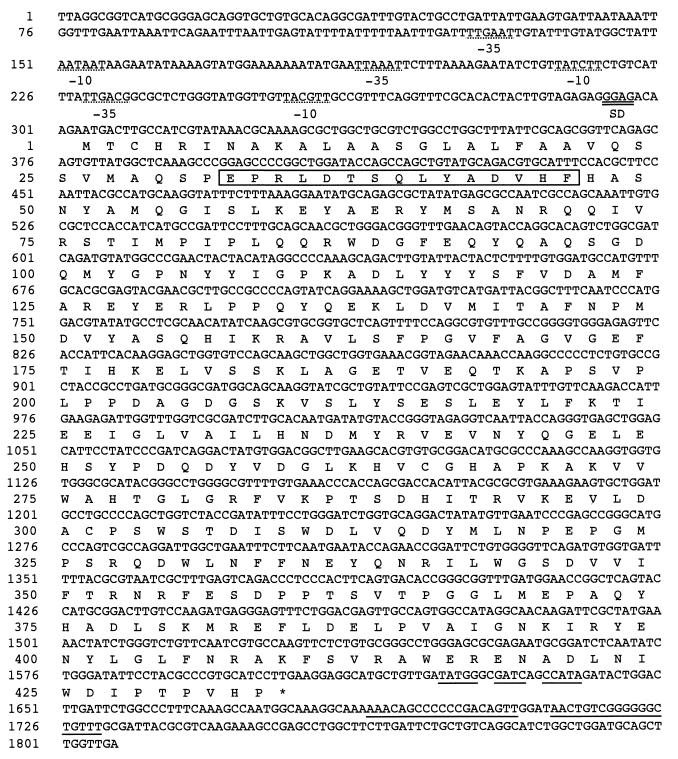
The nucleotide sequence of pB8-2R was determined. Figure 2 shows the nucleotide sequence and its deduced amino acid sequence. The N-38A enzyme gene was found in an open reading frame encoding 433 amino acid residues with a calculated molecular weight of 49,290.
FIG. 2.
Nucleotide sequence of the 5-oxoprolinase gene of A. faecalis N-38A and the deduced amino acid sequence of the enzyme. Numbering of amino acid residues starts at the amino terminus, Met, of the precursor protein. Three −35 and −10 regions of the putative promoter sequence are underlined with dashes. Double underlining shows the potential Shine-Dalgarno sequence (SD). The putative transcription terminator is underlined. The amino acid sequence of the amino terminal region of the authentic enzyme determined by Edman degradation is shown as a specified box. The termination codon is indicated by an asterisk.
Expression of recombinant enzyme.
The enzyme activity of E. coli JM109 harboring pB8 was very low. Therefore, we constructed an expression vector for the N-38A enzyme gene, pKK/N38A. Cultivation conditions for recombinant N-38A enzyme production were optimized. When E. coli JM109 cells harboring pKK/N38A were cultured in a TB medium at 25°C with 0.1 mM IPTG, the transformant showed potent activity (105 mU/ml), and its enzyme production was about 120-fold greater than that of the transformant harboring pB8 (0.9 mU/ml).
Purification of the recombinant enzyme.
The recombinant enzyme was purified from the cell extracts of E. coli JM109 cells harboring pKK/N38A. From 248 g of wet cell paste, 60.4 mg of purified recombinant enzyme was obtained, with a final yield of 24.6% (Table 1). The amount of enzyme production in E. coli cells was estimated to be 16.4 mg per liter of culture medium. This value was 5.3-fold greater than that of A. faecalis cells (3.1 mg/liter) (10). The specific activity of the purified recombinant enzyme was 2,520 mU/mg, which is comparable to that of authentic enzyme from A. faecalis (10).
TABLE 1.
