Abstract
Obesity is a term that has recently been referred to describe a condition in which a person has become a diseased vessel. Obesity’s internal pathology is too mysterious as it has a close resemblance with fatal diseases pathology. Obesity and coronavirus disease 2019 (COVID-19) are simultaneous epidemics declared by many organizations after observing their rampage in the recent world. Oxidative stress, cytokine storm, interleukin, and their contribution to the internal adipocyte environment implicated in the cascades of inflammatory pathology are portrayed here. Major determinants like angiotensin-converting enzyme 2 (ACE2) and renin–angiotensin–aldosterone system (RAAS) axis are highly sensitive molecular factors. Data from various countries suggested a clinical overview of how greater body mass index (BMI) is related to greater COVID-19 risk. It also gives insight into how obese individuals are obligately getting admitted and combating COVID-19 in intensive care unit including children less than 13 years of age under ultimate therapeutic options. There are numerous studies currently taking place for finding a cure for obesity which are mainly focused on natural resources and novel therapies like photobiomodulation (PBM) consisting of laser treatment, infrared treatment, etc. as current pharmacological treatments are reported to have fatal adverse effects. Finally, it is discussed how attenuating obesity will be a solution for future combat strategy. This review gives light on the areas of coagulation, inflammatory parameters, cardiometabolic complications, endothelial dysfunctions, immunological infirmity due to COVID-19 in obese individuals. A conceptual outline about correlation between the inflammatory pathophysiological steps triggering the aggravation of fatal consequences has been drawn in this review.
Graphical abstract
Keywords: Obesity, COVID-19, Cytokine storm, PBM, ACE2, D-dimer, WAT
Introduction
Over the past decade, there are numerous strides in understanding the underlying pathophysiology of obesity which is being reported in journals (Schwartz et al. 2017). Obesity is the condition resulting from excess consuming food or hampered energy expenditure and interlinked with various mortal diseases. Pathophysiology is implicated in many diseases like hepatic steatosis, atherosclerosis, various metabolic syndromes, non-alcoholic fatty liver, and insulin resistance precipitating type-2 diabetes mellitus diseases. As per various literatures, obesity is considered not just a risk factor but an initiator of diseases. Hitherto, the established pathophysiology suggests the involvement of oxidative stress and immunological reactions (Shoelson et al. 2007). This review is aimed towards detecting the relationship of coronavirus disease 2019 (COVID-19) pathology with obesity and how it is causing severity in complications for obese individual. As per numerous reports, it is expected that a huge extent of obese patients with a body mass index (BMI) greater than 25 suffered severe COVID complications. It is also reported that mostly the obese individuals were taken to intensive care units with many challenges of management (Bernard 1995). Obese patients have been reported with possessing more adipose tissues in the areas that cover the larynx and segments of the pharynx. Various literatures reported this as the main cause of bronchoconstriction to a greater extent than non-obese COVID individuals. Managing these patients was challenging in terms of intubation. Due to the limited extension of the truncal region in obese patients, airway flow is easily obstructed (Horner et al. 1989; Yu et al. 2021). Clinical literature reported that obese pregnant patients with COVID-19 caused greater resistance towards nursing staff to perform proning positions as a part of respiratory management due to their immobility caused by obesity (Saraya and Balkwill 1993). On the other hand, there is one “obesity paradox” which is observed and reported in COVID-19 cases that patients with acute respiratory distress syndrome (ARDS) reported to have decreased mortality rate in obese people rather than non-obese COVID-19 individuals (Dana et al. 2021). This review is also aimed to give an overview of relationships at the molecular level. From the established mechanism of action, it is reported that severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2) enters the host cell and triggers a functional downregulation of angiotensin-converting enzyme 2 (ACE2) (Gheblawi et al. 2020). The whole phenomenon is shifted towards triggering pro-inflammatory cascades due to the lack of ACE2/angiotensin (Ang-1–7)/mitochondrial assembly receptor (MasR) axis. A pro-inflammatory cascade explains the phenomena of cytokine storm and it shows synergistic action when ACE2 is compromised by viral infection. The main knotty factor is the presence of ACE2 in adipose tissue in higher amounts; it implies that the higher the adipose tissue, the higher the viral load. Obesity is familiar to trigger chronic inflammation and increase inflammatory factors like interleukin 6 (IL-6) and cytokines; those are reported to cause severity in COVID-19 complications. As per various reports, COVID-19 and obesity share the same pathology, so both cause huge amplification in the severity of disease (Fager and Freidberg 1980; Petrakis et al. 2020; Sanchis-Gomar et al. 2020). Possessing a higher BMI not only means an increase in the risk of infection and complication but also causes an increase in the chance of the appearance of other virulent viral strains. Obesity may result in a parallel pandemic by COVID-19-induced pneumonia and higher mortality in the future. Endothelial dysfunction is caused by obesity such as microvascular thrombosis; in COVID-19, it is interpreted as an increase in D-dimer which implies blood clot formation (Yan et al. 2021; Popkin et al. 2020; Gómez-Mesa et al. 2021). All the parameters in terms of evidence and how they are related are discussed in a sequential algorithm with some molecular level approach.
Obesity and COVID-19—crisscrossing pandemics
Obesity has always been a key factor for many chronic diseases like hypertension, diabetes, dyslipidemia, and cardiovascular complications like atherosclerotic block. Obesity is always a prevailing factor for malignancy and the growth of tumors (Pi-Sunyer 2009). It is established that obesity plays with various pathways of immunological response. Thus, it is obvious that it will trigger many forms of severity in COVID-19. As per various reports to date, it was reported that obesity takes part in the pathogenesis and severity of COVID-19 through altering the BMI (Jiang et al. 2016; Gao et al. 2021). Comorbidities are reported which are related to obesity in COVID-19 via aforesaid complications. As per the WHO report, the USA ranks first on the basis of morbidity and mortality due to obesity. Data from a study in New York City large academic hospital stated that 3615 individuals were tested with COVID-19 out of which 775 are having a BMI of 30–34 kg/m2 and 595 have greater than 35 kg/m2 (Singh and Misra 2020). Diabetes plays an inducer role along with obesity in the severity of COVID-19 as per reports from Mexico. Reports from France and Italy also revealed that increased BMI is the major risk factor for mortality and morbidity in COVID-19 by aggravating the symptoms (Hernández-Galdamez et al. 2020; Mohammad et al. 2021). The complete data related to the reports are given in Table 1. Therefore, obesity is considered a major key factor for severity in COVID-19. As per the aforementioned reports, we can outline dependent factors, so this review will deduce the equation of how the severity of COVID-19 is related to obesity factors from adipose tissue biology and metabolic dysfunctions as well as vascular and immunological point of view (Goossens et al. 2020). Associated risks are presented schematically in Fig. 1.
Table 1.
Data of COVID and BMI relation from many countries
| Country name | COVID-positive patients | Patients with BMI values within (30–34 kg/m2) | Patients with BMI values greater than 35 kg/m2 | References |
|---|---|---|---|---|
| USA | 3615 | 775 (21%) | 595 (16%) |
Singh and Misra (2020) Hernández-Galdamez et al. (2020) Mohammad et al. (2021) Goossens et al. (2020) |
| France | 124 (ICU admitted) | Not reported | 28.2% |
Singh and Misra (2020) Hernández-Galdamez et al. (2020) Mohammad et al. (2021) Goossens et al. (2020) |
| Spain | 48 (ICU admitted) | 48% | 44% (severe) |
Singh and Misra (2020) Hernández-Galdamez et al. (2020) Mohammad et al. (2021) Goossens et al. (2020) |
| Italy | Not known | Not reported | 7.2% |
Singh and Misra (2020) Hernández-Galdamez et al. (2020) Mohammad et al. (2021) Goossens et al. (2020) |
| Korea | 28 (hospitalized) | Not reported | 17.9% (severe) |
Singh and Misra (2020) Hernández-Galdamez et al. (2020) Mohammad et al. (2021) Goossens et al. (2020) |
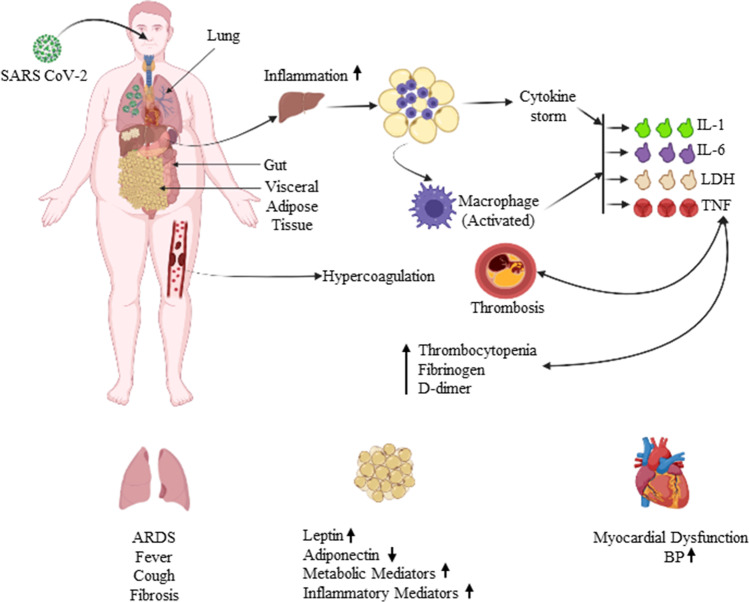
Fig. 1.
