Abstract
The significance of classical risk factors in coronary artery disease (CAD) remains unclear in older age due to possible changes in underlying disease pathologies. Therefore, we conducted Mendelian Randomization approaches to investigate the causal relationship between classical risk factors and primary CAD in different age groups. A Mendelian Randomization study was conducted in European-ethnicity individuals from the UK Biobank population. Analyses were performed using data of 22,313 CAD cases (71.6% men) and 407,920 controls (44.5% men). Using logistic regression analyses, we investigated the associations between standardized genetic risk score and primary CAD stratified by age of diagnosis. In addition, feature importance and model accuracy were assessed in different age groups to evaluate predictive power of the genetic risk scores with increasing age. We found age-dependent associations for all classical CAD risk factors. Notably, body mass index (OR 1.22 diagnosis < 50 years; OR 1.02 diagnosis > 70 years), blood pressure (OR 1.12 < 50 years; OR 1.04 > 70 years), LDL cholesterol (OR 1.16 < 50 years; OR 1.02 > 70 years), and triglyceride levels (OR 1.11 < 50 years; 1.04 > 70 years). In line with the Mendelian Randomization analyses, model accuracy and feature importance of the classical risk factors decreased with increasing age of diagnosis. Causal determinants for primary CAD are age dependent with classical CAD risk factors attenuating in relation with primary CAD with increasing age. These results question the need for (some) currently applied cardiovascular disease risk reducing interventions at older age.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11357-021-00498-9.
Keywords: Mendelian Randomization, Coronary artery disease, Risk factors, LDL cholesterol, Aging
Introduction
Current preventive strategies, especially for the prevention of a primary coronary artery disease (CAD) event, are mainly focused on reduction of body weight, blood LDL-cholesterol concentration, blood pressure, and to a lesser extent blood triglycerides concentration [1–4]. However, observational studies showed that total cholesterol levels are not as strongly associated with vascular events in older individuals as compared with middle-aged individuals [5] with similar observations for hypertension [6, 7].
In a randomized clinical trial comprising older people, the reduction of vascular events risk was only observed in users of pravastatin therapy with a history of vascular disease, suggesting predominantly effectiveness in secondary prevention in older people [8]. In addition, the effectiveness of cholesterol-lowering treatment irrespective of disease history was shown to decrease with increasing age in a large meta-analysis [9]. The comparison of the association of other cardiovascular risk factors with vascular events between younger and older individuals has not been studied to a large extent, although there seem to be indications that the effect of antihypertensive treatment on CAD risk is also reduced in old age [10]. Changes in the association of cardiovascular risk factors with events could be due to physiological/pathophysiological changes associated with increasing age, which include changes in DNA methylation [11, 12], mRNA expression profiles [13], and age-associated arterial stiffness [14]. Most importantly, randomized clinical trials are frequently only performed in individuals of younger age because of safety concerns and/or concomitant disease, and if performed, they usually contain a highly selected relatively healthy subpopulation, which limits direct translation of the study results to clinical practice[15].
Mendelian Randomization (MR) [16] has been successful in linking classical CAD risk factors to CAD in the absence of most confounding and reverse causation [17–19]. We hypothesized that classical causal primary CAD risk factors are not or weakly associated with primary CAD in older age due to age-associated changes in underlying disease pathologies. Consequently, this would mean that targeting these risk factors with pharmacological and/or lifestyle interventions might not be as efficient in older age for the prevention of primary CAD. To test this hypothesis, we assessed the associations between classical risk factors and CAD in different age groups in European individuals from the large UK Biobank using MR.
Materials and methods
Study setting and population
The UK Biobank cohort is a prospective general population cohort. Baseline assessments took place between 2006 and 2010 in 22 different assessment centers across the UK [20]. A total of 502,628 participants between the age of 40 and 70 years were recruited from the general population. Invitation letters were sent to eligible adults registered to the National Health Services (NHS) and living within a 25 miles distance from one of the study assessment centers. All participants from the UK Biobank cohort provided written informed consent, and the study was approved by the medical ethics committee. The project was completed under project number 56340.
For the present study, we restricted the analyses to the UK Biobank participants who reported to be of European ethnicity to minimize possible bias due to population stratification, and who were in the full release imputed genotyped datasets (N = 430,223). As the non-European-ancestry sample of the UK Biobank is heterogenous, we did not perform analyses restricted to this subpopulation.
The present study used an individual-level Mendelian Randomization design as summary-level data from genome-wide association studies (for conducting two-sample Mendelian Randomization) on coronary artery disease (CAD) stratified by age of diagnosis is lacking.
Genotyping and genetic imputations
UK Biobank genotyping was conducted by Affymetrix using a bespoke BiLEVE Axium array for approximately 50,000 participants; the remaining participants were genotyped using the Affymetrix UK Biobank Axiom array. All genetic data were quality controlled centrally by UK Biobank resources. More information on the genotyping processes can be found online (https://www.ukbiobank.ac.uk).
Based on the genotyped SNPs, UK Biobank resources performed centralized imputations on the autosomal SNPs using the UK10K haplotype [21], 1000 Genomes Phase 3 [22], and Haplotype Reference Consortium reference panels [23]. Based on the independent SNPs on the autosomal chromosomes, genetic principal components were calculated as a measure to be able to correct for possible present population substructures in the same ancestry group.
