Abstract
The escalation of life expectancy is accompanied by an increase in the prevalence of age-related conditions, such as sarcopenia. Sarcopenia, a muscle condition defined by low muscle strength, muscle quality or quantity, and physical performance, has a high prevalence among the elderly and is associated to increased mortality. The neuromuscular system has been emerging as a key contributor to sarcopenia pathogenesis. Indeed, the age-related degeneration of the neuromuscular junction (NMJ) function and structure may contribute to the loss of muscle strength and ultimately to the loss of muscle mass that characterize sarcopenia. The present mini-review discusses important signaling pathways involved in the function and maintenance of the NMJ, giving emphasis to the ones that might contribute to sarcopenia pathogenesis. Some conceivable biomarkers, such as C-terminal agrin fragment (CAF) and brain-derived neurotrophic factor (BDNF), and therapeutic targets, namely acetylcholine and calcitonin gene–related peptide (CGRP), can be retrieved, making way to future studies to validate their clinical use.
Keywords: BDNF, CAF, Denervation, Muscle wasting, Neuromuscular junction, Neurotrophins
Introduction
The global population is aging, with about 13.5% being 60 years of age or older in 2021, a number that is expected to increase during the next decades [1]. This rise in longevity exposes age-related conditions, such as sarcopenia. Sarcopenia is a progressive and generalized skeletal muscle disease defined by low muscle strength, muscle quantity or quality, and physical performance [2]. It affects 5–13% of people between 60 and 70 years old, and 11–50% of the adults older than 80 years [3–5]. In addition, sarcopenic subjects have a higher mortality risk than non-sarcopenic ones, with this risk being greater in people older than 79 years [6]. Sarcopenia etiology is multifactorial, with many systemic factors being related to its development [7, 8]; however, the local neuromuscular environment is emerging as an important contributor to sarcopenia pathogenesis [9]. Voluntary movement and the maintenance of muscle mass and strength require an efficient communication between the nervous and muscular systems through a normal innervation [10]. Age-related changes in the neuromuscular system manifest functionally as loss of muscle strength and coordination, which precedes the loss of muscle mass [11]. Indeed, in older people, the loss of muscle strength is significantly more rapid than the loss of muscle mass, and maintaining or gaining muscle mass does not avert the aging-related decrease in muscle strength [12]. More specifically, after 75 years, strength is lost at a rate of 2.5–3% per year in women and 3–4% in men, while muscle mass is lost at a rate of 0.64–0.70% per year in women and 0.80–0.98% in men [13]. The early identification of the age-related impairment of the neuromuscular system will create the opportunity to clinically intervene and to avoid the irreversible loss of muscle mass and related negative health outcomes [2, 14], envisioning the improvement of the clinical management of sarcopenic subjects.
This mini-review discusses the current knowledge on key neuromuscular system–related mechanisms and players that may be responsible for the age-related impairment of the neuromuscular junction (NMJ), and so, that may contribute to the loss of muscle strength and mass with aging. The reader, however, should keep in mind that most of the available data on this topic come from experiments in animal models given the invasiveness underlying skeletal muscle collection, and that among the available clinical studies, most of them enrolled healthy older adults. Regarding the data provided from human muscle biopsies, it is important to be aware that it is difficult to obtain several samples per subject [15], and one tissue sample represents less than 0.01% of the whole muscle and contains only about hundreds of fibers of even less motor units. This means that the data obtained from biochemical, histochemical, and morphometric analyses may not be representative of the whole muscle [16]. This mini-review hopes to make way to future studies, aiming to identify biomarkers and therapeutic targets of sarcopenia.
Neuromuscular junction aging
The NMJ is a highly specialized synapse that ensures the efficient transmission of electric impulses from the presynaptic innervating motoneuron to the postsynaptic innervated muscle fibers to stimulate contraction. Briefly, when the motor nerve action potential arrives, calcium enters the presynaptic terminal. Consequently, acetylcholine (ACh) is released from their synaptic vesicles into the synaptic cleft through the presynaptic active zones [17, 18]. ACh binds to its receptors (AChRs), which are tightly clustered in the postsynaptic membrane of the muscle fiber (also known as motor endplate), depolarizing it, which initiates the muscle action potential, resulting in muscle contraction [19]. Besides ACh, other mediators, namely neurotrophins (NTs), agrin, and calcitonin gene–related peptide (CGRP), are also released by the motoneuron. On another hand, signals derived from the skeletal muscle influence neuron survival, axonal growth, and maintenance of synaptic connections [20]. This bidirectional communication is vital for the survival of both muscle and nerve and for their mechanism of action [20]. Since both presynaptic and postsynaptic mediators are fundamental to the function of the neuromuscular system, the functional impairment of the NMJ may be a causative factor to the age-related loss of muscle strength [21, 22]. Nonetheless, there is a slight chance that the opposite, i.e., the sarcopenia-associated alterations (e.g., inactivity), may promote maladaptive changes in the NMJ.
Age-related morphological/structural changes
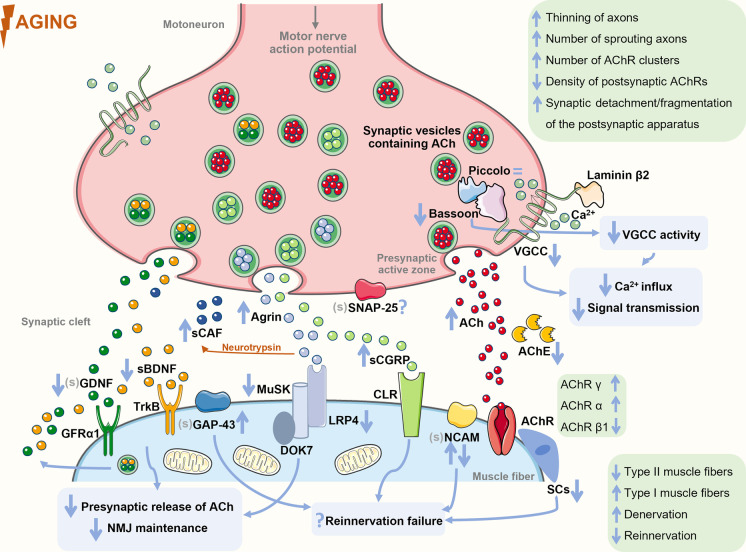
Classic morphological features of NMJ impairment, like significant thinning of axons, increased number of sprouting axons and AChR clusters, lower density of postsynaptic AChRs, and synaptic detachment and fragmentation of the postsynaptic apparatus, were observed in aged extensor digitorum longus (30 months) [23] and tibialis anterior (24 months) of mice [24] (Fig. 1). Comparative to rodents (mice and rats), human NMJs are significantly smaller and more fragmented, with smaller axon diameter and average area of AChR clusters [25]. In some human studies [26, 27], the age-related features of NMJs observed were similar to those found in animals. For instance, in intercostal muscles (4–77 years), postsynapses were fragmented with increased length and degeneration of the junctional folds. Moreover, a higher number of smaller conglomerates of AChRs were found in the postsynaptic side, suggesting a possible degeneration of the NMJs and muscle fibers. Nonetheless, a recent study challenged these findings by declaring that human NMJs remain remarkably stable across the adult lifespan (34–92 years) [25]. The disparity between these studies may be explained by, for instance, different methodologies used and the analysis of fresh versus postmortem tissue. Clearly, further studies are needed to better understand human NMJ age-related plasticity.
Fig. 1.
The age-induced alterations that seem to preclude sarcopenia. Changes in the levels of key neuromuscular system–related mediators associated to the impairment of the neuromuscular junction function and morphology are highlighted as well as alterations in signal transmission between the motoneurons and muscle fibers, and of the reinnervation process that culminate in impaired muscle function. The mediators analyzed in the serum in aging or sarcopenia contexts are preceded by “s”, while the ones not analyzed in these contexts (but in other conditions) are preceded by “(s)”. Figure produced with Servier Medical Art. Abbreviations: ACh: acetylcholine; AChE: acetylcholinesterase; AChR: acetylcholine receptor; BDNF: brain-derived neurotrophic factor; CAF: C-terminal agrin fragment; CGRP: calcitonin gene–related protein; CLR: calcitonin-like receptor; DOK7: downstream of kinase 7; GAP-43: growth-associated protein; GDNF: glial-cell-line-derived neurotrophic factor; GFRα1: glial-cell-line-derived neurotrophic factor family receptor alpha 1; LRP4: low-density lipoprotein receptor–related protein 4; MuSK: muscle-specific kinase; NCAM: neural cell adhesion molecule; SCs: satellite cells; SNAP-25: synaptosomal-associated protein of 25 kDa; TrkB: tropomyosin-related kinase; VGCC: voltage-gated calcium channel
With chronological aging is observed a process of remodeling of motor units, resulting mostly in denervation of type II muscle fibers (fast-twitch and glycolytic) that tend to be reinnervated by small neurons that innervate type I motor units [21, 28–31]. This remodeling results in the co-expression of multiple myosin heavy chain (MHC) isoforms and large fiber type clustering, i.e., an unusual number of adjacent fibers having the same fiber type [21, 32]. Since type II fibers are the ones capable of generating higher maximum force levels [33], changes in motor unit composition may be the main cause responsible for the loss of muscle strength that is reported in adults over 55 years [29], as shown in [34].