Schematic representation of the enhancement of complications and risks associated with respiratory and cardiovascular system precipitated in obese COVID-affected patients. TNF, tumor necrosis factor; LDH, lactic acid dehydrogenase; IL-1, interleukin-1; IL-6, interleukin-6; ARDS, acute respiratory distress syndrome; BP, blood pressure; D-dimer, protein fragment and diagnostic marker for blood clot formation
Impact of adipose tissue in SARS-CoV-2 infection
It is reported that the patients with increased white adipose tissue (WAT) and decreased brown adipose tissue (BAT) are diagnosed with chronically activated RAAS which explains a set of dysfunctions in various systems like the heart and kidney. Molecularly, it is explained as increased reactive oxygen species which is the main culprit not only in vascular dysfunctions as well as insulin resistance followed by diabetes (type 2) (Pahlavani et al. 2017). ROS is responsible for the immune system’s unregulated firing and thus inflammatory action over the pancreas and the death of beta cells. So, obesity indirectly implements cytotoxic activity in the body (Zorov et al. 2014; Echtay et al. 2002). Abnormal activation of RAAS pathway due to aforesaid reasons which is also induced by Ang II and aldosterone leads to cascades of vascular pathogenesis (Ma et al. 2010). Progression of metabolic disorders and immunological disorders is the major outcome of obesity as reported. COVID-19 is chiefly related to the activation and intrinsic activity of ACE2 receptors. So hampered RAAS pathway implies hampered ACE2 activity, which is in turn related to comorbidities. From the reports, it is concluded that increased WAT and decreased BAT (brown adipose tissue) result in severity of symptoms in COVID-19 (Iannelli et al. 2020; Vaduganathan et al. 2020). RAAS component ACE2 is well expressed in adipocytes and plays a major role in the metabolism of glucose and lipid as per many studies performed in vivo obese mice which were on high-fat diet (HFD) (Gupte et al. 2008). Apart from that, it is also reported some drugs which are used to treat obesity-induced complications like antihypertensives, statins, and fenofibrate can upregulate the activity of ACE2 consequently resulting in increased viral load. One of the most important factors is the cytokine storm of various anomalies in COVID-19 progression (Kaur et al. 2020; Ritter et al. 2020). And as per the report, it is stated that white adipocytes are responsible for pro-inflammatory cytokine release (Coppack 2001). Obesity also results in decreased type 1 interferon (IFN) release which is important for antiviral immunity. In a study, it is reported that WAT is responsible for bronchial asthma as it gets deposited on the airway walls and increases thickness so it leads to bronchoconstriction. Apart from that, it also results in increased infiltration of white blood cells (e.g., neutrophil) in the airway. Increased aggregation of immune mediator cells leads to increased cytokine release and increased tissue damage (Teran-Cabanillas et al. 2013; Tian et al. 2019). The whole phenomenon is followed by the incidence of fibrosis and increased comorbidity and severity of COVID-19. Excessive adipose tissue gives rise to a generation of lip fibroblasts which are also called lipofibroblast (LiFs). Lipofibroblast affects lung function and, in the worst-case scenario, it gets transformed and proliferated as myofibroblasts. Myofibroblast triggers collagen deposition and thereby pulmonary fibrosis. Lipofibroblast contains perilipin-2 inside its lipid vesicles at cytoplasm (Kendall and Feghali-Bostwick 2014). Alveolar interstitium-located cells express the excess of ACE2-type 2 epithelial cells. LiFs may produce pulmonary fibrosis (PF) by degeneration of the surfactant-producing cells and collagen deposition. Thus, obesity has a close relation with the severity of COVID-19 in this way (Engin et al. 2020; Hung et al. 2016). Furthermore, it has been revealed that LiFs express ACE2, implying that in the case of COVID-19, it may promote viral load. The extracellular homolog of ACE2 (angiotensin-converting enzyme 2) is anticipated to be the SARS-CoV-2 receptor, which will be coupled through the use of the spike (S) protein. SARS-CoV-2 entrance is dependent on the receptors transmembrane serine protease 2 (TMPRSS2) and ACE2. TMPRSS2 is a transmembrane protease that is triggered by androgens (Gkogkou et al. 2020). The RAS system (renin-angiotensin system), which consists of a cascade of enzymatic events leading up to the generation of many angiotensin peptides, has been linked to obesity in several investigations. Renin promotes proteolytic cleavage of angiotensinogen to form angiotensin-1, which is then transformed to angiotensin-2 by angiotensin-converting enzyme (ACE). Angiotensin-2 attaches to two types of receptors: angiotensin type 1 receptor (AT1R) and angiotensin type 2 receptor (AT2R). However, there are two types of ACEs: ACE1 and ACE2 (Fountain and Lappin 2021; Sztechman et al. 2018). The action of ACE1 leads to inflammation, vasoconstriction, fibrosis, and proliferation; meanwhile, the action of ACE2 causes dilatation functions as an anti-fibrotic agent, and anti-inflammatory even protects against sepsis by stimulating Mas receptors. ACE2 is widely distributed in human bodies and expressed broadly in adipocytes; its expression is upregulated in mice with obesity caused by a high-fat diet. The ACE/Ang II/AT1 receptor (AT1R) axis is downregulated by the ACE2/Ang-1–7/MasR axis (Sztechman et al. 2018). In both human and experimental animal models, mature adipocytes express angiotensinogen and release it intermittently from fat tissue. The angiotensin type 1 (AT1R) and type 2 (AT2R) receptors may mediate the impact of Ang II, causing an increase in adipose tissue lipogenesis (mediated by AT2R) and a decrease in lipolysis (mediated by AT1R) (mediated via AT1R). The RAS pathways were downregulated in obese patients with hypertension. Obesity can cause a condition of moderate chronic inflammation, with TNF and IL-6 levels persistently increased in obese human and mice models (Del Valle et al. 2020; Caci et al. 2020; Hernández-Galdamez et al. (2020)). Obese human subjects and mice had elevated amounts of angiotensinogen and ACE1. Different studies highlight the importance of angiotensin (Ang)-1–7 in metabolic control since Ang-1–7 is likely to play an important role in obesity prevention (Takahashi et al. 2007). Through the synthesis of Ang-1–7 and the Mas receptor, ACE2 has an anti-obesity impact. As evidenced by the following evidence, Ang-(1–7) has an anti-obesity impact. (i) Transgenic rats with elevated levels of Ang-(1–7) are slimmer and do not exhibit diet-induced obesity. (ii) Ang-(1–7) is given orally putting down weight and fat mass in mice. (iii) Hypercholesterolemia, abdominal fat accumulation, glucose intolerance, and decreased insulin sensitivity were all detected in Mas-deficient animals. In ACE2 knockout mice, insulin resistance and inflammation are increased, as well as there is an increase in pro-inflammatory profile in macrophages and lung disease. Natural killer (NK) cells are innate immune system effector lymphocytes that belong to the innate lymphoid cell (ILC) family. They are obligated for the elimination of cells with low or undetectable levels of high expression of stress ligands or major histocompatibility complex (MHC I), both of which are associated with viral infections. Obese patients have a considerable reduction in NK cell cytotoxic activity, which has been linked to increased virus transmission in human and animal models (Kawabe et al. 2019; Cantoni et al. 2020; Kosaraju et al. 2017). In vitro, antibody-dependent cellular cytotoxicity (ADCC) in NK cells was reduced in overweight and obese patients. COVID-19 patients, particularly those who required intensive care (ICU), had smaller percentages of circulating T CD8 + cells and T CD4 + , as well as a decreased capacity to produce antiviral cytokines. Increased blood IL-6 levels were also seen in these obese COVID-affected ICU patients, which was associated with a lower frequency of granzyme-expressing NK cells and a lower cytotoxic capacity. Tocilizumab, which is an anti-IL-6 monoclonal antibody, was used off-label to restore the cytotoxic activity of NK cells. Obesity causes a decrease in T-cell receptors and has also been linked to a reduction in lymph node size. As a result, the immunological response is compromised. In obese people, adipocytes suppress the anti-inflammatory response. T-cell fatigue and persistent inflammation are eventually precipitated by this cascade of downregulated pathways (Mazzoni et al. 2020; Magnuson et al. 2017; Smith et al. 2009). As a result, we now have a foundation to establish a relationship between obesity and worsening inflammatory and immunological severity in COVID patient infection, and the relation with adipose tissue is depicted in Fig. 2.