For the present study, we extracted, from published genome-wide association studies in which the UK Biobank did not contribute, the independent lead variants (p-value < 5 × 10−8) previously identified in relation to body mass index (339,224 individuals; 76 SNPs) [24], LDL cholesterol level (188,577 individuals; 15 SNPs) [25], triglycerides (188,577 individuals; 20 SNPs) [25], and systolic blood pressure (200,000 individuals; 42 SNPs) [26]. The selection of only SNPs that were genome-wide significant is conventional in the field of Mendelian Randomization and prevents bias from weak instrumental variables [27]. Using the beta estimates of the independent lead variants, we calculated weighted genetic risk scores per participant. To limit bias by pleiotropy, we did not allow overlap in independent lead variants between LDL cholesterol and triglyceride levels in the genetic risk scores.
Assessment of exposure variables at baseline
Body mass index was measured at the study center using the Tanita BC418MA body composition analyzer (Tanita, Inc. Manchester, UK). Systolic blood pressure was measured at the study center using an automated device (Omron device) in resting sitting position. Measurement was performed twice; the average of the two measurements was used for the analyses. LDL cholesterol was measured directly in mmol/L (analytical range: 0.26–10.3) using Enzymatic Selective Protection analysis methodology using the Beckman Coulter AU5800 platform (Beckman Coulter (UK), Ltd). Triglycerides were measured in mmol/L (analytical range: 0.1–11.3) using enzymatic methodology using the Beckman Coulter AU5800 platform (Beckman Coulter (UK), Ltd). When participants reported the use of cholesterol-lowering medication during the assessment, LDL cholesterol levels were divided by 0.7; when participants reported the use of blood pressure-lowering medication, 15 mmHg was added to the measured systolic blood pressure. These correction factors have been frequently used in large collaborative genetic association studies for reasons of data harmonization.
Cardiovascular disease outcomes
Information on incident cardiovascular disease was collected through information from the data provided by the NHS record systems. Diagnoses were coded according to the International Classification of Diseases (ICD) [20]. Here, the study outcome was CAD which we defined as angina pectoris (I20), myocardial infarction (I21 and I22), and acute and chronic ischemic heart disease (I24 and I25). We additionally defined subgroups of primary CAD on the basis of the age of diagnosis, notably CAD with age of diagnosis < 50 years, 50–60 years, 60–70 years, and > 70 years. Control participants were defined as having no CAD event prior to or during the follow-up, and we used the same control population for all analyses. For sensitivity analyses, we selected only the control subjects that remained free of primary CAD and had follow-up time available until the age of 70 years. For the age-specific analyses, primary CAD cases who did not fulfill the age criterion were set missing.
Statistical analyses
Population characteristics
Characteristics were presented for the total population free of CAD at baseline, and stratified in subgroups based on the age of enrolment, notably 40–50 years, 50–60 years, and 60–70 years. In addition, for the Mendelian Randomization analyses, characteristics of the study population were examined at enrolment and expressed as means (standard deviations) and proportions, and stratified for cases based on the age of diagnosis and controls (those not developing CAD during before and during the study period).
Multivariable-adjusted cox proportional hazard analyses
As first (descriptive) analyses, we associated baseline measured exposures (notably, body mass index, systolic blood pressure, LDL cholesterol, and triglyceride levels) with incident CAD in a cohort free of history of CAD using cox proportional hazard models (implemented in the survival package in R) adjusted for age at enrolment and sex. For these analyses, participants were followed until the end of follow-up, loss-to-follow up, mortality, or the date that the participant became a case, whichever came first. Analyses were additionally stratified based on the age at enrolment, notably 40–50 years, 50–60 years, and 60–70 years, and were repeated after the exclusion of cases who developed CAD during the first 2 years of follow-up to limit potential effects of reverse causation in these analyses. Furthermore, we additionally excluded participants either taking blood pressure-lowering medication (for the blood pressure analyses only) or LDL cholesterol-lowering medication (for the LDL cholesterol-lowering analyses only) at the moment of enrolment.
Mendelian Randomization analyses
For the Mendelian Randomization analyses (e.g., considering the lifelong exposure to high levels of the examined risk factors), which is an instrumental variable method that is free from most confounding and from reverse causation, we standardized the genetic risk scores to a standard normal distribution (mean = 0, s.d. = 1) to be able to compare the results of the different risk factors on a similar unit scale. For this analysis, we made use of a case–control design and included both past (prior to study enrolment) and incident cases (after enrolment until the end of follow-up) of CAD.
Before doing the main Mendelian Randomization analyses, we first validated the associations between the genetic risk scores and the exposures in the UK Biobank, and in groups dependent on the age at enrolment, notably 40–50 years, 50–60 years, 60–70 years, using linear regression models adjusted for age, sex, and the first 10 genetic principal components (to be able to correct for possible population substructures). Subsequently, we used logistic regression models to study the associations between the genetic risk scores and primary CAD stratified in age groups, adjusted for sex and the first 10 genetic principal components. In addition, we included all four genetic risk scores simultaneously in the logistic regression model to investigate whether the genetic risk scores are independent from each other (as we also attempted to perform by excluding genetic instruments associated with both LDL and TG). In addition, we repeated all statistical analyses stratified analyses by sex. These analyses were performed using the glm statistical package in R (version 3.6.1) [28], and the results could be interpreted as the odds ratio per 1 s.d. increased exposure to one of the classical primary CAD risk factors with accompanying 95% confidence interval.
