Abstract
Chronic subdural hematoma (CSH) affects mostly elderly subjects. Previously, pathophysiological concepts suggested that CSH is secondary to degradation of subdural collections of blood and its products exerting merely a mass effect on the underlying brain. During the last decades, however, new insights into the pathogenetic mechanisms urge us to reconsider such a simplistic view. Here, we critically review novel pathophysiological, imaging, interventional, and medical treatment aspects and establish an integrative concept of the pathogenesis of CSH stressing the role of age as key factor. Trauma is considered a trigger event that unleashes a cascade of immunological and angiogenic age-dependent responses. These are associated with hypervascularization of the outer hematoma membrane, rebleeding, and exsudation which are crucial determinants for further development and propagation of CSH. Neurosurgical evacuation of the hematoma has long been thought the only viable treatment option, and it is still the method of choice in the majority of cases. Only more recently, embolization of the middle meningeal artery has been introduced as an alternative to surgery, and pharmacological treatment options are being investigated. Persons with advanced age trauma and other trigger events encounter a repair system with characteristics of senescence. This repair system implies a dysfunctional secretory phenotype of senescent cells and results in an insufficient repair process including chronic inflammation and fibrosis. Increased knowledge about the pathomechanisms of CSH will inform future studies and open new perspectives for its treatment and possibly also for its prevention.
Keywords: Chronic subdural hematoma, Pathophysiology, Age, Rebleeding, Exsudation, Hypervascularization
Introduction
Chronic subdural hematoma (CSH) affects mostly the elderly with increasing relevance due to aging of the population both in Western and Asian countries [1]. Apart from the mechanistic view that CSH is simply a posttraumatic subdural collection of blood and its degradation products becoming symptomatic because of its space-occupying effect, new insights including molecular-biological aspects are increasingly gaining acceptance. The latter indicate that immunological and angiogenic mechanisms are pivotal for CSH development and enlargement which is fundamentally different from the pathophysiology of acute subdural hematoma [2–9].
The diagnosis of CSH is of utmost clinical importance as it is a treatable entity affecting the elderly and manifesting with various neurological symptoms and dementia [10–12]. In fact, it is one of the most common disorders seen in neurosurgical practice [13]. The typical CSH patient is a male in his seventh decade. Obviously, age may predispose to the development of CSH in a multifaceted fashion: older patients suffer falls more often [14], they are frequently under anticoagulation treatment [15], and brain atrophy—an independent risk factor for CSH—is correlated with advanced age [16–19]. However, it becomes evident that various other factors need to be considered which have been neglected or underestimated thus far. On closer inspection, CSH is not just the chronic version of an acute subdural hematoma. Trauma is not the single leading cause of CSH [20, 21] and acute subdural bleeding is not an indispensable prerequisite for the development of CSH (Fig. 1) [4, 22–24]. Only a minority of acute subdural hematomas turn into the chronic form [4, 25–27] and CSH is not an obligatory transient phase during the healing process after an acute bleeding. In contrast, CSH constitutes a unique entity and both trauma and bleeding can be seen as triggering events which are followed by a cascade of immunological and angiogenic responses.
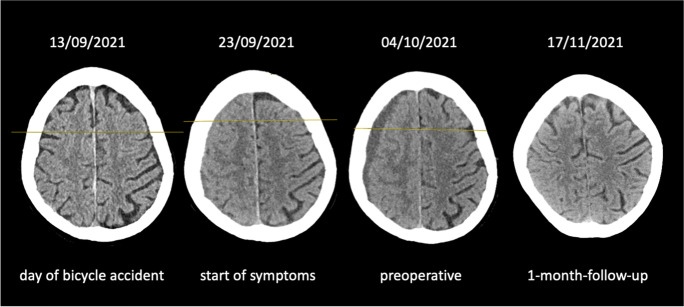
Fig. 1.
CT scans of an 85-year-old woman. A Few hours after a bicycle accident without loss of consciousness. There is not any intracranial lesion. B Ten days after the accident slight speech disturbance is noted. C Twenty-one days after the accident. The patient suffers from continuous headache, mild hemiparesis on the left, and psychomotor slowness. D One month after surgery the patient has recovered completely
The clarification of these mechanisms is currently considered the most promising approach to develop non-surgical treatment modalities. Such treatment options would be desirable since mortality rates and unfavorable functional outcomes are still relatively high after surgery, most probably associated with the advanced age of the patients [28–30]. Therapeutic options had not changed substantially for decades until recently [13] with the introduction of embolization of the middle meningeal artery evolving as a new therapeutic concept for patients with CSH (for review, see Haldrup et al. [31]). Treatment by embolization is based directly on the angiogenic concept of CSH development and propagation [2, 32–35] and may serve as further evidence for the relevance of new insights.
Moreover, the age profile of CSH patients strongly points to age-dependent alterations of the involved physiological immune responses and angiogenic pathways which may involve genomic instability, epigenetic defects, dysregulation of metabolic pathways, increased cell senescence, impaired cell regeneration, increased reactive oxygen species by mitochondria, and loss of proteostasis [36]. A closer look at these issues may yield crucial rationales for future research. Therefore, we here aim to shed light on age-dependent cellular and molecular particularities of immune responses and angiogenic mechanisms in older patients and we will unravel possible relationships between experimental findings in CSH and age-associated alterations.
Methods
To provide a critical review of pathophysiological, imaging, interventional, and medical treatment aspects of CSH concerning immunological and molecular-biological processes, a systematic literature search was performed using the PubMed database. The following keywords were applied in combination with the term chronic subdural hematoma: “pathophysiology,” “etiology,” “computed tomography,” “CT,” “magnetic resonance imaging,” “MRI,” “inflammation,” “inflammatory,” “angiogenesis,” and “angiogenic.”
As a second step, research and review articles on CSH were screened for additional references.
All original articles were screened for content concerning the topics under investigation in the present review. Findings were attributed to the following categories: imaging studies, molecular factors involved in the pathogenesis of CSH, and non-surgical treatment studies.
Results
Imaging studies
CT morphology
Contemporary CT- and MRI-based imaging quality allows detection of a CSH in clinically suspected cases with a near 100% sensitivity. Because of its broad availability, the short acquisition time, and its relative insusceptibility for movement artifacts in potentially noncompliant patients, CT-based imaging is still the primary method of choice [37]. In cases of intracranial hemorrhage, fresh blood with its high content of cell nuclei and protein is hyperdense compared to brain tissue. With ongoing degradation of these elements, the appearance becomes iso- and finally hypodense. However, these characteristics of intracerebral hemorrhages cannot simply be seen as equivalents of fluid accumulation in CSH. Obviously, there are mechanisms involved in CSH that can cause different chronological scenarios paralleling encapsulation of the hematoma, rebleeding, and exsudation [38].
Ito and colleagues had demonstrated in the 1980s that high and mixed density hematomas represent recent rebleeding [39] whereas Kao showed that hematomas of the layering type have a high tendency to rebleed [40]. Different attempts have been made to categorize the appearance of CSH cases in order to estimate the extent of rebleeding or exsudation. Both may influence the need for surgery due to hematoma enlargement or the risk of recurrence after decompression. Nomura et al. used a classification that differentiated 5 types of CSH: hyperdense, isodense, hypodense, mixed, and layering type. They found that in mixed density hematomas and in the layering type the tendency to rebleed was highest. Additionally, the layering type had a higher fibrinolytic activity. According to their findings, they draw the conclusion that enlargement of the hematoma is the result of both rebleeding and plasma exsudation [41]. It had already been shown before that the concentration of the plasmin α2-plasmin inhibitor complex reflecting local hyperfibrinolysis was highest, also in the latter type of hematomas [42]. Subsequently, it was shown that hematomas of the layering type have a higher tendency to recur [43, 44]. In contrast to this, Nakamura et al. showed that hematomas which resolve spontaneously are hypodense [45]. Tokmak and colleagues demonstrated that the exsudation rate is related to CT appearance and was highest in hyperdense and mixed density hematomas including hematomas from the layering type. On the basis of Nomura’s CT classification, our group showed that the exsudation rate was significantly correlated with the concentration of vascular endothelial growth factor (VEGF) in the hematoma [38]. A similar correlation was found also on the basis of a MRI classification [46]. High rates of VEGF were found to be combined with high rates of exsudation and leaky vessels.
Another classification postulated 4 different types of CSH according to the internal architecture of the hematomas. Based on serial imaging of a series of 18 patients, Nakaguchi and colleagues draw the conclusion that CSH evolves from a so-called homogenous type of hematoma and matures via a laminar type to a separated type and finally to a trabecular type. Spontaneous resolution of hematomas would occur in the trabecular stage. The lowest rate of recurrence was found in this group of hematomas. Subsumed in the homogenous type of hematomas are those with homogenous hypodense or isodense appearance [44]. This is in line with the descriptions of Lee and colleagues who reported that up to 58% of hygromas (a watery collection of fluid in the subdural space without encapsulation) evolve into CSH [47]. The highest rate of recurrence was seen in the separated type that corresponds to the layering type of Nomura.
MRI morphology
The physical background of MRI is much more complex and the prediction of resolution of the CSH or its recurrence is much more challenging [43]. The image characteristics of CSH are dependent on the magnetic properties of its content. It seems that the appearance of hematoma fluid is mainly a function of hemoglobin and its degradation products. Oxygenated hemoglobin is diamagnetic, appearing hypointense on T1- and hyperintense on T2-weighted images. With deoxygenation after bleeding, it becomes hypointense on T1 and T2. Thereafter, deoxyhemoglobin is degraded to methemoglobin which appears hyperintense on T1 and hypointense on T2. After lysis of cells, methemoglobin accumulates extracellularly and causes a hypertense signal in T1 and T2. Finally, hemosiderin deposits are superparamagnetic and are hypointense in T1 and T2 [48]. MRI may help to distinguish CSH from hygromas which have properties similar to CSF and show low signal intensity on proton weighted images whereas the high protein content of CSH results in a high signal intensity [43]. It was found that T2*-gradient-echo sequences (GRE) are highly sensitive to the susceptibility effects of the paramagnetic and superparamagnetic properties of hemoglobin and its breakdown products [48]. They allow showing even small bleedings in brain parenchyma which was confirmed by histological studies [49]. Imaizumi and colleagues described a black band corresponding to the inner membrane of the hematoma as a characteristic finding in T2* images [50]. They suggested that it would indicate deoxyhemoglobin or hemosiderin deposits within macrophages. The band vanished in cases that resolved spontaneously and in those who healed after decompression whereas continuance of the band was indicative of recurrence. From a histological point of view, these findings are difficult to explain because the inner membrane is not well vascularized and is probably not the location of major bleeding.
Tsutsumi et al. described 5 different types of hematomas according to their characteristics on T1-weighted images [43]. Low intense or isointense hematomas would reflect recent rebleeding and had the highest rate of recurrence. They indicated that a higher concentration of methemoglobin would correlate with a higher intensity of the hematoma and with a longer period since the last rebleeding. Consecutively, they concluded that high intense hematomas reflect the lowest risk for rebleeding. On serial images, Kaminogo et al. confirmed that rebleeding was an important factor in hematoma enlargement and neurological deterioration [51]. At 1.5 Tesla, fresh rebleeding appeared as low-density lesions on T1 and high-density lesions on T2. A recent metanalysis on the importance of low signal intensity in T1-weighted images seems to confirm an increased recurrence rate in these cases [52]. Moreover, Hua and colleagues correlated the concentration of VEGF and matrix metalloproteinase (MMP) 2 and 9 [53] which are important proangiogenic factors that trigger an angiogenic switch in an otherwise quiescent vascular bed [54, 55]. They used a MRI classification that considered T1 and T2 characteristics of hematomas. Hematomas with low intensity on both sequences indicated a high risk of rebleed and were significantly related to a high concentration of VEGF. High concentrations of VEGF however were paralleled by high concentrations of MMP-2 and MMP-9 which may confirm the notion that VEGF is an important factor for hematoma enlargement due to rebleeding and exsudation [38].
Molecular factors involved in the pathogenesis of CSH
Inflammatory pathways
Chronic subdural hematoma had originally been described as an inflammatory disorder under the name pachymeningitis hemorrhagica interna [56]. Subsequently, several direct and indirect markers of inflammation were found to be locally elevated within the hematoma compared to serum including fibrinogen degradation products [57], platelet activation factor (PAF) [58], tissue plasminogen activator (tPA) [59], bradykinin [60], tumor necrosis factor (TNF) [61], VEGF [35, 62], interleukin 6 (IL-6), and IL-8 [61]. Infiltration of inflammatory cells has been described early in histological studies, especially the high number of eosinophils in the outer membrane awakened interest [63–67]. Eosinophils can secrete plasminogen and induce fibrinolysis which starts the cascade that ends with wound healing or fibrosis [66]. These cells are considered to be important effector cells of fibrosis in eosinophil-associated allergic diseases like asthma [68], idiopathic pulmonary fibrosis [69], obstructive nephropathy [70], and pediatric eosinophilic esophagitis [71] by releasing transforming growth factor β (TGF-β) and activating the TGF-β/Smad pathway. More recently, Osuka et al. found that eotaxin 3, an eosinophil-specific chemoattractant, is present in high concentrations within the hematoma [72]. Eosinophils are also the source of TGF-β, the concentration of which is also elevated in hematoma fluid. Furthermore, the Smad pathway was shown to be activated in fibroblasts of the neomembranes [72].
