Abstract
The pathognomonic hallmark of Parkinson’s disease (PD), α-synuclein, has been observed in the retina of PD patients. We investigated whether biomarkers in the tears and retinal microvascular changes associate with PD risk and progression. This prospective study enrolled 49 PD patients and 45 age-matched healthy controls. The α-synuclein and neurofilament light chain (NfL) levels were measured using an electrochemiluminescence immunoassay. Retinal vessel density was assessed using optical coherence tomography angiography (OCT-A). The Movement Disorder Society-Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) and Mini-Mental State Examination score were used to assess motor and cognitive progression. The α-synuclein and NfL levels in the tears were higher in PD patients than in controls (α-synuclein: 55.49 ± 8.12 pg/mL vs. 31.71 ± 3.25 pg/mL, P = 0.009; NfL: 2.89 ± 0.52 pg/mL vs. 1.47 ± 0.23 pg/mL, P = 0.02). The vessel densities in the deep plexus of central macula and the radial peripapillary capillary layer of disc region were lower in PD patients with moderate-stage compared with early-stage PD (P < 0.05). The accuracy of predicting PD occurrence using age and sex alone (area under the curve [AUC] 0.612) was significantly improved by adding α-synuclein and NfL levels and retinal vascular densities (AUC 0.752, P = 0.001). After a mean follow-up of 1.5 ± 0.3 years, the accuracy of predicting motor or cognitive progression using age, sex, and baseline motor severity as a basic model was increased by incorporating retinal microvascular and biofluid markers as a full model (P = 0.001). Our results showed that retinal microvascular densities combined with α-synuclein and NfL levels in tears are associated with risk and progression of PD.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11357-022-00576-6.
Keywords: Parkinson’s disease, Optical coherence tomography angiography, α-Synuclein, Neurofilament light chain, Tears
Introduction
Parkinson’s disease (PD) is a common neurodegenerative disorder, affecting > 1% of the population ≥ 60 years of age and with a prevalence set to double by 2030 [1]. The neuropathological hallmarks of PD are intraneuronal α-synuclein accumulations and dopaminergic neuronal loss in the substantia nigra. Although dopaminergic treatments provide symptomatic benefits for motor symptoms of PD, the disease course is progressive and the expected mortality is 2- to threefold higher than that of the general population [2]. Patients with PD deteriorate not only in their motor aspects but also in non-motor features, including cognitive function, which is one of the most disabling non-motor symptoms of PD [3].
Visual deficits are among the non-motor features of PD and such deficits can manifest as deterioration in visual acuity, low sensitivity to contrast, and disturbed color vision [4]. The retina is part of the central nervous system and contains dopaminergic amacrine cells within the inner nuclear layer [5]. Retinal dopamine and its metabolites were found to be significantly lower in patients with PD [6], and one recent postmortem analysis demonstrated α-synuclein deposits in the retinas of PD patients, including in early stages prior to the development of clinical signs of motor dysfunction [7]. Consistently, the retinas of patients with PD display swelling of retinal ganglion cells and morphological deterioration of the perifoveal dopaminergic neuronal plexus, with thinning of the retinal nerve fiber layer [8–10]. Thinning of the peripapillary retinal nerve fiber layer on optical coherence tomography (OCT) may discriminate individuals with PD from healthy control individuals, but the results are conflicting [11–13], suggesting that other retina-based imaging or biofluid markers are needed to clarify whether retina is a surrogate window through which to glimpse the PD neurodegeneration process [14]. Given the likely entry into early human clinical trials of several classes of mechanism-targeted therapies, such as those targeting the propagation of α-synuclein between cells, the identification of non-invasive and easily accessible markers that could identify PD patients at an early stage and reflect disease severity in PD is urgently needed.
Neurodegenerative disorders have been reported to associate with vascular changes in capillary integrity and structure [15, 16]. White matter capillary changes were observed to dilate during dementia process in a post-mortem neuropathology study [15]. As human brain capillaries cannot be directly visualized in vivo, reduced retinal capillary densities in early disease stage of dementia and even in asymptomatic cognitively normal APOE ε4 gene carriers were reported [16], suggesting changes in the retinal capillary densities may precede neurodegeneration in the central nervous system. Aside from dementia syndrome, decreased retinal capillary branching as well as capillary fragmentation and shortening in the substantia nigra and basal ganglia of patients with PD have been observed [17]. Optical coherence tomography angiography (OCT-A) provides a noninvasive tool with which to assess the changes in the retinal microvasculature that are observed in individuals with PD and suggest that retinal microcapillary changes may correlate with brain degeneration [18, 19]. Furthermore, tears are the biofluids secreted by the lacrimal gland. The lacrimal gland is innervated by both cholinergic and dopaminergic neurons, which degenerate in PD [20]. This, combined with the recent evidence of α-synuclein deposits in the retinas of patients with PD [7], suggests that PD neurodegeneration may impact tear composition. Simultaneous assessments of retinal microvascular changes and PD-related biomolecules in tear fluids are scarce. Here, we applied an integrated approach combining OCT-A and measurements of α-synuclein and neurofilament light chain (NfL), a sensitive marker reflecting neuroaxonal degeneration, to compare retinal microvascular changes and biofluid markers in tear fluids in PD patients and healthy controls to delineate their interrelations and link to PD risk and disease progression.
Methods
Participants and clinical evaluation
A total of 94 participants, including 49 patients with PD and 45 age-matched healthy controls, were recruited from National Taiwan University Hospital. PD was diagnosed according to the United Kingdom PD Society Brain Bank Clinical Diagnostic Criteria [21]. Controls were neurologically unaffected participants who were spouses or accompanying friends of the PD patients. Participants were excluded if they had a history of diabetes, glaucoma, optic neuropathy, retinopathy, dense cataract, or had received ocular surgery. Motor symptom severity was evaluated using the motor subscale of the Movement Disorder Society-Unified Parkinson’s Disease Rating Scale (MDS-UPDRS part III) [22] and Hoehn-and-Yahr (H-Y) staging [23]. Cognition was examined with the Mini-Mental State Examination (MMSE) [24]. Patients taking anti-cholinergic medications were held for at least 12 h, and all the examinations were done in the “on” state of the disease. We assessed motor and cognition progression on the basis of changes in the MDS-UPDRS part III motor score and MMSE score during the follow-up period. Motor progression was defined as a sustained increase of at least 2 points in the MDS-UPDRS part III score in the “off” state at follow-up [25]. Cognition progression was defined as a sustained decrease of at least 2 points in the MMSE score during follow-up [26]. The study protocol was approved by the institutional review board of National Taiwan University Hospital. All participants signed written informed consent.
