Abstract
The debris-rich basal ice layers of a high Arctic glacier were shown to contain metabolically diverse microbes that could be cultured oligotrophically at low temperatures (0.3 to 4°C). These organisms included aerobic chemoheterotrophs and anaerobic nitrate reducers, sulfate reducers, and methanogens. Colonies purified from subglacial samples at 4°C appeared to be predominantly psychrophilic. Aerobic chemoheterotrophs were metabolically active in unfrozen basal sediments when they were cultured at 0.3°C in the dark (to simulate nearly in situ conditions), producing 14CO2 from radiolabeled sodium acetate with minimal organic amendment (≥38 μM C). In contrast, no activity was observed when samples were cultured at subfreezing temperatures (≤−1.8°C) for 66 days. Electron microscopy of thawed basal ice samples revealed various cell morphologies, including dividing cells. This suggests that the subglacial environment beneath a polythermal glacier provides a viable habitat for life and that microbes may be widespread where the basal ice is temperate and water is present at the base of the glacier and where organic carbon from glacially overridden soils is present. Our observations raise the possibility that in situ microbial production of CO2 and CH4 beneath ice masses (e.g., the Northern Hemisphere ice sheets) is an important factor in carbon cycling during glacial periods. Moreover, this terrestrial environment may provide a model for viable habitats for life on Mars, since similar conditions may exist or may have existed in the basal sediments beneath the Martian north polar ice cap.
Microbial activity has been found in ice-sediment communities in the surface layers of perennial and permanent lake ice at a depth of 2 m (30, 31). The lake ice microbial consortia are dependent on photosynthesis to provide energy for growth. In subglacial environments bacterial populations have been found beneath alpine glaciers (35) and in subglacially accreted ice above Lake Vostok, Antarctica (20, 29). However, the biogeochemical function of these populations has not been determined, and it remains to be demonstrated that they are active at in situ subglacial temperatures and under in situ conditions. Clearly, active subglacial microbial populations would have to be chemotrophic (nonphotosynthetic). Demonstrating the viability and geochemical function of subglacial microbial populations has a number of important consequences.
First, the presence of viable chemotrophic microbial activity at low temperatures (0 to 4°C) has important implications for global carbon cycling calculations. Traditionally, it has been thought that continental glaciation results in cessation of geochemical processes beneath the ice (13), and the impact of subglacial microbially mediated weathering has received little consideration. However, during the last glacial maximum, ice sheets covered approximately 20% of the continental northern hemisphere (16, 23, 24), including the area that is currently covered by the boreal forest, the world's largest store of soil carbon, whose size is estimated to be 330 Pg (40). A similar distribution of vegetation has been proposed for the last interglacial period (Eemian), as recorded in glacially overridden soils and peat (10, 32) and paleosols in unglaciated terrain beyond the ice margin (26). These overridden soils and peat deposits provide a large source of organic carbon beneath the midlatitude ice sheets. A number of the current carbon cycle models assume that the carbon accumulated during the Eemian in areas covered by the ice sheets was returned to the atmosphere by the last glacial maximum (1, 12, 40), but there has been no explanation as to how this was achieved. Ice is an efficient erosive agent (2, 15), and, therefore, it is likely that some of the carbon may be moved by physical transport to the ice sheet margins, either in ice, in deforming sediments, or in meltwater. However, active biogeochemical oxidation or reduction of the remaining carbon beneath warm-based sectors of midlatitude ice sheets may have a significant impact on carbon budget calculations for continental regions during the glacial phase of a glacial-interglacial cycle. Moreover, low-temperature respiration and/or fermentation of organic carbon in the subsurface environments of periglacial (33, 41) soils in unglaciated midlatitude regions and ice marginal zones is potentially an important process that previously has been given little consideration on glacial-interglacial time scales.
Second, extreme polar terrestrial environments are being investigated as analogues of viable extraterrestrial habitats (3, 14). Currently, water on Mars has been observed only as ice in the two polar caps (8). Given that all known bacteria on Earth require liquid water to be metabolically active, some of the most likely potential habitats for microbial life on Mars are the polar environments (36). Moreover, subglacial environments could provide shelter from the harsh conditions at the planet's surface, including large diurnal and seasonal temperature fluctuations and strong UV radiation. Some models suggest that during times of high obliquity on Mars, surficial melting may have occurred in the north polar ice cap (28). Under these conditions, the ice cap may have exhibited polythermal conditions and may have been more dynamic (19), and thus basal melting may have occurred, producing sediment-rich basal ice. Hence, debris-rich ice exposed at the margins of the north polar ice cap or as part of polar layered deposits may provide a record of dormant or possibly extant microbial life that is relatively accessible.
In this study we performed a number of laboratory experiments in which we assessed the diversity and viability of microbes from a high Arctic glaciated environment and examined the effects of microbial activity on biogeochemical processes. In the experiments we also compared microbial population diversity in surficial glacier environments (supraglacial waters and glacier ice, which are characterized by low solute concentrations [<10 μS cm−1] and low sediment concentrations [<0.01 g liter−1]) with microbial population diversity in subglacial environments (subglacial meltwaters and basal ice, which are characterized by high solute concentrations [>100 μS cm−1] and high sediment concentrations [>0.1 g liter−1]).
MATERIALS AND METHODS
Field site.
The study was performed with samples collected from John Evans Glacier (79°38′N, 74°23′W) on eastern Ellesmere Island, Nunavut, Canada. The local climate is a polar desert, and the mean annual air temperature at the glacier terminus is −14.5°C (4). The glacier has polythermal characteristics; cold (subfreezing) ice at the surface, margins, and terminus of the glacier surrounds a core zone where ice at the glacier bed is at the pressure melting point and basal melting occurs. This core zone begins ca. 7 km upglacier from the glacier snout, where the ice is ca. 400 m thick. Between 4 and 7 km from the glacier snout, temperate ice occurs only at the glacier bed, but in the lowest 4 km of the glacier, where the ice is up to 200 m deep, there is a layer of temperate basal ice up to 20 m thick (L. Copland and M. J. Sharp, submitted for publication). Debris-rich basal ice up to 0.5 m thick is widely exposed at the glacier margin. During the summer melt season, meltwaters which have acquired solutes from subglacial chemical weathering reactions drain from the glacier at a number of locations (35).
Definition of ice and water types.
