Abstract
Tomato is one of the most important crops grown under both greenhouse and field conditions throughout the world. Its production is highly challenged by infestation of leaf miner insect, Tuta absoluta Meyrick regardless of excessive insecticide application. The chemical insecticides results insect resistance, environmental pollution, and health problems and there is urgent need for management options such as integrated pest management (IPM) to obviate these problems. Thus, the present study aims to evaluate the effectiveness of single and combination treatments of entomopathogens; Beauveria bassiana, Metarhizium anisopliae, Bacillus thuringeinsis, and an insecticide against T. absoluta under greenhouse and field conditions. Two varieties (Awash and Venes) of tomato for greenhouse experiment and one (Gellila) variety for field experiment were used with Tutan36%SC (insecticide with active ingredient of Chlorphenapyr 36%SC) and untreated plots as positive and negative controls, respectively. The results showed significant leaf and fruit damage reduction in all the treatments. B. bassiana-AAUB03, M. anisopliae-AAUM78, and B. thuringiensis-AAUF6 showed the highest (93.4%, 89.7% and 90.1%) leaf and (93.5%, 94.4% and 95%) fruit protection under greenhouse condition. The combined treatments improved leaf protection efficacy up to 95.3% under field condition. When the entomopathogens were combined with half or quarter reduced concentrations of Tutan36% SC, it showed 94.4% of pest protection. In all the treatments, 72–96% of marketable fruit was obtained as par insecticide treatment scored 85–93%. All the entomopathogens did not cause any adverse effect on the growth of tomato rather improved shoot length, shoot branching, leaf and fruit numbers. Therefore, application of entomopathogens in single, consortium or in combination reduced the recommended concentration of Tutan36%SC to control T. absoluta.
Keywords: Biopesticide, Entomopathogens, Integrated pest management, Tuta absoluta
Biopesticide; Entomopathogens; Integrated pest management; Tuta absoluta
1. Introduction
Tomato (Solanum lycopersicum L.) is one of the horticultural plants affected by insect pests, microbial diseases, and nematode infections [1, 2]. According to Santana et al. [3], tomato leaf miner, Tuta absoluta Meyrick is one of the emerging insect pests that severely affect tomato production. The larval stages attack the aerial parts (leaves, stems and fruits) of tomato and destroy 80–100% of production [4]. The mouthpart and antenna of the insect have gustatory and olfactory sense organs (different types of sensilla) to select host plants [5].
Most domesticated and cultivated tomato varieties are vulnerable to the insect infestations. However, wild species of tomato, such as, S. habrochaites and S. cheesmaninae that are capable of synthesizing biomolecules, methyl-ketones (2-tridecanone), sesquiterpenes (zingiberene) and acyl sugar (acylglucose and acylsucrose) showed high resistance against T. absoluta [6, 7, 8]. This gives insight to develop pest resistant cultivars against the insect pests [6].
Although there are various methods to control insects, currently, chemical insecticides are principally used in the management of leaf miner. Several studies, however, showed that continuous use of the chemicals are implicated with destruction of natural enemies, buildup of insecticide residues on tomato fruits, and the environment, rapid development of insecticide resistance, and reduced profits from high insecticide costs [9, 10, 11]. This necessitates a shift from conventional pest control practices to integrated pest management (IPM), in order to reduce application of pesticides and produce healthy food [12].
Microbial entomopathogens are a natural component of IPM to control insect pests and naturally regulated by ecological factors with negligible effects on biodiversity [13]. Beauveria bassiana, Metarhizium anisopliae, and Bacillus thuringiensis are the most important microorganisms that are widely used to protect plants from insect pests for horticultural production [14, 15, 16, 17].
These microorganisms establish themselves endophytically in the plant tissue and augment synergistic pest control [18], promote plant growth, and crop productivity [19]. The hitherto studies showed successful endophytic colonization of tomato plants with entomopathogens [20]. The authors reiterated that B. bassiana showed significant growth improvement in tomato plants with direct (87.5%) and indirect (72.5%) protection activity against T. absoluta. Other studies showed that B. bassiana produce large amount of bioactive compounds of flavonoids, phenols and alkaloids that have antagonistic effect against several types of insects and microbial pathogens [21, 22, 23]. Similarly, Metarhizium spp. produce plant growth promoting hormones, cell division stimulators, and nutrient bioavailability modifiers to improve host plant growth besides management of insect pests [24].