Measurements of α-synuclein and NfL in tear fluids
Tear fluid samples were collected from both eyes using Schirmer test strips (Ophtechnics unlimited, Haryana, India). Strips were placed at the inferior eye lid margin. Participants’ eyes were closed for 5 min. After 5 min, the participants were asked to open their eyes and look upward, and the strips were removed. The results of the Schirmer test were determined by the length of the moistened area of the strips. The strips were immediately put into polypropylene tubes and stored at − 80 °C until further analysis. Tear fluid protein was eluted by adding RIPA buffer (Thermo Fisher Scientific, Waltham, MA) with protease and phosphatase inhibitors (cOmplete and PhosStOP; Roche, Basel, Switzerland) on ice overnight, with subsequent centrifugation at 36,000 g for 1 h.
Total protein content was determined by bicinchoninic acid assay (Thermo Fisher Scientific). The concentrations of total form of α-synuclein and NfL were quantified using a single-molecule array (SIMOA) detection system (SR-X, Quanterix, Billerica, MA) as previously described [26, 27]. All measurements were performed by research assistants who were blinded to the clinical diagnosis.
Optical coherence tomography angiography
All enrolled patients underwent a complete ophthalmic evaluation of best-corrected visual acuity, tonometry, autorefractometry, and fundus ophthalmoscopy as well as a slit-lamp examination. OCT-A imaging was performed with an AngioVue OCT (RTVue XR Avanti, Optovue, Fremont, CA, USA) after pupillary dilation. Vessel density was calculated as the percentage area occupied by flowing blood vessels in the segmented region. With a 3 × 3 mm2 field of view centered on the fovea, the foveal avascular zone, vessel density within the superficial vascular complex (VDsM), and deep vascular complex (VDdM) were determined using device software. The foveal and parafoveal regions were defined as circles of 1 mm and 3 mm, respectively, according to the standard Early Treatment Diabetic Retinopathy Study grid. Also using device software, with a 4.5 × 4.5 mm2 field of view centered on the disc, vessel density in the radial peripapillary capillary layer of the disc region (VDrID), and vessel density in the radial peripapillary capillary layer of the peripapillary region (VDrPP) were measured. Automatic segmentation was verified by two ophthalmologists (C.W.L. and T.T.L.). Images of insufficient quality (scan quality < 7/10) or affected by artifacts were excluded from analysis.
Statistical analysis
Numerical variables were expressed as the mean ± standard deviation of the mean or median with 95% confidence interval. For variables following a Gaussian distribution, data were compared using the 2-tailed t test, and multiple comparisons were performed using analysis of variance (ANOVA). We tested the homogeneity of variances by using the Levene test. For variables that violated the assumptions of normality or homoscedasticity, the groups were compared with the nonparametric Mann–Whitney U test (for 2 groups) or Kruskal–Wallis test (for > 2 groups). To compare the OCT-A variables among different stages of PD, we used a generalized estimating equation to adjust the intercorrelation between the two eyes and included age as a cofactor. Spearman’s rank-sum test was applied to determine the correlation between tear fluid biomarkers (α-synuclein and NfL) and the variables of microvascular density from OTC-A adjusting for age and sex. The diagnostic accuracy of tear fluid biomarkers and retinal microvascular densities from OTC-A were assessed with receiver operating characteristic curve (ROC) analyses. The diagnostic performance of the models was quantified using the area under a receiver operating characteristic curve (AUC) to explore the ability of individual or combined biomarkers to predict PD risk or disease progression in terms of motor and cognitive function. The predictive ability was determined using Nagelkerke’s R2 index, and we tested calibration using the Hosmer–Lemeshow test for goodness of fit. A P value < 0.05 was considered to indicate significance. The statistical analyses were performed in SPSS Version 25 (IBM, Armonk, NY, USA) and Stata (StataCorp LP, College Station, USA), and the figures were created in Prism 8 (GraphPad Software, La Jolla, CA, USA).
Results
Characteristics of the study population
The analyses were performed in 49 PD patients and 45 healthy controls. The demographic characteristics of both groups are summarized in Table 1. The average age was comparable between groups, but more male participants was noted in the PD group than in the control group (P = 0.05). MMSE scores were lower in the patients with PD than in the control participants (P = 0.06). Among PD patients, the mean disease duration was 5.32 ± 3.12 years, and the mean Hoehn-and-Yahr stage in the “off” state was 2.3 ± 1.1.
Table 1.
