Abstract
The search for effective vaccines to stop the COVID-19 pandemic has led to an unprecedented amount of global scientific production and activity. This study aimed to analyze global scientific production on the different vaccine types (mRNA and conventional) that were validated for COVID-19 during the years 2020-2021. The scientific production generated on COVID-19 vaccines during the period 2020-2021 totaled the enormous amount of 20,459 studies published. New mRNA vaccines clearly showed higher production levels than conventional vaccines (viral and inactivated vectors), with 786 and 350 studies, respectively. The USA is the undisputed leader in the global production on COVID-19 vaccines, with Israel and Italy also playing an important role. Among the journals publishing works in this field, the New England Journal of Medicine, the British Medical Journal, and Vaccines stand out from the rest as the most important. The keyword ‘immunogenicity’ and its derivatives have been more researched for the new mRNA vaccines, while thrombosis has been more studied for conventional vaccines. The massive scientific production generated on COVID-19 vaccines in only two years has shown the enormous gravity of the pandemic and the extreme urgency to find a solution. This high scientific production and the main keywords found for the mRNA vaccines indicate the great potential that these vaccines have against COVID-19 and future infectious diseases. Moreover, this study provides valuable information for guiding future research lines and promoting international collaboration for an effective solution.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11356-022-21553-8.
Keywords: vaccines, COVID-19, mRNA, viral vectors, inactivated virus, scientific production
Introduction
COVID-19 (Coronavirus disease of 2019) is an infectious respiratory disease caused by the recently discovered SARS-CoV-2 coronavirus species. Coronavirus was firstly identified in December 2019 in a group of patients in Wuhan (China), who were diagnosed with acute pneumonia. Its name is derived from its similarity with the already known SARS-CoV (Severe Acute Respiratory Syndrome Coronavirus), which was previously discovered in 2002 in the city of Guangdong (China). COVID-19 has caused a global pandemic, with there having been significant health, economic, and political consequences to date. SARS-CoV-2 is still in circulation and has caused 5,745,032 deaths between 2019 and the time of writing this paper, according to the World Health Organization (WHO) [accessed February, 2022]. The enormous impact of COVID-19 has resulted in the need to develop safe and effective vaccines, which has subsequently resulted in novel techniques and intense activity for vaccine development, global cooperation, and new research lines at a scale that has never been seen before (Gordon et al. 2021). Proof of this can be seen in the unprecedented speed with which the human clinical testing of the first vaccine candidates in March 2020 began; just 3 months after SARS-CoV-2 was discovered (Thanh Le et al. 2020). As of April 2020, a total of 115 vaccine candidates had been included in the vaccine research landscape (Thanh Le et al. 2020). From these trials, only the most advanced vaccines, including Pfizer-BioNTech, AstraZeneca, Moderna, and Johnson & Johnson’s Janssen, were selected for their use months later, starting from December 2020 in some countries. Vaccines help to prepare the body to fight against the infection of pathogens by introducing the harmless carrier molecules of a specific virus or bacterium, or the whole weakened bacterial cell or viral particle. These inoffensive molecules and live attenuated pathogens use the mechanisms of the host cell to produce harmless pathogen proteins, causing an immune response. The system produces defense proteins called antibodies as a normal part of the immune response, which recognize and destroy the pathogen, protecting the body during infection. Pfizer-BioNTech and Moderna are mRNA vaccines, a new type of vaccine consisting of messenger RNA (ribonucleic acid) molecules that contain the genes necessary for viral protein production, and which trigger an immune response by the host. In contrast, viral vector vaccines, such as AstraZeneca and Johnson & Johnson’s Janssen, use a modified version of a different harmless virus that enters into our body and contains the genetic information to produce pieces of the virus that causes the disease, triggering an immune response. Less extensively, inactivated vaccines such as Sinovac, Covaxin, and Sinopharm are being researched for their efficacy against COVID-19. This kind of vaccine contains viruses that have genetic material that has been destroyed by chemicals, heat, or radiation, which stops them from infecting human cells but still allows for an immune response to be triggered (Gao et al. 2020). However, these vaccines have not yet provided a level of immunity protection that is as strong as the other vaccine types and, as such, several doses are needed over time. This has been shown by some studies which have demonstrated lower levels of antibody concentration in participants who had received inactivated vaccines (Sinovac) as compared to those who had received an mRNA vaccine (Pfizer-BioNTech) (Lim et al. 2021).
As a consequence of the massive investment in research to contain the pandemic and limit the associated health risk, the volume of scientific production on COVID-19 and vaccines has reached unprecedented levels in a very short period of time. Most of the scientific journals are publishing their content with open access to be able to facilitate collaboration and knowledge exchange between different research groups, institutions, and countries, with the aim of finding the best solution as soon as possible (Torres-Salinas 2020). Bibliometric analyses particularly help in the understanding of such huge quantities of information by classifying it according to topic, journal, country, institution, etc., and could be of great importance in the development of both ongoing and new research on COVID-19.
The aim of the present study is to analyze international scientific publications using bibliometric indicators on the main vaccines (Pfizer-BioNTech, AstraZeneca, Moderna, Johnson & Johnson’s Janssen, Sinovac, Covaxin, and Sinopharm) that were used against COVID-19 during the years 2020 and 2021. Previous studies have analyzed the implications of scientific production regarding the vaccines used against COVID-19 for the scientific community during 2020 and part of 2021 (Ahmad et al. 2021). However, here we present global and updated quantitative data for each one of the most relevant vaccines for a more extended period, comprising of the first two years of the pandemic (from January 2020 to December 2021). Specifically, this paper focuses on the differences in the total scientific production between new mRNA vaccine technology and conventional vaccines (viral vectors and inactivated virus) against COVID-19. The results of this study show which vaccine type has had the greatest scientific impact during the pandemic, and also evaluate global research trends in terms of the vaccination technology used against this disease. In addition, this work provides novel, updated, quantitative, and comparable data that will be useful in the promotion of international collaborations between countries and research institutions, the search for a global clinical response to the pandemic, and the provision of support for the development of new research.
