Abstract
BACKGROUND:
Suicide is among the leading causes of death in children and adolescents. There are well-known risk factors of suicide, including childhood abuse, family conflicts, social adversity, and psychopathology. While suicide risk is also known to be heritable, few studies have investigated genetic risk in younger individuals.
METHODS:
Using polygenic risk score analysis, we examined whether genetic susceptibility to major psychiatric disorders is associated with suicidal behaviors among 11,878 children enrolled in the ABCD (Adolescent Brain Cognitive Development) Study. Suicidal ideation and suicide attempt data were assessed using the youth report of the Kiddie Schedule for Affective Disorders and Schizophrenia for DSM-5. After performing robust quality control of genotype data, unrelated individuals of European descent were included in analyses (n = 4344).
RESULTS:
Among 8 psychiatric disorders we examined, depression polygenic risk scores were associated with lifetime suicide attempts both in the baseline (odds ratio = 1.55, 95% CI = 1.10–2.18, p = 1.27 × 10−2) and in the follow-up year (odds ratio = 1.38, 95% CI = 1.08–1.77, p = 1.05 × 10−2), after adjusting for children’s age, sex, socioeconomic backgrounds, family history of suicide, and psychopathology. In contrast, attention-deficit/hyperactivity disorder polygenic risk scores were associated with lifetime suicidal ideation (odds ratio = 1.15, 95% CI = 1.05–1.26, p = 3.71 × 10−3), suggesting a distinct contribution of the genetic risk underlying attention-deficit/hyperactivity disorder and depression on suicidal behaviors of children.
CONCLUSIONS:
The largest genetic sample of suicide risk data in U.S. children suggests a significant genetic basis of suicide risk related to attention-deficit/hyperactivity disorder and depression. Further research is warranted to examine whether incorporation of genomic risk may facilitate more targeted screening and intervention efforts.
Suicide is the second leading cause of death in children and adolescents worldwide (1–3). Tragically, more than a half of adolescents who die by suicide have previous records of suicide attempts and self-harming behaviors (4), which start during childhood and persist over several years (5). Suicidality in children, here defined as an umbrella term that includes both suicidal ideation (SI) and suicide attempts (SAs) (6–8), is also significantly associated with psychiatric disorders in later life, suggesting intrinsic etiological connections between suicide risk and mental health that start at earlier developmental periods. Understanding the etiological basis of suicidality in children may facilitate prevention and early intervention strategies (9,10).
Twin, family, and adoption studies have consistently reported that suicidal behaviors are heritable (11,12). The latest population-based Swedish cohort study of more than 2.7 million offspring reported that 70% of the correlation between maternal and offspring suicidal behaviors was attributed to genetic factors shared across the generations, while the remaining was due to adverse environmental factors specific to the exposure to maternal suicidal behavior (13). Deciphering specific genetic risk mechanisms underlying suicide, however, has met with limited success. Several genome-wide association studies (GWASs) (14–19) have reported significant genetic correlations of self-harm and SAs with psychiatric disorders, with major depression (MD) showing the most extensive genetic overlap. Yet, few suicide-specific risks have been identified with conclusive evidence, possibly owing to the extensive polygenic nature of suicidal behaviors and the challenges of identifying samples of adequate sizes given the relatively lower base rate of suicide compared with psychiatric illness.
The primary aim of this study was to examine whether genetic risk for major psychiatric disorders is associated with SI and/or SAs in children. Emerging evidence in psychiatric genomics supports genetic overlap between SAs and major psychiatric disorders in adults. Yet, it is unknown whether genetic risk underlying these conditions is associated with suicidality in children. To test this hypothesis, we investigated data from the ABCD (Adolescent Brain Cognitive Development) Study, a population-based longitudinal study of more than 11,000 U.S. children enrolled at 9 to 10 years old. A wide range of measures encompassing social, familial, physical, mental, and behavioral aspects have been collected longitudinally since 2016, along with the genome-wide genotype data of the participants. As of now, several reports of suicidality data have been published using the initial release of the ABCD cohort (6–8,20–23). These studies have confirmed distinct characteristics of children who reported suicidality, including child psychopathology, family conflicts, and a parental history of suicide. Yet, few if any studies to date have incorporated genome-wide genetic data of ABCD participants in suicide research.
Harnessing the power of the ABCD cohort, we specifically aimed 1) to examine the association of common genetic variation underlying 8 major psychiatric disorders with SI and/or SAs in children; 2) to examine the association of genetic risk for psychiatric disorders with known sociodemographic and clinical risk factors of suicide, including age, sex, parental education, household income, marital status, child psychopathology, and family history of suicide; and 3) to examine whether genetic risk for psychiatric disorders improves prediction performance of SI and/or SAs in children independent of known suicide risk factors. To quantify the genetic liability of children to psychiatric disorders, we used polygenic risk scores (PRSs), which estimate the genome-wide aggregated effects of common genetic risk alleles in an individual based on independent genome-wide association studies of the target phenotype. Figure 1 summarizes the overview of our study design.
Figure 1.
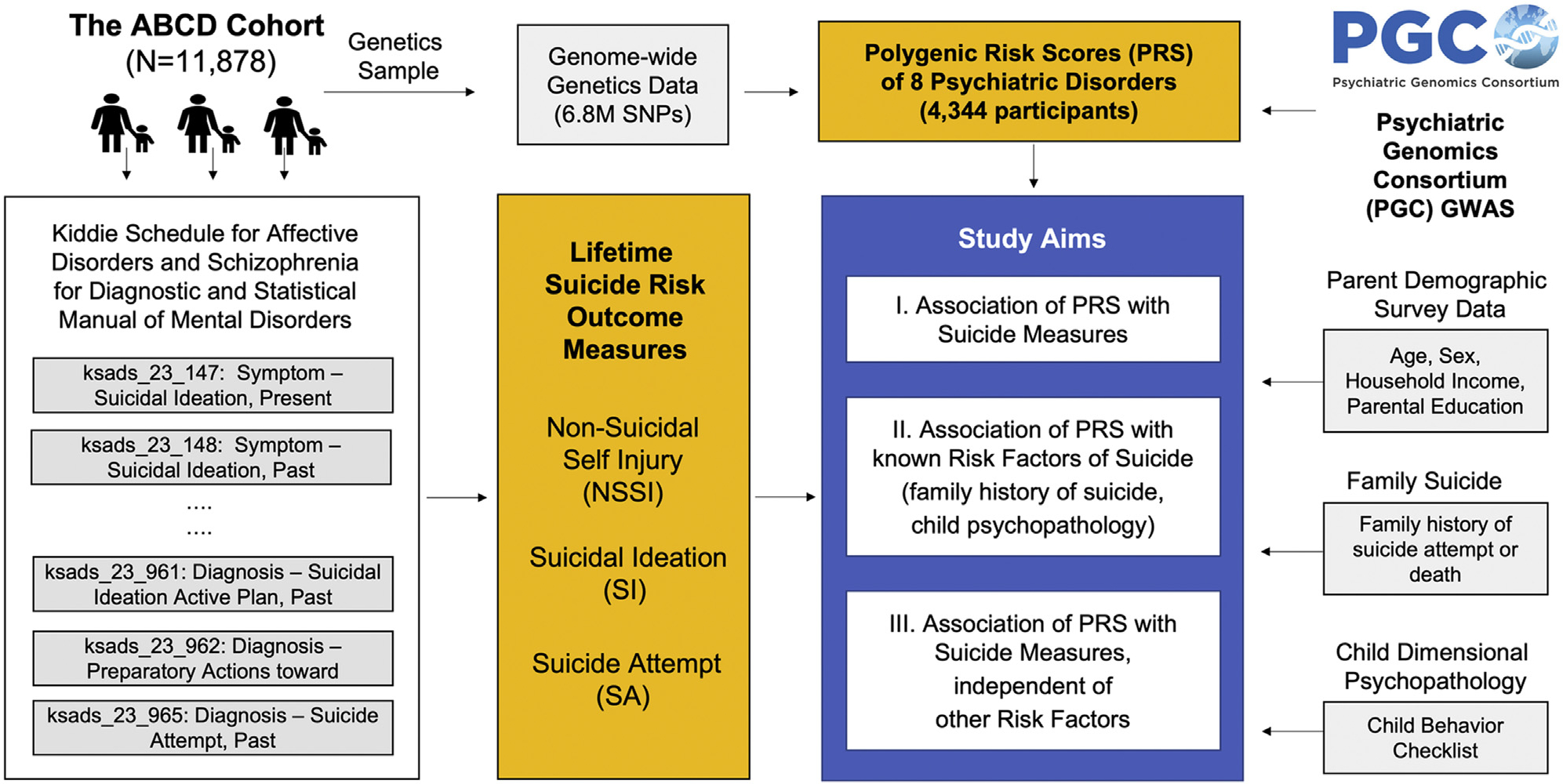
Study outline of the polygenic risk score (PRS) analyses. Our study used ABCD (Adolescent Brain Cognitive Development) Study data release 3.0, which included genetics and phenotypic data collected in the baseline and the first follow-up year for 11,877 participants. Suicidal data were collected using the youth version of the computerized Kiddie Schedule for Affective Disorders and Schizophrenia for DSM-5. Using the individual item data, we generated 3 lifetime suicide-related measures, encompassing nonsuicidal self-injury (NSSI), suicidal ideation (SI), and suicide attempt (SA). Well-known risk factors of suicide were included in the analysis using the ABCD Study survey data. For PRS data generation, 8 psychiatric disorder genome-wide association studies (GWASs) of the largest available sample size were applied to 4344 ABCD participants of European ancestry. SNP, single nucleotide polymorphism.
