Abstract
Previously, we reported isolation and characterization of mutacin III and genetic analysis of mutacin III biosynthesis genes from the group III strain of Streptococcus mutans, UA787 (F. Qi, P. Chen, and P. W. Caufield, Appl. Environ. Microbiol. 65:3880–3887, 1999). During the same process of isolating the mutacin III structural gene, we also cloned the structural gene for mutacin I. In this report, we present purification and biochemical characterization of mutacin I from the group I strain CH43 and compare mutacin I and mutacin III biosynthesis genes. The mutacin I biosynthesis gene locus consists of 14 genes in the order mutR, -A, -A′, -B, -C, -D, -P, -T, -F, -E, -G, orfX, orfY, orfZ. mutA is the structural gene for mutacin I, while mutA′ is not required for mutacin I activity. DNA and protein sequence analysis revealed that mutacins I and III are homologous to each other, possibly arising from a common ancestor. The mature mutacin I is 24 amino acids in size and has a molecular mass of 2,364 Da. Ethanethiol modification and peptide sequencing of mutacin I revealed that it contains six dehydrated serines, four of which are probably involved with thioether bridge formation. Comparison of the primary sequence of mutacin I with that of mutacin III and epidermin suggests that mutacin I likely has the same bridging pattern as epidermin.
Lantibiotics are lanthionine-containing small-peptide antibiotics that are produced by gram-positive bacteria (11, 32). The lantibiotics are ribosomally synthesized and posttranslationally modified (34). The modification reactions include dehydration of serine and threonine residues and addition of thiol groups from cysteine residues to the double bond to form lanthionines and β-methyllanthionines, respectively. Some dehydrated serine or threonine residues may remain as such in the mature lantibiotic peptide.
Based on the secondary structures, Jung assigned lantibiotics into two classes, types A (linear) and B (globular) (11). de Vos et al. (6) and Sahl and Bierbaum (31) further divided each class into subgroups according to their primary peptide sequences. Thus, subgroup AI contains the nisin-like lantibiotics, with nisin, subtilin, epidermin, and pep5 as the most thoroughly characterized members (1, 7, 8, 12, 38). Subgroup AII consists of lacticin 481, SA-FF22, salivaricin, and variacin (10, 25, 26, 30). The genes responsible for biosynthesis of lantibiotics are organized in operon-like structures. The biosynthesis locus of all members in the subgroup AI lantibiotics consists of lanA, the structural gene for the lantibiotic; lanB and lanC, the modifying enzyme genes for posttranslational modification of the preprolantibiotic; lanP, the protease gene for processing of the prelantibiotic; and lanT, the ABC transporter for secretion of the lantibiotic. In addition, epidermin and gallidermin have an extra gene, lanD, which is responsible for the C-terminal oxidative decarboxylation of the lantibiotic (17, 18). In comparison, subgroup AII lantibiotics have simpler genomic organizations. In subgroup AII, lanB and lanC are combined into one gene, lanM, and lanP and lanT are combined into lanT (3, 27, 29). All lantibiotic loci also contain a set of immunity genes, which are responsible for self-protection of the producer strains (33). Moreover, expression of the lantibiotic genes is usually regulated either by a single transcription regulator (24, 27) or by a two-component signal transduction system (5, 15, 16).
Previously, our group reported isolation, biochemical, and genetic characterizations of mutacin II, produced by a group II strain of the oral bacterium Streptococcus mutans (3, 22, 23, 27). Mutacin II belongs to subgroup AII in the lantibiotic family. Recently we reported isolation and genetic characterization of mutacin III from the group III S. mutans strain UA787 (28). The mature mutacin III is 22 amino acids in size and shows striking similarity with another lantibiotic, epidermin, produced by Staphylococcus epidermidis (1). The mutacin III biosynthesis gene locus consists of 11 genes in the order mutR, -A, -A′, -B, -C, -D, -P, -T, -F, -E, -G. The genomic organization and primary sequence of mutacin III place it in subgroup AI, with epidermin and gallidermin as its closest neighbors. In this communication, we report the biochemical and genetic characterization of another subgroup AI lantibiotic, mutacin I. Comparison of the biosynthesis genes between mutacins I and III revealed striking similarities as well as important differences. These differences may help to unravel the mechanism of lantibiotic modification, processing, and antimicrobial specificity.
MATERIALS AND METHODS
Bacterial strains and media.
The group I S. mutans strain CH43 originated from a Chinese schoolchild as part of a natural history study of human caries (Y. Li and P. W. Caufield, unpublished data). Strain CH43 contains a cryptic plasmid similar to other 5.6-kb plasmids within the S. mutans group I strains (P. W. Caufield and X. Zou, unpublished data). S. sanguis strain NY101 was used as the indicator for mutacin activity assays. CH43 and NY101 were grown on Todd-Hewitt (TH) plates with 1.6% agar (Difco Laboratories, Detroit, Mich.) unless indicated otherwise.
Cloning and sequencing of the mutacin I biosynthesis genes.
Cloning and sequencing of the mutacin I biosynthesis genes were performed exactly as described previously (28).
Insertional inactivation.
The mutA and mutA′ genes were inactivated separately by inserting a kanamycin resistance gene cassette exactly as described for mutacin III (28).
Isolation and purification of mutacin I.
For mutacin production, CH43 was grown on TH-agar plate for 1 day under anaerobic conditions. The cells were then spread on a PHWP membrane with 0.3-μm pore size (Millipore Corp., Bedford, Mass.) on top of a TH plate containing 0.3% agarose. The plate was incubated at 37°C for 2 days anaerobically. The membrane was transferred to a new plate for continued incubation every 2 days, and the old plate was frozen at −70°C. For mutacin isolation, the plates were thawed quickly in a 60°C water bath. The liquid medium was separated from the agarose debris by centrifugation, and the supernatant was passed through a membrane with 0.45-μm pore size. Mutacin I was extracted with an equal volume of chloroform as previously described (22). The precipitate was dried under a stream of air and washed once with double-distilled H2O. The water-insoluble material (crude extract) was dissolved in 6 M urea and tested for antimicrobial activity by a plate assay after serial dilution with double-distilled H2O. One arbitrary unit of activity was defined as the highest dilution that showed a clear zone of inhibition of the indicator strain NY101.
