Abstract
Introduction:
Adenoid and tonsillar hypertrophy in children often leads to adverse respiratory symptoms and obstructive sleep apnea (OSA). Current clinical guidelines from the American Academy of Pediatrics and American Academy of Otolaryngology–Head and Neck Surgery recommend tonsillectomy as the first line of pediatric OSA treatment for children with tonsillar hypertrophy. Rapid palatal expansion (RPE) performed by orthodontists improves obstructive sleep apnea in children by reducing nasal airway resistance, increasing nasal volume, raising tongue posture, and enlarging pharyngeal airway. However, the role of RPE in alleviating adenoid and tonsillar hypertrophy remains elusive. In this study, we aim to evaluate the changes in adenoid and palatine tonsil sizes following RPE using 3D volumetric analysis of cone beam computational tomography (CBCT) imaging.
Materials and methods:
In this retrospective cohort study, a total of 60 pediatric patients (mean age: 8.00, range: 5–15, 32 females and 28 males) who had tonsillar hypertrophy (size 3 and 4) were included and divided into the control group (n = 20) and expansion group (n = 40). The control group did not undergo any treatment. The expansion group underwent RPE using a conventional Hyrax expander, activated 0.25 mm per day for 4–6 weeks. Final CBCT scans (T2) were performed 13.8 ± 6.5 months after the initial scan (T1). Pediatric sleep questionnaire (PSQ) and BMI were obtained at each timepoint. Volumetric analysis of adenoid and palatine tonsils was performed using a combination of bony and soft tissue landmarks in CBCT scans through Anatomage Invivo 6 imaging software. Paired t-tests were used to evaluate the difference between the initial and final adenoid and tonsil volumes. p values less than 0.05 were considered statistically significant.
Results:
Compared to the control group, the expansion group experienced a statistically significant decrease in both adenoid and tonsil volume. There was non-statistically significant increase in volume from T1 to T2 for the control group. For the expansion group, 90.0% and 97.5% of patients experienced significant reduction in adenoid and tonsil volume, respectively. The average volume decrease of adenoids was 16.8% while that of tonsils was 38.5%. The patients had up to 51.6% and 75.4% reduction in adenoid and tonsil size, respectively, following RPE orthodontic treatment. Pearson correlation ranged from 0.88 to 0.99 for each measurement, representing excellent internal consistency. There was a significant reduction in the PSQ scores from 5.81 ± 3.31 to 3.75 ± 2.38 in expansion group (p < 0.001).
Conclusions:
Our results demonstrated that RPE significantly reduced the size of both adenoid and palatine tonsils and revealed another long-term benefit of RPE treatment. To our knowledge, this is the first study to quantify the changes of adenoids and tonsils following RPE. RPE treatment can be considered as a valid and effective treatment option for pediatric OSA population with narrow high arch palate and adenotonsillar hypertrophy.
Keywords: Tonsillar hypertrophy, Adenoid hypertrophy, Sleep disordered breathing, Rapid palatal expansion, Pediatric obstructive sleep apnea, Narrow high arch palate
1. Introduction
Adenoid and tonsil hypertrophy is a prevalent pediatric condition that can lead to adverse health conditions [1]. The prevalence of adenoid hypertrophy was reported to be 34.46% [2], while the prevalence of tonsillar hypertrophy was found to be 11% in school children [3]. Their hypertrophy can occur with or without infection. While infectious causes include viral and bacterial pathogens, non-infectious causes include allergies, gastroesophageal reflux and irritants such as secondhand smoke and pollution in the air [4]. Adenoid and tonsil hypertrophy often leads to upper airway obstruction with sleep disordered breathing (SDB) that ranges from mouth breathing, snoring, upper airway resistance syndrome to severe obstructive sleep apnea (OSA).
When SDB is left untreated, cardiovascular, metabolic, and neurocognitive comorbidities can subsequently develop at an earlier age [5]. Cognitive deficits associated with OSA may present as poor school performance, learning difficulties, poor language and/or verbal skills, hyperactivity, low general intelligence, and diminished adaptive function [6,7]. Furthermore, studies have revealed that upper airway obstruction can induce craniofacial anomalies suggesting that continuation of SDB impacts craniofacial growth and development year by year [8]. Therefore, it is imperative to detect and treat children with SDB and craniofacial/dental anomalies early on.
Current clinical guidelines from the American Academy of Pediatrics and the American Academy of Otolaryngology–Head and Neck Surgery recommend tonsillectomy as the first line of pediatric OSA treatment for children with tonsillar hypertrophy [9,10]. Adenotonsillectomy is generally believed to be a safe and effective procedure which cures 47–80% of children with SDB [8]. However, due to associated dentofacial deformities and recurrence of SDB at adolescence, other treatment options have been proposed.
Common anatomic characteristics in OSA, particularly in the pediatric population, include narrow palate, dental crowding, retrognathic mandible, steep occlusal plane and high mandibular plane angle [11]. Rapid palatal expansion (RPE) is a traditional orthodontic treatment modality to alleviate maxillary transverse deficiency in children. RPE has been shown to both modify the craniofacial structure and improve pediatric OSA. Our group has previously demonstrated that RPE provides subjective improvement utilizing the Nasal Obstruction Septoplasty Effectiveness (NOSE) scores as well as structural widening of the internal nasal valve (INV) angle and cross-sectional area [12,13]. Additionally, RPE reduces nasal airway resistance and raises the tongue posture in pediatric patients, ultimately enlarging the pharyngeal airway as evaluated using cone beam computational tomography (CBCT) scans [14]. Similarly, RPE is an effective OSA treatment in children with maxillary constriction and a 12-year follow up study has confirmed long-term stability of the airway [15].