METHODS AND MATERIALS
ABCD Study Participants
Our study used ABCD Data Release 3.0, which included data collected between September 2016 and February 2020 for 11,878 participants. The ABCD data were downloaded from the National Institute of Mental Health Data Archive (https://nda.nih.gov/abcd). In this study, we focused on the baseline and the first-year follow-up data, for which we had information of the full cohort. Detailed information about the selection of participants and assessment data has been published elsewhere (24–26).
Demographic and Family Socioeconomic Status
Demographic and family socioeconomic information was obtained from ABCD Parent Demographics Survey data. Indicator variables for 4 races (Asian, Black, Other, White) were created by combining the information from multiple race-related questions. “Other” includes study participants who endorsed multiple races, American Indian and Alaska Native, Native Hawaiian and Other Pacific Islander, or others. Ethnicity represented Hispanic and non-Hispanic descendants. For parental education, 5 categories were defined following Huber et al. (6): 1) less than high school diploma or General Educational Development Test; 2) high school diploma or General Educational Development Test; 3) some college, including associate degree; 4) bachelor’s degree; and 5) postgraduate degree. Yearly household income was categorized into 3 groups: 1) less than $50,000, 2) between $50,000 and $100,000, and 3) greater than $100,000.
Suicide Risk Outcome Measure
Lifetime measures of SI, SAs, and nonsuicidal self-injuries (NSSIs) were generated using the youth report of the computerized Kiddie Schedule for Affective Disorders and Schizophrenia for DSM-5 (K-SADS-5). The K-SADS-5 survey includes a suicide module, which consists of 35 items asking about the participants’ experiences of self-injuries, passive or active thoughts of suicide, or suicide attempts at present or in the past. Table S1 summarizes the details of the youth report–based K-SADS-5 items used for generating the lifetime SI, SA, and NSSI measures. The parent report of K-SADS-5 was available only for the baseline. For comparison, we provided the case numbers for NSSI, SI, and SA based on the youth and the caregiver report and their concordance in Table S1.
Risk Factors of Suicidality
In the ABCD Study, the Child Behavior Checklist (CBCL) was used to assess dimensional psychopathology spectrums of children (27,28). We examined the normalized T-scores of 11 CBCL syndromes representing anxious/depressed, withdrawn/depressed, somatic complaints, social problems, thought problems, attention problems, rule-breaking behavior, aggressive behavior, internalizing problems, externalizing problems, and total problems score. We also included a family history of suicide in the analyses. The parents’ report of the presence or absence of a blood relative of the youth ever making an SA or dying by suicide was used to generate a measure of a family history of suicidality. Socioeconomic backgrounds of children were added using household income, parental marital status, poverty, and highest parental education. To assess distinct characteristics of suicide risk factors between different groups (e.g., full cohort vs. genetics sample), we used Welch’s two-sample t test for quantitative measures and proportion tests for categorial variables in R version 4.0.5 (R Foundation for Statistical Computing).
Genotyping, Data Quality Control, and Imputation
Genotyping of ABCD samples was performed using the Affymetrix NIDA SmokeScreen Array at Rutgers University Cell and DNA Repository. The array included 733,293 single nucleotide polymorphisms (SNPs). We applied standard quality control of genome-wide data as we previously described (29). The quality-controlled data contained 516,598 genetic variants for 11,099 individuals. We excluded related individuals, retaining 9109 independent participants. Principal component analysis with the 1000 Genomes Project samples identified 4344 subjects of European ancestry. Imputation was conducted using the Michigan Imputation Server version 1.5.7 (https://imputationserver.sph.umich.edu/index.html#!) and minimac version 4–1.0.2 (https://genome.sph.umich.edu/wiki/Minimac) based on the Haplotype Reference Consortium panel (http://www.haplotype-reference-consortium.org). Haplotype phasing was conducted using Eagle version 2.4 (https://alkesgroup.broadinstitute.org/Eagle/) with r2 filtering of 0.8. Correlation of allele frequencies between the ABCD samples and the Haplotype Reference Consortium was 0.984. Quality-controlled imputation data included 6.7 million SNPs.
Polygenic Risk Scores
We generated PRSs for 8 psychiatric disorders: MD, bipolar disorder, schizophrenia, attention-deficit/hyperactivity disorder (ADHD), autism spectrum disorder, posttraumatic stress disorder, anorexia nervosa, and anxiety disorder. Table S2 summarizes the details of the GWAS datasets. PRSs were calculated using PRSice-2 software (30). To the best of our knowledge, these summary statistics represent the largest publicly available GWAS for these disorders. First, linkage disequilibrium–independent SNPs were identified using clumping implemented in PLINK (window of 250 kb, linkage disequilibrium clump-r2 = 0.1). We used a standard weighted sum scoring approach, representing each child’s additive genome-wide genetic risk to a target phenotype. As expected, PRSs showed a normal distribution (Shapiro test in R p > .05) and were standardized.
Statistical Analysis
To examine association of PRSs with lifetime suicide risk measures, we used multiple logistic regression glm in R. Each outcome measure was used as a binary dependent variable, while PRS was used as an independent variable along with 10 principal components of genetic ancestry as covariates. The effect of PRS on the outcome measure was presented using the odds ratio (OR), which was derived as the exponential of the logistic regression beta coefficient estimate. The OR represents the odds of inclusion in the suicidal group with an increase of one standard deviation change in the PRS. For multiple testing correction, we used the false discovery rate q value of .05, considering the number of youth report–based outcome measures and investigation of both the baseline and the first-year follow-up data for 8 PRSs. To measure the unique proportion of variance explained by PRSs, we calculated Nagelkerke’s pseudo-R2 (31).
To examine association of PRSs with child psychopathology, we calculated partial correlation between the two, adjusting for ancestry principal components, using the R ppcor package. Along with Pearson’s correlation estimates, t-statistics were used to represent a standardized relationship of the two variables. For family history of suicide, we conducted an unpaired t test to examine the differences of PRSs between the participants with and without a family history of suicide.
Lastly, we assessed predictive improvement of PRSs on each outcome measure independent of known risk factors using two logistic regression models in R. The first model included known risk factors of suicide as independent variables (i.e., base model), while the second model included PRS as an additional variable (i.e., genetic risk model). In all analyses, we included 10 genetic ancestry principal components as covariates to account for potential population substratification. Along with Nagelkerke’s pseudo-R2, the likelihood ratio test was performed to assess whether a genetic risk model significantly improves the prediction of suicidality compared with a base model in R version 4.0.5.
Sensitivity Analysis
For PRSs of significant association with a target outcome measure, we conducted sensitivity analyses to assess whether the identified association varies as the p-value threshold for constructing PRS changes. A total of 10 p values (p = 5 × 10−8, p = 5 × 10−6, p = 5 × 10−4, p = 5 × 10−2, p = .1, p = .2, p = .3, p = .4, p = .5, and p = 1.0) were used in sensitivity analysis. We also tested a two-PRS model to examine whether association of one PRS remained significant when the second PRS was added to the model. Here, in addition to PRSs for psychiatric disorder GWASs, we also assessed the impact of a suicide PRS constructed based on the latest GWAS of SA (17).