For purification, the crude extract of mutacin I was applied to a Source 15RPC column and eluted with a fragmented gradient of A (0.1% trifluoroacetic acid [TFA]) and B (0.085% TFA in 60% acetonitrile) using a LKB Purifier (Amersham Pharmacia Biotech, Piscataway, N.J.). The active fractions were pooled and dried in a lyophilizer. The pellet was redissolved in 0.25% TFA and subjected to a second round of purification using a fragmented gradient of buffer A (0.1% TFA) and B (0.085% TFA in 80% methanol). The single active peak fraction was collected, dried in a lyophilizer, and used for sequence analysis and electrospray ionization mass spectrometry (EIMS).
Chemical modification of mutacin I.
Fifty-microgram aliquots of purified mutacin I were dried under vacuum and resuspended in 90 μl of a derivatization mixture consisting of 280 μl of ethanol, 200 μl of water, 65 μl of 5 M sodium hydroxide, and 60 μl of ethanethiol as described elsewhere (20). The reaction proceeded at 50°C for 1 h under nitrogen and was then stopped by the addition of 2 μl of acetic acid. The reaction mixture was dried under vacuum and washed three times with 50% ethanol. The pellet was resuspended in 10 μl of 50% acetonitrile with 1% formic acid for EIMS analysis and peptide sequencing by Edman degradation.
Nucleic acid accession numbers.
The sequences reported have been submitted to GenBank with accession no. AF207710 (for mutA to mutT genes) and AF267498 (for mutFEG and orfXYZ genes).
RESULTS
Cloning and sequencing of the mutacin I biosynthesis genes.
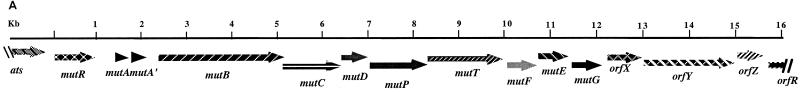
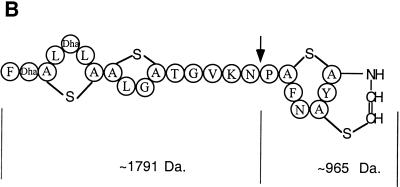
As described previously (28), while isolating mutacin III biosynthesis genes by PCR amplification using a pair of primers designed based on the conserved sequences among LanA and LanB proteins, we also isolated the mutacin I biosynthesis genes with the same primers. Sequencing of the isolated PCR fragment revealed a striking similarity between mutacin I and mutacin III genes. By chromosomal walking, the major part of mutacin I biosynthesis operon was cloned and sequenced. Sequence analysis revealed eight genes in the order mutR, -A, -A′, -B, -C, -D, -P, -T, preceded by an alanine tRNA synthetase gene (ats), which may mark the upstream border of the mutacin locus.
Cloning of the downstream genes was facilitated by the S. mutans genome database (University of Oklahoma). A search for the ats gene returned a contig (contig 257) which contained the ats gene followed by three ABC transporters and then a homolog of the mutF gene in mutacin III. Further downstream were genes similar to spaF and spaG of the subtilin biosynthesis locus (14), which were followed by a putative fimbrial assembly protein and a pair of two-component signal transduction protein genes (orfR/K) that were similar to the pair of histidine kinase/response regulator genes in the nisin (nisK/nisR) and subtilin (spaK/spaR) operons (15, 16) (data not shown). Using the sequence information, primers from the spaG and spaR homolog regions were synthesized and used with an upstream primer in mutT to PCR amplify the intervening region. Cloning and sequencing of the PCR products revealed genes in the order mutF, -E, -G, orfX, orfY, orfZ, orfR (Fig. 1A). Interestingly, this genomic organization is different in the region between mutG and the response regulator orfR than that in the S. mutans genome database, which is from a mutacin-nonproducing strain, UA159. In UA159, the intergenic region harbors a gene encoding a protein similar to fimbrial assembly protein in Dichelobacter nodosus (9); in CH43, the same region harbors three genes, orfX, -Y, and -Z.
FIG. 1.
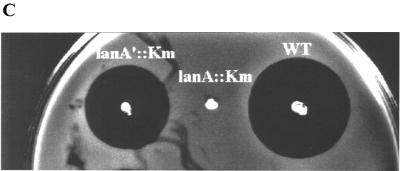
(A) The mutacin I biosynthesis genes. The orientations and relative sizes of the genes are shown. ats is the alanine tRNA synthetase gene, mutR encodes the transcription regulator for the mutacin operon, mutA is the structural gene for prepromutacin I, and mutA′ has no known function. mutB and -C encode the enzymes for dehydration and thioether bridge formation of premutacin I; mutD encodes a flavoprotein possibly responsible for oxydative decarboxylation of the C-terminal cysteine in premutacin I; mutP and -T code for the protease and ABC transporter, respectively, which are responsible for the processing and transportation of premutacin I. mutF, -E, and -G possibly code for proteins in the immunity complex. orfX encodes another ABC transporter of unknown function. OrfY is similar to YtsD in B. subtilis, which shows similarity to NADH dehydrogenase. OrfZ is a hypothetical protein whose function is unknown. OrfR is a response regulator in a two-component signal transduction system, which is not required for mutacin production. (B) Similarity between MutA and MutA′. The middle row shows the identical amino acids and the conserved changes (+). The arrow indicates the processing site in MutA. The leader peptide and the mature peptide moieties were determined based on MutA. (C) Effects of mutA and mutA′ mutations on mutacin I production. Cells from an overnight culture plate were stabbed on a TH-agar plate and incubated at 37°C for 24 h. The plate was heated at 80°C for 1 h to kill the producing bacteria and then overlaid with an overnight culture of the indicator strain NY101. The plate was inspected after an overnight incubation at 37°C. WT, wild type.
