Abstract
Elexacaftor, tezacaftor, ivacaftor (ETI) have been associated with marked clinical improvements in adults with CF, which appears to be associated with increased fertility. However, maternal and fetal effects of therapy continued during pregnancy are not well understood. We collected maternal blood, infant blood, cord blood, and breast milk from 3 mother-infant pairs from women who elected to remain on ETI therapy while pregnant. Our results demonstrated relatively high levels of ETI in cord blood, suggesting placental transfer of these compounds, as well as low levels of ETI in breast milk and infant blood, suggesting further transfer of these compounds to breast-fed infants in the post-natal period. These data underscore the need for larger studies on the effects of modulator surrounding reproduction.
Keywords: Pregnancy, elexacaftor, tezacaftor, ivacaftor, breast milk
Introduction
As life expectancy and quality of life has improved for people with CF with the advent of highly effective modulator treatment (HEMT) [A,B], women with CF are more frequently seeking pregnancy[1-3]. Furthermore, emerging evidence suggests that ETI may also improve fertility among women with CF[4]. A risk-benefit assessment of continuing therapies such as ETI is a critical part of clinical care before and during pregnancy, but data are lacking to inform these decisions. Reports of clinical deterioration in persons with CF upon discontinuation of HEMT favor continuing ETI during pregnancy to protect maternal health, however there is insufficient evidence to support ETI continuation during pregnancy as standard therapy[5]. Fortner and colleagues recently reported a homozygous Phe508del infant born to a mother with CF on ETI therapy throughout pregnancy who had a false-negative newborn screen, normal pancreatic function, and lower-than-expected sweat chloride levels[6]. These findings suggest fetal exposure to ETI rescued CFTR function in utero, which is consistent with a previous case report showing significant lumacaftor and ivacaftor concentrations in cord blood [7]. While animal studies have not suggested a strong association between ETI and adverse fetal outcomes, there is a known association between ivacaftor exposure and cataract formation[8]. In addition, the risk of breastfeeding infants while on ETI therapy is not well defined. Animal models suggest the individual components of ETI cross into breast milk, and the prior case report found significant lumacaftor and ivacaftor levels in breast milk [7]. To address fetal and neonatal exposure to ETI with mothers on therapy, we assessed maternal, fetal, and infant ETI exposure in three pregnancies through concentrations of ETI in cord blood, breast milk, and maternal and infant plasma.
1. Methods
This observational study was performed at the Adult Cystic Fibrosis Clinic at Oregon Health & Science University (OHSU) in Portland, Oregon and the Robert C. Schwartz Cystic Fibrosis Center at SUNY Upstate University Hospital in Syracuse, New York, and was approved by the institutional review board at OHSU, and subjects provided informed consent prior to participation. A pre-natal sample of maternal blood was obtained as a baseline comparison, and samples of maternal blood, cord blood, and newborn blood were obtained in the perinatal period when available. In the post-partum period, infant blood and breast milk samples were obtained whenever the infant’s pediatrician deemed clinical labs advisable for monitoring the infant’s health. A detailed clinical summary of the third mother-infant subject pair was previously described in a case report by Fortner et al[6], and a single complication of the second subject pair was reported in a prior study[5], though neither of these reports include measurement of ETI concentrations.
For analysis of concentrations via mass-spectroscopy, 100 uL of each sample was prepared by acetonitrile crash, with supernatants lyophilized to dryness and resuspended in 20% methanol. Individual component concentrations were assessed via liquid chromatography-mass spectroscopy using a previously described method[7] modified to add the selected reaction monitoring conditions for tezacaftor (m/z 521.5 -> 449.1) and elexacaftor (m/z 598.3 -> 422.3) determined empirically from analysis of standards. Peak area was extracted using automated processing software (Xcalibur®), and concentrations were determined though analysis of samples spiked with known concentrations of modulators.
No statistical methods were planned due to the pilot nature of this observational report and low sample size. Furthermore, due to the experimental nature of this assay, results were to be reported in aggregate a priori so as to minimize influencing clinical care for individuals.
2. Cohort report and results
A total of three mother and infant dyads were recruited for this study (see Table 1). Two women were identified and enrolled pre-partum from OHSU (subject pair A and B) and one subject pair enrolled from Syracuse post-partum following diagnosis of CF in the infant (subject pair C). All mothers were Phe508del homozygous. Two mothers started ETI prior to conception, whereas one mother started therapy during the second trimester after ETI became available. There were no interruptions in therapy. Subject pair A’s delivery was complicated by difficult delivery and use of vacuum extraction causing cephalohematoma that contributed to newborn jaundice. The pregnancy of subject pair B was notable for maternal cholecystitis requiring cholecystectomy but an unremarkable delivery. This complication was included in an series which has been previously reported[5]. Subject C’s pregnancy and delivery were unremarkable. All mothers breastfed, although with variable levels of formula supplementation.
Table 1.