As we recognize that the sample sizes for the case subgroups were different, we repeated the analyses in 1000 random subsamples of 1000 cases and 25,000 controls each. To be able to formally test whether the association between the genetically influenced level of exposure and primary CAD attenuated with increased age, we performed linear regression analyses with the maximum age without CAD (age at diagnosis for cases; maximum known age for controls) as outcome and an interaction term between the genetic risk score and CAD as independent variable, adjusted for sex and the first 10 principal components, as we have done previously [29]. As a last analysis, we explored the associations between the genetic risk scores and incident CAD in a population free of CAD at baseline in 10-year age bins. This analysis was conducted to explore the possible impact of immortal time bias since the oldest age group used in the main analyses only consisted of incident cases after study enrolment. These analyses were performed using cox proportional hazard models, adjusted for sex and the first 10 genetic principal components; effect attenuation was formally tested by including a multiplicative interaction term between age and the genetic risk score in the cox proportional hazard model.
We also explored the predictive ability of the genetic risk scores on primary CAD in 5-year age bins. To avoid imbalance between the two classes, the number of control participants was equalized with the number of participants with a primary CAD event. A logistic regression model for each of the age segments was specified, which uses a linear solver (LIBLINEAR library) and k-fold (k = 10) cross-validation to obtain the optimal hyperparameters [30]. The logistic regression models contained the genetic risk scores and sex as input. The output variable of the model indicates if the participant became a case. The binomial regression models were estimated and evaluated using the machine learning library Scikit learn for the Python programming language [31]. To determine the quality of the model, the area under the curve (AUC) was calculated for each model. The predictive power of the model can be deduced from this metric, with larger areas signifying higher predictive power. Along with AUC, the accuracy and recall were calculated from classification tables and used to evaluate the predictive power of the models. The accuracy is defined as (, which refers to the percentage of predictions the model calculated correctly. Recall refers to the percentage of total relevant results correctly classified by the algorithm defined as ( [32].
Previously, it has been shown that selection on age in MR can introduce survival/collider stratification bias with false-positive observations as its main consequence [33]. We attempted this issue by performing the following sensitivity analyses: First, we assessed the mean genetic risk scores within the different age bin to test for survival effects by the genetic risk scores. Second, we tested the association between the first ten principal components and CAD and age of first CAD occurrence to assess whether the genetic structure differs in cases and controls and in younger and older cases. And third, we repeated our main MR analyses by using only controls who were at least 70 years at the end of follow-up without developing CAD.
Results
Associations between measured exposures and incident CAD in a cohort without a history of CAD
In a cohort of 433,163 participants free of a history of CAD at enrolment, 9617 cases developed primary CAD during follow-up (Table 1). Without exception, a higher measured BMI, LDL cholesterol, triglycerides, or higher blood pressure at the moment of enrolment was associated with a higher risk of primary CAD during follow-up (Supplementary Table 1). However, in general, associations, adjusted for sex, were stronger in the subgroup age 40–50 years at enrolment and attenuated with increasing age. Results remained similar when we excluded cases from the analyses that developed CAD in the first 2 years of follow-up as well as when we excluded participants taking either blood pressure-lowering or cholesterol-lowering medication.
Table 1.
Baseline characteristics for the multivariable-adjusted analyses on incident coronary artery disease
| All | Age 40–50 years | Age 50–60 years | Age 60–70 years | |
|---|---|---|---|---|
| N | 433,163 | 99,001 | 144,920 | 187,213 |
| Number of cases | 9617 | 710 | 2572 | 6247 |
| Sex (% men) | 45% | 45% | 43% | 46% |
| Body mass index (kg/m2) | 27.3 (4.8) | 26.9 (4.9) | 27.4 (4.9) | 27.5 (4.5) |
| Blood pressure (mmHg) | 141 (21) | 130 (17) | 139 (19) | 148 (21) |
| LDL cholesterol (mmol/L) | 3.8 (0.9) | 3.5 (0.8) | 3.8 (0.9) | 3.9 (0.9) |
| Triglycerides (mmol/L), median (IQR) | 1.49 (1.05, 2.15) | 1.31 (0.91, 1.99) | 1.48 (1.04, 2.15) | 1.57 (1.14, 2.21) |
| Use of blood pressure medication (%) | 19% | 5.5% | 15% | 29% |
| Use of cholesterol-lowering medication (%) | 15% | 3.6% | 11% | 24% |
Data presented as means with standard deviations, or as indicated otherwise. A total 2029 participants were either younger than 40 years or older than 70 years at enrolment, and were not included in the age-stratified analyses
Abbreviations: IQR, interquartile range; LDL, low-density lipoprotein; mmHg, millimeter mercury
Mendelian Randomization analyses
Characteristics of the study population
Our study sample of the Mendelian Randomization analyses (Table 2) consisted of 22,313 primary CAD cases (61.5 [6.0] years of age at study inclusion, 71.6% men) and 407,920 controls (56.6 [8.0] years of age at study inclusion, 44.5% men). Of the primary CAD cases, 2241 cases were diagnosed before age 50 years and 1598 cases after age 70 years. In cases, the percentage of men diagnosed before age 50 years was 75.4% and this was 66.6% after the age of 70 years. The BMI at the day of study enrolment was 30.1 (5.5) kg/m2 when diagnosed before the age of 50 years, and 28.2 (4.3) kg/m2 when diagnosed after the age of 70 years.
Table 2.