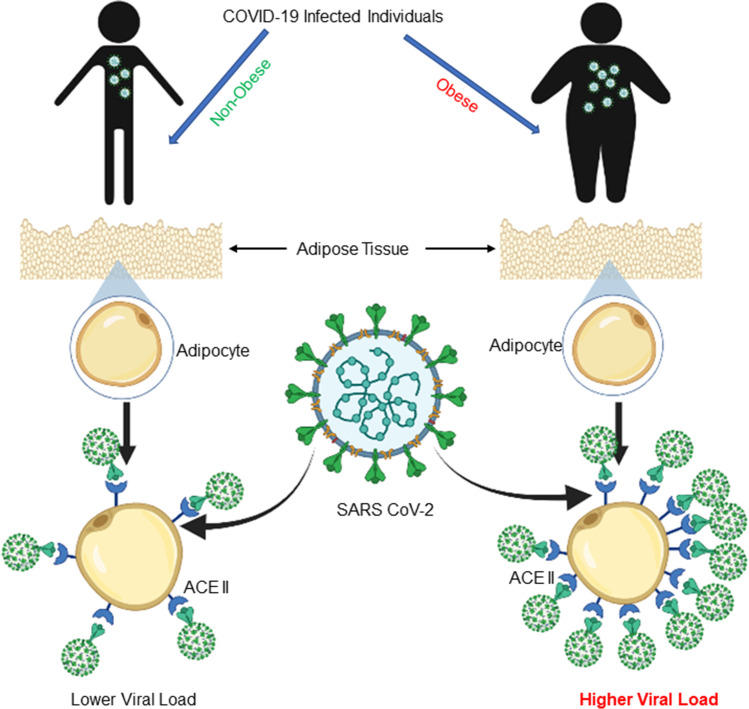
Fig. 2.
Schematic depiction of obesity being a cause of increment of viral load due to possessing higher expression of ACE2. ACE2 receptor, angiotensin-converting enzyme 2 receptor
Inflammatory alterations in obesity and COVID-19
The inflammatory response is one of the important determinants in the progression of COVID complications. Complications lead to chronic disease progress and death of COVID-affected patients. Inflammatory pathogenesis starts with the activation of IL-17, IL-1, IL-6, C-reactive protein, IL-18, and interferon (Tanaka et al. 2014; Gleeson et al. 2021). COVID-19 is associated with multiple forms of inflammatory reactions. All the mortality and morbidity were observed for these inflammatory responses. Various immunological parameters that have been seen in patients with COVID have much more similarities with the immunological parameters seen in obesity (Albashir 2020). From this point of view, COVID-19 and obesity can be correlated with each other. Unlike COVID-19, obesity shows low-grade inflammation; furthermore, this will lead to aggravated immunological cascade and metabolic disorders in chronic disorders. White adipose tissues (WAT) are the significant indicator that orchestrates obesity; it contains adipocytes, immune, and epithelial cells. WAT is also a production hub for cytokines; thus, unregulated entries in the number and contents of WAT lead to cellular necrosis, activating the local cellular immune response, and hypoxia. On the other hand, IL6 and TNF-alpha are the triggering factors of inflammation linked to COVID-19 comorbidities (Longo et al. 2019; Gubernatorova et al. 2020). Macrophages are attracted by monocyte chemoattractant protein-1 (MCP-1) towards the inflammatory sites due to an abundance of IL6 and TNF-alpha. Studies have been shown that there is infirmity of interferon production in obese individuals. IFN is the most important combating factor for viral infection. It implies that the complication in obese individuals will be severe and there will be a higher risk of comorbidities and mortality (Deshmane et al. 2009; García-Sastre 2017). Molecularly, it is shown in the following diagram (Fig. 3).
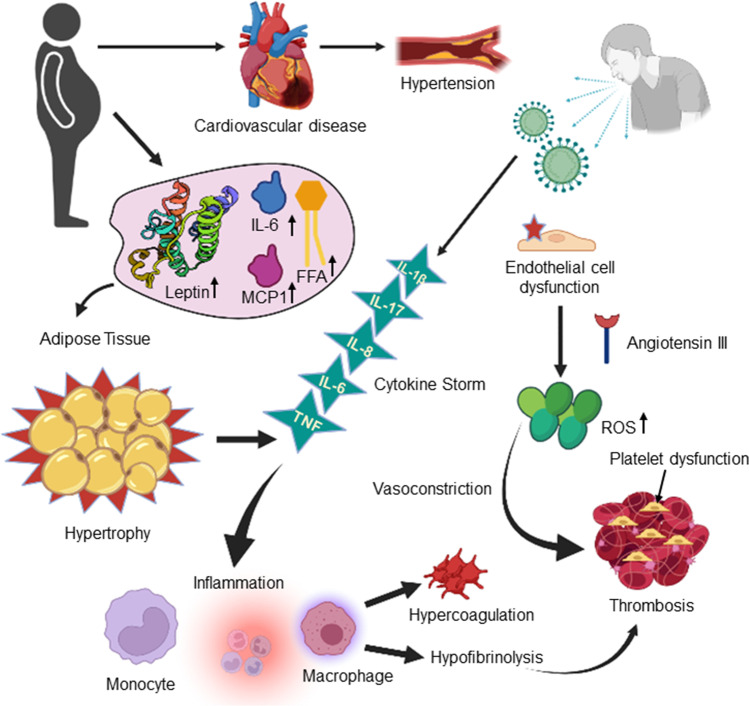
Fig. 3.
Pictorial illustration of altered inflammatory cascades and outcomes causing severity in obese COVID-affected individuals. ROS, reactive oxygen species; MCP-1, monocyte chemoattractant protein-1; IL, interleukin; TNF, tumor necrosis factor; FFA, free fatty acids
Coagulation alterations in obesity and COVID-19
Blood coagulation parameters have been reported to be altered increasingly which implies COVID-19 severity. As per recent studies, it is concluded that COVID-19 complication is linked with coagulation disorders and thrombus or embolus formation which is called disseminated intravascular coagulation (DIC). Obese COVID-19 patients who are hospitalized frequently have higher D-dimer levels than non-obese COVID-19 patients, followed by fibrin/fibrinogen disintegration, partial thromboplastin time, anomalies of prothrombin time, and atherosclerotic pathologies (Tang et al. 2020). Likely in the obesity scenario, there is a close similarity of hypercoagulopathy seen in COVID. The reason is that excess body weight, especially abdominal fat that leads to vascular dysfunction, releases adipokines which result in pro-inflammatory, prothrombotic, and embolization states. Hypertension, dyslipidemia, insulin resistance, cellular necrosis, thrombocytopenia, DM-II, and systemic oxidative stress are the contributions of obesity which in turn contributes to the severity of COVID complications (Abou-Ismail et al. 2020; Redinger 2007; Hayden 2020). Adipokines/inflammatory factors such as IL6, IL8, and TNF-alpha lead to the secretion of von Willebrand factor (vWF) from the endothelium layer followed by the activation of platelet adherence and aggregation which leads to thrombotic events. As per various reports, it is stated that shear stress produced by excess fat increases the expression ACE-II which promotes the production of NO to increase vascular permeation. As a consequence, SARS-CoV2 has greater access to increase viral load (Bernardo et al. 2004; Rajendran et al. 2013). Therefore, to prevent thromboembolic events, anticoagulation drugs such as heparin and enoxaparin are reported to have a preference for treating typically ill hospitalized obese COVID-19 patients (Barnes et al. 2020). Coagulation hamperment is depicted in Fig. 3.