Clinical comparison of cases.
| Subject pair A | Subject pair B | Subject pair C | |
|---|---|---|---|
| Location | OHSU | OHSU | Syracuse, previously discussed in Fortner paper[4] |
| Genotype | Phe508del homozygous | Phe508del homozygous | Phe508del homozygous |
| ETI start | 2nd trimester | Prior to conception | Prior to conception |
| ETI interruptions | No | No | No |
| Maternal complications | None | Maternal cholecystitis requiring cholecystectomy[3] | None |
| Delivery complications | Vacuum assisted delivery causing cephalohematoma and newborn jaundice | Unremarkable delivery | Unremarkable delivery |
| Breastfeeding | Yes + formula supplementation | Yes + formula supplementation | Yes + formula supplementation |
| Infant details | Healthy Temporary cardiac murmur | Healthy Temporary ALT elevation | F508del homozygous CF |
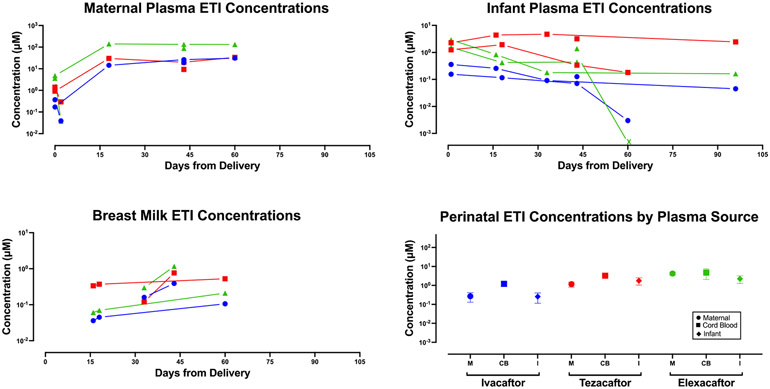
ETI levels in maternal serum, cord blood, and infant serum are illustrated in Figure 1. Umbilical cord blood levels of each component of ETI were similar to or higher than perinatal maternal serum levels at the time of delivery, with newborn levels comparable to maternal levels. Interestingly, perinatal maternal concentrations appeared lower than maternal concentrations collected at other time points including at 15 days prior to delivery, despite continued dosing of ETI therapy during labor and in the perinatal period.
Figure 1.
Aggregate maternal plasma, infant plasma, and breast milk concentrations of ETI from all 3 subject pairs on logarithmic sale. Samples from the same subject are denoted with connecting lines. Since perinatal samples were obtained from only two subjects, the range of these two values are denoted with error bars.
Analysis of serum from infants outside of the immediate neonatal period showed continued presence of all drug components, though at lower concentrations relative to cord blood levels and non-perinatal maternal levels. Similarly, all three ETI components were detectable in breast milk analysis though at much lower levels than maternal serum. Interestingly, tezacaftor concentrations were higher than the other two drugs in breast milk and infant serum, whereas elexacaftor was at highest relative concentrations in maternal serum and cord blood.
The clinical course of infants in this case series were generally benign. Infant A was healthy, including ophthalmologic exam, other than a heart murmur that self-resolved. Infant B had slowly increasing ALT values through 90 days of age, peaking at 65 U/L (upper limit of normal 30 U/L). Liver ultrasound and ophthalmologic exams were normal. Evaluation by a pediatric gastroenterologist did not identify a cause of increase ALT other than exposure to ETI, and levels normalized by age 158 days. Infant C was diagnosed with F508del homozygous CF by genetic testing but had normal newborn screen, normal pancreatic function, and lower-than-expected sweat chloride levels as previously reported [6]. The infant had serial fecal elastase measurements during the first year of life, which were all within the normal range, and the patient remained without clinical signs of pancreatic insufficiency. At age 292 days, the infant had amylase and lipase levels measured in peripheral blood, which were 105 and 2680 U/L respectively (normal ranges: 30-110 U/L, and <300 U/L respectively). These were obtained for surveillance purposes without any clinical signs of pancreatitis.
3. Discussion
Our findings suggest that elexacaftor, tezacaftor and ivacaftor cross the placenta leading to substantial fetal exposure in women continuing ETI therapy in pregnancy. The measured drug concentrations in cord blood being higher than or similar to maternal serum levels suggest that fetal drug concentrations in utero are within the therapeutic range. Physiologic changes in pregnancy may alter drug pharmacokinetics, however, it is not clear why perinatal maternal serum concentrations were lower than values measured earlier in pregnancy or post-partum. Given the small number of subjects in this study, more data is needed to determine the impact of pregnancy and delivery on maternal ETI concentrations.
