Abstract
As there is a continuous need for novel anti-infectives, the present study aimed to fuse two modes of action into a novel 3-nitroimidazo[1,2-b]pyridazine scaffold to improve antiparasitic efficacy. For this purpose, we combined known structural elements of phosphodiesterase inhibitors, a target recently proposed for Trypanosoma brucei and Giardia lamblia, with a nitroimidazole scaffold to generate nitrosative stress. The compounds were evaluated in vitro against a panel of protozoal parasites, namely Giardia lamblia, Trypanosoma brucei, T. cruzi, Leishmania infantum and Plasmodium falciparum and for cytotoxicity on MRC-5 cells. Interestingly, selective sub-nanomolar activity was obtained against G. lamblia, and by testing several analogues with and without the nitro group, it was shown that the presence of a nitro group, but not PDE inhibition, is responsible for the low IC50 values of these novel compounds. Adding the favourable drug-like properties (low molecular weight, cLogP (1.2–4.1) and low polar surface area), the key compounds from the 3-nitroimidazo[1,2-b]pyridazine series can be considered as valuable hits for further anti-giardiasis drug exploration and development.
Keywords: 3-nitroimidazo[1,2-b]pyridazine; Synthesis; Giardia lamblia; 3′,5′-cyclic nucleotide phosphodiesterase; In vitro
Graphical abstract
Highlights
-
•
Analogues fusing a nitroimidazole moiety and a PDE inhibitor scaffold were prepared.
-
•
These compounds were tested in vitro against a panel of protozoal parasites.
-
•
Against Giardia lamblia, sub-nanomolar IC50 values were determined.
-
•
PDE inhibition provided no significant contribution to the anti-Giardia potency.
-
•
High potency with drug-like properties warrants further study of this hit series.
1. Introduction
Antibiotic, antiviral, antifungal and antiprotozoal resistance is on the rise worldwide and poses a formidable challenge in achieving a Universal Health Coverage, as highlighted in a recent report from the Interagency Coordination Group on Antimicrobial resistance to the UN Secretary-General. In a worst-case scenario, this is projected to lead to 10 million deaths annually by 2050 (World Health Organisation, 2019). As such, there is a continuous need for novel antimicrobials, which are not only valid for bacterial infections but also for parasitic diseases. An estimated disease burden for sixteen of the most common parasitic infections, most of them on the WHO list of neglected tropical diseases, came to about 3 billion cases with 1 out of every 6 persons worldwide experiencing 1 or more infections each year. In DALYs (Disability Adjusted Life Years), this adds up to 25 million with over 500,000 deaths annually (Herricks et al., 2017). The high disease burden combined with the threat of drug resistance calls for urgent and continuous drug discovery efforts for parasitic diseases (Cui et al., 2015; Nabarro et al., 2015; Vanaerschot et al., 2014).
Heterocyclic aromatic nitro-compounds (imidazoles, furans and thiazoles, Fig. 1) are known for their potential in treating parasitic infections and their mode-of-action is considered to be the radical damage caused by the reactive and toxic species that are obtained from reduction of the nitro group by parasitic reductases (Ang et al., 2017). Benznidazole (1) and nifurtimox (2) are in clinical use for Chagas disease (Ribeiro et al., 2020). Fexinidazole (3) and nifurtimox combination with eflornithine (NECT), are used for treating the African sleeping sickness (de Koning, 2020) while metronidazole (4), tinidazole (5) and nitazoxanide (6) have a role in the treatment of giardiasis (Mørch and Hanevik, 2020). The compound DNDi-VL-2098 (7) is in development for visceral leishmaniasis (Gupta et al., 2015), while nitroimidazopyridazines (8) have been reported to be effective against a broad range of parasites growing under anaerobic or semi aerobic conditions (Tomcufcik et al., 1974).
Fig. 1.
Nitro-group containing heterocyclic aromatic compounds with antiparasitic activities.
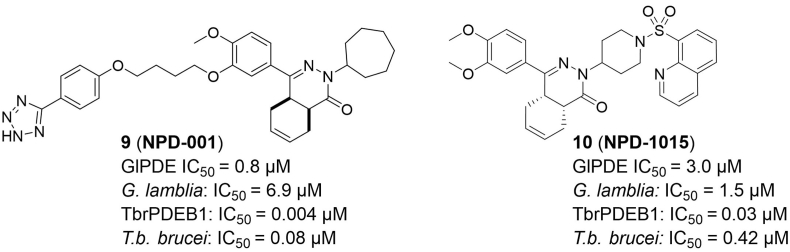
The 3′,5′-cyclic nucleotide phosphodiesterases (PDEs) have a critical role in cellular signal transduction: they hydrolyse the second messengers cyclic adenosine-3′,5′-monophosphate (cAMP) and cyclic guanosine-3′,5′-monophosphate (cGMP) to AMP and GMP, respectively. So far, eleven families of class-I PDEs were found (Maurice et al., 2014) and have been described as potential targets for the treatment of several parasitic diseases such as malaria (Howard et al., 2015) and Chagas disease (Sharon et al., 2010). Moreover, this class of enzymes has been proposed as potential therapeutic targets for sleeping sickness (de Koning et al., 2012; Oberholzer et al., 2007) and more recently also for giardiasis (Kunz et al., 2017a). For these last two parasitic diseases, tetrahydrophthalazinones were identified as an interesting hit series (9 and 10, Fig. 2), and initial hit optimization strategies towards parasitic PDE-selective compounds have been reported (Blaazer et al., 2018; Kunz et al., 2017a).
Fig. 2.
Tetrahydrophthalazinones 9, 10 and their reported IC50 values against G. lamblia and T. b. brucei and relevant parasitic PDEs (Blaazer et al., 2018; Kunz et al., 2017b).
Previous studies have shown hybrid antimalarial compounds as a promising strategy to overcome the emergence of resistant parasite strains (Oliveira et al., 2015). Moreover, nitro-drugs have been reported to suffer from parasite resistance (Patterson and Wyllie, 2014), whereas resistance for hybrid anti-parasitic drugs has not been reported for PDE inhibitor scaffolds so far. In this study, we hypothesized that by merging a tetrahydrophthalazinone scaffold with a nitroimidazopyridazine (8), compounds with a putative dual mode-of-action could be generated, making them potentially more effective against a range of parasites. To test this hypothesis, we synthesized a series of hybrid molecules that were tested against the protozoal parasites including G. lamblia, T. brucei, T. cruzi, L. infantum and P. falciparum.
2. Materials and methods
2.1. Synthetic procedures
All chemicals were obtained from commercial suppliers without further purification. Reaction progress was monitored using thin-layer chromatography (TLC) and LC-MS analysis. For TLC analysis, Merck F254 alumina silica plates were used and visualized using UV. Silicycle UltraPure silica gel was used for manual purification columns. Automatic columns were performed using Biotage equipment. LC-MS analysis was performed on a Shimadzu LC-20AD liquid chromatograph pump system, equipped with an Xbridge (C18) 5 μm column (50 mm, 4.6 mm), connected to a Shimadzu SPD-M20A diode array detector, and MS detection using a Shimadzu LC-MS-2010EV mass spectrometer. The LC-MS conditions were as follows: solvent B (acetonitrile with 0.1% formic acid) and solvent A (water with 0.1% formic acid), flow rate of 1.0 mL/min, start with 5% B, linear gradient to 90% B in 4.5 min, then 1.5 min at 90% B, then linear gradient to 5% B in 0.5 min, then 1.5 min at 5% B; total run time of 8 min. Reverse-phase column chromatography purifications were performed using Buchi PrepChrom C-700 equipment with a discharge deuterium lamp ranging from 200 to 600 nm to detect compounds using solvent B (acetonitrile with 0.1% formic acid), solvent A (water with 0.1% formic acid), flow rate of 15.0 mL/min, and a gradient (start 95% A for 3.36 min, then linear gradient to 5% A in 30 min, then at 5% A for 3.36 min, then linear gradient to 95% A in 0.5 min, and then 1.5 min at 95% A). The purity of a compound was determined by calculating the peak area percentage by UV detection at 254 nm and was in all cases >95% except for some intermediates (indicated in the characterization). A Bruker 500 or 600 MHz spectrometer was used to record 1H, 13C and 2D NMR spectra. Chemical shifts (δ in ppm) and coupling constants (J in Hz) are reported with residual solvent as internal standard (δ 1H NMR, CDCl3 7.26, CD2Cl2 5.32, DMSO-d6 2.50; δ 13C NMR, CDCl3 77.16, CD2Cl2 53.84, DMSO-d6 39.52). Abbreviations used for 1H NMR descriptions are as follows: s = singlet, d = doublet, t = triplet, q = quintet, hept = heptet, dd = doublet of doublets, dt = doublet of triplets, tt = triplet of triplets, m = multiplet, app = apparent, br = broad signal. For HRMS analysis, a Bruker micrOTOF mass spectrometer was used using ESI in positive ion mode. All reactions were carried out under an inert nitrogen atmosphere.
2.1.1. General methods
Method A: A mixture of 6-chloro-3-nitroimidazo[1,2-b]pyridazine (2.1 mmol, 0.42 g), an amine (2.0 mmol) and K2CO3 (4.7 mmol, 0.65 g) in DMF (20 mL) was heated for 2–24 h at 60 °C after which the mixture was poured into water. The aqueous mixture was extracted with DCM and the combined organic layers were dried over MgSO4 and evaporated. The residue was purified by silica gel column chromatography (eluents of EtOAc and n-heptane) or reverse phase chromatography.