In previous studies, Osuka et al. had described activation of the JAK/STAT pathway in fibroblasts of the outer membrane which is an activator of transcription and is also related to pathological fibrosis activated by IL-6 [73]. Both IL-6 and IL-8 are potent agents of the inflammatory response and are found in hematoma fluid in high concentrations [6, 61, 74–78]. Even more important is the fact that concentrations are higher in those patients who suffer recurrence [6, 74]. The proinflammatory cytokine IL-6 modulates cell–cell contacts by enlarging gap junctions and increasing vascular permeability [79]. It is part of the acute phase reaction during inflammation. IL-8 (nowadays called CXCL-8) is a chemotactic chemokine which has an impact on migratory immune cells. It supports angiogenesis and is involved in angiogenesis-dependent processes like formation of granulation tissue and wound healing [80]. Bradykinin, thrombin, and PAF fuel secretion of IL-6 and IL-8 from fibroblasts, endothelial cells, and immune cells within the neomembranes [6].
Comparing the concentration of proinflammatory and anti-inflammatory cytokines in the hematoma, there is an imbalance to the disadvantage of the latter which has led to the speculation of a poorly coordinated innate immune response within the hematoma [77].
Angiogenic pathways
Knowledge about the molecular mechanisms of angiogenesis has increased tremendously during the last 30 years [81]. The sequential steps of vessel branching are well orchestrated by various types of biological factors which are also active in CSH development. Remarkably, the majority of processes involving angiogenesis are strictly related to inflammation. When quiescent vessels sense an angiogenic stimulus like VEGF, bFGF, or ANG 2, pericytes liberate from the shared basement membrane by proteolytic degradation. This process is mediated by MMPs. Its histological characteristics have already been described for formation of the neomembranes in CSH in publications in the 1970s [82–86]. In line with this, we detected specific patterns of growth factor distribution within the hematoma with increases in VEGF and bFGF and a decrease in platelet-derived growth factor (PDGF) [62]. In addition to elevated concentrations of VEGF within the hematoma [35, 74, 87, 88], also synthesis of VEGF within the neomembranes was described [89]. However, since the concentration of VEGF within the hematoma can be excessively high, it is doubtful whether there is just a simple secretion from the neomembranes. Furthermore, the occurrence of postoperative cures despite the presence of relatively high residual amounts of diluted fluid within the hematoma cavity in postoperative imaging studies may indicate that healing is not primarily a problem of hematoma volume but rather of content. Cells floating within the hematoma could be additional sites of synthesis. Also, the ANG1/ANG2 ratio was shown to be indicative of ongoing angiogenesis [90]. As outlined, the involvement of MMPs was described both in the formation of neomembranes [91] and in the development of hematoma itself [53]. Tissue inhibitors of MMPs (TIMP) as well as plasminogen activation inhibitors (PAI) inactivate MMPs and cause the deposition of a basement membrane. The latter is a prerequisite for competent vessels and indicates maturation of newly sprouted vessels. Remarkably, the concentration of PAI was shown to be highest in low-density hematomas which are considered to have the lowest rate of rebleeding; hence, the neomembranes are maturated and have the lowest angiogenic activity [92].
Placental growth factor (PlGF) is another potent stimulus for induction of angiogenesis which in contrast to VEGF is exclusively associated with pathological angiogenesis [93]. It has been found in high concentrations within the hematoma [88]. In this context, it is of interest that the ratio of PlGF to its counterpart, the soluble VEGF receptor 1 (sVEGFR-1), is decreased in the hematoma fluid compared to serum. High concentrations of VEGF and PlGF but low concentration of sVEGF-1 lead to vessel overgrowth [94], which might explain the vascular characteristics of the neomembranes. The proangiogenic properties of hematoma fluid are underlined by the fact that they activate the mitogen-activated protein kinases (MAPK) which transduce the signals generated from growth factors, cytokines, and stressor agents [95]. Under laboratory conditions, for example, the extracellular signal-regulated kinase complex (MEK/ERK) was activated within 5 min in the endothelium of vessels from the neomembranes by the hematoma fluid. Blocking VEGF or IL-6 interrupted activation of MEK/ERK [96]. Since VEGF increases vascular permeability via this pathway [97], it may explain exsudation which is one of the main mechanisms of hematoma enlargement.
PI3/Akt/mTOR is another important signaling pathway for the control of cell cycle and proliferation that is activated in the ECs of hematoma membranes [98]. To date, this pathway is considered to play a role in different organ cancers [99]. Under physiological circumstances, extravasation of plasma constituents provides a provisional extracellular matrix (ECM) which changes to an angio-competent milieu by liberating angiogenic molecules from extracellular stores via proteases like MMPs [81].
One of the intriguing questions is which factors turn on the angiogenic switch in CSH. Local hypoxia might be an important candidate since it induces the secretion of IL-6, TNF-α, Cox-2, and hypoxia-inducible factor (HIF). When tissue sensors detect hypoxia, the heterodimeric transcriptional factor HIF is induced which mediates the expression of VEGF on the transcriptional level [100]. HIF-1α is oxygen labile and degrades under aerobic conditions. Throughout the neomembranes, it is constantly expressed in conjunction with VEGF and it has been suggested that HIF is one of the main inducers of VEGF expression in CSH [101]. Other potential candidates for VEGF induction or VEGF release from extracellular matrix stores in CSH include IL-6 [6, 61, 75, 77], TNF [76], COX2/PGE2 [102, 103], bFGF [2], and MMPs [91, 104].
A prerequisite for maturation of branching vessels is covering the EC lines with pericytes and deposition of a basement membrane. This is in part mediated by the release of platelet-derived growth factor (PDGF). However, compared to serum, the concentration of PDGF is reduced within the hematoma [2].
Non-surgical treatment studies
Interventional
New insights into the pathophysiology of CSH suggest that exsudation and rebleeding from the outer membrane are important factors for recurrence and probably for hematoma enlargement, too. According to these angiogenic theories, the constituents of the hematoma and the outer membrane interact within a vicious cycle. Reduction of blood supply to the outer membrane via embolization of the middle meningeal artery (EMMA) should interrupt this vicious cycle. First success of this concept was documented in a case report in 2000 [105]. Since then, several small case series but also randomized controlled trials (RCT) evaluated the impact of EMMA within nonoperative and operative treatment algorithms (for review, see Srivatsan [106]). Further studies are under way and may suggest additional treatment options in the near future at least in oligosymptomatic or asymptomatic patients who actually follow a watch-and-wait or wait-and-scan policy [107].
Pharmacological
As CSH is significantly influenced by inflammatory and angiogenic mechanisms, the use of corticosteroids appears to be a logical consequence. Steroids inhibit IL-6 and IL-8 and reduce the expression of VEGF [108–111]. Indeed, back in 1976, Glover and Labadie demonstrated under laboratory conditions that dexamethasone inhibits the formation of a neomembrane [112]. However, the animal model used was based on the assumption that CSH derives from chronification of an acute hematoma which did not prove true. A few case series or retrospective series in patients with CSH followed since then [113–118]. Several national surveys on the treatment of CSH, however, revealed a controversy about its use [119–122]. Overall, two strategies of cortisone treatment were recognized: (1) cortisone as a monotherapy as part of a non-surgical treatment and (2) cortisone in addition to surgery in order to prevent recurrence (for review, see Berghauser Pont et al. [123]). Preliminary results of a prospective study with dexamethasone could not find any beneficial effect of standalone cortisone treatment but revealed more serious side effects in the verum group [124]. A recent prospective randomized trial of dexamethasone as an adjunct to surgery demonstrated a beneficial effect concerning the number of recurrences but an increased number of adverse events and an overall reduced outcome [125]. The authors therefore did not emphasize the regular use of dexamethasone. On the basis of the available evidence that corticosteroids can reduce VEGF concentrations in certain entities and that VEGF concentration is highest in the layering type of hematomas which corresponds to the very high recurrence rate in this type of hematoma, its use may be justified especially in this subgroup of hematomas [126]. Further controlled randomized trials are currently underway to clarify some of the issues [127].
Since PAF has been shown to be a potent mediator of inflammation and is thought to be involved in the formation of the neomembranes [128], the PAF receptor antagonist etizolam deserves interest. There is evidence that treatment with etizolam reduces the risk of recurrence [129], and it is negatively correlated with the need for surgery [130].
Atorvastatin is another possibly attractive pharmacon for conservative treatment of CSH with its anti-inflammatory properties having been demonstrated in vitro and in vivo [131, 132]. In a rat model of acute subdural hematoma, atorvastatin modulated the inflammatory reaction and fueled shrinkage of hematomas. The authors ascribed the positive effects of the statin to reduction of the concentration of inflammatory cytokines and maturation of newly sprouted vessels of the neomembrane [133]. In a small size retrospective analysis, atorvastatin given as an adjunct to surgery reduced significantly the rate of postoperative recurrence [134]. A prospective trial demonstrated that atorvastatin in adjunct to surgery significantly reduced the risk for recurrence and prolonged the time to recurrence [135]. Unfortunately, the manuscript reporting the trial was retracted from publication because of irregularities in follow-up in one of the collaborating centers. Recently, a beneficial effect of atorvastatin on recurrence and also on outcome was confirmed by a meta-analysis according to PRISMA criteria including six original publications [136].
Angiotensin-converting enzyme (ACE) inhibitors [90] have been shown to interfere with tumor angiogenesis [137] and attenuate pathological angiogenesis in diabetic retinopathy [137, 138]. We have shown previously that ACE inhibitors used for the treatment of arterial hypertension reduced the risk of recurrence if not the overall occurrence of CSH [90]. Furthermore, a reduced concentration of VEGF in the hematoma fluid was found in patients with ACE inhibitor treatment. This indicated that elevation of VEGF could be a causative factor in CSH. However, neither a prospective randomized study with the ACE inhibitor perindopril could demonstrate an advantage regarding recurrence or residual hematoma size 6 weeks after surgery [139] nor two retrospective studies [140, 141].
Furthermore, COX-2 inhibition was tested in a prospectively designed multicenter study [102]. The synthesis of VEGF and other proangiogenic factors is dependent on COX-2 mediated eicosanoids like prostaglandins and thromboxanes. Selective COX-2 inhibition revealed anti-angiogenic effects in vivo and in vitro [142, 143]. Although theoretically attractive, the study was discontinued due to recruitment problems.
A different treatment concept was tested using tranexamic acid which interrupts fibrinolysis through inhibition of plasmin synthesis. Preliminary results from 5 RCTs suggest both reductions of hematoma volume when used in a non-surgical treatment concept and lowering of recurrence rates when used as an adjunct to surgical treatment (for review, see Edlmann et al. [144]).
Finally, cure from recurrent CSH in a patient with rheumatic arthritis was anecdotally reported after the patient started treatment with infliximab, a TNF-α inhibitor [145].
Discussion
Since the first description of a case of CSH by Johann Jacob Wepfer in 1675 [146], different theories on the pathophysiology of CSH have emerged (for review, see Weigel et al. [147]). Historically, Virchow had favored an inflammatory condition [56] whereas Gardner had postulated an osmotic gradient as the driving force for CSH development and growth [148]. Later on, Ito and coworkers developed a theory of local hyperfibrinolysis [149] and demonstrated repetitive bleeding episodes inside the hematoma cavity with labeled erythrocytes [39]. Furthermore, pathological vascularization of the outer membrane was studied in more detail [85, 150, 151], and exsudation was identified as an important mechanism of hematoma enlargement [7, 152]. Subsequently, it was postulated that CSH can be interpreted as an angiogenic disorder [2]. The limited body of scientific research provides evidence in support of each of these aspects [3, 6, 41, 42, 61, 74–76, 78, 87–89, 104, 152–156]. Until recently, no valid experimental model was available to test former and current disease concepts and therapeutic approaches in the laboratory [157]. Only in 2021, an encouraging new animal model using old Sprague Dawley rats showing the typical features of CSH in humans was published [158].