When the denervation rate outpaces the reinnervation one, due, for instance, to the age-related impairment of the reinnervation process, the resulting denervated muscle fibers [11] will atrophy [28] and be lost [11]. Some examples of this impairment were reported. On one hand, aging muscle has a blunted neural cell adhesion molecule (NCAM) response to denervation. This molecule, which is nearly absent in adult muscles [35], reappears in the extrasynaptic region of the adult muscle fibers in response to denervation to help in the reinnervation process [35–37]. For instance, the partial denervation of the extensor digitorum longus of aged mice (22–30 months) did not produce a NCAM response, while in young mice (2 months), an enhanced NCAM response was observed [37]. On another hand, in older humans (65–94 years), the vastus lateralis had small muscle fibers co-stained with NCAM and MHC neonatal isoform (MHCn) [38]. These small fibers may be long-term denervated fibers that have atrophied over time and reverted to an immature MHC configuration. In fact, it has been suggested that the presence of either MHCn or embryonic (MHCe) isoforms indicates denervated muscle fibers [38, 39]. In aged rat soleus (30 months), the number of MHCe-positive fibers increased with the degree of sarcopenia, so being associated with a poor outcome [39].
The maintenance and function of the NMJs involve highly synchronized and fine-tuned actions of, for instance, (i) mediators involved in the organization of presynaptic active zones, fundamental for ACh release [40]; (ii) presynaptic mediators, such as agrin and ACh, and of their postsynaptic receptors and downstream proteins [22]; and (iii) neurotrophins [41]. Hence, the disruption of the action of any of the mediators involved in these processes may play a role in age-related neuromuscular dysfunction, and therefore, in sarcopenia.
Age-related changes in presynaptic signaling
Active zones
Upon arrival of an action potential, the synaptic transmission at the adult NMJs initiates by the opening of P/Q-type voltage-gated calcium channels (VGCCs) in the active zones, causing local calcium influx, fusion of the synaptic vesicles with the presynaptic membrane, and the release of the neurotransmitter ACh into the synaptic cleft [42, 43]. Presynaptic active zones are sites of accumulation and release of synaptic vesicles, and harbor specialized multidomain scaffold proteins, such as Bassoon, CAST/Erc2, Munc13, Piccolo, and Rim1 [19, 44]. These proteins seem to function as structural organizers of the active zone and as regulators of the exocytosis of ACh, being also involved in the recruitment of VGCCs [45, 46]. Specifically, Bassoon and Piccolo have been implicated in the local regulation of protein ubiquitination and proteasome-mediated proteolysis at presynapse, which may contribute to short-term presynapse plasticity [46]. In addition, these two scaffold proteins seem to connect presynaptic activity to neuronal gene expression reprogramming, potentially contributing to long-term alterations of the presynaptic function. This demonstrates the importance of the regulation of the levels of active zone proteins to ensure a normal NMJ function. The organization and maintenance of NMJ active zones rely on cues provided by the synaptic basal lamina, a layer of extracellular matrix placed at the synaptic cleft [43]. A key component of this lamina is laminin-β2 that binds to the presynaptic receptor P/Q-type VGCC, which is concentrated at the presynaptic terminus, initiating active zone assembly [47]. The VGCC β1b- or β4-subunit binds to the cytosolic active zone protein Bassoon or CAST/Erc2 [44].
Changes in the levels of these active zone proteins were observed during aging (Fig. 1). For instance, in old rodents (24 and 29 months old), the levels of P/Q-type VGCC and Bassoon were significantly lower than in adult ones [42, 48]. The low levels of Bassoon led to an attenuation of P/Q-type VGCC activity, and consequently, to an attenuation of calcium influx, signal transmission, and muscle contraction [42] (Table 1). The decrease of these levels seems to precede denervation, since the presence of the neuronal proteins Bassoon and Piccolo in aged NMJs indicates that nerve terminals are present [48]. Indeed, Bassoon levels were decreased at innervated NMJs (of sternomastoid) of old mice (27 months), while in denervated ones, a complete absence of Bassoon was observed [40]. Interestingly, the levels of the protein Piccolo were not decreased [48], suggesting a selective reduction of active zone proteins during aging. The correct function of both Bassoon and Piccolo seems to be supported by integrin-α3, a key adhesion receptor of the presynaptic active zone [43]. The NMJs of integrin-α3-knockout mice presented a reduction of the localization of Bassoon and Piccolo and typical structural changes that have been related with aging, namely fragmentation, less AChRs clusters, terminal nerve sprouting, and excessive axon branching, which can result in a deficient synaptic transmission [43]. Therefore, integrin-α3 may contribute to NMJ dysfunction and consequent denervation in aging, but the effects of aging on presynaptic active zone proteins in humans are still unknown.
Table 1.
Effect of aging on key neuromuscular junction (NMJ) mediators and the possible outcomes in the NMJ that may contribute to sarcopenia
| Mediator | Effect of aging | Possible outcomes in NMJ | References |
|---|---|---|---|
| Presynaptic signaling | |||
| P/Q-type VGCC | ↓ | • ↓ P/Q-type VGCC activity | [48] |
| • ↓ Calcium influx | |||
| Bassoon | ↓ | • ↓ Signal transmission | [40, 42, 48] |
| • ↓ Muscle contraction | |||
| ACh | ↑ | • NMJ fragmentation | [58] |
| • Denervation | |||
| • Axon sprouting | |||
| Agrin | ↑ | • NMJ fragmentation | [77] |
| • Nerve terminal branching | |||
| • Denervation | |||
| • Smaller AChRs clusters | |||
| LRP4 | ↓ | • ↓ Agrin-LRP4-MuSK signaling | [68] |
| p-MuSK | ↓ | ||
| BDNF (serum) | ↓ | • Denervation (?) | [89–91] |
| • ↓ ACh release (?) | |||
| • ↓ Synapse maintenance (?) | |||
| BDNF (mRNA) | = | • ——————— | [67] |
| GDNF (mRNA) | ↓ | • ↓ ACh release (?) | [95] |
| GDNF | =/↓ | • ↓ Postsynaptic maintenance (?) | |
| Postsynaptic signaling | |||
| CGRP | ↑ | • ↓ Reinnervation (?) | [77] |
| GAP-43 | ↑ | [77, 113] | |
ACh, acetylcholine; AChRs, acetylcholine receptors; BDNF, brain-derived neurotrophic factor; CGRP, calcitonin gene–related protein; GAP-43, growth-associated protein; GDNF, glial-cell-line-derived neurotrophic factor; LRP4, low-density lipoprotein receptor–related protein 4; p-MuSK, phosphorylated muscle-specific kinase; VGCC, voltage-gated calcium channel
Another fundamental active zone protein is the synaptosomal-associated protein of 25 kDa (SNAP-25), a component of the soluble N-ethylmaleimide sensitive factor attachment protein receptor (SNARE) protein complex. SNAP-25 is presented in motor nerve endings, being responsible for the fusion of ACh-containing vesicles with the plasma membrane during synaptic transmission [49, 50]. Moreover, SNAP-25 also inhibits VGCC function, controlling calcium responsiveness to depolarization [51, 52]. However, the effects of aging on the levels of SNAP-25 in the NMJ are still unknown. Despite this, some studies suggest that SNAP-25 may be a target of reactive oxygen species (ROS), which leads to dysfunction in neurotransmitter release [53, 54]. Therefore, it is plausible to think that the age-related increase in oxidative stress [55, 56] may be involved, at some extent, in the deficient synaptic transmission observed in sarcopenia. It would be important to investigate this possible association.
Acetylcholine signaling
The principal mediator to NMJ function is ACh, which activates the AChRs located in the postsynaptic membrane, allowing sodium ions to enter the muscle and the generation of the endplate potential (EPP). If the EPP reaches the threshold, the sodium channels located in the borders of the motor endplate open, allowing a higher influx of positive ions, initiating the muscle action potential, which spreads in a wave-like effect throughout the sarcolemma [17, 18]. The action potential activates the voltage-gated dihydropyridine receptors (DHPRs) in the t-tubule membrane, and by induction, the ryanodine receptors (RyRs), releasing calcium from the sarcoplasmic reticulum and culminating in force production and muscle contraction [57]. It was observed an increase in the basal levels of ACh in the gastrocnemius of old mice (28 months vs. 8 weeks), which may indicate a compensation for decreased NMJ signals, i.e., denervation [58] (Table 1). Moreover, the mRNA levels of acetylcholinesterase, the enzyme that catalyzes the breakdown of ACh, was decreased in these animals, which might have contributed to the ACh levels observed [58]. In line with these results, it was demonstrated that increased levels of ACh at the synaptic cleft prematurely result in age-related structural alterations in the NMJs, including fragmentation, denervation, and axon sprouting, prior to muscle atrophy [59] (Table 1), which may indicate that ACh modulates the NMJ in a quantity-dependent manner. In fact, moderately reducing ACh levels promoted several positive effects on aged NMJs (17 months mice) and muscle fibers, such as an increase in NMJ area, muscle fiber cross-sectional area (CSA), and in the number of satellite cells (SCs) [60]. Therefore, moderately reducing ACh may constitute a therapeutic strategy to sarcopenia, as already suggested by [60].