It is established that success in pest control and plant promotion by entomopathogens depends on strain selection, appropriate formulation, proper application, suitable environmental condition for application, and application timing [25, 26]. To this end, entomopathogens are screened for effective control of insect pests and plant growth promotion function.
In Ethiopia, one study showed that some strains of B. bassiana and M. anisopliae reduced 84 and 76% of T. absoluta infestation under field conditions [27]. However, there is still a dearth of information on the synergistic role of entomopathogenic fungi (EPF) and bacteria (B. thuringiensis) against T. absoluta under greenhouse and field conditions. Therefore, in this study, effective strains of B. bassiana, M. anisopliae, and B. thuringiensis, those previously screened under laboratory condition [28, 29, 30] were evaluated under greenhouse and field conditions.
2. Materials and methods
2.1. Tomato varieties
Three tomato varieties were selected for this experiment. The newly released “Awash” variety with large fruit size and good aroma for consumption and better marketing [31], and the heat tolerant and disease resistant “Venes” tomato variety were collected from Merkamba PLc., Addis Ababa, Ethiopia. These two varieties were used for greenhouse experiment which was conducted at Addis Ababa University (AAU), Collage of Natural and Computational Sciences.
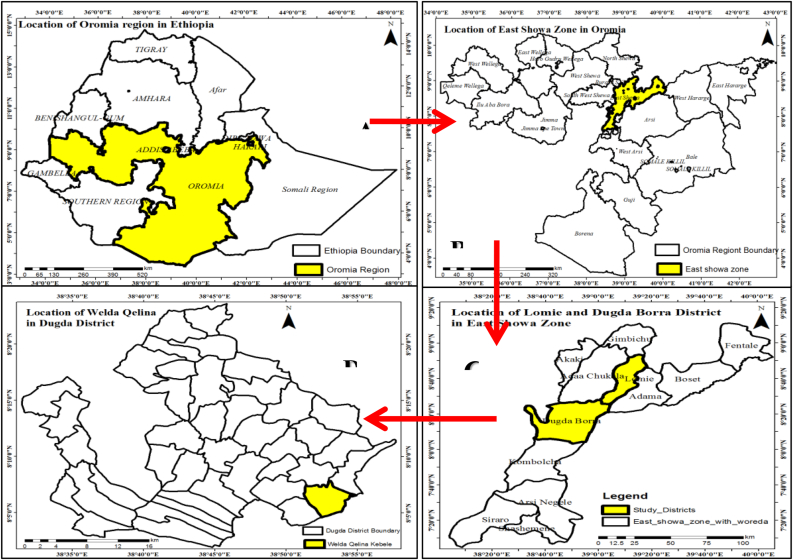
For the field trial, the high yielding commercial hybrid “Gellila” variety were bought from Florensis PLc., Addis Ababa, Ethiopia. The experiment was conducted between January 12 to May 26, 20/2021 under irrigation cropping system at Walda Qelina Kebele, Dugda Borra (Meki) district, Oromia Regional State, Ethiopia (Figure 1).
Figure 1.
Map of field experiment sites in the East Showa Zone, Oromia Regional State, Ethiopia.
2.2. Microbial bioinsecticides
Four entomopathogenic fungi; B. bassiana-AAUB03 (MT588402), B. bassiana-AAUB28 (MT588414), M. anisopliae-AAUM39 (MT463525), M. anisopliae-AAUM78 (MT463530), and two entomopathogenic bacteria; B. thuringiensis-AAUF6 (MW250834), and B. thuringiensis-AAUMF9 (MW250856) were selected on the basis of their effective larvicidal activity against T. absoluta under laboratory conditions [28, 29, 30] for greenhouse tests. From which, B. bassiana-AAUB03, M. anisopliae-AAUM78, and B. thuringiensis-AAUF6 were selected for the trial under field conditions.