Theoretical Framework
A large number of bibliometrics analyses on article production about COVID-19 have been published in the last 2 years. All these analyses could be of great help to clarify their evolution and dynamics and to compare with the results here presented. From a general point of view, it is worth mentioning the bibliometric studies of Lou et al. (2020); Atlasi et al. (2021); Chahrour et al. (2020); Herrera-Viedma et al. (2020); Hossain (2020); Pal (2021); Liu et al. (2020); Belli et al. (2020); Roshani et al. (2021); Homolak et al. (2020); Nowakowska et al. (2020). These studies provided valuable information from several indicators such as production, trends, researchers, countries, institutions, and research topics, and revealed a massive and unprecedented scientific production on COVID-19 in a very short period in comparison with other fields throughout history. Di Girolamo and Reynders (2020) suggested most of the works contained preliminary results that were published urgently due to the pandemic crisis and the need for valuable data. It should be noted that the most powerful publishers in the world (Elsevier, Springer, Taylor and Francis, etc.), and the major biomedical journals (Science, BMJ, JAMA, New England, Oxford, etc.) published their work in open access to facilitate the visibility of research results as a basis for the generation of new knowledge and the search for solutions (Torres-Salinas 2020). In addition, the works of Coccia (2021a, b, c) were very significant to know the research fields most affected by the pandemic crisis, the investments made in public health, and the environmental threats related to COVID-19. They demonstrated that the evolution and dynamics of research fields could be deeply influenced by crisis periods. Specifically, these unpredictable threats can accelerate scientific production to solve urgent and unknown problems.
From an economic–social point of view, the works of Verma and Gustafsson (2020); Nova-Reyes et al. (2020) concluded that COVID-19 global crisis has made a huge impact in many business, economical, and political aspects, promoting several socio-political short- and long-term changes. Another approach that has been widely discussed is related to pharmacological treatments against COVID-19. On this topic, numerous Systematic Reviews (Review Articles) have been published on the scientific literature produced (Menzella et al. (2020); Sanders et al. (2020); Scavone et al. (2020); Serafina et al. (2020); Wu et al. (2020); Jin et al. (2020)). These reviews give us an idea of the importance and speed with which research on the field is occurring and the need to analyze and systematize them. The classifications of drugs and therapeutic agents provided by these works and the evaluation of their efficacy have proved to be particularly useful in treating and combating the disease. Research on effective drugs against COVID-19 was crucial during the beginning of the pandemic to treat the symptoms, while the more tedious process of developing vaccines to eradicate the disease was taking place.
Methods
Data source and sample
We gathered our data from the scientific production indexed in the Web of Science Core Collection database (WoS 2022). This multi-disciplinary international source references the most prestigious scientific publications in the world and is an essential starting point for bibliometric studies, providing indicators of production and scientific impact. WoS has been found to match the current pace of publishing by rapidly indexing the specific COVID-19 sections that journals have created (Online articles, Articles in the press, Early Access, Latest issue, etc.), thus enhancing their dissemination and visibility. We carried out our searches from 01-01-2020 to 31-12-2021, which coincided with the first two years of the pandemic. The search strategies used to recover the scientific production indexed in WOS on the subject of study, as well as the treatment and analysis of the data obtained, are described in Supplementary Material.
Data analysis procedure
We tabulated the data obtained from the search ‘Combine #1 AND #2’ (see Supplementary Material) and produced a table to represent the scientific production and impact of the different COVID-19 vaccine types during 2020-2021. The 20,459 complete bibliographic records resulting from the search ‘Combine #1 AND #2’ were processed and standardized in Excel. For the individual analysis of each vaccine resulting from the search ‘Combine #1 AND #3,’ ‘Combine #1 AND #4,’ ‘Combine #1 AND #5,’ ‘Combine #1 AND #6,’ ‘Combine #1 AND #7,’ ‘Combine #1 AND #8,’ and ‘Combine #1 AND #9,’ we designed a database to analyze the production and impact of the studies recorded about vaccines against COVID-19, which was organized by TSP (Total Studies Produced), CR (Citations Received), MCS (Mean Citations/Study), CS (Citing Studies), +CS (Citations received by the most cited work), and H-index (Number of studies that have received the same or a higher number of citation). Additionally, we designed a database to analyze the production of the studies recorded, which was organized by institution, producer country, journal, and keyword co-occurrence. To visualize the bibliometric networks, we used the VOS-viewer software (https://www.vosviewer.com/), which worked with units of analysis (authors, organizations, keywords, etc.) and units of measurement (links, frequency, centrality, distance) to illustrate our results by grouping similarities in clusters. All documents were previously repaired using bibexcel (https://homepage.univie.ac.at/juan.gorraiz/bibexcel/), which enabled us to unify term entries. To build the co-occurrence networks, we generated vectors, which were pre-displayed in PAJEK (http://mrvar.fdv.uni-lj.si/pajek/) with definitive drawings created in the VOS-viewer software. We used this process because the VOS-viewer software is limited in that it labels nodes based on an internal, non-modifiable schedule. We labeled as many nodes as possible while guaranteeing that the sets were correctly displayed.
Results and discussion
General overview of scientific production on vaccines against COVID-19 during 2020-2021
According to our results, a total of 20,459 studies were published internationally on vaccines and COVID-19 during the first two years of the pandemic (2020-2021). This particularly high number of documents has no precedence in history, has been confirmed by many other studies related to COVID-19, and is the result of the intense research carried out to combat the very serious world health crisis caused by this disease. In comparison with other published studies, Ahmad et al. (2021) obtained a total of 1,093 studies during the first year of the pandemic (2020), while Sarirete (2021) obtained a total of 663 documents for 2020 and 1,446 for 2021. However, our search resulted in a much higher quantity (20,459 documents) in two years (2020 and 2021), of which 4,447 were published in 2020 and 16,012 in 2021. This may be due to our method being less restrictive through its use of the strategy TS=Topic (which includes Title, Abstract, and Keywords) and its use of a wider variety of terms to name each vaccine (see 7). As a result of this, our study is novel and all-encompassing. It is worth noting that, according to our results, more than three times as many papers were published during 2021, when COVID-19 vaccines started to be administered to the population. Of these 20,459 published studies, more than half (11,694) were published as articles. This indicates the clear experimental nature and the urgency of the research topic in question. The number of publications overtime here presented (2020-2021) followed the typical dynamics and evolution of crisis-driven research characterized by an incomparable speed in scientific production (Coccia 2020, 2021a). Most probably, many of the early works published during the beginning of COVID-19 pandemic presented tentative results due to the need of publish them urgently to be helpful for the scientific and medical community. Undoubtedly, notes, short papers, pre-print servers, and open access publications play a major contributing role in that massive production (Coccia 2021a; Torres-Salinas 2020).