Characteristics of the study population used for the Mendelian Randomization analyses
| Controls | Cases | Cases (< 50 years) | Cases (50–60 years) | Cases (60–70 years) | Cases (> 70 years) | |
|---|---|---|---|---|---|---|
| N | 407,920 | 22,313 | 2241 | 8615 | 9859 | 1598 |
| Age at inclusion, years | 56.6 (8.0) | 61.5 (6.0) | 51.1 (5.5) | 60.1 (5.3) | 64.2 (3.4) | 67.6 (1.5) |
| Sex (% men) | 44.5 | 71.6 | 75.4 | 73.6 | 69.7 | 66.6 |
| Body mass index* (kg/m2) | 27.3 (4.7) | 29.0 (4.8) | 30.1 (5.5) | 29.4 (4.9) | 28.6 (4.5) | 28.2 (4.3) |
*Information on body mass index was missing in 8838 individuals. Data of the continuous traits presented as means with standard deviations
Assessment of the association between the genetic risk scores and CAD by age of diagnosis
All genetic risk scores were associated with higher exposure levels at baseline (Supplementary Table 2) as well as when we stratified for the age at enrolment.
Irrespective of the age of diagnosis (Table 3), a higher genetically influenced BMI (OR: 1.10 [95%CI: 1.08, 1.12] per s.d.), blood pressure (OR: 1.08 [1.06, 1.10] per s.d.), r LDL cholesterol (OR: 1.11 [1.10, 1.13] per s.d.), and triglyceride (OR: 1.06 [1.05, 1.08] per s.d.) were associated with a higher risk of primary CAD.
Table 3.
Association between genetically influenced classical cardiovascular risk factors and atherogenic cardiovascular disease at different ages
| CAD | CAD (case < 50 years) | CAD (case 50–60 years) | CAD (case 60–70 years) | CAD (case > 70 years) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Body mass index | 1.10 | 1.08, 1.12 | 1.22 | 1.17, 1.28 | 1.14 | 1.11, 1.16 | 1.06 | 1.03, 1.08 | 1.02 | 0.97, 1.08 |
| Blood pressure | 1.08 | 1.06, 1.10 | 1.12 | 1.06, 1.17 | 1.09 | 1.07, 1.12 | 1.07 | 1.04, 1.09 | 1.04 | 0.98, 1.10 |
| LDL cholesterol | 1.11 | 1.10, 1.13 | 1.16 | 1.06, 1.22 | 1.15 | 1.12, 1.18 | 1.09 | 1.06, 1.11 | 1.02 | 0.97, 1.08 |
| Triglycerides | 1.06 | 1.05, 1.08 | 1.11 | 1.06, 1.16 | 1.06 | 1.03, 1.08 | 1.06 | 1.04, 1.09 | 1.04 | 0.99, 1.10 |
Analyses adjusted for sex and the first 10 principal components. Results presented as the increased odds in atherogenic cardiovascular disease per standard deviation increase in genetically determined body mass index, blood pressure, LDL cholesterol, or triglycerides with corresponding 95% confidence interval
Abbreviations: CAD, coronary artery disease; CI, confidence interval; LDL, low-density lipoprotein; OR, odds ratio
Without exception, we observed (Table 3) a stepwise diminished risk of primary CAD by the genetically influenced CVD risk factor with increasing age of diagnosis. In detail, the risk of primary CAD diagnosed before age 50 years was 1.22 times higher (95%CI: 1.17, 1.28) per s.d. higher genetically influenced BMI, was 1.12 times higher (95%CI: 1.06, 1.17) per s.d. higher genetically influenced blood pressure, was 1.16 times higher (95%CI: 1.06, 1.12) per s.d. higher genetically influenced LDL cholesterol concentration, and was 1.11 times higher (95%CI: 1.06, 1.16) per s.d. higher genetically influenced triglyceride concentration. Alternatively, the risk of primary CAD diagnosed after age 70 years was not increased by a higher genetically influenced BMI (OR 1.02 [95%CI: 0.97, 1.08] per s.d.), blood pressure (OR 1.04 [95%CI: 0.98, 1.10] per s.d.), LDL cholesterol (OR 1.02 [95%CI: 0.97, 1.08] per s.d.), and triglycerides (OR 1.04 [95%CI: 0.99, 1.10] per s.d.).
A similar attenuation of the effects was observed when we repeated the analyses 1000 times in random subpopulations of 1000 cases and 25,000 controls each (Supplementary Fig. 1). However, statistical evidence supporting attenuation of the causal effect was only observed for BMI (p-value = 3.4e-6) and LDL cholesterol (p-value = 4.0e-5), and not for blood pressure (p-value = 0.22) and triglycerides (p-value = 0.23).
Results did not differ when we performed multivariable model analyses including all studied genetic risk scores. When we stratified by sex, results were somewhat more pronounced in women compared to men (Supplementary Table 3).
Sensitivity analyses
In the analyses where we matched primary CAD cases with controls based on age of diagnosis or age at study inclusion, we observed no differences in the genetic risk scores across the different age groups (Supplementary Table 4). In addition, we did not observe, when taking into account multiple testing, an association between the principal components with primary CAD nor with the age of primary CAD diagnosis (Supplementary Table 5). And last, we observed similar results as in the main MR analyses when we only selected controls that were at least 70 years of age at the end of follow-up without developing primary CAD (Supplementary Table 6). Furthermore, results were consistent when we conducted prospective analyses in a cohort free of CAD at baseline (Supplementary Table 7).