The AChR in adult skeletal muscle forms a heteropentamer that harbors two α, one β, one δ, and one ε subunit (the ε subunit in adult AChR replaces the γ subunit in fetal AChR) [61]. These subunits also suffer changes in their expression during aging [62]. For instance, in the vastus lateralis of older women (71–78 years) was observed a robust increase of AChR γ mRNA expression and a decrease of AChR β1, compared to young women (20–28 years) [63]. These older women had a great amount of denervated fibers [63], which may explain the increase in the γ subunit, since its levels increase in response to denervation and neurotransmitter blockage [63, 64]. In another study, however, age (65–94 years) was negatively associated with AChR γ mRNA in the vastus lateralis, but existed a large interindividual variation in the mRNA levels of the participants of this study [38]. Human muscle biopsies may be accompanied by variability in the levels of the studied mediators due to, for instance, sampling site selection [65]. Thus, preclinical studies were considered. The mRNA levels of AChR α and γ were increased in the gastrocnemius (24 months vs. 4 months; 28 months vs. 8 weeks) [58, 66] and vastus lateralis (35 months vs. 8 months) of aged rodents [67], as well as the protein levels of AChR δ on the diaphragm of old mice (24 months vs. 8 months) [68]. However, no differences were found in protein levels of AChR α, β, and ε subunits in the muscles of aged animals compared to young ones [66, 68]. It was also suggested that the simple activation of AChRs by the ACh released from motoneurons is sufficient to prevent alterations associated to denervation, like the de novo synthesis and incorporation of connexins hemichannels into the sarcolemma, which initiates a sequence of deleterious changes, including sarcolemma permeabilization, increased cytosolic calcium and sodium levels, and upregulation of atrogenes, which leads to protein catabolism and muscle atrophy [69]. Moreover, AChR subunits may play a critical role in NMJ stability after denervation with the main goal of maintaining NMJ function [70]. However, in old animals and contrarily to what occurs in young ones, the protein levels of AChR α did not increase after nerve injury [66], which may jeopardize NMJ functions.
Agrin-LRP4-MuSK signaling
Data suggest that agrin-low-density lipoprotein receptor–related protein 4 (LRP4)-muscle-specific kinase (MuSK) signaling pathway may be impaired during aging (Fig. 1). Neural agrin, which is released from motoneurons and accumulates at the synaptic basal lamina, binds to its co-receptor LRP4 on the cell surface of muscle fibers, activating the tyrosine kinase domain of the receptor MuSK to self-phosphorylate [71, 72]. Activated MuSK is transported along the axons and released into the synaptic basal lamina of the NMJs, transmitting the extracellular signal into the myotubes, where it triggers the assembly of the postsynaptic apparatus, including AChRs clustering and stabilization of presynaptic structures [73]. In muscle fibers, activated MuSK also recruits and phosphorylates downstream of kinase 7 (DOK7), promoting DOK7 dimerization that, in turn, enhances MuSK phosphorylation through a positive feedback loop that has to be finely controlled for normal NMJ structure and function [71]. Activated DOK7 leads to the recruitment of two adapter proteins Crk and Crk-L [74]. This signaling cascade stimulates the cytoplasmatic membrane structural protein rapsyn to self-aggregate and interact with agrin and MuSK, which also induces AChRs clustering and postsynaptic specialization [74–76].
With aging, the activity of this signaling pathway in the NMJs appears to become dysregulated. A significant increase of the expression of agrin in the NMJs of the tibialis anterior, extensor digitorum longus, and soleus of old mice (24–30 months) was observed [77]. These animals had a deficient locomotor activity and the NMJs presented typical age-related structural alterations, namely fragmentation, nerve terminal branching, denervation, and smaller AChRs clusters [77] (Table 1). However, LRP4 levels were found markedly reduced in the diaphragm of old mice (24 months vs. 3 months), which may be explained by the enhanced LRP4 ubiquitination observed [68]. Accordingly, MuSK phosphorylation was found reduced in aged diaphragm (24 months vs. 3 months) [68], despite its mRNA levels being increased in the vastus lateralis of old mice (35 months) comparatively to young animals (8 months) [67]. These results suggest a compromised agrin-LRP4-MuSK activation with aging (Table 1), which may contribute to the alterations in NMJ function and structure. On the other hand, the levels of the intracellular adaptor protein rapsyn were increased in the vastus lateralis of old rats (35 months vs. 8 months) [67], but mutations in human rapsyn gene were already associated with NMJ-related diseases [78].
Another cause to age-related NMJ impairment may be the augment of the proteolytic cleavage of agrin by the neuronal serine protease neurotrypsin (Fig. 1) [79]. This agrin inactivation occurs by its cleavage at two homologous, highly conserved sites and results in the release of the soluble 22-kDa C-terminal agrin fragment (CAF) into circulation making it detectable in serum [80], and therefore a great candidate as sarcopenia biomarker. The increase in the serum levels of CAF with aging [73, 81, 82] translates an enhanced agrin degradation that may symbolize reduced levels of agrin at the NMJs, compromising agrin role in the maintenance of a functional NMJ. Actually, overexpression of neurotrypsin in motoneurons of young adult mice established the sarcopenic phenotype (reduced number of muscle fibers, increased heterogeneity of fiber thickness, fiber type grouping, and increased proportion of type I fibers) and led to an excessive fragmentation of the NMJ [83]. A neurotrypsin inhibitor (NT-1474) showed efficacy in vivo (mice) in reducing CAF serum levels by 44% [81]. Unfortunately, NMJs were not investigated. Nevertheless, in another study, muscular and junctional sarcopenic alterations were less pronounced in neurotrypsin-overexpressing aged mice (24 months; soleus) by transgenic co-expression of cleavage-resistant agrin, which seems to protect NMJs from disassembly and also exerts a survival-promoting effect for denervated fibers by enhancing reinnervation [83]. This was further strengthened in a posterior study where injection of neurotrypsin-resistant agrin activated agrin signaling and almost fully reversed the sarcopenia-like phenotype in neurotrypsin-overexpressing mice and also accelerated muscle reinnervation after nerve crush [84].
Neurotrophins
The skeletal muscle and nerves interact through electrical activity and neurotrophic regulation [41]. The latter occurs by the release of neurotrophic factors, like NTs [41]. The family of NTs harbors nerve growth factor (NGF), NT-3, NT-4, brain-derived neurotrophic factor (BDNF), and glial-cell-line-derived neurotrophic factor (GDNF), which regulate motoneuron survival, enhance presynaptic release of ACh, and promote the maintenance of the postsynaptic region [85, 86]. NTs are synthesized and released from both neurons and muscles [87]. NT signaling is mediated by the tropomyosin-related kinase (Trk) receptor and by the p75 neurotrophin receptor (p75NTR) [85]. BDNF is one of the most studied NTs and potentiates ACh release through TrkB phosphorylation and PKC activation [41]. In addition, it is involved in the regulation of synapse function and maintenance in the neuromuscular system and also in muscle development and metabolism [41]. Indeed, inhibition of BDNF-TrkB signaling at early old age (18 months) induced NMJ denervation [88]. Studies suggest that an age-related decrease in BDNF serum levels occurs [89–91] (Table 1). This may be explained by the hypothesis that BDNF is a contractile-inducible protein [92, 93] and older people tend to be more sedentary [94]. In the vastus lateralis of old mice (35 months), no changes were observed in the mRNA levels of NT-3, NT-4, BDNF, or GDNF compared to young animals (8 months), despite the presence of marked denervation and increased mRNA levels of the neurotrophic tyrosine kinase receptor type 3 (NTRK3) [67]. This may suggest that a normal neurotrophic signaling is insufficient to a successful maintenance of the neuromuscular system.
A decrease of GDNF mRNA was also observed in both aged rats’ gastrocnemius and soleus, but GDNF protein levels were maintained without alterations in the NMJs throughout adulthood [95] (Table 1). Nevertheless, the time analyzed was very low (birth to 3 months). In another study, 17-week rats showed lower GDNF levels on the soleus and extensor digitorum longus comparatively to 4-week animals [96].