Obesity and therapeutics against COVID-19
Obesity-related disorders enhance the probability of SARS-CoV-2 infection severity. The administration of multiple medications daily is required to regulate such illnesses, and their impact on the body’s ability to respond to infections is being widely debated around the world. Studies on this interaction have led to the development of prospective therapeutic targets aimed at reducing or preventing the severity of the symptoms. In the absence of a COVID-19 vaccine, research into existing potential medications is critical for battling COVID-19 and lowering the high mortality rate associated with obesity. As previously mentioned, chronic inflammation in the lungs caused by obesity triggers a cytokine storm by overexpressing pro-inflammatory mediators like TNF-alpha and IL-6. This is one of the mechanisms by which imbalanced inflammation in the host worsens the prognosis of COVID-19-affected persons with obesity. As a result, anti-inflammatory medications may play an essential role in safeguarding patients with obesity, particularly those with COVID-19 multi-organ damage, because inflammation is a common feature of COVID-19 and obesity aggravation. However, this must be carefully considered because lowering the pan-inflammatory response might also lengthen the time it takes for successful viral clearance. Anti-inflammatory medicines that are effective against COVID-19 are a concern. The use of non-steroidal anti-inflammatory medicines (NSAIDs) and corticosteroids has been linked to an increased risk of developing severe COVID-19. Corticosteroids are the ultimate option for COVID treatment to give a jerk in the body’s combat mechanism as we have seen in ICU patients. COVID with obesity has proven to have more steroidal drugs in a prescription for disease management; as a result, it leads to transient immune compromisation in those patients followed by a second opportunity for the viral manifestation and superinfections (Woods et al. 2020; Moore et al. 2020; Costela-Ruiz et al. 2020). On the other hand, this type of medication has the potential to synergize the diabetes precipitation action. Schematically, how steroidal medications are affecting immune response is shown in Fig. 4.
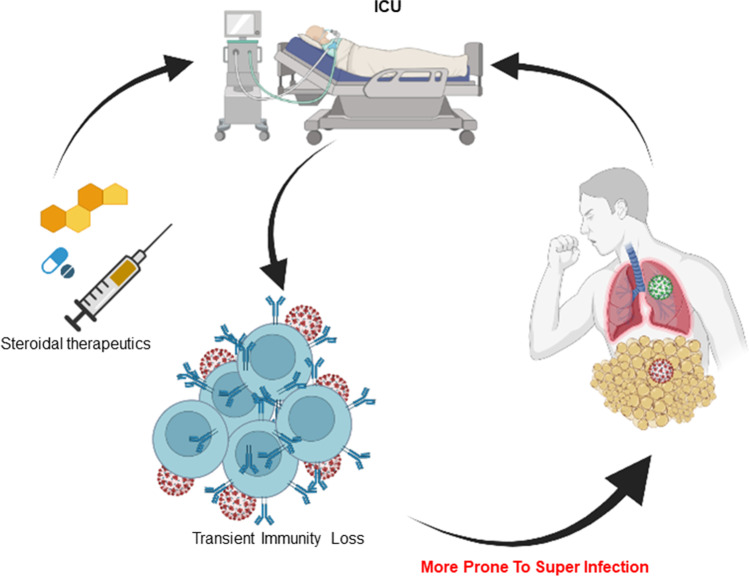
Fig. 4.
Therapeutics and drawbacks in ICU-admitted obese COVID individual with the chances of having superinfection; ICU, intensive care unit
COVID-19 severity associated with cardiometabolic complications in obese individuals
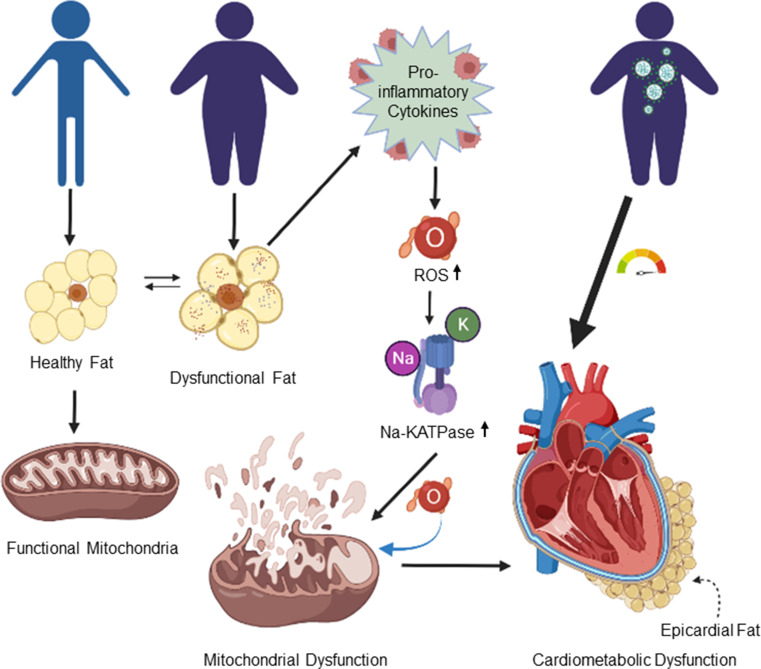
In endangered individuals, the causal agent of COVID-19 triggers a significant reaction, resulting in hyper-inflammation, cytokine storm syndrome, and acute heart damage, all of which are linked to disease severity and outcome. According to the literature, different cardiometabolic risk factors are associated with increasing the severity of COVID-19 leading to a high mortality rate (Costela-Ruiz et al. 2020; Moazzami et al. 2020). According to recent observational data, visceral adipose tissue, rather than total body mass, may play a role in predicting COVID-19 severity. In between the myocardial and the visceral layer of the pericardium, the heart’s visceral adipose tissue is a possible source of inflammatory mediators, i.e., interleukin (IL)-1β, tumor necrosis factor (TNF)-a, and IL-6. Epicardial adipose tissue (EAT) has recently been suggested as a major causative agent for myocardial inflammation in association with COVID-19 (Iacobellis and Bianco 2011). Cardiometabolic complications and linking other parameters are reproduced in Fig. 5.
Fig. 5.
Pictorial demonstration of cardiometabolic complication that arises from mitochondrial degeneration in molecular overview. ROS, reactive oxygen species; Na-KATPase, sodium–potassium ATPase enzyme; ATP, adenosine triphosphate
Post-hoc study—analyzing the epicardia adipose tissue
A cohort study was performed between the 25th of February and the 19th of April in the year 2020 among 652 COVID-19 patients, aimed to analyze the characteristics of EAT in obese individuals. The current study comprised a total of 192 patients with specific characteristics. The median age of the patients was 60 years of which 76% were men. This study comprised of the patients where overall 70% were obese. The total group’s median EAT volume was 2510 (1561; 3539) mm3 followed by 95.8 (99.1; 93.0) HU median EAT-At. EAT volume was found to be linked with BMI and EAT-At with systemic inflammation. The two groups had identical BMI and EAT volume, but EAT-At was much higher in patients with severe illness (Calder et al. 2011; Ellulu et al. 2017). It can be established that on a chest CT, increased EAT attenuation was a sign of EAT inflammation. The first and most important event that leads to whole-body metabolic disorders is visceral adipose tissue inflammation which can further lead to coronary microvascular inflammation and its dysfunctions. Infection with SARS-CoV-2 may cause EAT inflammation. The angiotensin-converting enzyme 2 (ACE2) receptor, which is abundantly expressed throughout the circulatory system, including EAT, allows SARS-CoV-2 to enter cells. SARS-CoV-2 binding to ACE2 lowers ACE2 expression on the surface, perhaps leading to EAT inflammation (Camici et al. 2020; Fontana et al. 2007). Although EAT-derived inflammatory mediators may have systemic consequences, EAT is more likely to act as a “fuel for cardiac inflammation” during COVID-19, boosting the release of cytokines like leptin, IL-1, IL-6, and TNF-alpha as well as free fatty acids. As we know, in obese individuals, there are high chances of leptin resistance and it acts as a cytokine itself; hence, it may induce lipotoxicity in obese COVID-19 individuals (Fain 2006). Therefore, in the population of this particular study, systemic inflammation, hyperglycemia, and impaired respiratory function were all significantly linked to the likelihood of ICU admission of the COVID-19 patients, invasive ventilation, or mortality (Shang et al. 2020). Future research should look into factors linked to an increased risk of SARS-CoV-2 infection in obese people, the role of ectopic fat deposition, and the mechanisms driving EAT inflammation and heart damage in COVID-19.