Accuracy and feature importance models
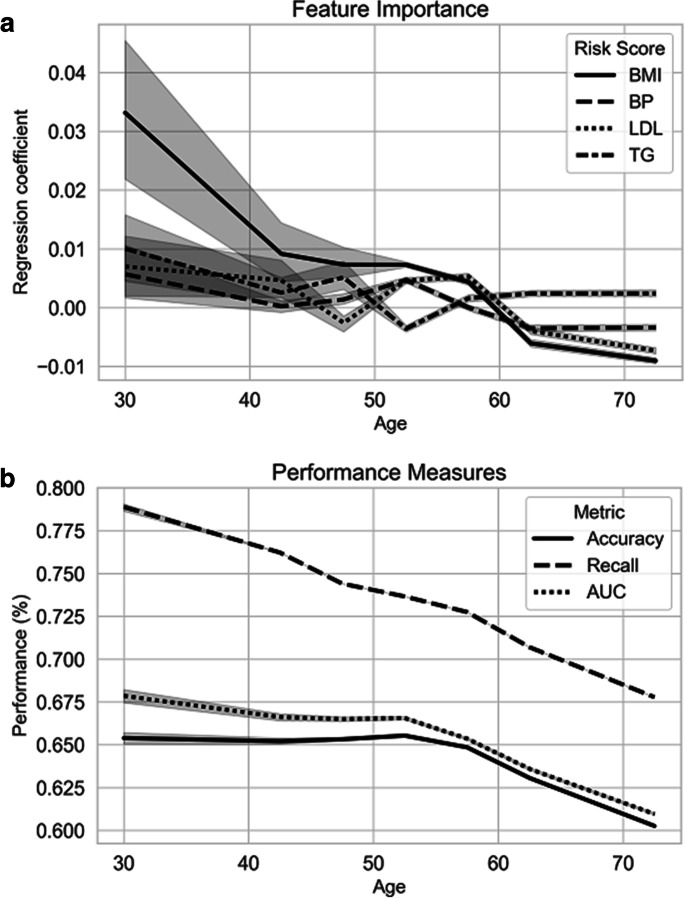
We observed (Fig. 1a) that while all individual genetic risk scores were specifically associated with primary CAD at younger age (in particular, the genetic risk score for BMI), these did not show associations with an increased primary CAD risk in older age, specifically after the age of 60 years. Similarly (Fig. 1b), the weighted genetic risk scores collectively showed a lower accuracy, recall, and AUC on primary CAD dependent on the age of diagnosis. More specifically, the AUC decreased from 0.65 at the age of 30 years to 0.60 at the age of 70 years, and specifically decreased after the age of 60 years. Model recall decreased from 0.79 at the age of 30 years to 0.68 at the age of 70 years, and showed a steady decrease with increasing age.
Fig. 1.
Feature importance and model performance dependent on the age of diagnosis. Analyses were performed using a balanced sample dependent on the age of diagnosis (for primary cases, cases of coronary artery disease) or age at study inclusion (for controls). The following age bins were used: 30–40 years (N = 210), 40–45 years (N = 711), 45–50 years (N = 1833), 50–55 years (N = 3828), 55–60 years (N = 6012), 60–65 years (N = 6376), and 65–80 years (N = 6336). a Presentation of the importance of the different features across age. b Performance measures of the combined genetic risk scores dependent on the age of diagnosis. Abbreviations: AUC, area under the curve; BMI, body mass index; BP, blood pressure; LDL, low-density lipoprotein; TG, triglycerides
Discussion
For the present study, we aimed to investigate whether classical cardiovascular risk factors gave a similar increase in risk of primary coronary artery disease in different age groups using MR. Without exception, we observed that higher levels of the measured exposures at the moment of enrolment were associated with an increased risk of incident CAD, but this association attenuated in subgroups with a higher age at enrolment. Using data from European individuals contributing to UK Biobank, we replicated the overall associations between genetically determined BMI, systolic blood pressure, LDL cholesterol, and triglycerides and risk of primary CAD that have been previously published [17–19]. Importantly, we observed that these associations were driven by cases diagnosed at younger age, while the associations between the genetically influenced exposures for the cardiovascular risk factors and primary CAD attenuated with increasing age. Although attenuation of these associations was visually observed for all investigated exposures, we only observed statistical evidence supporting an attenuation of effect for BMI and LDL cholesterol. Nevertheless, these results do indicate that the causal risk of primary CAD by classical risk factors is dependent on age.
Performing Mendelian Randomization analyses in older people is considered complicated given possible survival/selection or collider stratification bias [33]. However, simulations showed this effect was specifically present beyond age 80 years, and therefore minimally affecting the results from the present study [33]. Furthermore, in our sensitivity analyses, we found no difference in mean genetic risk scores between different age groups (meaning that there was no impact of the risk scores on survival/high age participation) nor did we observe an associations between the principal components and CAD and age of primary CAD.