Clinical characteristics of study participants
| Control participants (n = 45) | PD patients (n = 49) | P value | |
|---|---|---|---|
| Age, years (mean ± SD) | 64.33 ± 11.78 | 66.41 ± 7.37 | 0.32 |
| Sex, male, n (%) | 11 (24.4) | 22 (44.9) | 0.05 |
| Schirmer (mm) | 11.10 ± 6.12 | 10.26 ± 8.04 | 0.58 |
| Disease duration (years) | N.A | 5.32 ± 3.12 | |
| Hoehn-and-Yahr stage (off) | N.A | 2.3 ± 1.1 | |
| MDS-UPDRS part III (off) | N.A | 29.1 ± 17.3 | |
| MMSE | 28.8 ± 2.1 | 25.7 ± 4.8 | 0.06 |
Data are expressed as means ± standard deviations
Schirmer’s values are the sums of wetting length from both eyes
MDS-UPDRS, Movement Disorder Society Unified PD Rating Scale; MMSE, Mini-Mental State Examination; N.A., not available
Levels of α-synuclein and NfL in tears in PD patients and controls
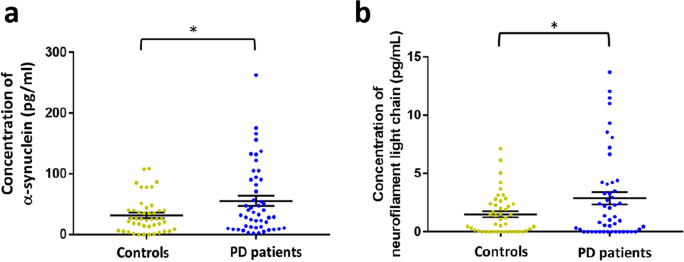
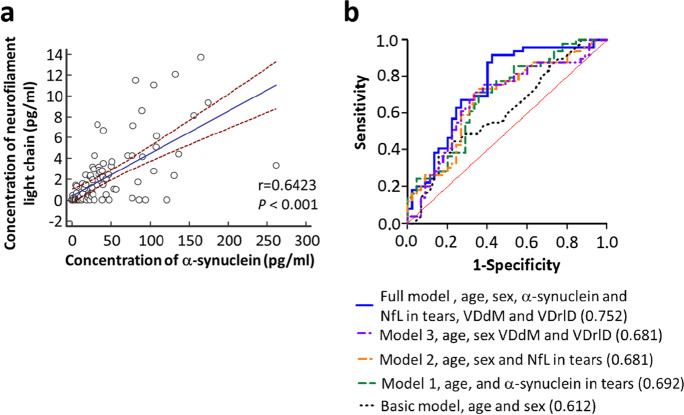
As the percentage of male participants was higher in the PD group than in the controls, we first examined whether sex might affect the levels of α-synuclein and NfL in tear fluids. The results showed there were no significant differences in α-synuclein and NfL levels between male and female participants in both groups (P = 0.51 for α-synuclein, P = 0.38 for NfL; Supplementary Fig. 1a, b). After adjusting for age and sex, the levels of α-synuclein and NfL were both significantly higher in PD patients than in controls (α-synuclein: 55.49 ± 8.12 pg/mL vs. 31.71 ± 3.25 pg/mL, P = 0.009; NfL: 2.89 ± 0.52 pg/mL vs. 1.47 ± 0.23 pg/mL, P = 0.02; Fig. 1a, b). Furthermore, the level of α-synuclein correlated with NfL in tear fluids (r = 0.64, P < 0.001, Fig. 2a). However, there was no significant difference in either α-synuclein or NfL levels in PD patients with different H-Y stages of PD (P = 0.237 for α-synuclein, P = 0.55 for NfL; Supplementary Fig. 2a, b).
Fig. 1.
Expression levels of α-synuclein and NfL in tear fluids in PD patients and healthy controls. The scatterplots display data density and means with standard deviations of tear fluid levels of α-synuclein (a), and NfL (b) in PD patients and healthy controls. PD, Parkinson’s disease; NfL, neurofilament light chain. * P < 0.05
Fig. 2.
Spearman correlation between each marker in tears and ROC curves for distinguishing PD patients from controls. a Spearman correlation of the tear fluid levels of α-synuclein and NfL in all participants. b The prediction accuracy for the occurrence of PD, as expressed by the area under the curve (AUC), improved from 0.61 in the basic model containing age and sex only (P = 0.062) to 0.69 with the addition to the model of either tear fluid levels or the retinal vascular densities VDdM or VDrID. The AUC further increased to 0.75 in the full model containing age, sex, tear fluid levels of α-synuclein and NfL, and the retinal vascular densities VDdM and VDrID (P < 0.001)
OCT-A parameters in PD patients and controls
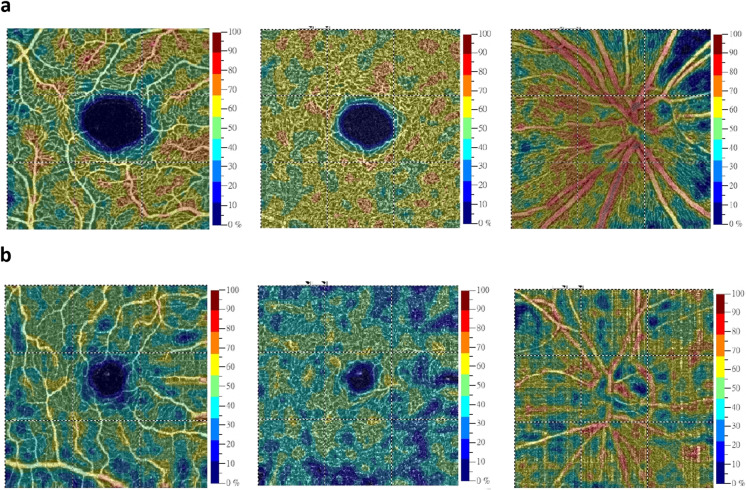
The OCT-A variables were comparable between PD patients and controls (Table 2). We then further divided PD patients into early-stage (H-Y stage I) and moderate-stage (Hoehn-Yahr stage II and III) subgroups. After excluding patients who could not complete the OCT-A examination due to motor disability or poor cooperation, analyses were conducted on 20 patients with early-stage PD and 22 patients with moderate-stage PD (Table 2). After adjustment for age, the vessel density in the deep plexus of the central macula (VDdM) and vessel density in the radial peripapillary capillary layer of the disc region (VDrID) were lower in PD patients with moderate-stage disease than in those with early-stage PD (VDdM: 50.50 ± 5.49% vs. 52.83 ± 4.95%, P = 0.04; VDrID: 48.91 ± 6.49% vs. 52.50 ± 6.70%, P = 0.03) (Table 2, representative images are shown in Fig. 3a, b).
Table 2.
OCT-A parameters in control participants and PD patients with different Hoehn-Yahr stages
| Control participants (n = 45) | PD patients | P value# | ||
|---|---|---|---|---|
| Stage I (n = 20) | Stage II + III (n = 22) | |||
| Age, years (mean ± SD) | 64.33 ± 11.78 | 64.20 ± 6.05 | 66.41 ± 7.00 | 0.29 |
| Sex, male, n (%) | 11 (24.4) | 8 (40.0) | 12 (54.5) | 0.35 |
| FAZ (mm2) | 0.28 ± 0.10 | 0.28 ± 0.09 | 0.27 ± 0.10 | 0.77 |
| VDsM, fovea (%) | 18.11 ± 6.95 | 17.85 ± 6.81 | 18.37 ± 7.07 | 0.50 |
| VDsM, parafovea (%) | 46.77 ± 5.19 | 46.92 ± 5.65 | 46.64 ± 4.71 | 0.50 |
| VDdM, fovea (%) | 32.66 ± 6.39 | 32.12 ± 6.00 | 33.17 ± 6.70 | 0.34 |
| VDdM, parafovea (%) | 51.63 ± 5.36 | 52.83 ± 4.95 | 50.50 ± 5.49 | 0.04* |
| VDrID (%) | 50.65 ± 6.83 | 52.50 ± 6.70 | 48.91 ± 6.49 | 0.03* |
| VDrPP (%) | 49.36 ± 6.52 | 48.19 ± 8.78 | 50.47 ± 2.69 | 0.26 |
Data are expressed as means ± standard deviation
OCT-A, optical coherence tomography angiography; FAZ, foveal avascular zone; VDsM, vessel density in the superficial plexus of the central macula; VDdM, vessel density in the deep plexus of the central macula; VDrID, vessel density in the radial peripapillary capillary layer of the disc region; VDrPP, vessel density in the radial peripapillary capillary layer of the peripapillary region
#Comparison between PD patients with Hoehn-Yahr stage I and those with Hoehn-Yahr stage II and III. *P < 0.05
Fig. 3.