Method B: A mixture of the benzyl chloride (1.0 mmol), 12c (1.0 mmol, 0.52 g) and K2CO3 (2.0 mmol, 0.28 g) in DMF (15 mL) was heated for 4 h at 60 °C, after which the mixture was poured into water. The aqueous mixture was extracted with DCM and the combined organic layers were dried over MgSO4 and evaporated. The residue was purified by silica gel column chromatography (eluents of EtOAc and n-heptane).
2.1.1.1. The intermediates are presented as supplemental data
cis-6-(3,4-Dimethoxybenzoyl)cyclohex-3-ene-1-carboxylic acid (11a) and (1R,6S)-6-(3,4-dimethoxybenzoyl)cyclohex-3-ene-1-carboxylic acid (11a’) Prepared as described (van der Mey et al., 2002).
(4aS,8aR)-4-(3,4-Dimethoxyphenyl)-2-(piperidin-4-yl)-4a,5,8,8a-tetrahydrophthalazin-1(2H)-one·HCl (11b) Prepared as described (Sterk et al., 2004a).
cis-6-(3-(Cyclopentyloxy)-4-methoxybenzoyl)cyclohex-3-ene-1-carboxylic acid (12a) Prepared as described (van der Mey et al., 2002).
cis-4-(3-(Cyclopentyloxy)-4-methoxyphenyl)-2-(piperidin-4-yl)-4a,5,8,8a-tetrahydrophthalazin-1(2H)-one·HCl (12b) Prepared from 12a (20 mmol, 6.9 g) and 4-hydrazinopiperidine·2HCl (20 mmol, 3.8 g) as described previously (van der Mey et al., 2002). Yield: 74%; LC-MS-ESI+ m/z 424 [M+H]+; purity 92%.
cis-4-(3-Hydroxy-4-methoxyphenyl)-2-(1-(3-nitroimidazo[1,2-b]pyridazin-6-yl)piperidin-4-yl)-4a,5,8,8a-tetrahydrophthalazin-1(2H)-one (12c) Prepared from 12 by hydrolysing the cyclopentyl ether with 4-toluene sulfonic acid in a Dean-Stark apparatus as described previously (van der Mey et al., 2002). Yield 74%; LC-MS-ESI+ m/z 518 [M+H]+; purity 94%.
4-(3,4-Dimethoxyphenyl)-4-oxobutanoic acid (18a) Prepared as described (van der Mey et al., 2001).
6-(3,4-Dimethoxyphenyl)-2-(piperidin-4-yl)-4,5-dihydropyridazin-3(2H)-one·HCl (18b) Prepared as described (Sterk et al., 2004b).
4-(3-Chloro-4-methoxyphenyl)-4-oxobutanoic acid (19a) Prepared from 2-chloroanisole and succinic anhydride as described for the 3,4-dimethoxy analogue (van der Mey et al., 2002). Yield: 84%; LC-MS-ESI+ m/z 243 [M+H]+; purity 94%.
6-(3-Chloro-4-methoxyphenyl)-2-(piperidin-4-yl)-4,5-dihydropyridazin-3(2H)-one·HCl (19b) Prepared from 4-(3-chloro-4-methoxyphenyl)-4-oxobutanoic acid and 4-hydrazinopiperidine·2HCl as described for the 3,4-dimethoxy analogue (Sterk et al., 2004a); Yield: 67%; LC-MS-ESI+ m/z 322 [M+H]+; purity 91%.
2.1.1.2. Final compounds (chemical analysis spectra shown in Figs. S1–S66)
(4aS,8aR)-4-(3,4-Dimethoxyphenyl)-2-(1-(3-nitroimidazo[1,2-b]pyridazin-6-yl)piperidin-4-yl)-4a,5,8,8a-tetrahydrophthalazin-1(2H)-one (11) Prepared from 11b (2.0 mmol, 0.81 g) by method A; Yield 72%. 1H NMR (500 MHz, CDCl3) δ 8.39 (s, 1H), 7.85 (d, J = 10.0 Hz, 1H), 7.39–7.35 (m, 1H), 7.29–7.24 (m, 1H), 7.16 (d, J = 10.1 Hz, 1H), 6.85 (d, J = 8.4 Hz, 1H), 5.81 (app d, J = 9.9 Hz, 1H), 5.70 (app d, J = 10.0 Hz, 1H), 4.96 (tt, J = 11.4, 3.9 Hz, 1H), 4.53–4.39 (m, 2H), 3.90 (s, 3H), 3.82 (s, 3H), 3.37–3.30 (m, 1H), 3.25–3.12 (m, 2H), 3.06–2.98 (m, 1H), 2.80 (app t, J = 5.8 Hz, 1H), 2.32–2.16 (m, 3H), 2.10–1.95 (m, 2H), 1.91–1.84 (m, 2H). 13C NMR (126 MHz, CDCl3) δ 167.1, 155.7, 154.5, 151.0, 149.3, 137.7, 134.5, 134.3, 127.7, 126.5, 126.1, 124.0, 119.4, 113.9, 110.7, 108.4, 56.1, 56.0, 52.4, 45.61, 45.57, 34.9, 31.3, 29.5, 28.8, 23.5, 22.5. HR-MS: calc. for [M+H]+. 532.2303, found 532.2311.
cis-4-(3-(Cyclopentyloxy)-4-methoxyphenyl)-2-(1-(3-nitroimidazo[1,2-b]pyridazin-6-yl)piperidin-4-yl)-4a,5,8,8a-tetrahydrophthalazin-1(2H)-one (12) Prepared from 12b (2.2 mmol, 0.92 g) by method A. Purified by crystallization from MeOH. Yield: 84%; 1H NMR (500 MHz, CDCl3) δ 8.40 (s, 1H), 7.90 (d, J = 10.1 Hz, 1H), 7.36 (d, J = 2.0 Hz, 1H), 7.26–7.22 (m, 1H), 7.19 (d, J = 10.1 Hz, 1H), 6.84 (d, J = 8.4 Hz, 1H), 5.84–5.78 (m, 1H), 5.76–5.66 (m, 1H), 4.97 (tt, J = 11.6, 4.2 Hz, 1H), 4.77–4.69 (m, 1H), 4.52–4.40 (m, 2H), 3.86 (s, 3H), 3.31 (dt, J = 11.6, 5.8 Hz, 1H), 3.27–3.14 (m, 2H), 3.06–2.97 (m, 1H), 2.79 (app t, J = 5.9 Hz, 1H), 2.31–2.15 (m, 3H), 2.11–1.95 (m, 4H), 1.91–1.70 (m, 6H), 1.55–1.42 (m, 2H). 13C NMR (126 MHz, CDCl3) δ 167.1, 155.6, 154.6, 152.1, 147.8, 137.3, 133.4, 127.5, 126.2, 126.1, 124.1, 119.2, 114.1, 112.3, 111.3, 80.9, 56.2, 52.3, 45.6, 34.9, 32.91, 32.88, 31.3, 29.6, 28.9, 24.19, 24.17, 23.5, 22.5. HR-MS: calc. for [M+H]+. 586.2772, found 586.2775.
cis-4-(4-Methoxy-3-((3-nitrobenzyl)oxy)phenyl)-2-(1-(3-nitroimidazo[1,2-b]pyridazin-6-yl)piperidin-4-yl)-4a,5,8,8a-tetrahydrophthalazin-1(2H)-one (13) Prepared from 3-nitrobenzylchloride (1.0 mmol, 0.17 g) by method B; Yield 45%. 1H NMR (500 MHz, CDCl3) δ 8.38 (s, 1H), 8.31 (s, 1H), 8.14 (d, J = 8.1 Hz, 1H), 7.89 (d, J = 10.0 Hz, 1H), 7.76 (d, J = 7.6 Hz, 1H), 7.55 (d, J = 7.9 Hz, 1H), 7.42 (s, 1H), 7.34–7.29 (m, 1H), 7.24 (d, J = 10.1 Hz, 1H), 6.91 (d, J = 8.5 Hz, 1H), 5.81 (app d, J = 9.3 Hz, 1H), 5.70 (app d, J = 9.5 Hz, 1H), 5.23 (s, 2H), 4.94 (ddd, J = 15.2, 11.2, 3.8 Hz, 1H), 4.53–4.40 (m, 2H), 3.95 (s, 3H), 3.29 (dt, J = 11.5, 5.7 Hz, 1H), 3.25–3.10 (m, 2H), 3.02 (app d, J = 17.1 Hz, 1H), 2.78 (app t, J = 5.8 Hz, 1H), 2.33–2.09 (m, 4H), 2.03–1.80 (m, 3H). 13C NMR (126 MHz, CDCl3) δ 167.0, 155.8, 154.0, 151.8, 148.5, 147.7, 139.3, 137.7, 134.5, 134.1, 133.4, 129.8, 127.7, 126.4, 126.1, 123.9, 123.2, 122.3, 120.6, 114.1, 112.0, 111.4, 70.4, 56.2, 52.3, 45.6, 45.5, 34.8, 31.2, 29.6, 28.9, 23.4, 22.4. HR-MS: calc. for [M+H]+. 653.2467, found 653.2477.