Visceral fat accumulation and COVID-19—a cohort study
The renin–angiotensin–aldosterone system (RAAS) includes the angiotensin-converting enzyme (ACE) 2 receptor for the severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2) (RAAS). It is found in a variety of tissues, including white adipose tissue (WAT). In patients with extreme or morbid obesity, visceral fat (VF) has a higher expression of ACE2 than subcutaneous fat (SCF). The cohort study was aimed to show that visceral fat accumulation, rather than SCF levels or BMI, is a better predictor of COVID-19 severity (Bourgonje et al. 2020; Smith 2015). This cohort included the combined study of 46 patients from Nice and 119 patients from Paris with symptomatic COVID-19. It mainly aimed among the non-obese patients to compare the severity of the disease. The average age of the combined cohort (patients from Paris and Nice) was 64 years and 17 months, with a mean SCF of 152.8 ± 103.4 cm2, a mean VF of 131.7 ± 101.3 cm2, and a mean BMI of 26.1 ± 5.4 kg/m2. The SCF/VF ratio was lower in patients with severe COVID-19 (p = 0.010), but there was no difference in subcutaneous fat between patients with mild and severe COVID-19. According to the ROC curve, a VF area of 128.5 cm2 provided the greatest predictive value for severe COVID-19. A high VF quantity of 128.5 cm2 was strongly linked with the severity of COVID-19 in each patient cohort (Yordanov et al. 2021; Huang et al. 2020; Kuiken et al. 2003). Because subcutaneous fat is unrelated to COVID-19 severity, the visceral to subcutaneous fat ratio is also irrelevant. Only high VF was linked to COVID-19 severity in a multivariate analysis, but not age or gender. Given that adults and men have more visceral fat than children and women, the amount of VF is likely to account for the link between illness severity, age, and sex. COVID-19 kills men and the elderly more often than it kills women and the young. Excess food consumption limits the ability of white adipose tissue to expand. WAT accepts extra calories above a certain threshold by producing pro-inflammatory adipocytokines in the VF and ectopic fat storage, which leads to insulin resistance. As a result, severe COVID-19 is linked to fatty liver, elevated epicardial adipose tissue, and increased intramuscular fat. Hence, it can be concluded that COVID-19 severity may be influenced by mechanisms other than the RAAS imbalance found in the WAT. ACE inhibitor or angiotensin receptor blocker use to manage hypertension in COVID-19 patients does not reduce the risk of developing severe COVID-19. By this cohort study, it was also believed that constitutive upregulation of ACE2 is the receptor of SARS-CoV-2 for attachment in VF which may contribute to the cytokine storm, although more research into the mechanisms is needed (Savoia et al. 2021; Vincent and Taccone 2020; Phua et al. 2020; Ye et al. 2020).
Future perspectives
As discussed throughout the review, obesity is a familiar, very serious, and expensive chronic disorder that triggers many mortal pathologies or aggravates normal diseases to a fatal one. In this current world, the main scene is played by COVID and its complications (Sanyaolu et al. 2020). As we have seen literature reported the major drawbacks of obesity in the purview of COVID complications those includes increment in a cytokine storm, having the triple risk of hospitalization for obese individuals, triggering impairment in immunological reactions, obesity reduces lung capacity and reserve volume these leads to respiratory failure followed by a painful death, many studies reported the huge ICU admission, invasive mechanical ventilation, and deaths for the people with higher BMI even in under 65 age (Demeulemeester et al. 2021; Favre et al. 2021). Children were reported to get diagnosed with obesity and COVID and suffered worse outcomes from COVID-19. In a report of COVID-affected cases, in patients of 18 years and younger than those, possessing obesity was 3.07 times more prone for hospitalization and 1.42 times greater risk of severe illness (ICU admission) than that of normal individuals (Kompaniyets et al. 2021; Tsankov et al. 2021; Kim et al. 2020; Alsaied et al. 2020). So, attenuating obesity or staying healthy and maintaining COVID precautions are the keys to avoiding such worse outcomes. As we know, for a healthy human being, COVID can be cured without any complications if it gets diagnosed in the very first place. Numerous researches are going on to attenuate obesity, with major areas focusing on the extraction of agents from natural sources for exploration or looking for a cure by PBM (photobiomodulation) therapies (Liebert et al. 2019). The established drugs for anti-obesity actions are showing severe side effects on long-term use (Cheung et al. 2013), so scientists are looking for promising natural agents which can show the desired action with lesser or no adverse effects (Sun et al. 2016). In this simultaneous epidemic (COVID and obesity), we all have to maintain good physical and mental health.
Conclusion
COVID-19 and its relationship to adiposity are key predictors of severe illness. Endothelial dysfunction, immunological dysregulation, hypercytokinemia, and cardiovascular abnormalities are all possible mechanisms by which an overabundance of adipose tissue can cause the acute hyper-inflammatory state that is characteristic of extreme SARS-CoV-2 infections and is responsible for their outcomes. Increased circulating levels of the pro-inflammatory adipokine leptin, combined with lower expression of the anti-inflammatory-acting ACE2 receptors in the lung epithelium of infected individuals, prevent the innate immune response from being cleared, resulting in catastrophic repercussions for the patients. Due to increased cytokine secretion by adipose tissue and associated immune cells, as well as elevated ferritin levels, the immune system can potentially overreact as a consequence of pro-inflammatory “priming,” resulting in a cytokine storm. As a result of the immune system’s inability to deliver a sufficient immunological response, virus clearance is hampered. Nevertheless, high-risk patients, such as the geriatric and those with obesity, may suffer from a less effective immune response and a lower lasting immunological memory, limiting vaccine effectiveness. The relevance of a healthier life in influencing the trajectory of COVID-19 disease is one of the most significant experiences gained from the catastrophe. There are numerous factors associated with COVID that may be proved to be future drug targets for obesity attenuation followed by reducing the chance of insulin resistance, thereby precipitating diabetes and other immunological and coagulable complications propagated by obesity. In order to create effective prophylactic and therapeutic strategies, future research must understand the sources of severity and complications. The current paper reviews the anatomical, immunological, and molecular alterations linked with obesity, which render obese people more susceptible to COVID-19 infection and make managing its comorbidities more difficult.
Acknowledgements
The authors are thankful to the Department of Pharmaceutical Sciences & Technology, Birla Institute of Technology, Mesra, Ranchi, for providing literature review facility and essential data for preparing the manuscript.
Abbreviations
- COVID-19
Coronavirus disease 2019
- ACE2
Angiotensin-converting enzyme 2
- RAAS
Renin–angiotensin–aldosterone system
- IL-6
Interleukin 6
- ARDS
Acute respiratory distress syndrome
- ANG-1–7
Angiotensin (1–7)
- MasR
Mas (mitochondrial assembly) receptor
- BMI
Body mass index
- WAT
White adipose tissue
- BAT
Brown adipose tissue
- HFD
High-fat diet
- IFN
Interferon
- LIF
Lipofibroblast
- PFL
Pulmonary fibrosis
- SARS-CoV-2
Severe acute respiratory syndrome-coronavirus 2
- TMPRSS2
Transmembrane serine protease 2
- AT1R
Angiotensin II type 1 receptor
- ACE
Angiotensin-converting enzyme
- NK
Natural killer cell
- ILC
Innate lymphoid cell
- MHC
Major histocompatibility complex
- ADCC
Antibody-dependent cellular cytotoxicity
- MCP1
Monocyte chemoattractant protein-1
- TNF-α
Tumor necrosis factor-α
- DIC
Disseminated intravascular coagulation
- DM2
Type 2 diabetes mellitus
- vWF
von Willebrand factor
- EAT
Epicardial adipose tissue
Author contribution
All the authors contributed to the work. Payel Mal, Tuhin Mukherjee, and Abhay K Upadhyay conducted the literature analysis and prepared the original draft. Satyajit Mohanty made the revisions and editing. Ashok Pattnaik conceptualized, edited, and critically revised the work. All the authors have read and approved the final manuscript.