Age-related changes in postsynaptic signaling
Calcitonin gene–related peptide
CGRP is a neuropeptide present in motoneurons where it is stored in dense-core vesicles, possibly coexisting with ACh, being released upon nerve stimulation [97–99]. In the skeletal muscle, CGRP binds to calcitonin-like receptor (CLR), a Gs protein–coupled receptor highly presented in the NMJs, and increases the levels of intracellular cyclic adenosine monophosphate (cAMP) that leads to the activation of cAMP-dependent protein kinase (PKA) and phosphorylation of cAMP response element-binding protein (CREB) [97], suggesting that CGRP regulates gene expression. It is known that CGRP increases the number of AChRs and the rate of AChR desensitization and decreases the expression of acetylcholinesterase, potentiating muscle contraction [97, 100]. This may be indicative that this neuropeptide acts on skeletal muscle to regulate the elements of the postsynaptic apparatus [100]. Despite CGRP levels being high during NMJ development, they markedly decrease in mature NMJs [101]; however, CGRP is upregulated following nerve injury, suggesting a function in NMJs during the process of reinnervation and nerve sprouting, with its levels declining with the morphological and functional recovery of the NMJ [77, 102]. Recent data also suggest that upon muscle denervation, CGRP is involved in NMJ stabilization through the transcriptional inhibition of forkhead box O (FoxO)/transcription factor EB (TFEB)–regulated autophagic genes, stimulation of mammalian target of rapamycin complex 1 (mTORC1), and inhibition of the calpain system. Hence, CGRP may be used as both a potential biomarker for age-induced denervation and a therapeutic target [103]. Indeed, mammalian target of rapamycin (mTOR) signaling must be fine-tuned to maintain NMJ function. A role for mTORC1 in NMJ morphology and function has been discussed. Typical morphological features of age-related NMJ instability like significant thinning of axons, increased number of sprouting axons, higher number of AChR clusters, and lower density of postsynaptic AChRs were observed in mice with sustained activation of mTORC1 (TSCmKO, 9 months, extensor digitorum longus) [23]. In contrast, mTORC1 inhibition (10 months mice, tibialis anterior) also resulted in denervation, NMJ fragmentation, and higher number of AChRs clusters [104]. Furthermore, partial mTORC1 inhibition (via RAD001, a rapalog, administrated at a low dose) reduced the age-induced transcriptional upregulation of denervation-associated gene markers in aged tibialis anterior (24 months); however, no differences were verified in the gastrocnemius or plantaris muscles [105]. Still, more studies are needed to clarify the role of mTOR signaling in NMJ function.
A significant increase in CGRP immunoreactivity was observed in the NMJs of old tibialis anterior, soleus, extensor digitorum longus, and gracilis muscles (24–30 months vs. 4 months) [77]. This continuous increase with aging without a subsequent decline may symbolize the occurrence of denervation without reinnervation, which may indicate failure in the functional recovery of the NMJs (Fig. 1 and Table 1). In fact, those animals exhibited frequent endplate denervation and classic age-related structural changes on their NMJs [77]. More studies on the effect of aging in the function of this signaling pathway are necessary, given that CGRP levels can be measured in human plasma [106], which makes it an attractive biomarker. Additionally, in vivo application of CGRP on the soleus of adult rats induced a local accumulation of AChRs on the extrajunctional surface where the AChRs are usually absent [98], indicating CGRP as a possible therapeutic strategy to counteract the impaired neuromuscular transmission observed with aging.
Growth-associated protein
Another mediator that is upregulated upon denervation is growth-associated protein (GAP-43) that is present in virtually all neurons during axonal growth, being particularly abundant in axonal growth cones [77]. GAP-34 is widely expressed in the central nervous system during the perinatal period, with its levels decreasing as maturation progresses [107, 108]. Mature motoneurons and their corresponding nerve terminals display low levels of GAP-43 [77]. Nonetheless, GAP-43 seems to be involved in the muscle regeneration process [109] with an upregulation of its levels following denervation [110], and also in the modulation of calcium dynamics and its intracellular roles [111, 112]. Indeed, GAP-43−/− mice showed, among others, decreased body weight, reduced muscle strength, altered myofiber ultrastructure, and dysregulated calcium homeostasis in the muscle [111], possible by the lack of the interaction of GAP-43 with DHPR and RyR [111, 112]. In old animals, a significant increase of GAP-43 levels was observed in the NMJs of the tibialis anterior, soleus, extensor digitorum longus, and gracilis muscles (24–30 months vs. 4 months) [77]. Moreover, aged (30 months vs. 2–3 months; rat) motoneurons revealed increased GAP-43 immunoreactivity and GAP-43 levels seem to be associated to the severity of the neuromuscular dysfunction [113] (Fig. 1). Thus, the increased levels of GAP-43 observed in old age may be indicative of denervated muscle fibers (Table 1). Indeed, increased muscle levels of this protein are present in denervation [114] and myopathies [115], and no immunoreactivity was found in the absence of those conditions; thus, GAP-43 may be a good candidate as sarcopenia biomarker. Hence, its study in age and sarcopenia contexts would be important.
Satellite cells
Muscle regeneration also relies on muscle stem cells designated SCs [116] that under resting conditions are quiescently located underneath the basal lamina [117]. Upon tissue injury, SCs exit quiescence and proliferate to myoblasts that later differentiate to myocytes (muscle cells) to regenerate the damaged tissue [117]. This regenerative capacity of skeletal muscle is greatly affected by aging, since SCs abundance declines with age in both human (22–76 years; vastus lateralis) [118] and animal muscles (3–33 months; soleus, extensor digitorum longus, tibialis anterior) [119, 120] (Fig. 1), which may suggest that SC population is not adequately replenished throughout life. Despite this decline in SC pool, some studies demonstrated that the myogenic potential of SCs does not decline with aging, despite a slower rate of SCs activation after an injury in both old (22–30 months) and geriatric (29–33 months) muscles [119, 121]; however, it was also suggested that in geriatric mice (28–32 months), resting SCs lost their reversible quiescence state and switch to an irreversible pre-senescence state, being unable to activate and repair the muscle upon injury [122]. Specifically, it was also demonstrated that SCs frequency declines in both synaptic and extra-synaptic regions in old age (18 and 24 months) [123]. In line with this, depletion of SCs resulted in an impaired NMJ reinnervation, reduction in postsynaptic morphology, and loss of post-synaptic myonuclei in response to denervation [124]. Moreover, SCs depletion at 12 months accelerated the onset of age-related impairment of NMJ integrity, and a decline in postsynaptic myonuclei and myofiber size (vs. 18 and 24 months) [123]. Therefore, the important role that SCs have in maintaining NMJ function is noteworthy, a role that may be impaired as a result of aging and that is important to explore in future studies. Immunostaining of muscle sections with SCs markers has been the methodological approach used to evaluate the age effect on SCs remodeling. In human skeletal muscles, SCs have been identified using the NCAM/CD56 antigen [125]. Despite being considered a reliable molecular marker to identify SCs, NCAM is also expressed in myoblasts, myotubes, and muscle fibers during regeneration, as described before [125]. Thus, other markers are used, namely M-cadherin (M-Cad), c-Met, and Pax7, which are thought to be exclusively expressed in SCs of mature muscles [125].
Conclusions
The study of the effect of aging on the NMJ morphology and function is challenging, particularly in the clinical set. Muscle collection from aged subjects may raise ethical questions and the obtained samples may not be representative of the whole muscle. Besides, the NMJs may not be collected in the biopsy, and if so, may have a negative impact on the older subjects’ muscle function, potentially aggravating an already impaired muscle. Hence, preclinical studies allow the generation of data from whole muscle that cannot be collected from humans. Despite all these constrains in the study of NMJs, the analysis of the literature highlights several interesting findings. With aging, the NMJ structure alters, jeopardizing the communication between the nervous and muscle systems. The reinnervation rate in older ages is not sufficient to counterweight the rate of the appearance of denervated fibers, possible due to an age-induced incapacity to enhance reinnervation-related processes, such as the NCAM response. The maintenance and function of the NMJ rely on different signaling pathways involving players that ensure the stability of the pre- and postsynaptic apparatus and a proper signal transmission through ACh and reinnervation following denervation. Agrin, ACh, and NTs (such as BDNF and GDNF) signaling is impaired at old ages, resulting in a deficient neuromuscular transmission. Moreover, the role of SCs and proteins involved in the reinnervation process, namely CGRP and GAP-43, is compromised during aging, contributing to the accumulation of denervated muscle fibers. The effect of aging on these key mediators and the consequent outcomes on the NMJ are overviewed in Table 1. The evidence provided here suggests a key role of NMJ-related players to sarcopenia pathogenesis and highlights potential biomarkers and therapeutic targets, paving the way to future studies. Nonetheless, it remains elusive the relative contribution of NMJ dysfunction to sarcopenia pathogenesis, a question that deserves to be explored to improve sarcopenia management. Future investigations should consider analyzing older individuals (animals, at least) and evaluate the mediators that have the potential to be analyzed in serum, such as CAF, BDNF, and CGRP, envisioning the translation to the clinical set.
Author contribution
A.M.P. conducted the literature search and drafted the manuscript, and R.F., P.A.O., and J.A.D. critically revised the work.