Representative optical coherence tomography angiography (OCT-A) scanning images in the macular and peripapillary regions from patients with different Hoehn-Yahr stages of PD. a The vessel density maps of the superficial retinal capillary plexus (left column), deep retinal capillary plexus (middle column), and radial peripapillary capillary layer in the peripapillary and disc regions (right column) in the right eye of a PD patient in Hoehn-Yahr stage I. b The vessel density maps of the superficial retinal capillary plexus (left column), deep retinal capillary plexus (middle column), and radial peripapillary capillary layer in the peripapillary and disc regions (right column) in the right eye of a PD patient in Hoehn-Yahr stage III
We then examined whether there was a correlation between the biofluid markers in tears and the vascular density changes observed from OCT-A. After adjusting for age, we observed a modest negative correlation between NfL in tears and the vessel density in the radial peripapillary capillary layer of the peripapillary region (VDrPP) (adjusted ρ = − 0.31, P = 0.06) (Table 3). Otherwise, there were no significant correlations between the OCT-A variables and the expression of α-synuclein or NfL in the tear fluids.
Table 3.
Correlations between the OCT-A paramers and levels of α-synuclein and NfL in tears
| FAZ | VDsM, fovea | VDsM, parafovea | VDdM, fovea | VDdM, parafovea | VDrID | VDrPP | |
|---|---|---|---|---|---|---|---|
| α-synuclein | |||||||
| ρ | − 0.10 | 0.04 | − 0.21 | 0.10 | 0.08 | 0.08 | − 0.21 |
| P value | 0.55 | 0.82 | 0.19 | 0.55 | 0.62 | 0.64 | 0.19 |
| Adjusted ρ# | − 0.10 | 0.03 | − 0.19 | 0.11 | 0.15 | 0.09 | − 0.20 |
| Adjusted P value# | 0.57 | 0.84 | 0.26 | 0.51 | 0.37 | 0.61 | 0.22 |
| NfL | |||||||
| ρ | 0.14 | − 0.13 | − 0.19 | − 0.16 | − 0.13 | 0.09 | − 0.33 |
| P value | 0.41 | 0.44 | 0.24 | 0.31 | 0.42 | 0.59 | 0.04* |
| Adjusted ρ# | 0.15 | − 0.14 | − 0.05 | − 0.14 | 0.01 | 0.12 | − 0.31 |
| Adjusted P value# | 0.36 | 0.38 | 0.78 | 0.39 | 0.94 | 0.47 | 0.06 |
#Adjustment of age
OCT-A, optical coherence tomography angiography; FAZ, foveal avascular zone; VDsM, vessel density in the superficial plexus of the central macula; VDdM, vessel density in the deep plexus of the central macula; VDrID, vessel density in the radial peripapillary capillary layer of the disc region; VDrPP, vessel density in the radial peripapillary capillary layer of the peripapillary region. *P < 0.05
Integrated retinal microvascular densities combined with tear fluid markers in predicting PD occurrence and progression
We then examined whether integrated ocular markers, including retinal microvascular densities combined with α-synuclein and NfL levels in tear fluids, might differentiate PD patients from control participants and predict disease progression in PD. The prediction accuracy for the occurrence of PD, as expressed by area under the curve (AUC), improved from 0.61 in the basic model containing age and sex only (P = 0.062) to 0.69 with the addition to the model of either tear fluid levels or the retinal vascular density VDdM or VDrID. The AUC further increased to 0.75 in the full model containing age, sex, tear fluid levels of α-synuclein and NfL, and the retinal vascular densities VDdM and VDrID (P < 0.001) (Fig. 2b and Table 4).
Table 4.
Receiver operating characteristic curve analysis for differentiating patients with Parkinson’s disease from controls
| Covariates | AUC (95% CI) | P value |
|---|---|---|
| Basic model, age and sex | 0.612 (0.498–0.726) | 0.062 |
| Model 1, basic model and α-synuclein | 0.692 (0.585–0.799) | 0.001** |
| Model 2, basic model and NfL | 0.681 (0.571–0.790) | 0.003** |
| Model 3, basic model and VDdM_parafovea and VDrID | 0.681 (0.570–0.791) | 0.003** |
| Full model, basic model and α-synuclein, NfL, VDdM_parafovea and VDrlD | 0.752 (0.651–0.852) | < 0.001** |
AUC, area under the curve; CI, confidence interval; NfL, neurofilament light chain
*Statistically significant, P < 0.05; **P < 0.01
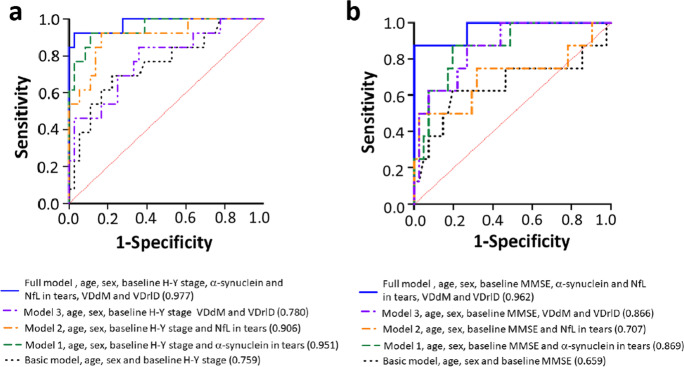
After a mean follow-up of 1.5 ± 0.3 years, 13 of 49 patients (26.5%) with PD displayed a sustained increase of at least 2 points in the MDS-UPDRS part III scores during the “off” state. The accuracy of predicting motor symptom progression improved from 0.75 in the basic model containing age, sex, and baseline H-Y stage (P = 0.006) to 0.95 with the addition to the model of α-synuclein in tear fluid ((P < 0.001); to 0.91 with the addition to the model of NfL in tear fluid (P < 0.001); and to 0.78 with the addition to the basic model of the retinal vascular density VDdM or VDrID (P = 0.003). The AUC further increased to 0.97 in the full model containing age, sex, baseline H-Y stage, tear fluid levels of α-synuclein and NfL, and the retinal vascular densities VDdM and VDrID (P < 0.001) (Fig. 4a and Table 5).