Intervention studies and randomized clinical trials aiming to reduce body weight (although with some mixed results), LDL cholesterol, systolic blood pressure, and triglycerides have been shown to effectively reduce the risk of coronary artery disease [1–4, 34]. In addition to a number of meta-analyses of observational studies that showed that the risk of classical CAD risk factors was dependent on age [5–7], our analyses suggest that the classical primary CAD risk factors predominantly affect those at young age. These results are in line with some recent recommendations for the older population [35]. However, these results deviate to some extent from the results of the randomized clinical trial of pravastatin treatment in individuals over 70 years of age [8]. However, subsequent stratification based on cardiovascular disease history revealed that the pravastatin-induced decrease in vascular event rate was only observed in those with a vascular event in the history [8]. This suggests treatment with cholesterol-lowering therapy does not yield clinical benefit on primary CAD prevention in older people. For blood pressure reduction, results from randomized clinical trials are more difficult to translate to the present findings with the HYVET trial showing clinical benefit of blood pressure-lowering medication on cardiovascular disease outcomes in individuals aged 80 years and older, although primary CAD was not examined [36]. However, no clinical benefit on cardiovascular outcomes was observed in the meta-analysis of the INDANA group possibly due to differences in antihypertensive drugs being used in the trials and dosing regimens[37]. As antihypertensive drugs are also prescribed for indications other than hypertension, our results should therefore only be interpreted in the light of management of high blood pressure. Also, additional studies and efforts are required to make clear distinction in causal risk factors based on disease history.
Obesity has been shown to raise ischemic heart disease risk [17], and rises the CAD risk factors lipid levels and systolic blood pressure [17], which therefore act as mediators. However, investigating the genetic risk scores simultaneously did not give different results, suggesting that BMI has additional (possibly direct) mechanisms through which it increases primary CAD risk, independent of LDL cholesterol and blood pressure.
CAD risk management is now also targeted at the lowering of blood triglyceride levels [34]. The so-called 2 × 2 factorial MR approach showed that decreased blood triglyceride levels by enhanced LPL activity was associated with a lower risk of CAD on top of the genetically determined lower CAD risk by lower LDL cholesterol [38]. Although we did not particularly focus on the modification of LPL activity in the present study, the lack of an association between triglyceride and primary CAD in individuals over 70 years suggests that reducing blood triglyceride levels is likely not to be effective as a primary preventive strategy in older individuals, and only younger individuals will likely show clinical benefit. However, this hypothesis should be explored in greater detail in subsequent studies.
The lack of significant causal determinants for CAD in individuals above the age of 70 years raises the question what physiological factors contribute the CAD onset in this particular population and what preventive strategies could be applied in the increasing number of older individuals in societies. It is important to stress that aging is associated with increased cardiac hypertrophy [39] to compensate for lower cardiac output. It is therefore likely that elderly have less compensatory mechanisms to handle increased CAD risk and will develop CAD in an earlier pathological stage than younger individuals, which occurs independently of the classical risk factors being examined in the present study. Interestingly, there seems to be an intersection of considered main biological mechanisms of aging (e.g., oxidative stress management, growth hormone signaling, sirtuins) and cardiovascular disease risk [40, 41], which might contribute to the observations from the present study, but warrant additional studies.
Although the present study was conducted in a large study sample with a relatively large number of primary cases of coronary artery disease allowing to stratify the cases in different subgroups dependent on the age of diagnosis, some limitations should be addressed. For example, despite the large numbers, the number of cases in the extreme groups (notably the youngest and oldest age stratum) was limited and could have resulted in false-negative results. Although a similar trend in the attenuation of the effect was observed in the equally sized random subgroups, which support the robustness of our findings, the results of our study still warrant replication in an independent cohort. However, it is important to stress that not only did the associations not reach the level of statistical significance, but the effect estimates were also considerably lower and do not provide a clinically relevant effect. In addition, this study only studied healthy individuals of European ethnicity; translation of our findings to other ancestry groups should be done with caution. Furthermore, the present study assumed that the SNP-exposure association, for use as instrumental variable in the MR analysis, remained similar in different age groups. Although our genetic risk scores were associated with higher levels of the measured exposure at the moment of enrolment in the UK Biobank, we were not able to validate these scores beyond age 70 years.
In summary, the results of the present study showed that specifically, the increased causal risk of primary CAD, conferred by the classical cardiovascular risk factors, high body mass index, high blood pressure, high LDL cholesterol, and high triglycerides, attenuated with increasing age. This study highlights the potential need of a dynamic approach based on age in primary cardiovascular disease risk factor management and questions the need for (some) currently applied cardiovascular disease risk reducing interventions at older age.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This research was conducted using the UK Biobank study under Application Number 56340.
Author contribution
Study design: SAJ, BH, DvH, RN. Data acquisition: RN. Data interpretation: SAJ, BH, JWJ, ST, SPM, KWvD, DvH, RN. Drafting the manuscript: SAJ, RN. Critical comments on the manuscript: all authors. Final approval of the manuscript: RN. Guarantator of the study: RN.
Funding
This work was supported by an innovation grant from the Dutch Heart Foundation (grant number 2019T103 to R.N.).
Data availability
Data of the UK Biobank is available upon acceptance of a research proposal submitted to UK Biobank Resources (https://www.ukbiobank.ac.uk/).