Ethical Approval
All caregivers and the participants of the ABCD Study provided written informed consent for general research. The University of California San Diego Institutional Review Board, which is responsible for the oversight of the ABCD Study, noted that analyses using publicly released ABCD Study data are not human subject research and did not require its approval. The present study obtained approval from the Massachusetts General Hospital Institutional Review Board as the secondary data analysis of the publicly available ABCD Study.
RESULTS
Prevalence and Sociodemographic Characteristics of Suicidality in ABCD Children
Table 1 describes the demographic, socioeconomic, and family characteristics of ABCD children based on the youth report of lifetime SI and SAs in the baseline. The mean (SD) age of the children at the enrollment was 9.91 (0.62) years; 47.83% of participants were female. In the baseline, we had suicide-related survey data separately reported by children and caregivers (Table S1). Overall, 8.63% of children reported SI, while the incidence was 7.5% based on caregivers. Youth-reported SAs were approximately 3 times more prevalent than when reported by caregivers (1.31% vs. 0.44%). Concordance between the youth and caregiver reports was low; approximately 25% of SI reported by youths (255/1025) was recognized by the caregivers, while the rate reduced to 15% (14/156) for SAs.
Table 1.
Demographic, Socioeconomic, and Family History of ABCD Participants Based on Youth-Reported Suicidal Ideation and Suicide Attempts at Baseline
| Variables | ABCD | Suicidal Ideation | Suicide Attempts | ||
|---|---|---|---|---|---|
| All Samples (N = 11,878) | Cases (n = 1025) | Controls (n = 10,853) | Cases (n = 156) | Controls (n = 11,722) | |
| Age, Years, Mean (±SD) | 9.91 (±0.62) | 9.91 (±0.63) | 9.92 (±0.62) | 9.93 (±0.62) | 9.91 (±0.62) |
| Sex, Male, n (%) | 6196 (52.17) | 595 (57.99) | 5601 (51.62) | 89 (57.05) | 6107 (52.1) |
| Race, n (%) | |||||
| African American | 2269 (19.1) | 199 (19.4) | 2070 (19.08) | 47 (30.13) | 2222 (18.96) |
| Asian | 1113 (9.37) | 119 (11.6) | 994 (9.16) | 15 (9.62) | 1098 (9.37) |
| Othera | 800 (6.74) | 71 (6.92) | 729 (6.72) | 9 (5.77) | 791 (6.75) |
| White | 7695 (64.79) | 637 (62.09) | 7058 (65.04) | 85 (54.49) | 7610 (64.93) |
| Ethnicity, Hispanic, n (%) | 2410 (20.29) | 195 (19.01) | 2215 (20.41) | 40 (25.64) | 2370 (20.22) |
| Parental Education, n (%) | |||||
| < HS diploma/GED | 593 (4.99) | 45 (4.39) | 548 (5.05) | 8 (5.13) | 585 (4.99) |
| HS diploma/GED | 1132 (9.53) | 88 (8.58) | 1044 (9.62) | 22 (14.1) | 1110 (9.47) |
| Some college | 3079 (25.92) | 307 (29.92) | 2772 (25.55) | 62 (39.74) | 3017 (25.74) |
| Bachelor’s degree | 3029 (25.5) | 258 (25.15) | 2771 (25.54) | 37 (23.72) | 2992 (25.53) |
| Postgraduate degree | 4044 (34.05) | 328 (31.97) | 3716 (34.25) | 27 (17.31) | 4017 (34.27) |
| Household Income, n (%) | |||||
| < $50,000 | 3223 (27.14) | 297 (28.95) | 2926 (26.97) | 76 (48.72) | 3147 (26.85) |
| > $50,000 and < $100,000 | 4089 (34.43) | 377 (36.74) | 3712 (34.21) | 52 (33.33) | 4037 (34.44) |
| > $100,000 | 4565 (38.44) | 352 (34.31) | 4213 (38.83) | 28 (17.95) | 4537 (38.71) |
| Marital Status of Parents, Married, n (%) | 8087 (68.09) | 651 (63.45) | 7436 (68.53) | 77 (49.36) | 8010 (68.34) |
| Family History of Suicide, n (%) | 1827 (15.38) | 198 (19.3) | 1629 (15.01) | 42 (26.92) | 1785 (15.23) |
Demographic and family socioeconomic information was obtained from the ABCD Parent Demographics Survey data. Suicidality data were generated based on the youth version of the computerized Kiddie Schedule for Affective Disorders and Schizophrenia for DSM-5; detailed information for individual items is summarized in Table S1.
ABCD, Adolescent Brain Cognitive Development; GED, General Educational Development Test; HS, high school.
“Other” includes study participants who endorsed multiple races, American Indian and Alaska Native, Native Hawaiian and Other Pacific, or others.
Principal component analyses revealed the diverse racial and ethnic backgrounds of the ABCD children (Figure S1). To minimize potential complications related to population stratification, our primary PRS analysis focused on 4344 children of European ancestry. Overall, the European genetics sample showed distinct socioeconomic status compared with the full cohort: higher parental education, higher household income, more married parents, and less poverty (all p < 2.2 × 10−16) (Table S3). This trend was consistent when we compared the European genetics sample with suicidality with the same group from the full cohort (Figure S2). For age, sex, family history of suicide, and child psychopathology measures, participants with suicidality did not differ significantly between the European genetics sample and the full cohort (Table S4).
Association of PRSs and Suicidality
For 4344 children with genotype data, PRSs for 8 psychiatric disorders were calculated using GWAS summary statistics representing the largest available sample size of the disorders (Table S2). We used a standard weighted sum scoring strategy, representing each child’s additive genome-wide genetic risk to a target phenotype. We first examined the association between the 8 PRSs and each lifetime suicide risk measure reported by children, adjusting for age, sex, and 10 principal components reflecting population substratification. Figure 2 summarizes the results (see Tables S5 and S6 for full results). It is notable that depression PRSs showed significant association with SAs both in the baseline (OR = 1.85, 95% CI = 1.32–2.60, uncorrected p = 3.21 × 10−4, false discovery rate q = 5.14 × 10−3) and in the follow-up year (OR = 1.63, 95% CI = 1.28–2.08, uncorrected p = 6.95 × 10−5, q = 1.67 × 10−3), while ADHD PRSs showed a significant association with SI in the baseline (OR = 1.17, 95% CI = 1.05–1.3, uncorrected p = 4.75 × 10−3, q = 3.80 × 10−2) and in the follow-up year (OR = 1.23, 95% CI = 1.12–1.34, uncorrected p = 1.01 × 10−5, q = 4.85 × 10−4). In contrast to youth report data, we found no statistically significant associations between PRSs and caregiver-reported outcome measures (Table S7).
Figure 2.