Purification of the recombinant N-38A enzyme from E. coli JM109 harboring pKK/N-38A cells
| Purification step | Total protein (mg) | Total activity (mU) | Specific activity (mU/mg) | Purification (fold) | Yield (%) |
|---|---|---|---|---|---|
| Cell extract | 33,100 | 619,000 | 18.7 | 1 | 100 |
| Butyl-Toyopearl 650M | 1,000 | 263,000 | 263 | 14 | 42.5 |
| Butyl-Toyopearl 650M | 114 | 221,000 | 1,940 | 104 | 35.7 |
| DEAE-Sepharose fast flow | 60.4 | 152,000 | 2,520 | 135 | 24.6 |
Enzymatic properties of the recombinant enzyme.
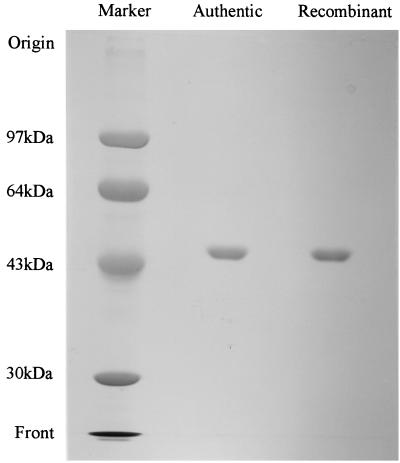
As shown in Fig. 3, the purified enzyme showed a single protein band on SDS-PAGE, which had the same mobility as the authentic enzyme. The amino-terminal amino acid sequence of the recombinant enzyme was determined to be NH2-QSPEPRLDTSQLYADVHFHA. This sequence corresponded to the amino acid sequence starting from the amino-terminal 29th position of the N-38A enzyme precursor (Fig. 2). Both NH2-EPRLD and NH2-LDTSQ sequences were also detected slightly in the same sample. The purified recombinant enzyme had enzymatic properties identical to those of the authentic enzyme (10). As shown in Table 2, the enzyme was inhibited by p-chloromercuribenzoic acid (pCMB), EDTA, o-phenanthroline, HgCl2, and CuSO4. According to the ICP analysis, 1.25 mg/ml of the recombinant enzyme solution contained 1.9 ppm of zinc and 0.11 ppm of magnesium. Cobalt was not detected. Assuming that the molecular weight of the enzyme is 47,000, the enzyme contains 1 g-atom of zinc per mole of protein. The reactivation of EDTA-inactivated enzyme by several divalent metal ions was examined. The addition of either Zn2+ or Co2+ almost completely restored activity of the EDTA-inactivated enzyme. It was also partially reactivated by Mg2+.
FIG. 3.
SDS-PAGE of the recombinant and authentic 5-oxoprolinases. A sample (about 20 μg of protein) was loaded onto a 7.5% gel after denaturation with 4% SDS and 10% mercaptoethanol, and the protein was stained with Coomassie brilliant blue R-250. Phosphorylase (94 kDa), bovine serum albumin (67 kDa), ovalbumin (43 kDa), and carbonic anhydrase (30 kDa) were used as protein markers.
TABLE 2.