In contrast to the nisR/K and spaR/K pairs of nisin and subtilin, which are part of the nisin and subtilin operons, respectively, the orfR/K pair in CH43 as well as in UA159 is present in every S. mutans strain that we tested, regardless of mutacin production (data not shown). Furthermore, unlike nisR/K and spaR/K, insertional inactivation of either gene had no effect on mutacin production (P. Chen et al., unpublished data). Therefore, we concluded that the mutacin I locus consists of 14 genes flanked by the upstream ats gene and the downstream response regulator gene of unknown function (Fig. 1A).
In accordance with the mutacin III operon, MutR was probably the positive regulator for the expression of the mutacin I operon (27, 28). MutA and MutA′ showed strong similarity to each other, especially in the leader peptide region and at the C-terminal part of the mature peptide (Fig. 1B). Insertional inactivation of mutA abolished mutacin production, while inactivation of mutA′ did not (Fig. 1C). This result suggested that, like mutA in the mutacin III operon, mutA in the mutacin I operon was likely the structural gene encoding prepromutacin I. The function of mutA′ is not clear. The small reduction in the zone of inhibition in the mutA′ mutant strain (Fig. 1C) may result from the effect of kanamycin resistance gene insertion, or it may suggest some regulatory role for mutA′. MutB, -C, and -D possibly constituted the modification apparatus for prepromutacin I, and MutT and -P are the ABC transporter and protease for transportation and processing of premutacin I, respectively. MutF, -E, and -G are probably the immunity proteins for mutacin I. The function of OrfX, -Y, and -Z proteins in mutacin production is unknown. OrfX showed strong similarity to other ABC transporters, and OrfY was similar to YtsD in Bacillus subtilis (19), which is similar to NADH dehydrogenase. No similar protein in the database was found for OrfZ.
Similarity between mutacin I and mutacin III biosynthesis genes.
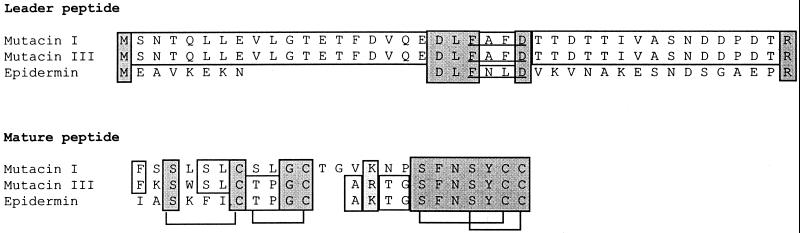
The overall similarity between mutacin I and mutacin III biosynthesis genes was ∼94% at the nucleotide level over a 10-kb region. However, differences between the two operons were not distributed evenly among the different genes. For example, from mutR to the region immediately upstream of mutA, the similarity was 99%, while in the mutA and mutA′ coding regions, the similarity was only 88 and 97%, respectively (data not shown). At the amino acid level, the two MutAs shared 84% identical residues (Fig. 2), and the two MutA′s shared 93% identical residues (data not shown). For MutB and MutC, the similarity was 93 and 95%, respectively (data not shown). An even higher similarity (99%) existed in MutP and -T between the two mutacins (data not shown). The region downstream of mutT also seemed to be similar in mutacin III as in mutacin I (data not shown). These results suggested that the two mutacin loci probably originated from a common ancestor.
FIG. 2.
Comparison of prepropeptides of mutacins I and III, using the sequence of preepidermin as a reference. Amino acids shared by all three lantibiotics are labeled with gray boxes, and those shared by any of two lantibiotics are in open boxes. The conserved sequence FNLD, which is shared by all lantibiotics in subgroup AI (31), is underlined. Brackets indicate the pairs of amino acid residues involved with thioether bridge formation in epidermin (1).
Purification of mutacin I.
To biochemically characterize mutacin I, a sufficient amount of starting material is required. Our first attempt to isolate mutacin I from liquid culture failed because no mutacin I was produced in any of the liquid cultures that we tested. We then tried a stab culture on a TH-agarose plate as described for mutacin III (28). Mutacin I was produced on such a plate; however, the production level was still too low for satisfactory isolation. Based on the observation that mutacin I could be produced on all solid medium plates regardless of medium composition, we reasoned that production of mutacin I may be regulated by a cell density-mediated control mechanism similar to quorum sensing (13, 36). If this was the case, then increasing cell density on the plate may increase mutacin production. Based on this rationale, we used a membrane transfer technique as described in Materials and Methods, which resulted in a satisfactory level of mutacin I production (∼1 mg/liter of supernatant).