Even though the number of studies published about the vaccines against COVID-19 has been enormous, previous studies carried out by our group show that medical research has also developed with great intensity on other fronts such as the use of drugs against COVID-19; a subject regarding which a total of 6,533 papers were published during 2020, that is, around 2,000 more papers than on vaccines (4,447 papers) (Ruiz-Fresneda et al. 2022). This could be due to the fact that during the first few months of the epidemic, research was focused on the search for treatments to prevent the serious symptoms in the short term. This was due to the manufacturing of vaccines being a slower process, which resulted in the later publication of studies. However, from the moment that results began to be published, growth began to be equally explosive. Scientific production on drugs against COVID-19, as well as the present study on vaccines, clearly followed the evolution and dynamics characteristic of crisis period and pandemic threats. As expected, the USA and England are at the top of the list of countries by the number of papers published on vaccines and COVID-19, with 6,344 and 2,042, respectively (Table S1). The USA has produced three times more studies than the country in second place (England), accounting for 30% of the total studies published in the world on the topic. Somewhat unexpectedly, India is the third-largest producer of studies, with 1,852 publications. These data reveal the power that is being acquired in recent years in this country in terms of research and scientific development, as well as the potential of its pharmaceutical industry. In fourth place is China, the birthplace of COVID-19, with 1,797 studies. Following, in fifth position is Italy, one of the most affected countries in Europe, especially at the beginning of the pandemic, with a total of 1,560 studies. Regarding institutions, as expected considering the previous data, the USA and England lead in terms of scientific production (Table S1). The University of London is at the top of the leaderboard with a total of 638 studies and is closely followed by Harvard University, with 628 studies. The University of California System has published 505 studies, the University of Oxford has published 371, and Johns Hopkins University has published 356 documents. As can be seen, there is an absolute predominance of Anglo-American universities. It is also clear how these universities, which are recognized as some of the most prestigious in the world, have decisively taken the lead in research.
In terms of the funding agencies reported in the published papers, more than 6,000 organizations were identified, including the Health Human Services, the National Institutes of Health, the National Science Foundation, and the National Institute of Allergy Infectious Diseases, from the USA. Together they account for more than 15% of global funding. The European Union is the second largest funder if we include the joint funding from the European Commission (2.19%) and individual contributions from countries such as the UK, where various agencies such as UK Research Innovation, the Medical Research Council and the Wellcome Trust are prominent. The German Research Foundation, the French National Research Agency and the ministry if Health Italy also had an important role. In terms of funding from private sources or non-governmental organizations, the top 25 includes the Bill Melinda Gates Foundation, which has funded 109 research projects on vaccines. Interesting is the presence of Pfizer, the US pharmaceutical company responsible for one of the most successful vaccines with m-RNA technology (Pfizer-BioNTech), which financed more than 50 studies. In contrast, viral vector vaccines did not receive as much funding as m-RNA vaccines. J&J Janssen and AstraZeneca companies highlighted with 12 and 7 works, respectively. The greater funding obtained by m-RNA vaccines could be related to the production of a larger number of studies produced. This is logical since these vaccines have never been administered to the population and therefore require more research.
Differences between COVID-19 vaccine types in global scientific production: mRNA, viral vectors, and inactivated virus vaccines
Given the analysis of the international scientific production on the studied vaccines against COVID during the period 2020-2021 (8), a specific study was conducted out on each of the main vaccines that have been validated by the WHO as of November 30, 2021 (Pfizer-BioNTech, Moderna, AstraZeneca, J&J Janssen, Sinopharm, Covaxin-Bharat, and Sinovac). This study was carried out with the objective of understanding, for the first time, the differences and similarities in the previous studies about the different types of vaccines (mRNA, viral vector, and inactivated virus). More specifically, an analysis was carried out regarding the international scientific production and impact; production at the level of institutions, countries, and scientific journals; and finally, research topics addressed, by analyzing the co-occurrence of keywords.
Scientific production and impact
In terms of scientific production and impact, Pfizer-BioNTech (mRNA) is the vaccine with the most published studies (TSP) with a total of 576 studies published and 9,835 citations (Table 1). It is followed by AstraZeneca (viral vector) with 223 studies published and a total of 2,002 citations, and Moderna (mRNA) which, despite having a similar number of published studies (210) as AstraZeneca, has three times more citations (6,523) (Table 1). In fourth position is the J&J Janssen vaccine with 56 studies and 1,118 citations (Table 1). Finally, and at a much lower level than the other vaccines, we find the vaccines based on inactivated SARS-CoV2 viruses. These vaccines have been subsequently manufactured, studied, and validated. This would partly explain their lesser scientific visibility when compared with other mRNA and viral vector vaccines. The three vaccines approved by the WHO in November 2021 (Sinopharm, Covaxin, and Sinovac) collectively reach only 71 studies and 269 citations in total (Table 1). Of these three vaccines, Sinovac is clearly noted as the most relevant in terms of impact and scientific production.
Table 1.
Scientific production and impact of the different COVID-19 vaccine types during 2020-2021.
| Vaccine type | TSP | CR | MCS | CS | +CR | H-index | |
|---|---|---|---|---|---|---|---|
| mRNA | Pfizer-BioNTech | 576 | 9,835 | 17.07 | 5,672 | 3,160 | 41 |
| Moderna | 210 | 6,523 | 31.06 | 4,590 | 2,052 | 26 | |
| Total | 786 | 16,358 | - | 10,262 | - | - | |
| Viral vectors | AstraZeneca | 223 | 2,002 | 8.98 | 1,538 | 285 | 18 |
| J&J Janssen | 56 | 1,118 | 19.96 | 920 | 329 | 11 | |
| Total | 279 | 3,120 | - | 2,458 | - | - | |
| Inactivated virus | Sinopharm | 20 | 23 | 1.15 | 20 | 8 | 2 |
| Covaxin-Bharat | 8 | 34 | 4.25 | 33 | 15 | 3 | |
| Sinovac | 43 | 212 | 4.93 | 195 | 110 | 7 | |
| Total | 71 | 269 | - | 250 | 133 | - | |
TSP (Total Studies Produced), CR (Citations Received), MCS (Mean Citations/Study), CS (Citing Studies), +CR (Citations received by the most cited work), H-index (Number of studies that have received the same or a higher number of citations).