Fig. 4.
ROC curves for predicting motor and cognitive symptom progression in patients with PD. a The accuracy of predicting motor symptom progression improved from 0.75 in the basic model containing age, sex, and baseline H-Y stage (P = 0.006) to 0.95 with the addition to the model of α-synuclein in tear fluid (P < 0.001); to 0.91 with the addition to the model of NfL in tear fluid (P < 0.001); and to 0.78 with the addition to the basic model of the retinal vascular density VDdM or VDrID (P = 0.003). The AUC further increased to 0.97 in the full model containing age, sex, baseline H-Y stage, tear fluid levels of α-synuclein and NfL, and the retinal vascular densities VDdM and VDrID (P < 0.001). b The accuracy of predicting cognitive symptom progression improved from 0.65 in the basic model containing age, sex, and baseline MMSE scores (P = 0.16); to 0.86 with the addition to the model of α-synuclein in tear fluid (P = 0.001); to 0.71 with the addition to the model of NfL in tear fluid (P = 0.06); to 0.86 with the addition to the basic model of the retinal vascular density VDdM or VDrID (P = 0.001). The AUC further increased to 0.96 in the full model containing age, sex, baseline H-Y stage, tear fluid levels of α-synuclein and NfL, and the retinal vascular densities VDdM and VDrID (P < 0.001)
Table 5.
Receiver operating characteristic curve analyses for predicting motor or cognitive progression in patients with Parkinson’s disease
| Covariates | Motor progression | Cognitive progression | ||
|---|---|---|---|---|
| AUC (95% CI) | p value | AUC (95% CI) | p value | |
| Basic model (age, sex, and baseline H-Y stage or MMSE score) | 0.759 (0.601–0.916) | 0.006** | 0.659 (0.403–0.915) | 0.160 |
| Model 1, basic model and α-synuclein | 0.951 (0.886–1.000) | < 0.001** | 0.869 (0.746–0.992) | 0.001** |
| Model 2, basic model and NfL | 0.906 (0.806–1.000) | < 0.001** | 0.707 (0.464–0.950) | 0.066 |
| Model 3, basic model and VDdM_parafovea and VDrID | 0.780 (0.629–0.931) | 0.003** | 0.866 (0.744–0.988) | 0.001** |
| Full model, basic model and α-synuclein, NfL, VDdM_parafovea and VDrID | 0.977 (0.933–1.000) | < 0.001** | 0.962 (0.901–1.000) | < 0.001** |
AUC, Area under the curve; CI, confidence interval; H-Y stage, Hoehn-and-Yahr stage; NfL, neurofilament light chain
*Statistically significant, P < 0.05; **P < 0.01
Among PD patients, 8 of 49 patients (16.3%) with PD displayed a sustained decrease of at least 2 points in the MMSE scores. The accuracy of predicting cognitive symptom progression improved from 0.65 in the basic model containing age, sex, and baseline MMSE scores (P = 0.16) to 0.86 with the addition to the model of α-synuclein in tear fluid (P = 0.001); to 0.71 with the addition to the model of NfL in tear fluid (P = 0.06); and to 0.86 with the addition to the basic model of the retinal vascular density VDdM or VDrID (P = 0.001). The AUC further increased to 0.96 in the full model containing age, sex, baseline H-Y stage, tear fluid levels of α-synuclein and NfL, and the retinal vascular densities VDdM and VDrID (P < 0.001) (Fig. 4b and Table 5).
Discussion
Our study demonstrated that the levels of α-synuclein and NfL in tear fluids were increased in PD patients compared with controls. Among PD patients, the vascular densities in the deep plexus of the central macula (VDdM) and vessel density in the radial peripapillary capillary layer of the disc region (VDrID) were reduced in PD patients with moderate-stage of disease in comparison with those in early-stage PD. The integrated α-synuclein and NfL levels in tear fluids combined with the retinal microvascular densities, VDdM and VDrID, associate with increased PD risk and symptom progression.
We observed that α-synuclein could be detected in the tears of participants. The level of α-synuclein was increased in PD patients compared with controls and was also associated with increased disease progression. Consistent with our findings, one recent study using the same SIMOA platform to measure levels of α-synuclein in tears found increased expression of total form of soluble α-synuclein in PD patients and detected no significant differences in α-synuclein levels among patients with different severities [27]. Another two studies using an enzyme-linked immunosorbent assay (ELISA) observed increased oligomeric but reduced total form of α-synuclein in both basal tears and reflex tears in PD patients compared with controls [28, 29]. One possible contributor to the conflicting results may be the methods used to detect the ultra-low concentration of α-synuclein in tears. Currently, most biofluid biomarkers, including cerebrospinal fluid (CSF), serum, and plasma biomarkers, are mainly analyzed using ELISA. These assays are often performed manually and are therefore difficult to standardize. This has resulted in substantial variability in measurements between clinical centers and laboratories [30]. SIMOA is an ultrasensitive digital ELISA technology that uses antibody-coated beads and a fluorescently conjugated detection antibody to detect ultralow concentrations of proteins in biofluids. It has been shown to be more sensitive than traditional immunoassays [31]. The source of α-synuclein in tear fluids is unclear. The lacrimal gland, the principal source of tear proteins, is an exocrine gland innervated by parasympathetic nerves originating in the brainstem, which is in the trajectory of α-synuclein transmission as proposed by Braak’s hypothesis [32, 33]. The detected α-synuclein and NfL may be released from innervating parasympathetic nerve terminals in the lacrimal glands into tear fluids in the course of neuronal degeneration and α-synuclein cell-to-cell transmission [32]. Another possible source of the biomolecules in the tears maybe derived from blood, as an ultrafiltrate of plasma. Several studies using SIMOA for the detection of total soluble α-synuclein or NfL in plasma samples have shown results similar to our findings in tear fluids [26, 34]. Compared to the CSF and blood samples, tears have the advantages of noninvasive and could be applied for serial check-up in a large-population scale. Further validation in independent and larger cohorts including PD patients with more severe disease, including H-Y stages IV and V, is needed to clarify the correlation between a-synuclein levels in tears and PD disease severity.