cis-4-(4-Methoxy-3-((3-methoxybenzyl)oxy)phenyl)-2-(1-(3-nitroimidazo[1,2-b]pyridazin-6-yl)piperidin-4-yl)-4a,5,8,8a-tetrahydrophthalazin-1(2H)-one (14) Prepared from 3-methoxybenzylchloride (1.0 mmol, 0.16 g) by method B; Yield 51%. 1H NMR (500 MHz, CDCl3) δ 8.39 (s, 1H), 7.93 (d, J = 9.8 Hz, 1H), 7.41 (d, J = 1.9 Hz, 1H), 7.29–7.21 (m, 3H), 7.00–6.93 (m, 2H), 6.88 (d, J = 8.4 Hz, 1H), 6.80 (d, J = 8.0 Hz, 1H), 5.84–5.78 (m, 1H), 5.72–5.66 (m, 1H), 5.17–5.09 (m, 2H), 4.94 (tt, J = 11.4, 4.1 Hz, 1H), 4.55–4.42 (m, 2H), 3.93 (s, 3H), 3.78 (s, 3H), 3.30–3.13 (m, 3H), 3.06–2.98 (m, 1H), 2.77 (app t, J = 6.0 Hz, 1H), 2.28–2.17 (m, 2H), 2.15–2.06 (m, 1H), 2.04–1.78 (m, 4H). 13C NMR (126 MHz, CDCl3) δ 167.1, 160.0, 155.7, 154.2, 151.5, 148.2, 138.6, 137.5, 134.5, 133.7, 129.7, 127.5, 126.3, 126.0, 124.0, 119.7, 119.6, 114.1, 113.5, 113.0, 111.5, 111.1, 71.3, 56.2, 55.4, 52.2, 45.6, 45.5, 34.8, 31.2, 29.6, 28.9, 23.4, 22.4. HR-MS: calc. for [M+H]+. 638.2722, found 638.2731.
cis-4-(4-Methoxy-3-(pyridin-3-ylmethoxy)phenyl)-2-(1-(3-nitroimidazo[1,2-b]pyridazin-6-yl)piperidin-4-yl)-4a,5,8,8a-tetrahydrophthalazin-1(2H)-one (15) Prepared from 3-(chloromethyl)pyridine·HCl (1.0 mmol, 0.16 g) by method B; Yield 42%. 1H NMR (500 MHz, CDCl3) δ 8.71 (s, 1H), 8.56 (d, J = 6.2 Hz, 1H), 8.36 (s, 1H), 7.98 (d, J = 7.9 Hz, 1H), 7.83 (d, J = 10.0 Hz, 1H), 7.51–7.46 (m, 1H), 7.43 (d, J = 2.0 Hz, 1H), 7.32–7.27 (m, 2H), 6.89 (d, J = 8.5 Hz, 1H), 5.83–5.77 (m, 1H), 5.71–5.65 (m, 1H), 5.19 (s, 2H), 4.93 (tt, J = 11.4, 4.2 Hz, 1H), 4.54–4.41 (m, 2H), 3.91 (s, 3H), 3.32–3.26 (m, 1H), 3.23–3.12 (m, 2H), 3.05–2.98 (m, 1H), 2.77 (app t, J = 5.8 Hz, 1H), 2.27–2.11 (m, 3H), 2.06–1.92 (m, 3H), 1.88–1.82 (m, 1H). 13C NMR (126 MHz, CDCl3) δ 167.0, 155.8, 153.9, 151.7, 147.6, 146.8, 146.1, 138.1, 137.9, 134.9, 134.6, 134.3, 127.7, 126.7, 126.1, 124.6, 123.9, 120.7, 114.1, 112.1, 111.4, 68.6, 56.2, 52.4, 45.6, 34.8, 31.2, 29.7, 28.9, 23.4, 22.4. HR-MS: calc. for [M+H]+. 609.2568, found 609.2587.
cis-4-(4-Methoxy-3-((2-methoxybenzyl)oxy)phenyl)-2-(1-(3-nitroimidazo[1,2-b]pyridazin-6-yl)piperidin-4-yl)-4a,5,8,8a-tetrahydrophthalazin-1(2H)-one (16) Prepared from 2-methoxybenzylchloride (1.0 mmol, 0.16 g) by method B; Yield 72%. 1H NMR (500 MHz, CDCl3) δ 8.37 (s, 1H), 7.89 (d, J = 10.1 Hz, 1H), 7.40 (d, J = 7.4 Hz, 1H), 7.35 (s, 1H), 7.32 (d, J = 8.5 Hz, 1H), 7.25–7.16 (m, 2H), 6.92 (app t, J = 7.5 Hz, 1H), 6.90–6.82 (m, 2H), 5.81 (d, J = 9.7 Hz, 1H), 5.69 (d, J = 9.7 Hz, 1H), 5.26–5.17 (m, 2H), 4.93 (tt, J = 11.3, 3.8 Hz, 1H), 4.50 (app d, J = 13.4 Hz, 1H), 4.41 (app d, J = 13.3 Hz, 1H), 3.92 (s, 3H), 3.81 (s, 3H), 3.29–3.23 (m, 1H), 3.23–3.11 (m, 2H), 3.02 (app d, J = 16.7 Hz, 1H), 2.78 (app t, J = 5.8 Hz, 1H), 2.28–2.08 (m, 4H), 2.05–1.80 (m, 3H). 13C NMR (126 MHz, CDCl3) δ 167.0, 156.8, 155.6, 154.4, 151.5, 148.4, 137.5, 134.5, 133.8, 129.1, 128.9, 127.5, 126.3, 126.0, 125.1, 124.1, 120.8, 119.4, 113.8, 111.7, 111.3, 110.4, 66.2, 56.2, 55.6, 52.2, 45.41, 45.39, 34.8, 31.2, 29.5, 28.9, 23.4, 22.4. HR-MS: calc. for [M+H]+. 638.2722, found 638.2737.
cis-4-(4-Methoxy-3-(pyridin-2-ylmethoxy)phenyl)-2-(1-(3-nitroimidazo[1,2-b]pyridazin-6-yl)piperidin-4-yl)-4a,5,8,8a-tetrahydrophthalazin-1(2H)-one (17) Prepared from 2-chloromethylpyridine·HCl (1.0 mmol, 0.16 g) by method B; Yield 62%. 1H NMR (500 MHz, CD2Cl2) δ 8.49 (d, J = 4.7 Hz, 1H), 8.31 (s, 1H), 7.83 (d, J = 10.1 Hz, 1H), 7.74 (app t, J = 6.5 Hz, 1H), 7.54 (d, J = 6.8 Hz, 1H), 7.47 (d, J = 2.0 Hz, 1H), 7.30 (dd, J = 8.5, 2.0 Hz, 1H), 7.23 (app d, J = 10.0 Hz, 2H), 6.89 (d, J = 8.5 Hz, 1H), 5.79–5.74 (m, 1H), 5.71–5.65 (m, 1H), 5.20 (s, 2H), 4.90 (tt, J = 11.5, 4.0 Hz, 1H), 4.52–4.40 (m, 2H), 3.88 (s, 3H), 3.35–3.27 (m, 1H), 3.22–3.10 (m, 2H), 2.97–2.89 (m, 1H), 2.76 (app t, J = 5.9 Hz, 1H), 2.26–2.08 (m, 3H), 2.04–1.89 (m, 3H), 1.84–1.78 (m, 1H). 13C NMR (126 MHz, CD2Cl2) δ 167.1, 157.1, 156.1, 154.1, 151.6, 149.0, 148.3, 138.5, 137.8, 134.9, 134.8, 128.0, 126.9, 126.2, 124.4, 123.3, 122.2, 120.1, 114.2, 111.44, 111.40, 71.6, 56.3, 52.5, 45.8, 45.7, 35.1, 31.3, 29.7, 29.1, 23.5, 22.7. HR-MS: calc. for [M+H]+. 609.2568, found 609.2583.
6-(3,4-Dimethoxyphenyl)-2-(1-(3-nitroimidazo[1,2-b]pyridazin-6-yl)piperidin-4-yl)-4,5-dihydropyridazin-3(2H)-one (18) Prepared from 18b (2.0 mmol, 0.71 g) by method A; Yield 72%. 1H NMR (500 MHz, CDCl3) δ 8.39 (s, 1H), 7.83 (d, J = 10.1 Hz, 1H), 7.32 (d, J = 2.3, 1H), 7.22 (dd, J = 8.4, 2.3 Hz, 1H), 7.16 (d, J = 9.9 Hz, 1H), 6.85 (d, J = 8.5 Hz, 1H), 4.94 (tt, J = 11.6, 4.2 Hz, 1H), 4.46 (d, J = 13.4 Hz, 2H), 3.90 (s, 3H), 3.82 (s, 3H), 3.24–3.13 (m, 3H), 2.97–2.86 (m, 2H), 2.61 (app t, J = 8.2 Hz, 2H), 2.15 (app qd, J = 12.6, 4.4, 2H), 1.93 (app dd, J = 13.5, 3.9 Hz, 2H). 13C NMR (126 MHz, CDCl3) δ 165.3, 155.6, 150.9, 150.6, 149.1, 137.9, 134.7, 128.6, 126.6, 119.4, 113.8, 110.6, 108.6, 77.2, 56.1, 56.0, 52.1, 45.6, 29.2, 27.6, 22.4. HR-MS: calc. for [M+H]+. 480.1990, found 480.1990.
