Abstract
Recent studies using dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) with gadolinium-based contrast agents (GBCA) have demonstrated subtle blood–brain barrier (BBB) leaks in the human brain during normal aging, in individuals with age-related cognitive dysfunction, genetic risk for Alzheimer’s disease (AD), mild cognitive impairment, early AD, cerebral small vessel disease (SVD), and other neurodegenerative disorders. In these neurological conditions, the BBB leaks, quantified by the unidirectional BBB GBCA tracer’s constant Ktrans maps, are typically orders of magnitude lower than in brain tumors, after stroke and/or during relapsing episodes of multiple sclerosis. This puts extra challenges for the DCE-MRI technique by pushing calculations towards its lower limits of detectability. In addition, presently, there are no standardized multivendor protocols or evidence of repeatability and reproducibility. Nevertheless, subtle BBB leaks may critically contribute to the pathophysiology of cognitive impairment and dementia associated with AD or SVD, and therefore, efforts to improve sensitivity of detection, reliability, and reproducibility are warranted. A larger number of participants scanned by different MR scanners at different clinical sites are sometimes required to detect differences in BBB integrity between control and at-risk groups, which impose additional challenges. Here, we focus on these new challenges and propose some approaches to normalize and harmonize DCE data between different scanners. In brief, we recommend specific regions to be used for the tracer’s vascular input function and DCE data processing and how to find and correct negative Ktrans values that are physiologically impossible. We hope this information will prove helpful to new investigators wishing to study subtle BBB damage in neurovascular and neurodegenerative conditions and in the aging human brain.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11357-022-00571-x.
Keywords: Blood–brain barrier permeability, Aging population, Cognitive impairment, Alzheimer’s disease, Dementia, Small vessel disease, Dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI)
Introduction
Vascular contributions to dementia and Alzheimer’s disease (AD) are increasingly recognized [1–15]. According to the neurovascular hypothesis of AD, changes in the cerebrovascular system contribute to cognitive impairment, dementia, and AD. Besides other vascular pathologies such as white matter changes, lacunes, microinfarcts, and ischemic changes [7, 13], early loss of cerebrovascular integrity or leaks in a protective blood–brain barrier (BBB) has been linked to human cognitive impairment, dementia, and AD [15, 16].
Blood–brain barrier
The BBB is positioned centrally within the neurogliovascular unit (NGVU) which is composed of neurons, glial cells (e.g., astrocytes, microglia), and vascular cells (e.g., endothelial cells, pericytes) [9, 12, 17–19]. Anatomically, the BBB is formed by a tightly sealed endothelial monolayer that not only is primarily localized at the level of brain capillaries, but also extends along the endothelium of the arterial and arteriolar tree as well as along the venous and venular segments [12, 15]. One of the key functions of the BBB is to prevent entry into the brain of blood-derived toxic molecules, cells, and microorganisms and of excess edema fluid that is toxic to neurons and glia [12, 19]. As reviewed in detail elsewhere [12, 20, 21], the BBB integrity at the molecular level is maintained by several tight junction (TJ) proteins such as, to name a few, zonula occludens (ZO-1, ZO-2, and ZO-3); occludin; claudin-1, claudin-3, claudin-5, and claudin-12; cadherins; catenins and other adhesion molecules; and adherens junction (AJ) proteins such as VE-cadherin and platelet cell adhesion molecule 1 (PECAM-1), which connect the neighboring endothelial cells.
The TJs appear on electron microscopy as hollow tubes of approximately 10 nm in diameter that connect neighboring endothelial cells which, in contrast to fenestrated endothelium of peripheral organs, precludes free paracellular exchanges of solutes from blood to brain and brain to blood. The TJs are connected to intracellular actin and vinculin cytoskeletal filaments via scaffolding proteins of the membrane-associated guanylate kinase family ZO-1, ZO-2, and ZO-3 cyclases [12, 20, 21]. These ZO proteins play a critical role in regulation of cytoskeletal processes essential for the integrity of cell junctions. ZO-1 and/or other ZO family protein deficiencies, as well as loss of occludin and claudins, are associated with BBB breakdown in many neurodegenerative and acute neurological brain disorders [12] as well as in small vessel disease (SVD) [22].
AJs form homophilic endothelial-to-endothelial cell contacts roughly 20 nm wide and are also connected to cytoskeleton to modulate receptor signaling and regulate transendothelial migration of lymphocytes, monocytes, and neutrophils [12, 20, 21]. In addition to restricted paracellular transport, low rates of transcellular endothelial bulk flow transcytosis importantly limit solute transport exchanges between blood and brain compartments. Altogether, these molecular processes maintain normal barrier integrity of the endothelial monolayer in the brain which keeps optimal chemical composition of brain interstitial fluid (ISF), necessary for proper functioning of neuronal circuits and synapses.
In addition to rejecting blood-derived toxic molecules, cells, and microorganisms, the BBB also regulates blood-to-brain transport across the capillary wall of essential energy metabolites, amino acids, hormones, fatty acids, nucleotides, inorganic anions, choline, vitamins, some peptides and proteins, fluid (i.e., water), and controls clearance of brain endogenous neurotoxins and metabolic end products from brain to blood [12, 20, 21]. In this sense, the BBB acts as a semipermeable selective barrier.
Recent cell and nuclear RNA sequencing (RNAseq) studies of the mouse brain vasculature [23, 24] and human brain vasculature [25, 26] have revealed the presence of about 10,000 transcripts in both murine and human BBB endothelium including genes encoding for multiple TJ and AJ proteins, multiple substrate-specialized transporters and receptors, ion channels, and other proteins with biological functions at the BBB, as reviewed in detail in these publications. The link between BBB and AD is also supported by a recent nuclear RNAseq study, which revealed that 30 of the top 45 AD genes identified by genome wide association studies (GWAS) are expressed in human brain vasculature [26]. Vascular GWAS genes were mapped to endothelial protein transport, adaptive immune, and extracellular matrix pathways.
The BBB-associated mural cells, pericytes, enwrap brain capillaries and critically maintain BBB integrity [27–30]. Although the exact molecular pathways enabling pericytes to control functional integrity of the endothelial monolayer have not been yet completely elucidated, it has been proposed that pericyte loss in pericyte-deficient mice leads to a significant decrease in the levels of major facilitator superfamily domain containing 2A (MSFD2A) gene in brain endothelium which encodes an orphan transporter for essential omega-3 fatty acids, which in turn inhibits non-specific bulk flow fluid transcytosis protecting barrier integrity. Lack of MFSD2A leads to BBB breakdown by increasing the rate of transcytosis [31]. More recently, it has been suggested that pericyte-derived vitronectin interacts with endothelial integrin α5 receptor which results in a protective BBB signaling and maintains barrier integrity by inhibiting fluid phase transcytosis [32]. Strikingly, dysfunction in each of the cellular components of the NGVU has been linked to evolution of dementia and AD in experimental, imaging, pathological, and epidemiological studies [9–12, 17–19, 33].
That intact BBB is critical for normal brain function is best illustrated by examples of about 20 rare human monogenic neurologic diseases when neurologic symptoms are not caused by primary defects in neurons, but are caused by genetic defects in individual cell types within the BBB leading to BBB breakdown and NGVU disruption [19]. These monogenic diseases, to name only a few, include, for example, inactivating mutations in the solute carrier family 2 member 1 (SLC2A1) gene encoding GLUT1 glucose transporter in brain endothelial cells, which in turn leads to GLUT1 deficiency syndrome that is characterized with BBB breakdown causing early-onset microcephaly and seizures [34]. Similarly, inactivating mutations in the MSFD2A gene–encoding BBB omega-3 fatty acids transporter leads to BBB breakdown [31, 35] and microcephaly [36, 37]. Mutations in genes encoding TJs proteins can lead to uncontrolled leaks of blood into brain parenchyma eliciting neuroinflammation, microbleeds, seizures, headache, and/or even ischemic strokes [12].