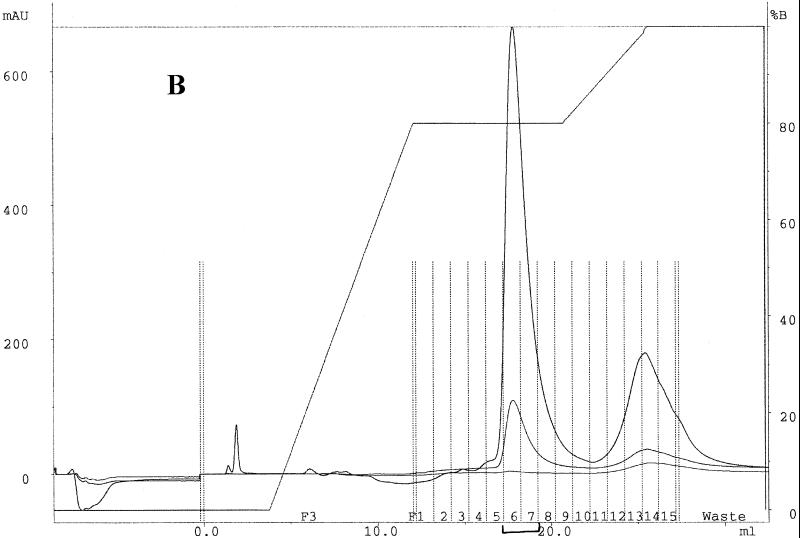
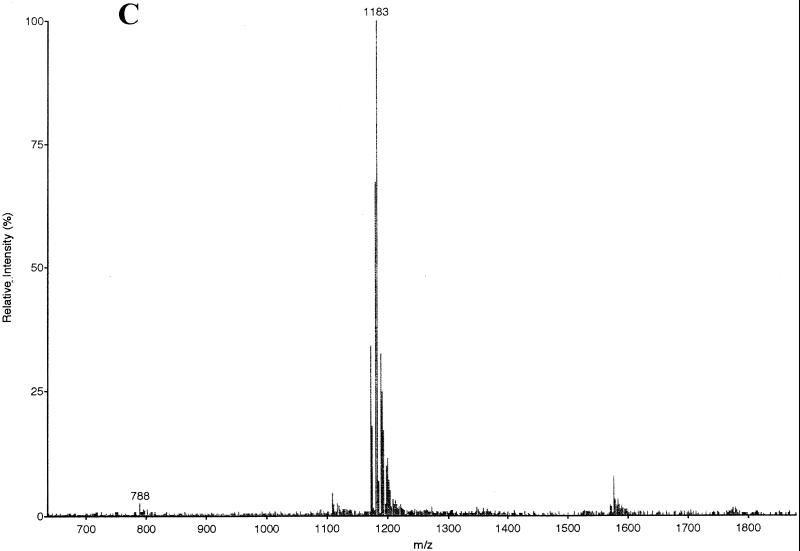
Mutacin I was purified by reverse-phase high-pressure liquid chromatography (HPLC) (Fig. 3). The active fraction (fraction 6) from the first pass (Fig. 3A) was collected and subjected to a second round of purification using a different buffer B and a different gradient (Fig. 3B). The active fractions (fractions 6 and 7) from the second pass were dried under vacuum and tested for purity by EIMS analysis. As shown in Fig. 3C, mutacin I was purified to near homogeneity, as judged by the lack of significant background peaks in the MS chromatogram.
FIG. 3.
Purification and EIMS analyses of mutacin I. (A) Elution profile of the first-round purification of crude extract of mutacin I by reverse-phase HPLC. One-milliliter fractions were collected along the course of elution and tested for antimicrobial activity (insert). MAU, milli-absorption units. (B) Elution profile of the second-round purification using pooled fraction 6 from the first pass as starting material. Fractions 6 and 7 were active. (C) EIMS of the purified mutacin I. The mass-to-charge ratios (m/z) for the doubly charged molecule (1,183) and the triply charged molecule (788) are labeled. The estimated molecular mass was 2,364 Da.
Characterization of mutacin I by ethanethiol derivatization and MS analyses.
The molecular weight of mutacin I was measured by EIMS. The mass-to-charge ratio for the doubly charged molecule was 1,183, and that for the triply charged molecule was 788 (Fig. 3C). Thus, the measured molecular mass was 2,364 Da. This value was in a good agreement with the calculated value of 2,516 Da for the unmodified mutacin I minus six molecules of water (108 Da) and one molecule of carboxy residue (45 Da from decarboxylation at the C-terminal cysteine residue).
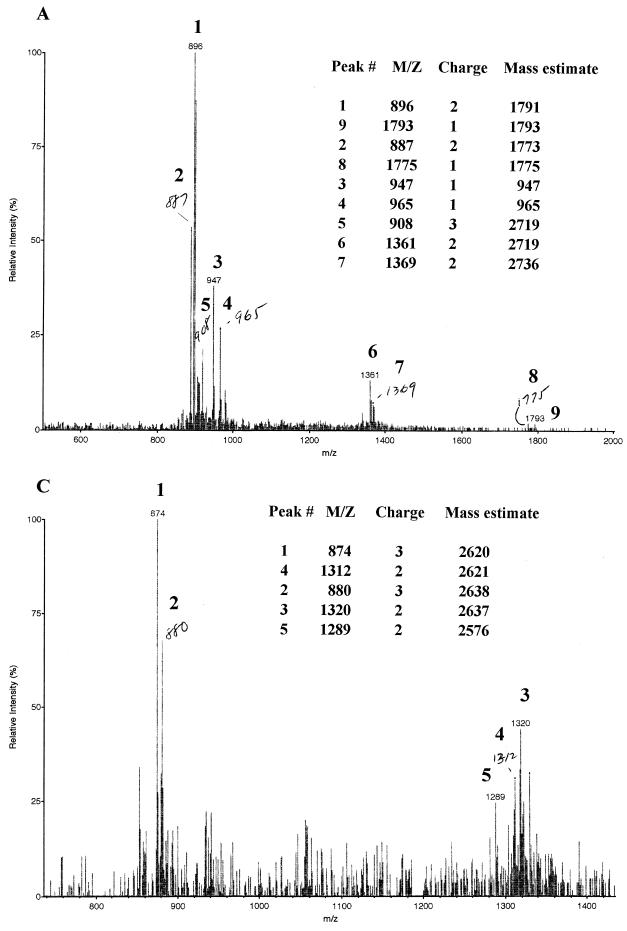
The primary sequence of mutacin I contained six serine residues and one threonine residue, all of which were potential sites for posttranslational dehydration. To confirm that there were indeed six dehydrated residues in the mature mutacin I, we performed an ethanethiol modification of mutacin I under alkaline conditions. In this reaction, one molecule of ethanethiol could insert into the thioether bridge, resulting in a S-ethylcysteine and a cysteine, or it could insert into the double bond of a dehydrated serine or threonine to form an S-ethylcysteine or β-methyl-S-ethylcysteine (20, 23). Ethanethiol derivatization of lantibiotics has been used prior to sequencing of the other lantibiotics gallidermin and pep5 (20) and for determination of the number of dehydrated amino acid residues in mutacin II (23). The expected molecular mass of mutacin I after each addition of an ethanethiol molecule is listed in Table 1. Quite surprisingly, none of the major peaks generated after ethanethiol modification of mutacin I had the expected molecular mass (Fig. 4A). A very small portion of the molecules showed a mass of 2,736 Da (peak 7), which could account for mutacin I plus six molecules of ethanethiol (2,364 + 62 × 6); the rest of the molecules were all much smaller than expected. With close inspection and calculations, we finally determined the identity of the small molecules. As shown in Fig. 4B, it appeared that the majority of mutacin I molecules broke into two fragments after the addition of six molecules of ethanethiol. The larger fragment with a mass of 1,791 Da was the N-terminal part from F-1 to N-16, and the smaller fragment (965 Da) was the C-terminal part from P-17 to C-24. This finding was of interest because the closely related mutacin III molecule remained intact after the same modification reaction under the same conditions (Fig. 4C).