Acknowledgments
This work was supported by CIAFEL (UIDB/00617/2020), LAQV (UIDB/50006/2020), and CITAB (UIDB/04033/2020) research units and by A.M.P.’s fellowship (SFRH/BD/144396/2019) through national funds by the Portuguese Foundation for Science and Technology (FCT) and co-financed by the European Regional Development Fund (FEDER), within the PT2020 Partnership Agreement.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization . Decade of healthy ageing: baseline report. Summary. Geneva: World Health Organization; 2021. [Google Scholar]
- 2.Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: revised european consensus on definition and diagnosis. Age Ageing. 2019;48:16–31. doi: 10.1093/ageing/afy169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rossi AP, Rubele S, D’Introno A, Zoico E, Brandimarte P, Amadio G, et al. An update on methods for sarcopenia diagnosis: from bench to bedside. Ital J Med. 2018;12:97–107. doi: 10.4081/itjm.2018.995. [DOI] [Google Scholar]
- 4.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: european consensus on definition and diagnosis. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yazar T, Olgun YH. Prevalance of sarcopenia according to decade. Clin Nutr ESPEN. 2019;29:137–141. doi: 10.1016/j.clnesp.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 6.Beaudart C, Zaaria M, Pasleau F, Reginster J-Y, Bruyère O. Health outcomes of sarcopenia: a systematic review and meta-analysis. PLoS ONE. 2017;12:e0169548. doi: 10.1371/journal.pone.0169548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wiedmer P, Jung T, Castro JP, Pomatto LCD, Sun PY, Davies KJA, et al. Sarcopenia – molecular mechanisms and open questions. Ageing Res Rev. 2021;65:101200. doi: 10.1016/j.arr.2020.101200. [DOI] [PubMed] [Google Scholar]
- 8.Ibebunjo C, Chick JM, Kendall T, Eash JK, Li C, Zhang Y, et al. Genomic and proteomic profiling reveals reduced mitochondrial function and disruption of the neuromuscular junction driving rat sarcopenia. Mol Cell Biol. 2013;33:194–212. doi: 10.1128/mcb.01036-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deschenes MR, Gaertner JR, O’Reilly S. The effects of sarcopenia on muscles with different recruitment patterns and myofiber profiles. Curr Aging Sci. 2013;6:266–272. doi: 10.2174/18746098113066660035. [DOI] [PubMed] [Google Scholar]
- 10.Rygiel KA, Picard M, Turnbull DM. The ageing neuromuscular system and sarcopenia: a mitochondrial perspective. J Physiol. 2016;594:4499–4512. doi: 10.1113/JP271212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hepple RT, Rice CL. Innervation and neuromuscular control in ageing skeletal muscle. J Physiol. 2016;594:1965–1978. doi: 10.1113/JP270561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delmonico MJ, Harris TB, Visser M, Park SW, Conroy MB, Velasquez-Mieyer P, et al. Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am J Clin Nutr. 2009;90:1579–1585. doi: 10.3945/ajcn.2009.28047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mitchell WK, Williams J, Atherton P, Larvin M, Lund J, Narici M. Sarcopenia, dynapenia, and the impact of advancing age on human skeletal muscle size and strength; a quantitative review. Front Physiol. 2012;3:260. doi: 10.3389/fphys.2012.00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Santilli V, Bernetti A, Mangone M, Paoloni M. Clinical definition of sarcopenia. Clin Cases Miner Bone Metab. 2014;11:177–180. [PMC free article] [PubMed] [Google Scholar]
- 15.Hayot M, Michaud A, Koechlin C, Caron M-A, LeBlanc P, Préfaut C, et al. Skeletal muscle microbiopsy: a validation study of a minimally invasive technique. Eur Respir J. 2005;25:431–440. doi: 10.1183/09031936.05.00053404. [DOI] [PubMed] [Google Scholar]
- 16.Baguet A, Everaert I, Hespel P, Petrovic M, Achten E, Derave W. A new method for non-invasive estimation of human muscle fiber type composition. PLoS ONE. 2011;6:e21956. doi: 10.1371/journal.pone.0021956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodríguez Cruz PM, Cossins J, Beeson D, Vincent A. The neuromuscular junction in health and disease: molecular mechanisms governing synaptic formation and homeostasis. Front Mol Neurosci. 2020;13:610964. doi: 10.3389/fnmol.2020.610964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ham DJ, Rüegg MA. Causes and consequences of age-related changes at the neuromuscular junction. Curr Opin Physiol. 2018;4:32–39. doi: 10.1016/j.cophys.2018.04.007. [DOI] [Google Scholar]
- 19.Mukund K, Subramaniam S. Skeletal muscle: a review of molecular structure and function, in health and disease. Wiley Interdiscip Rev Syst Biol Med. 2020;12:e1462. doi: 10.1002/wsbm.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lepore E, Casola I, Dobrowolny G, Musarò A. Neuromuscular junction as an entity of nerve-muscle communication. Cells. 2019;8:906. doi: 10.3390/cells8080906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rudolf R, Khan MM, Labeit S, Deschenes MR. Degeneration of neuromuscular junction in age and dystrophy. Front Aging Neurosci. 2014;6:00099. doi: 10.3389/fnagi.2014.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Punga AR, Ruegg MA. Signaling and aging at the neuromuscular synapse: lessons learnt from neuromuscular diseases. Curr Opin Pharmacol. 2012;12:340–346. doi: 10.1016/j.coph.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 23.Ham DJ, Börsch A, Lin S, Thürkauf M, Weihrauch M, Reinhard JR, et al. The neuromuscular junction is a focal point of mTORC1 signaling in sarcopenia. Nat Commun. 2020;11:4510. doi: 10.1038/s41467-020-18140-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Valdez G, Tapia JC, Kang H, Clemenson GD, Gage FH, Lichtman JW, et al. Attenuation of age-related changes in mouse neuromuscular synapses by caloric restriction and exercise. Proc Natl Acad Sci U S A. 2010;107:14863–14868. doi: 10.1073/pnas.1002220107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones RA, Harrison C, Eaton SL, Llavero Hurtado M, Graham LC, Alkhammash L, et al. Cellular and molecular anatomy of the human neuromuscular junction. Cell Rep. 2017;21:2348–2356. doi: 10.1016/j.celrep.2017.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wokke JHJ, Jennekens FGI, van den Oord CJM, Veldman H, Smit LME, Leppink GJ. Morphological changes in the human end plate with age. J Neurol Sci. 1990;95:291–310. doi: 10.1016/0022-510X(90)90076-Y. [DOI] [PubMed] [Google Scholar]
- 27.Oda K. Age changes of motor innervation and acetylcholine receptor distribution on human skeletal muscle fibres. J Neurol Sci. 1984;66:327–338. doi: 10.1016/0022-510X(84)90021-2. [DOI] [PubMed] [Google Scholar]
- 28.da Orssatto LBR, Wiest MJ, Diefenthaeler F. Neural and musculotendinous mechanisms underpinning age-related force reductions. Mech Ageing Dev. 2018;175:17–23. doi: 10.1016/j.mad.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 29.Roberts BM, Lavin KM, Many GM, Thalacker-Mercer A, Merritt EK, Bickel CS, et al. Human neuromuscular aging: sex differences revealed at the myocellular level. Exp Gerontol. 2018;106:116–124. doi: 10.1016/j.exger.2018.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.St-Jean-Pelletier F, Pion CH, Leduc-Gaudet JP, Sgarioto N, Zovilé I, Barbat-Artigas S, et al. The impact of ageing, physical activity, and pre-frailty on skeletal muscle phenotype, mitochondrial content, and intramyocellular lipids in men. J Cachexia Sarcopenia Muscle. 2017;8:213–228. doi: 10.1002/jcsm.12139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kwon YN, Yoon SS. Sarcopenia: neurological point of view. J Bone Metab. 2017;24:83–89. doi: 10.11005/jbm.2017.24.2.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Joanisse S, Nederveen JP, Snijders T, McKay BR, Parise G. Skeletal muscle regeneration, repair and remodelling in aging: the importance of muscle stem cells and vascularization. Gerontology. 2016;63:91–100. doi: 10.1159/000450922. [DOI] [PubMed] [Google Scholar]
- 33.Li M, Larsson L. Force-generating capacity of human myosin isoforms extracted from single muscle fibre segments. J Physiol. 2010;588:5105–5114. doi: 10.1113/jphysiol.2010.199067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Verdijk LB, Snijders T, Beelen M, Savelberg HHCM, Meijer K, Kuipers H, et al. Characteristics of muscle fiber type are predictive of skeletal muscle mass and strength in elderly men. J Am Geriatr Soc. 2010;58:2069–2075. doi: 10.1111/j.1532-5415.2010.03150.x. [DOI] [PubMed] [Google Scholar]