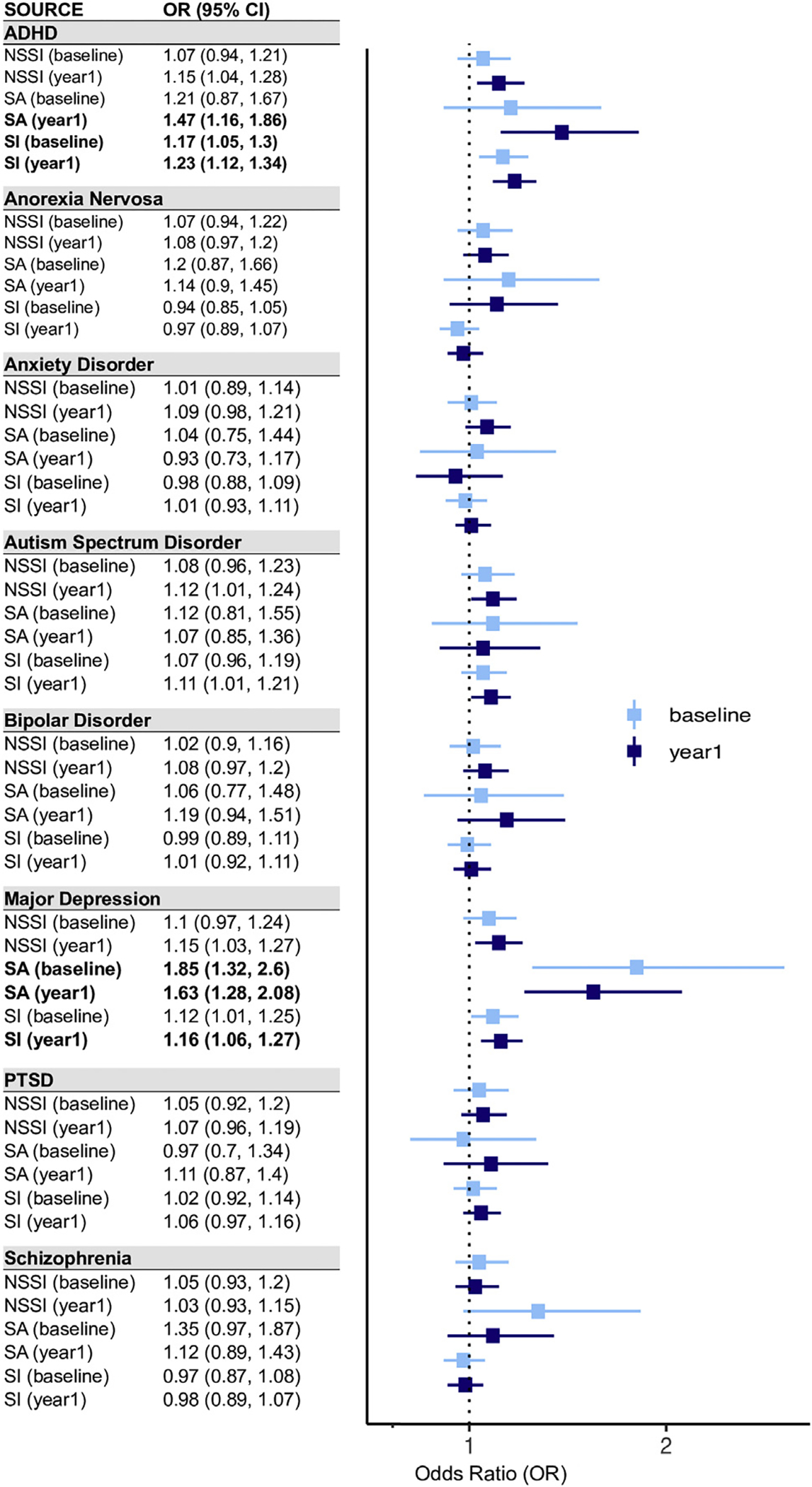
Logistic regression analysis results for testing the association between 8 psychiatric disorder polygenic risk scores and lifetime suicide risk outcome measures. Odds ratio (OR) represents the exponential of the logistic regression estimates. Error bars represent 95% confidence intervals of ORs. ADHD, attention-deficit/hyperactivity disorder; PTSD, posttraumatic stress disorder; NSSI, nonsuicidal self-injury; SA, suicide attempt; SI, suicidal ideation.
Association of PRSs and Known Risk Factors of Suicide
We next examined whether the above associations of ADHD and MD PRSs with suicide risk measures reflect etiological relationships between genetic susceptibility of the disorders and known clinical risk factors for suicide, such as child psychopathology. Specifically, we hypothesized that depression PRSs may be associated with participants’ internalizing problems, while ADHD PRSs may be associated with externalizing problems. Table S8 summarizes the association analysis results of ADHD and MD PRSs with 11 CBCL measures of children’s emotional and behavioral problems assessed in the ABCD Study. Unexpectedly, MD PRSs were significantly associated with all domains of children’s problematic behaviors, encompassing somatic, internalizing, and externalizing problems. ADHD PRSs showed an association with all examined measures except a few internalizing problems (false discovery rate q > .01). Overall, we found stronger correlations between PRSs and CBCL measures in the follow-up year compared with the baseline (one-sided paired t test p = 2.16 × 10−4) (Figure 3). We also found significantly higher ADHD and MD PRSs of the participants when stratified by family history of suicide or disadvantageous socioeconomic status (Figure 3).
Figure 3.
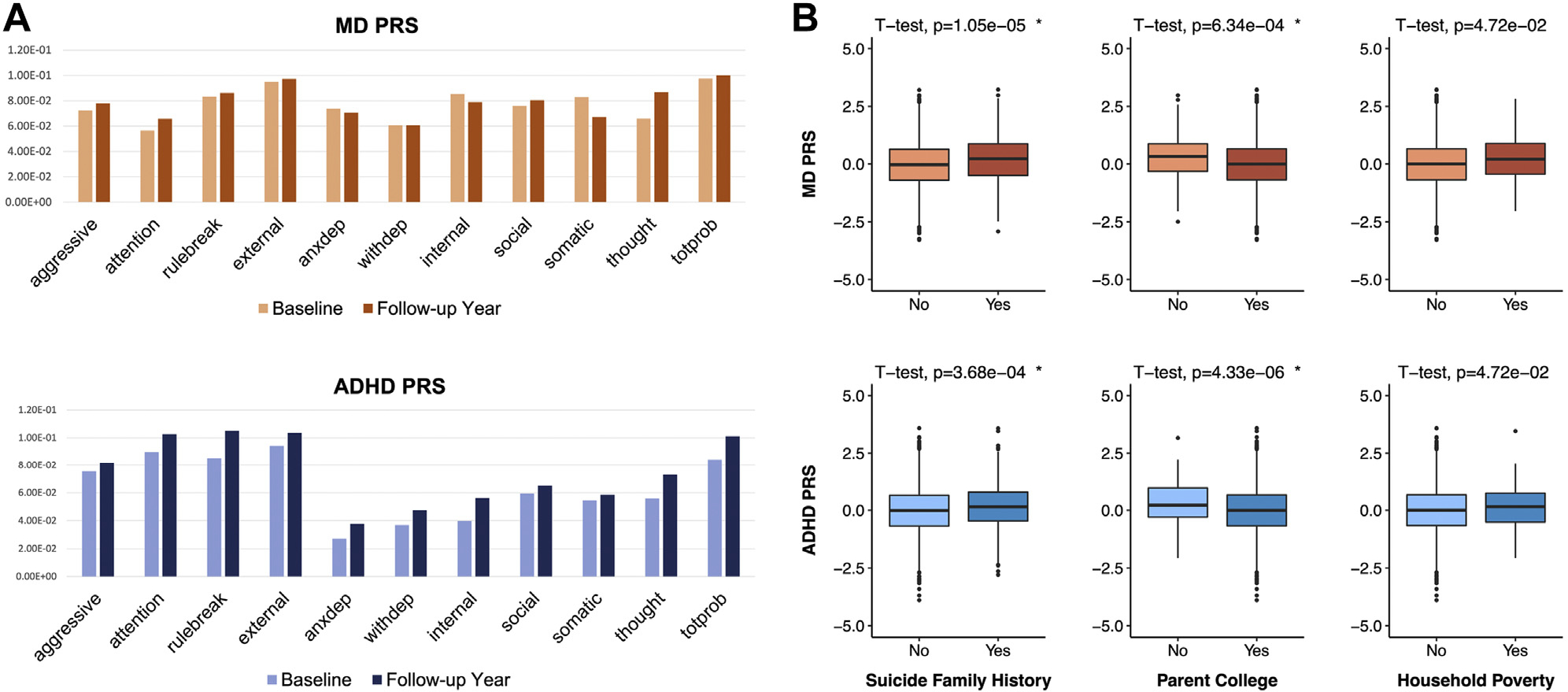
Association analysis results of major depression (MD) and attention-deficit/hyperactivity disorder (ADHD) polygenic risk scores (PRSs) with known risk factors of suicide. (A) Partial correlations were measured between PRSs and 11 Child Behavior Checklist items, conditioning on age, sex, and 10 genetic principal components to control for potential population substratification within Europeans. The y-axis represents the t-statistics of partial correlation measures. (B) The t-test results for comparing ADHD and MD PRSs between 2 groups of participants stratified by various risk factors. Participants were divided into two groups based on family history of suicide, parent college education, and poverty. Poverty was set at yes if the household income of the participants was less than $20,000 annually. The top panels display MD PRS scores on the y-axis, while the bottom panels show ADHD PRS scores. aggressive = aggressive behavior; anxdep = anxious/depressed; attention = attention problems; external = externalizing problems; internal = internalizing problems; rulebreak = rule-breaking behavior; social = social problems; somatic = somatic complaints; thought = thought problems; totprob = total problem score; withdep = withdrawn/depressed.