Similar to our previous findings with lumacaftor and ivacaftor [7], we identified detectable concentrations of ETI in breast milk. The relative concentrations of tezacaftor were higher in breast milk than in maternal serum, suggesting that this drug is transported into breast milk with greater affinity than the other CFTR modulators. The presence of ETI in breast milk likely contributed to the low but measurable levels of ETI in infant serum, in which tezacaftor was also relatively higher than the other drug components. The low concentrations of ETI in infants did not lead to significant complications other than mild elevations in ALT that ultimately resolved.
However, the true safety of ETI in infants cannot be assessed in this small study. Infant C poses a special case, given her diagnosis of CF. While she was born with normal pancreatic function as evidenced by clinical features as well as fecal elastase and IRT, our findings would suggest that her total exposure to ETI diminished with time, despite breastfeeding. Although she has not developed clinical pancreatic insufficiency, her elevated lipase levels at 9 months of life could suggest slowly developing pancreatic dysfunction.
These findings underscore the need for additional data on effects of HEMT use with pregnancy, particularly in a prospective, multi-centered fashion, as well as provide proof-of-concept for pharmacokinetic studies of modulators in pregnancy. The Maternal and Fetal Outcomes in the Era of Modulators (MAYFLOWERS) study sponsored by the CF Foundation and CF Therapeutics Development Network is currently starting, which we hope will provide more robust results to better assist clinical providers and patients prepare for and manage pregnancy in CF.
Highlights.
Concentrations of Elexacaftor, tezacaftor, ivacaftor (ETI) in cord blood were reported when ETI was continued during pregnancy.
Concentrations of ETI in breast milk and infant blood suggest ongoing exposure in early life.
Acknowledgements:
The authors would like to thank the mothers and their families for allowing access to these samples during major life events. Also, we would like to thank the local hospitals and clinic which helped facilitate sample collection.
Funding source:
Dr. Esther reports from the National Institutes of Health (NIH/NIEHS P30-ES10126, NIH/NHLBI P30-DK065988 and P01 HL108808), and Cystic Fibrosis Foundation (RDP R026-CR11). Dr. Trimble reports funding from the Cystic Fibrosis Foundation (TRIMBL19AC0).
Footnotes
Declarations of interest:
Drs. Trimble and Fortner report the following personal financial relationships with commercial interests relevant to this article within the past 3 years: As faculty in institutions within the CF Therapeutics Development Network, Dr. Trimble and Fortner have been site Principal Investigator on studies for Vertex. Dr. Fortner reports receiving compensation for speaking engagements for Vertex.
Credit author statement
B Collins: Writing Original Draft, Review, & Editing C Fortner: Methodology, Investigation. A Cotey: Investigation. CR Esther Jr: Methodology, Validation, Investigation, Resources, Data Curation A Trimble: Conceptualization, Investigation, Visualization, Writing Original Draft, Review, & Editing, Supervision
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Kazmerski TM, Gmelin T, Slocum B, Borrero S, Miller E. Attitudes and decision making related to pregnancy among young women with cystic fibrosis. Maternal and child health journal. 2017;21(4):818–24. [DOI] [PubMed] [Google Scholar]
- 2.Middleton PG, Mall MA, Dřevínek P, Lands LC, McKone EF, Polineni D, et al. Elexacaftor–Tezacaftor–Ivacaftor for Cystic Fibrosis with a Single Phe508del Allele. New England Journal of Medicine. 2019;381(19):1809–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heijerman HGM, McKone EF, Downey DG, Van Braeckel E, Rowe SM, Tullis E, et al. Efficacy and safety of the elexacaftor plus tezacaftor plus ivacaftor combination regimen in people with cystic fibrosis homozygous for the F508del mutation: a double-blind, randomised, phase 3 trial. The Lancet. 2019;394(10212):1940–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O'Connor KE, Goodwin DL, NeSmith A, Garcia B, Mingora C, Ladores SL, et al. Elexacafator/tezacaftor/ivacaftor resolves subfertility in females with CF: A two center case series. Journal of Cystic Fibrosis. 2021;20(3):399–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taylor-Cousar JL, Jain R. Maternal and fetal outcomes following elexacaftor-tezacaftor-ivacaftor use during pregnancy and lactation. Journal of Cystic Fibrosis. 2021. [DOI] [PubMed] [Google Scholar]
- 6.Fortner CN, Seguin JM, Kay DM. Normal pancreatic function and false-negative CF newborn screen in a child born to a mother taking CFTR modulator therapy during pregnancy. Journal of Cystic Fibrosis. 2021. [DOI] [PubMed] [Google Scholar]
- 7.Trimble A, McKinzie C, Terrell M, Stringer E, Esther CR Jr. Measured fetal and neonatal exposure to Lumacaftor and Ivacaftor during pregnancy and while breastfeeding. Journal of Cystic Fibrosis. 2018;17(6):779–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vertex pharmacuticals. Trikafta® (elexacaftor,tezacaftor,ivacaftor; ivacaftor) [package insert]. U.S. Food and Drug Administration; website. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/212273s000lbl.pdf Revised October 2019. Accessed August 31, 2021. [Google Scholar]