Glacier ice is formed by firnification or superimposed ice generation (refreezing of percolating waters in near-surface snow and firn) at the glacier surface, and it has low solute concentrations (<10 μS cm−1) and low sediment concentrations (<0.01 g liter−1). Supraglacial meltwaters are generated by melting of snow and ice and flow on the surface of the glacier. They are also characterized by low solute and suspended-sediment concentrations. Subglacial waters flow at the bed of the glacier and originate as supraglacial waters which reach the bed through crevasses and moulins and mix with small amounts of groundwater and basal meltwater generated by frictional and geothermal melting of ice at the glacier base. Subglacial waters interact with the rock and sediments that underlie the ice and hence contain high solute concentrations (>100 μS cm−1) and high suspended-sediment concentrations (>0.1 g liter−1). Basal ice contains significant sediment concentrations and forms at the glacier bed by a number of mechanisms, which could include folding (17), regelation of ice into the substrate (18), and basal accretion of supercooled subglacial water (21). This ice provides a record of the geochemical and microbiological conditions at the glacier bed and lies stratigraphically unconformably beneath the glacier ice. At John Evans Glacier the basal ice layers were probably formed by either regelation of ice into the substrate or basal accretion of supercooled water since there is no structural evidence of folding.
Water samples collected in 1996.
In the first set of experiments undertaken in 1996 we examined the viability of microbes present in glacial meltwaters having different chemical compositions and suspended-sediment concentrations, and we also examined the relationship between suspended-sediment concentration and microbiological activity. In July 1996, 4-liter samples of subglacial and supraglacial meltwater were collected aseptically from the terminus of John Evans Glacier by using twice-autoclaved plastic carboys (Nalgene) with screw caps fitted with sterile air ports (0.2-μm pore-size Teflon PTFE filters; Nalge). These samples were amended in the field with 10× sterile R2A growth medium lacking agar so that the final concentration was 0.1× R2A medium (1× R2A medium contains [per liter of doubly glass-distilled water] 0.5 g of yeast extract [Difco], 0.5 g of Casamino Acids [Difco], 0.5 g of glucose, 0.5 g of soluble starch [Difco], 0.3 g of K2HPO4, 0.3 g of sodium pyrophosphate, 0.25 g of BiTek tryptone [Difco], 0.25 g of Bacto Peptone [Difco], and 24 mg of MgSO4; adapted from the medium described by Atlas [5]). Samples were stored on ice in the dark at ≤4°C for up to 1 month in the field, transported to the laboratory (72 h) at ≤4°C, and then incubated statically in the dark at ≤4°C. For comparison, other subglacial and supraglacial water samples were incubated without R2A medium. The pH values and dissolved oxygen concentrations of the samples were measured at the time of sampling and 7 weeks later by using an Orion model 290A pH meter fitted with a Ross Sure-flow pH electrode and an Orion dissolved oxygen probe (model 97-08), respectively. H2S concentrations were determined colorimetrically by using a Hach H2S test kit.
In the laboratory, a control was prepared by using 3 liters of heat-sterilized distilled water amended with 30 ml of the R2A medium stock used in the field. The control flask was incubated in parallel with the water samples. After incubation for 2 months at 4°C, 100-ml subsamples of this laboratory control were filtered through 0.2-μm-pore-size MicroFunnel filtration units (Gelman Sciences) to capture any viable microbes. Each unit was supplemented with an ampoule of R2A broth (Gelman Sciences) and incubated at 22°C for 1 month or at 4°C for 2 months to culture recovered microbes. No colonies were observed at either temperature, which showed that the amendment was sterile.
After 7 weeks of incubation at ≤4°C, 10-ml subsamples of the R2A medium-amended and unamended 4-liter water samples were inoculated into 50-ml portions of prechilled quarter-strength nitrate medium (25) or quarter-strength sulfate medium (7) (in which sodium lactate was replaced with sodium acetate and sodium pyruvate) that had been pregassed with sterile 10% CO2–90% N2 in 125-ml serum bottles fitted with gas-tight septa, and the preparations were incubated statically at 4°C in the dark. All manipulations were carried out in a biosafety cabinet with HEPA-filtered airflow. Growth was revealed by increasing turbidity and browning of the nitrate medium or blackening of the sulfate medium from sulfide precipitation compared with parallel uninoculated media. Subsamples of the same subglacial and supraglacial waters were diluted with cold 3 mM phosphate buffer (pH 7), inoculated onto prechilled quarter-strength plate count agar (Difco), and incubated aerobically at 4 or 22°C.
Ice samples collected in 1997.
In the experiments in which we used ice samples collected in 1997 we examined both microbial diversity and rates of biogeochemical activity in thawed samples of glacier ice and basal ice. The chemical and microbiological characteristics of the thawed basal ice also provided information about the nature of subglacial environments. In May 1997, samples of basal ice and glacier ice were collected aseptically from the glacier terminus by using ethanol-flame-sterilized ice axes. The surface few centimeters of ice were scraped away prior to sampling to avoid atmospheric contamination. Samples were then chipped into flame-sterilized metal collection trays and transferred without handling into sterile plastic bags (Whirl-Pak; Nasco Plastics, New Hamburg, Ontario, Canada). Samples remained frozen until they were used in the experiments. Subsequent manipulations were carried out aseptically in a biosafety cabinet with HEPA-filtered airflow.
(i) Anaerobic incubation.
Ice was thawed at 4°C in sterile containers and transferred into 168-ml bottles that were fitted with gas-impermeable septa and contained 50-ml portions of half-strength growth media, as follows: nitrate medium (25) and sulfate medium (7) pregassed with sterile 10% CO2–90% N2; and R2A medium pregassed with sterile 0.5% CO2–99.5% N2. The prepared bottles containing media were precooled on ice prior to inoculation. For glacier ice samples, the inoculum consisted of 50 ml of meltwater, whereas for basal ice 5 g of sediment plus 45 ml of associated meltwater were used. Parallel uninoculated controls containing 100 ml of each growth medium were also prepared. Inoculated, poisoned controls containing 20 mM Na2MoO4 and 500 μM HgCl2 were also included to evaluate abiotic contributions to the aqueous geochemistry. Unamended background controls containing melted glacier ice (100 ml) or basal ice (10 g of sediment plus 90 ml of meltwater) were also prepared. All cultures were sealed with the appropriate sterile headspace gases and incubated statically in the dark at 4°C for 90 days. At 14-day intervals, 0.5-ml samples were withdrawn aseptically through the septa by using fine-gauge needles and syringes. Basal ice samples were clarified by using a 0.45-μm-pore-size filter membrane (Millipore) prior to analysis. The concentrations of Cl−, NO3−, and SO42− in the samples were determined with a Dionex model DX500 ion chromatograph by using a Dionex Ionpac AS4 column and an eluent consisting of 1.7 mM Na2CO3 and 1.8 mM NaHCO3. Headspace concentrations of CO2 and CH4 were determined with a Hewlett-Packard model 5890 Series II gas chromatograph equipped with a thermal conductivity detector and with a VarianStar model 3400 or Hewlett-Packard model 5700A gas chromatograph fitted with flame ionization detectors, respectively. The δ13C values of headspace gases were determined by continuous-flow–isotope ratio mass spectrometry by using a Hewlett-Packard model 5890 Series II gas chromatograph connected to a Finigan Mat 252 mass spectrometer and are reported relative to VPDB.