Independent Predictive Effects of PRSs on Suicidality
Considering the significant association of ADHD and MD PRSs with child psychopathology and family history of suicide, we examined whether PRSs could improve prediction of suicidality in children, independent of known risk factors of suicide. Multiple logistic regression analysis confirmed that MD PRSs are independently associated with youth-reported lifetime SAs, after accounting for age, sex, family history of suicide, socioeconomic status, and child psychopathology using the CBCL total score (Table 2; Table S9). With the addition of MD PRSs, Nagelkerke’s R2 indicated an improvement of 1.87% (PRS OR = 1.55, 95% CI = 1.10–2.18, p = 1.27 × 10−2) in the baseline and 1.17% (PRS OR = 1.38, 95% CI = 1.08–1.77, p = 1.05 × 10−2) in the follow-up year for the prediction model. We also confirmed the independent contribution of ADHD PRSs with SI symptom in the follow-up year. In the prediction model, child psychopathology was the most significant predictor of SI (OR = 1.70, SE = 0.05, p < 2 × 10−16). Association of ADHD PRSs followed in the second place (OR = 1.15, 95% CI = 1.05–1.26, p = 3.71 × 10−3). Nagelkerke’s R2 was estimated as 0.39% with the addition of ADHD PRSs. Using both ADHD and MD PRSs in the same model yielded similar results, with little improvement in prediction performance (likelihood ratio test p > .05) (Table S10).
Table 2.
Logistic Regression Analysis Results of ADHD and Major Depression PRSs on Suicidality While Accounting for Demographic, Socioeconomic, and Family Risk Factors
| Outcome/Independent Variable | OR | 95% CI | Beta | SE | p Value | R 2 |
|---|---|---|---|---|---|---|
| Suicide Attempts (Baseline) | 1.87% | |||||
| Age | 0.92 | 0.66–1.29 | −0.08 | 0.17 | 6.32 × 10−1 | |
| Sex | 1.02 | 0.51–2.01 | 0.02 | 0.35 | 9.62 × 10−1 | |
| Marital status | 0.61 | 0.28–1.35 | −0.49 | 0.40 | 2.24 × 10−1 | |
| Parental education | 0.85 | 0.59–1.21 | −0.17 | 0.18 | 3.56 × 10−1 | |
| Household income | 0.70 | 0.47–1.04 | −0.36 | 0.20 | 7.53 × 10−2 | |
| Poverty | 0.84 | 0.29–2.47 | −0.17 | 0.55 | 7.54 × 10−1 | |
| Child psychopathology | 2.67 | 1.89–3.77 | 0.98 | 0.18 | 2.21 × 10−8c | |
| Family history of suicide | 1.12 | 0.86–1.46 | 0.11 | 0.14 | 4.14 × 10−1 | |
| Major depression PRS | 1.55 | 1.10–2.18 | 0.44 | 0.17 | 1.27 × 10−2a | |
| Suicide Attempts (Year 1) | 1.17% | |||||
| Age | 1.10 | 0.87–1.41 | 0.10 | 0.12 | 4.26 × 10−1 | |
| Sex | 1.04 | 0.64–1.71 | 0.04 | 0.25 | 8.65 × 10−1 | |
| Marital status | 0.64 | 0.36–1.15 | −0.44 | 0.30 | 1.40 × 10−1 | |
| Parental education | 0.83 | 0.64–1.07 | −0.19 | 0.13 | 1.47 × 10−1 | |
| Household income | 0.88 | 0.66–1.17 | −0.13 | 0.15 | 3.69 × 10−1 | |
| Poverty | 0.94 | 0.38–2.33 | −0.06 | 0.46 | 8.96 × 10−1 | |
| Child psychopathology | 2.61 | 2.03–3.35 | 0.96 | 0.13 | 7.73 × 10−14c | |
| Family history of suicide | 1.15 | 0.95–1.40 | 0.14 | 0.10 | 1.58 × 10−1 | |
| Major depression PRS | 1.38 | 1.08–1.77 | 0.32 | 0.13 | 1.05 × 10−2a | |
| Suicidal Ideation (Baseline) | 0.13% | |||||
| Age | 0.90 | 0.81–1.00 | −0.11 | 0.06 | 5.30 × 10−2 | |
| Sex | 0.83 | 0.67–1.04 | −0.18 | 0.11 | 1.07 × 10−1 | |
| Marital status | 0.75 | 0.56–1.00 | −0.29 | 0.15 | 4.78 × 10−2a | |
| Parental education | 0.95 | 0.84–1.07 | −0.06 | 0.06 | 3.65 × 10−1 | |
| Household income | 0.89 | 0.78–1.01 | −0.12 | 0.07 | 7.58 × 10−2 | |
| Poverty | 0.71 | 0.41–1.25 | −0.34 | 0.28 | 2.36 × 10−1 | |
| Child psychopathology | 1.59 | 1.43–1.77 | 0.46 | 0.05 | < 2 × 10−16c | |
| Family history of suicide | 1.00 | 0.90–1.11 | 0.00 | 0.05 | 9.69 × 10−1 | |
| ADHD PRS | 1.09 | 0.98–1.22 | 0.09 | 0.06 | 1.30 × 10−1 | |
| Suicidal Ideation (Year 1) | 0.39% | |||||
| Age | 0.98 | 0.89–1.07 | −0.02 | 0.05 | 6.46 × 10−1 | |
| Sex | 0.98 | 0.82–1.19 | −0.02 | 0.10 | 8.70 × 10−1 | |
| Marital status | 0.84 | 0.65–1.08 | −0.17 | 0.13 | 1.72 × 10−1 | |
| Parental education | 0.97 | 0.88–1.08 | −0.03 | 0.05 | 5.83 × 10−1 | |
| Household income | 0.92 | 0.82–1.03 | −0.08 | 0.06 | 1.46 × 10−1 | |
| Poverty | 0.92 | 0.57–1.49 | −0.08 | 0.24 | 7.43 × 10−1 | |
| Child psychopathology | 1.70 | 1.55–1.87 | 0.53 | 0.05 | < 2 × 10−16c | |
| Family history of suicide | 0.99 | 0.90–1.08 | −0.01 | 0.05 | 8.20 × 10−1 | |
| ADHD PRS | 1.15 | 1.05–1.26 | 0.14 | 0.05 | 3.71 × 10−3b |
Beta and SE represent the logistic regression beta coefficient and its standard error. OR was calculated as the exponential of the logistic regression beta coefficient. 95% CI is the 95% confidence interval of OR. p value represents the significance of estimated beta using z-statistic. R2 represents Nagelkerke’s pseudo R2, which estimates the unique proportion of variance explained by PRSs.
ADHD, attention-deficit/hyperactivity disorder; OR, odds ratio; PRS, polygenic risk score.
p < .05.
p < .01.
p < .001.
To assess the robustness of the main findings, we examined whether the independent predictive effects of PRSs are observed consistently as the p-value threshold for generating PRS changes. This sensitivity analysis tested 10 different p values (p = 5 × 10−8, p = 5 × 10−5, p = 5 × 10−3, p = 5 × 10−2, p = .1, p = .2, p = .3, p = .4, p = .5, and p = 1.0). We observed significant associations of genome-wide ADHD and MD PRSs with SI and SAs, respectively, across multiple p-value ranges (Table S11). We also found independent effects of MD and ADHD PRSs when predicting new-onset cases in the follow-up year (e.g., participants who did not endorse SI in the baseline but did in year 1) (Table S12) or when suicide PRSs were added in the prediction model (Table S13).
DISCUSSION
Despite significant heritability and familial aggregation of suicidal behaviors, defining features of genetic risk underlying suicide have been elusive, especially in children and adolescents. Recent GWASs now offer the possibility to quantify heritable risk for a range of phenotypes that are relevant to this construct. The potential for such information to serve as a tool for risk stratification is particularly important to study in relation to children, given the rising rates of suicide in youth and the dearth of empirical studies in this age group (32).
In the largest U.S. sample of children characterized with in-depth phenotype and genome-wide genetic variation data, our study shows robust evidence that common genetic variants underlying ADHD and MD are significant predictors of suicidality in children. Importantly, higher genetic susceptibility to MD is associated with increased SAs in children, while genome-wide genetic risk to ADHD is associated with SI in children, suggesting distinctive contributions of these clinical conditions to children’s suicide risk. These associations remain significant independent of established clinical, familial, and demographic risk factors of suicide.