TABLE 1.
Expected molecular masses of ethanethiol derivatives of mutacins I and III
| Mutacin | Expected mass (Da)
|
||||||
|---|---|---|---|---|---|---|---|
| 0a | 1 | 2 | 3 | 4 | 5 | 6 | |
| I | 2,364 | 2,426 | 2,487 | 2,549 | 2,611 | 2,673 | 2,738 |
| III | 2,264 | 2,318 | 2,390 | 2,452 | 2,514 | 2,576 | 2,638 |
Number of ethanethiol molecules added.
FIG. 4.
Biochemical characterization of mutacin I. (A) EIMS analysis of the ethanethiol-derivatized mutacin I. Peaks 1 and 2 are the doubly charged molecules of 1,791 and 1,774 Da, respectively. The 1,774-Da molecule may be a deaminated form of the 1,792-Da molecule. Peak 3 may be a deaminated form of peak 4, both of which are singly charged. Peak 5 and peak 6 are triply and doubly charged molecules of 2,719 Da, respectively. Peak 7 is a doubly charged molecule of 2,736 Da, which gives rise to the deaminated form of 2,719 Da (peaks 5 and 6). Peak 8 is a singly charged, deaminated form of peak 9, which has a molecular mass of 1,793 Da.The expected molecular mass of mutacin I after insertion of six molecules of ethanethiol is 2,736 Da (2,364 + 62 × 6), which correlated very well with the measured mass of 2,736 as shown by peak 7. Addition of the two molecular masses of 1,791 (peak 1) and 965 (peak 4) results in a molecular mass of 2,756 Da, which correlates well with the intact modified mutacin I of 2,736 Da plus one molecule of H2O (from breakage of the molecule). (B) Proposed structure of mutacin I based on the data presented in panel A and in Fig. 2. The arrow indicates the position where the peptide bond is broken in the ethanethiol-modified mutacin I. The calculated molecular mass for each fragment is labeled. (C) EIMS analysis of mutacin III derivatized with ethanethiol under the same conditions as for mutacin I. The expected molecular mass for fully derivatized mutacin III is 2,636 (Table 1), and the measured molecular mass is 2,638 from the doubly and triply charged peaks (peaks 2 and 3). The 2,620-Da molecule as shown by peaks 1 and 4 is probably the deaminated form of the 2,638-Da molecule. The 2,576-Da molecule as shown in peak 5 resulted from addition offive molecules of ethanethiol (Table 1).
Peptide sequencing of unmodified and ethanethiol modified mutacin I.
Comparison of mutacin I and mutacin III revealed that mutacin I had seven potential dehydration sites (six serines and one threonine), while mutacin III had six (four serines and two threonines). Interestingly, both mutacins had six ethanethiol additions after ethanethiol modification (Fig. 4A and C), suggesting that all serine and threonine residues in mutacin III were dehydrated. To determine which serine or threonine residue was not dehydrated in mutacin I, the purified mutacin I was subjected to peptide sequencing by Edman degradation. With native mutacin I, Edman degradation was blocked after the first F residue, suggesting that the second serine residue was dehydrated (data not shown). Dehydrated amino acids were shown to block Edman degradation in other lantibiotics (8, 21, 22).
To obtain the complete sequence of mutacin I, the ethanethiol-derivatized mutacin I had to be used. Ethanethiol derivatization of lantibiotics was shown to allow Edman degradation to proceed through the dehydrated serine and threonine residues and thioether bridges in other lantibiotics (20, 21). Since the majority of mutacin I molecules was broken into two fragments (Fig. 4) during ethanethiol modification, we had to eliminate the C-terminal fragment to solve the problem of having two N termini in the reaction mixture. After several trials, we succeeded by washing the reaction mixture with 30% acetonitrile. The pellet fraction after 30% acetonitrile wash contained mostly the full-length modified mutacin I and the N-terminal fragment. The pellet fraction was found to have the sequence F1-SEC2-SEC3-L4-SEC5-L6-SEC7-SEC8-L9-G10- SEC11-T12-G13-V14-K15-N16-P17-SEC18-F19-N20-SEC21-Y22- SEC23. S-Ethylcysteine (SEC) was the product of ethanethiol insertion into the double bond of dehydrated serine or the thioether bridge in lanthionine. Our results revealed that all six serine residues in the mutacin I molecule were dehydrated and that T-12 remained nondehydrated. In addition, a closer look at the HPLC chromatogram of the sequencing reaction of mutacin I revealed minor peaks in the sequence P-x-F-N-x-Y. This sequence correlated with the C-terminal fragment of mutacin I: P17-S18-F19-N20-S21-Y22-C23-C24. This result corroborated our previous assignment for the two peptide fragments generated during ethanethiol modification (Fig. 4B).