(ii) Aerobic incubation.
Samples consisting of 100 ml of melted glacier ice or 30 ml of melted basal ice were amended with 50 ml of half-strength R2A medium. A parallel uninoculated control containing 50 ml of medium was included. All cultures received portions of a filter-sterilized (pore size, 0.2 μm) solution of sodium [2-14C]acetate which resulted in final acetate concentrations of ≤50 μM and 98,000 dpm per flask. The culture flasks were sealed with neoprene stoppers with an aerobic headspace and were incubated at 8°C in the dark with gyratory shaking at 200 rpm for 270 days. At intervals, subsamples of liquid plus headspace were removed aseptically by using a needle and a syringe, acidified, and sparged with N2 to trap and quantify the evolved 14CO2 (11).
Ice samples collected in 1998.
In the experiments performed in 1998 and 1999 we recreated in situ subglacial conditions as closely as possible in order to establish whether the microbes were viable and active under such conditions. The rates of microbial respiration were measured for both thawed glacier ice and basal ice at temperatures below and just above 0°C. All manipulations were carried out on ice, and the media used were prechilled to ensure that culture temperatures remained as close to 0°C as possible. Experiments were conducted by using fresh samples of basal ice, which was collected aseptically in July 1998 (as described above) at a site 3 km from the 1997 collection site, and glacier ice collected in 1997, which had been stored frozen and undisturbed in the original sterile bags. Samples were melted at 4°C in sterile containers, and subsamples were transferred with HEPA-filtered airflow to sterile 250-ml bottles fitted with gas-tight septa. Unamended samples consisted of 200 ml of melted glacier ice or 150 g of basal ice sediment plus 75 ml of associated meltwater. Another set of samples was amended by replacing 20 ml of melted ice with 20 ml of R2A medium, which resulted in a final concentration of 0.1× R2A medium. Heat-sterilized controls were also prepared consisting of basal ice containing 100 g of twice-autoclaved sediment plus 28 ml of associated meltwater, glacier ice containing 200 ml of meltwater, and an uninoculated control containing 200 ml of sterile medium. Each culture was supplemented with a filter-sterilized solution of sodium [2-14C]acetate so that it contained 195,000 dpm and an acetate concentration of 50 to 130 μM depending on the total volume of the sample, and then it was sealed under an aerobic headspace. One set of unamended samples was incubated at 4°C in the dark. The contents of the remaining bottles were frozen by immersion in glycol at −4.8°C; the bottles were shaken every 15 min during freezing to ensure even distribution of any sediment within the ice that was forming. These cultures were incubated in the dark and were submerged in a refrigerated glycol circulator bath (Cole Parmer Polystat) at −4.8°C for 31 days, then at −1.8°C for 35 days, and finally at 0.3°C for 91 days. The bath temperature was monitored by using a thermistor (Campbell Scientific, model 107B) connected to a model CR10 datalogger (Campbell Scientific) and remained within ±0.3°C of the desired temperature. After 66 days the frozen samples were melted at 0.3°C, and the 14CO2 that evolved was measured by aseptically removing a subsample of liquid and quantifying the 14CO2 (11); sediment-laden samples were first clarified by filtration through a Millipore Millex GS filter unit (pore size, 0.2 μm). The thawed samples were incubated for an additional 91 days at 0.3°C, and subsamples were removed and used for 14CO2 analysis at intervals. Samples of the unfrozen (4°C liquid) cultures were obtained after 97, 122, and 157 days and were analyzed to determine the evolved 14CO2 content as described above (11). Extraction of all subsamples was performed in a biosafety cabinet, and all cultures were kept on ice during the procedure.
Microscopy.
Transmission electron microscopy (TEM) was performed with both cultured and uncultured samples of glacier ice and basal ice in order to examine microbial morphology. Drops of melted ice or amended cultures were placed on Formvar-coated copper grids, briefly dried in vacuo, and then negatively stained with filter-sterilized (pore size, 0.2 μm) 2% phosphotungstic acid and examined with a Philips model 201 electron microscope.
OC concentrations.
To examine the availability of organic carbon (OC) in catchment materials, 20 sediment samples were obtained from a variety of soil or sediment environments in the proglacial and ice marginal zones. A subsample of basal ice was also obtained and used for a total OC (TOC) determination. All sediment samples were dried at 105°C for 24 h and allowed to cool in a desiccator prior to weighing. The samples were combusted at 550°C for 16 h and then allowed to cool in a desiccator prior to weighing. The TOC content (expressed as a percentage, by weight) was calculated from 0.4× organic matter content (the weight lost between the drying stage and the combustion stage) (6).
The dissolved OC (DOC) concentrations of the basal ice and glacier ice and the TOC concentration of the glacier ice were determined as follows. All glassware and filters were precombusted at 550°C. Ice samples were melted and passed through 0.7-μm-pore-size Whatman GF/F filters, and the filtrates were analyzed to determine DOC contents. Melted samples were acidified to pH 2 with HCl and sparged with CO2-free N2 gas to remove dissolved inorganic carbon. A 100-μl sample was dried and flash combusted in a microvolume disposable quartz furnace which was continuously purged with He gas at a low flow rate. The combustion gases were passed through a series of furnaces containing CuO (850°C), CuO plus Ag (450°C), and Cu (680°C) in order to oxidize all CO to CO2, remove sulfur oxides, and reduce nitrogen oxides to N2. The gas stream was dried by passage through a Nafion (DuPont) dryer, and the purified, dehumidified CO2 was directed to a Finigan model MAT 252 isotope ratio mass spectrometer for quantification (22). Unfiltered samples of glacier ice were analyzed to determine their TOC contents by using the procedure described above (22). The particulate OC content was determined by subtracting the DOC content from the TOC content.
RESULTS
Water samples collected in 1996.