BBB leaks during normal aging and cognitive impairment
Several neuropathological studies have shown regional leaks of neurotoxic blood-derived proteins into brain parenchyma in the cortex and hippocampus of elderly patients diagnosed with cognitive impairment, dementia, and AD suggesting BBB breakdown [10]. Moreover, recent neuroimaging studies using dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) with gadolinium-based contrast agents (GBCA) have demonstrated subtle BBB breakdown in individuals along the AD continuum that becomes evident at the stage of early cognitive impairment and is accelerated in individuals with mild cognitive impairment (MCI) and AD-type dementia. Interestingly, early neuroimaging studies using computed tomography (CT) [38, 39], positron emission tomography (PET) with [68 Ga]EDTA [40], and DCE-MRI semiquantitative analysis [41, 42] were not able to detect BBB breakdown in AD patients. In contrast, recent neuroimaging studies from different groups have shown BBB breakdown during normal aging [43–50], in MCI [44–46, 51–53], AD [54–57], cerebral SVD [58–69], and in other neurodegenerative disorders [70, 71]. The ability of recent studies to detect subtle regional BBB leaks could be attributed to more advanced approaches and sophisticated analysis that includes but is not limited to use of MRI sequences with higher spatial and temporal resolution [44, 45, 51, 54, 72], measurements of individual vascular arterial input [44–46, 51] or venous input [43, 47–50, 52, 53, 55–58, 60, 61, 64, 66, 68, 69] functions, and use of quantification methods to calculate the unidirectional BBB transport constant Ktrans for GBCA with the Patlak analysis [73, 74], which were not applied in previous studies [38–42].
In terms of regionality, accelerated BBB breakdown by DCE-MRI has been detected initially in the hippocampus, a center for learning and memory, during normal aging and in individuals with MCI [44], which has been confirmed by elevated levels of biochemical biomarkers of BBB breakdown such as cerebrospinal fluid (CSF)/serum albumin ratio, Qalb, elevated CSF fibrinogen and CSF plasminogen levels [44, 51, 75], and increased CSF levels of soluble platelet-derived growth factor β (sPDGFRβ), a biomarker of pericyte injury [44, 51, 75]. The follow-up DCE-MRI studies reported a more widespread BBB breakdown in MCI in both gray matter and normal-appearing white matter [55]. BBB breakdown in gray and white matter was also found in the elderly population associated with age-related cognitive decline [45, 47, 48], particularly in those with the loss of memory retrieval [49].
Limitations and challenges of DCE-MRI technique
GBCA are injected approximately 30 million times annually for clinical evaluation of BBB integrity in patients treated for multiple sclerosis, brain tumors, and/or some other neurological disorders [76]. However, in contrast to large BBB leakages seen in brain tumors, after acute ischemic stroke, large arterial infracts, and/or during relapsing episodes in multiple sclerosis patients, the BBB leaks detected by DCE-MRI during normal aging, in MCI, AD, SVD, and/or other neurodegenerative disorders, are almost one to two orders of magnitude lower and much more difficult to detect [77, 78]. This pushes the DCE-MRI technique to the lower limits of detectability close to background noise, emphasizing the importance of optimal technique at all stages of study design. In addition, a lack of standardized multivendor protocols also contributes to problems with repeatability and reproducibility. Nevertheless, subtle chronic leakages in the BBB that persist over years or decades during preclinical decline and/or clinical progression to dementia, AD, and SVD may importantly contribute to cognitive impairment.
To more accurately detect these changes, often a large number of participants must be scanned to achieve adequate power, and this may require multicenter participation using different MR scanners at different sites which impose an additional set of challenges. Alternatively, recruiting participants in multiple centers has advantages including increased diversity of participants, generalizability of the results, dissemination of the methodology, and may allow participation of uncommon alongside common subcategories of a disorder in the same protocol, thus enabling direct comparison (such as rare genetic forms of SVD alongside the more common sporadic type) [79] and should therefore be encouraged in the future.
Here, we describe how DCE data between different scanners can be normalized and/or harmonized, how to find the best possible region for the tracer’s vascular input function and DCE data processing, and possible strategies to correct for negative Ktrans values that are physiologically impossible and represents artifact. These approaches with some technical details and examples and our recommendations are discussed next.
SPGR- and VIBE-derived T1 mapping
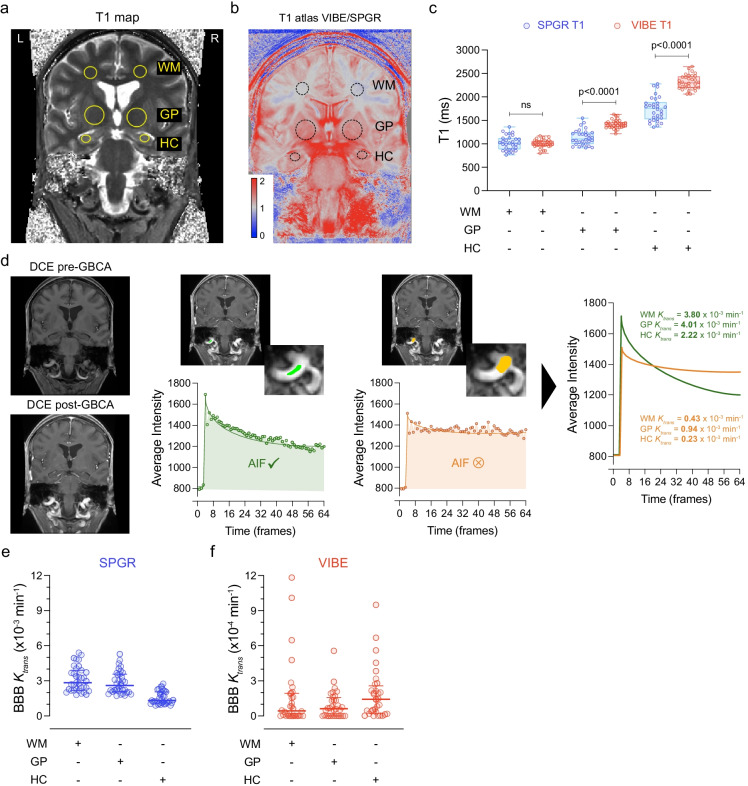
To begin addressing data harmonization between different scanners, here we discuss an example of 62 scanned participants for the assessment of BBB permeability using a pre-contrast brain T1-mapping VFA method followed by a 16-min DCE-MRI protocol with injection of Gadolinium contrast (gadobenate dimeglumine or gadoterate meglumine) at 30 s [44–46, 51]. In this example, we compared 30 cognitively unimpaired participants who were scanned on a GE 3 T HDXT MR scanner using a T1-weighted 3D spoiled gradient echo (SPGR) pulse sequence to 32 cognitively unimpaired participants who were scanned on a Siemens 3 T Prisma scanner using a T1-weighted 3D volumetric interpolated breath-hold examination (VIBE) sequence (Supp Table 1). We focused our analysis on three brain regions that are relevant to normal aging and pathological aging when it comes to vascular disease, namely the prefrontal subcortical watershed white matter (WM), globus pallidus (GP), and hippocampus (HC) (Fig. 1a). The SPGR pulse sequence uses a time repetition (TR) of 8 ms, time echo (TE) of 2 ms, flip angle (FA) of 15°, 64 frames, and 12 slices [44], and the VIBE sequence uses TR = 5 ms, TE = 2 ms, FA = 15°, 64 frames, and 14 slices [45].