The results clearly reflect the greater scientific relevance of the mRNA-type vaccines (Pfizer-BioNTech, and Moderna) with respect to the other types, with a total of 786 published studies, 16,358 citations, and more than 10,000 citing papers (Table 1), in only a two-year period (2020-2021). Among the different bibliometric parameters, it is worth noting the high impact indexes (H-index) of 41 for the papers on Pfizer and 26 for those on Moderna (Table 1). Moreover, it is relevant to highlight the high number of citations (almost a third of the total citations) regarding the most cited papers for each of these vaccines, with 3,160 and 2,052 citations for the most cited papers on Pfizer and Moderna (Table 1). At much lower levels, we can observe the viral vector vaccines (AstraZeneca and Janssen) with a total of 279 TSP and 3,120 citations (Table 1). However, it is important to emphasize that production in terms of published studies has been enormous as well for this type of vaccine. Interestingly, the fact that Pfizer and Moderna are the vaccines with the most scientific impact coincides with the number of administered doses in places such as the USA (CDS 2022a) and Europe (ECDC 2022), where the mRNA vaccines (Pfizer and Moderna) are the most used in comparison with the other types of vaccines [accessed February, 2022]. In fact, the CDC (Centers for Disease Control and Prevention) recommends the use of mRNA vaccines against COVID-19 (CDS 2022b).
The above data clearly indicate a change regarding the global trends in the use of vaccines, with there now being the implementation of this recent mRNA technology as opposed to conventional vaccines that are based on the use of parts of the virus or inactivated strains of the virus that causes the disease. The increased scientific impact of the mRNA vaccines that was identified in this study could be related to their greater efficacy against COVID-19. Many studies have been published on this matter. Rotshild et al. (2021) showed that mRNA technology (specifically, Pfizer’s BNT162b2 and Moderna’s mRNA-1273 vaccines) has greater efficacy in the prevention of COVID-19 symptoms when compared to other vaccines. Moreover, the production of neutralizing antibodies in blood seems to be greater in people who have been vaccinated with the mRNA Pfizer vaccine than in people with the inactivated virus vaccines Coronavac and Sinovac, indicating the lower efficacy of the latter vaccine type (Lim et al. 2021). The rapid development that mRNA vaccines have had and the uncertainty caused by the possible adverse effects in the long term have led to a certain level of doubt regarding this new technology, especially when the vaccination process first began in 2021. However, the data obtained to date clearly show that the mRNA vaccines, which have been approved for their use against COVID-19, are safe and effective for the wide majority of people and are essential for ending this global pandemic, which is causing an extremely high number of deaths (Anand and Stahel 2021). As such, we can say that this new mRNA vaccination technology has been successful in combating the COVID-19 pandemic and could be a very useful tool for future infectious diseases. The enormous quantity of published studies with positive results is a testimony to that.
Scientific production by Institutions, Countries, and Journals
Table 2 shows the total scientific production of the four vaccines with the highest impact to date, by institution, country, and journal. Regarding the mRNA Pfizer-BioNTech vaccine, it stands out that three institutions with the highest number of published studies on Pfizer and COVID19 are from Israel: Tel Aviv University with 57, Sackler Faculty of Medicine with 51, and Chaim Sheba Medical Center with 25 (Table 2). This is due to this country making a clear commitment to combatting this epidemic by purchasing and using a large number of vaccines, as well as funding many research projects on them. At the end of 2020 (December 31), when the majority of countries still had not administered a single vaccine, Israel had vaccinated a total of 949,112 people from a total population of 9.3 million inhabitants, which equated to an average of 10.97 doses per 100 inhabitants and 10.2% people vaccinated in only one month (Rosen et al. 2021). In these same months, the other countries that had begun the vaccination process found themselves very much behind. In these same months, the other countries that had begun the vaccination process found themselves very much behind. From these countries, two stand out: the USA, for having just over 2.7 million people vaccinated and an average of 0.84 doses per 100 inhabitants, and China, with 4.5 million total doses and an average of 0.31 doses per 100 inhabitants (Rosen et al. 2021). The fact that Israel has clearly committed itself to science, as reflected by how much of its GDP is allocated to science and research (4.9% of GDP) (World Bank Open Data 2022), has greatly aided the publication of these studies and the rapid deployment of vaccinations. This can also be seen in the scientific production data when organized by country, where Israel reached third place with 84 studies by Israeli authors, behind the USA and Italy.
Table 2.
Scientific production on the different COVID-19 vaccine types by institution, country, and journal (2020-2021).
| Vaccine type | Institutions | TSP | %TSP | Country | TSP | %TSP | Journals | TSP | %TSP | |
|---|---|---|---|---|---|---|---|---|---|---|
| mRNA | Pfizer-BioNTech | Tel Aviv University | 57 | 9.9 | USA | 119 | 20.7 | Vaccines | 47 | 8.2 |
| Sackler Faculty of Medicine | 51 | 8.6 | Italy | 96 | 16.7 | BMJ British Medical Journal | 23 | 4 | ||
| Chaim Sheba Medical Center | 25 | 4.3 | Israel | 84 | 14.6 | New England Journal of Medicine | 20 | 3.5 | ||
| Institut National de la Santé et de la Recherche Médicale (Inserm) | 21 | 3.6 | England | 50 | 8.7 | Vaccine | 14 | 2.4 | ||
| University of London | 21 | 3.6 | Germany | 48 | 8.3 | Frontiers in Immunology | 12 | 2.1 | ||
| Moderna | University of California System | 18 | 8.6 | USA | 111 | 52.9 | New England Journal of Medicine | 22 | 10.5 | |
| Harvard University | 17 | 8.1 | Spain | 14 | 6.7 | Cureus | 14 | 6.7 | ||
| Emory University | 16 | 7.6 | Germany | 13 | 6.2 | BMJ British Medical Journal | 7 | 3.3 | ||
| National Institutes of Health (NIH USA) | 16 | 7.6 | Canada | 12 | 5.7 | Frontiers in Immunology | 6 | 2.9 | ||
| National Institute of Allergy Infectious Diseases (NIAID) | 14 | 6.7 | Italy | 10 | 4.8 | Vaccines | 5 | 2.4 | ||
| Viral vectors | AstraZeneca | University of Oxford | 21 | 9.4 | England | 43 | 19.3 | Vaccines | 17 | 7.6 |
| Imperial College London | 10 | 4.5 | USA | 22 | 9.9 | BMJ British Medical Journal | 16 | 7.2 | ||
| University of London | 9 | 4 | India | 20 | 9 | Frontiers in Immunology | 5 | 2.2 | ||
| National and Kapodistrian University of Athens | 6 | 2.7 | Italy | 19 | 8.5 | BMJ Case Reports | 4 | 1.8 | ||
| Public Health England | 6 | 2.7 | Australia | 14 | 6.3 | Cureus | 4 | 1.8 | ||
| J&J Janssen | Harvard University | 9 | 16.1 | USA | 34 | 60.7 | New England Journal of Medicine | 9 | 16.1 | |
| Janssen Vaccines | 9 | 16.1 | Netherlands | 13 | 23.2 | JAMA Journal of the American Medical Association | 4 | 7.1 | ||
| Beth Israel Deaconess Medical Center | 8 | 14.3 | Belgium | 10 | 17.9 | JAMA Network Open | 3 | 5.4 | ||
| Janssen Pharmaceuticals | 8 | 14.3 | Italy | 4 | 7.1 | American Journal of Emergency Medicine | 2 | 3.6 | ||
| Johnson &Johnson | 8 | 14.3 | England | 3 | 5.4 | Cureus | 2 | 3.6 | ||
TSP (Total Studies Produced). %TSP (Percentage of total studies produced)
Regarding Moderna, the five institutions with the highest number of published studies on COVID-19 are from the USA: The University of California System (18 studies), Harvard University (17 studies), Emory University (16 studies), National Institutes of Health, NIH USA (16 studies), and NIH National Institute of Allergy Infectious Diseases, NIAID (14 studies) (Table 2). Consequently, the USA is the country with the most authors publishing studies on this vaccine (111 studies) with there being an abysmal difference with the second place, Spain, which has a total of 14 studies published by Spanish authors (Table 2).