Integrative concept of the pathogenesis of CSH
The subdural space is not present under physiological conditions. In the following, we summarize the pathophysiological mechanisms according to current knowledge. Shear stress even from a minor trauma dissolves the loosely connected border cells of the inner layer of the dura. This constitutes also a signal for fibroblast growth. A cascade of cellular angiogenic and inflammatory responses follows which results in the formation of a richly vascularized external neomembrane. The subdural space is filled with blood, blood degradation products, and extravasation fluid. Chemotaxis of immune cells occurs and is driven by fibrinogen degradation products, eotaxin, PAF, and CXCL-8 (IL-8). Increased levels of IL-6, HIF, TNF-α, and Cox-2 might induce VEGF secretion. Additionally, proteases like MMPs liberate angiogenic molecules stored in the provisional extracellular matrix (ECM). In parallel oversecretion of TGF-β activates intracellular messenger systems like Smat, which sensitizes cells for external stimulation via growth factors and other cytokines. High levels of VEGF and PlGF and overexpression of ANG-2 within the neomembranes keep newly sprouted vessels leaky by activation of the MEK/ERK signal transducers. Together with constantly high levels of IL-6 that increase vascular permeability via the JAK/STAT pathway, extravasation of plasma proteins perpetuates and leads to enlargement of the hematoma volume. Finally, anti-inflammatory cytokines like IL-10 and IL-13 or factors that could establish a patent vessel network like PDGF are inadequately represented within the hematoma which facilitates chronification.
The role of age and further perspectives
The immune response in CSH is unrewarding leading to a condition which may best be described as a chronic proinflammatory state characterized by hypervascularization, exsudation, and rebleeding (Fig. 2). The concentrations of proangiogenic and proinflammatory cytokines and chemokines are elevated. In general, a prolonged inflammatory response delays wound healing and probably promotes tissue fibrosis reducing the chance of true regeneration [159, 160]. Increased inflammatory responses are also characteristic features of aging cells and aging tissues [161, 162].
Fig. 2.
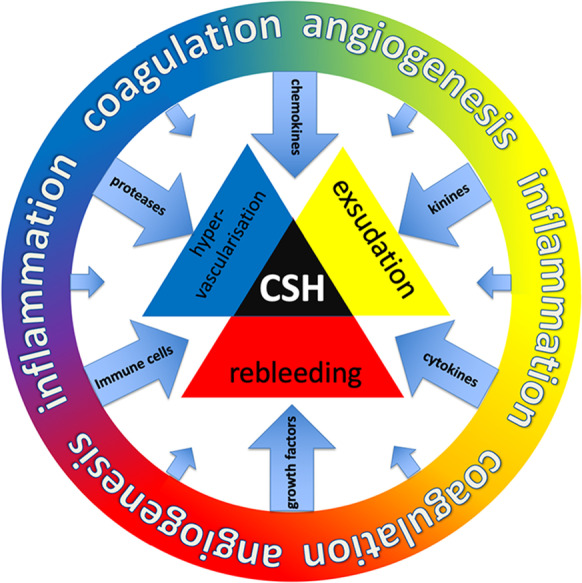
Schematic illustration of the pathogenesis of CSH. The outer ring represents different biological processes that are involved: angiogenesis, coagulation, and inflammation. Subsequent processes are chemotaxis of immune cells and secretion of growth factors, chemokines, cytokines, kinins, and proteases. The passages between these processes are fluent. They cause exsudation, hypervascularization, and rebleeding. As a result, chronic subdural hematoma (CSH) develops and is maintained
With regard to the data available presented thus far, the demographic background of CSH may suggest a “protective” effect of sex hormones, a dysregulated immune response of the senescent immune system, and a dysregulated angiogenetic response of an aging vascular bed.
It was early discussed in the CSH literature that sex hormones might exert a protective effect in minor head trauma [163]. Meanwhile, it has been demonstrated that estrogen has a potent anti-inflammatory effect [164, 165], it can accelerate wound healing [166], and it can improve systemic as well as cellular immune responses to traumatic injury [167]. Furthermore, estrogen has a vasoprotective effect in women [168]. Overall, it is feasible that there is a positive net effect of estrogen in patients at risk for the development of CSH.
Aging as an important prerequisite for the development of CSH might explain why experimental studies which were conducted with young rodents failed to produce experimental CSH thus far [157]. In general, aged individuals appear to be at higher risk for processes associated with pathological vessel formation. It has been shown that aging impacts virtually every angiogenic pathway identified thus far [169, 170]. With advancing age, endothelial cells have depleted anti-inflammatory and anti-oxidant defense mechanisms. Hence, they are subjected to augmented inflammatory and oxidative stress that impairs their number, morphology, and function [171]. Furthermore, changes in hemostatic function in the aging vasculature may have a profound effect on the induction of new vessels [172]. Angiogenesis in the aged often reveals impairment of maturation and stabilization of newly formed vessels [173] as it is seen also in CSH [82, 84, 85]. Such impaired angiogenesis may also be related to the production of reactive oxygen species (ROS) [174] or advanced glycation end products (AGE) [175]. The role of neither one has yet been elucidated in CSH. Notably, oxidative stress is increased in aging [176], and ROS can induce hyperstimulation of endothelial cells both by inducing VEGF production and by amplifying its intracellular effects [177].
There are several examples of age-dependent cellular and molecular disturbances of the immune response in the elderly (Table 1). In the following, we want to set such findings in perspective to the development of CSH in the elderly. Advanced age affects macrophage function, cytokine and chemokine secretion, infiltration, and wound repair [172]. It was shown that ocular macrophages in aged mice have impaired anti-angiogenic properties leading to pathological hypervascularization as seen in macular degeneration [193]. Furthermore, mRNA expression coding for the proinflammatory cytokines IL-6 and TNF-α has been shown to be increased in macrophages of elderly mice as compared to young animals [194]. Moreover, increased levels of IL-6 in IL-6 knockout mice are characteristic of the age-associated microenvironment of macrophages which play a role in the regulation of age-dependent defects of macrophages [167].
Table 1.
Mediators that are identified in the pathogenetic process of CSH and possible interference with age
| Mediator | CSH | Age |
|---|---|---|
| IL-6 | Higher concentration in CSH fluid compared to plasma [6] | Increase with age [178] |
| IL-8 | Higher concentration in CSH fluid compared to plasma [6] | No correlation of spontaneous levels with age; increased production in fibroblasts of the elderly after stimulation [179] |
| Eosinophils | Infiltration of eosinophils in outer membrane [64] | Age impairs effector functions [167] |
| Eotaxin 3 (CCL26) | Higher levels in CSH fluid compared to CSF [72] | Elevated levels in age- associated Neurodegenerative diseases [180] |
| TGF | Higher levels in CSH fluid compared to CSF [72] | Impairment of TGF signaling with age; upregulation of TGF-beta ligands [181] |
| VEGF | Higher levels in CSH fluid compared to plasma [35, 62] | Serum levels positively correlated with age [182] |
| bFGF | Higher levels in CSH fluid compared to plasma [2] | Expression of bFGF positively correlated with age in a mouse model of wound repair [183] |
| PDGF | Lower levels in CSH fluid compared to plasma [2] | Expression of PDGF delayed with increasing age in a mouse model of wound repair [183] |
| PlGF | Elevated levels in CSH fluid compared to plasma [88] | Role of PlGF restricted to pathological conditions [93] elevated in age-related retinal vasculopathies [184] |
| PAI | Lower levels in CSH fluid of layering and mixed density hematomas [92] | Elevated in the elderly [185] |
| MMP | Present in neomembranes [91] and hematoma [53] | Relevance in age-associated disorders [186, 187] |
| Bradykinin | Higher concentration in CSH fluid compared to plasma [60] | Downregulation of receptors in senescent cells [188] |
| TNF | Lower concentration in CSH fluid compared to plasma [104] | Increase with age [189–191] |
| tPA | Increased in CSH [59]; correlates with recurrence [92] | Reduced release with age [192] |
The term “inflammaging” stands for a chronic inflammatory state in elderly patients without an obvious underlying disease [195]. It is characterized by an elevated inflammatory response after trauma associated with increased levels of IL-6 [196]. Remarkably, increased IL-6 levels have been thought to be associated both with increased morbidity and disability [197, 198]. Such mechanisms may also be related to the increased IL-6 levels in CSH [6, 61, 74–77]. Another issue to consider is that in the elderly the number of peripheral eosinophils correlates with elevated serum levels of IL-6 [199]. The exact mechanism of the correlation has not yet been elucidated; however, a similar constellation is present within the neomembranes and the hematoma fluid of CSH patients [63, 64, 66, 200].
Inflammation is part of the wound healing process that induces further steps such as new tissue formation and tissue remodeling. Aging affects both the ability of cells to respond to injury and the physiology of the extracellular matrix (ECM) [201]. It is known that MMPs are involved in the remodeling of ECM and the expression and the activity of MMPs is dysregulated in the aging vasculature. This is a key factor of age-related vascular pathology and includes both excess or deficient activity [172]. Gingival repair is delayed in aged patients, and it has been shown that this is paralleled by an increase in the level of MMPs [202]. It was hypothesized that tissue repair is impaired in aged subjects secondary to increased levels of proteolytic activity [201]. With that regard, it has to be noticed that the involvement of different MMPs in the pathophysiological process of CSH was described both for the neomembranes and also for the hematoma itself [53, 91].
Aging also has been generally associated with increased intracellular levels and activity of the enzyme cyclooxygenase-2 (COX-2) [203]. In contrast to COX-1, it is synthesized on demand in cases of injury, inflammation, or cell proliferation, and it is involved in the induction of angiogenesis [204]. Notably, COX-2 was found in the outer membrane of CSH patients [103]. Furthermore, the concentration of prostaglandin-E2 (PG-E2) which is the product of COX-2 synthesis linearly correlates with the time period which precedes the manifestation of CSH.
While angiopoietin 1/TIE2 (ANG1/TIE2) supports the maturation of vascular structures and has a role in maintenance of vascular integrity through the recruitment of pericytes and endothelial cells, angiopoietin 2 (ANG2) interferes with the ANG1/TIE2 signal and loosens tight cellular connections [205]. Thus, it exposes the endothelium to inducers of angiogenesis like VEGF and other growth factors. The relative ratio of ANG1 and ANG2 is thought to be crucial for the regulation of angiopoietins. It was shown to be stable in a mouse model for aging of skeletal muscles whereas the TIE receptor mRNA was decreased [206]. When expression of the receptor TIE is reduced in aging vascular structures, a relative preponderance of the ANG2 signal will result and the vascular integrity will be reduced [206]. Likewise, it was demonstrated that in the neomembranes of CSH patients, the ratio of ANG1/ANG2 turned towards a preponderance of the ANG2 signal. Both mechanisms might explain the unmatured phenotypic characteristics of the majority of vessels within the neomembranes [3]. Recently, a similar preponderance of ANG2 was verified at the protein level within the hematoma [98].
The expressions of the VEGF receptors fms-like tyrosine kinase 1 (flt-1) and fetal liver kinase 1 (flk-1) also change with aging. In young individuals, there is a preponderance of the flt-1 receptor which plays an essential role in the development of embryonic vasculature, the regulation of angiogenesis, cell survival, and migration, as well as macrophage function and chemotaxis. In aged vessels, the expression of flk-1 predominates. It was shown that in vessels of the neomembranes of CSH, flk-1 is the principal receptor [207]. flk-1 is responsible for transmitting VEGF signals in the vascular endothelium including proliferation, migration, survival, and permeability [208]. With that regard, it is also of interest that tumor growth and diabetic retinopathy are associated with pathological signaling of flk-1 characterized by hyperpermeable neovascularization as it is seen in CSH [85].
Experimental models have demonstrated impairments in the expression and function of the angiogenic factor PDGF in aging [209]. PDGF is an important cytokine during the last step of angiogenesis where the newly sprouted endothelial tubes mature. PDGF steers both coverings of the EC lines with pericytes and deposition of a basement membrane. Compared to the high concentrations of VEGF and to a lesser degree of basic fibroblast growth factor (bFGF) in hematoma fluid, the concentration of PDGF is only marginal which might be another mechanism that hinders newly formed endothelial tubes to mature into patent vessels in CSH patients [2].
A higher expression of TNF-a, in general, is also observed with advancing age [189, 210]. This results in NADPH-dependent free radical production [211] and the biotransformation of NO into tissue-reactive and harmful nitrogenous species including peroxynitrite (OONO−) [212]. Subsequent to NO depletion and upregulation of proinflammatory genes, the vasculature becomes vulnerable to inflammation and fibrosis. Remarkably, TNF-a is a proinflammatory cytokine which is controlled by estrogen [213]. This might be one of the mechanisms why women are more rarely afflicted by CSH than men.