However, the impact of RPE on adenoid and tonsillar size is not yet known. The aim of this study is to evaluate volumetric changes in adenoid and palatine tonsil sizes following RPE in a pediatric patient population using 3D volumetric analysis of CBCT imaging. CBCT is a variation of the traditional CT technique where a single rotation of a ‘cone-shaped’ x-ray beam around the region of interest captures data and reconstructs the data into a three-dimensional (3D) image of the patient’s anatomy [16]. The radiation dose to the patient is significantly lower with shorter scan time and submillimeter spatial resolution when compared to traditional CT systems [17]. In this study, we demonstrate the efficacy of early orthodontic palatal expansion treatment in reducing adenoid and tonsil tissue.
2. Materials and Methods
A retrospective review of pediatric patients with a high arched narrow palate undergoing RPE as a part of orthodontic treatment in two orthodontic offices (A.Y. and R.B.) was conducted after the approval from the Stanford University institutional review board (#59392). A total of 60 pediatric patients (mean age: 8.00, range: 5–15, 32 females and 28 males) were divided into the control (n = 20) and expansion (n = 40) groups. During the orthodontic assessment, these children underwent the assessment of the grade of adenoid and tonsil hypertrophy on a scale ranging from 1 to 4. Inclusion criteria include patients with clinical indications for RPE and with adenoid and tonsil hypertrophy of grade 3 and 4. Indications for RPE treatment are narrow maxilla, posterior crossbite, arch length discrepancy in maxilla, decreased maxillary intermolar width, and skeletal transverse discrepancy between maxilla and mandible. Patients with craniofacial anomalies such as cleft lip and palate were excluded from this study.
Although the control group patients also presented with indications for RPE treatment as patients in the expansion group, the control group did not undergo any orthodontic treatment due to patients’ personal and financial reasons. The expansion group underwent RPE using a conventional Hyrax expander, activated 0.25 mm per day for 4–6 weeks.
CBCT scans of the maxillofacial region were acquired using an i-CAT device (Imaging Sciences International, Hatfield, Pa). An initial scan (T1) was performed before RPE and a final CBCT scan (T2) was performed 13.8 ± 6.5 months after the initial scan (T1). Pediatric Sleep Questionnaire (PSQ) and BMI were obtained at each timepoint. Volumetric analysis of adenoid and palatine tonsils was performed using a combination of bone and soft tissue landmarks in CBCT images using Invivo 6 Advanced 3D Imaging Software (version 6.0; Anatomage, San Jose, California). Patients who were excluded from the study included those with severe hypertrophy of either the adenoids or the tonsils leading to obliteration of essential anatomical landmarks to perform the measurements. Additionally, patients who are 15 years or older, with history of tonsillectomy and/or adenoidectomy, or underwent a surgical or mini-implant assisted palatal expansion were excluded. Data was screened, reviewed and analyzed by two calibrated, blinded, and independent reviewers (A.V. and K.L.). The CBCT images were oriented using the Frankfort Horizontal Plane, zygomaticotemporal suture, and zygomaticofrontal suture. Each measurement was performed twice by each of the two observers, for calculating the inter-rater reliability.
2.1. Volumetric assessment of adenoids
The volume of adenoid tissue was measured using a combination of both bony and soft tissue landmarks with the InVivo software. The area measurement tool in InVivo was used to isolate the periphery of the adenoidal mass. The coronal and axial CBCT slices were used to accurately correlate the location of the Fossa of Rosenmüller, used as the lateral limit of the adenoidal tissue both on the right and left sides. Superiorly, the area was bound by the inferior surface of the sphenoid bone and the inferior limit of the adenoid outline was the plane along the inferior plane of atlas (C1) vertebra. All adenoid tissue projecting anteriorly into the airway up to the vomer was included for the measurement. The midsagittal slice was used to isolate the periphery and quantify the volume of the adenoid tissue. The volume of the outlined area was calculated using the Cavalieri’s principle [18–21]. In alignment with the Cavalieri’s principle, the area measurement tool was used to measure the surface area of each section of the adenoid gland at 0.5 mm interval and then summed up to obtain the total volume. The following formula, which has been used in previous reports [18–21] was used for calculation to estimate the volume of the adenoid gland: V = t × ∑A where t is the section thickness and the interval of consecutive sections while ∑A is the total sectional area of the consecutive sections. Please refer to Fig. 1 (A, B, C) for the methodology of measuring the adenoids.
Fig. 1.