The initial experiments were primarily qualitative and were intended to determine whether viable microbes were present in subglacial and/or supraglacial waters. After 7 weeks of incubation at ≤4°C, a 4-liter subglacial sample amended with 0.1× R2A medium had a decreased dissolved oxygen level (1.7 versus 10.9 ppm of O2) and a decreased pH (pH 6.5 versus 7.8) compared to an unamended sample and contained 0.7 ppm of H2S (H2S was not detected in the unamended sample). By contrast, there was no change in the geochemistry of the amended supraglacial sample. When subsamples of R2A medium-amended subglacial waters were incubated with anaerobic nitrate or sulfate medium at 4°C, the media became brown (nitrate medium) or black (sulfate medium) and turbid within 6 weeks. Subsamples of unamended subglacial waters were slower to show positive results (4 months at 4°C), whereas unamended supraglacial waters did not yield positive results within 4 months. Parallel bottles inoculated with the laboratory control medium remained sterile, demonstrating that the anaerobes did not come from the R2A medium. These subculture experiments were repeated several times, and the same results were obtained each time, which confirmed the observation that viable respiring anaerobes were present in subglacial waters. We noted that the appearance of turbid cultures was qualitatively proportional to the amount of mineral sediment associated with the water sample.
Aerobic plating of unamended subglacial meltwaters onto quarter-strength plate count agar incubated at 22°C yielded 102 CFU ml of water−1, whereas parallel plates incubated at 4°C yielded >103 CFU ml−1; many of the colonies were pigmented yellow or orange. In general, colonies purified from 4°C plates were not able to grow when they were subcultured at 22°C, suggesting that they were true psychrophiles, whereas organisms isolated on plates incubated at 22°C were able to grow when they were cultured at 4°C. R2A medium-amended subglacial waters yielded >106 CFU ml−1 regardless of the plate incubation temperature. The colonies were predominantly nonpigmented, and they could be subcultured at either temperature. Studies are in progress to determine the identity and diversity of the populations. The unamended supraglacial water sample also yielded 103 CFU ml−1 on plates incubated at 4°C (predominantly pigmented colonies) and 10-fold-lower values on plates incubated at 22°C. The laboratory medium control (0.5 ml of undiluted medium) did not yield any colonies at either temperature after 3 months of incubation.
Ice samples collected in 1997.
The qualitative results obtained with subglacial and supraglacial water samples described above suggested that the subglacial environment harbored viable populations of both aerobes and anaerobes and thus that direct study of the ice originating from the glacier base was merited. Experiments performed with basal ice showed that aerobic and anaerobic microbial activities were detectable at 8 and 4°C, respectively, if a sample was amended with low levels of organic carbon (i.e., growth medium) or a terminal electron acceptor, such as NO3− or SO42−. Microbial activity was observed in cultured basal ice but not in cultured glacier ice, again suggesting that there was an association with the sediment. The absence of microbial activity in the glacier ice confirmed that we were using a suitable aseptic technique to prevent contamination of the samples during sampling and analysis.
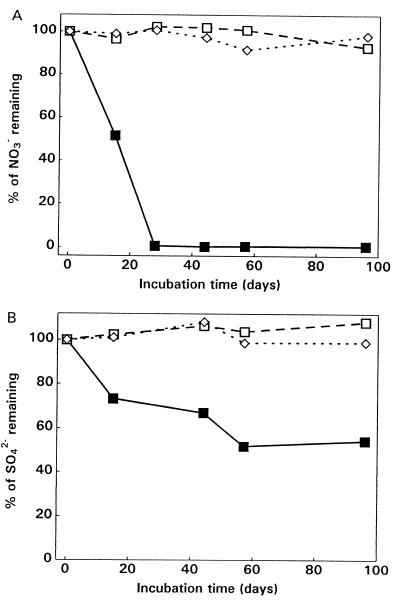
Significant depletion of NO3− and SO42− occurred during anaerobic incubation of amended basal ice (Fig. 1). In contrast, thawed glacier ice samples and uninoculated medium exhibited no reduction of NO3− and SO42− in the 90-day incubation period (Fig. 1). In the anaerobic cultures containing thawed basal ice amended with dilute R2A medium, both CO2 and CH4 were produced (Table 1) at concentrations that were 10 and 104 times higher, respectively, than the concentrations produced in samples when we used thawed glacier ice or uninoculated medium. The δ13C-CH4 of the thawed basal ice sample was −73.3‰, which clearly demonstrated that the origin of the CH4 was microbial. With poisoned controls there was neither depletion of NO3− and SO42− nor production of CO2 and CH4, indicating that abiotic processes were not responsible for the observations. These results indicate that viable, metabolically diverse anaerobic bacteria, including nitrate reducers, sulfate reducers, and methanogens, were present in the basal ice and active in cultures incubated at 4°C.
FIG. 1.
Anaerobic incubation of thawed ice samples at 4°C in the dark. Samples were amended with dilute nitrate medium (5 mM NO3−) (A) or dilute sulfate medium (14 mM SO42−) (B). Symbols: ■, basal ice; □, glacier ice; ◊, uninoculated medium.
TABLE 1.
Concentrations of headspace gases generated during 12 months of incubation at 4°C by 1997 thawed ice samples amended with 0.25× R2A medium
| Sample | Concn (ppmv) ofa:
|
|
|---|---|---|
| CO2 | CH4 | |
| Basal ice (debris rich) | 58,000 ± 2,700 | 16,000 ± 100 |
| Glacier ice (debris poor) | 5,500 ± 150 | 1.7 ± 0.1 |
| Uninoculated medium | 8,600 ± 300 | 1.4 ± 0.2 |
Values are means ± standard deviations based on the results of duplicate analyses.
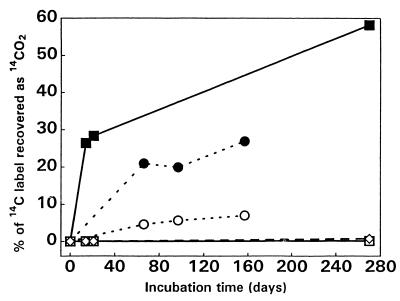
In the experiment performed with 1997 ice samples under aerobic conditions, a significant amount of 14CO2 was generated from the thawed basal ice amended with [14C]acetate but not from the amended thawed glacier ice or a parallel uninoculated control (Fig. 2). Again, this implies that microbial activity was associated with the presence of sediment in the ice.
FIG. 2.