Knowledge of the molecular mechanisms which are involved in the development of CSH has considerably increased during the last decades. While the presence of some of these mechanisms per se indicates a pathological condition, it remains unclear whether or not some findings are truly pathological or they simply represent a physiological process. Nevertheless, also physiological mechanisms can be exaggerated or diminished and as such become pathological. Age probably has an impact on all of these eventualities. It is reasonable to assume that trauma or other trigger events encounter a repair system with characteristics of senescence. This repair system implies a dysfunctional secretory phenotype of senescent cells and results in an insufficient repair process including chronic inflammation and fibrosis. Increased knowledge about the pathomechanisms of CSH may also open perspectives for prevention of its occurrence in the future.
Author contribution
R.W. and L.S. had the idea for the article. R.W. performed the literature search and the data analysis. R.W. and L.S. drafted the first manuscript. J.K.K. critically revised the work. All authors read and approved the final manuscript.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ducruet AF, Grobelny BT, Zacharia BE, Hickman ZL, DeRosa PL, Anderson K, et al. The surgical management of chronic subdural hematoma. Neurosurg Rev. 2012;35(2):155–69. doi: 10.1007/s10143-011-0349-y. [DOI] [PubMed] [Google Scholar]
- 2.Weigel R, Schilling L, Schmiedek P. Specific pattern of growth factor distribution in chronic subdural hematoma (CSH): evidence for an angiogenic disease. Acta Neurochir. 2001;143(8):811–818. doi: 10.1007/s007010170035. [DOI] [PubMed] [Google Scholar]
- 3.Hohenstein A, Erber R, Schilling L, Weigel R. Increased mRNA expression of VEGF within the hematoma and imbalance of angiopoietin-1 and-2 mRNA within the neomembranes of chronic subdural hematoma. J Neurotrauma. 2005;22(5):518–528. doi: 10.1089/neu.2005.22.518. [DOI] [PubMed] [Google Scholar]
- 4.Edlmann E, Whitfield P, Kolias AG, Hutchinson PJ. Pathogenesis of chronic subdural haematoma: a cohort evidencing de novo and transformational origins. J Neurotrauma. 2021 doi: 10.1089/neu.2020.7574. [DOI] [PubMed] [Google Scholar]
- 5.Edlmann E, Giorgi-Coll S, Whitfield PC, Carpenter KLH, Hutchinson PJ. Pathophysiology of chronic subdural haematoma: inflammation, angiogenesis and implications for pharmacotherapy. J Neuroinflammation. 2017;14(1):108. doi: 10.1186/s12974-017-0881-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frati A, Salvati M, Mainiero F, Ippoliti F, Rocchi G, Raco A, et al. Inflammation markers and risk factors for recurrence in 35 patients with a posttraumatic chronic subdural hematoma: a prospective study. J Neurosurg. 2004;100(1):24–32. doi: 10.3171/jns.2004.100.1.0024. [DOI] [PubMed] [Google Scholar]
- 7.Tokmak M, Iplikcioglu AC, Bek S, Gokduman CA, Erdal M. The role of exudation in chronic subdural hematomas. J Neurosurg. 2007;107(2):290–295. doi: 10.3171/JNS-07/08/0290. [DOI] [PubMed] [Google Scholar]
- 8.Funai M, Osuka K, Usuda N, Atsuzawa K, Inukai T, Yasuda M, et al. Activation of PI3 kinase/Akt signaling in chronic subdural hematoma outer membranes. J Neurotrauma. 2011;28(6):1127–1131. doi: 10.1089/neu.2010.1498. [DOI] [PubMed] [Google Scholar]
- 9.Holl DC, Volovici V, Dirven CMF, Peul WC, van Kooten F, Jellema K, et al. Pathophysiology and nonsurgical treatment of chronic subdural hematoma: from past to present to future. World Neurosurg. 2018;116:402–11.e2. doi: 10.1016/j.wneu.2018.05.037. [DOI] [PubMed] [Google Scholar]
- 10.Muangpaisan W, Petcharat C, Srinonprasert V. Prevalence of potentially reversible conditions in dementia and mild cognitive impairment in a geriatric clinic. Geriatr Gerontol Int. 2012;12(1):59–64. doi: 10.1111/j.1447-0594.2011.00728.x. [DOI] [PubMed] [Google Scholar]
- 11.Ishikawa E, Yanaka K, Sugimoto K, Ayuzawa S, Nose T. Reversible dementia in patients with chronic subdural hematomas. J Neurosurg. 2002;96(4):680–683. doi: 10.3171/jns.2002.96.4.0680. [DOI] [PubMed] [Google Scholar]
- 12.Gill M, Maheshwari V, Narang A, Lingaraju TS. Impact on cognitive improvement following burr hole evacuation of chronic subdural hematoma: a prospective observational study. J Neurosci Rural Pract. 2018;9(4):457–460. doi: 10.4103/jnrp.jnrp_126_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weigel R, Schmiedek P, Krauss JK. Outcome of contemporary surgery for chronic subdural haematoma: evidence based review. J Neurol Neurosurg Psychiatry. 2003;74(7):937–943. doi: 10.1136/jnnp.74.7.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ha VT, Nguyen TN, Nguyen TX, Nguyen HTT, Nguyen TTH, Nguyen AT, et al. Prevalence and factors associated with falls among older outpatients. Int J Environ Res Public Health. 2021;18(8). 10.3390/ijerph18084041. [DOI] [PMC free article] [PubMed]
- 15.Bartolazzi F, Ribeiro ALP, de Sousa W, Vianna MS, da Silva JLP, Martins MAP. Relationship of health literacy and adherence to oral anticoagulation therapy in patients with atrial fibrillation: a cross-sectional study. J Thromb Thrombolysis. 2021 doi: 10.1007/s11239-021-02432-4. [DOI] [PubMed] [Google Scholar]
- 16.Nyberg L, Boraxbekk CJ, Sörman DE, Hansson P, Herlitz A, Kauppi K, et al. Biological and environmental predictors of heterogeneity in neurocognitive ageing: evidence from Betula and other longitudinal studies. Ageing Res Rev. 2020;64:101184. doi: 10.1016/j.arr.2020.101184. [DOI] [PubMed] [Google Scholar]
- 17.Liu H, Yang Y, Xia Y, Zhu W, Leak RK, Wei Z, et al. Aging of cerebral white matter. Ageing Res Rev. 2017;34:64–76. doi: 10.1016/j.arr.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Macaron T, Giudici KV, Bowman GL, Sinclair A, Stephan E, Vellas B, et al. Associations of Omega-3 fatty acids with brain morphology and volume in cognitively healthy older adults: a narrative review. Ageing Res Rev. 2021;67:101300. doi: 10.1016/j.arr.2021.101300. [DOI] [PubMed] [Google Scholar]
- 19.Rauhala M, Helén P, Huhtala H, Heikkilä P, Iverson GL, Niskakangas T, et al. Chronic subdural hematoma-incidence, complications, and financial impact. Acta Neurochir (Wien) 2020;162(9):2033–2043. doi: 10.1007/s00701-020-04398-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marshman LA, Manickam A, Carter D. Risk factors for chronic subdural haematoma formation do not account for the established male bias. Clin Neurol Neurosurg. 2015;131:1–4. doi: 10.1016/j.clineuro.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 21.Mehta V, Harward SC, Sankey EW, Nayar G, Codd PJ. Evidence based diagnosis and management of chronic subdural hematoma: a review of the literature. J Clin Neurosci. 2018;50:7–15. doi: 10.1016/j.jocn.2018.01.050. [DOI] [PubMed] [Google Scholar]
- 22.Lee KS. The pathogenesis and clinical significance of traumatic subdural hygroma. Brain Inj. 1998;12(7):595–603. doi: 10.1080/026990598122359. [DOI] [PubMed] [Google Scholar]
- 23.Olivero WC, Wang H, Farahvar A, Kim TA, Wang F. Predictive (subtle or overlooked) initial head CT findings in patients who develop delayed chronic subdural hematoma. J Clin Neurosci. 2017;42:129–133. doi: 10.1016/j.jocn.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 24.Komiyama K, Tosaka M, Shimauchi-Ohtaki H, Aihara M, Shimizu T, Yoshimoto Y. Computed tomography findings after head injury preceding chronic subdural hematoma. Neurosurg Focus. 2019;47(5):E12. doi: 10.3171/2019.8.Focus19535. [DOI] [PubMed] [Google Scholar]
- 25.Son S, Yoo CJ, Lee SG, Kim EY, Park CW, Kim WK. Natural course of initially non-operated cases of acute subdural hematoma : the risk factors of hematoma progression. J Korean Neurosurg Soc. 2013;54(3):211–219. doi: 10.3340/jkns.2013.54.3.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laviv Y, Rappaport ZH. Risk factors for development of significant chronic subdural hematoma following conservative treatment of acute subdural hemorrhage. Br J Neurosurg. 2014:1–6. 10.3109/02688697.2014.918578. [DOI] [PubMed]
- 27.Ahmed E, Aurangzeb A, Khan SA, Maqbool S, Ali A, Zadran KK, et al. Frequency of conservatively managed traumatic acute subdural haematoma changing into chronic subdural haematoma. J Ayub Med Coll Abbottabad. 2012;24(1):71–74. [PubMed] [Google Scholar]
- 28.Rauhala M, Helén P, Seppä K, Huhtala H, Iverson GL, Niskakangas T, et al. Long-term excess mortality after chronic subdural hematoma. Acta Neurochir (Wien) 2020;162(6):1467–1478. doi: 10.1007/s00701-020-04278-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miranda LB, Braxton E, Hobbs J, Quigley MR. Chronic subdural hematoma in the elderly: not a benign disease. J Neurosurg. 2011;114(1):72–76. doi: 10.3171/2010.8.JNS10298. [DOI] [PubMed] [Google Scholar]
- 30.Uno M, Toi H, Hirai S. Chronic subdural hematoma in elderly patients: is this disease benign? Neurol Med Chir (Tokyo) 2017;57(8):402–409. doi: 10.2176/nmc.ra.2016-0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haldrup M, Ketharanathan B, Debrabant B, Schwartz OS, Mikkelsen R, Fugleholm K, et al. Embolization of the middle meningeal artery in patients with chronic subdural hematoma-a systematic review and meta-analysis. Acta Neurochir (Wien) 2020;162(4):777–784. doi: 10.1007/s00701-020-04266-0. [DOI] [PubMed] [Google Scholar]
- 32.Weigel R, Krauss JK. Chronic subdural hematoma in the elderly. In: Sinha KK, Chandra P, editors. Advances in clinical neurosciences. Ranchi: East Zone Neuro DME; 2004. pp. 231–252. [Google Scholar]
- 33.Leibovitz A, Baumohl Y, Segal R, Habot B. Age-associated neovasculopathy with recurrent bleeding. Med Hypotheses. 2001;57(5):616–618. doi: 10.1054/mehy.2001.1429. [DOI] [PubMed] [Google Scholar]
- 34.Nakamura S, Tsubokawa T. Extraction of angiogenesis factor from chronic subdural haematomas. Significance in capsule formation and haematoma growth. Brain Inj. 1989;3(2):129–36. doi: 10.3109/02699058909004543. [DOI] [PubMed] [Google Scholar]
- 35.Suzuki K, Takano S, Nose T, Doi M, Ohashi N. Increased concentration of vascular endothelial growth factor (VEGF) in chronic subdural hematoma [letter] J Trauma. 1999;46(3):532–3. doi: 10.1097/00005373-199903000-00040. [DOI] [PubMed] [Google Scholar]
- 36.Hayashi SI, Rakugi H, Morishita R. Insight into the role of angiopoietins in ageing-associated diseases. Cells. 2020;9(12). 10.3390/cells9122636. [DOI] [PMC free article] [PubMed]
- 37.Krauss JK, Marshall LF, Weigel R. Medical and surgical management of subdural hematomas. In: Winn HR, editor. Youmans Neurological Surgery. 6th ed.: Elsevier Saunders; 2011. p. 535–43.