Regarding viral vector vaccines, we notice that four out of the five institutions with the highest number of published studies on AstraZeneca and COVID-19 are English: University of Oxford (21 studies), Imperial College London (10 studies), University of London (9 studies), and Public Health England (6 studies) (Table 2). England also leads in the number of works published with 43 studies, followed by the USA with a total of 22 studies. With regard to the studies published on the Janssen vaccine and COVID-19, it should be noted that most of the institutions with the most papers belong to the company itself: Janssen Vaccines (9 studies), Janssen Pharmaceuticals (8 studies), Johnson &Johnson (8 studies) (Table 2). Outside of this, the North American Harvard University and the Israeli Beth Israel Deaconess Medical Center are the first and third institutions with 9 and 8 published papers, respectively.
The USA is the indisputable leader in terms of scientific production on vaccines against COVID-19 for both the new mRNA vaccines and conventional vaccines as it is the country with the most published studies on Pfizer-BioNTech, Moderna, and J&J Janssen. It is also the second leading country with the most studies published about AstraZeneca (Table 2). This fact was already evident since the USA is one of the countries that invests the most in science and research in the world. Moreover, eight out of ten of the best institutions and universities, according to the Shanghai Ranking, are from the USA (data accessed: February, 2022) and have been intensely involved in this research. The fact that the companies Pfizer-BioNTech and Moderna are from the USA may have had an influence on the majority of the research being developed there. However, the high scientific production from the USA on vaccines manufactured by companies from other countries, such as AstraZeneca or Janssen, demonstrates not only the scientific but also the economic power of this country. Something similar can be seen to happen with the company AstraZeneca, which has its headquarters in England; a country that is ahead with the number of published studies on COVID-19 and this vaccine with there being 43 papers (Table 2). Along with the previously mentioned role of Israel, it is important to highlight Italy as one of the countries with the highest number of scientific contributions, as noted by its presence in the top five of the most relevant vaccines to treat COVID-19. Italy is the second leading country with the most publications on the Pfizer vaccine with 96 published studies, the fifth leading in terms of Moderna with 10 published studies, the fourth leading in terms of AstraZeneca with 19 published studies, and is also the fourth leading country with studies on the Janssen vaccine, with 4 published studies (Table 2). This high amount of scientific production and impact could be due to the fact that Italy was one of the first European countries to detect and inform about the presence of SARS-CoV2 in Europe, and has been one of the countries with the highest rate of infection and death, especially at the onset of the pandemic in 2020.
Regarding the scientific journals that are linked to publications about the four main vaccines, there are four that stand out from the rest. Vaccines, the BMJ-British Medical Journal, the New England Journal of Medicine, and Cureus are in the top five journals with the most papers published about three of the four vaccines (Table 2). The New England Journal of Medicine leads in terms of the number of publications about Moderna, Janssen, and COVID-19 with 22 and 9 papers, respectively, while Vaccines also leads in terms of the number of publications about Pfizer and AstraZeneca with 47 and 17 studies, respectively (Table 2). The presence of two particularly prestigious international journals such as the New England Journal of Medicine and the BMJ demonstrates the enormous relevance of the results in the research of these vaccines and the dimensions and urgency of this global pandemic. Moreover, the presence of journals with less scientific impact at the international level but which are very specialized in the subject, such as Vaccines and Cureus, has also been of vital importance. These journals facilitated a quicker publication of results, which has allowed for the collaboration and the exchange of results to provide a more rapid response to the pandemic.
Analysis of keyword co-occurrence
Constructing network visualization maps for the co-occurrence of keywords allowed us to evaluate the different global trends between mRNA vaccines and the other types. For all the network maps, the minimum number of occurrences of a keyword was set at 3. The intensity in the co-relation between the different keywords was expressed as TLS (Total Link Strength) by the VOS-viewer software. As expected, for all vaccines, the co-occurrence of keyword mapping showed ‘COVID-19,’ ‘SARS-CoV2,’ ‘vaccine/s,’ and ‘vaccination’ as the most dominant keywords (Figs. 1, 2, 3 and 4).