Aerobic incubation of thawed ice samples in the dark. Samples collected in 1997 were supplemented with 98,000 dpm of sodium [2-14C]acetate and incubated at 8°C for 270 days with gyratory shaking at 200 rpm (■, basal ice plus dilute R2A medium; □, glacier ice plus dilute R2A medium; ◊, dilute R2A medium). Samples collected in 1998 were supplemented with 195,000 dpm of sodium [2-14C]acetate and incubated statically at 4°C for 157 days (●, basal ice without R2A medium; ○, glacier ice without R2A medium).
Ice samples collected in 1998.
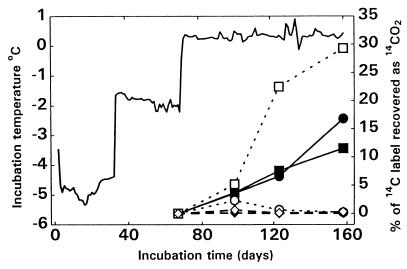
Whereas the 1997 ice samples were incubated at temperatures higher than those expected in situ and were modestly amended with nutrients, the 1998 ice samples were incubated under conditions as close as possible to those found at the base of the glacier (temperature, ca. 0°C) with minimal organic carbon amendment that was sufficient to detect respiration. The experimental temperature was set at 0.3°C in order to ensure that the entire slurry remained in liquid form (>0°C) due to the tolerances (±0.3°C) of the temperature bath. The experiments were designed to demonstrate not only that viable microbes were present in the basal ice but also that they were active under simulated in situ subglacial conditions. Activity was assessed simply by quantifying aerobic mineralization of radiolabeled acetate to 14CO2. [14C]acetate incorporation into biomass and 14CO2 depletion by autotrophy were not assessed, nor was oxygen in the sealed headspace replaced during incubation. Thus, the activity measured should be considered a conservative estimate of aerobic activity.
Unamended and 0.1× R2A medium-amended samples were incubated under a sealed aerobic headspace in the presence of sodium [2-14C]acetate at progressively higher temperatures ranging from subfreezing (−4.8 and −1.8°C) to just above freezing (0.3°C). At subfreezing temperatures no mineralization of the radiolabel was detected after 66 days of incubation (Fig. 3) even though there was probably up to 0.3% liquid water present as brine pockets at the grain boundaries of the ice crystals (G. M. Marion and S. A. Grant, Proc. Int. Symp. Phys. Chem. Ecol. Frozen Soils 1998, p. 349–356.). 14CO2 was produced only when the entire sample was in liquid form. Mineralization of the labeled acetate was observed in both the unamended thawed basal ice and, to a much lesser extent, in the unamended thawed glacier ice. Amendment with 0.1× R2A medium significantly increased the acetate mineralization rate in the glacier ice sample but had little effect on the mineralization rate in the basal ice sample. This implies that the microbes in the thawed glacier ice were more nutrient limited than those in the thawed basal ice. Unamended samples incubated at 4°C produced results similar to those obtained previously at 8°C, and the rates of acetate mineralization were higher in the thawed basal ice than in the thawed glacier ice (Fig. 2).
FIG. 3.
Aerobic incubation of 1998 ice samples in the dark at increasing temperatures (−4.8, −1.8, and 0.3°C). All samples were supplemented with 195,000 dpm of sodium [2-14C]acetate. Symbols: ■, basal ice plus dilute R2A medium; □, glacier ice plus dilute R2A medium; ●, basal ice without R2A medium; ○, glacier ice without R2A medium; ⧫, basal ice sterile control; ◊, glacier ice sterile control. Incubation temperatures are also shown.
Microscopy.
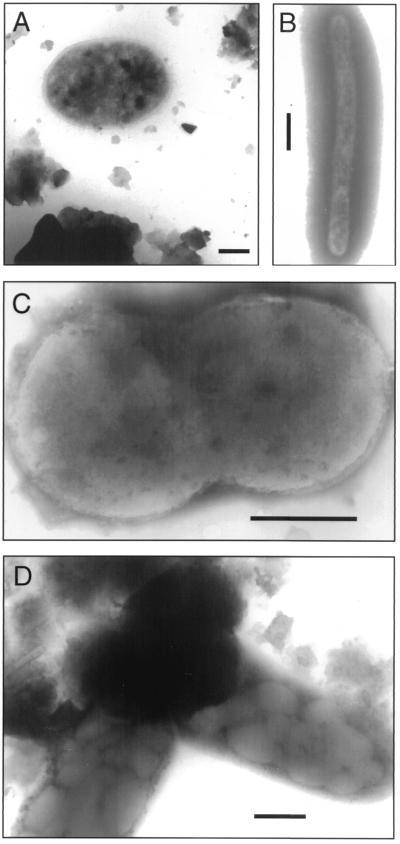
Microscopic studies revealed that microbes were present in both freshly thawed uncultured basal ice samples and cultured basal ice samples taken in 1998. Typical prokaryotic cell morphologies, including bacilli, cocci, and cells undergoing division, were observed in both cultured and uncultured basal ice samples when TEM was used (Fig. 4). Cells were not exclusively associated with sediment in basal ice samples and were often observed singly rather than in microcolonies or obvious biofilms.
FIG. 4.
TEM images of bacteria in meltwater from basal ice samples. Scale bars = 0.5 μm. (A) Coccus associated with sediment from uncultured basal ice immediately after thawing. (B and C) Long thin rod (B) and actively dividing cocci (C) from an aerobic stationary culture incubated at 0.3°C without R2A medium. (D) Short, fat rods with inclusions, associated with sediment from an aerobic culture incubated at 4°C without R2A medium.
Allochthonous OC.
Uncultured 1998 basal ice samples had sediment concentrations of 2,000 g liter−1 whereas the glacier ice did not contain visible particulates. The particulate OC concentrations in the basal ice were 0.53 to 0.97 M, compared with 34 μM in the glacier ice. Basal ice had DOC concentrations of 100 μM, compared with 24 μM in the glacier ice. This indicates that there was a much larger source of OC in the basal ice. Sediment samples obtained in the proglacial and ice marginal zones contained cyanobacterial mats, mosses, and plant remains that comprised 0.3 to 4.1% (wt/wt) OC. This confirmed that glacially overridden sediments can provide a source of allochthonous OC that serves as a carbon and energy source in the subglacial environment.
DISCUSSION
The 1996 experiments clearly demonstrated that viable microbes are present in both the supraglacial and subglacial environments of a polythermal Arctic glacier. We found that microbial activity increased as the suspended sediment concentration in the water samples increased, which was similar to findings obtained for alpine subglacial environments (35).