- 38.Weigel R, Hohenstein A, Schilling L. Vascular endothelial growth factor concentration in chronic subdural hematoma fluid is related to computed tomography appearance and exudation rate. J Neurotrauma. 2014;31(7):670–673. doi: 10.1089/neu.2013.2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ito H, Yamamoto S, Saito K, Ikeda K, Hisada K. Quantitative estimation of hemorrhage in chronic subdural hematoma using the 51Cr erythrocyte labeling method. J Neurosurg. 1987;66(6):862–864. doi: 10.3171/jns.1987.66.6.0862. [DOI] [PubMed] [Google Scholar]
- 40.Kao MC. Sedimentation level in chronic subdural hematoma visible on computerized tomography. J Neurosurg. 1983;58(2):246–251. doi: 10.3171/jns.1983.58.2.0246. [DOI] [PubMed] [Google Scholar]
- 41.Nomura S, Kashiwagi S, Fujisawa H, Ito H, Nakamura K. Characterization of local hyperfibrinolysis in chronic subdural hematomas by SDS-PAGE and immunoblot. J Neurosurg. 1994;81(6):910–913. doi: 10.3171/jns.1994.81.6.0910. [DOI] [PubMed] [Google Scholar]
- 42.Saito K, Ito H, Hasegawa T, Yamamoto S. Plasmin-alpha 2-plasmin inhibitor complex and alpha 2-plasmin inhibitor in chronic subdural hematoma. J Neurosurg. 1989;70(1):68–72. doi: 10.3171/jns.1989.70.1.0068. [DOI] [PubMed] [Google Scholar]
- 43.Tsutsumi K, Maeda K, Iijima A, Usui M, Okada Y, Kirino T. The relationship of preoperative magnetic resonance imaging findings and closed system drainage in the recurrence of chronic subdural hematoma [see comments] J Neurosurg. 1997;87(6):870–875. doi: 10.3171/jns.1997.87.6.0870. [DOI] [PubMed] [Google Scholar]
- 44.Nakaguchi H, Tanishima T, Yoshimasu N. Factors in the natural history of chronic subdural hematomas that influence their postoperative recurrence. J Neurosurg. 2001;95(2):256–262. doi: 10.3171/jns.2001.95.2.0256. [DOI] [PubMed] [Google Scholar]
- 45.Nakamura N, Ogawa T, Hashimoto T, Yuki K, Kobayashi S. Reevaluation on resolving subdural hematoma (author’s transl) Neurol Med Chir (Tokyo) 1981;21(5):491–500. doi: 10.2176/nmc.21.491. [DOI] [PubMed] [Google Scholar]
- 46.Li F, Hua C, Feng Y, Yuan H, Bie L. Correlation of vascular endothelial growth factor with magnetic resonance imaging in chronic subdural hematomas. J Neurol Sci. 2017;377:149–154. doi: 10.1016/j.jns.2017.04.013. [DOI] [PubMed] [Google Scholar]
- 47.Lee KS, Bae WK, Doh JW, Bae HG, Yun IG. Origin of chronic subdural haematoma and relation to traumatic subdural lesions. Brain Inj. 1998;12(11):901–910. doi: 10.1080/026990598121972. [DOI] [PubMed] [Google Scholar]
- 48.Kidwell CS, Wintermark M. Imaging of intracranial haemorrhage. Lancet Neurol. 2008;7(3):256–267. doi: 10.1016/s1474-4422(08)70041-3. [DOI] [PubMed] [Google Scholar]
- 49.Fazekas F, Kleinert R, Roob G, Kleinert G, Kapeller P, Schmidt R, et al. Histopathologic analysis of foci of signal loss on gradient-echo T2*-weighted MR images in patients with spontaneous intracerebral hemorrhage: evidence of microangiopathy-related microbleeds. AJNR Am J Neuroradiol. 1999;20(4):637–642. [PMC free article] [PubMed] [Google Scholar]
- 50.Imaizumi T, Horita Y, Honma T, Niwa J. Association between a black band on the inner membrane of a chronic subdural hematoma on T2*-weighted magnetic resonance images and enlargement of the hematoma. J Neurosurg. 2003;99(5):824–830. doi: 10.3171/jns.2003.99.5.0824. [DOI] [PubMed] [Google Scholar]
- 51.Kaminogo M, Moroki J, Ochi A, Ichikura A, Onizuka M, Shibayama A, et al. Characteristics of symptomatic chronic subdural haematomas on high-field MRI. Neuroradiology. 1999;41(2):109–116. doi: 10.1007/s002340050714. [DOI] [PubMed] [Google Scholar]
- 52.Sherrod BA, Baker C, Gamboa N, McNally S, Grandhi R. Preoperative MRI characteristics predict chronic subdural haematoma postoperative recurrence: a meta-analysis. Br J Neurosurg. 2021:1–5. 10.1080/02688697.2021.1903391. [DOI] [PubMed]
- 53.Hua C, Zhao G, Feng Y, Yuan H, Song H, Bie L. Role of matrix metalloproteinase-2, matrix metalloproteinase-9, and vascular endothelial growth factor in the development of chronic subdural hematoma. J Neurotrauma. 2016;33(1):65–70. doi: 10.1089/neu.2014.3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3(6):401–410. doi: 10.1038/nrc1093. [DOI] [PubMed] [Google Scholar]
- 55.van Hinsbergh VW, Engelse MA, Quax PH. Pericellular proteases in angiogenesis and vasculogenesis. Arterioscler Thromb Vasc Biol. 2006;26(4):716–728. doi: 10.1161/01.atv.0000209518.58252.17. [DOI] [PubMed] [Google Scholar]
- 56.Virchow R. Das Hämatom der Dura mater. Verh Phys Med Ges Würzburg. 1857;7:134–142. [Google Scholar]
- 57.Nomura S, Kashiwagi S, Ito H, Mimura Y, Nakamura K. Degradation of fibrinogen and fibrin by plasmin and nonplasmin proteases in the chronic subdural hematoma: evaluation by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblot. Electrophoresis. 1993;14(12):1318–1321. doi: 10.1002/elps.11501401202. [DOI] [PubMed] [Google Scholar]
- 58.Hirashima Y, Endo S, Hayashi N, Karasawa K, Nojima S, Takaku A. Platelet-activating factor (PAF) and the formation of chronic subdural haematoma. Measurement of plasma PAF levels and anti-PAF immunoglobulin titers. Acta Neurochir (Wien) 1995;137(1–2):15–8. doi: 10.1007/BF02188773. [DOI] [PubMed] [Google Scholar]
- 59.Ito H, Saito K, Yamamoto S, Hasegawa T. Tissue-type plasminogen activator in the chronic subdural hematoma. Surg Neurol. 1988;30(3):175–179. doi: 10.1016/0090-3019(88)90269-8. [DOI] [PubMed] [Google Scholar]
- 60.Fujisawa H, Ito H, Kashiwagi S, Nomura S, Toyosawa M. Kallikrein-kinin system in chronic subdural haematomas: its roles in vascular permeability and regulation of fibrinolysis and coagulation. J Neurol Neurosurg Psychiatry. 1995;59(4):388–394. doi: 10.1136/jnnp.59.4.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Suzuki M, Endo S, Inada K, Kudo A, Kitakami A, Kuroda K, et al. Inflammatory cytokines locally elevated in chronic subdural haematoma. Acta Neurochir (Wien) 1998;140(1):51–55. doi: 10.1007/s007010050057. [DOI] [PubMed] [Google Scholar]
- 62.Weigel R, Schilling L, Schmiedek P. Analysis of vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF) and platelet derived growth factor (PDGF) in chronic subdural hematoma. Zentralbl Neurochir. 1999;60(supp):84. [Google Scholar]
- 63.Fujioka S, Matsukado Y, Kuratsu J, Kaku M. Significance of eosinophilic infiltration in chronic subdural hematoma, with special reference to the relationship with estrogens. Neurol Med Chir (Tokyo) 1983;23(2):145–151. doi: 10.2176/nmc.23.145. [DOI] [PubMed] [Google Scholar]
- 64.Muller W, Firsching R. Significance of eosinophilic granulocytes in chronic subdural hematomas. Neurosurg Rev. 1990;13(4):305–308. doi: 10.1007/BF00346370. [DOI] [PubMed] [Google Scholar]
- 65.Ueda Y, Matsumoto T, Nagai H, Nakamura T. Eosinophilic infiltration in the neomembrane of chronic subdural hematoma. Neurol Med Chir (Tokyo) 1988;28(3):236–240. doi: 10.2176/nmc.28.236. [DOI] [PubMed] [Google Scholar]
- 66.Yamashima T, Kubota T, Yamamoto S. Eosinophil degranulation in the capsule of chronic subdural hematomas. J Neurosurg. 1985;62(2):257–260. doi: 10.3171/jns.1985.62.2.0257. [DOI] [PubMed] [Google Scholar]
- 67.Yamashima T, Tachibana O, Hasegawa M, Nitta H, Yamashita J. Liberation of eosinophil granules in the inner capsule of chronic subdural hematomas. Neurochirurgia (Stuttg) 1989;32(6):168–171. doi: 10.1055/s-2008-1054030. [DOI] [PubMed] [Google Scholar]
- 68.Kay AB. The role of eosinophils in the pathogenesis of asthma. Trends Mol Med. 2005;11(4):148–152. doi: 10.1016/j.molmed.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 69.Gharaee-Kermani M, Phan SH. The role of eosinophils in pulmonary fibrosis (Review) Int J Mol Med. 1998;1(1):43–53. [PubMed] [Google Scholar]
- 70.Sato M, Muragaki Y, Saika S, Roberts AB, Ooshima A. Targeted disruption of TGF-beta1/Smad3 signaling protects against renal tubulointerstitial fibrosis induced by unilateral ureteral obstruction. J Clin Investig. 2003;112(10):1486–1494. doi: 10.1172/jci19270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Aceves SS, Newbury RO, Dohil R, Bastian JF, Broide DH. Esophageal remodeling in pediatric eosinophilic esophagitis. J Allergy Clin Immunol. 2007;119(1):206–212. doi: 10.1016/j.jaci.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 72.Osuka K, Watanabe Y, Usuda N, Aoyama M, Takeuchi M, Takayasu M. Eotaxin-3 activates the Smad pathway through the transforming growth factor beta 1 in chronic subdural hematoma outer membranes. J Neurotrauma. 2014;31(16):1451–1456. doi: 10.1089/neu.2013.3195. [DOI] [PubMed] [Google Scholar]
- 73.Osuka K, Watanabe Y, Usuda N, Atsuzawa K, Shima H, Takeuchi M, et al. Activation of JAK-STAT3 signaling pathway in chronic subdural hematoma outer membranes. Neurosci Lett. 2013;534:166–170. doi: 10.1016/j.neulet.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 74.Hong HJ, Kim YJ, Yi HJ, Ko Y, Oh SJ, Kim JM. Role of angiogenic growth factors and inflammatory cytokine on recurrence of chronic subdural hematoma. Surg Neurol. 2009;71(2):161–5. doi: 10.1016/j.surneu.2008.01.023. [DOI] [PubMed] [Google Scholar]
- 75.Kitazono M, Yokota H, Satoh H, Onda H, Matsumoto G, Fuse A, et al. Measurement of inflammatory cytokines and thrombomodulin in chronic subdural hematoma. Neurol Med Chir (Tokyo) 2012;52(11):810–815. doi: 10.2176/nmc.52.810. [DOI] [PubMed] [Google Scholar]
- 76.Pripp AH, Stanisic M. The correlation between pro- and anti-inflammatory cytokines in chronic subdural hematoma patients assessed with factor analysis. PLoS ONE. 2014;9(2):e90149. doi: 10.1371/journal.pone.0090149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stanisic M, Aasen AO, Pripp AH, Lindegaard KF, Ramm-Pettersen J, Lyngstadaas SP, et al. Local and systemic pro-inflammatory and anti-inflammatory cytokine patterns in patients with chronic subdural hematoma: a prospective study. Inflamm Res. 2012 doi: 10.1007/s00011-012-0476-0. [DOI] [PubMed] [Google Scholar]
- 78.Wada T, Kuroda K, Yoshida Y, Ogasawara K, Ogawa A, Endo S. Local elevation of the anti-inflammatory interleukin-10 in the pathogenesis of chronic subdural hematoma. Neurosurg Rev. 2006;29(3):242–245. doi: 10.1007/s10143-006-0019-7. [DOI] [PubMed] [Google Scholar]
- 79.Maruo N, Morita I, Shirao M, Murota S. IL-6 increases endothelial permeability in vitro. Endocrinology. 1992;131(2):710–714. doi: 10.1210/endo.131.2.1639018. [DOI] [PubMed] [Google Scholar]
- 80.Baggiolini M. CXCL8 - the first chemokine. Front Immunol. 2015;6:285. doi: 10.3389/fimmu.2015.00285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473(7347):298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Friede RL, Schachenmayr W. The origin ofsubdural neomembranes. II. Fine structural of neomembranes. Am J Pathol. 1978;92(1):69–84. [PMC free article] [PubMed] [Google Scholar]