Our findings are in agreement with previous epidemiological studies that showed a significant association between MD and suicide (11,33). Genome-wide genetics studies have reported significant genetic overlap of MD PRSs with a range of suicide risk phenotypes in adults, including SAs (17), severity of SAs (14), and suicide death (16). Our results extend previous evidence showing that increased risk of SAs in children may also be driven at least in part by genetic susceptibility to depression. Moreover, compared with previous PRS studies of adults with suicidal behaviors, the variance explained for the ABCD participants was larger. In Mullins et al. (17), depression PRSs explained up to 0.42% of SAs in adult patients with psychiatric disorders. In Levey et al. (14), depression PRSs explained up to 0.7% of phenotypic variance for the severity of SAs in adults, while our study shows the highest variance of 3.3%.
We also found that higher genetic risk for ADHD is specifically associated with increased SI in children. ADHD is one of the most common child-onset psychiatric disorders, with significantly increased risk of SI and SAs (34–36). There are multiple mechanisms through which ADHD may increase the risk of suicide. Several groups suggested a mediating role of depression between ADHD and suicidality based on the elevated comorbidity of MD among youths with ADHD (37,38). Our findings, however, suggest a potentially distinct etiological role of the two disorders in suicidality during development. Higher levels of impulsivity and irritability have also been attributed to increasing suicide risk in children with ADHD (39). Further studies will be essential to clarify genetic relationships between ADHD and SI and to identify potential environmental stressors that may trigger the transition from SI to action.
Another notable finding is that associations of ADHD and MD PRSs with suicidality in children were observed only for the youth report–based outcome measures. Considering the high level of discordance between the caregiver and the youth reports, more attention needs to be directed toward mental health assessments of children starting as early as elementary school age. Furthermore, we found no association of psychiatric disorder PRSs with NSSIs. While several studies have reported a common genetic etiology between NSSIs and SI (40,41), our finding suggests that the genetic basis of NSSIs is distinct from that of SI and suicidal behaviors at least among children of this age group.
Our study has several strengths. To the best of our knowledge, this is the first genetic data analysis of suicidal phenotypes reported for the ABCD Study. Our conservative quality control and focus on the participants of European ancestry ensure that the results are robust to potential confounding from population genetic structure. Consistent association of PRSs across a range of SNP selection thresholds also substantiates that PRSs for ADHD and MD, one representing externalizing problems and the other representing internalizing psychopathology, contribute to SI and SAs in children. Our study is also based on the largest GWASs of ADHD and MD, each representing hundreds of thousands of cases and controls. Use of independent GWASs with sufficient statistical power is one of the most critical factors in polygenic scoring analysis (42).
The present findings should be interpreted in light of several limitations. First, although the addition of ADHD and MD PRSs clearly explains more variance of suicidality independent of other risk factors, clinical utility of the prediction model is still limited. The ORs of both child psychopathology and PRSs, the two strongest risk predictors, were modest, and overall discrimination of the prediction models remained poor. Our future research aims to improve the accuracy of the prediction models, which includes the investigation of additional predictor sets and advanced statistical analysis methods (9). Second, our PRS genetics data analysis is based on ABCD participants of European ancestry and thus may not be generalizable to other populations. Our decision to restrict genetic analyses to European ancestry was to ensure findings that are not confounded by population genetic structure (43,44). Furthermore, GWAS data of 8 psychiatric disorders were based on European descendants, which may bias PRS analysis when applied to non-European individuals owing to differences in causal variants, effect estimates, and linkage disequilibrium structure between populations (42,44,45). We call for more proactive efforts to create well-powered GWAS datasets for currently underrepresented populations in genetic studies. Third, we have applied p value–based selection strategies to generate PRS scores, which have room for improvement. While PRSs are powerful predictors over individual genome-wide significant variants, a considerable proportion of SNPs included in the calculation may not be related to a target phenotype and thus limit the statistical power. Improvement of PRS scores, for example, based on relevant biological knowledge or statistical techniques, merits further study. Fourth, we note that we controlled for child psychopathology in our analyses using the CBCL total score, which includes items such as “deliberately harms self or attempts suicide” and “talks of killing self.” Our choice to include this score in our analysis promotes the generalizability of our findings, given that the CBCL is commonly used in both clinical and research settings; however, the inclusion of suicide-related items in these scales may have underestimated relationships in relevant analyses and speaks to the strength of the associations that we found. Finally, we note that our sample targeted a relatively narrow age range in children. Further studies of young children and adolescents are needed to clarify the relevance of PRSs to predictors at different age epochs (46,47).
Despite these issues, our data advance the sparse empirical literature on suicide risk in children. There is a high level of interest in identifying risk factors for suicide that could open the door to targeted evidence-based prevention strategies (48,49). Despite their modest prediction and the fact that the majority of the variance in childhood suicidality is yet unaccounted for, our findings show that PRSs provide independent predictive value relevant to other risk variables of suicidality. The rationale for studying youth samples is augmented by the possibility, suggested indirectly by our data, that there may be a greater genetic contribution to children’s risk for suicidality compared with adults. The ABCD Study provides a rich collection of longitudinal neuroimaging datasets. Our future research includes the investigation of these datasets to understand how genetic susceptibility to ADHD and MD leads to structural or functional changes of brain development, which may contribute to suicidality in youth (50,51). In conclusion, coupled with the known genetic basis of suicidality and the growing evidence from other fields of medicine that PRSs may contribute to risk stratification, our data support further research into personalized suicide screening that incorporates genomic information.
Supplementary Material
KEY RESOURCES TABLE.
| Resource Type | Specific Reagent or Resource | Source or Reference | Identifiers | Additional Information |
|---|---|---|---|---|
| Downloaded Data; Public Website | GWAS summary statistics for ADHD | Psychiatric Genomics Consortium | https://doi.org/10.6084/m9.figshare.14671965 | |
| Downloaded Data; Public Website | GWAS summary statistics for autism spectrum disorder | Psychiatric Genomics Consortium | https://doi.org/10.6084/m9.figshare.14671989 | |
| Downloaded Data; Public Website | GWAS summary statistics for anxiety disorder | Psychiatric Genomics Consortium | https://doi.org/10.6084/m9.figshare.14102594 | |
| Downloaded Data; Public Website | GWAS summary statistics for anorexia nervosa | Psychiatric Genomics Consortium | https://doi.org/10.6084/m9.figshare.14671980 | |
| Downloaded Data; Public Website | GWAS summary statistics for bipolar disorder | Psychiatric Genomics Consortium | https://doi.org/10.6084/m9.figshare.14102594 | |
| Downloaded Data; Public Website | GWAS summary statistics for major depression | Psychiatric Genomics Consortium | https://datashare.ed.ac.uk/handle/10283/3203 | |
| Downloaded Data; Public Website | GWAS summary statistics for PTSD | Psychiatric Genomics Consortium | https://doi.org/10.6084/m9.figshare.14672133 | |
| Downloaded Data; Public Website | GWAS summary statistics for schizophrenia | Psychiatric Genomics Consortium | https://doi.org/10.6084/m9.figshare.14681220 | |
| Downloaded Data; Public Website | ABCD Study phenotype and genotype data | https://nda.nih.gov/abcd | ABCD v3.0 | |
| Software; Algorithm | PLINK | https://zzz.bwh.harvard.edu/plink/plink2.shtml | PLINK v2.0 | |
| Software; Algorithm | PRSice | https://www.prsice.info | PRSice v2.3.5 | |
| Software; Algorithm | R | https://www.r-project.org | R v4.04 |
ACKNOWLEDGMENTS AND DISCLOSURES
This work was supported by the National Institute of Mental Health (Grant Nos. R00 MH101367 and R01 MH119243 [to PHL]). The ABCD Study was supported by the National Institutes of Health (Grant Nos. U01DA041022, U01DA041025, U01DA041028, U01DA041048, U01DA041089, U01DA041093, U01DA041106, U01DA041117, U01DA041120, U01DA041134, U01DA041148, U01DA041156, U01DA041174, U24DA041123, and U24DA041147).
Statistical analyses were carried out on the Partner’s Research Computing Cluster servers and high-performance computing clusters hosted by the Broad Institute of MIT and Harvard.
We thank the ABCD research team for their great efforts in collecting data. We thank the ABCD research participants and their families for their continued support of the ABCD Study. We thank the participants who donated DNA samples for various genetics research of common and complex human diseases. We thank the clinical and scientific teams that processed, analyzed, and publicly shared the summary statistics of genome-wide association study datasets for pain and depression, including the Psychiatric Genomics Consortium and the UK Biobank. We thank the investigators for their dedication in genetics research and the open data science policy.