6-(3-Chloro-4-methoxyphenyl)-2-(1-(3-nitroimidazo[1,2-b]pyridazin-6-yl)piperidin-4-yl)-4,5-dihydropyridazin-3(2H)-one (19) Prepared from 19b (2.0 mmol, 0.72 g) by method A; Yield 72%. 1H NMR (500 MHz, CDCl3) δ 8.40 (s, 1H), 7.89 (d, J = 10.0 Hz, 1H), 7.78–7.74 (m, 1H), 7.56 (dd, J = 8.6, 1.7 Hz, 1H), 7.19 (d, J = 10.1 Hz, 1H), 6.91 (d, J = 8.7 Hz, 1H), 4.95 (tt, J = 11.5, 3.8 Hz, 1H), 4.48 (app d, J = 13.4 Hz, 2H), 3.92 (s, 3H), 3.17 (app t, J = 12.5 Hz, 2H), 2.89 (t, J = 8.2 Hz, 2H), 2.62 (t, J = 8.2 Hz, 2H), 2.15 (app td, J = 12.5, 3.9 Hz, 2H), 1.92 (app d, J = 11.1 Hz, 2H). 13C NMR (126 MHz, CDCl3) δ 165.0, 156.2, 155.5, 149.1, 137.4, 133.7, 132.5, 129.0, 127.7, 126.3, 125.5, 122.9, 113.9, 111.6, 56.3, 52.0, 45.3, 29.1, 27.3, 22.2. HR-MS: calc. for [M+H]+. 484.1495, found 484.1500.
6-(3,4-Dimethoxyphenyl)-2-(1-(imidazo[1,2-b]pyridazin-6-yl)piperidin-4-yl)-4,5-dihydropyridazin-3(2H)-one (20) Prepared from 18b (2.0 mmol, 0.71 g) and 6-chloroimidazo[1,2-b]pyridazine (2.1 mmol, 0.32 g) by method A, but heating at 60 °C for 24 h; Yield 19%. 1H NMR (600 MHz, CDCl3) δ 7.89 (d, J = 9.9 Hz, 1H), 7.69 (s, 1H), 7.58 (s, 1H), 7.35 (d, J = 1.9 Hz, 1H), 7.24 (dd, J = 8.4, 2.0 Hz, 1H), 6.97 (d, J = 10.0 Hz, 1H), 6.87 (d, J = 8.4 Hz, 1H), 4.94 (tt, J = 11.6, 4.1 Hz, 1H), 4.29 (app d, J = 13.4 Hz, 2H), 3.92 (s, 3H), 3.79 (s, 3H), 3.16–3.05 (m, 2H), 2.93 (t, J = 8.2 Hz, 2H), 2.69–2.58 (m, 2H), 2.17 (app qd, J = 12.6, 4.1 Hz, 2H), 1.94–1.85 (m, 2H). 13C NMR (151 MHz, CDCl3) δ 165.3, 155.2, 150.9, 150.4, 149.2, 135.6, 129.9, 128.6, 125.3, 119.3, 116.7, 111.6, 110.7, 108.5, 56.1, 55.9, 52.2, 46.1, 29.1, 27.6, 22.3. HR-MS: calc. for [M+H]+. 435.2139, found 435.2153.
N,N-Dimethyl-3-nitroimidazo[1,2-b]pyridazin-6-amine (21) Prepared from dimethylamine hydrochloride by method A; Yield 71%. 1H NMR (500 MHz, CDCl3) δ 8.37 (s, 1H), 7.82 (d, J = 10.0 Hz, 1H), 7.03 (d, J = 10.0 Hz, 1H), 3.22 (s, 6H). 13C NMR (126 MHz, CDCl3) δ 155.8, 137.7, 134.5, 134.2, 126.3, 112.8, 38.7. HR-MS: calc. for [M+H]+. 208.0829, found 208.0829.
3-Nitro-6-(pyrrolidin-1-yl)imidazo[1,2-b]pyridazine (22) Prepared from pyrrolidine by method A; Yield 65%. 1H NMR (500 MHz, CDCl3) δ 8.35 (s, 1H), 7.78 (d, J = 9.9 Hz, 1H), 6.86 (d, J = 9.9 Hz, 1H), 3.63–3.54 (m, 4H), 2.13–2.04 (m, 4H). 13C NMR (126 MHz, CDCl3) δ 153.8, 138.0, 134.4, 134.0, 126.2, 113.8, 47.4, 25.5. HR-MS: calc. for [M+H]+. 234.0986, found 234.0996. Spectral data are in agreement with a previous report (Sridhar et al., 2017).
3-Nitro-6-(piperidin-1-yl)imidazo[1,2-b]pyridazine (23) Prepared from piperidine by method A; Yield 61%. 1H NMR (600 MHz, CDCl3) δ 8.37 (s, 1H), 7.81 (d, J = 10.0 Hz, 1H), 7.12 (d, J = 10.0 Hz, 1H), 3.65 (app s, 4H), 1.72 (app s, 6H). 13C NMR (151 MHz, CDCl3) δ 156.1, 138.0, 134.8, 134.4, 126.4, 114.2, 47.3, 25.7, 24.6. HR-MS: calc. for [M+H]+. 248.1142, found 248.1149. Spectral data are in agreement with a previous report (Sridhar et al., 2017).
6-(4-Methylpiperazin-1-yl)-3-nitroimidazo[1,2-b]pyridazine formate (24) Prepared from N-methylpiperazine by method A; Yield 45%. 1H NMR (500 MHz, CDCl3) δ 8.41 (s, 1H), 8.24 (s, 1H), 7.88 (d, J = 10.0 Hz, 1H), 7.10 (d, J = 10.0 Hz, 1H), 5.42 (s, 1H), 3.81 (t, J = 5.2 Hz, 4H), 2.84 (t, J = 5.1 Hz, 4H), 2.51 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 165.5, 155.5, 138.0, 134.9, 133.9, 127.0, 113.4, 53.5, 45.0, 44.8. HR-MS: calc. for [M+H]+. 263.1251, found 263.1257.
3-Nitro-6-(4-(pyrrolidin-1-yl)piperidin-1-yl)imidazo[1,2-b]pyridazine formate (25) Prepared from 4-(pyrrolidin-1-yl)-piperidine by method A; Yield 46%. 1H NMR (500 MHz, CDCl3) δ 8.43 (s, 1H), 8.39 (s, 1H), 7.84 (d, J = 10.0 Hz, 1H), 7.11 (d, J = 10.0 Hz, 1H), 6.11 (s, 1H), 4.41 (app d, J = 13.6 Hz, 2H), 3.17 (app s, 4H), 3.02 (app t, J = 12.1 Hz, 3H), 2.20 (app d, J = 12.2 Hz, 2H), 2.09–1.90 (m, 6H). 13C NMR (126 MHz, CDCl3) δ 167.9, 155.4, 138.0, 135.0, 134.6, 126.9, 113.7, 61.5, 50.6, 44.9, 28.5, 23.4. HR-MS: calc. for [M+H]+. 317.1721, found 317.1732.
3-Nitro-6-(4-phenylpiperidin-1-yl)imidazo[1,2-b]pyridazine (26) Prepared from 4-phenylpiperidine by method A; Yield 46%. 1H NMR (500 MHz, CDCl3) δ 8.41 (s, 1H), 7.87 (d, J = 10.0 Hz, 1H), 7.35 (t, J = 7.6 Hz, 2H), 7.27–7.23 (m, 3H), 7.20 (d, J = 10.1 Hz, 1H), 4.50 (app d, J = 13.4 Hz, 2H), 3.16 (app td, J = 13.1, 2.1 Hz, 2H), 2.86 (tt, J = 12.2, 3.4 Hz, 1H), 2.06 (app d, J = 13.1 Hz, 2H), 1.90–1.79 (m, 2H). 13C NMR (126 MHz, CDCl3) δ 155.8, 145.1, 137.6, 134.5, 133.8, 128.8, 126.9, 126.8, 126.3, 114.2, 46.9, 42.6, 32.8. HR-MS: calc. for [M+H]+. 324.1455, found 324.1440.
3-Nitro-6-(4-phenylpiperazin-1-yl)imidazo[1,2-b]pyridazine (27) Prepared from N-phenylpiperazine by method A; Yield 49%. 1H NMR (500 MHz, CDCl3) δ 8.41 (s, 1H), 7.87 (d, J = 10.0 Hz, 1H), 7.32 (app t, J = 7.8 Hz, 2H), 7.16 (d, J = 10.0 Hz, 1H), 7.02 (app d, J = 6.2 Hz, 2H), 6.95 (t, J = 6.9 Hz, 1H), 3.86 (app s, 4H), 3.41–3.33 (m, 4H). 13C NMR (126 MHz, CDCl3) δ 155.7, 150.9, 138.1, 134.9, 134.6, 129.5, 126.9, 120.8, 116.7, 113.4, 49.2, 45.9. HR-MS: calc. for [M+H]+. 325.1408, found 325.1405. Spectral data are in agreement with a previous report (Sridhar et al., 2017).