Data availability
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors have approved the manuscript and agree with submission to Environmental Science and Pollution Research (ESPR).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abou-Ismail MY, Diamond A, Kapoor S, Arafah Y, Nayak L. The hypercoagulable state in COVID-19: incidence, pathophysiology, and management. Thromb Res. 2020;194:101–115. doi: 10.1016/j.thromres.2020.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albashir A. The potential impacts of obesity on COVID-19. Clin Med (lond) 2020;20(4):e109–e113. doi: 10.7861/clinmed.2020-0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsaied T, Aboulhosn JA, Cotts TB, Daniels CJ, Etheridge SP, Feltes TF, Gurvitz MZ, Lewin MB, Oster ME, Saidi A. Coronavirus disease 2019 (COVID-19) pandemic implications in pediatric and adult congenital heart disease. J Am Heart Assoc. 2020;9(12):e017224. doi: 10.1161/JAHA.120.017224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes GD, Burnett A, Allen A, Blumenstein M, Clark NP, Cuker A, Dager WE, Deitelzweig SB, Ellsworth S, Garcia D, Kaatz S, Minichiello T. Thromboembolism and anticoagulant therapy during the COVID-19 pandemic: interim clinical guidance from the anticoagulation forum. J Thromb Thrombolysis. 2020;50(1):72–81. doi: 10.1007/s11239-020-02138-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard RW. The sombrero technique for stenting a nipple reconstruction. Plast Reconstr Surg. 1995;95(5):942–943. [PubMed] [Google Scholar]
- Bernardo A, Ball C, Nolasco L, Moake JF, Dong JF. Effects of inflammatory cytokines on the release and cleavage of the endothelial cell-derived ultralarge von Willebrand factor multimers under flow. Blood. 2004;104(1):100–106. doi: 10.1182/blood-2004-01-0107. [DOI] [PubMed] [Google Scholar]
- Bourgonje AR, Abdulle AE, Timens W, Hillebrands JL, Navis GJ, Gordijn SJ, Bolling MC, Dijkstra G, Voors AA, Osterhaus AD, van der Voort PH, Mulder DJ, van Goor H. Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19) J Pathol. 2020;251(3):228–248. doi: 10.1002/path.5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caci G, Albini A, Malerba M, Noonan DM, Pochetti P, Polosa R. COVID-19 and obesity: dangerous liaisons. J Clin Med. 2020;9(8):2511. doi: 10.3390/jcm9082511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calder PC, Ahluwalia N, Brouns F, Buetler T, Clement K, Cunningham K, Esposito K, Jönsson LS, Kolb H, Lansink M, Marcos A, Margioris A, Matusheski N, Nordmann H, O'Brien J, Pugliese G, Rizkalla S, Schalkwijk C, Tuomilehto J, Wärnberg J, … Winklhofer-Roob BM (2011) Dietary factors and low-grade inflammation in relation to overweight and obesity. The British journal of nutrition, 106 Suppl 3, S5–S78. 10.1017/S0007114511005460 [DOI] [PubMed]
- Camici PG, Tschöpe C, Di Carli MF, Rimoldi O, Van Linthout S. Coronary microvascular dysfunction in hypertrophy and heart failure. Cardiovasc Res. 2020;116(4):806–816. doi: 10.1093/cvr/cvaa023. [DOI] [PubMed] [Google Scholar]
- Cantoni C, Granata S, Bruschi M, Spaggiari GM, Candiano G, Zaza G. Recent advances in the role of natural killer cells in acute kidney injury. Front Immunol. 2020;11:1484. doi: 10.3389/fimmu.2020.01484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung BM, Cheung TT, Samaranayake NR. Safety of antiobesity drugs. Therapeutic Advances in Drug Safety. 2013;4(4):171–181. doi: 10.1177/2042098613489721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppack SW. Pro-inflammatory cytokines and adipose tissue. Proc Nutr Soc. 2001;60(3):349–356. doi: 10.1079/pns2001110. [DOI] [PubMed] [Google Scholar]
- Costela-Ruiz VJ, Illescas-Montes R, Puerta-Puerta JM, Ruiz C, Melguizo-Rodríguez L. SARS-CoV-2 infection: the role of cytokines in COVID-19 disease. Cytokine Growth Factor Rev. 2020;54:62–75. doi: 10.1016/j.cytogfr.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dana R, Bannay A, Bourst P. Obesity and mortality in critically ill COVID-19 patients with respiratory failure. Int J Obes. 2021;45:2028–2037. doi: 10.1038/s41366-021-00872-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Valle DM, Kim-Schulze S, Huang HH, Beckmann ND, Nirenberg S, Wang B, Lavin Y, Swartz TH, Madduri D, Stock A, Marron TU, Xie H, Patel M, Tuballes K, Van Oekelen O, Rahman A, Kovatch P, Aberg JA, Schadt E, Jagannath S, … Gnjatic S (2020) An inflammatory cytokine signature predicts COVID-19 severity and survival. Nature medicine, 26(10), 1636–1643. 10.1038/s41591-020-1051-9 [DOI] [PMC free article] [PubMed]
- Demeulemeester F, de Punder K, van Heijningen M, van Doesburg F. Obesity as a risk factor for severe COVID-19 and complications: a review. Cells. 2021;10(4):933. doi: 10.3390/cells10040933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): an overview. Journal of Interferon & Cytokine Research : the Official Journal of the International Society for Interferon and Cytokine Research. 2009;29(6):313–326. doi: 10.1089/jir.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echtay KS, Roussel D, St-Pierre J, Jekabsons MB, Cadenas S, Stuart JA, Harper JA, Roebuck SJ, Morrison A, Pickering S, Clapham JC, Brand MD. Superoxide activates mitochondrial uncoupling proteins. Nature. 2002;415(6867):96–99. doi: 10.1038/415096a. [DOI] [PubMed] [Google Scholar]
- Ellulu MS, Patimah I, Khaza'ai H, Rahmat A, Abed Y. Obesity and inflammation: the linking mechanism and the complications. Archives of Medical Science : AMS. 2017;13(4):851–863. doi: 10.5114/aoms.2016.58928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engin AB, Engin ED, Engin A. Two important controversial risk factors in SARS-CoV-2 infection: obesity and smoking. Environ Toxicol Pharmacol. 2020;78:103411. doi: 10.1016/j.etap.2020.103411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fager CA, Freidberg SR. Analysis of failures and poor results of lumbar spine surgery. Spine. 1980;5(1):87–94. doi: 10.1097/00007632-198001000-00015. [DOI] [PubMed] [Google Scholar]
- Fain JN. Release of interleukins and other inflammatory cytokines by human adipose tissue is enhanced in obesity and primarily due to the nonfat cells. Vitam Horm. 2006;74:443–477. doi: 10.1016/S0083-6729(06)74018-3. [DOI] [PubMed] [Google Scholar]
- Favre G, Legueult K, Pradier C, Raffaelli C, Ichai C, Iannelli A, Redheuil A, Lucidarme O, Esnault V (2021) Visceral fat is associated to the severity of COVID-19. Metabolism: clinical and experimental, 115, 154440. 10.1016/j.metabol.2020.154440 [DOI] [PMC free article] [PubMed]
- Fontana L, Eagon JC, Trujillo ME, Scherer PE, Klein S. Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes. 2007;56(4):1010–1013. doi: 10.2337/db06-1656. [DOI] [PubMed] [Google Scholar]
- Fountain JH, Lappin SL (2021). Physiology, renin angiotensin system. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK470410/ [PubMed]
- Gao, Min , Piernas,Carmen, Astbury Nerys M, Julia Hippisley-Cox(2021) “Associations between body-mass index and COVID-19 severity in 6·9 million people in England: a prospective, community-based, cohort study”. The Lancet Diabetes & Endocrinology, vol 9, no. 6, pp. 350–359. Elsevier BV, 10.1016/s2213-8587(21)00089-9. Accessed 18 Nov. [DOI] [PMC free article] [PubMed]
- García-Sastre A. Ten strategies of interferon evasion by viruses. Cell Host Microbe. 2017;22(2):176–184. doi: 10.1016/j.chom.2017.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gheblawi M, Wang K, Viveiros A, Nguyen Q, Zhong JC, Turner AJ, Raizada MK, Grant MB, Oudit GY. Angiotensin-converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system: celebrating the 20th anniversary of the discovery of ACE2. Circ Res. 2020;126(10):1456–1474. doi: 10.1161/CIRCRESAHA.120.317015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gkogkou E, Barnasas G, Vougas K, Trougakos IP. Expression profiling meta-analysis of ACE2 and TMPRSS2, the putative anti-inflammatory receptor and priming protease of SARS-CoV-2 in human cells, and identification of putative modulators. Redox Biol. 2020;36:101615. doi: 10.1016/j.redox.2020.101615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleeson LE, Roche HM, Sheedy FJ. Obesity, COVID-19 and innate immunometabolism. Br J Nutr. 2021;125(6):628–632. doi: 10.1017/S0007114520003529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Mesa JE, Galindo-Coral S, Montes MC, Muñoz Martin AJ. Thrombosis and coagulopathy in COVID-19. Curr Probl Cardiol. 2021;46(3):100742. doi: 10.1016/j.cpcardiol.2020.100742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goossens GH, Dicker D, Farpour-Lambert NJ, Frühbeck G, Mullerova D, Woodward E, Holm J-C (2020) Obesity and COVID-19: a perspective from the European association for the study of obesity on immunological perturbations, therapeutic challenges, and opportunities in obesity. Obes Facts;13:439–452. 10.1159/000510719 [DOI] [PMC free article] [PubMed]