2.3. Preparation of entomopathogenic fungi (EPF)
The fungal strains were produced in diphasic (liquid and solid state) fermentations following standard methods [32]. They were grown on potato dextrose agar (PDA) medium at 25 °C until conidiation for 20 days. The conidia were scraped into sterile test tube containing 20 mL of water and 0.01% Tween 80 to produce 1 × 106 conidial suspensions per milliliter. Of which, 15 mL of suspension from each strain was inoculated into 150 mL of potato dextrose broth (PDB) in 250 mL Erlenmeyer flasks and incubated at 28 °C for 7 days with manual agitation every two days to improve aeration. Then, 120 mL of blastoconidia was transferred into 1 kg of autoclaved wheat bran substrate and moistened with sterile water in 1.5 kg capacity of polyethylene bags having pores to allow free flow of air for solid-state fermentation. The culture was incubated at 30 °C for one month, and mixed in a two-day interval at the first week to profuse conidiation.
2.4. Conidial harvesting
The conidia were harvested from the culture by using a manual sifter method [33]. The culture substrates were air-dried, squashed and sieved using a pore size of 500 μm fine wire mesh. The conidia were weighed, collected in sterile 100 mL reagent bottle, and stored at 4 °C for further work.
2.5. Conidial viability test
Conidial viability of the entomopathogens was evaluated by germination test on PDA medium through incubating at 28 °C for 24 h [34]. Percent germination of conidia was determined after having treated the medium with 70% ethanol to halt germination. The number of germinated conidia was detected based on the formation of conidia germ tubes under 40× magnification of a compound microscope. The percentage germination was calculated using the following formula;
where, present of G: germinated conidia; NG: non-germinated conidia and TC: total count of conidia.
Of these, an appropriate amount of conidia were suspended into sterile water containing 0.01% of Tween 80 and the concentration was adjusted to 1 × 108 conidia mL−1 to use for greenhouse and field applications.
2.6. Culturing of Bacillus thuringiensis
Pure isolates of B. thuringiensis-AAUF6, and B. thuringiensis-AAUMF9 were cultured on nutrient agar medium adjusted at pH of 7.1 [35]. A portion of an active colony was inoculated in to 250 mL of nutrient broth medium prepared in 500 mL Erlenmeyer flask and incubated at 28 °C with subsequent hand shaking for 84 h. The bacterial growth was checked through visual observation of cloud formation in culture medium and the cell concentration was adjusted to 1 × 108 cfu (colony forming units) mL−1 using serial dilution techniques for greenhouse and field applications.
2.7. Design of greenhouse experiments
For greenhouse experiment, seeds from each of Awash and Venes tomato varieties were surface sterilized using sodium hypochlorite (3%) and ethanol (70%) for 3 min and rinsed with sterile water five times [36]. Five seeds of each variety were sown in 3 kg capacity pots filled with a 2:1 ratio of sandy soil and compost mixture. They were thinned down into three after a week of germination. All the seedlings were grown and watered as required and fertilized twice monthly with nitrogen (0.05% NPK).
The greenhouse experimental design includes six single entomopathogen applications such as, B. bassiana-AAUB03, B. bassiana-AAUB28, M. anisopliae-AAUM39, M. anisopliae-AAUM78, B. thuringiensis-AAUF6, and B. thuringiensis-AAUMF9, and positive (Tutan 36% SC (insecticide with active ingredient of chlorphenapyr 36% SC)) and negative (untreated) control. The treatments were arranged in a complete randomized design (CRD) with three replications. Four pots with tomato plantation were arranged separately by enclosing with cotton meshed net to prevent insect transfer from one treatment in the greenhouse with photoperiod of 12/12 h of day and night at College of Natural Sciences, Addis Ababa University.
Then, after four days of hatching, three pairs of T. absoluta moths (male and female) were released on each treatment replica using wet cheese close wrap method. The formulated fungal conidia and bacterial culture cells were sprayed using a hand sprayer nozzle after five days of insect release after one month of plantation with a frequency treatment twice a week in the first month and once per week thereafter for a period of three months.