DISCUSSION
In this study, we cloned and sequenced mutacin I biosynthesis genes from the group I strain of S. mutans CH43. DNA and protein sequence analysis revealed that mutacins I and III are homologous to each other, likely arising from a common gene ancestor. Mutacin I was produced by a membrane transfer technique and purified to homogeneity by reverse-phase HPLC. The mature mutacin I is 24 amino acids in size and has a molecular mass of 2,364 Da. Ethanethiol modification of mutacin I revealed that it contains six dehydrated amino acids. Sequencing of the native and ethanethiol-derivatized mutacin I by Edman degradation demonstrated that mutacin I is encoded by mutA and that the six serine residues in the primary sequence of mutacin I, four of which are possibly involved with thioether bridge formation, are dehydrated. Comparison of the primary sequence of mutacin I with that of mutacin III and epidermin suggests that mutacin I likely possesses the same bridging pattern as epidermin.
A closer inspection of differences between the homologous genes of mutacin I and mutacin III revealed that they are not all distributed evenly. For MutR, -D, -P, and -T, the similarity is 99% between the two mutacins, while for MutA, -A′, -B, and -C, the similarity varies from 88 to 95%. The distribution of the variations within a protein also is not even. For example, in MutA, the leader peptide regions were identical between the two mutacins. However, the mature peptide regions differed by 37.5% (Fig. 2). More interestingly, the sequence of the mature mutacin III is closer to that of epidermin (77% similarity) than to that of mutacin I (62.5% similarity), while the sequences of the leader peptides of mutacin III and epidermin are dramatically different (Fig. 2). For MutB, -C, -D, -P, and -T, mutacins I and III are much closer to each other than to epidermin. The question of what determines the specificity of modification and processing of the prelantibiotics then arises (see below).
The biosynthesis of lantibiotics involves several posttranslational modification steps (2, 6, 31). The first step is the translation of the structural mRNA into a prepropeptide. The prepropeptide is then modified by dehydration of serine and threonine residues and subsequent formation of thioether bridges between cysteine and the dehydrated amino acid residues. The prepeptide is then translocated across the cell membrane, where the leader peptide is cleaved off and the mature peptide is released to the outside medium. The mechanism of how this happens and what determines the specificity of modification and processing are not fully understood. It was demonstrated with nisin that the leader peptide is involved in biosynthesis, especially the conserved residues, S-6, F-18 to D-15, which appeared to be essential for modification, secretion, or both (6, 37). Using chimeras constructed from nisin and subtilin, two closely related lantibiotics, Chakicherla and Hansen (2) demonstrated that correct processing requires a specific recognition between the prelantibiotic peptide and the processing machinery. They propose that the leader peptide region is primarily responsible for engaging the prepeptide with the processing machinery, while the overall folding of the prepeptide determines the pattern of thioether bridge formation. On the other hand, dehydration of serine and threonine residues does not seem to be sequence specific. Our finding that the leader peptide and the modification enzymes of mutacin III are either identical or highly similar to those of mutacin I, while the mature peptide is closer to epidermin, suggests that the leader peptide of mutacins may play a more important role in determining substrate specificity for the modification and processing machinery. Alternatively, it may suggest that, although different in primary sequence, the mature peptide moiety in the prepeptide of the two mutacins can fold into similar secondary structures.
One advantage of lantibiotics over classical antibiotics is their gene-encoded nature, which means that lantibiotics can be altered with ease by manipulating the structural genes through mutagenesis. In reality, however, the number of mutations that one can make is limited because the production of active lantibiotics depends on correct posttranslational modification and processing. Any disruption of the normal modification or processing reaction will render the production of an active product less likely. For future construction of analogs of lantibiotics by rational design, it is critical to understand the mechanism of modification and processing and the role that each domain of the proteins plays in the process. The pair of mutacins (mutacins I and III) with similar yet not identical sequences and structures will prove to be a valuable addition to the pool of lantibiotics in facilitating studies of lantibiotic modification and processing. As has been done with nisin and subtilin, swapping the structural or modifying enzyme genes between mutacin I and mutacin III, or constructing chimeric proteins, will enable us to pinpoint the critical residues in the prepeptide or in the modification enzymes that determine the specificity and efficiency of modification and processing.
Mutacins I and III are closely related to each other at both nucleotide and amino acid levels. Comparison of the mature peptide sequences of mutacins I and III suggest that they may also have the same pattern of thioether bridge formation. Despite the many similarities, some important differences exist between the two mutacins. For example, ethanethiol modification of mutacin I cleaved the molecule into two fragments between N-16 and P-17 (Fig. 4B), while the same reaction did not affect the integrity of mutacin III (Fig. 4C). Comparison of the two mutacins revealed that the major difference is at the linker region (T-12 to P-17), where mutacin I has the sequence T-G-V-K-N-P, and mutacin III has the sequence A-R-T-G (Fig. 2). These different amino acid residues, according to the statistical figures of Creighton (4), have different tendencies in forming different secondary structures in proteins. For example, N-16 and P-17 in mutacin I are more likely to be involved in forming β turns, while A-12 in mutacin III is more likely to participate in α-helix formation (35). More importantly, N-16 and P-17 are absent in mutacin III. Although the implication of these features in the secondary structure and stability of mutacin I is not clear at present, their presence may make a difference in tertiary structure between the two mutacins. In fact, our mutagenesis studies indicate that most of the different residues between the two mutacins are not interchangeable (P. Chen et al., unpublished).
In accordance with the possible difference in secondary and tertiary structures, mutacins I and III differ in hydrophobicity and antimicrobial activity. In reverse-phase HPLC analysis, mutacin I is eluted at a higher acetonitrile concentration than mutacin III (data not shown), suggesting a higher hydrophobicity for mutacin I. In antimicrobial spectrum assays with a limited set of pathogens, mutacin III is more potent than mutacin I against Staphylococcus aureus and Staphylococcus epidermidis, while the two mutacins have equal activities against other pathogens such as enterococci, pneumococci, and group A streptococci (F. Qi et al., unpublished data). These observations suggest that the mutacin I and mutacin III system can be used to study the mechanism determining antimicrobial specificity and activity of lantibiotics. Understanding this mechanism will provide much insight into the rational design of lantibiotics to target specific pathogens in the future.