Footnotes
All authors declare that they have no known competing financial interests or personal relationships that could have influenced the work reported in this article. RHP has received fees for consulting or service on scientific advisory boards for Genomind, Psy Therapeutics, Outermost Therapeutics, RID Ventures, and Takeda. He has received patent royalties from Massachusetts General Hospital. He holds equity in Psy Therapeutics and Outermost Therapeutics. In the past 3 years, RCK was a consultant for Datastat, Inc., Holmusk, RallyPoint Networks, Inc., and Sage Pharmaceuticals. He has stock options in Mirah, PYM, and Roga Sciences. MF has lifetime research support from Abbott Laboratories, Acadia Pharmaceuticals, Alkermes, Inc., American Cyanamid, Aspect Medical Systems, AstraZeneca, Avanir Pharmaceuticals, AXSOME Therapeutics, BioClinica, Inc, Biohaven, BioResearch, BrainCells Inc., Bristol-Myers Squibb, CeNeRx BioPharma, Cephalon, Cerecor, Clarus Funds, Clexio Biosciences, Clintara, LLC, Covance, Covidien, Eli Lilly and Company, EnVivo Pharmaceuticals, Inc., Euthymics Bioscience, Inc., Forest Pharmaceuticals, Inc., FORUM Pharmaceuticals, Ganeden Biotech, Inc., Gentelon, LLC, GlaxoSmithKline, Harvard Clinical Research Institute, Hoffman-LaRoche, Icon Clinical Research, Indivior, i3 Innovus/Ingenix, Janssen R&D, LLC, Jed Foundation, Johnson & Johnson Pharmaceutical Research & Development, Lichtwer Pharma GmbH, Lorex Pharmaceuticals, Lundbeck Inc., Marinus Pharmaceuticals, MedAvante, Methylation Sciences Inc, Brain & Behavior Research Foundation (formerly National Alliance for Research on Schizophrenia & Depression), National Center for Complementary and Alternative Medicine, National Coordinating Center for Integrated Medicine, National Institute on Drug Abuse, National Institutes of Health, National Institute of Mental Health, Neuralstem, Inc., NeuroRx, Novartis AG, Organon Pharmaceuticals, Otsuka Pharmaceutical Development, Inc., PamLab, LLC., Pfizer Inc., Pharmacia-Upjohn, Pharmaceutical Research Associates., Inc., Pharmavite LLC, PharmoRx Therapeutics, Photothera, Premiere Research International, Protagenic Therapeutics, Inc., Reckitt Benckiser, Relmada Therapeutics Inc., Roche Pharmaceuticals, RCT Logic, LLC (formerly Clinical Trials Solutions, LLC), Sanofi-Aventis US LLC, Shenox Pharmaceuticals, LLC, Shire, Solvay Pharmaceuticals, Inc., Stanley Medical Research Institute, Synthélabo, Taisho Pharmaceuticals, Takeda Pharmaceuticals, Tal Medical, VistaGen, and Wyeth-Ayerst Laboratories. He also has equity holdings at Compellis and Psy Therapeutics. All other authors report no biomedical financial interests or potential conflicts of interest.
Supplementary material cited in this article is available online at https://doi.org/10.1016/j.biopsych.2021.11.026.
Contributor Information
Phil H. Lee, Center for Genomic Medicine, Massachusetts General Hospital Department of Psychiatry, Massachusetts General Hospital; Stanley Center for Psychiatric Research, Broad Institute of MIT and Harvard, Cambridge, Massachusetts.
Alysa E. Doyle, Center for Genomic Medicine, Massachusetts General Hospital Department of Psychiatry, Massachusetts General Hospital.
Xuyang Li, Center for Genomic Medicine, Massachusetts General Hospital.
Micah Silberstein, Center for Genomic Medicine, Massachusetts General Hospital.
Jae-Yoon Jung, Department of Pediatrics, Stanford University, Stanford, California.
Randy L. Gollub, Department of Psychiatry, Massachusetts General Hospital
Andrew A. Nierenberg, Department of Psychiatry, Massachusetts General Hospital Dauten Family Center for Bipolar Treatment Innovation, Massachusetts General Hospital.
Richard T. Liu, Department of Psychiatry, Massachusetts General Hospital Depression Clinical and Research Program, Massachusetts General Hospital.
Ronald C. Kessler, Department of Health Care Policy, Harvard Medical School, Boston
Roy H. Perlis, Center for Genomic Medicine, Massachusetts General Hospital Department of Psychiatry, Massachusetts General Hospital; Stanley Center for Psychiatric Research, Broad Institute of MIT and Harvard, Cambridge, Massachusetts.
Maurizio Fava, Department of Psychiatry, Massachusetts General Hospital; Stanley Center for Psychiatric Research, Broad Institute of MIT and Harvard, Cambridge, Massachusetts.
REFERENCES
- 1.Xu J, Murphy SL, Kockanek KD, Arias E (2020): Mortality in the United States, 2018. NCHS Data Brief, no. 355. Hyattesville, MD: National Center for Health Statistics. [PubMed] [Google Scholar]
- 2.World Health Organization (2014): Global Health Estimates 2013: Deaths by Cause, Age and Sex, Estimates for 2000–2012. Geneva: World Health Organization. [Google Scholar]
- 3.World Health Organization: WHO Suicide data. Available at: https://www.who.int/data/gho/data/themes/mental-health/suicide-rates. Accessed February 7, 2022.
- 4.Appleby L, Kapur N, Shaw J, Rodway C, Turnbull P, Ibrahim S, et al. (2017): Suicide by children and young people. National Confidential Inquiry into Suicide and Homicide by People with Mental Illness (NCISH). Manchester: University of Manchester. [Google Scholar]
- 5.Hawton K, Bale L, Brand F, Townsend E, Ness J, Waters K, et al. (2020): Mortality in children and adolescents following presentation to hospital after non-fatal self-harm in the Multicentre Study of Self-harm: A prospective observational cohort study. Lancet Child Adolesc Health 4:111–120. [DOI] [PubMed] [Google Scholar]
- 6.Huber RS, Sheth C, Renshaw PF, Yurgelun-Todd DA, McGlade EC (2020): Suicide ideation and neurocognition among 9- and 10-year old children in the Adolescent Brain Cognitive Development (ABCD) Study [published online ahead of print Sep 28]. Arch Suicide Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Janiri D, Doucet GE, Pompili M, Sani G, Luna B, Brent DA, et al. (2020): Risk and protective factors for childhood suicidality: A US population-based study. Lancet Psychiatry 7:317–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aguinaldo L, Goldstone A, Hasler BP, Brent DA, Coronado C, Jacobus J (2021): Preliminary analysis of low-level alcohol use and suicidality with children in the Adolescent Brain and Cognitive Development (ABCD) baseline cohort. Psychiatry Res 299:113825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kessler RC, Bossarte RM, Luedtke A, Zaslavsky AM, Zubizarreta JR (2020): Suicide prediction models: A critical review of recent research with recommendations for the way forward. Mol Psychiatry 25:168–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Connor RC, Robb KA (2020): Identifying suicide risk factors in children is essential for developing effective prevention interventions. Lancet Psychiatry 7:292–293. [DOI] [PubMed] [Google Scholar]
- 11.Franklin JC, Ribeiro JD, Fox KR, Bentley KH, Kleiman EM, Huang X, et al. (2017): Risk factors for suicidal thoughts and behaviors: A meta-analysis of 50 years of research. Psychol Bull 143:187–232. [DOI] [PubMed] [Google Scholar]
- 12.Zai CC, de Luca V, Strauss J, Tong RP, Sakinofsky I, Kennedy JL (2012): Genetic factors and suicidal behavior. In: Dwivedi Y, editor. The Neurobiological Basis of Suicide. Boca Raton, FL: CRC Press/Taylor & Francis. [PubMed] [Google Scholar]
- 13.O’Reilly L, Kuja-Halkola R, Rickert ME, Class QA, Larsson H, Lichtenstein P, D’Onofrio BM (2020): The intergenerational transmission of suicidal behavior: An offspring of siblings study. Transl Psychiatry 10:173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levey DF, Polimanti R, Cheng Z, Zhou H, Nunez YZ, Jain S, et al. (2019): Genetic associations with suicide attempt severity and genetic overlap with major depression. Transl Psychiatry 9:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perlis RH, Huang J, Purcell S, Fava M, Rush AJ, Sullivan PF, et al. (2010): Genome-wide association study of suicide attempts in mood disorder patients. Am J Psychiatry 167:1499–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Docherty AR, Shabalin AA, DiBlasi E, Monson E, Mullins N, Adkins DE, et al. (2020): Genome-wide association study of suicide death and polygenic prediction of clinical antecedents. Am J Psychiatry 177:917–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mullins N, Bigdeli TB, Børglum AD, Coleman JRI, Demontis D, Mehta D, et al. (2019): GWAS of suicide attempt in psychiatric disorders and association with major depression polygenic risk scores. Am J Psychiatry 176:651–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mullins N, Kang J, Campos AI, Coleman JRI, Edwards AC, Galfalvy H, et al. (2022): Dissecting the shared genetic architecture of suicide attempt, psychiatric disorders and known risk factors. Biol Psychiatry 91:313–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Willour V, Seifuddin F, Mahon PB, Jancic D, Pirooznia M, Steele J, et al. (2021): Nonsuicidal self-injury, suicide ideation, and suicide attempts among sexual minority children. J Consult Clin Psychol 89:73–80. [DOI] [PubMed] [Google Scholar]