Effect of various chemical reagents and metal ions on N-38A enzyme activity
| Addition (1 mM) | Inhibition (%)
|
|
|---|---|---|
| Recombinant | Authentic | |
| pCMB | 92.5 | 89.6 |
| Iodoacetamide | 10.7 | 10.3 |
| N-Ethylmaleimide | 25.4 | 23.8 |
| EDTA | 80.6 | 85.9 |
| o-Phenanthroline | 85.3 | 82.8 |
| KCN | 0 | 0 |
| NaN3 | 0 | 0 |
| SDS | 43.9 | 50.6 |
| HgCl2 | 92.6 | 90.9 |
| FeCl2 | 12.2 | 10.2 |
| FeCl3 | 8.5 | 8.6 |
| CoSO4 | 36.9 | 38.9 |
| ZnCl2 | 0 | 0 |
| MnCl2 | 10.9 | 9.5 |
| CaCl2 | 2.1 | 2.9 |
| MgSO4 | 35.6 | 38.9 |
| CuSO4 | 90.5 | 92.1 |
DISCUSSION
By comparing the amino-terminal amino acid sequence of authentic enzyme with that deduced from the N-38A gene, the N-38A enzyme was deduced to be synthesized as a precursor composed of an amino-terminal Pro region and a mature N-38A enzyme region. These results indicate that mature protein consists of 402 amino acid residues having a molecular weight of 46,163. The additional sequence was presumed to be a signal peptide. Two Met (ATG) residues of the 1st and 27th positions were presented in the upstream sequence of the amino terminus. There was a GAGG (nucleotide 294 to 297) sequence which is a putative ribosome-binding sequence at six bases upstream from the first Met residue. This sequence agreed with that of the d-aminoacylase gene from Alcaligenes xylosoxidans (18). Therefore, the first Met residue is presumed to be the initiation codon. A stop codon, TGA, follows the final proline codon (codon 433) of the precursor protein. In the 5′ noncoding region of the N-38A enzyme gene, three putative promoter-like sequences in the −35 region (TTGAAT [nucleotide 127 to 132], TTAAAT [nucleotide 188 to 193], and TTGACG [nucleotide 229 to 234]) and in the −10 region (AATAAT [nucleotide 151 to 156], TATCTT [nucleotide 213 to 218], and TACGTT [nucleotide 254 to 259]) were searched for with GENETYX genetic information processing software. Two palindromic sequences that are potential terminator sequences were located farther downstream from the stop codon. E. coli cells harboring pB8 and pB8-5 were expressed as an active enzyme without IPTG induction. This indicates that the promoter system of A. faecalis is active in E. coli cells. A computer search of the protein data bank (SWISSPLOT, SWISSPLOT-upd, pir, pdbstr, prf, nr-aa, and genes) detected no significant homology between the N-38A enzyme and other proteins, including the rat kidney enzyme and the bacterial hydantoinases HyuA and HyuB (19). The result suggests that the N-38A enzyme is assigned to the 5-oxoprolinase family as a member with an original structure.
To clarify the details of enzymatic properties, a superior expression system for the N-38A enzyme was essential. A plasmid, pKK/N38A, was derived from pKK223-3, which was constructed for an efficient expression of the N-38A enzyme. The expressed recombinant enzyme was purified by three steps of column chromatography. Enzymatic properties of the recombinant enzyme, such as optimum pH, molecular weight, specific activity, and sensitivity to inhibitors, were identical to those of an authentic enzyme, except for the amino-terminal amino acid sequence (10). The recombinant enzyme had three extra amino acids (Gln-Ser-Pro) at the amino terminal of the mature enzyme. In the case of blue copper protein (25), polyhydroxybutylate depolymerase (13), penicillin G amidase (17), and copper nitrate reductase (11) produced by A. faecalis, these precursor proteins were cleaved at the carboxyl side of the alanine or glycine residues. The N-38A enzyme precursor was cleaved at the carboxyl side of the proline. The differences in these cleavage sites of recombinant and authentic enzymes can be explained as follows: (i) the substrate specificity of signal peptidase in A. faecalis N-38A is different from that in E. coli, and (ii) the aminopeptidases hydrolyzing the three residues at the amino terminus exist in A. faecalis N-38A. The results described above suggest that the recombinant enzyme is identical to the authentic enzyme.
Rat kidney and Pseudomonas enzymes required sulfhydryl groups for their catalytic functions. Another enzyme produced by Alcaligenes sp. F-137 was inactivated by N-methylmaleimide, pCMB, EDTA, and o-phenanthroline (3). The N-38A enzyme was also inhibited by pCMB, HgCl2, EDTA, and o-phenanthroline. In terms of their characteristics, these enzymes may be regarded as metal-activated or sulfhydryl. In the case of the N-38A enzyme, activity of the EDTA-inactivated enzyme was restored almost completely by an addition of Zn2+ or Co2+. This enzyme also required no metal ions for its activity. Furthermore, it was revealed that the N-38A enzyme contained 1 g-atom of zinc per mol of protein. Based on these results, we concluded that the N-38A enzyme is a zinc metalloenzyme.