Fig. 1.
T1 mapping and dynamic contrast-enhanced (DCE) MRI: differences across scanners and importance of the arterial input function. a Representative unenhanced coronal T1 map computed from a fast spoiled gradient echo (SPGR) variable flip angle scan before administration of a gadolinium-based contrast agent (GBCA). The yellow circles represent the main regions-of-interest (ROIs) for both hemispheres: subcortical white matter (WM), globi pallidi (GP), and hippocampi (HC). b T1 map atlas made by averaging 30 volumetric interpolated breath-hold examination (VIBE)- and 32 SPGR-derived T1 maps, followed by a division VIBE/SPGR atlas. Pixels > 1 (red) represent T1 values VIBE > SPGR and pixels < 1 (blue) represent T1 values VIBE < SPGR. Pixels = 1 (gray) means no difference between T1 VIBE and SPGR. c Quantification of T1 values in WM, GP, and HC from subject scanned with SPGR (blue) and VIBE (red). Box plots indicate median values, boxes indicate interquartile range (IQR), and whiskers indicate minimum and maximum values; 30 SPGR and 32 VIBE subjects; unpaired two-tailed Student’s t-tests were used. d Left panels represent a DCE maximum intensity projection image prior GBCA administration (top) and after (bottom). The middle panels represent a “good” arterial input function (AIF) ROI (green) and a “bad” AIF ROI (orange) taken from the common carotid artery with the associated AIF dynamics underneath. The right panel shows the “good” (green) and “bad” (orange) AIF curves superimposed and the associated BBB Ktrans outputs for WM, GP, and HC. e, f Quantification of BBB Ktrans values in SPGR (e, blue) and VIBE (f, red) subjects in WM, GP, and HC regions. The horizontal lines represent the medians, and error bars are IQR; 30 SPGR and 32 VIBE subjects. See associated Supplementary Fig. 1
Although no change in T1 values were noticed in WM when comparing VIBE- to SPGR-derived T1 mapping (Fig. 1b, c), significant 28% and 31% increases were found in GP and HC, respectively (Fig. 1b, c). Overall, there is a substantial difference between SPGR- and VIBE-derived whole brain T1 mapping with the most drastic changes in deeper brain regions including basal ganglia, hippocampal and parahippocampal subregions, and the pons and medulla (Fig. 1b). The median whole brain T1 fluctuates around 1200 ms for SPGR datasets as opposed to 1600 ms for VIBE datasets (Supp Fig. 1a−d). As abnormally high T1 values may lead to severe underestimation of BBB Ktrans, we then examined and processed the SPGR and VIBE dynamic scans to compute BBB Ktrans maps (Supp Fig. 1e,f). As suspected, VIBE-derived BBB Ktrans values were substantially lower as discussed below.
Vascular input function and DCE data processing
The measurement of the vascular input function is a critical step that can have a large effect on the final Ktrans values, as previously shown [69, 80]. Briefly, it can be challenging to make accurate measurements for a variety of reasons, including motion artifacts of the head or in flowing blood, the small size of vessels causing partial volume errors, inflow of fresh blood that has seen few radiofrequency pulses and has not reached steady state, and insufficient temporal resolution to accurately measure the first pass of the contrast bolus. While each of these can be a source of considerable error, most can be minimized by careful region selection.
In our studied cases of coronal DCE, choosing a large neck vessel with a sharp bolus peak during the first pass is not sufficient to obtain a good vascular input function. Indeed, we showed that a slight difference in the region used for the arterial input function (AIF) from the same common carotid artery can considerably decrease the final BBB Ktrans values by up to tenfold (Fig. 1d) due to the inclusion of partial volume edge voxels. Therefore, to avoid potential confounding factors, we recommend:
To select a large vessel which presents a large signal intensity change upon gadolinium injection and is returning towards the baseline quickly
To draw the region-of-interest (ROI) in the center of the vessel
To avoid the vessel edges in order to limit partial volume effects
To ensure the vessel is centered in the slice and larger than the slice thickness to limit partial volume effect in the slice direction
To check the whole dynamic series and make sure that the ROI does not land outside the vessel due to motion which will help to limit partial volume effects; and
To avoid selecting a vessel that is near the edge of the excitation volume as the inflow of fresh blood will not have reached a steady state
Using optimal AIF criteria followed by Patlak modelling, we found that SPGR-derived BBB Ktrans values (Fig. 1e) are on average higher than VIBE-derived BBB Ktrans values (Fig. 1f). Furthermore, we noticed that a significant number of VIBE participants had very low BBB Ktrans values close to null (Fig. 1f). This poses an extra challenge for multicenter studies and makes further data harmonization strategies useful as discussed in the next section.
In our examples here, two different GBCA were used for VIBE-derived and GE-derived Ktrans values on different scanners with different relaxivity properties (Supp Table 1). Of note, differences in contrast agents could also be a possible source of variation in Ktrans values, especially since some contrast agents are known to have different partial binding to albumin in the blood [81]. However, our published data on a different cohort of participants scanned with a VIBE sequence and gadobenate dimeglumine [82] observed a similar pattern of negative Ktrans values on a limited number of subjects and similar median Ktrans (0.5 × 10−3/min) values as obtained with the VIBE sequence with gadoterate meglumine in the present cohort (0.4 × 10−3/min; Fig. 2c). That the median Ktrans values with VIBE and gadobenate dimeglumine are nearly identical to VIBE and gadobenate meglumine suggests that choice of GBCA, in this case gadobenate dimeglumine (SPGR-derived Ktrans) or gadoterate meglumine (VIBE-derived Ktrans), likely did not have a major influence on the Ktrans values computed in the present study.
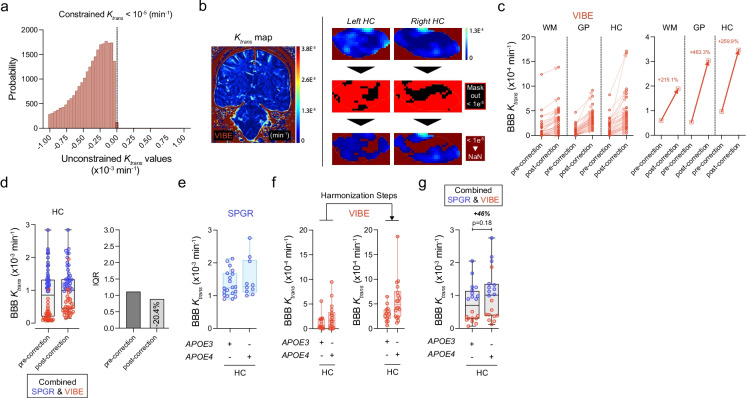
Fig. 2.