The 1997 experiments confirmed that there are culturable microbial populations in the debris-rich basal ice. Moreover, the basal ice harbored numerous types of aerobic and anaerobic bacteria, including heterotrophs, nitrate and sulfate reducers, and methanogens. It is particularly interesting that methanogens were viable in cultures because the samples were not obtained or stored in an anaerobic environment. Moreover, the dissolved oxygen content was close to 100% of the saturation value in the subglacial meltwaters. However, there is considerable variability in the sensitivity of methanogens to oxygen, and viable methanogens have been detected in nominally aerobic environments where anaerobic microenvironments or transient anaerobic conditions occur (42). The presence of methanogens suggests that although the subglacial meltwaters are essentially aerobic, the underlying thawed subglacial sediments contain anaerobic microenvironments which are retained as the basal ice forms.
The 1998 culture experiments showed that aerobic microbial growth can occur in the thawed basal ice under conditions as close to in situ conditions as could be achieved in the laboratory, with minimal amendment of OC and without additional nutrients. Addition of 0.1× R2A growth medium had no effect on the respiration rates in the thawed basal ice samples but resulted in significant increases in the respiration rates in the thawed glacier ice. The latter finding suggests that microbes are present in the glacier ice but are more nutrient and carbon limited than the microbes in the basal ice, probably because of the low solute content of the thawed glacier ice. Hence, the microbes are not necessarily physically associated with sediment particles. Our experiments also revealed no sign of aerobic respiration at subfreezing temperatures; however, it is likely that longer experiments would be required to confirm these observations.
The DOC concentration in the basal ice was at least twice the DOC concentration used for amendment in the culture experiments, and the TOC concentrations were orders of magnitude greater. This confirms that there is sufficient OC available in the subglacial sediments to facilitate subglacial microbial metabolism. The source of the OC in subglacial sediments is most likely permafrozen soils that are overridden by the advancing glacier and then finely ground by subglacial abrasion processes. The organic material in present-day proglacial and ice marginal sediments, which are probably similar to the source material for the subglacial sediments, consists of cyanobacterial mats, plant material, and roots. These types of carbonaceous material are readily biodegradable by microbial activity.
Based on the experiments performed with waters and ice it is evident that there is a clear link between the presence of sediment and the level of microbial activity. The sediment is probably the primary source of the microbes, which are present in the soils and sediments that the glacier overrides. As determined by TEM (Fig. 4A and D), microbes are clearly associated with the sediment, although they may not be physically attached. Additionally, the sediment provides a source of carbon, and weathered sediments produce aqueous nutrients for the subglacial microbial community. Microbes may also be washed into the subglacial environment from the glacier surface; however, it appears from the culture experiments that the combination of microbes, nutrients, and carbon necessary to support viable microbial activity is present only in the subglacial environment. Similarly, in the ice-sediment microbial communities in perennial Antarctic lake ice (30) there is a link between the concentration of sediment in the ice and microbial activity. The sediment provides nutrient-enriched microzones that support the microbial community, and there is evidence that the bacteria are physically attached to the sediment in these microzones. However, the highest concentrations of bacterial cells and DOC are not directly correlated with high sediment concentrations.
The experimental cultures and DOC-TOC results indicate that there are sufficient nutrients and carbon to support aerobic microbial activity in unfrozen basal sediments beneath John Evans Glacier. Therefore, to maintain the aerobic respiration processes in the subglacial environment, a supply of oxygen is clearly required. The potential oxygen sources in the glacier base include oxygenated surficial waters that reach the bed via crevasses or subglacial melting of glacier ice. Assuming that glacier ice contains 0.09 cm3 air of standard composition g of ice−1 (39) and the basal rate of melting is 12 mm year−1 (6 mm year−1 due to geothermal heating and 6 mm year−1 due to friction, assuming that ice slides at a rate of 20 m year−1) (27), basal melting delivers O2 to the glacier bed at a rate determined by the product of the basal melt rate and the O2 content of the ice, i.e., 1 mM O2 m−2 year−1. If surficial waters reach the bed, the O2 supply would be significantly higher. If the oxygen supply to the glacier base is completely exhausted, then it is evident that there is a diverse population of anaerobic bacteria in the unfrozen sediments. These anaerobic bacteria are viable at 4°C, and it is highly likely that they would also function at 0.3°C, albeit at lower metabolic rates. Hence, both aerobic and anaerobic microbial activities are tenable processes in unfrozen sediments beneath a high Arctic polythermal glacier. The implications of these findings are relatively wide ranging and are outlined below.
Glacial-interglacial carbon cycling.
Microbial populations are found in a range of perennially frozen environments (14, 30, 31, 33), including subglacial environments (20, 29, 35; this study) and subzero marine sediments (34). These environments contain water, nutrients, carbon, and oxygen or a suitable electron acceptor and thus provide the necessary constituents to support microbial activity. Thus, it seems likely that microbial populations were active in the temperate-based sectors of the Pleistocene midlatitude ice sheets, in which water and OC (in overridden boreal soils and peat) were present (32; Copland and Sharp, submitted). Viable aerobic and anaerobic bacteria that are functionally similar to the bacteria cultured from thawed basal ice at John Evans Glacier have been resuscitated from ancient (≤3-million-year-old) permafrozen boreal soils from Siberia and cultured at −8 to 4°C (14, 33). Hence, reactivation of microbes during sub-ice sheet thawing of permafrozen soils and peat is a tenable process.
The impact of subglacial microbial activity on carbon cycling models remains unquantified. However, a simple calculation suggests that aerobic respiration of OC, constrained by the supply of oxygen to the ice sheet beds by basal melting alone (1 mM O2 m−2 year−1), could convert 8.1 Pg of C to CO2 over a glacial cycle. Aerobic CO2 production beneath ice sheets is calculated with the following assumptions: (i) basal melting delivers O2 to the glacier bed at a rate determined by the product of the basal melt rate and the O2 content of the ice; (ii) all of the O2 is converted to CO2 by microbial processes (CH2O + O2 → CO2 + H2O); and (iii) total CO2 production is calculated by determining the integral over time of CO2 production rates in the warm-based sectors of ice sheets. The area of warm-based ice beneath the Laurentide ice sheet and its evolution over time have been described previously (24), and the area is multiplied by 1.13 to allow for warm-based ice beneath the Fennoscandian ice sheet (16).