We have cloned and sequenced the 5-oxoprolinase gene from A. faecalis N-38A and developed efficient expression systems in E. coli cells. Catalytic residues of the 5-oxoprolinase family have not yet been identified. We are trying to clone the 5-oxoprolinase gene from other sources. Based on sequence similarity, it may be possible to identify the catalytic residues of the N-38A enzyme by a molecular biological approach.
ACKNOWLEDGMENTS
We appreciate Takahiro Matsuda and Marie Uchida (Department of Applied Microbial Technology, Kumamoto Institute of Technology) for their technical assistance. We also thank Yoshie Ueno (Kyoto Management and Technology, Kyoto Prefecture) for ICP analysis of the recombinant enzyme.
REFERENCES
- 1.Brosius J, Holy A. Regulation of ribosomal RNA promoters with a synthetic lac operator. Proc Natl Acad Sci USA. 1984;81:6929–6933. doi: 10.1073/pnas.81.22.6929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Griffith O W, Meister A. 5-Oxo-l-prolinase (l-pyroglutamate hydrolase), study of the chemical mechanism. J Biol Chem. 1981;256:9981–9985. [PubMed] [Google Scholar]
- 3.Koyama H. Purification and characterization of 5-oxo-l-prolinase (l-pyroglutamate hydrolase) from Alcaligenes sp. F-137. Agric Biol Chem. 1988;52:735–741. [Google Scholar]
- 4.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 5.Li L, Seddon A P, Meister A. Interaction of the protein components of 5-oxoprolinase. J Biol Chem. 1988;263:6495–6501. [PubMed] [Google Scholar]
- 6.Li L Y, Seddon A P, Meister A. 18O studies on the 5-oxoprolinase reaction. Evidence for a phosphorylated tetrahedral intermediate. J Biol Chem. 1987;262:11020–11025. [PubMed] [Google Scholar]
- 7.Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987;262:10035–10038. [PubMed] [Google Scholar]
- 8.Mazelis M, Creveling R K. 5-Oxoprolinase (l-pyroglutamate hydrolase) in higher plants. Plant Physiol. 1978;62:798–801. doi: 10.1104/pp.62.5.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murao S, Nishimura A, Ozaki Y, Oyama H, Shin T. Isolation and characterization of a novel 5-oxoprolinase (without ATP-hydrolyzing) from Alcaligenes faecalis N-38A. Biosci Biotechnol Biochem. 1995;59:2010–2012. doi: 10.1271/bbb.59.2010. [DOI] [PubMed] [Google Scholar]
- 10.Nishimura A, Ozaki Y, Oyama H, Shin T, Murao S. Purification and characterization of a novel 5-oxoprolinase (without ATP hydrolyzing activity) from Alcaligenes faecalis N-38A. Appl Environ Microbiol. 1999;65:712–717. doi: 10.1128/aem.65.2.712-717.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nishiyama M, Suzuki J, Kukimoto M, Ohnuki T, Horinouchi S, Beppu T. Cloning and characterization of a nitrite reductase gene from Alcaligenes faecalis and its expression in Escherichia coli. J Gen Microbiol. 1993;63:725–733. doi: 10.1099/00221287-139-4-725. [DOI] [PubMed] [Google Scholar]
- 12.Saito H, Miura K. Preparation of transforming deoxynucleic acid by phenol treatment. Biochim Biophys Acta. 1963;72:619–629. [PubMed] [Google Scholar]