Combining VIBE- and SPGR-derived DCE datasets: possible post-processing step towards harmonization. a Histogram of voxels to be removed from analysis due to having a constrained Patlak value Ktrans < 10−5. The histogram shows Ktrans values calculated with an unconstrained Patlak fitting that allows negative Ktrans. The vast majority (approximately 99.55%) of these voxels are artifacts having physiologically impossible negative Ktrans values. b Representative whole brain VIBE-derived BBB Ktrans map (left), and hippocampi BBB Ktrans maps before (top right) and after (bottom right) masking out and eliminating negative Ktrans values < 1e−5 min−1. c Quantification of BBB Ktrans values before and after eliminating artifacts with negative Ktrans values (< 1e−5 min−1) in WM, GP, and HC regions. The graph on the left side represents the 32 individual VIBE datasets prior to- and post-correction for each ROI, and the graph on the right side represents the median values prior to- and post-correction for each ROI. d Combined SPGR- (blue) and VIBE (red)-derived BBB Ktrans values pre- and post-correction (i.e., elimination of negative voxel artifacts < 1e−5 min−1 in VIBE datasets) for HC. Box plots indicate median values, boxes indicate interquartile range (IQR), and whiskers indicate minimum and maximum values; 30 SPGR and 32 VIBE subjects. The second graphs represent the associated IQR pre- (dark gray) and post- (light gray) correction for HC. e SPGR-derived BBB Ktrans values (blue) for HC in apolipoprotein E ε4 carriers (APOE4) and non-carrier (APOE3). f VIBE-derived BBB Ktrans values (red) for HC in APOE4 carriers and non-carriers, before and after harmonization steps (i.e., “good” AIF ROI and elimination of voxels < 1e−5 min−1). g Combined SPGR- (blue; 10 APOE3 and 10 APOE4) and VIBE (red; 10 APOE3 and 10 APOE4)-derived BBB Ktrans values post-correction (i.e., elimination of negative artifact voxels < 1e−5 min−1 in VIBE datasets) for HC in APOE4 carriers and non-carriers. Box plot indicates median values, boxes IQR, and whiskers indicate minimum and maximum values; 30 SPGR (blue; 20 APOE3, 10 APOE4) and 32 VIBE (red; 14 APOE3, 18 APOE4) subjects. In panels c and f, unpaired two-tailed Student’s t-tests were used
In axial imaging, which might be preferred when white matter areas are the main tissue of interest, it is preferable to place the ROI to sample VIF in the posterior end of the sagittal sinus for a couple of reasons [69]. First, there is much less problem due to head motion since the posterior sagittal sinus is close to the pivot point in the occiput; therefore, the VIF ROI is less likely to move out of the sinus if the patient does move than does an ROI placed on the internal carotid artery (ICA) or middle carotid artery (MCA) which are much further from the fulcrum at the back of the head. Second, the sagittal sinus is generally larger than either ICA or MCA making it easier to place the ROI within the vessel lumen.
Negative Ktrans
Ktrans is very sensitive to several parameters including signal drift, the AIF utilized, and T1 values of the blood and tissue. Some of the Ktrans maps (more often in the VIBE-derived dataset) contain voxel clusters with Ktrans = 10−7/min. These voxels were confirmed to converge on negative Ktrans values with unconstrained Patlak fitting and represent physiologically impossible values. Various techniques may be used to correct for these artifacts. Alternatively, no correction could be applied. In some datasets, these voxels may just represent statistical noise when the true Ktrans is near zero. If this were the case, the negative Ktrans values should be distributed based on biological factors, e.g., more often in younger subjects, or more often in the white matter. In the present dataset, the cases were not clustered based on biological factors, but on scanner and sequence type. This suggests that the negative Ktrans values are more likely due to a technical issue with the acquisition; thus, a correction of some sort is appropriate.
If the source of error is known, such as scanner drift, a specific correction factor can be applied. Scanner drift is known to occur and have a significant effect on measured Ktrans values [83]. If a large source of constant signal is available, this can be used to correct for scanner drift on individual scans, as we and others reported [84, 85]. This correction is generally more useful in animal scans where it is easier to include a large water reference phantom [84]. The tight fitting of most human head coils limits the options for including a large enough water tube as a source of constant signal. Errors in the measurement of the constant signal can easily induce more error so this approach may not be suitable for all experiments and/or studies.
Alternatively, if the source of error is unknown, or it is not possible to perform accurate drift correction, a more general statistical correction can be used. Previous work has done this by defining a lower noise threshold and excluding values below it [86, 87] and/or explicitly modelling negative values as part of an assumed noise distribution [55, 58, 63]. Both approaches have been successfully used and do not require extra data acquisition, can be applied to existing datasets, and can correct for various technical errors.
Based on the present example, we recommend a more general correction approach of masking out voxels that converge on negative Ktrans values. This is a slightly more conservative approach than previous statistical corrections, as only voxels with known errors are modified. As illustrated in the histogram in Fig. 2a, a threshold value of 10−5/min was selected to efficiently eliminate the negative Ktrans values. Namely, at the level of 10−5/min, > 99% of Ktrans values were negative when applying an unconstrained Patlak fitting (Fig. 2a), whereas at the level of 10−6/min and 10−7/min, all Ktrans values were negative (data not shown). There are many different factors that may influence Ktrans calculations, but identifying the main cause of these negative values is presently unknown and beyond the scope of this paper. However, poorly calibrated RF pulses on some patients may play a role and could also explain the differences seen in the T1 maps. Signal intensity drift of the MRI scanner that was previously shown to have similar effects could also play a role [74].
By eliminating these negative clusters (Fig. 2b), the average BBB Ktrans values were significantly increased in WM, GP, and HC by 215%, 463%, and 260%, respectively (Fig. 2c). This correction step may be important to consider for harmonization purposes across different DCE sequences and different scanners. The desired endpoint is reduction of interquartile range across the sample of subjects used in the study. The current sample was selected to be as similar as possible, with minimal differences in MRI protocol, subject age, and health. By applying the proposed correction to a combined SPGR and VIBE DCE datasets, for example, in the HC, we were able to achieve a 20% reduction of the interquartile range (Fig. 2d). In general, what variance across sites will be tolerable for different types of studies will depend on the details of the study being performed. However, we suggest that the correction step outlined here will provide significant improvement for most multicenter studies which are necessary to recruit a sufficient number of participants and sample a wider variety of subjects.
Although not powered enough for subgroup analysis, there is a trend towards greater BBB Ktrans values in the HC in apolipoprotein E ε4 carriers (APOE4) when compared to non-carriers (APOE3 ε3 homozygotes) in both SPGR (Fig. 2e) and VIBE (Fig. 2f) datasets, as previously reported [45]. Discarding the hippocampal negative voxels from VIBE-derived Ktrans maps helped to increase the Ktrans values (Fig. 2f), which in turn permitted to combine SPGR and VIBE DCE datasets (Fig. 2g). The analysis of combined datasets indicated a 46% increase in hippocampal BBB permeability in APOE4 carriers compared to non-carriers (Fig. 2g), which was within the range of what has been recently reported by different groups [45, 47]. When we conducted power analyses for a two-sided, independent sample t-tests in R based on our prior studies [45], the results indicated that n = 55 per group (total N = 110) would be needed to detect a significant difference between APOE4 carriers and APOE3 homozygotes at 80% power with alpha set at 0.05. However, the purpose of the present analysis was not to repeat or confirm the results of the prior work at the level p < 0.05 [45, 47], but to show that the datasets between different scanners can be more successfully harmonized with a reduced interquartile range and used for statistical analysis after carefully defining the AIF curves as per recommendations above and eliminating negative non-physiological Ktrans value artifacts.
In addition, careful set up of protocols and evaluation of scanners during the study are important to identify scanner drift and other performance features. Since studies may take several years to acquire the necessary sample of participants, regular phantom and human volunteer scanning are invaluable for monitoring for any drift in scanner performance across the study as well as quantifying the drift magnitude within individual scanning sessions. Scanning of phantoms to assess geometry and T1 values should not only be part of routine quality assurance but can also provide important data to facilitate correction of drift. Secondly, scanning of human volunteers with the DCE sequence (but without the contrast injection) provides data enabling assessment of drift across the study.
Despite every effort to match the studied groups in the current analysis, there remain still a few differences which represent potential shortcomings. As noted above, a different contrast agent was used and there are slightly more APOE4 participants in the VIBE group compared to SPGR. The differences in APOE status have a much smaller effect than the noted differences between sequences, and with the present groups would tend to decrease the median Ktrans in the SPGR group, which is the opposite of what is observed. As discussed above, while we believe the contrast agent has minimal to no effect on Ktrans, any effect would similarly be to decrease Ktrans in the SPGR group. Since both of these potential differences are acting in the opposite direction of the observed differences, we do not believe they confound the current analysis. However, future studies to directly validate the role of contrast agents and the effects of different number of participants at different sites may be of value.