2.8. Design of field experiments
2.8.1. Preparation of land, seedling transplant and treatments
The field site was ploughed and prepared according to the cultural practices of the tomato farming system in the area. A total of 392 m2 (14 m × 28 m) land was divided into six blocks or replications separated by a canal with a width of 0.75 m. Each block was sub-divided into nine plots (each 3 by 45 cm apart separated by 0.5 m canal) corresponding to nine treatments (Table 1S). Treatments were designed in complete randomized block design (CRBD) with six replications. Three rows were made on each plot and one month old seedlings of Gellila variety was obtained from greenhouse of FlorensisPLc. located around Awash River, center for seed propagation following the standards of tomato seedling propagation requirements [37]. They were transplanted in 40 cm apart from each other in a row (Fig S1). All tomato plants were fertilized twice monthly with recommended rate (32 kg ha−1) of the fertilizer.
Table 1.
Production of conidia and conidial viability of B. bassiana and M. anisopliae strains.
| EPF strains | Conidia per kg of substrate in g | Conidial density g−1 | % of conidial germination |
|---|---|---|---|
| B. bassiana-AAUB03 | 10.5 ± 0.67a | 5.2 × 1010a | 95. 5 ± 0.91a |
| B. bassiana-AAUB28 | 7.8 ± 0.89b | 6.2 × 109b | 89.2 ± 0.23c |
| M. anisopliae-AAUM39 | 5.9 ± 1.23c | 1.3 × 108c | 88.5 ± 0.67d |
| M. anisopliae-AAUM78 | 8.7 ± 0.98b | 3.1 × 1010a | 92. 7 ± 1.02b |
Same superscript letters within the column indicate no significant differences between strains (p <0.05).
The transplanted tomato plants were left open for natural infestation of T. absoluta and after a week, the presence of eggs, larvae, adult insects as well as lesion on plant leaves were checked. Following the detection of insect manifestation, the formulated fungal conidia, and bacterial culture were sprayed on the plants in single and in combination using a knapsack sprayer. The combined treatments were composed of IPM components that included single, two or three entomopathogens together or entomopathogens with 25% or 50% of the full dose of Tutan36% SC (insecticide). Full dose of Tutan36% SC was sprayed on plots as positive control, whereas plots without any spray were served as negative control. The application of treatments on the field experiments follows the same procedures used for greenhouse experiment.
2.8.2. Data collection
In order to determine damages caused by T. absoluta in tomato seedling, twelve plants (one plant per each pot per replica) from the greenhouse experiment and eighteen plants (one plant per each line per replica) from the field experiments were randomly selected and total leaves, leaf lesions, total fruits and fruit bores were counted weekly. Leaf lesion data were collected right after a week of the first application of treatments, whereas fruit bores data were gathered just after one week of tomato fruit sets. The damaged leaves and fruits were recorded weekly until the time of harvest.
The biocontrol effectiveness (Ebiocon) of the treatments was calculated by using the following formula in order to determine healthy leaves or fruits:
where TNL, the total mean number of leaves; NIL, the mean number of infected leaves; TNF, the total mean number of fruits; NIF, the mean number of infected fruits per plant.
The percentage of fruit marketability (Fmark) per plant was assessed by using formula of;
where BF, the mean number of bored fruits per plant; TF, mean number of total fruits counted per plant.
2.8.3. Data analysis
All the data were analyzed by using SPSS software version 25 with one way ANOVA and compared with positive control. Means separation of treatments and control was calculated using Tukey's Least Significant Difference (LSD) test at p ≤ 0.05.
3. Results and discussion
3.1. Conidial production of entomopathogenic fungi
The entomopathogenic fungi showed significant (p <0.05) variation in the production of conidia, conidial density and germination rate when grown on the wheat bran (Table 1). B. bassiana-AAUB03 produced the highest conidial biomass (10.5 g conidia kg−1 of substrate) with the largest density (5.2 × 1010 conidia g−1) and germination rate (95.5%), followed by M. anisopliae-AAUM78 with production of 8.7 g conidia kg−1 of wheat bran, 3.1 × 1010 conidia g−1 (density) and conidial germination rate of 92.7% (Table 1). Although the two isolates displayed much lower conidial biomass than produced (40.5 g kg−1 of substrate) from optimized rice [38], and lower conidial density (8.7 × 107 conidia g−1) of B. bassiana strain grown on brewer's spent grain [39], their germination rate was as good as those obtained from both substrates. This deviation may come from substrate difference since the latter differ in their nitrogen, carbon and amino acids contents that significantly enhance growth and conidial production of entomopathogens [32, 40].