- Gubernatorova EO, Gorshkova EA, Polinova AI, Drutskaya MS. IL-6: Relevance for immunopathology of SARS-CoV-2. Cytokine Growth Factor Rev. 2020;53:13–24. doi: 10.1016/j.cytogfr.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupte M, Boustany-Kari CM, Bharadwaj K, Police S, Thatcher S, Gong MC, English VL, Cassis LA (2008) ACE2 is expressed in mouse adipocytes and regulated by a high-fat diet. American journal of physiology. Regulatory, integrative and comparative physiology, 295(3), R781–R788. 10.1152/ajpregu.00183.2008 [DOI] [PMC free article] [PubMed]
- Hayden MR. An immediate and long-term complication of COVID-19 may be type 2 diabetes mellitus: the central role of β-cell dysfunction, apoptosis and exploration of possible mechanisms. Cells. 2020;9(11):2475. doi: 10.3390/cells9112475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Galdamez DR, González-Block MÁ, Romo-Dueñas DK, Lima-Morales R, Hernández-Vicente IA, Lumbreras-Guzmán M, Méndez-Hernández P. Increased risk of hospitalization and death in patients with COVID-19 and pre-existing noncommunicable diseases and modifiable risk factors in Mexico. Arch Med Res. 2020;51(7):683–689. doi: 10.1016/j.arcmed.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner RL, Mohiaddin RH, Lowell DG, Shea SA, Burman ED, Longmore DB, Guz A. Sites and sizes of fat deposits around the pharynx in obese patients with obstructive sleep apnoea and weight matched controls. Eur Respir J. 1989;2(7):613–622. [PubMed] [Google Scholar]
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, … Cao B (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet (London, England), 395(10223), 497–506. 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed]
- Hung YH, Hsieh WY, Hsieh JS, Liu FC, Tsai CH, Lu LC, Huang CY, Wu CL, Lin CS. Alternative roles of STAT3 and MAPK signaling pathways in the MMPs activation and progression of lung injury induced by cigarette smoke exposure in ACE2 knockout micE. Int J Biol Sci. 2016;12(4):454–465. doi: 10.7150/ijbs.13379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacobellis G, Bianco AC. Epicardial adipose tissue: emerging physiological, pathophysiological and clinical features. Trends Endocrinol Metab. 2011;22(11):450–457. doi: 10.1016/j.tem.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannelli A, Favre G, Frey S, Esnault V, Gugenheim J, Bouam S, Schiavo L, Tran A, Alifano M. Obesity and COVID-19: ACE 2, the missing tile. Obes Surg. 2020;30(11):4615–4617. doi: 10.1007/s11695-020-04734-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang SZ, Lu W, Zong XF, Ruan HY, Liu Y. Obesity and hypertension. Exp Ther Med. 2016;12(4):2395–2399. doi: 10.3892/etm.2016.3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur U, Acharya K, Mondal R, Singh A, Saso L, Chakrabarti S, Chakrabarti SS. Should ACE2 be given a chance in COVID-19 therapeutics: a semi-systematic review of strategies enhancing ACE2. Eur J Pharmacol. 2020;887:173545. doi: 10.1016/j.ejphar.2020.173545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawabe Y, Mori J, Morimoto H, Yamaguchi M, Miyagaki S, Ota T, Tsuma Y, Fukuhara S, Nakajima H, Oudit GY, Hosoi H (2019) ACE2 exerts anti-obesity effect via stimulating brown adipose tissue and induction of browning in white adipose tissue. American journal of physiology. Endocrinology and metabolism, 317(6), E1140–E1149. 10.1152/ajpendo.00311.2019 [DOI] [PubMed]
- Kendall RT, Feghali-Bostwick CA. Fibroblasts in fibrosis: novel roles and mediators. Front Pharmacol. 2014;5:123. doi: 10.3389/fphar.2014.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim L, Whitaker M, O'Halloran A, Kambhampati A, Chai SJ, Reingold A, Armistead I, Kawasaki B, Meek J, Yousey-Hindes K, Anderson EJ, Openo KP, Weigel A, Ryan P, Monroe ML, Fox K, Kim S, Lynfield R, Bye E, Shrum Davis S, … COVID-NET Surveillance Team (2020) Hospitalization rates and characteristics of children aged <18 years hospitalized with laboratory-confirmed COVID-19 - COVID-NET, 14 States, March 1-July 25, 2020. MMWR. Morbidity and mortality weekly report, 69(32), 1081–1088. 10.15585/mmwr.mm6932e3 [DOI] [PMC free article] [PubMed]
- Kompaniyets L, Agathis NT, Nelson JM, Preston LE, Ko JY, Belay B, Pennington AF, Danielson ML, DeSisto CL, Chevinsky JR, Schieber LZ, Yusuf H, Baggs J, Mac Kenzie WR, Wong KK, Boehmer TK, Gundlapalli AV, Goodman AB. Underlying medical conditions associated with severe COVID-19 illness among children. JAMA Netw Open. 2021;4(6):e2111182. doi: 10.1001/jamanetworkopen.2021.11182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosaraju R, Guesdon W, Crouch MJ, Teague HL, Sullivan EM, Karlsson EA, Schultz-Cherry S, Gowdy K, Bridges LC, Reese LR, Neufer PD, Armstrong M, Reisdorph N, Milner JJ, Beck M, Shaikh SR (2017) B cell activity is impaired in human and mouse obesity and is responsive to an essential fatty acid upon murine influenza infection. Journal of immunology (Baltimore, Md. : 1950), 198(12), 4738–4752. 10.4049/jimmunol.1601031 [DOI] [PMC free article] [PubMed]
- Kuiken T, Fouchier RA, Schutten M, Rimmelzwaan GF, van Amerongen G, van Riel D, Laman JD, de Jong T, van Doornum G, Lim W, Ling AE, Chan PK, Tam JS, Zambon MC, Gopal R, Drosten C, van der Werf S, Escriou N, Manuguerra JC, Stöhr K, … Osterhaus AD (2003) Newly discovered coronavirus as the primary cause of severe acute respiratory syndrome. Lancet (London, England), 362(9380), 263–270. 10.1016/S0140-6736(03)13967-0 [DOI] [PMC free article] [PubMed]
- Liebert A, Bicknell B, Johnstone DM, Gordon LC, Kiat H, Hamblin MR. “Photobiomics”: can light, including photobiomodulation, alter the microbiome? Photobiomodulation, Photomedicine, and Laser Surgery. 2019;37(11):681–693. doi: 10.1089/photob.2019.4628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo M, Zatterale F, Naderi J, Parrillo L, Formisano P, Raciti GA, Beguinot F, Miele C. Adipose tissue dysfunction as determinant of obesity-associated metabolic complications. Int J Mol Sci. 2019;20(9):2358. doi: 10.3390/ijms20092358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma TK, Kam KK, Yan BP, Lam YY. Renin-angiotensin-aldosterone system blockade for cardiovascular diseases: current status. Br J Pharmacol. 2010;160(6):1273–1292. doi: 10.1111/j.1476-5381.2010.00750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnuson AM, Regan DP, Fouts JK, Booth AD, Dow SW, Foster MT. Diet-induced obesity causes visceral, but not subcutaneous, lymph node hyperplasia via increases in specific immune cell populations. Cell Prolif. 2017;50(5):e12365. doi: 10.1111/cpr.12365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzoni A, Salvati L, Maggi L, Capone M, Vanni A, Spinicci M, Mencarini J, Caporale R, Peruzzi B, Antonelli A, Trotta M, Zammarchi L, Ciani L, Gori L, Lazzeri C, Matucci A, Vultaggio A, Rossi O, Almerigogna F, Parronchi P, … Cosmi L (2020) Impaired immune cell cytotoxicity in severe COVID-19 is IL-6 dependent. J Clin Investig 130(9):4694–4703. 10.1172/JCI138554 [DOI] [PMC free article] [PubMed]
- Moazzami B, Chaichian S, Kasaeian A, Djalalinia S, Akhlaghdoust M, Eslami M, Broumand B. Metabolic risk factors and risk of Covid-19: A systematic review and meta-analysis. PLoS ONE. 2020;15(12):e0243600. doi: 10.1371/journal.pone.0243600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammad S, Aziz R, Al Mahri S. Obesity and COVID-19: what makes obese host so vulnerable? Immun Ageing. 2021;18:1. doi: 10.1186/s12979-020-00212-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore N, Carleton B, Blin P, Bosco-Levy P, Droz C. Does ibuprofen worsen COVID-19? Drug Saf. 2020;43(7):611–614. doi: 10.1007/s40264-020-00953-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahlavani M, Kalupahana NS, Ramalingam L, Moustaid-Moussa N. Regulation and functions of the renin-angiotensin system in white and brown adipose tissue. Compr Physiol. 2017;7(4):1137–1150. doi: 10.1002/cphy.c160031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrakis D, Margină D, Tsarouhas K, Tekos F, Stan M, Nikitovic D, Kouretas D, Spandidos DA, Tsatsakis A. Obesity - a risk factor for increased COVID-19 prevalence, severity and lethality (Review) Mol Med Rep. 2020;22(1):9–19. doi: 10.3892/mmr.2020.11127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phua J, Weng L, Ling L, Egi M, Lim CM, Divatia JV, Shrestha BR, Arabi YM, Ng J, Gomersall CD, Nishimura M, Koh Y, Du B, Asian Critical Care Clinical Trials Group (2020) Intensive care management of coronavirus disease 2019 (COVID-19): challenges and recommendations. The Lancet. Respiratory medicine, 8(5), 506–517. 10.1016/S2213-2600(20)30161-2 [DOI] [PMC free article] [PubMed]