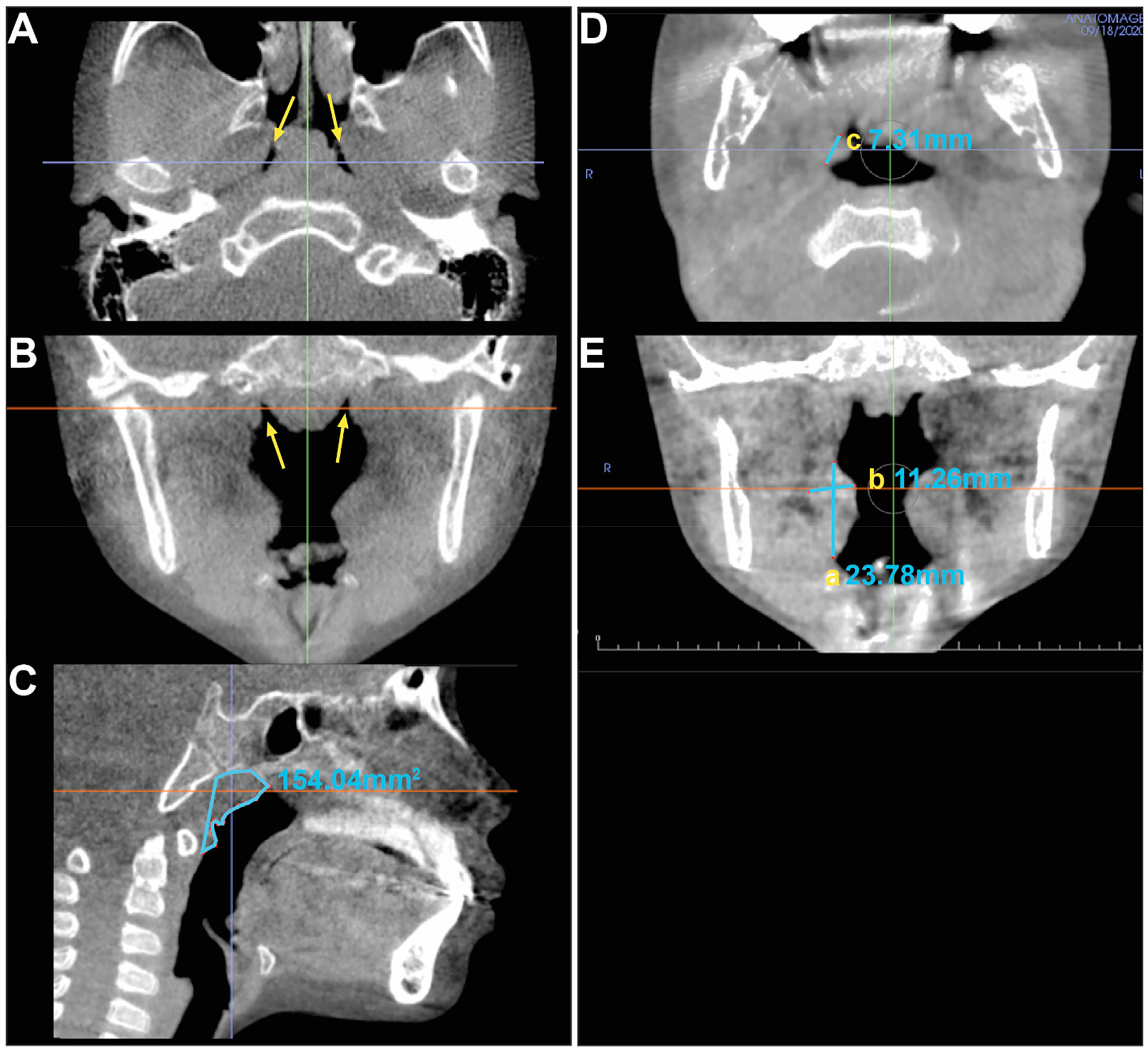
CBCT slices illustrating the methodology for volumetric measurement of the adenoids and of the palatine tonsils. For adenoid measurements, the Fossae of Rosenmüller in the axial (A) and coronal (B) slices at the arrows mark the lateral limit of the measurements. The sagittal slice (C) at midsagittal plane shows the outlined area of the surface area measurement of the adenoid. For palatine tonsil measurements, the axial slice (D) illustrates the depth (c) of the right tonsil and the coronal slice (E) illustrates the vertical (a) and transverse measurements (b) of the right palatine tonsil.
2.2. Volumetric assessment of palatine tonsils
The palatine tonsillar volume was quantified using soft tissue landmarks in the coronal, axial, and sagittal views on the CBCT. A mathemati cal formula to measure tonsillar volume was calculated using the maximum vertical (“a”), transverse (“b”), and depth (“c”) dimensions. Tonsillar volume was previously quantified with a high reproducibility and validity utilizing the formula: a*b*c*0.523 [22]. The volume equation was used individually for the right and left anatomical tonsils, and then subsequently added together. The vertical (a) and transverse (b) dimensions of the tonsils were measured on the coronal view, and the depth (c) on the axial view. These measurements were made of the right and left palatine tonsils in the region of maximum hypertrophy. The vertical dimension of the palatine tonsil (a) was measured between the upper and lower indentation of the airway into the lateral pharyngeal wall at the level of the upper and lower border of the tonsil respectively. The transverse dimension (b) was measured between the parapharyngeal fat pad laterally to the tip of the convexity of the tonsil’s medial periphery. The depth (c) was measured between the anterior and posterior airway indentation into the lateral pharyngeal wall.
Additionally, for further validation, the tonsillar volume was further quantified and confirmed utilizing formula V = thickness × surface area [23]. Using the CBCT anatomical landmarks in the method described above, the surface area of the tonsils (right and left) was measured using area measurement tool in InVivo 6 corresponding to maximum hypertrophy. Please refer to Fig. 1 (D, E) for the methodology of measuring the tonsils.
2.3. Statistical analysis
We used ICC (intraclass correlation coefficient) for evaluating inter-rater reliability between the two independent investigators. Paired t-test was used to evaluate the differences in PSQ and volume between the initial and final adenoid and tonsil measurements for both groups. Statistical significance was set at 0.05 (p < 0.05).
2.4. Ethics
This study was approved by the Institutional Review Board of Stanford University (#59392).