In this study, we developed a membrane transfer technique for the production of mutacin I, based on the premise that mutacin I production may be controlled by a cell density-mediated control mechanism. This technique worked better than the stab culture technique used for mutacin III production (28). A more important aspect of this technique is that it will provide a basis for the development of an immobilized cell culture apparatus for large-scale production of mutacin I in an industrial setting.
ACKNOWLEDGMENTS
We thank W. H. Benjamin for providing the pathogenic strains, K. Morrison for assistance in sequencing mutacin I, and M. Kirk for assistance with EIMS.
This work was supported by NIH grant RO1 DE09082.
REFERENCES
- 1.Allgaier H, Jung G, Werner R G, Schneider U, Zahner H. Epidermin: sequencing of a heterodetic tetracyclic 21-peptide amide antibiotic. Eur J Biochem. 1986;160:9–22. doi: 10.1111/j.1432-1033.1986.tb09933.x. [DOI] [PubMed] [Google Scholar]
- 2.Chakicherla A, Hansen J N. Role of the leader and structural regions of prelantibiotic peptides as assessed by expressing nisin-subtilin chimeras in Bacillus subtilis 168, and characterization of their physical, chemical, and antimicrobial properties. J Biol Chem. 1995;270:23533–23539. doi: 10.1074/jbc.270.40.23533. [DOI] [PubMed] [Google Scholar]
- 3.Chen P, Qi F, Novak J, Caufield P W. The unique genes required for mutacin II biosynthesis in Streptococcus mutans T8 are clustered and can be transferred en bloc. Appl Environ Microbiol. 1999;65:1356–1360. doi: 10.1128/aem.65.3.1356-1360.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Creighton T G. Proteins: structures and molecular principles. New York, N.Y: W. H. Freeman and Company; 1983. p. 235. [Google Scholar]
- 5.de Ruyter P G G A, Kuipers O P, Beerthuyzen M M, van Alan-Boerrigter I, de Vos W M. Functional analysis of promoters in the nisin gene cluster of Lactococcus lactis. J Bacteriol. 1996;178:3434–3439. doi: 10.1128/jb.178.12.3434-3439.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Vos W M, Kuipers O P, van der Meer J R, Siezen R J. Maturation pathway of nisin and other lantibiotics: post-translationally modified antimicrobial peptides exported by Gram-positive bacteria. Mol Microbiol. 1995;17:427–437. doi: 10.1111/j.1365-2958.1995.mmi_17030427.x. [DOI] [PubMed] [Google Scholar]
- 7.Gross E, Morell J L. The number and nature of α,β-unsaturated amino acids in nisin. FEBS Lett. 1968;2:61–64. doi: 10.1016/0014-5793(68)80101-2. [DOI] [PubMed] [Google Scholar]
- 8.Gross E, Morell J L. The structure of nisin. J Am Chem Soc. 1971;93:4634–4635. doi: 10.1021/ja00747a073. [DOI] [PubMed] [Google Scholar]
- 9.Hobbs M, Dalrymple B P, Cox P T, Livingstone S P, Delaney S F, Mattick J S. Organization of the fibrial gene region of Bacteroides nodosus: class I and class II strains. Mol Microbiol. 1991;5:543–560. doi: 10.1111/j.1365-2958.1991.tb00726.x. [DOI] [PubMed] [Google Scholar]
- 10.Hynes W L, Ferretti J P, Tagg J R. Cloning of the gene encoding streptococcin A-FF22, a novel lantibiotic produced by Streptococcus pyogenes, and determination of its nucleotide sequence. Appl Environ Microbiol. 1993;59:1969–1971. doi: 10.1128/aem.59.6.1969-1971.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jung G. Lantibiotics: a survey. In: Jung G, Sahl H G, editors. Nisin and novel lantibiotics. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1991. pp. 1–34. [Google Scholar]
- 12.Kaletta C, Entian K D, Kellner R, Jung G, Reis M, Sahl H-G. Pep5, a new lantibiotic: structural gene isolation and prepeptide sequence. Arch Microbiol. 1989;152:16–19. doi: 10.1007/BF00447005. [DOI] [PubMed] [Google Scholar]
- 13.Kleerebezem M, Quadri L E N, Kuipers O P, De Vos W M. Quorum sensing by peptide pheromones and two-component signal-transduction systems in Gram-positive bacteria. Mol Microbiol. 1997;24:895–904. doi: 10.1046/j.1365-2958.1997.4251782.x. [DOI] [PubMed] [Google Scholar]
- 14.Klein C, Entian K D. Genes involved in self-protection against the lantibiotic subtilin produced by Bacillus subtilis ATCC 6633. Appl Environ Microbiol. 1994;60:2793–2801. doi: 10.1128/aem.60.8.2793-2801.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klein C, Kaletta C, Entian K D. Biosynthesis of the lantibiotic subtilin is regulated by a histidine kinase/response regulator system. Appl Environ Microbiol. 1993;59:296–303. doi: 10.1128/aem.59.1.296-303.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuipers O P, Beerthuyzen M M, de Ruyter P G G A, Luesink E J, de Vos W M. Autoregulation of nisin biosynthesis in Lactococcus lactis by signal transduction. J Biol Chem. 1995;270:27295–27304. doi: 10.1074/jbc.270.45.27299. [DOI] [PubMed] [Google Scholar]
- 17.Kupke T, Kempter C, Gnau V, Jung G, Gotz F. Mass spectroscopic analysis of a novel enzymatic reaction: oxidative decarboxylation of the lantibiotic precursor peptide EpiA catalyzed by the flavoprotein EpiD. J Biol Chem. 1994;269:5653–5659. [PubMed] [Google Scholar]
- 18.Kupke T, Kempter C, Jung G, Gotz F. Oxidative decarboxylation of peptides catalyzed by flavoprotein EpiD: determination of substrate specificity using peptide libraries and neutral loss mass spectrometry. J Biol Chem. 1995;270:11282–11289. doi: 10.1074/jbc.270.19.11282. [DOI] [PubMed] [Google Scholar]