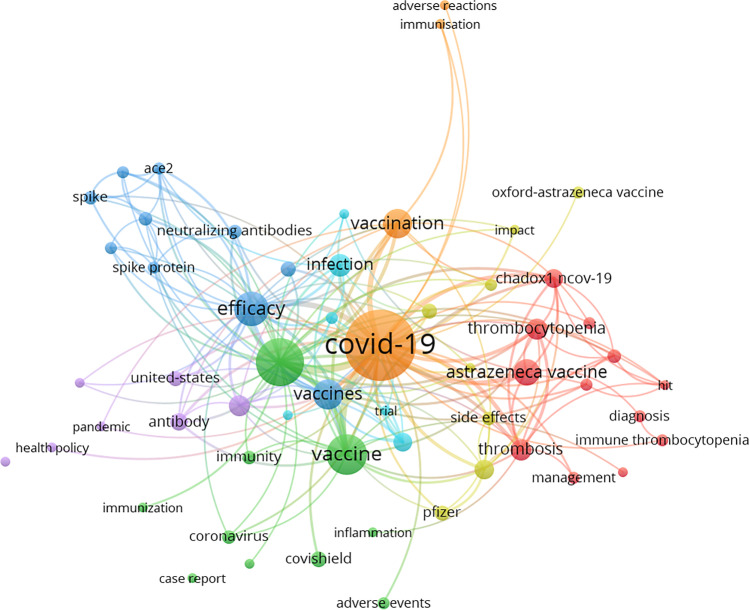
Fig. 1.
Network visualization map for the co-occurrence of keywords for all the works reported for the Pfizer-BioNTech vaccine and COVID-19. The size of the spheres is proportional to the number of co-occurrences for each keyword. Lines represent the total link strength and co-relation between the keywords.
Fig. 2.
Network visualization map for the co-occurrence of keywords for all the works reported for the Moderna vaccine and COVID-19. The size of the spheres is proportional to the number of co-occurrences for each keyword. Lines represent the total link strength and co-relation between the keywords.
Fig. 3.
Network visualization map for the co-occurrence of keywords for all the works reported for the AstraZeneca vaccine and COVID-19. The size of the spheres is proportional to the number of co-occurrences for each keyword. Lines represent the total link strength and co-relation between the keywords.
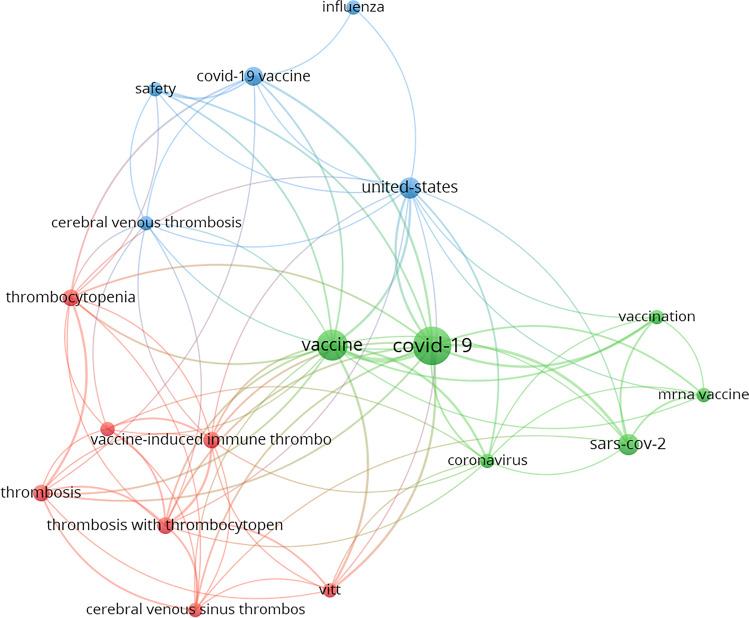
Fig. 4.
Network visualization map for the co-occurrence of keywords for all the documents reported for the Janssen vaccine and COVID-19. The size of the spheres is proportional to the number of co-occurrences for each keyword. Lines represent the total link strength and co-relation between the keywords.
The maps for the mRNA vaccines (Pfizer and Moderna) showed a higher co-occurrence for keywords related to immune response (‘immunogenicity,’ ‘immunization,’ ‘immune response,’ ‘antibody/ies,’ ‘antibody/ies response,’ ‘neutralizing antibodies’) (Figs. 1 and 2). For example, for Pfizer, the keywords ‘immunogenicity’ and ‘antibodies’ presented 30 and 26 co-occurrences and a TLS of 105 and 93, respectively. However, for AstraZeneca (viral vector) the keyword ‘immunogenicity’ reported 11 co-occurrences, with a TLS of 44. The Janssen vaccine (viral vector) did not even present immunology-related keywords with a relevant co-occurrence. This fact could indicate that this new vaccination technology was more studied from an immunologic point of view, suggesting a higher concern and research on the efficacy and safety evaluation of these new types of vaccines, which had never been administered before the COVID outbreak.
Among the keywords, ‘thrombosis’ was the main side effect produced by the vaccines in terms of co-occurrence appearance. Interestingly, the number of keyword co-occurrences related to thrombosis (‘thrombosis,’ ‘thrombocytopenia,’ ‘cerebral venous thrombosis,’ ‘vaccine-induced immune thrombosis’) was considerably higher for the conventional vaccines AstraZeneca and Janssen in comparison with mRNA vaccines (Figs. 3 and 4). For AstraZeneca, the keyword ‘thrombosis’ reported 11 co-occurrences with a TLS of 46, while ‘thrombocytopenia’ reported 9, with a TLS of 40. In the case of Janssen, ‘thrombosis’ presented 4 co-occurrences (TLS=19), ‘thrombocytopenia’ presented 4 co-occurrences (TLS=16), and ‘cerebral venous thrombosis’ presented 3 co-occurrences (TLS=12). No relevant co-occurrences were observed for Moderna, and only 3 were observed for ‘thrombocytopenia,’ which appeared for Pfizer with a TLS of 6. Our results indicate that thrombosis was the main side effect related to the administration of AstraZeneca and Janssen. However, for mRNA vaccines, thrombosis was not researched as much as for conventional vaccines. These data could indicate that there is greater safety with new mRNA vaccines and note the wide concern that occurred about thrombosis cases during the beginning of vaccination. However, ultimately, all vaccines have been shown to be safe over time.