3. Results
A total of 60 patients were included and divided into the control (n = 20) and expansion (n = 40) groups. The patient demographic is shown in Table 1. The average age of the control group was 7.85 years old while the average age of the expansion group was 8.08 years old. There was no statistically significant difference in age and sex between control and expansion groups. The mean body mass index (BMI) among the cohort was 17.37 ± 3.10 kg/m2 and the mean BMI-for-age percentile was 58.57 ± 29.55%. The control group was composed of 13 females and 7 males and the expansion group was composed of 19 females and 21 males. An excellent interrater reliability was found with ICC greater than 0.9 between the two independent investigators. In addition, there was an excellent correlation between the two independent methods for palatine tonsil measurement, validating our methodology used for this study (R > 0.8).
Table 1.
Patient demographics.
| Patient Demographics | ||||
|---|---|---|---|---|
| All | Study Group | Control | ||
| Gender | Male | 28 (46.7%) | 21 (52.5%) | 7 (35.0%) |
| Female | 32 (53.3%) | 19 (47.5%) | 13 (65.0%) | |
| Age (y) | Mean | 8.00 | 8.08 | 7.85 |
| Std dev | 2.20 | 1.99 | 2.62 | |
| Weight (lb) | Mean | 67.03 | 68.04 | 65.83 |
| Std dev | 28.89 | 29.47 | 28.88 | |
| Height (inches) | Mean | 51.00 | 51.92 | 49.90 |
| Std dev | 6.90 | 6.55 | 7.31 | |
| BMI (kg/m2) | Mean | 17.37 | 16.98 | 17.85 |
| Std dev | 3.10 | 2.73 | 3.50 | |
| Percentile | Mean | 58.57 | 54.58 | 63.35 |
| Std dev | 29.55 | 26.75 | 32.64 | |
| Ethnicity | White | 20 (33.3%) | 18 (45.0%) | 2 (10.0%) |
| Hispanic | 20 (33.3%) | 9 (22.5%) | 11 (55.0%) | |
| Asian | 15 (25.0%) | 9 (22.5%) | 6 (30.0%) | |
| Black | 3 (5.0%) | 2 (5.0%) | 1 (5.0%) | |
| Persian | 2 (3.3%) | 2 (5.0%) | 0 (0.0%) | |
There was a significant reduction in the PSQ scores from 5.81 ± 3.31 to 3.75 ± 2.38 in expansion group (p < 0.001). The expansion group, upon visualization, revealed adenoid and tonsil size decrease (Fig. 2). Compared to the control group, the expansion group experienced a statistically significant decrease in both adenoid (p < 0.001) and tonsil volume (p < 0.001) (Tables 2 and 3). Overall, within the expansion group, the average volume reduction of adenoids was 16.8% (486.9 mm3) and that of tonsils was 38.5% (2470.0 mm3) (Fig. 3). Thirty-six out of forty patients (90.0%) in the expansion group showed a reduction in adenoid volume. Of these responders, the average volume reduction reached 20.1%, with the greatest percentage reduction being 51.6%. Thirty-nine out of forty patients (97.5%) in the expansion group showed tonsil volume reduction. Of these responders, the average volume percent decrease was 40.2%, with the greatest percentage decrease being 75.4%. There was no statistically significant difference in age and sex between responders and non-responders. Compared to the expansion group, the control group experienced an average increase of 6.4% (131.2 mm3) and 24.8% (913.6 mm3) in adenoid and tonsil size, respectively (Table 2). However, these changes were not statistically significant (Fig. 3).