A higher level of CO2 production is likely because the calculation described above is based on conservative assumptions about basal melting rates and ignores O2 input from surface waters. In addition, anaerobic decomposition of OC could also contribute to CO2 evolution under depleted oxygen conditions. Although it is hard to quantify the rate at which this might occur, we note that SO42− reduction rates of 0.9 to 1.2 mM m−2 day−1 have been reported for Arctic marine sediments at −1.7 to 0.2°C (34). Such rates would produce CO2 at 1,000 times the rate calculated on the basis of aerobic respiration. Thus, it would be useful to consider the effects of subglacial microbial activity in carbon cycling models on glacial-interglacial time scales.
Ice core gases.
In this study we found that strictly anaerobic methanogenic bacteria are present in thawed basal ice samples. These bacteria are viable in low-temperature cultures, which suggests that within the unfrozen basal sediments there are anaerobic microenvironments that have the potential to generate methane subglacially. This provides a possible alternative interpretation for the high greenhouse gas load observed in debris-rich basal ice from both the Dye 3 (38) and GRIP (39) ice cores from the Greenland ice sheet (Dye 3, 40,000 parts per million by volume (ppmv) of CO2; GRIP, 130,000 ppmv of CO2 and 6,000 ppmv of CH4). It is thought that the high greenhouse gas load in the basal ice results from incorporation of ground ice into the glacier ice by glacier flow-induced mixing during ice sheet buildup (38, 39). Our results suggest that the gases in these ancient samples may include products of in situ microbial activity at the base of the ice sheet, so that preglacial CH4 production and subsequent flow mixing may not be required to explain the observed CH4 values.
Martian polar analogue.
In this study we demonstrated that the subglacial environment beneath a polythermal glacier provides a viable habitat for microbial life. There is access to liquid water and sediment (nutrients), and sub-ice microbial communities are protected from diurnal and seasonal temperature fluctuations. Therefore, this environment may provide a model for viable habitats for life on Mars, since similar conditions may exist or may have existed in the basal sediments beneath the Martian north polar ice cap (9).
ACKNOWLEDGMENTS
This research was supported by Canadian Circumpolar Institute and Geological Society of America grants to M. L. Skidmore and by NSERC operating grants to J. M. Foght and M. J. Sharp. Logistical field support was provided by the Polar Continental Shelf Project (PCSP), Canada.
Fieldwork was carried out with permission from the Nunavut Research Institute and the hamlets of Grise Fjord and Resolute Bay. R. Young, J. Barker, L. Copland, W. Davis, and D. Glowacki assisted in the collection of field samples, S. Ebert provided technical help with the culture work, and R. Bhatnagar assisted with the TEM imaging. DOC and TOC analyses of the ice samples were kindly performed by K. Leckrone, Biogeochemical Laboratories, Indiana University. We are grateful to K. Muehlenbachs for helpful discussions and to two anonymous referees for their comments.
Footnotes
This is Polar Continental Shelf Project contribution 00199.
REFERENCES
- 1.Adams J M, Faure H, Faure-Denard L, McGlade J M, Woodward F I. Increases in the terrestrial carbon storage from the Last Glacial Maximum to the present. Nature. 1990;348:711–714. [Google Scholar]
- 2.Alley R B, Cuffey K M, Evenson E B, Strasser J C, Lawson D E, Larson G J. How glaciers entrain and transport basal sediment: physical constraints. Quat Sci Rev. 1997;16:1017–1038. [Google Scholar]
- 3.Andersen D T, Pollard W H, McKay C T, Omelon C. Perennial springs in the Canadian High Arctic, analogs of past Martian liquid water habitats. Eos Trans. 1998;79(45):59. [Google Scholar]
- 4.Arendt A. M. S. thesis. Alberta, Edmonton, Canada: University of Alberta; 1997. [Google Scholar]
- 5.Atlas R M. Handbook of microbiological media for environmental microbiology. Boca Raton, Fla: CRC Press; 1995. [Google Scholar]
- 6.Bengtsson L, Enell M. Chemical analysis. In: Berglund B E, editor. Handbook of Holocene palaeoecology and palaeohydrology. Chichester, United Kingdom: Wiley-Interscience; 1986. pp. 423–451. [Google Scholar]
- 7.Butlin K R, Adams M E, Thomas M. The isolation and cultivation of sulfate-reducing bacteria. J Gen Microbiol. 1949;3:46–59. doi: 10.1099/00221287-3-1-46. [DOI] [PubMed] [Google Scholar]
- 8.Cantor B A, James P B. Proceedings of the 1st International Conference on Mars Polar Science and Exploration. Lunar and Planetary Institute contribution no. 953. Houston, Tex: Lunar and Planetary Institute; 1998. Review of the 1990–1997 Hubble space telescope observations of the Martian polar caps; pp. 5–6. [Google Scholar]
- 9.Clifford S M. Polar basal melting on Mars. J Geophy Res. 1987;92:9135–9152. [Google Scholar]
- 10.Dredge L A, Morgan A V, Nielsen E. Sangamon and pre-Sangamon interglaciations in the Hudson Bay lowlands of Manitoba. Geogr Phys Quat. 1990;44:319–336. [Google Scholar]
- 11.Fedorak P M, Foght J M, Westlake D W S. A method for monitoring mineralization of 14C-labeled compounds in aqueous samples. Water Res. 1982;16:1285–1290. [Google Scholar]
- 12.Francois L M, Godderis Y, Warnant P, Ramstein G, de Noblet N, Lorenz S. Carbon stocks and isotopic budgets of the terrestrial biosphere at mid-Holocene and last glacial maximum times. Chem Geol. 1999;159:163–189. [Google Scholar]
- 13.Gibbs M T, Kump L R. Global chemical erosion during the last glacial maximum and the present: sensitivity to changes in lithology and hydrology. Paleoceanography. 1994;9:529–543. [Google Scholar]