Conclusions
Recent studies from different groups have suggested that subtle BBB leaks in the aging human may importantly contribute to cognitive impairment, MCI, AD-type dementia, and/or vascular dementia. The BBB leaks quantified by the unidirectional BBB GBCA tracer’s constant Ktrans maps in those neurological conditions are typically orders of magnitude lower than in brain tumors, after stroke and/or during relapse of multiple sclerosis. To be accurately detected, a large number of participants may be scanned at different MR scanners and at different sites. This in turn mandates development of approaches such as how to normalize and/or better harmonize DCE datasets between different scanners, how to minimize artifacts in DCE data processing caused by inadequately defined tracer’s vascular input function, and how to eliminate artifacts caused physiologically impossible negative Ktrans values. We hope that the recommendations provided in this perspective will help new investigators wishing to study the role of BBB damage in cognitive impairment during normal aging, MCI, and in different types of dementias including AD and vascular dementia.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary Fig. 1. Representative SPGR- and VIBE-derived T1 mapping and dynamic contrast-enhanced MRI datasets. (a) Representative unenhanced coronal fast spoiled gradient echo (SPGR) T1 variable flip angle (2°, 5°, and 10°) and its associated T1 map before administration of a gadolinium-based contrast agent (GBCA). (b) Histogram illustrating the distribution of the T1 values within the whole brain (blue line) of a SPGR subject. (c) Representative unenhanced coronal volumetric interpolated breath-hold examination (VIBE) T1 variable flip angle (2°, 5°, 10°, 12°, and 15°) and its associated T1 map before administration of a GBCA. (d) Histogram illustrating the distribution of the T1 values within the whole brain (red line) of a VIBE subject. (e) Representative 64-frame SPGR DCE (yellow dot represents the GBCA injection time), maximum intensity projection (MIP) of the 64 frames, and its associated BBB Ktrans map. (f) Representative 64-frame VIBE DCE (yellow dot represents the GBCA injection time), maximum intensity projection (MIP) of the 64 frames, and its associated BBB Ktrans map. (a-d) Linked to main Fig. 1a-c; (e,f) Linked to main Fig. 1d-f.
Supplementary file1 (PDF 1594 KB)
Acknowledgements
We thank Drs. Joanna Wardlaw and Michael Thrippleton for their most helpful comments and discussion.
Author contribution
A.M., S.R.B., and B.V.Z. performed literature search and designed the study. A.M. and S.R.B. performed MRI data analyses and interpreted the data. D.A.N. helped with statistical analyses. A.W.T. provided critical reading and additional information of the manuscript. K.K. helped with manuscript writing. A.M. and S.R.B. contributed to manuscript writing, and B.V.Z. supervised all data analysis and interpretation and wrote the manuscript.
Funding
The work of B.V.Z. is supported by the National Institutes of Health (grant nos. R01AG023084, R01NS090904, R01NS034467, R01AG039452, 1R01NS100459, 5P01AG052350, and P30AG06653), in addition to the Alzheimer’s Association (strategic 509279 grant) and the Foundation Leducq Transatlantic Network of Excellence for the Study of Perivascular Spaces in Small Vessel Disease (reference no. 16 CVD 05). The work of A.M. is supported by the UK Dementia Research Institute (MRC, Alzheimer’s Society, ARUK) and the UKRI Medical Research Council (Career Development Award MR/V032488/1). The work of D.A.N. is supported by the National Institutes of Health (grant nos. P01AG052350, R01AG064228, R01AG060049, P30AG066519) and Alzheimer’s Association (grant AARG-17–532905). The work of A.W.T. is supported by the National Institutes of Health (grant nos. P01AG052350, P41EB015922 and P30AG066530).
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Axel Montagne, Email: axel.montagne@ed.ac.uk.
Samuel R. Barnes, Email: sabarnes@llu.edu
Berislav V. Zlokovic, Email: zlokovic@usc.edu
References
- 1.Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke J Cereb Circ. 2011;42(9):2672–2713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vickrey BG, Brott TG, Koroshetz WJ, Stroke Research Priorities Meeting Steering Committee and the National Advisory Neurological Disorders and Stroke Council, National Institute of Neurological Disorders and Stroke Research priority setting: a summary of the 2012 NINDS Stroke Planning Meeting Report. Stroke. 2013;44(8):2338–42. [DOI] [PubMed]
- 3.Iadecola C. The neurovascular unit coming of age: a journey through neurovascular coupling in health and disease. Neuron. 2017;96(1):17–42. doi: 10.1016/j.neuron.2017.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Montine TJ, Koroshetz WJ, Babcock D, Dickson DW, Galpern WR, Glymour MM, et al. Recommendations of the Alzheimer’s disease-related dementias conference. Neurology. 2014;83(9):851–860. doi: 10.1212/WNL.0000000000000733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Snyder HM, Corriveau RA, Craft S, Faber JE, Greenberg SM, Knopman D, et al. Vascular contributions to cognitive impairment and dementia including Alzheimer’s disease. Alzheimers Dement J Alzheimers Assoc. 2015;11(6):710–717. doi: 10.1016/j.jalz.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12(8):822–838. doi: 10.1016/S1474-4422(13)70124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kapasi A, DeCarli C, Schneider JA. Impact of multiple pathologies on the threshold for clinically overt dementia. Acta Neuropathol (Berl) 2017;134(2):171–186. doi: 10.1007/s00401-017-1717-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iturria-Medina Y, Sotero RC, Toussaint PJ, Mateos-Pérez JM, Evans AC. Alzheimer’s Disease Neuroimaging Initiative. Early role of vascular dysregulation on late-onset Alzheimer’s disease based on multifactorial data-driven analysis. Nat Commun. 2016;7:11934. doi: 10.1038/ncomms11934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kisler K, Nelson AR, Montagne A, Zlokovic BV. Cerebral blood flow regulation and neurovascular dysfunction in Alzheimer disease. Nat Rev Neurosci. 2017;18(7):419–434. doi: 10.1038/nrn.2017.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sweeney MD, Sagare AP, Zlokovic BV. Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat Rev Neurol. 2018;14(3):133–150. doi: 10.1038/nrneurol.2017.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sweeney MD, Kisler K, Montagne A, Toga AW, Zlokovic BV. The role of brain vasculature in neurodegenerative disorders. Nat Neurosci. 2018;21(10):1318–1331. doi: 10.1038/s41593-018-0234-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sweeney MD, Zhao Z, Montagne A, Nelson AR, Zlokovic BV. Blood-brain barrier: from physiology to disease and back. Physiol Rev. 2019;99(1):21–78. doi: 10.1152/physrev.00050.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wardlaw JM, Smith C, Dichgans M. Small vessel disease: mechanisms and clinical implications. Lancet Neurol. 2019. 10.1016/S1474-4422(19)30079-1 [DOI] [PubMed]
- 14.Cortes-Canteli M, Iadecola C. Alzheimer’s disease and vascular aging: JACC Focus Seminar. J Am Coll Cardiol. 2020;75(8):942–951. doi: 10.1016/j.jacc.2019.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barisano G, Montagne A, Kisler K, Schneider JA, Wardlaw JM, Zlokovic BV. Blood–brain barrier link to human cognitive impairment and Alzheimer’s disease. Nat Cardiovasc Res. 2022;1(2):108–115. doi: 10.1038/s44161-021-00014-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaufer D, Friedman A. Damage to a protective shield around the brain may lead to Alzheimer’s and other diseases. Sci Am. 2021;43–7.