Fig. 2.
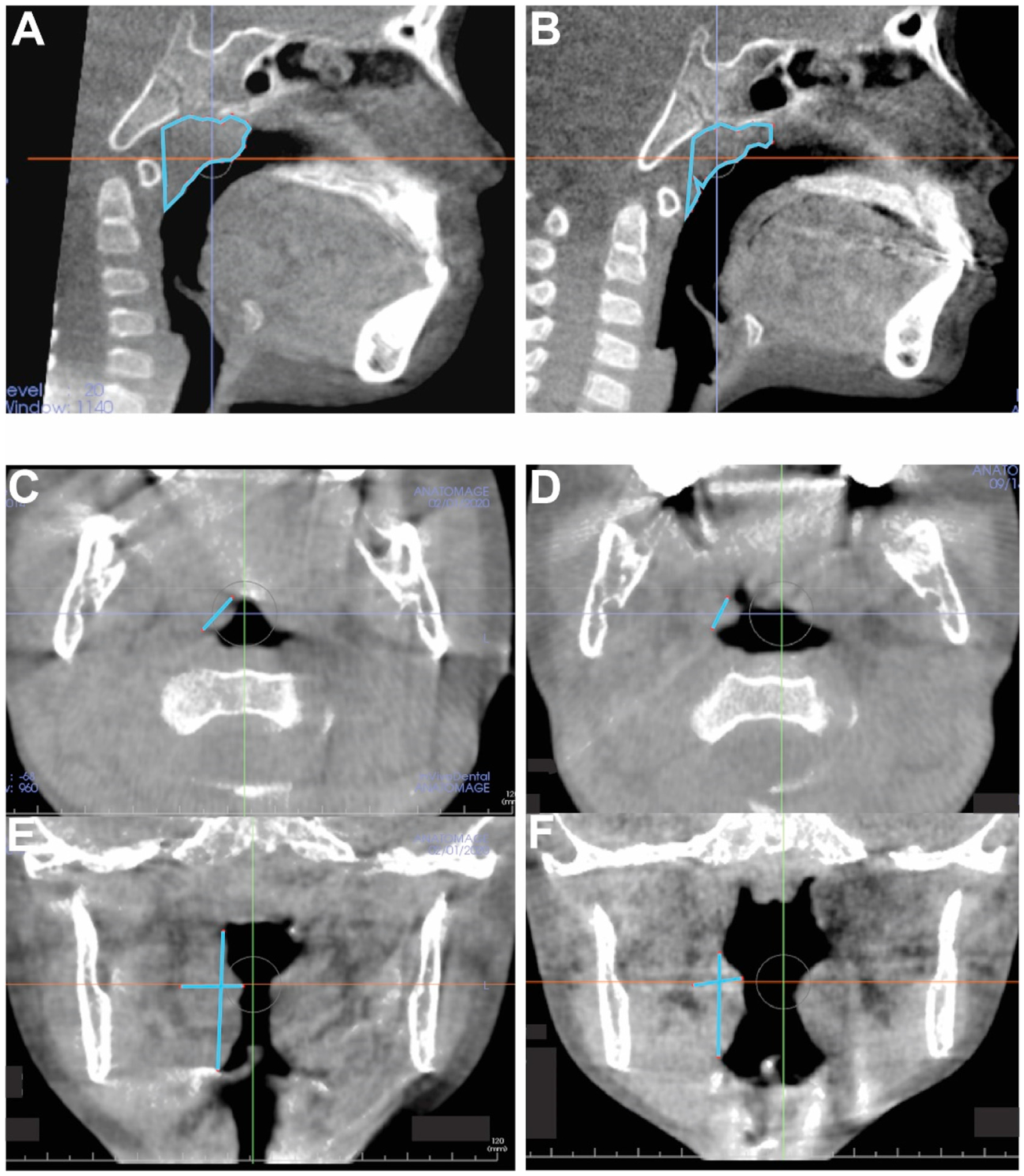
Representative CBCT sagittal slices of T1 (A) and T2 (B) for adenoid volume changes. CBCT axial slices of T1 (C) and T2 (D) and coronal slices of T1 (E) and T2 (F) for palatine tonsil volume changes.
Table 2.
Control group: evaluating adenoid and tonsil size using CBCT.
| Control Group Adenoid and Tonsil Size | ||||||
|---|---|---|---|---|---|---|
| Adenoid | Tonsil | |||||
| T1 | T2 | Δ | T1 | T2 | Δ | |
| Average (mm3) | 2655.12 | 2786.28 | 131.16 | 4916.34 | 5829.93 | 913.59 |
| SD | 1173.53 | 1195.44 | 365.79 | 2541.29 | 2778.69 | 2015.64 |
| Minimum Value | 1195.32 | 667.62 | −613.75 | 1756.43 | 2206.25 | −5161.91 |
| Maximum Value | 5131.96 | 5105.42 | 909.84 | 11313.67 | 11534.00 | 5846.38 |
| P-Value | 0.13 | 0.06 | ||||
Table 3.
Expansion group: evaluating adenoid and tonsil size using CBCT.
| Expansion Group Adenoid and Tonsil Size | ||||||
|---|---|---|---|---|---|---|
| Adenoid | Tonsil | |||||
| T1 | T2 | Δ | T1 | T2 | Δ | |
| Average (mm3) | 2728.27 | 2241.34 | −486.93 | 6001.65 | 3531.65 | −2470.01 |
| SD | 922.96 | 887.66 | 499.67 | 2276.04 | 1287.05 | 1909.27 |
| Minimum Value | 986.66 | 1096.51 | −1879.92 | 3211.32 | 1614.56 | −8083.46 |
| Maximum Value | 4051.85 | 4257.00 | 509.20 | 13974.37 | 7133.75 | 1855.87 |
| P-Value | <0.001 | <0.001 | ||||
Fig. 3.
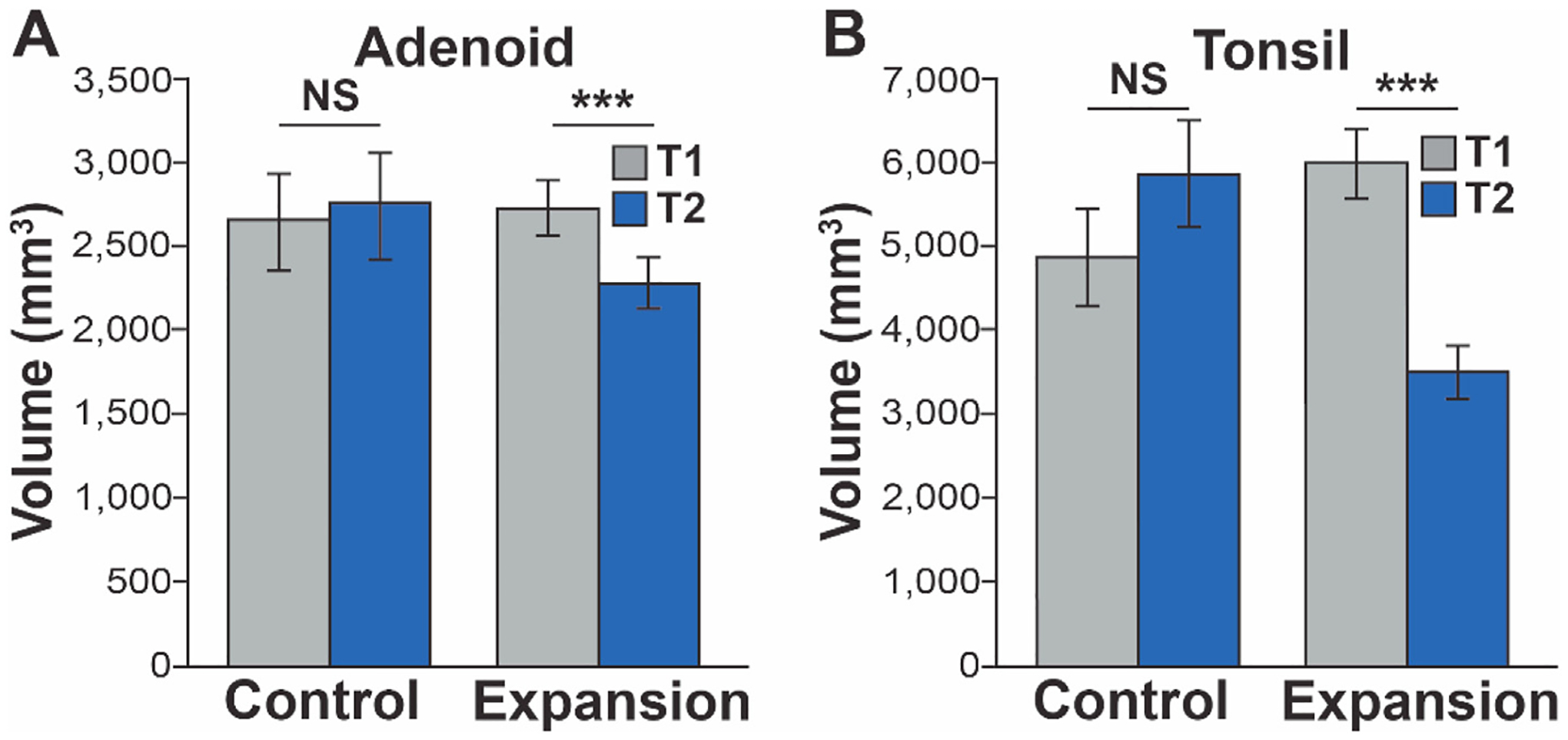
Adenoid (A) and tonsil (B) volumetric measurements at (T1) and (T2) in both groups.
4. Discussion
In children with OSA and narrow maxillae, RPE can be an effective treatment option [24]. A recent meta-analysis showed that RPE leads to 70% AHI reduction and improvement in the lowest O2 saturation to up to 5.7% in children with OSA [25]. The otolaryngology and orthodontic literature is sparse in reviewing changes in the airway lymphoid tissue after maxillary expansion [24,26,27]. In the study by Villa et al. authors discussed their observation of reduction in the size of tonsils and adenoids following RPE, however, they didn’t report objective measurements for these changes [28]. Our study demonstrated that 90.0% of patients, who underwent RPE, had a significant reduction in adenoid volume and 97.5% of patients had a reduction in palatine tonsil volume. Moreover, to our knowledge this is the first study to use low-radiation cone beam CT scans for volumetric assessment of soft tissue structures in the head and neck. This study also validated 3D radiological tools for measuring adenoids and tonsils.