Conclusions
This study demonstrated that the scientific production on vaccines and COVID-19 has been enormous, with a total of 20,459 documents having been published in a very short period of time (2020-2021). These impressive and unprecedented numbers clearly fit dynamics characteristic of research fields driven by crises and environmental threats and show how extreme the consequences of the pandemic have been, as well as the urgent need to find a solution. A higher number of works regarding the new mRNA vaccines (Pfizer and Moderna) have been published in comparison with vaccines using more conventional technology (AstraZeneca, Janssen, Sinopharm, Sinovac, etc.) during this period. This indicated a major shift in global trends in the use of vaccines with this new technology, which had never been used prior to this pandemic, as well as indicate their great potential, not only against COVID-19 but also against future infectious diseases. The results presented herein evidenced the importance of research prior to safely administration of medical and pharmaceutical products and demonstrated the need for government funding of these projects. At a general level, the USA excelled as the leading contributor with major contributions in scientific production for both m-RNA and traditional vaccines, indicating how the USA is still one of the world’s economic powers that invest the most in science and technology, even above other emerging powers such as China. The remarkable role of Italy in global scientific production showed how the most affected countries during the pandemic greatly financed investigations on COVID-19. Most of the studies were published in journals specialized in vaccination research and medicine, being Vaccines and BMJ the journal with the highest number of works. Our analysis of the scientific production by journal provides the most relevant journals in the field and, thus, could be useful for authors during publication and journal selection process. Finally, the mapping of keyword co-occurrence networks revealed a special focus on mRNA vaccines in immunologic studies when compared with conventional vaccines, indicating a major concern and more research on the efficacy and safety evaluation of this new type of vaccines. In addition, conventional vaccines showed higher co-occurrence values for keywords related to thrombosis. This result outlined thrombosis as the main side effect related to vaccines, particularly in the cases of AstraZeneca and Janssen. These data could indicate an increased safety of the new mRNA vaccines and may highlight thrombosis as the main concern related to the potential adverse effects of the COVID-19 vaccination process. The bibliometric analysis presented herein comparing new and conventional vaccine technologies could be of great use for guiding future research lines and promoting international collaboration for an effective solution.
Supplementary Information
(DOC 43 kb)
Acknowledgements
This work was supported by the PAIDI (Plan Andaluz de Investigación, Desarrollo e Innovación 2020) program (Junta de Andalucía. Spain. HUM.777-EC3 Research Group). The authors acknowledge English–Spanish Translation & Communication® for the revision of the English language.
Author contribution
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Miguel Angel Ruiz-Fresneda, Rafael Ruiz-Pérez, Carlos Ruiz-Fresneda, and Evaristo Jiménez-Contreras. The first draft of the manuscript was written by Miguel Angel Ruiz-Fresneda, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the PAIDI (Plan Andaluz de Investigación, Desarrollo e Innovación 2020) program (Junta de Andalucía. Spain. HUM.777-EC3 Research Group).
Data availability
All data and materials as well as software application support their published claims and comply with field standards.
Declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
The authors have not submitted the manuscript to a preprint server before submitting it to Environmental Science and Pollution Research. We confirm that this manuscript has not been published elsewhere and is not under consideration by another journal. All authors have approved the manuscript and agreed with its submission to Environmental Science and Pollution Research.
Competing interest
The authors have no relevant financial or non-financial interests to disclose.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ahmad T, Murad MA, Baig M, Hui J. Research trends in COVID-19 vaccine: a bibliometric analysis. Hum Vaccin Immunother. 2021;17(8):2367–2372. doi: 10.1080/21645515.2021.1886806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand P, Stahel VP. The safety of Covid-19 mRNA vaccines: a review. Patient Saf Surg. 2021;15(1):20. doi: 10.1186/s13037-021-00291-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atlasi R, Noroozi Chakoli A, Ramezani A, et al. Scientometric analyzing the output of researchers and organizations on COVID-19 for better conducting the scientific efforts: With a glance to endocrinology. J Diab Metab Disorders. 2021;20:107–118. doi: 10.1007/s40200-020-00718-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belli S, Mugnaini R, Balta J, Abadal E. Coronavirus mapping in scientific publications: When science advances rapidly and collectively, is access to this knowledge open to society? Scientometrics. 2020;124(3):2661–2685. doi: 10.1007/s11192-020-03590-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDS 24/7. Centers for Disease Control and Prevention (2022a) COVID Data Tracker. Maps, charts, and data provided by CDC. https://covid.cdc.gov/covid-data-tracker/#vaccinations_vacc-total-admin-rate-total. Accessed March 2022
- CDS 24/7. Centers for Disease Control and Prevention (2022b) Vaccines & Immunizations. Vaccines for COVID-19. COVID-19 vaccines are safe, effective, and free. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/index.html. Accessed March 2022
- Chahrour M, Assi S, Bejjani M, et al. (2020) A Bibliometric Analysis of COVID-19 Research Activity: A Call for Increased Output. Cureus 12(3): e7357. 10.7759/cureus.7357 [DOI] [PMC free article] [PubMed]
- Coccia M. The evolution of scientific disciplines in applied sciences: dynamics and empirical properties of experimental physics. Scientometrics. 2020;124:451–487. doi: 10.1007/s11192-020-03464-y. [DOI] [Google Scholar]
- Coccia M. Evolution and structure of research fields driven by crises and environmental threats: the COVID-19 research. Scientometrics. 2021;126(12):9405–9429. doi: 10.1007/s11192-021-04172-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccia M. High health expenditures and low exposure of population to air pollution as critical factors that can reduce fatality rate in COVID-19 pandemic crisis: a global analysis. Environ Res. 2021;199:111339. doi: 10.1016/j.envres.2021.111339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccia M. Effects of the spread of COVID-19 on public health of polluted cities: results of the first wave for explaining the dejà vu in the second wave of COVID-19 pandemic and epidemics of future vital agents. Environ Sci Pollut Res. 2021;28(15):19147–19154. doi: 10.1007/s11356-020-11662-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Girolamo N, Reynders RM. Characteristics of scientific articles on COVID-19 published during the initial three months of the pandemic: a meta-epidemiological study. Scientometrics. 2020;125:795–812. doi: 10.1007/s11192-020-03632-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECDC. European Centre for Disease Prevention and Control (2022) COVID-19 Vaccine Tracker. https://vaccinetracker.ecdc.europa.eu/public/extensions/COVID-19/vaccine-tracker.html#distribution-tab. Accessed March 2022