To our knowledge, this study is the first to examine the level of NfL in tear fluids. Our results show that tear NfL is elevated in PD patients compared with controls and that baseline NfL could be integrated as one of the ocular markers associated with PD occurrence and progression in terms of motor and cognitive decline. NfL is a protein that is highly expressed in large-caliber myelinated axons and is the main byproduct of neurodegeneration [35]. The high correlation between CSF and plasma or serum NfL [36] prompted the following studies showing that NfL is a sensitive but not disease-specific marker of the extent of neurodegeneration [37]. Previous studies have shown that NfL levels are elevated in either the CSF or blood in patients with PD as compared with controls and that the level increases over time and with age and correlates with clinical measures of PD severity [38, 39]. Our results extend current knowledge by demonstrating that NfL can also be detected in tear fluids. Our findings may serve as a foundation for further investigation of other biomarkers in tear fluids to reflect central nervous system neurodegeneration in PD. A similar concept has been applied to another common neurodegenerative disorder, Alzheimer’s disease. One recent study found elevated levels of tear amyloid-beta (Aβ) 40 and tear total tau in patients with cognitive impairment, and those levels increased with aggravated disease severity [40]. These observations, combined with our findings, are in line with other efforts identifying tear biomarkers for different neurological conditions, such as TNF-α [41] for PD, and alpha-1 anti-chymotrypsin for multiple sclerosis [42]. In those studies, the levels of TNF-α and alpha-1 antichymotrypsin were also elevated in the CSF of Parkinsonian patients and multiple sclerosis patients, respectively. Together, these studies support the hypothesis that tear fluid may mirror pathophysiological changes in the central nervous system. A larger study incorporating both disease-specific and neurodegeneration-sensitive markers—namely α-synuclein and NfL—in CSF, blood, and tears, is needed to clarify this concept in PD.
In addition to the biofluid marker changes in the tears of PD patients, we also observed that OTC-A changes in the vascular densities VDdM and VDrID were reduced in PD patients with moderate- vs. early-stage disease. The integrated retinal microvascular densities VDdM and VDrID combined with α-synuclein and NfL levels in tear fluids were associated with increased risk and symptom progression in PD patients. Two studies using OTC-A have shown that vessel density in the retinal capillary plexus is significantly lower in early-stage PD patients compared with controls [19, 43]. Another study also reported decreased retinal capillary complexity in their PD group, using fractal dimension analysis [18]. However, the correlation between retinal microvascular abnormality and disease severity was not previously validated. Our results revealed that vessel density in the deep plexus of the parafoveal region and radial peripapillary capillary layer of the disc region was significantly lower in PD patients with moderate- vs. early-stage disease. Our findings are in line with previous studies demonstrating that the deep retinal microvascular network is more sensitive to cerebellar ataxia, ischemic stroke, and diabetic neuropathy than is the superficial microvascular network [44–46]. Furthermore, one study revealed that reduced retinal capillary complexity of the deep capillary plexus is significantly correlated with the duration of PD [18]. Retinal trans-synaptic degeneration in PD may manifest as retinal microvascular capillary changes. This expression can occur first in the deep microvascular network [18], which was also observed in our study. Notably, longitudinal live retina imaging in a transgenic PD rodent model revealed that the GFP-tagged α-synuclein accumulated not only in the retinal ganglion cell layer but also in the edges of the retinal arterial blood vessels [47], which could partly explain the retinal microvascular changes observed in PD patients. These in vivo findings combined with the observations in PD patients further support the notion that retinal microvascular changes are another potential surrogate marker to the progression of PD. Further study of a large cohort with a long longitudinal follow-up is warranted to confirm our findings.
Our study has several limitations. First, the number of PD patients was relatively small. The lack of advanced stage PD patients, such as in H-Y stage IV and V, limited our investigation of the correlation of biofluid markers in tears or changes in the retinal microvasculature with the disease severity of PD. Second, the cross-sectional study design precludes any inferences of a causal relationship between tear biomarkers or retinal microvascular changes and the disease process in PD. Further longitudinal follow-up studies with serial measurements of both tear fluid marker and retinal vascular changes using OTC-A are needed. Finally, the diagnosis of PD was based on clinical diagnostic criteria and lacks neuropathology confirmation.
In conclusion, our findings suggest that integrated ocular markers, including α-synuclein and NfL in tears and changes in the deep retinal microvascular network, may provide a non-invasive view that differentiates PD from controls and reflects disease progression. Further, large-cohort longitudinal studies are needed to strengthen the possible surrogate marker role of integrated ocular markers in PD progression.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank all the participants in this study. They also thank the staff of the Second Core Laboratory, Department of Medical Research, National Taiwan University Hospital for their technical support of the study.
Author contribution
Conceptualization: CW Lin and CH Lin; data curation: CW Lin, TT Lai, SJ Chen, and CH Lin; formal analysis: CW Lin, TT Lai, and SJ Chen; funding acquisition: CH Lin. investigation: CW Lin, TT Lai, and CH Lin; methodology: CW Lin, TT Lai, SJ Chen, and CH Lin; resources: CW Lin, TT Lai, and CH Lin; supervision: CH Lin. validation: CW Lin and CH Lin; writing — original draft: CW Lin; writing — review and editing: CH Lin.
Funding
The authors are grateful to the funding support from the Academia Sinica (AS-HLGC-110–03) and National Taiwan University Hospital (111-UN0036).