- 83.Sato S, Suzuki J. Ultrastructural observations of the capsule of chronic subdural hematoma in various clinical stages. J Neurosurg. 1975;43:569–578. doi: 10.3171/jns.1975.43.5.0569. [DOI] [PubMed] [Google Scholar]
- 84.Schachenmayr W, Friede RL. The origin of subdural neomembranes. I. Fine structure of the dura-arachnoid interface in man. Am J Pathol. 1978;92(1):53–68. [PMC free article] [PubMed] [Google Scholar]
- 85.Yamashima T, Yamamoto S, Friede RL. The role of endothelial gap junctions in the enlargement of chronic subdural hematomas. J Neurosurg. 1983;59(2):298–303. doi: 10.3171/jns.1983.59.2.0298. [DOI] [PubMed] [Google Scholar]
- 86.Yamashima T, Yamamoto S, Friede RL. A comparative study of the capsular vessels of acute subdural hematoma in the chronic healing stage and those of chronic subdural hematoma. Neurol Med Chir (Tokyo) 1983;23(6):428–436. doi: 10.2176/nmc.23.428. [DOI] [PubMed] [Google Scholar]
- 87.Shono T, Inamura T, Morioka T, Matsumoto K, Suzuki SO, Ikezaki K, et al. Vascular endothelial growth factor in chronic subdural haematomas. J Clin Neurosci. 2001;8(5):411–415. doi: 10.1054/jocn.2000.0951. [DOI] [PubMed] [Google Scholar]
- 88.Kalamatianos T, Stavrinou LC, Koutsarnakis C, Psachoulia C, Sakas DE, Stranjalis G. PlGF and sVEGFR-1 in chronic subdural hematoma: implications for hematoma development. J Neurosurg. 2013;118(2):353–357. doi: 10.3171/2012.10.jns12327. [DOI] [PubMed] [Google Scholar]
- 89.Vaquero J, Zurita M, Cincu R. Vascular endothelial growth-permeability factor in granulation tissue of chronic subdural haematomas. Acta Neurochir (Wien). 2002;144(4):343–6. doi: 10.1007/s007010200047. [DOI] [PubMed] [Google Scholar]
- 90.Weigel R, Hohenstein A, Schlickum L, Weiss C, Schilling L. Angiotensin converting enzyme inhibition for arterial hypertension reduces the risk of recurrence in patients with chronic subdural hematoma possibly by an antiangiogenic mechanism. Neurosurg. 2007;61(4):788–792. doi: 10.1227/01.NEU.0000298907.56012.E8. [DOI] [PubMed] [Google Scholar]
- 91.Nakagawa T, Kodera T, Kubota T. Expression of matrix metalloproteinases in the chronic subdural haematoma membrane. Acta Neurochir (Wien) 2000;142(1):61–66. doi: 10.1007/s007010050008. [DOI] [PubMed] [Google Scholar]
- 92.Lim DJ, Chung YG, Park YK, Song JH, Lee HK, Lee KC, et al. Relationship between tissue plasminogen activator, plasminogen activator inhibitor and CT image in chronic subdural hematoma. J Korean Med Sci. 1995;10(5):373–378. doi: 10.3346/jkms.1995.10.5.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Carmeliet P, Moons L, Luttun A, Vincenti V, Compernolle V, De Mol M, et al. Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. Nat Med. 2001;7(5):575–583. doi: 10.1038/87904. [DOI] [PubMed] [Google Scholar]
- 94.Fischer C, Mazzone M, Jonckx B, Carmeliet P. FLT1 and its ligands VEGFB and PlGF: drug targets for anti-angiogenic therapy? Nat Rev Cancer. 2008;8(12):942–956. doi: 10.1038/nrc2524. [DOI] [PubMed] [Google Scholar]
- 95.Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001;410(6824):37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- 96.Aoyama M, Osuka K, Usuda N, Watanabe Y, Kawaguchi R, Nakura T, et al. Expression of mitogen-activated protein kinases in chronic subdural hematoma outer membranes. J Neurotrauma. 2015;32(14):1064–1070. doi: 10.1089/neu.2014.3594. [DOI] [PubMed] [Google Scholar]
- 97.Breslin JW, Pappas PJ, Cerveira JJ, Hobson RW, 2nd, Duran WN. VEGF increases endothelial permeability by separate signaling pathways involving ERK-1/2 and nitric oxide. Am J Physiol Heart Circ Physiol. 2003;284(1):H92–h100. doi: 10.1152/ajpheart.00330.2002. [DOI] [PubMed] [Google Scholar]
- 98.Isaji T, Osuka K, Ohmichi Y, Ohmichi M, Naito M, Nakano T, et al. Expression of angiopoietins and angiogenic signaling pathway molecules in chronic subdural hematomas. J Neurotrauma. 2020;37(23):2493–2498. doi: 10.1089/neu.2020.7042. [DOI] [PubMed] [Google Scholar]
- 99.Sokolowski KM, Koprowski S, Kunnimalaiyaan S, Balamurugan M, Gamblin TC, Kunnimalaiyaan M. Potential molecular targeted therapeutics: role of PI3-K/Akt/mTOR inhibition in cancer. Anticancer Agents Med Chem. 2016;16(1):29–37. doi: 10.2174/1871520615666150716104408. [DOI] [PubMed] [Google Scholar]
- 100.Ziello JE, Jovin IS, Huang Y. Hypoxia-inducible factor (HIF)-1 regulatory pathway and its potential for therapeutic intervention in malignancy and ischemia. Yale J Biol Med. 2007;80(2):51–60. [PMC free article] [PubMed] [Google Scholar]
- 101.Nanko N, Tanikawa M, Mase M, Fujita M, Tateyama H, Miyati T, et al. Involvement of hypoxia-inducible factor-1alpha and vascular endothelial growth factor in the mechanism of development of chronic subdural hematoma. Neurol Med Chir (Tokyo) 2009;49(9):379–385. doi: 10.2176/nmc.49.379. [DOI] [PubMed] [Google Scholar]
- 102.Schaumann A, Klene W, Rosenstengel C, Ringel F, Tuttenberg J, Vajkoczy P. COXIBRAIN: results of the prospective, randomised, phase II/III study for the selective COX-2 inhibition in chronic subdural haematoma patients. Acta Neurochir (Wien) 2016;158(11):2039–2044. doi: 10.1007/s00701-016-2949-3. [DOI] [PubMed] [Google Scholar]
- 103.Hara M, Tamaki M, Aoyagi M, Ohno K. Possible role of cyclooxygenase-2 in developing chronic subdural hematoma. J Med Dent Sci. 2009;56(3):101–106. [PubMed] [Google Scholar]
- 104.Stanisic M, Lyngstadaas SP, Pripp AH, Aasen AO, Lindegaard KF, Ivanovic J, et al. Chemokines as markers of local inflammation and angiogenesis in patients with chronic subdural hematoma: a prospective study. Acta Neurochir (Wien) 2012;154(1):113–20. doi: 10.1007/s00701-011-1203-2. [DOI] [PubMed] [Google Scholar]
- 105.Mandai S, Sakurai M, Matsumoto Y. Middle meningeal artery embolization for refractory chronic subdural hematoma. Case report. J Neurosurg. 2000;93(4):686–688. doi: 10.3171/jns.2000.93.4.0686. [DOI] [PubMed] [Google Scholar]
- 106.Srivatsan A, Mohanty A, Nascimento FA, Hafeez MU, Srinivasan VM, Thomas A, et al. Middle meningeal artery embolization for chronic subdural hematoma: meta-analysis and systematic review. World Neurosurg. 2019;122:613–619. doi: 10.1016/j.wneu.2018.11.167. [DOI] [PubMed] [Google Scholar]
- 107.Soleman J, Nocera F, Mariani L. The conservative and pharmacological management of chronic subdural haematoma. Swiss Med Wkly. 2017;147:w14398. doi: 10.57187/smw.2017.14398. [DOI] [PubMed] [Google Scholar]
- 108.Colville-Nash PR, Alam CA, Appleton I, Brown JR, Seed MP, Willoughby DA. The pharmacological modulation of angiogenesis in chronic granulomatous inflammation. J Pharmacol Exp Ther. 1995;274(3):1463–1472. [PubMed] [Google Scholar]
- 109.Folkman J. Angiogenesis and its inhibitors. Import Adv Oncol. 1985:42–62. [PubMed]
- 110.Nauck M, Karakiulakis G, Perruchoud AP, Papakonstantinou E, Roth M. Corticosteroids inhibit the expression of the vascular endothelial growth factor gene in human vascular smooth muscle cells. Eur J Pharmacol. 1998;341(2–3):309–315. doi: 10.1016/s0014-2999(97)01464-7. [DOI] [PubMed] [Google Scholar]
- 111.Liu Z, Yuan X, Luo Y, He Y, Jiang Y, Chen ZK, et al. Evaluating the effects of immunosuppressants on human immunity using cytokine profiles of whole blood. Cytokine. 2009;45(2):141–147. doi: 10.1016/j.cyto.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 112.Glover D, Labadie EL. Physiopathogenesis of subdural hematomas. Part 2: inhibition of growth of experimental hematomas with dexamethasone. J Neurosurg. 1976;45(4):393–7. doi: 10.3171/jns.1976.45.4.0393. [DOI] [PubMed] [Google Scholar]
- 113.Bender MB, Christoff N. Nonsurgical treatment of subdural hematomas. Arch Neurol. 1974;31(2):73–79. doi: 10.1001/archneur.1974.00490380021001. [DOI] [PubMed] [Google Scholar]
- 114.Delgado-Lopez PD, Martin-Velasco V, Castilla-Diez JM, Rodriguez-Salazar A, Galacho-Harriero AM, Fernandez-Arconada O. Dexamethasone treatment in chronic subdural haematoma. Neurocirugia (Astur) 2009;20(4):346–359. doi: 10.1016/s1130-1473(09)70154-x. [DOI] [PubMed] [Google Scholar]
- 115.Drapkin AJ. Chronic subdural hematoma: pathophysiological basis for treatment. Br J Neurosurg. 1991;5(5):467–473. doi: 10.3109/02688699108998475. [DOI] [PubMed] [Google Scholar]
- 116.Sun TF, Boet R, Poon WS. Non-surgical primary treatment of chronic subdural haematoma: preliminary results of using dexamethasone. Br J Neurosurg. 2005;19(4):327–333. doi: 10.1080/02688690500305332. [DOI] [PubMed] [Google Scholar]
- 117.Parajua JL, Goni M, Gimenez M, Feijoo M. Medical treatment of chronic subdural hematoma. Med Clin. 1984;82(9):404–406. [PubMed] [Google Scholar]
- 118.Rudiger A, Ronsdorf A, Merlo A, Zimmerli W. Dexamethasone treatment of a patient with large bilateral chronic subdural haematomata. Swiss Med Wkly. 2001;131(25–26):387. doi: 10.4414/smw.2001.09745. [DOI] [PubMed] [Google Scholar]
- 119.Guenot M. Chronic subdural hematoma. Introduction and results of a survey by the French Society of Neurosurgery. Neurochirurgie. 2001;47(5):459–60. [PubMed] [Google Scholar]
- 120.Cenic A, Bhandari M, Reddy K. Management of chronic subdural hematoma: a national survey and literature review. Can J Neurol Sci. 2005;32(4):501–506. doi: 10.1017/s0317167100004510. [DOI] [PubMed] [Google Scholar]
- 121.Santarius T, Lawton R, Kirkpatrick PJ, Hutchinson PJ. The management of primary chronic subdural haematoma: a questionnaire survey of practice in the United Kingdom and the Republic of Ireland. Br J Neurosurg. 2008;22(4):529–534. doi: 10.1080/02688690802195381. [DOI] [PubMed] [Google Scholar]
- 122.Berghauser Pont LM, Dippel DW, Verweij BH, Dirven CM, Dammers R. Ambivalence among neurologists and neurosurgeons on the treatment of chronic subdural hematoma: a national survey. Acta Neurol Belg. 2013;113(1):55–59. doi: 10.1007/s13760-012-0130-1. [DOI] [PubMed] [Google Scholar]
- 123.Berghauser Pont LM, Dirven CM, Dippel DW, Verweij BH, Dammers R. The role of corticosteroids in the management of chronic subdural hematoma: a systematic review. Eur J Neurol. 2012;19(11):1397–1403. doi: 10.1111/j.1468-1331.2012.03768.x. [DOI] [PubMed] [Google Scholar]
- 124.Prud’homme M, Mathieu F, Marcotte N, Cottin S. A pilot placebo controlled randomized trial of dexamethasone for chronic subdural hematoma. Can J Neurol Sci. 2016;43(2):284–90. doi: 10.1017/cjn.2015.393. [DOI] [PubMed] [Google Scholar]
- 125.Hutchinson PJ, Edlmann E, Bulters D, Zolnourian A, Holton P, Suttner N, et al. Trial of dexamethasone for chronic subdural hematoma. N Engl J Med. 2020;383(27):2616–2627. doi: 10.1056/NEJMoa2020473. [DOI] [PubMed] [Google Scholar]
- 126.Nagatani K, Wada K, Takeuchi S, Nawashiro H. Corticosteroid suppression of vascular endothelial growth factor and recurrence of chronic subdural hematoma. Neurosurgery. 2012;70(5):E1334. doi: 10.1227/NEU.0b013e31824ae86a. [DOI] [PubMed] [Google Scholar]