- Gao Q, Bao L, Mao H, Wang L, Xu K, Yang M, et al. Development of an inactivated vaccine candidate for SARS-CoV-2. Science. 2020;369(6499):77–81. doi: 10.1126/science.abc1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon C, Porteous D, Unsworth J. COVID-19 vaccines and vaccine administration. Br J Nurs. 2021;30(6):344–349. doi: 10.12968/bjon.2021.30.6.344. [DOI] [PubMed] [Google Scholar]
- Herrera-Viedma E, López-Robles J, Guallar J, Cobo M. Global trends in coronavirus research at the time of Covid-19: A general bibliometric approach and content analysis using SciMAT. El profesional de la Información. 2020;29(3):e290322. doi: 10.3145/epi.2020.may.22. [DOI] [Google Scholar]
- Homolak J, Kodvanj I, Virag D. Preliminary analysis of COVID-19 academic information patterns: a call for open science in the times of closed borders. Scientometrics. 2020;124(3):2687–2701. doi: 10.1007/s11192-020-03587-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain MM. Current status of global research on novel coronavirus disease (Covid-19): A bibliometric analysis and knowledge mapping. F1000Research. 2020;9:374. doi: 10.12688/f1000research.23690.1. [DOI] [Google Scholar]
- Jin Z, Liu JY, Feng R, Ji L, Jin ZL, Li HB. Drug treatment of coronavirus disease 2019 (COVID-19) in China. Eur J Pharmacol. 2020;883:173326. doi: 10.1016/j.ejphar.2020.173326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le TT, Andreadakis Z, Kumar A, Román RG, Tollefsen S, Saville M, et al. The COVID-19 vaccine development landscape. Nat Rev Drug Discov. 2020;19(5):305–306. doi: 10.1038/d41573-020-00073-5. [DOI] [PubMed] [Google Scholar]
- Lim WW, Mak L, Leung GM, Cowling BJ, Peiris M. Comparative immunogenicity of mRNA and inactivated vaccines against COVID-19. Lancet Microbe. 2021;2(9):e423. doi: 10.1016/S2666-5247(21)00177-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N, Chee ML, Niu CL, Pek PP, Siddiqui FJ, et al. Coronavirus disease 2019 (COVID-19): an evidence map of medical literature. BMC Med Res Methodol. 2020;20:1. doi: 10.1186/s12874-020-01059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou J, Tian SJ, Niu SM, Kang XQ, Lian HX, Zhang LX, Zhang JJ. Coronavirus disease 2019: a bibliometric analysis and review. Eur Rev Med Pharmacol Sci. 2020;24(6):3411–3421. doi: 10.26355/eurrev_202003_20712. [DOI] [PubMed] [Google Scholar]
- Menzella F, Biava M, Barbieri C, Livrieri F, Facciolongo N. Pharmacological treatment of COVID-19: lights and shadows [Review] Drugs Context. 2020;9:4–6. doi: 10.7573/dic.2020-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nova-Reyes A, Munoz-Leiva F, Luque-Martinez T. The Tipping Point in the Status of Socially Responsible Consumer Behavior Research? A Bibliometric Analysis. Sustainability. 2020;12(8):3141. doi: 10.3390/su12083141. [DOI] [Google Scholar]
- Nowakowska J, Sobocinska J, Lewicki M, Lemanska Z, Rzymski P. When science goes viral: The research response during three months of the COVID-19 outbreak. Biomed Pharmacother. 2020;129:110451. doi: 10.1016/j.biopha.2020.110451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal JK. Visualizing the knowledge outburst in global research on COVID-19. Scientometrics. 2021;126:4173–4193. doi: 10.1007/s11192-021-03912-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen B, Waitzberg R, Israeli A. Israel’s rapid rollout of vaccinations for COVID-19. Isr J Health Policy Res. 2021;10(1):1–14. doi: 10.1186/s13584-021-00440-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roshani S, Bagheri R, Mosleh M, Coccia M. What is the relationship between research funding and citation-based performance? A comparative analysis between critical research fields. Scientometrics. 2021;126:7859–7874. doi: 10.1007/s11192-021-04077-9. [DOI] [Google Scholar]
- Rotshild V, Hirsh-Raccah B, Miskin I, Muszkat M, Matok I. Comparing the clinical efficacy of COVID-19 vaccines: a systematic review and network meta-analysis. Sci Rep. 2021;11(1):22777. doi: 10.1038/s41598-021-02321-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Fresneda MA, Jiménez-Contreras E, Ruiz-Fresneda C, Ruiz-Pérez R. Bibliometric Analysis of International Scientific Production on Pharmacologic Treatments for SARS-CoV-2/COVID-19 During 2020. Front Public Health. 2022;9:778203. doi: 10.3389/fpubh.2021.778203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic Treatments for Coronavirus Disease 2019 (COVID-19): A Review. JAMA. 2020;323(18):1824–1836. doi: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- Sarirete A. A Bibliometric Analysis of COVID-19 Vaccines and Sentiment Analysis. Procedia Comput Sci. 2021;194:280–287. doi: 10.1016/j.procs.2021.10.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scavone C, Brusco S, Bertini M et al (2020) Current pharmacological treatments for COVID-19: What’s next?. [Review Article Themed Issue] British. Aust J Pharm:1–12. 10.1111/bph.15072 [DOI] [PMC free article] [PubMed]
- Serafina MB, Bottega A, Foletto VS, Da Rosa TF, Hörner A, Hörner R. Drug repositioning is an alternative for the treatment of coronavirus COVID-19. Int J Antimicrob Agents. 2020;55(6):105969. doi: 10.1016/j.ijantimicag.2020.105969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Salinas D. Ritmo de crecimiento diario de la producción científica sobre Covid-19. Análisis en bases de datos y repositorios en acceso abierto. EPI. 2020;29(2):e290215. doi: 10.3145/epi.2020.mar.15. [DOI] [Google Scholar]
- Verma S, Gustafsson A. Investigating the emerging COVID-19 research trends in the field of business and management: A bibliometric analysis approach. J Bus Res. 2020;118:253–261. doi: 10.1016/j.jbusres.2020.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Bank Open Data (2022) Research and development expenditure (% of GDP). https://data.worldbank.org/indicator/GB.XPD.RSDV.GD.ZS. Accessed March 2022
- WoS. Clarivate Analytics Web of Science (2022) Web of Science Core Collection. https://www.webofscience.com/wos/woscc/basic-search. Accessed Jan 2022
- Wu R, Wang L, Kuo HD, et al. An Update on Current Therapeutic Drugs Treating COVID-19. Curr Pharmacol Rep. 2020;6:56–70. doi: 10.1007/s40495-020-00216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 43 kb)
Data Availability Statement
All data and materials as well as software application support their published claims and comply with field standards.