- 17.Guo S, Lo EH. Dysfunctional cell-cell signaling in the neurovascular unit as a paradigm for central nervous system disease. Stroke. 2009;40(3 Suppl):S4–7. doi: 10.1161/STROKEAHA.108.534388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iadecola C. The pathobiology of vascular dementia. Neuron. 2013;80(4):844–866. doi: 10.1016/j.neuron.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao Z, Nelson AR, Betsholtz C, Zlokovic BV. Establishment and dysfunction of the blood-brain barrier. Cell. 2015;163(5):1064–1078. doi: 10.1016/j.cell.2015.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lochhead JJ, Yang J, Ronaldson PT, Davis TP. Structure, function, and regulation of the blood-brain barrier tight junction in central nervous system disorders. Front Physiol. 2020;11:914. doi: 10.3389/fphys.2020.00914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Banks WA, Reed MJ, Logsdon AF, Rhea EM, Erickson MA. Healthy aging and the blood–brain barrier. Nat Aging. 2021;1(3):243–254. doi: 10.1038/s43587-021-00043-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bailey EL, Wardlaw JM, Graham D, Dominiczak AF, Sudlow CLM, Smith C. Cerebral small vessel endothelial structural changes predate hypertension in stroke-prone spontaneously hypertensive rats: a blinded, controlled immunohistochemical study of 5- to 21-week-old rats: vascular changes in SHRSP rats. Neuropathol Appl Neurobiol. 2011;37(7):711–726. doi: 10.1111/j.1365-2990.2011.01170.x. [DOI] [PubMed] [Google Scholar]
- 23.Vanlandewijck M, He L, Mäe MA, Andrae J, Ando K, Del Gaudio F, et al. A molecular atlas of cell types and zonation in the brain vasculature. Nature. 2018;554(7693):475–480. doi: 10.1038/nature25739. [DOI] [PubMed] [Google Scholar]
- 24.Kalucka J, de Rooij LPMH, Goveia J, Rohlenova K, Dumas SJ, Meta E, et al. Single-cell transcriptome atlas of murine endothelial cells. Cell. 2020;180(4):764–779.e20. doi: 10.1016/j.cell.2020.01.015. [DOI] [PubMed] [Google Scholar]
- 25.Winkler EA, Kim CN, Ross JM, Garcia JH, Gil E, Oh I, et al. A single-cell atlas of the normal and malformed human brain vasculature. Science. 2022;eabi7377. [DOI] [PMC free article] [PubMed]
- 26.Yang AC, Vest RT, Kern F, Lee DP, Agam M, Maat CA, et al. A human brain vascular atlas reveals diverse mediators of Alzheimer’s risk. Nature. 2022. 10.1038/s41586-021-04369-3 [DOI] [PMC free article] [PubMed]
- 27.Armulik A, Genové G, Mäe M, Nisancioglu MH, Wallgard E, Niaudet C, et al. Pericytes regulate the blood-brain barrier. Nature. 2010;468(7323):557–561. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- 28.Daneman R, Zhou L, Kebede AA, Barres BA. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature. 2010;468(7323):562–566. doi: 10.1038/nature09513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bell RD, Winkler EA, Sagare AP, Singh I, LaRue B, Deane R, et al. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron. 2010;68(3):409–427. doi: 10.1016/j.neuron.2010.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nikolakopoulou AM, Montagne A, Kisler K, Dai Z, Wang Y, Huuskonen MT, et al. Pericyte loss leads to circulatory failure and pleiotrophin depletion causing neuron loss. Nat Neurosci. 2019;22(7):1089–1098. doi: 10.1038/s41593-019-0434-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ben-Zvi A, Lacoste B, Kur E, Andreone BJ, Mayshar Y, Yan H, et al. Mfsd2a is critical for the formation and function of the blood-brain barrier. Nature. 2014;509(7501):507–511. doi: 10.1038/nature13324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ayloo S, Lazo CG, Sun S, Zhang W, Cui B, Gu C. Pericyte-to-endothelial cell signaling via vitronectin-integrin regulates blood-CNS barrier. 2021. 10.1101/2021.04.22.441019 [DOI] [PMC free article] [PubMed]
- 33.Montagne A, Zhao Z, Zlokovic BV. Alzheimer’s disease: a matter of blood-brain barrier dysfunction? J Exp Med. 2017;214(11):3151–3169. doi: 10.1084/jem.20171406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Winkler EA, Nishida Y, Sagare AP, Rege SV, Bell RD, Perlmutter D, et al. GLUT1 reductions exacerbate Alzheimer’s disease vasculo-neuronal dysfunction and degeneration. Nat Neurosci. 2015;18(4):521–530. doi: 10.1038/nn.3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nguyen LN, Ma D, Shui G, Wong P, Cazenave-Gassiot A, Zhang X, et al. Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid. Nature. 2014;509(7501):503–506. doi: 10.1038/nature13241. [DOI] [PubMed] [Google Scholar]
- 36.Alakbarzade V, Hameed A, Quek DQY, Chioza BA, Baple EL, Cazenave-Gassiot A, et al. A partially inactivating mutation in the sodium-dependent lysophosphatidylcholine transporter MFSD2A causes a non-lethal microcephaly syndrome. Nat Genet. 2015;47(7):814–817. doi: 10.1038/ng.3313. [DOI] [PubMed] [Google Scholar]
- 37.Guemez-Gamboa A, Nguyen LN, Yang H, Zaki MS, Kara M, Ben-Omran T, et al. Inactivating mutations in MFSD2A, required for omega-3 fatty acid transport in brain, cause a lethal microcephaly syndrome. Nat Genet. 2015;47(7):809–813. doi: 10.1038/ng.3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Caserta MT, Caccioppo D, Lapin GD, Ragin A, Groothuis DR. Blood–brain barrier integrity in Alzheimer’s disease patients and elderly control subjects. J Neuropsychiatry Clin Neurosci. 1998;10(1):78–84. doi: 10.1176/jnp.10.1.78. [DOI] [PubMed] [Google Scholar]
- 39.Dysken MW, Nelson MJ, Hoover KM, Kuskowski M, McGeachie R. Rapid dynamic CT scanning in primary degenerative dementia and age-matched controls. Biol Psychiatry. 1990;28(5):425–434. doi: 10.1016/0006-3223(90)90410-4. [DOI] [PubMed] [Google Scholar]
- 40.Schlageter NL, Carson RE, Rapoport SI. Examination of blood–brain barrier permeability in dementia of the Alzheimer type with [68Ga]EDTA and positron emission tomography. J Cereb Blood Flow Metab. 1987;7(1):1–8. doi: 10.1038/jcbfm.1987.1. [DOI] [PubMed] [Google Scholar]
- 41.Wang H, Golob EJ, Su MY. Vascular volume and blood-brain barrier permeability measured by dynamic contrast enhanced MRI in hippocampus and cerebellum of patients with MCI and normal controls. J Magn Reson Imaging. 2006;24(3):695–700. doi: 10.1002/jmri.20669. [DOI] [PubMed] [Google Scholar]