The significant reduction in the volume of the adenoids and tonsils after RPE treatment can be attributed to the increase in nasal volume, particularly at the level of the internal nasal valve. This nasal volume expansion is known to decrease air velocity and resistance in nasal cavity [29], thereby improving the irritation of the lymphoid tissue. In addition, the improved nasal airflow results in the generation of lower sub-atmospheric inspiratory pressures which lessens the collapsibility of pharyngeal airway [11]. This enhancement in pharyngeal airway may also contribute to alleviating the irritation of the airway-associated lymphoid tissues. Furthermore, RPE treatment creates expanded oral cavity volume that helps in correcting the tongue posture and therefore, the retroglossal dimensions [14]. Lastly, the expansion of the posterior aspect of the maxillae may exert direct effects on soft palate tension and function. Overall, RPE reduces the collapsibility of upper airway and reduces inflammation.
Moreover, it is often observed that patients who underwent RPE experience improvements in nasal obstruction potentially leading to nasal breathing restoration [12]. The nasal passage is responsible for filtering, warming and humidifying the inspired air. Filtration, warming and humidification is achieved within channels formed by the turbinates of the nasal cavities [30]. Nasal breathing facilitates inhalation of nitric oxide and helps prevent infections as well as allergic reaction. In mouth breathers, foreign antigens presented to immune cells of the tonsils through the oral cavity trigger an immune response that subsequently initiates tonsillar hypertrophy given the lack of the nasal protective mechanisms [31]. Thus, with nasal breathing restoration after RPE, this mechanism may be reversed. Another potential explanation for adenoidal hypertrophy in constricted maxillae is the limited nasal airflow resulting in chronic hypoxemia irritating the lymphoid tissues [32]. Therefore, after RPE, the improved nasal airflow would reduce inflammation and irritation of the adenoids, alleviating adenoidal hypertrophy.
Previous studies that assessed age-related changes in the size of lymphoid tissues reported that the size of adenotonsillar tissues do not decrease in snoring children over 8 years compared to the age-matched non-snoring children [33]. In addition, the oropharyngeal lumen does not widen and nasopharyngeal lumen remains significantly narrower in snoring children when compared to the non-snoring population. Furthermore, amongst children who snore, it was found that there is a significant discrepancy in their maxillary width when compared to non-snoring children that is attributed to predominant lymphoid hypertrophy [34]. Therefore, children especially, those over 8 years with SDB, may benefit greatly from RPE treatment which leads to widening of oral and nasal cavity.