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cholesterol Treatment Trialists Collaboration. Fulcher J, O'Connell R, Voysey M, Emberson J, Blackwell L, et al. Efficacy and safety of LDL-lowering therapy among men and women: meta-analysis of individual data from 174,000 participants in 27 randomised trials. Lancet. 2015;385(9976):1397–405. doi: 10.1016/S0140-6736(14)61368-4. [DOI] [PubMed] [Google Scholar]
- 2.Ettehad D, Emdin CA, Kiran A, Anderson SG, Callender T, Emberson J, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. 2016;387(10022):957–967. doi: 10.1016/S0140-6736(15)01225-8. [DOI] [PubMed] [Google Scholar]
- 3.Ma C, Avenell A, Bolland M, Hudson J, Stewart F, Robertson C, et al. Effects of weight loss interventions for adults who are obese on mortality, cardiovascular disease, and cancer: systematic review and meta-analysis. BMJ. 2017;359:j4849. doi: 10.1136/bmj.j4849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kane JA, Mehmood T, Munir I, Kamran H, Kariyanna PT, Zhyvotovska A, et al. Cardiovascular risk reduction associated with pharmacological weight loss: a meta-analysis. Int J Clin Res Trials. 2019 4(1). 10.15344/2456-8007/2019/131.
- 5.Prospective Studies Collaboration. Lewington S, Whitlock G, Clarke R, Sherliker P, Emberson J, et al. Blood cholesterol and vascular mortality by age, sex, and blood pressure: a meta-analysis of individual data from 61 prospective studies with 55,000 vascular deaths. Lancet. 2007;370(9602):1829–39. doi: 10.1016/S0140-6736(07)61778-4. [DOI] [PubMed] [Google Scholar]
- 6.Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364(9438):937–952. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 7.van den Hoogen PC, van Popele NM, Feskens EJ, van der Kuip DA, Grobbee DE, Hofman A, et al. Blood pressure and risk of myocardial infarction in elderly men and women: the Rotterdam study. J Hypertens. 1999;17(10):1373–1378. doi: 10.1097/00004872-199917100-00003. [DOI] [PubMed] [Google Scholar]
- 8.Shepherd J, Blauw GJ, Murphy MB, Bollen EL, Buckley BM, Cobbe SM, et al. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet. 2002;360(9346):1623–1630. doi: 10.1016/s0140-6736(02)11600-x. [DOI] [PubMed] [Google Scholar]
- 9.Collaboration CTT. Efficacy and safety of statin therapy in older people: a meta-analysis of individual participant data from 28 randomised controlled trials. Lancet. 2019;393(10170):407–415. doi: 10.1016/S0140-6736(18)31942-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murad MH, Larrea-Mantilla L, Haddad A, Spencer-Bonilla G, Serrano V, Rodriguez-Gutierrez R, et al. Antihypertensive agents in older adults: a systematic review and meta-analysis of randomized clinical trials. J Clin Endocrinol Metab. 2019;104(5):1575–1584. doi: 10.1210/jc.2019-00197. [DOI] [PubMed] [Google Scholar]
- 11.Boks MP, Derks EM, Weisenberger DJ, Strengman E, Janson E, Sommer IE, et al. The relationship of DNA methylation with age, gender and genotype in twins and healthy controls. PLoS One. 2009;4(8):e6767. doi: 10.1371/journal.pone.0006767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Florath I, Butterbach K, Muller H, Bewerunge-Hudler M, Brenner H. Cross-sectional and longitudinal changes in DNA methylation with age: an epigenome-wide analysis revealing over 60 novel age-associated CpG sites. Hum Mol Genet. 2014;23(5):1186–1201. doi: 10.1093/hmg/ddt531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peters MJ, Joehanes R, Pilling LC, Schurmann C, Conneely KN, Powell J, et al. The transcriptional landscape of age in human peripheral blood. Nat Commun. 2015;6:8570. doi: 10.1038/ncomms9570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mitchell GF, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, et al. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation. 2010;121(4):505–511. doi: 10.1161/CIRCULATIONAHA.109.886655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mooijaart SP, Broekhuizen K, Trompet S, de Craen AJ, Gussekloo J, Oleksik A, et al. Evidence-based medicine in older patients: how can we do better? Neth J Med. 2015;73(5):211–218. [PubMed] [Google Scholar]
- 16.Lawlor DA, Harbord RM, Sterne JA, Timpson N, Davey SG. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med. 2008;27(8):1133–1163. doi: 10.1002/sim.3034. [DOI] [PubMed] [Google Scholar]
- 17.Dale CE, Fatemifar G, Palmer TM, White J, Prieto-Merino D, Zabaneh D, et al. Causal associations of adiposity and body fat distribution with coronary heart disease, stroke subtypes, and type 2 diabetes mellitus: a Mendelian Randomization analysis. Circulation. 2017;135(24):2373–2388. doi: 10.1161/CIRCULATIONAHA.116.026560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holmes MV, Asselbergs FW, Palmer TM, Drenos F, Lanktree MB, Nelson CP, et al. Mendelian Randomization of blood lipids for coronary heart disease. Eur Heart J. 2015;36(9):539–550. doi: 10.1093/eurheartj/eht571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nazarzadeh M, Pinho-Gomes AC, Smith Byrne K, Canoy D, Raimondi F, Ayala Solares JR, et al. Systolic blood pressure and risk of valvular heart disease: a Mendelian Randomization study. JAMA Cardiol. 2019;4(8):788–795. doi: 10.1001/jamacardio.2019.2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12(3):e1001779. doi: 10.1371/journal.pmed.1001779.PMEDICINE-D-12-02351[pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.UK10K Consortium. Walter K, Min JL, Huang J, Crooks L, Memari Y, et al. The UK10K project identifies rare variants in health and disease. Nature. 