- 35.Covault J, Sanes JR. Neural cell adhesion molecule (N-CAM) accumulates in denervated and paralyzed skeletal muscles. Proc Natl Acad Sci U S A. 1985;82:4544–4548. doi: 10.1073/pnas.82.13.4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hendrickse P, Galinska M, Hodson-Tole E, Degens H. An evaluation of common markers of muscle denervation in denervated young-adult and old rat gastrocnemius muscle. Exp Gerontol. 2018;106:159–164. doi: 10.1016/j.exger.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 37.Gillon A, Sheard P. Elderly mouse skeletal muscle fibres have a diminished capacity to upregulate NCAM production in response to denervation. Biogerontology. 2015;16:811–823. doi: 10.1007/s10522-015-9608-6. [DOI] [PubMed] [Google Scholar]
- 38.Soendenbroe C, Heisterberg MF, Schjerling P, Karlsen A, Kjaer M, Andersen JL, et al. Molecular indicators of denervation in aging human skeletal muscle. Muscle Nerve. 2019;60:453–463. doi: 10.1002/mus.26638. [DOI] [PubMed] [Google Scholar]
- 39.Edström E, Ulfhake B. Sarcopenia is not due to lack of regenerative drive in senescent skeletal muscle. Aging Cell. 2005;4:65–77. doi: 10.1111/j.1474-9728.2005.00145.x. [DOI] [PubMed] [Google Scholar]
- 40.Chen J, Mizushige T, Nishimune H. Active zone density is conserved during synaptic growth but impaired in aged mice. J Comp Neurol. 2012;520:434–452. doi: 10.1002/cne.22764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hurtado E, Cilleros V, Nadal L, Simó A, Obis T, Garcia N, et al. Muscle contraction regulates BDNF/TrkB signaling to modulate synaptic function through presynaptic cPKCα and cPKCβi. Front Mol Neurosci. 2017;10:147. doi: 10.3389/fnmol.2017.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nishimune H, Numata T, Chen J, Aoki Y, Wang Y, Starr MP, et al. Active zone protein Bassoon co-localizes with presynaptic calcium channel, modifies channel function, and recovers from aging related loss by exercise. PLoS ONE. 2012;7:e38029. doi: 10.1371/journal.pone.0038029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ross JA, Webster RG, Lechertier T, Reynolds LE, Turmaine M, Bencze M, et al. Multiple roles of integrin-α3 at the neuromuscular junction. J Cell Sci. 2017;130:1772–1784. doi: 10.1242/jcs.201103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen J, Billings SE, Nishimune H. Calcium channels link the muscle-derived synapse organizer laminin β2 to Bassoon and CAST/Erc2 to organize presynaptic active zones. J Neurosci. 2011;31:512–525. doi: 10.1523/JNEUROSCI.3771-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Waites CL, Leal-Ortiz SA, Okerlund N, Dalke H, Fejtova A, Altrock WD, et al. Bassoon and Piccolo maintain synapse integrity by regulating protein ubiquitination and degradation. EMBO J. 2013;32:954–969. doi: 10.1038/emboj.2013.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ivanova D, Dirks A, Fejtova A. Bassoon and piccolo regulate ubiquitination and link presynaptic molecular dynamics with activity-regulated gene expression. J Physiol. 2016;594:5441–5448. doi: 10.1113/JP271826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shi L, Fu AKY, Ip NY. Molecular mechanisms underlying maturation and maintenance of the vertebrate neuromuscular junction. Trends Neurosci. 2012;35:441–453. doi: 10.1016/j.tins.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 48.Nishimune H, Badawi Y, Mori S, Shigemoto K. Dual-color STED microscopy reveals a sandwich structure of Bassoon and Piccolo in active zones of adult and aged mice. Sci Rep. 2016;6:27. doi: 10.1038/srep27935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Casati M, Costa AS, Capitanio D, Ponzoni L, Ferri E, Agostini S, et al. The biological foundations of sarcopenia: established and promising markers. Front Med. 2019;6:184. doi: 10.3389/fmed.2019.00184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Antonucci F, Corradini I, Fossati G, Tomasoni R, Menna E, Matteoli M. SNAP-25, a known presynaptic protein with emerging postsynaptic functions. Front Synaptic Neurosci. 2016;8:7. doi: 10.3389/fnsyn.2016.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Simó A, Cilleros-Mañé V, Just-Borràs L, Hurtado E, Nadal L, Tomàs M, et al. nPKCε mediates SNAP-25 phosphorylation of Ser-187 in basal conditions and after synaptic activity at the neuromuscular junction. Mol Neurobiol. 2019;56:5346–5364. doi: 10.1007/s12035-018-1462-5. [DOI] [PubMed] [Google Scholar]
- 52.Islamov RR, Samigullin DV, Rizvanov AA, Bondarenko NI, Nikolskiy EE. Synaptosome-associated protein 25 (SNAP25) synthesis in terminal buttons of mouse motor neuron. Dokl Biochem Biophys. 2015;464:272–274. doi: 10.1134/S1607672915050026. [DOI] [PubMed] [Google Scholar]
- 53.Giniatullin AR, Darios F, Shakirzyanova A, Davletov B, Giniatullin R. SNAP25 is a pre-synaptic target for the depressant action of reactive oxygen species on transmitter release. J Neurochem. 2006;98:1789–1797. doi: 10.1111/j.1471-4159.2006.03997.x. [DOI] [PubMed] [Google Scholar]
- 54.Kaneai N, Arai M, Takatsu H, Fukui K, Urano S. Vitamin E inhibits oxidative stress-induced denaturation of nerve terminal proteins involved in neurotransmission. J Alzheimer’s Dis. 2012;28:183–189. doi: 10.3233/JAD-2011-111133. [DOI] [PubMed] [Google Scholar]
- 55.Liguori I, Russo G, Curcio F, Bulli G, Aran L, Della-Morte D, et al. Oxidative stress, aging, and diseases. Clin Interv Aging. 2018;13:757–772. doi: 10.2147/CIA.S158513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Baumann CW, Kwak D, Liu HM, Thompson LV. Age-induced oxidative stress: how does it influence skeletal muscle quantity and quality? J Appl Physiol. 2016;121:1047–1052. doi: 10.1152/japplphysiol.00321.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kalinkovich A, Livshits G. Sarcopenia - the search for emerging biomarkers. Ageing Res Rev. 2015;22:58–71. doi: 10.1016/j.arr.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 58.Uchitomi R, Hatazawa Y, Senoo N, Yoshioka K, Fujita M, Shimizu T, et al. Metabolomic analysis of skeletal muscle in aged mice. Sci Rep. 2019;9:10425. doi: 10.1038/s41598-019-46929-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sugita S, Fleming LL, Wood C, Vaughan SK, Gomes MPSM, Camargo W, et al. VAChT overexpression increases acetylcholine at the synaptic cleft and accelerates aging of neuromuscular junctions. Skelet Muscle. 2016;6:31. doi: 10.1186/s13395-016-0105-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vaughan SK, Sutherland NM, Valdez G. Attenuating cholinergic transmission increases the number of satellite cells and preserves muscle mass in old age. Front Aging Neurosci. 2019;11:262. doi: 10.3389/fnagi.2019.00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cetin H, Beeson D, Vincent A, Webster R. The structure, function, and physiology of the fetal and adult acetylcholine receptor in muscle. Front Mol Neurosci. 2020;13:581097. doi: 10.3389/fnmol.2020.581097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bao Z, Cui C, Chow SK-H, Qin L, Wong RMY, Cheung W-H. AChRs degeneration at NMJ in aging-associated sarcopenia – a systematic review. Front Aging Neurosci. 2020;12:597811. doi: 10.3389/fnagi.2020.597811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Soendenbroe C, Bechshøft CJL, Heisterberg MF, Jensen SM, Bomme E, Schjerling P, et al. Key components of human myofibre denervation and neuromuscular junction stability are modulated by age and exercise. Cells. 2020;9:893. doi: 10.3390/cells9040893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Witzemann V, Brenner H-R, Sakmann B. Neural factors regulate AChR subunit mRNAs at rat neuromuscular synapses. J Cell Biol. 1991;114:125–141. doi: 10.1083/jcb.114.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Caron M-A, Charette SJ, Maltais F, Debigaré R. Variability of protein level and phosphorylation status caused by biopsy protocol design in human skeletal muscle analyses. BMC Res Notes. 