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Collaborators GBDPsD Global, regional, and national burden of Parkinson’s disease, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018;17:939–953. doi: 10.1016/S1474-4422(18)30295-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Macleod AD, Taylor KS, Counsell CE. Mortality in Parkinson’s disease: a systematic review and meta-analysis. Mov Disord. 2014;29:1615–1622. doi: 10.1002/mds.25898. [DOI] [PubMed] [Google Scholar]
- 3.Hely MA, Reid WG, Adena MA, Halliday GM, Morris JG. The Sydney multicenter study of Parkinson’s disease: the inevitability of dementia at 20 years. Mov Disord. 2008;23:837–844. doi: 10.1002/mds.21956. [DOI] [PubMed] [Google Scholar]
- 4.Armstrong RA. Oculo-visual dysfunction in Parkinson’s disease. J Parkinsons Dis. 2015;5:715–726. doi: 10.3233/JPD-150686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frederick JM, Rayborn ME, Laties AM, Lam DM, Hollyfield JG. Dopaminergic neurons in the human retina. J Comp Neurol. 1982;210:65–79. doi: 10.1002/cne.902100108. [DOI] [PubMed] [Google Scholar]
- 6.Harnois C, Di Paolo T. Decreased dopamine in the retinas of patients with Parkinson's disease. Invest Ophthalmol Vis Sci. 1990;31:2473–2475. [PubMed] [Google Scholar]
- 7.Ortuno-Lizaran I, Beach TG, Serrano GE, Walker DG, Adler CH, Cuenca N. Phosphorylated alpha-synuclein in the retina is a biomarker of Parkinson’s disease pathology severity. Mov Disord. 2018;33:1315–1324. doi: 10.1002/mds.27392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee JY, Ahn J, Oh S, Shin JY, Kim YK, Nam H, Jeon B. Retina thickness as a marker of neurodegeneration in prodromal lewy body disease. Mov Disord. 2020;35:349–354. doi: 10.1002/mds.27914. [DOI] [PubMed] [Google Scholar]
- 9.Archibald NK, Clarke MP, Mosimann UP, Burn DJ. The retina in Parkinson’s disease. Brain. 2009;132:1128–1145. doi: 10.1093/brain/awp068. [DOI] [PubMed] [Google Scholar]
- 10.Moschos MM, Gonidakis F, Varsou E, Markopoulos I, Rouvas A, Ladas I, Papadimitriou GN. Anatomical and functional impairment of the retina and optic nerve in patients with anorexia nervosa without vision loss. Br J Ophthalmol. 2011;95:1128–1133. doi: 10.1136/bjo.2009.177899. [DOI] [PubMed] [Google Scholar]
- 11.Garcia-Martin E, Satue M, Fuertes I, Otin S, Alarcia R, Herrero R, Bambo MP, Fernandez J, Pablo LE. Ability and reproducibility of Fourier-domain optical coherence tomography to detect retinal nerve fiber layer atrophy in Parkinson's disease. Ophthalmology. 2012;119:2161–2167. doi: 10.1016/j.ophtha.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 12.Satue M, Garcia-Martin E, Fuertes I, Otin S, Alarcia R, Herrero R, Bambo MP, Pablo LE, Fernandez FJ. Use of Fourier-domain OCT to detect retinal nerve fiber layer degeneration in Parkinson’s disease patients. Eye (Lond) 2013;27:507–514. doi: 10.1038/eye.2013.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang L, Wang Y, Zhang R. Intravenous thrombolysis in patients with central retinal artery occlusion: a systematic review and meta-analysis. J Neurol. 2021 doi: 10.1007/s00415-021-10838-6. [DOI] [PubMed] [Google Scholar]
- 14.MacCormick IJ, Czanner G, Faragher B. Developing retinal biomarkers of neurological disease: an analytical perspective. Biomark Med. 2015;9:691–701. doi: 10.2217/bmm.15.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hase Y, Ding R, Harrison G, Hawthorne E, King A, Gettings S, Platten C, Stevenson W, Craggs LJL, Kalaria RN. White matter capillaries in vascular and neurodegenerative dementias. Acta Neuropathol Commun. 2019;7:16. doi: 10.1186/s40478-019-0666-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elahi FM, Ashimatey SB, Bennett DJ, Walters SM, La Joie R, Jiang X, Wolf A, Cobigo Y, Staffaroni AM, Rosen HJ, Miller BL, Rabinovici GD, Kramer JH, Green AJ, Kashani AH. Retinal imaging demonstrates reduced capillary density in clinically unimpaired APOE ε4 gene carriers. Alzheimers Dement (Amst) 2021;13(1):e12181. doi: 10.1002/dad2.12181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guan J, Pavlovic D, Dalkie N, Waldvogel HJ, O'Carroll SJ, Green CR, Nicholson LF. Vascular degeneration in Parkinson’s disease. Brain Pathol. 2013;23:154–164. doi: 10.1111/j.1750-3639.2012.00628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi C, Chen Y, Kwapong WR, Tong Q, Wu S, Zhou Y, Miao H, Shen M, Ye H. Characterization by fractal dimension analysis of the retinal capillary network in Parkinson disease. Retina. 2020;40:1483–1491. doi: 10.1097/IAE.0000000000002641. [DOI] [PubMed] [Google Scholar]
- 19.Robbins CB, Thompson AC, Bhullar PK, Koo HY, Agrawal R, Soundararajan S, Yoon SP, Polascik BW, Scott BL, Grewal DS, Fekrat S. Characterization of retinal microvascular and choroidal structural changes in Parkinson disease. JAMA Ophthalmol. 2021;139:182–188. doi: 10.1001/jamaophthalmol.2020.5730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dartt DA. Neural regulation of lacrimal gland secretory processes: relevance in dry eye diseases. Prog Retin Eye Res. 2009;28:155–177. doi: 10.1016/j.preteyeres.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez-Martin P, Poewe W, Sampaio C, Stern MB, Dodel R, Dubois B, Holloway R, Jankovic J, et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord. 2008;23:2129–2170. doi: 10.1002/mds.22340. [DOI] [PubMed] [Google Scholar]
- 23.Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17:427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- 24.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 25.Poewe W. Clinical measures of progression in Parkinson's disease. Mov Disord. 2009;24(Suppl 2):S671–676. doi: 10.1002/mds.22600. [DOI] [PubMed] [Google Scholar]