One of our study limitations is measuring soft tissue with CBCT as it has poor contrast resolution; therefore, we utilized hard tissue landmarks which can be consistently and reproducibly visualized. Given the landmarks used for the adenoid volume, it is possible that the total volume was overestimated by including additional soft tissue. Thus, it is possible that there was an even greater reduction in adenoid tissue itself than what we were able to discern from the data. Future studies can include MRI imaging for better soft tissue resolution to assess adenoid volume changes following RPE.
While age and sex were matched between the control and expansion groups, the outcomes of this study are also limited by the sample size and the lack of ethnic matching between expansion and control groups. Also in this cohort, patients were mainly receiving the RPE treatment for orthodontic corrections and not necessarily for the purpose of improving naso-respiratory blockage or sleep apnea. Future studies can attempt RPE in patients who explicitly present with naso-respiratory symptoms.
In the expansion group, one patient out of the forty did not show shrinkage in the tonsil size after RPE. Instead, the volume increased by 35.16%. Similarly, another four patients showed an increase in their adenoid volume by an average of 21.47%. There was no statistically significant difference between responders and non-responders in age and sex. We have conducted this study in children with significantly enlarged adenoids and tonsils and it is not unusual that these children have used medications including nasal sprays or anti-inflammatory medications during orthodontic treatment. We were not able to track daily usage of patient’s allergy medication and we couldn’t collect the complex variables such as the presence of infection or allergy and the exposure of irritants at each time point. In addition, genetic individual susceptibility may add the complexity to our results. Further studies using a larger population are needed in order to explore the genotypes of population who are associated with reduction in tonsil and adenoid volume.
5. Conclusion
This study demonstrated that RPE led to a significant reduction in the size of adenoids and palatine tonsils, revealing another long-term benefit of RPE treatment. To our knowledge, this is the first study to establish the methodology for CBCT volumetric analysis of adenoids and tonsils and capture the volumetric changes following RPE. Our results show the potential role of RPE treatment in reducing the size of the upper airway lymphoid tissues for the pediatric OSA population with narrow high arch palate and adenotonsillar hypertrophy.
Acknowledgements
We thank Angela Chan and Alex Jun for collecting patient’s data and Jinsook Suh and Megan Do for assisting with measurement softwares. We also thank Heeyeon Suh for statistics.
Financial support
This work was supported by NIH/NIDCR grant K08DE024603. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of interest
The Authors declare that they have no conflict of interest.
The ICMJE Uniform Disclosure Form for Potential Conflicts of Interest associated with this article can be viewed by clicking on the following link: https://doi.org/10.1016/j.sleep.2022.02.011.
All authors have seen and approved the manuscript.
References
- [1].Yonkers AJ, Spaur RC. Upper airway obstruction and the pharyngeal lymphoid tissue. Otolaryngol Clin 1987;20(2):235–9. [PubMed] [Google Scholar]
- [2].Pereira L, Monyror J, Almeida FT, et al. Prevalence of adenoid hypertrophy: a systematic review and meta-analysis. Sleep Med Rev 2018;38:101–12. [DOI] [PubMed] [Google Scholar]
- [3].Kara CO, Ergin H, Kocak G, et al. Prevalence of tonsillar hypertrophy and associated oropharyngeal symptoms in primary school children in Denizli, Turkey. Int J Pediatr Otorhinolaryngol 2002;66(2):175–9. [DOI] [PubMed] [Google Scholar]
- [4].Evcimik MF, Dogru M, Cirik AA, et al. Adenoid hypertrophy in children with allergic disease and influential factors. Int J Pediatr Otorhinolaryngol 2015;79(5):694–7. [DOI] [PubMed] [Google Scholar]
- [5].Li Z, Celestin J, Lockey RF. Pediatric sleep apnea syndrome: an update. J Allergy Clin Immunol Pract 2016;4(5):852–61. [DOI] [PubMed] [Google Scholar]
- [6].Perfect MM, Archbold K, Goodwin JL, et al. Risk of behavioral and adaptive functioning difficulties in youth with previous and current sleep disordered breathing. Sleep 2013;36(4):517–525B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bourke R, Anderson V, Yang JS, et al. Cognitive and academic functions are impaired in children with all severities of sleep-disordered breathing. Sleep Med 2011;12(5):489–96. [DOI] [PubMed] [Google Scholar]
- [8].Praud JP, Dorion D. Obstructive sleep disordered breathing in children: beyond adenotonsillectomy. Pediatr Pulmonol 2008;43(9):837–43. [DOI] [PubMed] [Google Scholar]
- [9].Mitchell RB, Archer SM, Ishman SL, et al. Clinical practice guideline: tonsillectomy in children (Update)-Executive summary. Otolaryngol Head Neck Surg 2019;160(2):187–205. [DOI] [PubMed] [Google Scholar]