6-(4-Benzylpiperidin-1-yl)-3-nitroimidazo[1,2-b]pyridazine (28) Prepared from 4-benzylpiperidine by method A; Yield 67%. 1H NMR (500 MHz, CDCl3) δ 8.37 (s, 1H), 7.79 (d, J = 10.0 Hz, 1H), 7.34–7.28 (m, 2H), 7.22 (t, J = 7.4 Hz, 1H), 7.16 (d, J = 7.4 Hz, 2H), 7.10 (d, J = 10.0 Hz, 1H), 4.31 (app d, J = 13.6 Hz, 2H), 3.02–2.90 (m, 2H), 2.59 (d, J = 6.8 Hz, 2H), 1.91–1.78 (m, 3H), 1.40–1.28 (m, 2H). 13C NMR (126 MHz, CDCl3) δ 155.7, 139.9, 137.7, 134.2, 129.2, 128.5, 126.31, 126.26, 114.0, 77.2, 46.4, 43.1, 38.1, 31.6. HR-MS: calc. for [M+H]+. 338.1612, found 338.1614.
(1-(3-Nitroimidazo[1,2-b]pyridazin-6-yl)piperidin-4-yl)(phenyl)methanone (29) Prepared from 4-benzoylpiperidine by method A; Yield 59%. 1H NMR (500 MHz, CDCl3) δ 8.39 (s, 1H), 7.97 (d, J = 7.3 Hz, 2H), 7.86 (d, J = 10.0 Hz, 1H), 7.60 (t, J = 7.4 Hz, 1H), 7.51 (t, J = 7.7 Hz, 2H), 7.17 (d, J = 10.1 Hz, 1H), 4.36 (app d, J = 13.5 Hz, 2H), 3.60 (tt, J = 10.8, 3.8 Hz, 1H), 3.32–3.20 (m, 2H), 2.12–2.02 (m, 2H), 2.00–1.87 (m, 2H). 13C NMR (126 MHz, CDCl3) δ 201.7, 155.7, 137.8, 135.7, 134.5, 134.3, 133.5, 129.0, 128.4, 126.5, 114.0, 45.8, 43.1, 28.0. HR-MS: calc. for [M+H]+. 352.1404, found 352.1417.
N-(4-Methoxybenzyl)-N-methyl-3-nitroimidazo[1,2-b]pyridazin-6-amine (30) Prepared from N-(4-methoxybenzyl)methylamine by method A; Yield 62%. 1H NMR (500 MHz, CDCl3) δ 8.38 (s, 1H), 7.79 (d, J = 10.0 Hz, 1H), 7.28 (d, J = 8.5 Hz, 2H), 6.99 (d, J = 10.0 Hz, 1H), 6.87 (d, J = 8.5 Hz, 2H), 4.74 (s, 2H), 3.79 (s, 3H), 3.26 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 159.3, 155.4, 137.7, 134.4, 134.1, 128.9, 128.6, 126.4, 114.3, 113.1, 55.4, 53.9, 37.3. HR-MS: calc. for [M+H]+. 314.1248, found 314.1247.
6-(Piperidin-1-yl)imidazo[1,2-b]pyridazine (31) Prepared from piperidine and 6-chloroimidazo[1,2-b]pyridazine instead of 6-chloro-3-nitroimidazo[1,2-b]pyridazine by method A, but heating at 60 °C for 24 h; Yield 21%. 1H NMR (500 MHz, CDCl3) δ 7.68 (s, 1H), 7.66 (s, 1H), 7.51 (s, 1H), 6.81 (d, J = 9.9 Hz, 1H), 3.47 (app s, 4H), 1.67 (app s, 6H). 13C NMR (126 MHz, CDCl3) δ 155.4, 136.4, 131.7, 125.8, 116.6, 110.7, 47.6, 25.5, 24.6. HR-MS: calc. for [M+H]+. 203.1291, found 203.1291.
2.2. In vitro tests
G. lamblia trophozoites (WBC6) were seeded into 96-well-plates in 0.2 mL Keister-medium containing 103 trophozoites per well in the presence of the compounds dissolved in dimethyl sulfoxide (DMSO, final concentration 0.25%) or with DMSO as a solvent control and incubated for 72 h at 37 °C in an anaerobic chamber (80% N2, 10% H2, 10% CO2). Then, plates were centrifuged (2 min, 1000 rpm), medium was removed, and cells were washed once with PBS containing 1 g/L glucose. Then, PBS-glucose containing 10 mg/L resazurin was added, and plates were incubated for up to 5 h at 37 °C. Fluorescence reading (ex 530 nm/em 590 nm) was performed at various time points, and the linear increase of fluorescence was used as a marker for cell viability. IC50 values were calculated using the logit-log algorithm and are given as mean values with 95%-confidence intervals (Müller et al., 2020; Müller and Hemphill, 2013; Müller and Müller, 2019). The assays for growth inhibition of T. b. brucei, T. cruzi, L. infantum, P. falciparum and MRC-5 (human lung fibroblasts MRC-5SV40) were previously described (Blaazer et al., 2014). PDE activity was determined exactly as described (de Heuvel et al., 2021; Kunz et al., 2005, 2017b). All assays were carried out in triplicates and no more than 20% substrate was hydrolyzed in all reactions. Compounds were dissolved in DMSO and the final DMSO concentration was 1% in all reaction mixes. Control reactions with DMSO alone were always included. Data were analyzed using the GraphPad Prism software package (v7.0, GraphPad, San Diego).
3. Results and discussion
3.1. Synthesis of 3-nitroimidazo[1,2-b]pyridazine analogues
Compounds 11-20 were prepared according to Scheme 1A. In the case R is MeO or Cl, X is H and the sequence started with a Friedel–Crafts acylation (step i) using succinic anhydride or tetrahydrophthalic anhydride. If R is a cyclopentyloxy group, X is Br and the sequence started with a Grignard reaction (step ii). In both cases γ-ketocarboxylic acids were obtained. Enantiomeric acid 11a’ was obtained through resolution with (S)-(−)-α-methylbenzylamine in EtOAc from 11a (step iii). The next step (iv), condensation with 4-hydrazinopiperidine, was performed in refluxing ethanol and resulted in the substituted piperidines, which were further N-substituted with an imidazopyridazine in DMF using K2CO3 to scavenge the HCl. As described previously (van der Mey et al., 2002), the cyclopentyl group of 12 can be hydrolyzed selectively using 4-toluenesulfonic acid in a Dean-Stark apparatus and the resulting phenol 12c was substituted with commercially available benzyl chlorides or pyridylmethyl chlorides under standard conditions. All final compounds were prepared as racemic mixtures of the cis-diastereomers (4aS,8aR and 4aR,8aS) except for compound 11 (4aS, 8aR). Scheme 1B depicts the synthesis of 6-amino-imidazopyridazine analogues (21-31) using conditions essentially identical to step v in Scheme 1A.
Scheme 1.
Synthesis of analogues 11-31. Reagents and conditions: i for 11a, 18a, 19a: AlCl3, DCM, rt, 4h; ii for 12a: Mg, THF, rt followed by 2 h reflux; iii: crystallization (×3) with (S)-(−)-α-methylbenzylamine from EtOAc; iv: 4-hydrazinopiperidine·2HCl, TEA, EtOH, reflux, 16 h; v: DMF, K2CO3, 60 °C, 2–24 h; vi: Dean-Stark, 4-toluenesulfonic acid, toluene, reflux, 4 h; vii: R2Cl, K2CO3, DMF, 60 °C, 4 h.
3.2. Antiparasitic activities of 3-nitroimidazo[1,2-b]pyridazine analogues
As PDE inhibitors and nitro heterocyclic compounds both have therapeutic potential in protozoal infections, we combined the tetrahydrophthalazinone scaffold with a nitroimidazopyridazine aiming for two modes of action into one hybrid molecule with improved efficacy. For this purpose, we first prepared the tetrahydrophthalazinones 11-17 with variations in R2, the meta position of the phenyl ring, and tested them against the panel of protozoa and for cytotoxicity on the human MRC-5 cell line (using tamoxifen as positive control) together with the benchmark compounds metronidazole, miltefosine, chloroquine, benznidazole and suramin. Activities of control antiparasitic agents were within previously described ranges (Bénéré et al., 2007; El Sayed et al., 2009; van Baelen et al., 2008; Venkatraj et al., 2014) and the results for 11-20 are summarized in Table 1 with representative dose-response curves shown in Fig. S67. All compounds show excellent anti-Giardia activity and no or very weak toxicity (Table 1) with high selectivity over other parasites (Table S1). Notably, all these novel tetrahydrophthalazinones have much higher anti-Giardia activity than the clinically used metronidazole (IC50 = 0.8 μM): a 40-fold (chlorine derivative 19, IC50 = 19.0 nM) to an exceptional >1,000-fold (methoxy and 2-pyridylmethoxy derivatives 11 and 17, IC50 = 0.5 nM for both).
Table 1.