- 42.Starr JM, Farrall AJ, Armitage P, McGurn B, Wardlaw J. Blood-brain barrier permeability in Alzheimer’s disease: a case-control MRI study. Psychiatry Res - Neuroimaging. 2009;171(3):232–241. doi: 10.1016/j.pscychresns.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 43.Ha IH, Lim C, Kim Y, Moon Y, Han S-H, Moon W-J. Regional differences in blood-brain barrier permeability in cognitively normal elderly subjects: a dynamic contrast-enhanced MRI-based study. Korean J Radiol. 2021;22(7):1152. doi: 10.3348/kjr.2020.0816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Montagne A, Barnes SR, Sweeney MD, Halliday MR, Sagare AP, Zhao Z, et al. Blood-brain barrier breakdown in the aging human hippocampus. Neuron. 2015;85(2):296–302. doi: 10.1016/j.neuron.2014.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Montagne A, Nation DA, Sagare AP, Barisano G, Sweeney MD, Chakhoyan A, et al. APOE4 leads to blood–brain barrier dysfunction predicting cognitive decline. Nature. 2020;581(7806):71–76. doi: 10.1038/s41586-020-2247-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Montagne A, Huuskonen MT, Rajagopal G, Sweeney MD, Nation DA, Sepehrband F, et al. Undetectable gadolinium brain retention in individuals with an age-dependent blood-brain barrier breakdown in the hippocampus and mild cognitive impairment. Alzheimers Dement. 2019;15(12):1568–1575. doi: 10.1016/j.jalz.2019.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moon W-J, Lim C, Ha IH, Kim Y, Moon Y, Kim H-J, et al. Hippocampal blood–brain barrier permeability is related to the APOE4 mutation status of elderly individuals without dementia. J Cereb Blood Flow Metab. 2021;41(6):1351–1361. doi: 10.1177/0271678X20952012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Verheggen ICM, de Jong JJA, van Boxtel MPJ, Gronenschild EHBM, Palm WM, Postma AA, et al. Increase in blood–brain barrier leakage in healthy, older adults. GeroScience. 2020;42(4):1183–1193. doi: 10.1007/s11357-020-00211-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Verheggen ICM, de Jong JJA, van Boxtel MPJ, Postma AA, Jansen JFA, Verhey FRJ, et al. Imaging the role of blood–brain barrier disruption in normal cognitive ageing. GeroScience. 2020. 10.1007/s11357-020-00282-1 [DOI] [PMC free article] [PubMed]
- 50.Li Y, Li M, Yang L, Qin W, Yang S, Yuan J, et al. The relationship between blood–brain barrier permeability and enlarged perivascular spaces: a cross-sectional study. Clin Interv Aging. 2019;14:871–878. doi: 10.2147/CIA.S204269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nation DA, Sweeney MD, Montagne A, Sagare AP, D’Orazio LM, Pachicano M, et al. Blood-brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat Med. 2019;25(2):270–276. doi: 10.1038/s41591-018-0297-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Freeze WM, Jacobs HIL, de Jong JJ, Verheggen ICM, Gronenschild EHBM, Palm WM, et al. White matter hyperintensities mediate the association between blood-brain barrier leakage and information processing speed. Neurobiol Aging. 2020;85:113–122. doi: 10.1016/j.neurobiolaging.2019.09.017. [DOI] [PubMed] [Google Scholar]
- 53.Li M, Li Y, Zuo L, Hu W, Jiang T. Increase of blood-brain barrier leakage is related to cognitive decline in vascular mild cognitive impairment. BMC Neurol. 2021;21(1):159. doi: 10.1186/s12883-021-02189-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Milikovsky DZ, Ofer J, Senatorov VV, Friedman AR, Prager O, Sheintuch L, et al. Paroxysmal slow cortical activity in Alzheimer’s disease and epilepsy is associated with blood-brain barrier dysfunction. Sci Transl Med. 2019;11(521):8954. doi: 10.1126/scitranslmed.aaw8954. [DOI] [PubMed] [Google Scholar]
- 55.Van De Haar HJ, Burgmans S, Jansen JFA, Van Osch MJP, Van Buchem MA, Muller M, et al. Blood-brain barrier leakage in patients with early Alzheimer disease. Radiology. 2016;281(2):527–535. doi: 10.1148/radiol.2016152244. [DOI] [PubMed] [Google Scholar]
- 56.Van de Haar HJ, Jansen JFA, van Osch MJP, van Buchem MA, Muller M, Wong SM, et al. Neurovascular unit impairment in early Alzheimer’s disease measured with magnetic resonance imaging. Neurobiol Aging. 2016;45:190–196. doi: 10.1016/j.neurobiolaging.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 57.Van De Haar HJ, Jansen JFA, Jeukens CRLPN, Burgmans S, Van Buchem MA, Muller M, et al. Subtle blood‐brain barrier leakage rate and spatial extent: considerations for dynamic contrast‐enhanced MRI. Med Phys. 2017;44(8):4112–25. [DOI] [PubMed]
- 58.Kerkhofs D, Wong SM, Zhang E, Uiterwijk R, Hoff EI, Jansen JFA, et al. Blood–brain barrier leakage at baseline and cognitive decline in cerebral small vessel disease: a 2-year follow-up study. GeroScience. 2021. 10.1007/s11357-021-00399-x [DOI] [PMC free article] [PubMed]
- 59.Shao X, Jann K, Ma SJ, Yan L, Montagne A, Ringman JM, et al. Comparison between blood-brain barrier water exchange rate and permeability to gadolinium-based contrast agent in an elderly cohort. Front Neurosci. 2020;14:571480. doi: 10.3389/fnins.2020.571480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li Y, Li M, Zhang X, Shi Q, Yang S, Fan H, et al. Higher blood–brain barrier permeability is associated with higher white matter hyperintensities burden. J Neurol. 2017;264(7):1474–1481. doi: 10.1007/s00415-017-8550-8. [DOI] [PubMed] [Google Scholar]
- 61.Li Y, Li M, Zuo L, Shi Q, Qin W, Yang L, et al. Compromised blood-brain barrier integrity is associated with total magnetic resonance imaging burden of cerebral small vessel disease. Front Neurol. 2018;9(APR). 10.3389/fneur.2018.00221 [DOI] [PMC free article] [PubMed]
- 62.Uchida Y, Kan H, Sakurai K, Arai N, Inui S, Kobayashi S, et al. Iron leakage owing to blood–brain barrier disruption in small vessel disease CADASIL. Neurology. 2020;95(9):e1188–e1198. doi: 10.1212/WNL.0000000000010148. [DOI] [PubMed] [Google Scholar]
- 63.Wong SM, Jansen JFA, Zhang CE, Hoff EI, Staals J, van Oostenbrugge RJ, et al. Blood-brain barrier impairment and hypoperfusion are linked in cerebral small vessel disease. Neurology. 2019;92(15):e1669–e1677. doi: 10.1212/WNL.0000000000007263. [DOI] [PubMed] [Google Scholar]
- 64.Zhang CE, Wong SM, Van De Haar HJ, Staals J, Jansen JFA, Jeukens CRLPN, et al. Blood–brain barrier leakage is more widespread in patients with cerebral small vessel disease. Neurology. 2017;88(5):426–32. [DOI] [PubMed]
- 65.Zhang CE, Wong SM, Uiterwijk R, Backes WH, Jansen JFA, Jeukens CRLPN, et al. Blood–brain barrier leakage in relation to white matter hyperintensity volume and cognition in small vessel disease and normal aging. Brain Imaging Behav. 2019;13(2):389. [DOI] [PMC free article] [PubMed]
- 66.Wardlaw JM, Makin SJ, Valdés Hernández MC, Armitage PA, Heye AK, Chappell FM, et al. Blood-brain barrier failure as a core mechanism in cerebral small vessel disease and dementia: evidence from a cohort study. Alzheimers Dement. 2017;13(6):634–643. [Google Scholar]