- [10].Section on Pediatric Pulmonology SoOSASAAoP. Clinical practice guideline: diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics 2002;109(4):704–12. [DOI] [PubMed] [Google Scholar]
- [11].Kikuchi M Orthodontic treatment in children to prevent sleep-disordered breathing in adulthood. Sleep Breath 2005;9(4):146–58. [DOI] [PubMed] [Google Scholar]
- [12].Yoon A, Abdelwahab M, Liu S, et al. Impact of rapid palatal expansion on the internal nasal valve and obstructive nasal symptoms in children. Sleep Breath 2021;25(2):1019–27. [DOI] [PubMed] [Google Scholar]
- [13].Abdelwahab M, Yoon A, Okland T, et al. Impact of distraction osteogenesis maxillary expansion on the internal nasal valve in obstructive sleep apnea. Otolaryngology–head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery 2019;161(2):362–7. [DOI] [PubMed] [Google Scholar]
- [14].Iwasaki T, Saitoh I, Takemoto Y, et al. Tongue posture improvement and pharyngeal airway enlargement as secondary effects of rapid maxillary expansion: a cone-beam computed tomography study. Am J Orthod Dentofacial Orthop 2013;143(2):235–45. [DOI] [PubMed] [Google Scholar]
- [15].Pirelli P, Saponara M, Guilleminault C. Rapid maxillary expansion (RME) for pediatric obstructive sleep apnea: a 12-year follow-up. Sleep Med 2015;16(8): 933–5. [DOI] [PubMed] [Google Scholar]
- [16].Scarfe WC, Farman AG. What is cone-beam CT and how does it work? Dent Clin 2008;52(4):707–30 [v]. [DOI] [PubMed] [Google Scholar]
- [17].Ludlow JB, Davies-Ludlow LE, Brooks SL, et al. Dosimetry of 3 CBCT devices for oral and maxillofacial radiology: CB Mercuray, NewTom 3G and i-CAT. Dentomaxillofacial Radiol 2006;35(4):219–26. [DOI] [PubMed] [Google Scholar]
- [18].Bayram M, Kayipmaz S, Sezgin OS, et al. Volumetric analysis of the mandibular condyle using cone beam computed tomography. Eur J Radiol 2012;81(8): 1812–6. [DOI] [PubMed] [Google Scholar]
- [19].Mazonakis M, Damilakis J, Maris T, et al. Comparison of two volumetric techniques for estimating liver volume using magnetic resonance imaging. J Magn Reson Imag 2002;15(5):557–63. [DOI] [PubMed] [Google Scholar]
- [20].Sahin B, Alper T, Kokcu A, et al. Estimation of the amniotic fluid volume using the Cavalieri method on ultrasound images. Int J Gynaecol Obstet 2003;82(1): 25–30. [DOI] [PubMed] [Google Scholar]
- [21].Acer N, Sahin B, Bas O, et al. Comparison of three methods for the estimation of total intracranial volume: stereologic, planimetric, and anthropometric approaches. Ann Plast Surg 2007;58(1):48–53. [DOI] [PubMed] [Google Scholar]
- [22].Prim MP, De Diego JI, Garcia-Bermudez C, et al. A method to calculate the volume of palatine tonsils. Anat Rec 2010;293(12):2144–6. [DOI] [PubMed] [Google Scholar]
- [23].Feczko E, Augustinack JC, Fischl B, et al. An MRI-based method for measuring volume, thickness and surface area of entorhinal, perirhinal, and posterior parahippocampal cortex. Neurobiol Aging 2009;30(3):420–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Machado-Junior AJ, Zancanella E, Crespo AN. Rapid maxillary expansion and obstructive sleep apnea: a review and meta-analysis. Med Oral Patol Oral Cir Bucal 2016;21(4):e465–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Camacho M, Chang ET, Song SA, et al. Rapid maxillary expansion for pediatric obstructive sleep apnea: a systematic review and meta-analysis. Laryngoscope 2017;127(7):1712–9. [DOI] [PubMed] [Google Scholar]
- [26].Marcus CL, Brooks LJ, Draper KA, et al. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics 2012;130(3):576–84. [DOI] [PubMed] [Google Scholar]
- [27].Owens J, Opipari L, Nobile C, et al. Sleep and daytime behavior in children with obstructive sleep apnea and behavioral sleep disorders. Pediatrics 1998;102(5):1178–84. [DOI] [PubMed] [Google Scholar]
- [28].Villa MP, Malagola C, Pagani J, et al. Rapid maxillary expansion in children with obstructive sleep apnea syndrome: 12-month follow-up. Sleep Med 2007;8(2):128–34. [DOI] [PubMed] [Google Scholar]
- [29].Iwasaki T, Yoon A, Guilleminault C, et al. How does distraction osteogenesis maxillary expansion (DOME) reduce severity of obstructive sleep apnea? Sleep Breath 2020;24(1):287–96. [DOI] [PubMed] [Google Scholar]
- [30].Kevin J, Sullivan HKC. Flow dynamics of the nasal passage. 1990.
- [31].Heimroth RD, Casadei E, Salinas I. Molecular drivers of lymphocyte organization in vertebrate mucosal surfaces: revisiting the TNF superfamily hypothesis. J Immunol 2020;204(10):2697–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Cistulli PA, Palmisano RG, Poole MD. Treatment of obstructive sleep apnea syndrome by rapid maxillary expansion. Sleep 1998;21(8):831–5. [DOI] [PubMed] [Google Scholar]
- [33].Papaioannou G, Kambas I, Tsaoussoglou M, et al. Age-dependent changes in the size of adenotonsillar tissue in childhood: implications for sleep-disordered breathing. J Pediatr 2013;162(2):269–274 e264. [DOI] [PubMed] [Google Scholar]
- [34].Hultcrantz E, Lofstrand-Tidestrom B, Ahlquist-Rastad J. The epidemiology of sleep related breathing disorder in children. Int J Pediatr Otorhinolaryngol 1995;32(Suppl):S63–6. [DOI] [PubMed] [Google Scholar]