Anti-protozoal activity and toxicity of pyridazines and tetrahydrophthalazinones.
| Cmpd | R1 | R2 | Y | M.W. | cLogPa | tPSAa | IC50 (nM) |
|
|---|---|---|---|---|---|---|---|---|
| G.l.b | MRC-5c | |||||||
| 11 | NO2 | MeO | Y1 | 531.6 | 3.3 | 127.7 | 0.5 ± 0.2 | >6.4 × 104 |
| 12 | NO2 | cyclopentyloxy | Y2 | 585.7 | 4.6 | 127.7 | 9.3 ± 0.6 | >6.4 × 104 |
| 13 | NO2 | 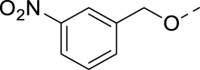 |
Y2 | 652.7 | 5.0 | 170.8 | 1.2 ± 0.4 | >6.4 × 104 |
| 14 | NO2 |  |
Y2 | 637.7 | 4.9 | 136.9 | 1.0 ± 0.5 | >6.4 × 104 |
| 15 | NO2 | 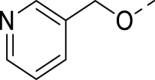 |
Y2 | 608.7 | 3.8 | 140.6 | 6.8 ± 1.5 | 15,000 ± 11,000 |
| 16 | NO2 | 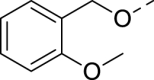 |
Y2 | 637.7 | 4.9 | 136.9 | 2.8 ± 0.8 | 49,000 ± 21,000 |
| 17 | NO2 |  |
Y2 | 608.7 | 3.9 | 140.6 | 0.5 ± 0.1 | >6.4 × 104 |
| 18 | NO2 | MeO | Y3 | 479.5 | 2.1 | 127.7 | 0.2 ± 0.1 | >6.4 × 104 |
| 19 | NO2 | Cl | Y3 | 483.9 | 2.9 | 118.5 | 19.0 ± 3.2 | >6.4 × 104 |
| 20 | H | MeO | Y3 | 434.5 | 2.1 | 84.5 | 930 ± 100 | >6.4 × 104 |
| 3 (MET) | - | - | - | 171.2 | −0.5 | 81.2 | 800 ± 100 | - |
| Tamoxifen | - | - | - | 371.5 | 6.8 | 12.5 | - | 11,000 ± 5,000 |
MET: metronidazole; G.l.: Giardia lamblia.
cLogP, tPSA are calculated using Collaborative Drug Discovery (CDD) Vault.
mean values ± standard errors are given for quadruplicates, the standard errors correspond to the 95% confidence intervals as calculated via the logit-log algorithm.
mean values ± standard deviations, n ≥ 2.
As mentioned earlier, tetrahydrophthalazinones are known PDE inhibitors of selected human (e.g. human PDE4) and parasitic enzymes (e.g. GlPDE). To investigate if PDE inhibition is contributing to the high anti-Giardia activity of 11, we determined its activity as a GlPDE inhibitor (Table 2). Moreover, in light of the known interaction of tetrahydrophthalazinones with human PDE4, also the activity against the off-target human PDE4 (Houslay et al., 2005; Manallack et al., 2005; Tenor et al., 2011) was measured. As can be seen in Table 2, 11 is a moderate inhibitor of GlPDE (Ki = 0.5 μM, Fig. S69), but is 3,000-fold more active against human PDE4 (Ki = 0.16 nM, Fig. S68). As such potent human PDE4 inhibition will prevent any further development because of foreseen unwanted adverse effects (Souness et al., 2000; Tenor et al., 2011), we aimed at modifying the scaffold in such a way that the anti-Giardia activity would be retained while the potency against human PDE4 would be strongly reduced. Based on expert knowledge from previous structure-activity relationship (SAR) studies for PDE4 inhibition (van der Mey et al., 2001, van der Mey et al., 2002; Veerman et al., 2016), we decided to remove the cyclohexene ring Y1/Y2 (Table 1) and replace one of the ethers (R2, Table 1) by a chlorine, resulting in the pyridazinones 18 and 19. Removing the cyclohexene ring as in 18 resulted in a 2.5-fold increase in anti-Giardia potency (Table 1, IC50 = 0.2 nM), while the hPDE4 affinity was 20-fold decreased (Table 2). An additional replacement of the meta-methoxy group by a chlorine (19) decreased the anti-Giardia activity 100-fold while the affinity for hPDE4B was 60-fold decreased compared to 18. In this series of tetrahydrophthalazonones/pyridazinones, 18 is our most potent anti-Giardia compound with an IC50 value on growth inhibition of ∼200 pM.
Table 2.
PDE inhibiting activity of selected compounds.
| Cmpd | R1 | R2 | Y | M.W. | cLogPa | tPSAa | G.l. IC50 (nM)b | GlPDE Ki (nM)c | hPDE4B Ki (nM)c |
|---|---|---|---|---|---|---|---|---|---|
| 11 | NO2 | MeO | Y1 | 531.6 | 3.3 | 127.7 | 0.5 ± 0.2 | 500 ± 300 | 0.16 ± 0.02 |
| 18 | NO2 | MeO | Y3 | 479.5 | 2.1 | 127.7 | 0.2 ± 0.1 | >104 | 3.2 ± 0.4 |
| 19 | NO2 | Cl | Y3 | 483.9 | 2.9 | 118.5 | 19.0 ± 3.2 | >106 | 200 ± 50 |
| 20 | H | MeO | Y3 | 434.5 | 2.1 | 84.5 | 930 ± 100 | >106 | 5.0 ± 1.0 |
cLogP, tPSA are calculated using CDD Vault.
mean values ± standard errors are given for quadruplicates, the standard errors correspond to the 95% confidence intervals as calculated via the logit-log algorithm.
mean values ± standard deviations, n ≥ 2.
For the compounds 11, 18–20 we also measured their GlPDE activities (Table 2). From these data, one can conclude that it is highly unlikely that inhibition of this PDE significantly contributes to the anti-parasitic efficacy of these compounds against Giardia. This is especially evident for our most potent compound 18, with only a Ki value of >10 μM on GlPDE, which is over 50,000-fold more than the IC50 value (0.2 nM) for Giardia growth inhibition.
To find out if this class of compounds has any other activity, independent of the nitro group, which might contribute to their anti-Giardia efficacy, we decided to prepare 20, an analogue of 18 lacking the nitro group. As 20 is 4,500-fold less active than its nitro analogue 18, we concluded that the main mode-of-action of 18 is directly related to the nitro group. The remaining anti-Giardia activity of 20 (IC50 = 0.93 μM) that is equipotent to metronidazole is also not related to GlPDE inhibition, since also 20 shows no GlPDE inhibition (Table 2), indicating that PDE inhibition should not be regarded as an additional mode-of-action of these anti-Giardia compounds. Meanwhile, imidazo[1,2-b]pyridazines have been previously reported as kinase inhibitors for P. falciparum and Toxoplasma gondii, which could serve as a potential mechanism for the remaining activity of 20 (Garrido et al., 2021).
To better understand the potential of nitroimidazopyridazines in designing novel and potent compounds with anti-Giardia activity, the relatively “simple” analogues in Table 3 lacking the tetrahydrophthalazinone part associated with the off-target PDE4 inhibition were prepared and tested for antiparasitic efficacy and cytotoxicity (Table 3). Surprisingly, also in this series analogues with nano- to picomolar IC50 values against G. lamblia were identified. None of the analogues showed relevant activity against the other protozoa (Table S1). Anti-Giardia activities range from 144 nM (24, NR3R4 = N-methylpiperazine) to 500 pM (28, NR3R4 = 4-benzylpiperidine). Also for this series, the importance of the nitro group was investigated. Compound 31, the non-nitro analogue of 23, was prepared and proved to be inactive (Table 3). The difference between both compounds in inhibiting growth of G. lamblia is larger than 2,500 folds, providing strong evidence that the nitro group is of main importance for the activity of this scaffold. Like the compounds in Table 1, also the compounds in Table 3 have negligible toxicity on the human cell line MRC-5.
Table 3.
Antiprotozoal activity and toxicity of 6-substituted imidazo[1,2-b]pyridazines.
| Cmpd | R1 | NR3R4 | M.W. | cLogPa | tPSAa | IC50 (nM) |
|
|---|---|---|---|---|---|---|---|
| G.l.b | MRC-5c | ||||||
| 21 | NO2 | 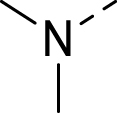 |
207.2 | 1.4 | 76.6 | 22.0 ± 3.0 | >6.4 × 104 |
| 22 | NO2 | 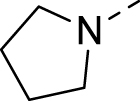 |
233.2 | 1.8 | 76.6 | 2.3 ± 0.4 | >6.4 × 104 |
| 23 | NO2 |  |
247.3 | 2.2 | 76.6 | 3.6 ± 0.8 | 28,100 ± 1,400 |
| 24d | NO2 | 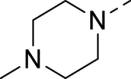 |
308.3 | 1.2 | 79.8 | 144.0 ± 31.0 | >6.4 × 104 |
| 25d | NO2 |  |
316.4 | 1.8 | 79.8 | 5.8 ± 0.9 | >6.4 × 104 |
| 26 | NO2 | 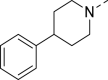 |
323.4 | 3.7 | 76.6 | 8.2 ± 1.0 | >6.4 × 104 |
| 27 | NO2 | 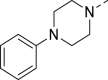 |
324.3 | 3.1 | 79.8 | 0.7 ± 0.1 | >6.4 × 104 |
| 28 | NO2 | 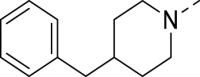 |
337.4 | 4.1 | 76.6 | 0.5 ± 0.1 | 7,960 ± 210 |
| 29 | NO2 | 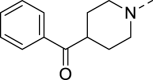 |
351.4 | 3.2 | 93.6 | 1.1 ± 0.2 | >6.4 × 104 |
| 30 | NO2 | 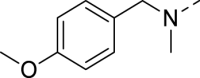 |
313.3 | 2.9 | 85.8 | 10.3 ± 1.5 | >6.4 × 104 |
| 31 | H |  |
202.3 | 2.2 | 33.4 | >104 | >6.4 × 104 |
| 2 (MET) | - | 171.2 | −0.5 | 81.2 | 800 ± 100 | - | |

cLogP, tPSA are calculated using CDD Vault.
mean values ± standard errors are given for quadruplicates, the standard errors correspond to the 95% confidence intervals as calculated via the logit-log algorithm.
mean values ± standard deviations, n ≥ 2.
formate.