- 14.Gilichinsky D A, Soina V S, Petrova V A. Cryoprotective properties of water in the earth cryolithosphere and its role in exobiology. Origins Life Evol Biosphere. 1993;23:65–75. doi: 10.1007/BF01581991. [DOI] [PubMed] [Google Scholar]
- 15.Hallet B, Hunter L, Bogen J. Rates of erosion and sediment evacuation by glaciers: a review of field data and their implications. Global Planet Change. 1996;12:213–235. [Google Scholar]
- 16.Holmlund P, Fastook J. A time dependent glaciological model of the Weichselian Ice Sheet. Quat Int. 1995;27:53–58. [Google Scholar]
- 17.Hubbard B, Sharp M J. Basal ice formation and deformation: a review. Prog Phys Geogr. 1989;13:529–558. [Google Scholar]
- 18.Iverson N R, Semmens D J. Intrusion of ice into porous media by regelation: a mechanism of sediment entrainment by glaciers. J Geophy Res. 1995;100:10219–10230. [Google Scholar]
- 19.Kargel J S. Proceedings of the 1st International Conference on Mars Polar Science and Exploration. Lunar and Planetary Institute contribution no. 953. Houston, Tex: Lunar and Planetary Institute; 1998. Possible composition of Martian polar caps and controls on ice-cap behaviour; pp. 22–23. [Google Scholar]
- 20.Karl D M, Bird D F, Bjorkman K, Houlihan T, Shackelford R, Tupas L. Microorganisms in the accreted ice of Lake Vostok, Antarctica. Science. 1999;286:2144–2147. doi: 10.1126/science.286.5447.2144. [DOI] [PubMed] [Google Scholar]
- 21.Lawson D E, Strasser J C, Evenson E B, Alley R B, Larson G J, Arcone S A. Glaciohydraulic supercooling: a freeze-on mechanism to create stratified, debris-rich ice. I. Field evidence. J Glaciol. 1999;44:547–562. [Google Scholar]
- 22.Leckrone K J. Ph.D. thesis. Bloomington: University of Indiana; 1997. [Google Scholar]
- 23.Ludwig W, Amiotte-Suchet P, Probst J-L. Enhanced chemical weathering of rocks during the last glacial maximum: a sink for atmospheric CO2? Chem Geol. 1999;159:147–161. [Google Scholar]
- 24.Marshall S J, Clarke G K C. Modeling North American fresh water runoff through the last glacial cycle. Quat Res. 1999;52:300–315. [Google Scholar]
- 25.Mikesell M D, Kukor J J, Olsen R H. Metabolic diversity of aromatic hydrocarbon-degrading bacteria from a petroleum-contaminated aquifer. Biodegradation. 1993;4:249–259. doi: 10.1007/BF00695973. [DOI] [PubMed] [Google Scholar]
- 26.Morozova T D, Velichko A A, Dlussky K G. Organic carbon content in the late Pleistocene and Holocene fossil soils (reconstruction for Eastern Europe) Global Planet Change. 1998;16–17:131–151. [Google Scholar]
- 27.Paterson W S B. The physics of glaciers. 1st ed. Oxford, United Kingdom: Pergamon; 1969. [Google Scholar]
- 28.Pathare A V, Paige D A. Proceedings of the 1st International Conference on Mars Polar Science and Exploration. Lunar and Planetary Institute contribution no. 953. Houston, Tex: Lunar and Planetary Institute; 1998. Recent liquid water in the polar regions of Mars; pp. 31–32. [Google Scholar]
- 29.Priscu J C, Adams E E, Berry Lyons W, Voytek M A, Mogk D W, Brown R L, McKay C P, Takacs C D, Welch K A, Wolf C F, Kirshtein J D, Avci R. Geomicrobiology of subglacial ice above Lake Vostok, Antarctica. Science. 1999;286:2141–2144. doi: 10.1126/science.286.5447.2141. [DOI] [PubMed] [Google Scholar]
- 30.Priscu J C, Fritsen C H, Adams E E, Giovannoni S J, Paerl H W, McKay C P, Doran P T, Gordon D A, Lanoil B D, Pinckney J L. Perennial Antarctic lake ice: an oasis for life in a polar desert. Science. 1998;280:2095–2098. doi: 10.1126/science.280.5372.2095. [DOI] [PubMed] [Google Scholar]
- 31.Psenner R, Sattler B. Life at the freezing point. Science. 1998;280:2073–2074. doi: 10.1126/science.280.5372.2073. [DOI] [PubMed] [Google Scholar]
- 32.Punkari M, Forsstrom L. Organic remains in Finnish subglacial sediments. Quat Res. 1995;43:415–425. [Google Scholar]
- 33.Rivkina E, Gilichinsky D, Wagener S, Tiedje J, McGrath J. Biogeochemical activity of anaerobic microorganisms from buried permafrost sediments. Geomicrobiology. 1998;15:187–193. [Google Scholar]
- 34.Sagemann J, Jorgensen B B, Greeff O. Temperature dependence and rates of sulfate reduction in cold sediments of Svalbard, Arctic Ocean. Geomicrobiol J. 1998;15:85–100. [Google Scholar]
- 35.Sharp M, Parkes J, Cragg B, Fairchild I J, Lamb H, Tranter M. Widespread bacterial populations at glacier beds and their relationship to rock weathering and carbon cycling. Geology. 1999;27:107–110. [Google Scholar]
- 36.Skidmore M, Foght J M, Sharp M J. Proceedings of the 1st International Conference on Mars Polar Science and Exploration. Lunar and Planetary Institute contribution no. 953. Houston, Tex: Lunar and Planetary Institute; 1998. Microbial experiments on basal ice from John Evans Glacier, eastern Ellesmere Island, N.W.T., Canada; pp. 34–35. [Google Scholar]
- 37.Skidmore M L, Sharp M J. Drainage behaviour of a high Arctic polythermal glacier. Ann Glaciol. 1999;28:209–215. [Google Scholar]
- 38.Souchez R, Bouzette A, Clausen H B, Johnsen S J, Jouzel J. A stacked mixing sequence at the base of the Dye 3 core, Greenland. Geophys Res Lett. 1998;25:1943–1946. [Google Scholar]
- 39.Souchez R, Lemmens M, Chappellaz J. Flow-induced mixing in the GRIP basal ice deduced from the CO2 and CH4 records. Geophys Res Lett. 1995;22:41–44. [Google Scholar]
- 40.Van Campo E, Guiot J, Peng C. A data-based reappraisal of the terrestrial carbon budget at the last glacial maximum. Global Planet Change. 1993;8:189–201. [Google Scholar]
- 41.Zimov S A, Zimova G M, Daviodov S P, Daviodova A I, Voropaev Y V, Voropaeva Z V, Prosiannikov S F, Prosiannikova O V, Semiletova I V, Semiletov I P. Winter biotic activity and production of CO2 in Siberian soils: a factor in the greenhouse effect. J Geophys Res. 1993;98:5017–5023. [Google Scholar]
- 42.Zinder S H. Physiological ecology of methanogens. In: Ferry J G, editor. Methanogenesis. New York, N.Y: Chapman and Hall; 1993. pp. 128–206. [Google Scholar]