2011;4:488. doi: 10.1186/1756-0500-4-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Apel PJ, Alton T, Northam C, Ma J, Callahan M, Sonntag WE, et al. How age impairs the response of the neuromuscular junction to nerve transection and repair: an experimental study in rats. J Orthop Res. 2009;27:385–393. doi: 10.1002/jor.20773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Aare S, Spendiff S, Vuda M, Elkrief D, Perez A, Wu Q, et al. Failed reinnervation in aging skeletal muscle. Skelet Muscle. 2016;6:29. doi: 10.1186/s13395-016-0101-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhao K, Shen C, Li L, Wu H, Xing G, Dong Z, et al. Sarcoglycan alpha mitigates neuromuscular junction decline in aged mice by stabilizing LRP4. J Neurosci. 2018;38:8860–8873. doi: 10.1523/JNEUROSCI.0860-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cisterna BA, Vargas AA, Puebla C, Fernández P, Escamilla R, Lagos CF, et al. Active acetylcholine receptors prevent the atrophy of skeletal muscles and favor reinnervation. Nat Commun. 2020;11:1073. doi: 10.1038/s41467-019-14063-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ma J, Shen J, Garrett JP, Lee CA, Li Z, Elsaidi GA, et al. Gene expression of myogenic regulatory factors, nicotinic acetylcholine receptor subunits, and GAP-43 in skeletal muscle following denervation in a rat model. J Orthop Res. 2007;25:1498–1505. doi: 10.1002/jor.20414. [DOI] [PubMed] [Google Scholar]
- 71.Chen A, Bai L, Zhong K, Shu X, Wang A, Xiao Y, et al. APC2CDH1 negatively regulates agrin signaling by promoting the ubiquitination and proteolytic degradation of DOK7. FASEB J. 2020;34:12009–12023. doi: 10.1096/fj.202000485R. [DOI] [PubMed] [Google Scholar]
- 72.Rimer M. Emerging roles for MAP kinases in agrin signaling. Commun Integr Biol. 2011;4:143–146. doi: 10.4161/psb.4.2.14357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Landi F, Calvani R, Lorenzi M, Martone AM, Tosato M, Drey M, et al. Serum levels of C-terminal agrin fragment (CAF) are associated with sarcopenia in older multimorbid community-dwellers: results from the ilSIRENTE study. Exp Gerontol. 2016;79:31–36. doi: 10.1016/j.exger.2016.03.012. [DOI] [PubMed] [Google Scholar]
- 74.Ohno K, Ohkawara B, Ito M. Agrin-LRP4-MuSK signaling as a therapeutic target for myasthenia gravis and other neuromuscular disorders. Expert Opin Ther Targets. 2017;21:949–958. doi: 10.1080/14728222.2017.1369960. [DOI] [PubMed] [Google Scholar]
- 75.Nishimune H, Shigemoto K. Pratical anatomy of the neuromuscular junction in health and disease. Neurol Clin. 2018;36:231–240. doi: 10.1016/j.ncl.2018.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Naguib M, Flood P, McArdle JJ, Brenner HR. Advances in neurobiology of the neuromuscular junction: implications for the anesthesiologist. Anesthesiology. 2002;96:202–231. doi: 10.1097/00000542-200201000-00035. [DOI] [PubMed] [Google Scholar]
- 77.Blasco A, Gras S, Mòdol-Caballero G, Tarabal O, Casanovas A, Piedrafita L, et al. Motoneuron deafferentation and gliosis occur in association with neuromuscular regressive changes during ageing in mice. J Cachexia Sarcopenia Muscle. 2020;11:1628–1660. doi: 10.1002/jcsm.12599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dunne V, Maselli RA. Identification of pathogenic mutations in the human rapsyn gene. J Hum Genet. 2003;48:204–207. doi: 10.1007/s10038-003-0005-7. [DOI] [PubMed] [Google Scholar]
- 79.Bolliger MF, Zurlinden A, Lüscher D, Bütikofer L, Shakhova O, Francolini M, et al. Specific proteolytic cleavage of agrin regulates maturation of the neuromuscular junction. J Cell Sci. 2010;123:3944–3955. doi: 10.1242/jcs.072090. [DOI] [PubMed] [Google Scholar]
- 80.Reif R, Sales S, Hettwer S, Dreier B, Gisler C, Wölfel J, et al. Specific cleavage of agrin by neurotrypsin, a synaptic protease linked to mental retardation. FASEB J. 2007;21:3468–3478. doi: 10.1096/fj.07-8800com. [DOI] [PubMed] [Google Scholar]
- 81.Hettwer S, Dahinden P, Kucsera S, Farina C, Ahmed S, Fariello R, et al. Elevated levels of a C-terminal agrin fragment identifies a new subset of sarcopenia patients. Exp Gerontol. 2013;48:69–75. doi: 10.1016/j.exger.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 82.Marzetti E, Calvani R, Lorenzi M, Marini F, D’Angelo E, Martone AM, et al. Serum levels of C-terminal agrin fragment (CAF) are associated with sarcopenia in older hip fractured patients. Exp Gerontol. 2014;60:79–82. doi: 10.1016/j.exger.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 83.Bütikofer L, Zurlinden A, Bolliger MF, Kunz B, Sonderegger P. Destabilization of the neuromuscular junction by proteolytic cleavage of agrin results in precocious sarcopenia. FASEB J. 2011;25:4378–4393. doi: 10.1096/fj.11-191262. [DOI] [PubMed] [Google Scholar]
- 84.Hettwer S, Lin S, Kucsera S, Haubitz M, Oliveri F, Fariello RG, et al. Injection of a soluble fragment of neural agrin (NT-1654) considerably improves the muscle pathology caused by the disassembly of the neuromuscular junction. PLoS ONE. 2014;9:e88739. doi: 10.1371/journal.pone.0088739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Colombo E, Bedogni F, Lorenzetti I, Landsberger N, Previtali SC, Farina C. Autocrine and immune cell-derived BDNF in human skeletal muscle: implications for myogenesis and tissue regeneration. J Pathol. 2013;231:190–198. doi: 10.1002/path.4228. [DOI] [PubMed] [Google Scholar]
- 86.Sakuma K, Yamaguchi A. The recent understanding of the neurotrophin’s role in skeletal muscle adaptation. J Biomed Biotechnol. 2011;2011:201696. doi: 10.1155/2011/201696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Leßmann V, Brigadski T. Mechanisms, locations, and kinetics of synaptic BDNF secretion: an update. Neurosci Res. 2009;65:11–22. doi: 10.1016/j.neures.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 88.Greising SM, Stowe JM, Sieck GC, Mantilla CB. Role of TrkB kinase activity in aging diaphragm neuromuscular junctions. Exp Gerontol. 2015;72:184–191. doi: 10.1016/j.exger.2015.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Miyazaki S, Iino N, Koda R, Narita I, Kaneko Y. Brain-derived neurotrophic factor is associated with sarcopenia and frailty in Japanese hemodialysis patients. Geriatr Gerontol Int. 2020 doi: 10.1111/ggi.14089. [DOI] [PubMed] [Google Scholar]
- 90.Shimada H, Makizako H, Doi T, Yoshida D, Tsutsumimoto K, Anan Y, et al. A large, cross-sectional observational study of serum BDNF, cognitive function, and mild cognitive impairment in the elderly. Front Aging Neurosci. 2014;6:69. doi: 10.3389/fnagi.2014.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kwak JY, Hwang H, Kim S-K, Choi JY, Lee S-M, Bang H, et al. Prediction of sarcopenia using a combination of multiple serum biomarkers. Sci Rep. 2018;8:8574. doi: 10.1038/s41598-018-26617-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gómez-Pinilla F, Ying Z, Roy RR, Molteni R, Edgerton VR. Voluntary exercise induces a BDNF-mediated mechanism that promotes neuroplasticity. J Neurophysiol. 2002;88:2187–2195. doi: 10.1152/jn.00152.2002. [DOI] [PubMed] [Google Scholar]
- 93.Matthews VB, Åström MB, Chan MHS, Bruce CR, Krabbe KS, Prelovsek O, et al. Brain-derived neurotrophic factor is produced by skeletal muscle cells in response to contraction and enhances fat oxidation via activation of AMP-activated protein kinase. Diabetologia. 2009;52:1409–1418. doi: 10.1007/s00125-009-1364-1. [DOI] [PubMed] [Google Scholar]
- 94.Gomes M, Figueiredo D, Teixeira L, Poveda V, Paúl C, Santos-Silva A, et al. Physical inactivity among older adults across Europe based on the SHARE database. Age Ageing. 2017;46:71–77. doi: 10.1093/ageing/afw165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nagano M, Suzuki H. Quantitative analyses of expression of GDNF and neurotrophins during postnatal development in rat skeletal muscles. Neurosci Res. 2003;45:391–399. doi: 10.1016/S0168-0102(03)00010-5. [DOI] [PubMed] [Google Scholar]