- 13.Saito T, Suzuki K, Yamamoto J, Fukui T, Miwa K, Tomita K, Nakanishi S, Odani S, Suzuki J, Ishikawa K. Cloning, nucleotide sequence, and expression in Escherichia coli of the gene for poly(3-hydroxybutyrate) depolymerase from Alcaligenes faecalis. J Bacteriol. 1989;171:184–189. doi: 10.1128/jb.171.1.184-189.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 15.Seddon A P, Li L, Meister A. Resolution of 5-oxoprolinase into a 5-oxo-l-proline-dependent ATPase and a coupling protein. J Biol Chem. 1984;259:8091–8094. [PubMed] [Google Scholar]
- 16.Seddon A P, Meister A. Trapping of an intermediate in the reaction catalyzed by 5-oxoprolinase. J Biol Chem. 1986;261:11538–11543. [PubMed] [Google Scholar]
- 17.Verhaert R M D, Riemens A M, van der Laan J-M, van Duin J, Quax W J. Molecular cloning and analysis of the gene encoding the thermostable penicillin G acylase from Alcaligenes faecalis. Appl Environ Microbiol. 1997;63:3412–3418. doi: 10.1128/aem.63.9.3412-3418.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wakayama M, Katsuno Y, Hayashi S, Miyamoto Y, Sakai K, Moriguchi M. Cloning and sequence of a gene encoding d-aminoacylase from Alcaligenes xylosoxydans subsp. xylosoxydans A-6 and expression of the gene in Escherichia coli. Biosci Biotechnol Biochem. 1995;59:2115–2119. doi: 10.1271/bbb.59.2115. [DOI] [PubMed] [Google Scholar]
- 19.Watabe K, Ishikawa T, Mukohara Y, Nakamura H. Cloning and sequencing of the genes involved in the conversion of 5-substituted hydantoins to the corresponding l-amino acids from the native plasmid of Pseudomonas sp. strain NS671. J Bacteriol. 1992;174:962–969. doi: 10.1128/jb.174.3.962-969.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Werf P V D, Meister A. Isolation of 5-oxoprolinase from a prokaryote. Biochem Biophys Res Commun. 1974;56:90–96. doi: 10.1016/s0006-291x(74)80319-0. [DOI] [PubMed] [Google Scholar]
- 21.Werf P V D, Orlowski M, Meister A. Enzymatic conversion of 5-oxo-l-proline (l-pyrrolidone carboxylate) to l-glutamate coupled with cleavage of adenosine triphosphate to adenosine diphosphate, a reaction in the γ-glutamyl cycle. Proc Natl Acad Sci USA. 1971;65:2982–2985. doi: 10.1073/pnas.68.12.2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Werf P V D, Griffith O W, Meister A. 5-Oxoprolinase (l-pyroglutamate hydrolase), purification and catalytic properties. J Biol Chem. 1975;250:6686–6692. [PubMed] [Google Scholar]
- 23.Werf P V D, Stephani R A, Meister A. Accumulation of 5-oxoproline in mouse tissues after inhibition of 5-oxoprolinase and administration of amino acids: evidence for function of the γ-glutamyl cycle. Proc Natl Acad Sci USA. 1974;71:1026–1029. doi: 10.1073/pnas.71.4.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Williamson J M, Meister A. Effect of sulfhydryl group modification on the activities of 5-oxo-l-prolinase. J Biol Chem. 1982;257:9161–9172. [PubMed] [Google Scholar]
- 25.Yamamoto K, Uozumi T, Beppu T. The blue copper protein gene of Alcaligenes faecalis S-6 directs secretion of blue copper protein from Escherichia coli cells. J Bacteriol. 1987;169:5648–5652. doi: 10.1128/jb.169.12.5648-5652.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ye G J, Breslow E B, Meister A, Guo-jie G E. The amino acid sequence of rat kidney 5-oxo-l-prolinase determined by cDNA cloning. J Biol Chem. 1996;271:32293–32300. doi: 10.1074/jbc.271.50.32293. [DOI] [PubMed] [Google Scholar]