2015;526(7571):82–90. doi: 10.1038/nature14962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.1000 Genomes Project Consortium. Auton A, Brooks LD, Durbin RM, Garrison EP, Kang HM, et al. A global reference for human genetic variation. Nature. 2015;526(7571):68–74. doi: 10.1038/nature15393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCarthy S, Das S, Kretzschmar W, Delaneau O, Wood AR, Teumer A, et al. A reference panel of 64,976 haplotypes for genotype imputation. Nat Genet. 2016;48(10):1279–1283. doi: 10.1038/ng.3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Locke AE, Kahali B, Berndt SI, Justice AE, Pers TH, Day FR, et al. Genetic studies of body mass index yield new insights for obesity biology. Nature. 2015;518(7538):197–206. doi: 10.1038/nature14177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Willer CJ, Schmidt EM, Sengupta S, Peloso GM, Gustafsson S, Kanoni S, et al. Discovery and refinement of loci associated with lipid levels. Nat Genet. 2013;45(11):1274–1283. doi: 10.1038/ng.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.International Consortium for Blood Pressure Genome-Wide Association Studies. Ehret GB, Munroe PB, Rice KM, Bochud M, Johnson AD, et al. Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature. 2011;478(7367):103–9. doi: 10.1038/nature10405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davies NM, Holmes MV, Davey SG. Reading Mendelian Randomisation studies: a guide, glossary, and checklist for clinicians. BMJ. 2018;362:k601. doi: 10.1136/bmj.k601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marschner IA. glm2: Fitting generalized linear models with convergence problems. The R Journal. 2011;3(2):12–15. doi: 10.32614/RJ-2011-012. [DOI] [Google Scholar]
- 29.Noordam R, Lall K, Smit RAJ, Laisk T, Estonian Biobank Research T. Metspalu A, et al. Stratification of type 2 diabetes by age of diagnosis in the UK Biobank reveals subgroup-specific genetic associations and causal risk profiles. Diabetes. 2021;70(8):1816–25. doi: 10.2337/db20-0602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fan RE, Chang KW, Hsieh CJ, Wang XR, Lin CJ. LIBLINEAR: a library for large linear classification. J Mach Learn Res. 2008;9:1871–1874. [Google Scholar]
- 31.Pedregosa F, Varoquaux G, Gramfort A, Michel V, Thirion B. Scikit-learn: machine learning in Python. J Mach Learn Res. 2011;12:2825–2830. [Google Scholar]
- 32.Powers DMW. Evaluation: from precision, recall and F-measure to ROC, informedness, markedness and correlation. J Mach Learn Res. 2011;2:37–63. [Google Scholar]
- 33.Smit RAJ, Trompet S, Dekkers OM, Jukema JW, le Cessie S. Survival bias in Mendelian Randomization studies: a threat to causal inference. Epidemiology. 2019;30(6):813–816. doi: 10.1097/EDE.0000000000001072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marston NA, Giugliano RP, Im K, Silverman MG, O'Donoghue ML, Wiviott SD, et al. Association between triglyceride lowering and reduction of cardiovascular risk across multiple lipid-lowering therapeutic classes: a systematic review and meta-regression analysis of randomized controlled trials. Circulation. 2019;140(16):1308–1317. doi: 10.1161/CIRCULATIONAHA.119.041998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van der Ploeg MA, Floriani C, Achterberg WP, Bogaerts JMK, Gussekloo J, Mooijaart SP, et al. Recommendations for (discontinuation of) statin treatment in older adults: review of guidelines. J Am Geriatr Soc. 2020;68(2):417–425. doi: 10.1111/jgs.16219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beckett NS, Peters R, Fletcher AE, Staessen JA, Liu L, Dumitrascu D, et al. Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008;358(18):1887–1898. doi: 10.1056/NEJMoa0801369. [DOI] [PubMed] [Google Scholar]
- 37.Gueyffier F, Bulpitt C, Boissel JP, Schron E, Ekbom T, Fagard R, et al. Antihypertensive drugs in very old people: a subgroup meta-analysis of randomised controlled trials INDANA Group. Lancet. 1999;353(9155):793–796. doi: 10.1016/s0140-6736(98)08127-6. [DOI] [PubMed] [Google Scholar]
- 38.Lotta LA, Stewart ID, Sharp SJ, Day FR, Burgess S, Luan J, et al. Association of genetically enhanced lipoprotein lipase-mediated lipolysis and low-density lipoprotein cholesterol-lowering alleles with risk of coronary disease and type 2 diabetes. JAMA Cardiol. 2018;3(10):957–966. doi: 10.1001/jamacardio.2018.2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Levy D, Anderson KM, Savage DD, Kannel WB, Christiansen JC, Castelli WP. Echocardiographically detected left ventricular hypertrophy: prevalence and risk factors. The Framingham Heart Study. Ann Intern Med. 1988;108(1):7–13. doi: 10.7326/0003-4819-108-1-7. [DOI] [PubMed] [Google Scholar]
- 40.Dai DF, Chen T, Johnson SC, Szeto H, Rabinovitch PS. Cardiac aging: from molecular mechanisms to significance in human health and disease. Antioxid Redox Signal. 2012;16(12):1492–1526. doi: 10.1089/ars.2011.4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.North BJ, Sinclair DA. The intersection between aging and cardiovascular disease. Circ Res. 2012;110(8):1097–1108. doi: 10.1161/CIRCRESAHA.111.246876. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data of the UK Biobank is available upon acceptance of a research proposal submitted to UK Biobank Resources (https://www.ukbiobank.ac.uk/).