The importance of physicochemical properties of potential new drugs has become a mainstay in drug development since the formulation of the Lipinski rule of 5 (Ro5) (Lipinski et al., 1997). Still, the use of this Ro5 should be limited to oral availability of systemically active drugs. Moreover, it was clearly stated by McKerrow and Lipinski that this Ro5 is not applicable to potential anti-parasitic drugs (McKerrow and Lipinski, 2017). Despite these limitations of the Ro5, for our project we are convinced that physicochemical properties for targeting gastrointestinal parasites are important. Though the optimal values are not known, to our knowledge, it seems plausible that low lipophilicity (cLogP), low polar surface area (tPSA) and low molecular weight must be important, e.g. for proper solubility and membrane passage to enter parasites. Therefore, we believe that the result from this study, compounds which combine high anti-parasite potency with good calculated physicochemical properties (cLogP: 1.2–4.1; tPSA: 77-94 Å; molecular weight: 202.3–351.4 Dalton) warrants detailed evaluation of this class of compounds for their potential in the treatment of giardiasis. Importantly, the known nitro-resistance of a number of parasites, including Giardia lamblia (Nabarro et al., 2015), might limit the ultimate usefulness of this class of compounds and warrants future studies with resistant parasite strains.
In summary, we have identified novel nitroimidazopyrimidine compounds that have nanomolar to picomolar activity against G. lamblia without significant toxicity against human MRC-5 cells. Though this study was set up to find novel compounds with dual activity, their potency seems to rely mainly on the nitro group. In view of their good drug-like properties, especially low lipophilicity, low polar surface area and the low molecular weight, 3-nitroimidazo[1,2-b]pyridazines are promising candidates for further lead optimization studies.
Declaration of competing interest
The authors declared that there is no conflict of interest.
Acknowledgment
This work was supported by the European Commission 7th Framework Program FP7-HEALTH-2013-INNOVATION-1 under project reference 602666 “Parasite-specific cyclic nucleotide phosphodiesterase inhibitors to target Neglected Parasitic Diseases” (PDE4NPD). YZ acknowledges the China Scholarship Council (CSC) for funding (Grant No. 201506220185). The authors thank Hans Custers and Andrea van de Stolpe for their technical assistance.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijpddr.2022.05.004.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Ang C.W., Jarrad A.M., Cooper M.A., Blaskovich M.A.T. Nitroimidazoles: molecular fireworks that combat a broad spectrum of infectious diseases. J. Med. Chem. 2017;60:7636–7657. doi: 10.1021/acs.jmedchem.7b00143. [DOI] [PubMed] [Google Scholar]
- Bénéré E., da Luz R.A.I., Vermeersch M., Cos P., Maes L. A new quantitative in vitro microculture method for Giardia duodenalis trophozoites. J. Microbiol. Methods. 2007;71:101–106. doi: 10.1016/J.MIMET.2007.07.014. [DOI] [PubMed] [Google Scholar]
- Blaazer A.R., Orrling K.M., Shanmugham A., Jansen C., Maes L., Edink E., Sterk G.J., Siderius M., England P., Bailey D., de Esch I.J.P., Leurs R. Fragment-based screening in tandem with phenotypic screening provides novel antiparasitic hits. J. Biomol. Screen. 2014;20:131–140. doi: 10.1177/1087057114549735. [DOI] [PubMed] [Google Scholar]
- Blaazer A.R., Singh A.K., de Heuvel E., Edink E., Orrling K.M., Veerman J.J.N., van den Bergh T., Jansen C., Balasubramaniam E., Mooij W.J., Custers H., Sijm M., Tagoe D.N.A., Kalejaiye T.D., Munday J.C., Tenor H., Matheeussen A., Wijtmans M., Siderius M., de Graaf C., Maes L., de Koning H.P., Bailey D.S., Sterk G.J., de Esch I.J.P., Brown D.G., Leurs R. Targeting a subpocket in trypanosoma brucei phosphodiesterase B1 (TbrPDEB1) enables the structure-based discovery of selective inhibitors with trypanocidal activity. J. Med. Chem. 2018;61:3870–3888. doi: 10.1021/acs.jmedchem.7b01670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui L., Mharakurwa S., Ndiaye D., Rathod P.K., Rosenthal P.J. Antimalarial drug resistance: literature review and activities and findings of the ICEMR network. Am. Soc. Trop. Med. Hyg. 2015;93:57–68. doi: 10.4269/ajtmh.15-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Heuvel E., Kooistra A.J., Edink E., van Klaveren S., Stuijt J., van der Meer T., Sadek P., Mabille D., Caljon G., Maes L., Siderius M., de Esch I.J.P., Sterk G.J., Leurs R. Discovery of diaryl ether substituted tetrahydrophthalazinones as TbrPDEB1 inhibitors following structure-based virtual screening. Front. Chem. 2021 doi: 10.3389/fchem.2020.608030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Koning H.P. The drugs of sleeping sickness: their mechanisms of action and resistance, and a brief history. Trop. Med. Infect. Dis. 2020;5:14. doi: 10.3390/tropicalmed5010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Koning H.P., Gould M.K., Sterk G.J., Tenor H., Kunz S., Luginbuehl E., Seebeck T. Pharmacological validation of trypanosoma brucei phosphodiesterases as novel drug targets. J. Infect. Dis. 2012;206:229–237. doi: 10.1093/infdis/jir857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Sayed I., van der Veken P., Steert K., Dhooghe L., Hostyn S., van Baelen G., Lemière G., Maes B.U.W., Cos P., Maes L., Joossens J., Haemers A., Pieters L., Augustyns K. Synthesis and antiplasmodial activity of aminoalkylamino-substituted neocryptolepine derivatives. J. Med. Chem. 2009;52:2979–2988. doi: 10.1021/jm801490z. [DOI] [PubMed] [Google Scholar]
- Garrido A., Vera G., Delaye P.O., Enguehard-Gueiffier C. Imidazo[1,2-b]pyridazine as privileged scaffold in medicinal chemistry: an extensive review. Eur. J. Med. Chem. 2021;226 doi: 10.1016/J.EJMECH.2021.113867. [DOI] [PubMed] [Google Scholar]
- Gupta S., Yardley V., Vishwakarma P., Shivahare R., Sharma B., Launay D., Martin D., Puri S.K. Nitroimidazo-oxazole compound DNDI-VL-2098: an orally effective preclinical drug candidate for the treatment of visceral leishmaniasis. J. Antimicrob. Chemother. 2015;70:518–527. doi: 10.1093/jac/dku422. [DOI] [PubMed] [Google Scholar]
- Herricks J.R., Hotez P.J., Wanga V., Coffeng L.E., Haagsma J.A., Basáñez M.-G., Buckle G., Budke C.M., Carabin H., Fèvre E.M., Fürst T., Halasa Y.A., King C.H., Murdoch M.E., Ramaiah K.D., Shepard D.S., Stolk W.A., Undurraga E.A., Stanaway J.D., Naghavi M., Murray C.J.L. The global burden of disease study 2013: what does it mean for the NTDs? PLoS Neglected Trop. Dis. 2017;11:1–21. doi: 10.1371/journal.pntd.0005424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houslay M.D., Schafer P., Zhang K.Y.J. Keynote review: phosphodiesterase-4 as a therapeutic target. Drug Discov. Today. 2005;10:1503–1519. doi: 10.1016/S1359-6446(05)03622-6. [DOI] [PubMed] [Google Scholar]
- Howard B.L., Harvey K.L., Stewart R.J., Azevedo M.F., Crabb B.S., Jennings I.G., Sanders P.R., Manallack D.T., Thompson P.E., Tonkin C.J., Gilson P.R. Identification of potent phosphodiesterase inhibitors that demonstrate cyclic nucleotide-dependent functions in apicomplexan parasites. ACS Chem. Biol. 2015;10:1145–1154. doi: 10.1021/cb501004q. [DOI] [PubMed] [Google Scholar]