- 96.McCullough MJ, Peplinski NG, Kinnell KR, Spitsbergen JM. Glial cell line-derived neurotrophic factor (GDNF) protein content in rat skeletal muscle is altered by increased physical activity in vivo and in vitro. Neuroscience. 2011;174:234–244. doi: 10.1016/j.neuroscience.2010.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Victoria Vega A, Avila G. CGRP, a vasodilator neuropeptide that stimulates neuromuscular transmission and EC coupling. Curr Vasc Pharmacol. 2010;8:394–403. doi: 10.2174/157016110791112287. [DOI] [PubMed] [Google Scholar]
- 98.Buffelli M, Pasino E, Cangiano A. In vivo acetylcholine receptor expression induced by calcitonin gene-related peptide in rat soleus muscle. Neuroscience. 2001;104:561–567. doi: 10.1016/S0306-4522(01)00090-2. [DOI] [PubMed] [Google Scholar]
- 99.Parnow A, Gharakhanlou R, Gorginkaraji Z, Rajabi S, Eslami R, Hedayati M, et al. Effects of endurance and resistance training on calcitonin gene-related peptide and acetylcholine receptor at slow and fast twitch skeletal muscles and sciatic nerve in male wistar rats. Int J Pept. 2012;2012:962651. doi: 10.1155/2012/962651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Machado J, Manfredi LH, Silveira WA, Gonçalves DAP, Lustrino D, Zanon NM, et al. Calcitonin gene-related peptide inhibits autophagic-lysosomal proteolysis through cAMP/PKA signaling in rat skeletal muscles. Int J Biochem Cell Biol. 2016;72:40–50. doi: 10.1016/j.biocel.2015.12.011. [DOI] [PubMed] [Google Scholar]
- 101.Matteoli M, Balbi S, Sala C, Chini B, Cimino M, Vitadello M, et al. Developmentally regulated expression of calcitonin gene-related peptide at mammalian neuromuscular junction. J Mol Neurosci. 1990;2:175–184. doi: 10.1007/BF02896842. [DOI] [PubMed] [Google Scholar]
- 102.Tarabal O. Regulation of motoneuronal calcitonin gene-related peptide (CGRP) during axonal growth and neuromuscular synaptic plasticity induced by botulinum toxin in rats. Eur J Neurosci. 1996;8:829–836. doi: 10.1111/j.1460-9568.1996.tb01269.x. [DOI] [PubMed] [Google Scholar]
- 103.Machado J, Silveira WA, Gonçalves DA, Schavinski AZ, Khan MM, Zanon NM, et al. α−calcitonin gene-related peptide inhibits autophagy and calpain systems and maintains the stability of neuromuscular junction in denervated muscles. Mol Metab. 2019;28:91–106. doi: 10.1016/j.molmet.2019.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Baraldo M, Geremia A, Pirazzini M, Nogara L, Solagna F, Türk C, et al. Skeletal muscle mTORC1 regulates neuromuscular junction stability. J Cachexia Sarcopenia Muscle. 2020;11:208–225. doi: 10.1002/jcsm.12496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Joseph GA, Wang SX, Jacobs CE, Zhou W, Kimble GC, Tse HW, et al. Partial inhibition of mTORC1 in aged rats counteracts the decline in muscle mass and reverses molecular signaling associated with sarcopenia. Mol Cell Biol. 2019;39:e00141–e219. doi: 10.1128/MCB.00141-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Edwards BJ, Perry HM, Kaiser FE, Morley JE, Kraenzle D, Stevenson R, et al. Relationship of age and calcitonin gene-related peptide to postprandial hypotension. Mech Ageing Dev. 1996;87:61–73. doi: 10.1016/0047-6374(96)01688-0. [DOI] [PubMed] [Google Scholar]
- 107.Holahan MR. A shift from a pivotal to supporting role for the growth-associated protein (GAP-43) in the coordination of axonal structural and functional plasticity. Front Cell Neurosci. 2017;11:1–19. doi: 10.3389/fncel.2017.00266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hesselmans LFGM, Jennekens FGI, van den Oord CJM, Oestreicher AB, Veldman H, Gispen WH. A light and electron microscopical study of B-50 (GAP-43) in human intramuscular nerve and neuromuscular junctions during development. J Neurol Sci. 1989;89:301–311. doi: 10.1016/0022-510X(89)90031-2. [DOI] [PubMed] [Google Scholar]
- 109.Aigner L, Arber S, Kapfhammer JP, Laux T, Schneider C, Botteri F, et al. Overexpression of the neural growth-associated protein GAP-43 induces nerve sprouting in the adult nervous system of transgenic mice. Cell. 1995;83:269–278. doi: 10.1016/0092-8674(95)90168-X. [DOI] [PubMed] [Google Scholar]
- 110.Woolf CJ, Reynolds ML, Chong MS, Emson P, Irwin N, Benowitz LI. Denervation of the motor endplate results in the rapid expression by terminal Schwann cells of the growth-associated protein GAP-43. J Neurosci. 1992;12:3999–4010. doi: 10.1523/jneurosci.12-10-03999.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Caprara GA, Morabito C, Perni S, Navarra R, Guarnieri S, Mariggiò MA. Evidence for altered Ca2+ handling in growth associated protein 43-knockout skeletal muscle. Front Physiol. 2016;7:493. doi: 10.3389/fphys.2016.00493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Guarnieri S, Morabito C, Paolini C, Boncompagni S, Pilla R, Fanò-Illic G, et al. Growth associated protein 43 is expressed in skeletal muscle fibers and is localized in proximity of mitochondria and calcium release units. PLoS ONE. 2013;8:e53267. doi: 10.1371/journal.pone.0053267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Johnson H, Mossberg K, Arvidsson U, Piehl F, Hökfelt T, Ulfhake B. Increase in α-CGRP and GAP-43 in aged motoneurons: a study of peptides, growth factors, and ChAT mRNA in the lumbar spinal cord of senescent rats with symptoms of hindlimb incapacities. J Comp Neurol. 1995;359:69–89. doi: 10.1002/cne.903590106. [DOI] [PubMed] [Google Scholar]
- 114.Verzè L, Buffo A, Rossi F, Oestreicher AB, Gispen WH, Strata P. Increase of B-50/GAP-43 immunoreactivity in uninjured muscle nerves of MDX mice. Neuroscience. 1996;70:807–815. doi: 10.1016/S0306-4522(96)83017-X. [DOI] [PubMed] [Google Scholar]
- 115.Heuß D, Engelhardt A, Göbel H, Neundörfer B. Light-microscopic study of phosphoprotein B-50 in myopathies. Virchows Arch. 1995;426:69–76. doi: 10.1007/BF00194700. [DOI] [PubMed] [Google Scholar]
- 116.Yoshimoto Y, Ikemoto-Uezumi M, Hitachi K, Fukada S, Uezumi A. Methods for accurate assessment of myofiber maturity during skeletal muscle regeneration. Front Cell Dev Biol. 2020;8:267. doi: 10.3389/fcell.2020.00267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sousa-Victor P, García-Prat L, Muñoz-Cánoves P. Control of satellite cell function in muscle regeneration and its disruption in ageing. Nat Rev Mol Cell Biol. 2021 doi: 10.1038/s41580-021-00421-2. [DOI] [PubMed] [Google Scholar]
- 118.Sajko Š, Kubínová L, Cvetko E, Kreft M, Wernig A, Eržen I. Frequency of M-cadherin-stained satellite cells declines in human muscles during aging. J Histochem Cytochem. 2004;52:179–185. doi: 10.1177/002215540405200205. [DOI] [PubMed] [Google Scholar]
- 119.Shefer G, Van de Mark DP, Richardson JB, Yablonka-Reuveni Z. Satellite-cell pool size does matter: defining the myogenic potency of aging skeletal muscle. Dev Biol. 2006;294:50–66. doi: 10.1016/j.ydbio.2006.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Arpke RW, Shams AS, Collins BC, Larson AA, Lu N, Lowe DA, et al. Preservation of satellite cell number and regenerative potential with age reveals locomotory muscle bias. Skelet Muscle. 2021;11:22. doi: 10.1186/s13395-021-00277-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Collins CA, Zammit PS, Ruiz AP, Morgan JE, Partridge TA. A population of myogenic stem cells that survives skeletal muscle aging. Stem Cells. 2007;25:885–894. doi: 10.1634/stemcells.2006-0372. [DOI] [PubMed] [Google Scholar]
- 122.Sousa-Victor P, Gutarra S, García-Prat L, Rodriguez-Ubreva J, Ortet L, Ruiz-Bonilla V, et al. Geriatric muscle stem cells switch reversible quiescence into senescence. Nature. 2014;506:316–321. doi: 10.1038/nature13013. [DOI] [PubMed] [Google Scholar]
- 123.Liu W, Klose A, Forman S, Paris ND, Wei-LaPierre L, Cortés-Lopéz M, et al. Loss of adult skeletal muscle stem cells drives age-related neuromuscular junction degeneration. Elife. 2017;6:e26464. doi: 10.7554/eLife.26464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Liu W, Wei-LaPierre L, Klose A, Dirksen RT, Chakkalakal JV. Inducible depletion of adult skeletal muscle stem cells impairs the regeneration of neuromuscular junctions. Elife. 2015;4:e09221. doi: 10.7554/eLife.09221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Snijders T, Nederveen JP, McKay BR, Joanisse S, Verdijk LB, van Loon LJC, et al. Satellite cells in human skeletal muscle plasticity. Front Physiol. 2015;6:283. doi: 10.3389/fphys.2015.00283. [DOI] [PMC free article] [PubMed] [Google Scholar]