- Pi-Sunyer X. The medical risks of obesity. Postgrad Med. 2009;121(6):21–33. doi: 10.3810/pgm.2009.11.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popkin BM, Du S, Green WD, Beck MA, Algaith T, Herbst CH, Alsukait RF, Alluhidan M, Alazemi N, Shekar M. Individuals with obesity and COVID-19: a global perspective on the epidemiology and biological relationships. Obesity Reviews : an Official Journal of the International Association for the Study of Obesity. 2020;21(11):e13128. doi: 10.1111/obr.13128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajendran P, Rengarajan T, Thangavel J, Nishigaki Y, Sakthisekaran D, Sethi G, Nishigaki I. The vascular endothelium and human diseases. Int J Biol Sci. 2013;9(10):1057–1069. doi: 10.7150/ijbs.7502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redinger RN. The pathophysiology of obesity and its clinical manifestations. Gastroenterology & Hepatology. 2007;3(11):856–863. [PMC free article] [PubMed] [Google Scholar]
- Ritter A, Kreis NN, Louwen F, Yuan J. Obesity and COVID-19: molecular mechanisms linking both pandemics. Int J Mol Sci. 2020;21(16):5793. doi: 10.3390/ijms21165793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchis-Gomar F, Lavie CJ, Mehra MR, Henry BM, Lippi G. Obesity and outcomes in COVID-19: when an epidemic and pandemic collide. Mayo Clin Proc. 2020;95(7):1445–1453. doi: 10.1016/j.mayocp.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanyaolu A, Okorie C, Marinkovic A, Patidar R, Younis K, Desai P, Hosein Z, Padda I, Mangat J, Altaf M (2020) Comorbidity and its impact on patients with COVID-19. SN comprehensive clinical medicine, 1–8. Advance online publication. 10.1007/s42399-020-00363-4 [DOI] [PMC free article] [PubMed]
- Saraya KA, Balkwill FR. Temporal sequence and cellular origin of interleukin-2 stimulated cytokine gene expression. Br J Cancer. 1993;67(3):514–521. doi: 10.1038/bjc.1993.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savoia C, Volpe M, Kreutz R. Hypertension, a moving target in COVID-19: current views and perspectives. Circ Res. 2021;128(7):1062–1079. doi: 10.1161/CIRCRESAHA.121.318054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MW, Seeley RJ, Zeltser LM, Drewnowski A, Ravussin E, Redman LM, Leibel RL (2017) Obesity pathogenesis: an endocrine society scientific statement. Endocr Rev 38(4):267–296 [DOI] [PMC free article] [PubMed]
- Shang Y, Pan C, Yang X, Zhong M, Shang X, Wu Z, Yu Z, Zhang W, Zhong Q, Zheng X, Sang L, Jiang L, Zhang J, Xiong W, Liu J, Chen D. Management of critically ill patients with COVID-19 in ICU: statement from front-line intensive care experts in Wuhan China. Annals of Intensive Care. 2020;10(1):73. doi: 10.1186/s13613-020-00689-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoelson SE, Herrero L, Naaz A (2007) Obesity, inflammation, and insulin resistance. Gastroenterology 132(6):2169–2180. 10.1053/j.gastro.2007.03.059 [DOI] [PubMed]
- Singh AK, Misra A. Impact of COVID-19 and comorbidities on health and economics: focus on developing countries and India. Diabetes & Metabolic Syndrome. 2020;14(6):1625–1630. doi: 10.1016/j.dsx.2020.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith U. Abdominal obesity: a marker of ectopic fat accumulation. J Clin Investig. 2015;125(5):1790–1792. doi: 10.1172/JCI81507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AG, Sheridan PA, Tseng RJ, Sheridan JF, Beck MA. Selective impairment in dendritic cell function and altered antigen-specific CD8+ T-cell responses in diet-induced obese mice infected with influenza virus. Immunology. 2009;126(2):268–279. doi: 10.1111/j.1365-2567.2008.02895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun NN, Wu TY, Chau CF. Natural dietary and herbal products in anti-obesity treatment. Molecules (basel, Switzerland) 2016;21(10):1351. doi: 10.3390/molecules21101351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sztechman D, Czarzasta K, Cudnoch-Jedrzejewska A, Szczepanska-Sadowska E, Zera T (2018) Aldosterone and mineralocorticoid receptors in regulation of the cardiovascular system and pathological remodelling of the heart and arteries. Journal of physiology and pharmacology : an official journal of the Polish Physiological Society, 69(6), 10.26402/jpp.2018.6.01 [DOI] [PubMed]
- Takahashi E, Kuranaga N, Satoh K, Habu Y, Shinomiya N, Asano T, Seki S, Hayakawa M. Induction of CD16+ CD56bright NK cells with antitumour cytotoxicity not only from CD16- CD56bright NK cells but also from CD16- CD56dim NK cells. Scand J Immunol. 2007;65(2):126–138. doi: 10.1111/j.1365-3083.2006.01883.x. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol. 2014;6(10):a016295. doi: 10.1101/cshperspect.a016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. Journal of Thrombosis and Haemostasis : JTH. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teran-Cabanillas E, Montalvo-Corral M, Caire-Juvera G, Moya-Camarena SY, Hernández J (2013) Decreased interferon-α and interferon-β production in obesity and expression of suppressor of cytokine signaling. Nutrition (Burbank, Los Angeles County, Calif.), 29(1), 207–212. 10.1016/j.nut.2012.04.019 [DOI] [PubMed]
- Tian Y, Jennings J, Gong Y, Sang Y. Viral infections and interferons in the development of obesity. Biomolecules. 2019;9(11):726. doi: 10.3390/biom9110726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsankov BK, Allaire JM, Irvine MA, Lopez AA, Sauvé LJ, Vallance BA, Jacobson K. Severe COVID-19 infection and pediatric comorbidities: a systematic review and meta-analysis. International Journal of Infectious Diseases : IJID : Official Publication of the International Society for Infectious Diseases. 2021;103:246–256. doi: 10.1016/j.ijid.2020.11.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaduganathan M, Vardeny O, Michel T, McMurray J, Pfeffer MA, Solomon SD. Renin-angiotensin-aldosterone system inhibitors in patients with Covid-19. N Engl J Med. 2020;382(17):1653–1659. doi: 10.1056/NEJMsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Taccone FS. Understanding pathways to death in patients with COVID-19. Lancet Respir Med. 2020;8(5):430–432. doi: 10.1016/S2213-2600(20)30165-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods JA, Hutchinson NT, Powers SK, Roberts WO, Gomez-Cabrera MC, Radak Z, Berkes I, Boros A, Boldogh I, Leeuwenburgh C, Coelho-Júnior HJ, Marzetti E, Cheng Y, Liu J, Durstine JL, Sun J, Ji LL. The COVID-19 pandemic and physical activity. Sports Medicine and Health Science. 2020;2(2):55–64. doi: 10.1016/j.smhs.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan T, Xiao R, Wang N, Shang R, Lin G. Obesity and severe coronavirus disease 2019: molecular mechanisms, paths forward, and therapeutic opportunities. Theranostics. 2021;11(17):8234–8253. doi: 10.7150/thno.59293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Q, Wang B, Mao J. The pathogenesis and treatment of the ‘cytokine storm’ in COVID-19. J Infect. 2020;80(6):607–613. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yordanov Y, Dinh A, Bleibtreu A, Mensch A, Lescure FX, Debuc E, Jourdain P, Jaulmes L, Dechartres A, AP-HP/Universities/Inserm COVID-19 research collaboration (2021) Clinical characteristics and factors associated with hospital admission or death in 43 103 adult outpatients with coronavirus disease 2019 managed with the Covidom telesurveillance solution: a prospective cohort study. Clinical microbiology and infection: the official publication of the European Society of Clinical Microbiology and Infectious Diseases, 27(8), 1158–1166. 10.1016/j.cmi.2021.04.010 [DOI] [PMC free article] [PubMed]
- Yu W, Rohli KE, Yang S, Jia P. Impact of obesity on COVID-19 patients. J Diabetes Complications. 2021;35(3):107817. doi: 10.1016/j.jdiacomp.2020.107817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol Rev. 2014;94(3):909–950. doi: 10.1152/physrev.00026.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.