- 127.Iorio-Morin C, Blanchard J, Richer M, Mathieu D. Tranexamic acid in chronic subdural hematomas (TRACS): study protocol for a randomized controlled trial. Trials. 2016;17(1):235. doi: 10.1186/s13063-016-1358-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hirashima Y, Endo S, Kato R, Ohmori T, Nagahori T, Nishijima M, et al. Platelet-activating factor (PAF) and the development of chronic subdural haematoma. Acta Neurochir (Wien) 1994;129(1–2):20–25. doi: 10.1007/BF01400868. [DOI] [PubMed] [Google Scholar]
- 129.Hirashima Y, Kuwayama N, Hamada H, Hayashi N, Endo S. Etizolam, an anti-anxiety agent, attenuates recurrence of chronic subdural hematoma–evaluation by computed tomography. Neurol Med Chir (Tokyo) 2002;42(2):53–5. doi: 10.2176/nmc.42.53. [DOI] [PubMed] [Google Scholar]
- 130.Hirashima Y, Kurimoto M, Nagai S, Hori E, Origasa H, Endo S. Effect of platelet-activating factor receptor antagonist, etizolam, on resolution of chronic subdural hematoma–a prospective study to investigate use as conservative therapy. Neurol Med Chir (Tokyo) 2005;45(12):621–6. doi: 10.2176/nmc.45.621. [DOI] [PubMed] [Google Scholar]
- 131.Jain MK, Ridker PM. Anti-inflammatory effects of statins: clinical evidence and basic mechanisms. Nat Rev Drug Discovery. 2005;4(12):977–987. doi: 10.1038/nrd1901. [DOI] [PubMed] [Google Scholar]
- 132.Verschuren L, Kleemann R, Offerman EH, Szalai AJ, Emeis SJ, Princen HM, et al. Effect of low dose atorvastatin versus diet-induced cholesterol lowering on atherosclerotic lesion progression and inflammation in apolipoprotein E*3-Leiden transgenic mice. Arterioscler Thromb Vasc Biol. 2005;25(1):161–167. doi: 10.1161/01.atv.0000148866.29829.19. [DOI] [PubMed] [Google Scholar]
- 133.Li T, Wang D, Tian Y, Yu H, Wang Y, Quan W, et al. Effects of atorvastatin on the inflammation regulation and elimination of subdural hematoma in rats. J Neurol Sci. 2014;341(1–2):88–96. doi: 10.1016/j.jns.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 134.Chan DY, Chan DT, Sun TF, Ng SC, Wong GK, Poon WS. The use of atorvastatin for chronic subdural haematoma: a retrospective cohort comparison study. Br J Neurosurg. 2017;31(1):72–77. doi: 10.1080/02688697.2016.1208806. [DOI] [PubMed] [Google Scholar]
- 135.Liu H, Luo Z, Liu Z, Yang J, Kan S. Atorvastatin may attenuate recurrence of chronic subdural hematoma. Front Neurosci. 2016;10:303. doi: 10.3389/fnins.2016.00303. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 136.He C, Xia P, Xu J, Chen L, Zhang Q. Evaluation of the efficacy of atorvastatin in the treatment for chronic subdural hematoma: a meta-analysis. Neurosurg Rev. 2021;44(1):479–484. doi: 10.1007/s10143-019-01218-w. [DOI] [PubMed] [Google Scholar]
- 137.Merchan JR, Chan B, Kale S, Schnipper LE, Sukhatme VP. In vitro and in vivo induction of antiangiogenic activity by plasminogen activators and captopril. J Natl Cancer Inst. 2003;95(5):388–399. doi: 10.1093/jnci/95.5.388. [DOI] [PubMed] [Google Scholar]
- 138.Gilbert RE, Kelly DJ, Cox AJ, Wilkinson-Berka JL, Rumble JR, Osicka T, et al. Angiotensin converting enzyme inhibition reduces retinal overexpression of vascular endothelial growth factor and hyperpermeability in experimental diabetes. Diabetologia. 2000;43(11):1360–1367. doi: 10.1007/s001250051539. [DOI] [PubMed] [Google Scholar]
- 139.Poulsen FR, Munthe S, Soe M, Halle B. Perindopril and residual chronic subdural hematoma volumes six weeks after burr hole surgery: a randomized trial. Clin Neurol Neurosurg. 2014;123:4–8. doi: 10.1016/j.clineuro.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 140.Bartek J, Jr, Sjåvik K, Schaible S, Gulati S, Solheim O, Förander P, et al. The role of angiotensin-converting enzyme inhibitors in patients with chronic subdural hematoma: a scandinavian population-based multicenter study. World Neurosurg. 2018;113:e555–e560. doi: 10.1016/j.wneu.2018.02.094. [DOI] [PubMed] [Google Scholar]
- 141.Neidert MC, Schmidt T, Mitova T, Fierstra J, Bellut D, Regli L, et al. Preoperative angiotensin converting enzyme inhibitor usage in patients with chronic subdural hematoma: associations with initial presentation and clinical outcome. J Clin Neurosci. 2016;28:82–86. doi: 10.1016/j.jocn.2015.09.022. [DOI] [PubMed] [Google Scholar]
- 142.Iniguez MA, Rodriguez A, Volpert OV, Fresno M, Redondo JM. Cyclooxygenase-2: a therapeutic target in angiogenesis. Trends Mol Med. 2003;9(2):73–78. doi: 10.1016/s1471-4914(02)00011-4. [DOI] [PubMed] [Google Scholar]
- 143.Masferrer JL, Leahy KM, Koki AT, Zweifel BS, Settle SL, Woerner BM, et al. Antiangiogenic and antitumor activities of cyclooxygenase-2 inhibitors. Can Res. 2000;60(5):1306–1311. [PubMed] [Google Scholar]
- 144.Edlmann E, Holl DC, Lingsma HF, Bartek J, Jr, Bartley A, Duerinck J, et al. Systematic review of current randomised control trials in chronic subdural haematoma and proposal for an international collaborative approach. Acta Neurochir (Wien) 2020;162(4):763–776. doi: 10.1007/s00701-020-04218-8. [DOI] [PubMed] [Google Scholar]
- 145.Ross D. Resolution of chronic subdural hematoma after treatment with tumor necrosis factor alpha inhibitor. Neurosci Med. 2011;2:347–50. [Google Scholar]
- 146.Wepfer JJ. Observationes anatomicae ex cadaveribus eorum, quos sustulit apoplexia cum exercitatione de eius loco affecto. Schaffhausen: Waldkirch Alexandri Riedingii; 1675.
- 147.Weigel R, Krauss JK, Schmiedek P. Concepts of neurosurgical management of chronic subdural haematoma: historical perspectives. Br J Neurosurg. 2004;18(1):8–18. doi: 10.1080/02688690410001660418. [DOI] [PubMed] [Google Scholar]
- 148.Gardner WJ. Traumatic subdural hematoma with particular reference to the latent interval. Arch Neurol Psychiat. 1932;27:847–858. [Google Scholar]
- 149.Ito H, Yamamoto S, Komai T, Mizukoshi H. Role of local hyperfibrinolysis in the etiology of chronic subdural hematoma. J Neurosurg. 1976;45(1):26–31. doi: 10.3171/jns.1976.45.1.0026. [DOI] [PubMed] [Google Scholar]
- 150.Yamashima T, Shimoji T, Komai T, Kubota T, Ito H, Yamamoto S. Growing mechanism of chronic subdural hematoma–light and electron microscopic study on outer membranes of chronic subdural hematoma (author’s transl) Neurol Med Chir (Tokyo) 1978;18(10 2):743–52. doi: 10.2176/nmc.18pt2.743. [DOI] [PubMed] [Google Scholar]
- 151.Yamashima T, Yamamoto S. How do vessels proliferate in the capsule of a chronic subdural hematoma? Neurosurgery. 1984;15(5):672–678. doi: 10.1227/00006123-198411000-00006. [DOI] [PubMed] [Google Scholar]
- 152.Fujisawa H, Nomura S, Tsuchida E, Ito H. Serum protein exudation in chronic subdural haematomas: a mechanism for haematoma enlargement? Acta Neurochir (Wien). 1998;140(2):161–5. doi: 10.1007/s007010050077. [DOI] [PubMed] [Google Scholar]
- 153.Weir B. Oncotic pressure of subdural fluids. J Neurosurg. 1980;53(4):512–515. doi: 10.3171/jns.1980.53.4.0512. [DOI] [PubMed] [Google Scholar]
- 154.Ito H, Shimoji T, Yamamoto S, Saito K, Uehara S. Colloidal osmotic pressure in chronic subdural hematoma. Neurol Med Chir (Tokyo) 1988;28(7):650–653. doi: 10.2176/nmc.28.650. [DOI] [PubMed] [Google Scholar]
- 155.Toyosawa M, Kashiwagi S, Pei W, Fujisawa H, Ito H, Nakamura K. Electrophoretic demonstration of high molecular weight fibrin degradation products persisting in chronic subdural hematomas. Electrophoresis. 1997;18(1):118–121. doi: 10.1002/elps.1150180122. [DOI] [PubMed] [Google Scholar]
- 156.Sajanti J, Majamaa K. High concentrations of procollagen propeptides in chronic subdural haematoma and effusion. J Neurol Neurosurg Psychiatry. 2003;74(4):522–524. doi: 10.1136/jnnp.74.4.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.D’Abbondanza JA, Loch MR. Experimental models of chronic subdural hematoma. Neurol Res. 2014;36(2):176–188. doi: 10.1179/1743132813Y.0000000279. [DOI] [PubMed] [Google Scholar]
- 158.Xu X, Wang D, Han Z, Wang B, Gao W, Fan Y, et al. A novel rat model of chronic subdural hematoma: induction of inflammation and angiogenesis in the subdural space mimicking human-like features of progressively expanding hematoma. Brain Res Bull. 2021;172:108–119. doi: 10.1016/j.brainresbull.2021.04.024. [DOI] [PubMed] [Google Scholar]
- 159.Arancibia R, Oyarzun A, Silva D, Tobar N, Martinez J, Smith PC. Tumor necrosis factor-alpha inhibits transforming growth factor-beta-stimulated myofibroblastic differentiation and extracellular matrix production in human gingival fibroblasts. J Periodontol. 2013;84(5):683–693. doi: 10.1902/jop.2012.120225. [DOI] [PubMed] [Google Scholar]
- 160.Pacios S, Kang J, Galicia J, Gluck K, Patel H, Ovaydi-Mandel A, et al. Diabetes aggravates periodontitis by limiting repair through enhanced inflammation. FASEB J. 2012;26(4):1423–1430. doi: 10.1096/fj.11-196279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Chung HY, Cesari M, Anton S, Marzetti E, Giovannini S, Seo AY, et al. Molecular inflammation: underpinnings of aging and age-related diseases. Ageing Res Rev. 2009;8(1):18–30. doi: 10.1016/j.arr.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Chung HY, Lee EK, Choi YJ, Kim JM, Kim DH, Zou Y, et al. Molecular inflammation as an underlying mechanism of the aging process and age-related diseases. J Dent Res. 2011;90(7):830–840. doi: 10.1177/0022034510387794. [DOI] [PubMed] [Google Scholar]
- 163.Giuffre R. Physiopathogenesis of chronic subdural hematomas: a new look to an old problem. Riv Neurol. 1987;57(5):298–304. [PubMed] [Google Scholar]
- 164.Flake NM, Hermanstyne TO, Gold MS. Testosterone and estrogen have opposing actions on inflammation-induced plasma extravasation in the rat temporomandibular joint. Am J Physiol Regul Integr Comp Physiol. 2006;291(2):R343–R348. doi: 10.1152/ajpregu.00835.2005. [DOI] [PubMed] [Google Scholar]
- 165.Kovacs EJ, Messingham KA, Gregory MS. Estrogen regulation of immune responses after injury. Mol Cell Endocrinol. 2002;193(1–2):129–135. doi: 10.1016/s0303-7207(02)00106-5. [DOI] [PubMed] [Google Scholar]
- 166.Ashcroft GS, Greenwell-Wild T, Horan MA, Wahl SM, Ferguson MW. Topical estrogen accelerates cutaneous wound healing in aged humans associated with an altered inflammatory response. Am J Pathol. 1999;155(4):1137–1146. doi: 10.1016/s0002-9440(10)65217-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Gomez CR, Nomellini V, Faunce DE, Kovacs EJ. Innate immunity and aging. Exp Gerontol. 2008;43(8):718–728. doi: 10.1016/j.exger.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Xing D, Nozell S, Chen YF, Hage F, Oparil S. Estrogen and mechanisms of vascular protection. Arterioscler Thromb Vasc Biol. 2009;29(3):289–295. doi: 10.1161/atvbaha.108.182279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Rivard A, Fabre JE, Silver M, Chen D, Murohara T, Kearney M, et al. Age-dependent impairment of angiogenesis. Circulation. 1999;99(1):111–120. doi: 10.1161/01.cir.99.1.111. [DOI] [PubMed] [Google Scholar]