- 20.Blashill AJ, Fox K, Feinstein BA, Albright CA, Calzo JP (2021): Nonsuicidal self-injury, suicide ideation, and suicide attempts among sexual minority children. J Consult Clin Psychol 89:73–80. [DOI] [PubMed] [Google Scholar]
- 21.Assari S (2020): Racial variation in the association between suicidal history and positive and negative urgency among American children. J Educ Cult Stud 4:39–53. [DOI] [PubMed] [Google Scholar]
- 22.Assari S, Boyce S, Bazargan M, Caldwell CH (2020): African Americans’ diminished returns of parental education on adolescents’ depression and suicide in the Adolescent Brain Cognitive Development (ABCD) Study. Eur J Investig Health Psychol Educ 10:656–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DeVille D, Whalen D, Breslin FJ, Morris AS, Khalsa SS, Paulus MP, Barch DM (2020): Prevalence and family-related factors associated with suicidal ideation, suicide attempts, and self-injury in children aged 9 to 10 years. JAMA Netw Open 3:e1920956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zucker RA, Gonzalez R, Feldstein Ewing SW, Paulus MP, Arroyo J, Fuligni A, et al. (2018): Assessment of culture and environment in the Adolescent Brain and Cognitive Development Study: Rationale, description of measures, and early data. Dev Cogn Neurosci 32:107–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barch DM, Albaugh MD, Avenevoli S, Chang L, Clark DB, Glantz MD, et al. (2018): Demographic, physical and mental health assessments in the adolescent brain and cognitive development study: Rationale and description. Dev Cogn Neurosci 32:55–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luciana M, Bjork JM, Nagel BJ, Barch DM, Gonzalez R, Nixon SJ, et al. (2018): Adolescent neurocognitive development and impacts of substance use: Overview of the Adolescent Brain Cognitive Development (ABCD) baseline neurocognition battery. Dev Cogn Neurosci 32:67–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Achenbach TM (2019): International findings with the Achenbach System of Empirically Based Assessment (ASEBA): Applications to clinical services, research, and training. Child Adolesc Psychiatry Ment Health 13:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Achenbach TM, Rescorla LA (2001): Manual for the ASEBA School-Age Forms & Profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, & Families. [Google Scholar]
- 29.Lee P, Anttila V, Won H, Feng YA, Rosenthal J, Zhu Z, et al. (2019): Genomic relationships, novel loci, and pleiotropic mechanisms across eight psychiatric disorders. Cell 179:1469–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choi S, Mak TS, O’Reilly PF (2020): Tutorial: A guide to performing polygenic risk score analyses. Nat Protoc 15:2759–2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nagelkerke N (1991): A note on a general definition of the coefficient of determination. Biometrika 78:691–692. [Google Scholar]
- 32.Ayer L, Colpe L, Pearson J, Rooney M, Murphy E (2020): Advancing research in child suicide: A call to action. J Am Acad Child Adolesc Psychiatry 59:1028–1035. [DOI] [PubMed] [Google Scholar]
- 33.Ribeiro J, Huang X, Fox KR, Franklin JC (2018): Depression and hopelessness as risk factors for suicide ideation, attempts and death: Meta-analysis of longitudinal studies. Br J Psychiatry 212:279–286. [DOI] [PubMed] [Google Scholar]
- 34.Shen Y, Chan BSMC, Cui X, Liu J, Lu J, et al. (2021): Suicidal behaviors and attention deficit hyperactivity disorder (ADHD): A cross-sectional study among Chinese medical college students. BMC Psychiatry 21:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fuller-Thomson E, Rivière RN, Carrique L, Agbeyaka S (2020): The dark side of ADHD: Factors associated with suicide attempts among those with ADHD in a national representative Canadian sample [published online ahead of print Dec 21]. Arch Suicide Res. [DOI] [PubMed] [Google Scholar]
- 36.Giupponi G, Giordano G, Maniscalco I, Erbuto D, Berardelli I, Conca A, et al. (2018): Suicide risk in attention-deficit/hyperactivity disorder. Psychiatr Danub 30:2–10. [DOI] [PubMed] [Google Scholar]
- 37.Yoshimasu K, Barbaresi WJ, Colligan RC, Voigt RG, Killian JM, Weaver AL, Katusic SK (2019): Psychiatric comorbidities modify the association between childhood ADHD and risk for suicidality: A population-based longitudinal study. J Atten Disord 23:777–786. [DOI] [PubMed] [Google Scholar]
- 38.Biederman J, Ball SW, Monuteaux MC, Mick E, Spencer TJ, McCreary M, et al. (2008): New insights into the comorbidity between ADHD and major depression in adolescent and young adult females. J Am Acad Child Adolesc Psychiatry 47:426–434. [DOI] [PubMed] [Google Scholar]
- 39.Garas P, Balazs J (2020): Long-term suicide risk of children and adolescents with attention deficit and hyperactivity disorder—a systematic review. Front Psychiatry 11:557909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Campos A, Verweij KJH, Statham DJ, Madden PAF, Maciejewski DF, Davis KAS, et al. (2020): Genetic aetiology of self-harm ideation and behaviour. Sci Rep 10:9713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maciejewski D, Creemers HE, Lynskey MT, Madden PA, Heath AC, Statham DJ, et al. (2014): Overlapping genetic and environmental influences on nonsuicidal self-injury and suicidal ideation: Different outcomes, same etiology? JAMA Psychiatry 71:699–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lewis C, Vassos E (2020): Polygenic risk scores: From research tools to clinical instruments. Genome Med 12:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tucker G, Price AL, Berger B (2014): Improving the power of GWAS and avoiding confounding from population stratification with PC-Select. Genetics 197:1045–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martin A, Kanai M, Kamatani Y, Okada Y, Neale BM, Daly MJ (2019): Clinical use of current polygenic risk scores may exacerbate health disparities. Nat Genet 51:584–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Duncan L, Shen H, Gelaye B, Meijsen J, Ressler K, Feldman M, et al. (2019): Analysis of polygenic risk score usage and performance in diverse human populations. Nat Commun 10:3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bridge J, Goldstein TR, Brent DA (2006): Adolescent suicide and suicidal behavior. J Child Psychol Psychiatry 47:372–394. [DOI] [PubMed] [Google Scholar]
- 47.Sheftall A, Asti L, Horowitz LM, Felts A, Fontanella CA, Campo JV, Bridge JA (2016): Suicide in elementary school-aged children and early adolescents. Pediatrics 138:e20160436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nierenberg A (2021): Precision psychiatry and a vision for the future. Psychiatr Ann 51:206. [Google Scholar]
- 49.Niculescu A, Le-Niculescu H, Levey DF, Phalen PL, Dainton HL, Roseberry K, et al. (2017): Precision medicine for suicidality: From universality to subtypes and personalization. Mol Psychiatry 22:1250–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schmaal L, van Harmelen AL, Chatzi V, Lippard ETC, Toenders YJ, Averill LA, et al. (2020): Imaging suicidal thoughts and behaviors: A comprehensive review of 2 decades of neuroimaging studies. Mol Psychiatry 25:408–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hibar D, Stein JL, Renteria ME, Arias-Vasquez A, Desrivières S, Jahanshad N, et al. (2015): Common genetic variants influence human subcortical brain structures. Nature 520:224–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