- Kunz S., Balmer V., Sterk G.J., Pollastri M.P., Leurs R., Müller N., Hemphill A., Spycher C. The single cyclic nucleotide-specific phosphodiesterase of the intestinal parasite Giardia lamblia represents a potential drug target. PLoS Neglected Trop. Dis. 2017;11:1–23. doi: 10.1371/journal.pntd.0005891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz S., Balmer V., Sterk G.J., Pollastri M.P., Leurs R., Müller N., Hemphill A., Spycher C. The single cyclic nucleotide-specific phosphodiesterase of the intestinal parasite Giardia lamblia represents a potential drug target. PLoS Neglected Trop. Dis. 2017;11 doi: 10.1371/journal.pntd.0005891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz S., Oberholzer M., Seebeck T. A FYVE-containing unusual cyclic nucleotide phosphodiesterase from Trypanosoma cruzi. FEBS J. 2005;272:6412–6422. doi: 10.1111/j.1742-4658.2005.05039.x. [DOI] [PubMed] [Google Scholar]
- Lipinski C.A., Lombardo F., Dominy B.W., Feeney P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 1997;23:3–25. doi: 10.1016/S0169-409X(96)00423-1. [DOI] [PubMed] [Google Scholar]
- Manallack D.T., Hughes R.A., Thompson P.E. The next generation of phosphodiesterase inhibitors: structural clues to ligand and substrate selectivity of phosphodiesterases. J. Med. Chem. 2005;48:3449–3462. doi: 10.1021/jm040217u. [DOI] [PubMed] [Google Scholar]
- Maurice D.H., Ke H., Ahmad F., Wang Y., Chung J., Manganiello V.C. Advances in targeting cyclic nucleotide phosphodiesterases. Nat. Rev. Drug Discov. 2014;13:290–314. doi: 10.1038/nrd4228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKerrow J.H., Lipinski C.A. The rule of five should not impede anti-parasitic drug development. Int. J. Parasitol.: Drugs Drug Resist. 2017;7:248–249. doi: 10.1016/J.IJPDDR.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mørch K., Hanevik K. Giardiasis treatment: an update with a focus on refractory disease. Curr. Opin. Infect. Dis. 2020;33 doi: 10.1097/QCO.0000000000000668. [DOI] [PubMed] [Google Scholar]
- Müller J., Braga S., Uldry A.-C., Heller M., Müller N. Comparative proteomics of three Giardia lamblia strains: investigation of antigenic variation in the post-genomic era. Parasitology. 2020;147:1008–1018. doi: 10.1017/S0031182020000657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller J., Hemphill A. International Review of Cell and Molecular Biology. Academic Press; 2013. New approaches for the identification of drug targets in Protozoan parasites; pp. 359–401. [DOI] [PubMed] [Google Scholar]
- Müller J., Müller N. Nitroreductases of bacterial origin in Giardia lamblia: potential role in detoxification of xenobiotics. Microbiologyopen. 2019;8 doi: 10.1002/mbo3.904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabarro L.E.B., Lever R.A., Armstrong M., Chiodini P.L. Increased incidence of nitroimidazole-refractory giardiasis at the hospital for tropical diseases, london: 2008–2013. Clin. Microbiol. Infect. 2015;21:791–796. doi: 10.1016/j.cmi.2015.04.019. [DOI] [PubMed] [Google Scholar]
- Oberholzer M., Marti G., Baresic M., Kunz S., Hemphill A., Seebeck T. The Trypanosoma brucei cAMP phosphodiesterases TbrPDEBl and TbrPDEB2: flagellar enzymes that are essential for parasite virulence. Faseb. J. 2007;21:720–731. doi: 10.1096/fj.06-6818com. [DOI] [PubMed] [Google Scholar]
- Oliveira R., Miranda D., Magalhães J., Capela R., Perry M.J., O'Neill P.M., Moreira R., Lopes F. From hybrid compounds to targeted drug delivery in antimalarial therapy. Bioorg. Med. Chem. 2015;23:5120–5130. doi: 10.1016/J.BMC.2015.04.017. [DOI] [PubMed] [Google Scholar]
- Patterson S., Wyllie S. Nitro drugs for the treatment of trypanosomatid diseases: past, present, and future prospects. Trends Parasitol. 2014;30:289–298. doi: 10.1016/J.PT.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro V., Dias N., Paiva T., Hagström-Bex L., Nitz N., Pratesi R., Hecht M. Current trends in the pharmacological management of Chagas disease. Int. J. Parasitol.: Drugs Drug Resist. 2020;12:7–17. doi: 10.1016/j.ijpddr.2019.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharon K.-K., Minyong L., Alyssa S., Shilong Z., Gurpreet K., Xiaochuan Y., Binghe W., Roberto D. Chemical validation of phosphodiesterase C as a chemotherapeutic target in trypanosoma cruzi, the etiological agent of Chagas' disease. Antimicrob. Agents Chemother. 2010;54:3738–3745. doi: 10.1128/AAC.00313-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souness J.E., Aldous D., Sargent C. Immunosuppressive and anti-inflammatory effects of cyclic AMP phosphodiesterase (PDE) type 4 inhibitors. Immunopharmacology. 2000;47:127–162. doi: 10.1016/S0162-3109(00)00185-5. [DOI] [PubMed] [Google Scholar]
- Sridhar P., Alagumuthu M., Ram B., Arumugam S., Reddy S.R. Drugs against neurodegenerative diseases: design and synthesis of 6-Amino–substituted imidazo[1,2-b]pyridazines as acetylcholinesterase inhibitors. ChemistrySelect. 2017;2:842–847. doi: 10.1002/slct.201601353. [DOI] [Google Scholar]
- Sterk G.J., Hatzelmann A., Barsig J., Marx D., Kley H.-P., Christiaans J.A.M., Menge W.M.P.B. 2004. Piperidine-pyridazones and Phthalazones as PDE4 Inhibitors. PCT WO2004017974A1. [Google Scholar]
- Sterk G.J., Hatzelmann A., Barsig J., Marx D., Kley H.-P., Christiaans J.A.M., Menge W.M.P.B. 2004. Pyridazinone-derivatives as PDE4 Inhibitors. PCT WO2004018451A1. [Google Scholar]
- Tenor H., Hatzelmann A., Beume R., Lahu G., Zech K., Bethke T.D. Springer; 2011. Pharmacology, Clinical Efficacy, and Tolerability of Phosphodiesterase-4 Inhibitors: Impact of Human Pharmacokinetics, Phosphodiesterases as Drug Targets. [DOI] [PubMed] [Google Scholar]
- Tomcufcik A.S., Izzo P.T., Fabio P.F. 1974. 6-substituted 3-Nitroimidazo(1,2-B)pyridazines and Method of Preparing Same. [Google Scholar]
- van Baelen G., Meyers C., Lemière G.L.F., Hostyn S., Dommisse R., Maes L., Augustyns K., Haemers A., Pieters L., Maes B.U.W. Synthesis of 6-methyl-6H-indolo[3,2-c]isoquinoline and 6-methyl-6H-indolo[2,3-c]isoquinoline: two new unnatural isoquinoline isomers of the cryptolepine series. Tetrahedron. 2008;64:11802–11809. doi: 10.1016/J.TET.2008.08.116. [DOI] [Google Scholar]
- van der Mey M., Boss H., Couwenberg D., Hatzelmann A., Sterk G.J., Goubitz K., Schenk H., Timmerman H. Novel selective phosphodiesterase (PDE4) inhibitors. 4. Resolution, absolute configuration, and PDE4 inhibitory activity of cis-tetra- and cis-hexahydrophthalazinones. J. Med. Chem. 2002;45:2523–2535. doi: 10.1021/jm0110338. [DOI] [PubMed] [Google Scholar]
- van der Mey M., Hatzelmann A., van der Laan I.J., Sterk G.J., Thibaut U., Timmerman H. Novel selective PDE4 inhibitors. 1. Synthesis, Structure−Activity relationships, and molecular modeling of 4-(3,4-dimethoxyphenyl)-2H-phthalazin-1-ones and analogues. J. Med. Chem. 2001;44:2511–2522. doi: 10.1021/jm010837k. [DOI] [PubMed] [Google Scholar]
- Vanaerschot M., Huijben S., van den Broeck F., Dujardin J.-C. Drug resistance in vectorborne parasites: multiple actors and scenarios for an evolutionary arms race. FEMS (Fed. Eur. Microbiol. Soc.) Microbiol. Rev. 2014;38:41–55. doi: 10.1111/1574-6976.12032. [DOI] [PubMed] [Google Scholar]
- Veerman J., van den Bergh T., Orrling K.M., Jansen C., Cos P., Maes L., Chatelain E., Ioset J.-R., Edink E.E., Tenor H., Seebeck T., de Esch I., Leurs R., Sterk G.J. Synthesis and evaluation of analogs of the phenylpyridazinone NPD-001 as potent trypanosomal TbrPDEB1 phosphodiesterase inhibitors and in vitro trypanocidals. Bioorg. Med. Chem. 2016;24:1573–1581. doi: 10.1016/j.bmc.2016.02.032. [DOI] [PubMed] [Google Scholar]
- Venkatraj M., Ariën K.K., Heeres J., Joossens J., Dirié B., Lyssens S., Michiels J., Cos P., Lewi P.J., Vanham G., Maes L., van der Veken P., Augustyns K. From human immunodeficiency virus non-nucleoside reverse transcriptase inhibitors to potent and selective antitrypanosomal compounds. Bioorg. Med. Chem. 2014;22:5241–5248. doi: 10.1016/J.BMC.2014.08.005. [DOI] [PubMed] [Google Scholar]
- World Health Organisation . World Health Organisation; Geneva: 2019. No Time to Wait: Securing the Future from Drug-Resistant Infections. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.