- 170.Lahteenvuo J, Rosenzweig A. Effects of aging on angiogenesis. Circ Res. 2012;110(9):1252–1264. doi: 10.1161/circresaha.111.246116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Brandes RP, Fleming I, Busse R. Endothelial aging. Cardiovasc Res. 2005;66(2):286–294. doi: 10.1016/j.cardiores.2004.12.027. [DOI] [PubMed] [Google Scholar]
- 172.Edelberg JM, Reed MJ. Aging and angiogenesis. Front Biosci. 2003;8:s1199–s1209. doi: 10.2741/1166. [DOI] [PubMed] [Google Scholar]
- 173.Reed MJ, Edelberg JM. Impaired angiogenesis in the aged. Sci Aging Knowl Environ SAGE KE. 2004;2004(7):e7. doi: 10.1126/sageke.2004.7.pe7. [DOI] [PubMed] [Google Scholar]
- 174.Abete P, Napoli C, Santoro G, Ferrara N, Tritto I, Chiariello M, et al. Age-related decrease in cardiac tolerance to oxidative stress. J Mol Cell Cardiol. 1999;31(1):227–236. doi: 10.1006/jmcc.1998.0862. [DOI] [PubMed] [Google Scholar]
- 175.Nishikawa T, Edelstein D, Brownlee M. The missing link: a single unifying mechanism for diabetic complications. Kidney Int Suppl. 2000;77:S26–30. doi: 10.1046/j.1523-1755.2000.07705.x. [DOI] [PubMed] [Google Scholar]
- 176.Muller FL, Lustgarten MS, Jang Y, Richardson A, Van Remmen H. Trends in oxidative aging theories. Free Radical Biol Med. 2007;43(4):477–503. doi: 10.1016/j.freeradbiomed.2007.03.034. [DOI] [PubMed] [Google Scholar]
- 177.Ushio-Fukai M, Alexander RW. Reactive oxygen species as mediators of angiogenesis signaling: role of NAD(P)H oxidase. Mol Cell Biochem. 2004;264(1–2):85–97. doi: 10.1023/b:mcbi.0000044378.09409.b5. [DOI] [PubMed] [Google Scholar]
- 178.Ershler WB, Keller ET. Age-associated increased interleukin-6 gene expression, late-life diseases, and frailty. Annu Rev Med. 2000;51:245–270. doi: 10.1146/annurev.med.51.1.245. [DOI] [PubMed] [Google Scholar]
- 179.Wolf J, Weinberger B, Arnold CR, Maier AB, Westendorp RG, Grubeck-Loebenstein B. The effect of chronological age on the inflammatory response of human fibroblasts. Exp Gerontol. 2012;47(9):749–753. doi: 10.1016/j.exger.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Huber AK, Giles DA, Segal BM, Irani DN. An emerging role for eotaxins in neurodegenerative disease. Clin Immunol. 2018;189:29–33. doi: 10.1016/j.clim.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 181.Tominaga K, Suzuki HI. TGF-β signaling in cellular senescence and aging-related pathology. Int J Mol Sci. 2019;20(20). 10.3390/ijms20205002. [DOI] [PMC free article] [PubMed]
- 182.Ruggiero D, Dalmasso C, Nutile T, Sorice R, Dionisi L, Aversano M, et al. Genetics of VEGF serum variation in human isolated populations of cilento: importance of VEGF polymorphisms. PLoS ONE. 2011;6(2):e16982. doi: 10.1371/journal.pone.0016982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Ashcroft GS, Horan MA, Ferguson MW. The effects of ageing on wound healing: immunolocalisation of growth factors and their receptors in a murine incisional model. J Anat. 1997;190(Pt 3):351–65. doi: 10.1046/j.1469-7580.1997.19030351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Mesquita J, Castro-de-Sousa JP, Vaz-Pereira S, Neves A, Passarinha LA, Tomaz CT. Vascular endothelial growth factors and placenta growth factor in retinal vasculopathies: current research and future perspectives. Cytokine Growth Factor Rev. 2018;39:102–115. doi: 10.1016/j.cytogfr.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 185.Yamamoto K, Takeshita K, Kojima T, Takamatsu J, Saito H. Aging and plasminogen activator inhibitor-1 (PAI-1) regulation: implication in the pathogenesis of thrombotic disorders in the elderly. Cardiovasc Res. 2005;66(2):276–285. doi: 10.1016/j.cardiores.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 186.Freitas-Rodríguez S, Folgueras AR, López-Otín C. The role of matrix metalloproteinases in aging: tissue remodeling and beyond. Biochim Biophys Acta Mol Cell Res. 2017;1864(11 Pt A):2015–25. doi: 10.1016/j.bbamcr.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 187.Rempe RG, Hartz AMS, Bauer B. Matrix metalloproteinases in the brain and blood-brain barrier: versatile breakers and makers. J Cereb Blood Flow Metab. 2016;36(9):1481–1507. doi: 10.1177/0271678x16655551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Nurmi L, Heikkilä HM, Vapaatalo H, Kovanen PT, Lindstedt KA. Downregulation of Bradykinin type 2 receptor expression in cardiac endothelial cells during senescence. J Vasc Res. 2012;49(1):13–23. doi: 10.1159/000329615. [DOI] [PubMed] [Google Scholar]
- 189.Kirwan JP, Krishnan RK, Weaver JA, Del Aguila LF, Evans WJ. Human aging is associated with altered TNF-alpha production during hyperglycemia and hyperinsulinemia. Am J Physiol Endocrinol Metab. 2001;281(6):E1137–E1143. doi: 10.1152/ajpendo.2001.281.6.E1137. [DOI] [PubMed] [Google Scholar]
- 190.Singh T, Newman AB. Inflammatory markers in population studies of aging. Ageing Res Rev. 2011;10(3):319–329. doi: 10.1016/j.arr.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Bruunsgaard H, Pedersen AN, Schroll M, Skinhoj P, Pedersen BK. TNF-alpha, leptin, and lymphocyte function in human aging. Life Sci. 2000;67(22):2721–2731. doi: 10.1016/s0024-3205(00)00851-1. [DOI] [PubMed] [Google Scholar]
- 192.Seals DR, Desouza CA, Donato AJ, Tanaka H. Habitual exercise and arterial aging. J Appl Physiol (1985) 2008;105(4):1323–32. doi: 10.1152/japplphysiol.90553.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Kelly J, Ali Khan A, Yin J, Ferguson TA, Apte RS. Senescence regulates macrophage activation and angiogenic fate at sites of tissue injury in mice. J Clin Investig. 2007;117(11):3421–3426. doi: 10.1172/jci32430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Wu D, Ren Z, Pae M, Guo W, Cui X, Merrill AH, et al. Aging up-regulates expression of inflammatory mediators in mouse adipose tissue. J Immunol (Baltimore, Md : 1950) 2007;179(7):4829–39. doi: 10.4049/jimmunol.179.7.4829. [DOI] [PubMed] [Google Scholar]
- 195.Franceschi C, Valensin S, Lescai F, Olivieri F, Licastro F, Grimaldi LM, et al. Neuroinflammation and the genetics of Alzheimer’s disease: the search for a pro-inflammatory phenotype. Aging (Milan, Italy) 2001;13(3):163–170. doi: 10.1007/BF03351475. [DOI] [PubMed] [Google Scholar]
- 196.De Martinis M, Franceschi C, Monti D, Ginaldi L. Inflamm-ageing and lifelong antigenic load as major determinants of ageing rate and longevity. FEBS Lett. 2005;579(10):2035–2039. doi: 10.1016/j.febslet.2005.02.055. [DOI] [PubMed] [Google Scholar]
- 197.Ferrucci L, Harris TB, Guralnik JM, Tracy RP, Corti MC, Cohen HJ, et al. Serum IL-6 level and the development of disability in older persons. J Am Geriatr Soc. 1999;47(6):639–646. doi: 10.1111/j.1532-5415.1999.tb01583.x. [DOI] [PubMed] [Google Scholar]
- 198.Kuller LH. Serum levels of IL-6 and development of disability in older persons. J Am Geriatr Soc. 1999;47(6):755–756. doi: 10.1111/j.1532-5415.1999.tb01604.x. [DOI] [PubMed] [Google Scholar]
- 199.Leng S, Xue QL, Huang Y, Semba R, Chaves P, Bandeen-Roche K, et al. Total and differential white blood cell counts and their associations with circulating interleukin-6 levels in community-dwelling older women. J Gerontol A Biol Sci Med Sci. 2005;60(2):195–199. doi: 10.1093/gerona/60.2.195. [DOI] [PubMed] [Google Scholar]
- 200.Golden J, Frim DM, Chapman PH, Vonsattel JP. Marked tissue eosinophilia within organizing chronic subdural hematoma membranes. Clin Neuropathol. 1994;13(1):12–16. [PubMed] [Google Scholar]
- 201.Smith PC, Caceres M, Martinez C, Oyarzun A, Martinez J. Gingival wound healing: an essential response disturbed by aging? J Dent Res. 2015;94(3):395–402. doi: 10.1177/0022034514563750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Benatti BB, Silverio KG, Casati MZ, Sallum EA, Nociti FH., Jr Influence of aging on biological properties of periodontal ligament cells. Connect Tissue Res. 2008;49(6):401–408. doi: 10.1080/03008200802171159. [DOI] [PubMed] [Google Scholar]
- 203.Hayek MG, Mura C, Wu D, Beharka AA, Han SN, Paulson KE, et al. Enhanced expression of inducible cyclooxygenase with age in murine macrophages. J Immunol (Baltimore, Md : 1950) 1997;159(5):2445–51. [PubMed] [Google Scholar]
- 204.Simmons DL, Botting RM, Hla T. Cyclooxygenase isozymes: the biology of prostaglandin synthesis and inhibition. Pharmacol Rev. 2004;56(3):387–437. doi: 10.1124/pr.56.3.3. [DOI] [PubMed] [Google Scholar]
- 205.Fam NP, Verma S, Kutryk M, Stewart DJ. Clinician guide to angiogenesis. Circulation. 2003;108(21):2613–2618. doi: 10.1161/01.cir.0000102939.04279.75. [DOI] [PubMed] [Google Scholar]
- 206.Wagatsuma A. Effect of aging on expression of angiogenesis-related factors in mouse skeletal muscle. Exp Gerontol. 2006;41(1):49–54. doi: 10.1016/j.exger.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 207.Hohenstein A. Faktoren der Angiogenese im Chronisch Subduralen Hämatom. Neurochirurgische Universitätsklinik Mannheim. Mannheim: Ruprecht Karls Universität Heidelberg, Fakultät für klinische Medizin Mannheim; 2003. p. 91.
- 208.Holmes K, Roberts OL, Thomas AM, Cross MJ. Vascular endothelial growth factor receptor-2: structure, function, intracellular signalling and therapeutic inhibition. Cell Signal. 2007;19(10):2003–2012. doi: 10.1016/j.cellsig.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 209.Sarzani R, Arnaldi G, Takasaki I, Brecher P, Chobanian AV. Effects of hypertension and aging on platelet-derived growth factor and platelet-derived growth factor receptor expression in rat aorta and heart. Hypertension. 1991;18(5 Suppl):III93–9. doi: 10.1161/01.hyp.18.5_suppl.iii93. [DOI] [PubMed] [Google Scholar]
- 210.Bruunsgaard H, Skinhoj P, Pedersen AN, Schroll M, Pedersen BK. Ageing, tumour necrosis factor-alpha (TNF-alpha) and atherosclerosis. Clin Exp Immunol. 2000;121(2):255–260. doi: 10.1046/j.1365-2249.2000.01281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Cave AC, Brewer AC, Narayanapanicker A, Ray R, Grieve DJ, Walker S, et al. NADPH oxidases in cardiovascular health and disease. Antioxid Redox Signal. 2006;8(5–6):691–728. doi: 10.1089/ars.2006.8.691. [DOI] [PubMed] [Google Scholar]
- 212.Ghebre YT, Yakubov E, Wong WT, Krishnamurthy P, Sayed N, Sikora AG, et al. Vascular aging: implications for cardiovascular disease and therapy. Transl Med (Sunnyvale, Calif). 2016;6(4). 10.4172/2161-1025.1000183. [DOI] [PMC free article] [PubMed]
- 213.Novella S, Heras M, Hermenegildo C, Dantas AP. Effects of estrogen on vascular inflammation: a matter of timing. Arterioscler Thromb Vasc Biol. 2012;32(8):2035–2042. doi: 10.1161/atvbaha.112.250308. [DOI] [PubMed] [Google Scholar]