- 26.Lin CH, Li CH, Yang KC, Lin FJ, Wu CC, Chieh JJ, Chiu MJ. Blood NfL: A biomarker for disease severity and progression in Parkinson disease. Neurology. 2019;93:e1104–e1111. doi: 10.1212/WNL.0000000000008088. [DOI] [PubMed] [Google Scholar]
- 27.Maass F, Rikker S, Dambeck V, Warth C, Tatenhorst L, Csoti I, Schmitz M, Zerr I, Leha A, Bahr M, Lingor P. Increased alpha-synuclein tear fluid levels in patients with Parkinson's disease. Sci Rep. 2020;10:8507. doi: 10.1038/s41598-020-65503-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hamm-Alvarez SF, Janga SR, Edman MC, Feigenbaum D, Freire D, Mack WJ, Okamoto CT, Lew MF. Levels of oligomeric alpha-Synuclein in reflex tears distinguish Parkinson's disease patients from healthy controls. Biomark Med. 2019;13:1447–1457. doi: 10.2217/bmm-2019-0315. [DOI] [PubMed] [Google Scholar]
- 29.Hamm-Alvarez SF, Okamoto CT, Janga SR, Feigenbaum D, Edman MC, Freire D, Shah M, Ghanshani R, Mack WJ, Lew MF. Oligomeric alpha-synuclein is increased in basal tears of Parkinson's patients. Biomark Med. 2019;13:941–952. doi: 10.2217/bmm-2019-0167. [DOI] [PubMed] [Google Scholar]
- 30.Atik A, Stewart T, Zhang J. Alpha-synuclein as a biomarker for Parkinson’s disease. Brain Pathol. 2016;26:410–418. doi: 10.1111/bpa.12370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Costa OR, Verhaeghen K, Roels S, Stange G, Ling Z, Pipeleers D, Gorus FK, Martens GA. An analytical comparison of three immunoassay platforms for subpicomolar detection of protein biomarker GAD65. PLoS ONE. 2018;13:e0193670. doi: 10.1371/journal.pone.0193670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee SJ, Desplats P, Lee HJ, Spencer B, Masliah E. Cell-to-cell transmission of alpha-synuclein aggregates. Methods Mol Biol. 2012;849:347–359. doi: 10.1007/978-1-61779-551-0_23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 34.Fyfe I. Ultrasensitive assay raises hope of plasma PD marker. Nat Rev Neurol. 2019;15:186–187. doi: 10.1038/s41582-019-0159-3. [DOI] [PubMed] [Google Scholar]
- 35.Petzold A. Neurofilament phosphoforms: surrogate markers for axonal injury, degeneration and loss. J Neurol Sci. 2005;233:183–198. doi: 10.1016/j.jns.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 36.Bacioglu M, Maia LF, Preische O, Schelle J, Apel A, Kaeser SA, Schweighauser M, Eninger T, Lambert M, Pilotto A, Shimshek DR, Neumann U, Kahle PJ, et al. Neurofilament light chain in blood and CSF as marker of disease progression in mouse models and in neurodegenerative diseases. Neuron. 2016;91:56–66. doi: 10.1016/j.neuron.2016.05.018. [DOI] [PubMed] [Google Scholar]
- 37.Bridel C, van Wieringen WN, Zetterberg H, Tijms BM, Teunissen CE, and the NFLG. Alvarez-Cermeno JC, Andreasson U, Axelsson M, Backstrom DC, Bartos A, Bjerke M, Blennow K, et al. Diagnostic value of cerebrospinal fluid neurofilament light protein in neurology: a systematic review and meta-analysis. JAMA Neurol. 2019;76:1035–1048. doi: 10.1001/jamaneurol.2019.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mollenhauer B, Dakna M, Kruse N, Galasko D, Foroud T, Zetterberg H, Schade S, Gera RG, Wang W, Gao F, Frasier M, Chahine LM, Coffey CS, et al. Validation of serum neurofilament light chain as a biomarker of Parkinson’s disease Progression. Mov Disord. 2020;35:1999–2008. doi: 10.1002/mds.28206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Backstrom D, Linder J, Jakobson Mo S, Riklund K, Zetterberg H, Blennow K, Forsgren L, Lenfeldt N. NfL as a biomarker for neurodegeneration and survival in Parkinson disease. Neurology. 2020;95:e827–e838. doi: 10.1212/WNL.0000000000010084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gijs M, Ramakers I, Visser PJ, Verhey FRJ, van de Waarenburg MPH, Schalkwijk CG, Nuijts R, Webers CAB. Association of tear fluid amyloid and tau levels with disease severity and neurodegeneration. Sci Rep. 2021;11:22675. doi: 10.1038/s41598-021-01993-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Comoglu SS, Guven H, Acar M, Ozturk G, Kocer B. Tear levels of tumor necrosis factor-alpha in patients with Parkinson's disease. Neurosci Lett. 2013;553:63–67. doi: 10.1016/j.neulet.2013.08.019. [DOI] [PubMed] [Google Scholar]
- 42.Salvisberg C, Tajouri N, Hainard A, Burkhard PR, Lalive PH, Turck N. Exploring the human tear fluid: discovery of new biomarkers in multiple sclerosis. Proteomics Clin Appl. 2014;8:185–194. doi: 10.1002/prca.201300053. [DOI] [PubMed] [Google Scholar]
- 43.Kwapong WR, Ye H, Peng C, Zhuang X, Wang J, Shen M, Lu F. Retinal microvascular impairment in the early stages of Parkinson’s disease. Invest Ophthalmol Vis Sci. 2018;59:4115–4122. doi: 10.1167/iovs.17-23230. [DOI] [PubMed] [Google Scholar]
- 44.Zhang Y, Shi C, Chen Y, Wang W, Huang S, Han Z, Lin X, Lu F, Shen M. Retinal Structural and microvascular alterations in different acute ischemic stroke subtypes. J Ophthalmol. 2020;5:2422. doi: 10.1155/2020/8850309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bhanushali D, Anegondi N, Gadde SG, Srinivasan P, Chidambara L, Yadav NK, Sinha RA. Linking retinal microvasculature features with severity of diabetic retinopathy using optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2016;57:519–525. doi: 10.1167/iovs.15-18901. [DOI] [PubMed] [Google Scholar]
- 46.Turski CA, Turski GN, Faber J, Teipel SJ, Holz FG, Klockgether T, Finger RP. Microvascular breakdown due to retinal neurodegeneration in ataxias. Mov Disord. 2022;37:162–170. doi: 10.1002/mds.28791. [DOI] [PubMed] [Google Scholar]
- 47.Price DL, Rockenstein E, Mante M, Adame A, Overk C, Spencer B, Duong-Polk KX, Bonhaus D, Lindsey J, Masliah E. Longitudinal live imaging of retinal alpha-synuclein::GFP deposits in a transgenic mouse model of Parkinson’s Disease/Dementia with Lewy Bodies. Sci Rep. 2016;6:29523. doi: 10.1038/srep29523. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.