- 67.Rosenberg GA, Prestopnik J, Adair JC, Huisa BN, Knoefel J, Caprihan A, et al. Validation of biomarkers in subcortical ischaemic vascular disease of the Binswanger type: approach to targeted treatment trials. J Neurol Neurosurg Psychiatry. 2015;86(12):1324–1330. doi: 10.1136/jnnp-2014-309421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Huisa BN, Caprihan A, Thompson J, Prestopnik J, Qualls CR, Rosenberg GA. Long-term blood-brain barrier permeability changes in Binswanger disease. Stroke. 2015;46(9):2413–2418. doi: 10.1161/STROKEAHA.115.009589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Heye AK, Thrippleton MJ, Armitage PA, Valdés Hernández M del C, Makin SD, Glatz A, et al. Tracer kinetic modelling for DCE-MRI quantification of subtle blood-brain barrier permeability. NeuroImage. 2016;125:446–55. [DOI] [PMC free article] [PubMed]
- 70.Al-Bachari S, Naish JH, Parker GJM, Emsley HCA, Parkes LM. Blood–brain barrier leakage is increased in Parkinson’s disease. Front Physiol. 2020;11. 10.3389/fphys.2020.593026 [DOI] [PMC free article] [PubMed]
- 71.Drouin-Ouellet J, Sawiak SJ, Cisbani G, Lagacé M, Kuan W-L, Saint-Pierre M, et al. Cerebrovascular and blood-brain barrier impairments in Huntington’s disease: potential implications for its pathophysiology. Ann Neurol. 2015;78(2):160–177. doi: 10.1002/ana.24406. [DOI] [PubMed] [Google Scholar]
- 72.Senatorov VV, Friedman AR, Milikovsky DZ, Ofer J, Saar-Ashkenazy R, Charbash A, et al. Blood-brain barrier dysfunction in aging induces hyperactivation of TGFβ signaling and chronic yet reversible neural dysfunction. Sci Transl Med. 2019;11(521):eaaw8283. doi: 10.1126/scitranslmed.aaw8283. [DOI] [PubMed] [Google Scholar]
- 73.Patlak CS, Blasberg RG. Graphical evaluation of blood to brain barrier transfer constants from multiple time uptake data. Generalizations J Cereb Blood Flow Metab. 1985;5(4):584–590. doi: 10.1038/jcbfm.1985.87. [DOI] [PubMed] [Google Scholar]
- 74.Barnes SR, Ng TSC, Montagne A, Law M, Zlokovic BV, Jacobs RE. Optimal acquisition and modeling parameters for accurate assessment of low K-trans blood-brain barrier permeability using dynamic contrast-enhanced MRI. Magn Reson Med. 2016;75(5):1967–1977. doi: 10.1002/mrm.25793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sweeney MD, Sagare AP, Pachicano M, Harrington MG, Joe E, Chui HC, et al. A novel sensitive assay for detection of a biomarker of pericyte injury in cerebrospinal fluid. Alzheimers Dement. 2020;16(6):821–830. doi: 10.1002/alz.12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gulani V, Calamante F, Shellock FG, Kanal E, Reeder SB. Gadolinium deposition in the brain: summary of evidence and recommendations. Lancet Neurol. 2017;16(7):564–570. doi: 10.1016/S1474-4422(17)30158-8. [DOI] [PubMed] [Google Scholar]
- 77.Thrippleton MJ, Backes WH, Sourbron S, Ingrisch M, van Osch MJP, Dichgans M, et al. Quantifying blood-brain barrier leakage in small vessel disease: review and consensus recommendations. Alzheimers Dement. 2019;15(6):840–858. doi: 10.1016/j.jalz.2019.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Raja R, Rosenberg GA, Caprihan A. MRI measurements of blood-brain barrier function in dementia: a review of recent studies. Neuropharmacology. 2018;134(Pt B):259–271. doi: 10.1016/j.neuropharm.2017.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Blair GW, Stringer MS, Thrippleton MJ, Chappell FM, Shuler K, Hamilton I, et al. Imaging neurovascular, endothelial and structural integrity in preparation to treat small vessel diseases. The INVESTIGATE-SVDs study protocol. Part of the SVDs@Target project. Cereb Circ - Cogn Behav. 2021;2:100020. [DOI] [PMC free article] [PubMed]
- 80.Chagnot A, Barnes SR, Montagne A. Magnetic resonance imaging of blood-brain barrier permeability in dementia. Neuroscience. 2021;474:14–29. doi: 10.1016/j.neuroscience.2021.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Port M, Corot C, Violas X, Robert P, Raynal I, Gagneur G. How to compare the efficiency of albumin-bound and nonalbumin-bound contrast agents in vivo: the concept of dynamic relaxivity. Invest Radiol. 2005;40(9):565–573. doi: 10.1097/01.rli.0000175388.98721.9b. [DOI] [PubMed] [Google Scholar]
- 82.Barnes S, Chowdhury S, Gatto NM, Fraser GE, Lee GJ. Omega‐3 fatty acids are associated with blood–brain barrier integrity in a healthy aging population. Brain Behav. 2021;11(8). 10.1002/brb3.2273 [DOI] [PMC free article] [PubMed]
- 83.Armitage PA, Farrall AJ, Carpenter TK, Doubal FN, Wardlaw JM. Use of dynamic contrast-enhanced MRI to measure subtle blood–brain barrier abnormalities. Magn Reson Imaging. 2011;29(3):305–314. doi: 10.1016/j.mri.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Montagne A, Nikolakopoulou AM, Zhao Z, Sagare AP, Si G, Lazic D, et al. Pericyte degeneration causes white matter dysfunction in the mouse CNS. Nat Med. 2018. 10.1038/nm.4482 [DOI] [PMC free article] [PubMed] [Retracted]
- 85.Barnes SR, Ng TSC, Santa-Maria N, Montagne A, Zlokovic BV, Jacobs RE. ROCKETSHIP: a flexible and modular software tool for the planning, processing and analysis of dynamic MRI studies. BMC Med Imaging. 2015;15:19. doi: 10.1186/s12880-015-0062-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Taheri S, Gasparovic C, Huisa BN, Adair JC, Edmonds E, Prestopnik J, et al. Blood-brain barrier permeability abnormalities in vascular cognitive impairment. Stroke J Cereb Circ. 2011;42(8):2158–2163. doi: 10.1161/STROKEAHA.110.611731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Taheri S, Gasparovic C, Shah NJ, Rosenberg GA. Quantitative measurement of blood-brain barrier permeability in human using dynamic contrast-enhanced MRI with fast T1 mapping. Magn Reson Med. 2011;65(4):1036–1042. doi: 10.1002/mrm.22686. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. 1. Representative SPGR- and VIBE-derived T1 mapping and dynamic contrast-enhanced MRI datasets. (a) Representative unenhanced coronal fast spoiled gradient echo (SPGR) T1 variable flip angle (2°, 5°, and 10°) and its associated T1 map before administration of a gadolinium-based contrast agent (GBCA). (b) Histogram illustrating the distribution of the T1 values within the whole brain (blue line) of a SPGR subject. (c) Representative unenhanced coronal volumetric interpolated breath-hold examination (VIBE) T1 variable flip angle (2°, 5°, 10°, 12°, and 15°) and its associated T1 map before administration of a GBCA. (d) Histogram illustrating the distribution of the T1 values within the whole brain (red line) of a VIBE subject. (e) Representative 64-frame SPGR DCE (yellow dot represents the GBCA injection time), maximum intensity projection (MIP) of the 64 frames, and its associated BBB Ktrans map. (f) Representative 64-frame VIBE DCE (yellow dot represents the GBCA injection time), maximum intensity projection (MIP) of the 64 frames, and its associated BBB Ktrans map. (a-d) Linked to main Fig. 1a-c; (e,f) Linked to main Fig. 1d-f.
Supplementary file1 (PDF 1594 KB)