- 19.Lapidus A, Galleron N, Sorokin A, Ehrlich S D. Sequencing and functional annotation of the Bacillus subtilis genes in the 200 kb rrnB-dnaB region. Microbiology. 1997;143:3431–3441. doi: 10.1099/00221287-143-11-3431. [DOI] [PubMed] [Google Scholar]
- 20.Meyer H E, Heber M, Eisermann B, Korte H, Metzger J W, Jung G. Sequence analysis of lantibiotics: chemical derivatization procedures allow a fast access to complete Edman degradation. Anal Biochem. 1994;223:185–190. doi: 10.1006/abio.1994.1571. [DOI] [PubMed] [Google Scholar]
- 21.Mota-Meira M, Lacroix C, LaPointe G, Lavoie M C. Purification and structure of mutacin B-Ny266: a new lantibiotic produced by Streptococcus mutans. FEBS Lett. 1997;410:275–279. doi: 10.1016/s0014-5793(97)00425-0. [DOI] [PubMed] [Google Scholar]
- 22.Novak J, Caufield P W, Miller E J. Isolation and biochemical characterization of a novel lantibiotic mutacin from Streptococcus mutans. J Bacteriol. 1994;176:4316–4320. doi: 10.1128/jb.176.14.4316-4320.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Novak J, Kirk M, Caufield P W, Barnes S, Morrison K, Baker J. Detection of modified amino acids in lantibiotic peptide mutacin II by chemical derivation and electrospray ionization-mass spectroscopic analysis. Anal Biochem. 1996;236:358–360. doi: 10.1006/abio.1996.0181. [DOI] [PubMed] [Google Scholar]
- 24.Peschel A, Augustin J, Kupke T, Stevanovic S, Götz F. Regulation of epidermin biosynthetic genes by EpiQ. Mol Microbiol. 1993;9:31–39. doi: 10.1111/j.1365-2958.1993.tb01666.x. [DOI] [PubMed] [Google Scholar]
- 25.Piard J-C, Kuipers O, Rollema H S, Desmazeaud M J, de Vos W M. Structure, organization, and expression of the lct gene for lacticin 481, a novel lantibiotic produced by Lactococcus lactis. J Biol Chem. 1993;268:16361–16368. [PubMed] [Google Scholar]
- 26.Pridmore D, Rekhif N, Pittet A-C, Suri B, Mollet B. Variacin, a new lanthionine-containing bacteriocin produced by Micrococcus varians: comparison to lacticin 481 of Lactococcus lactis. Appl Environ Microbiol. 1996;62:1799–1802. doi: 10.1128/aem.62.5.1799-1802.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qi F, Chen P, Caufield P W. Functional analyses of the promoters in the lantibiotic mutacin II biosynthetic locus in Streptococcus mutans. Appl Environ Microbiol. 1999;65:652–658. doi: 10.1128/aem.65.2.652-658.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qi F, Chen P, Caufield P W. Purification of mutacin III from the group III Streptococcus mutans UA787 and genetic analyses of mutacin III biosynthetic genes. Appl Environ Microbiol. 1999;65:3880–3887. doi: 10.1128/aem.65.9.3880-3887.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rince A, Dufour A, Le Pogam S, Thuault D, Bourgeois C M, Le Pennec J P. Cloning, expression, and nucleotide sequence of genes involved in production of lactococcin DR, a bacteriocin from Lactococcus lactis subsp. lactis. Appl Environ Microbiol. 1994;60:1652–1657. doi: 10.1128/aem.60.5.1652-1657.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ross K F, Ronson C W, Tagg J R. Isolation and characterization of the lantibiotic salivaricin A and its structural gene salA from Streptococcus salivarius 20P3. Appl Environ Microbiol. 1993;59:2014–2021. doi: 10.1128/aem.59.7.2014-2021.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sahl H-G, Bierbaum G. Lantibiotics: biosynthesis and biological activities of uniquely modified peptides from Gram-positive bacteria. Annu Rev Microbiol. 1998;52:41–79. doi: 10.1146/annurev.micro.52.1.41. [DOI] [PubMed] [Google Scholar]
- 32.Sahl H-G, Jack R W, Bierbaum G. Biosynthesis and biological activities of lantibiotics with unique post-translational modifications. Eur J Biochem. 1995;230:827–853. doi: 10.1111/j.1432-1033.1995.tb20627.x. [DOI] [PubMed] [Google Scholar]
- 33.Saris P, Immonen T, Reis M, Sahl H. Immunity to lantibiotics. Antonie Leewenhoek. 1996;69:151–159. doi: 10.1007/BF00399420. [DOI] [PubMed] [Google Scholar]
- 34.Schell N, Entian K D, Schneider U, Gotz F, Zahner H, Kellner R, Jung G. Prepeptide sequence of epidermin, a ribosomally synthesized polypeptide antibiotic containing four sulphide-rings. Nature. 1988;333:276–278. doi: 10.1038/333276a0. [DOI] [PubMed] [Google Scholar]
- 35.Stryer L. Biochemistry. New York, N.Y: W. H. Freeman and Company; 1988. p. 37. [Google Scholar]
- 36.Surette M G, Miller M B, Bassler B L. Quorum sensing in Escherichia coli, Salmonella typhimurium, and Vibrio harveyi: a new family of genes responsible for autoinducer production. Proc Natl Acad Sci USA. 1999;96:1639–1644. doi: 10.1073/pnas.96.4.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van der Meer J R, Rollema H S, Siezen R J, Beerthuyzen M M, Kuipers O P, de Vos W M. Influence of amino acid substitutions in the nisin leader peptide on biosynthesis and secretion of nisin by Lactococcus lactis. J Biol Chem. 1994;269:3555–3562. [PubMed] [Google Scholar]
- 38.Weil H P, Sickinger A G B, Metzger J, Stevanovic S, Jung G, Josten M, Sahl H G. Biosynthesis of the lantibiotic pep5. Eur J Biochem. 1990;194:217–223. doi: 10.1111/j.1432-1033.1990.tb19446.x. [DOI] [PubMed] [Google Scholar]