Although M. anisopliae-AAUM39 induced the lowest conidial density (1.3 × 108 conidia g−1) on wheat bran, its production was much higher than the conidial density of 4 × 106 conidia g−1 produced in the earlier study [41]. This variation in conidial production is due to many factors include; types of substrates, moisture content, incubation temperature, age of initial inoculums, initial concentration of conidia, inoculums size, incubation period, the presence of substrate degrading enzymes and aeration of culture during fermentation process [38, 42].
3.2. Greenhouse study
3.2.1. Leaf protection
The entomopathogens significantly (P < 0.05) reduced the leaf damage inflicted by the insect pest, T. absoluta ranged from 76% with B. thuringiensis-AAUFM9 to 93% with B. bassiana-AAUB03 on Awash tomato variety and 65–83% on Venes variety compared to the untreated control (Table 2). This is best performance compared to previous report of reduction in the insect infestation of 46–75% on tomato plants [43]. The entomopathogen treatments showed significant variation in suppressing the insect infestation to reduce the percent leaf damage of Awash variety that was not different from 96% of protection by chemical treatment (Table 2). They also showed the same pattern, but with less percentage reduction of leaf lesion (65–83%) on Venes variety compared to the insecticide that scored 87% of protection. Qayyum et al [44] indicated that variation in effectiveness is attributed to the difference in the inherent ability of entomopathogens to successfully colonize plant species and attack insect pests.
Table 2.
Effectiveness of the entomopathogens in reducing leaf damage of tomato by T. absoluta under greenhouse conditions.
| Treatments |
Awash variety |
Venes variety |
||
|---|---|---|---|---|
| Mean LI (%) | Protection (%) | Mean LI (%) | Protection (%) | |
| B. bassiana-AAUB03 | 6.6 ± 3.2g | 93.4ab | 16.5 ± 1.5e | 83.5b |
| B. bassiana-AAUB28 | 13.9 ± 2.0de | 86.1b | 19.3 ± 2.0ef | 80.2bc |
| M. anisopliae-AAUM39 | 20.8 ± 3.2c | 79.2c | 27.6 ± 1.7cd | 72.4d |
| M. anisopliae-AAUM78 | 10.3 ± 3.7ef | 89.7b | 19.8 ± 2.1ef | 80.7bc |
| B. thuringeinsis-AAUF6 | 9.9 ± 2.4f | 90.1ab | 22 ± 2.0de | 78c |
| B. thuringeinsis-AAUMF9 | 23.1 ± 1.7bc | 76.9c | 34.6 ± 2.2bc | 65.4e |
| Tutan36% SC (insecticide) | 4.0 ± 2.12hj | 96.0a | 13 ± 1.2gh | 87a |
| Untreated control Mean |
69.7 ± 4.1a | - 86 |
73.5 ± 10.2a | - 77 |
LI, Leaf infestation; same letters within the column indicate no significant differences p <0.05).
Among the entomopathogens, B. bassiana-AAUB03 showed slightly higher percentage leaf damage reduction (88%) on both tomato varieties, followed by percentage leaf damage decrease of plants treated with M. anisopliae-AAUM78 (83%) and B. thuringensis-AAUF6 (84%). This was similar to the previous report of 73 and 84% leaf damage reduction of T. absoluta on Coshoro variety treated with B. bassiana and M. anisopliae, respectively [45]. However, B. thuringensis-AAUF6 induced 78% of leaf damage reduction on the Venes variety which was slightly higher reduction in leaf damage of 67% reported by Hosseinzadeh et al. [46]. These isolates were also significantly more effective in reducing leaf damage than the commercially recommended dose of B. thuringiensis that only reduced 31% of leaf damage on tomato variety [47]. All the treatments showed significant (F 19, 32 = 10. 23, p <0.001) variation (4–34%) of leaf infestation (LI) by T. absoluta on both varieties of tomato compared to high damage (>70%) observed from untreated control plants (Table 2). In the other study infestation rate of T. absoluta was vary from cultivar to cultivar in tomato production [48].
Based on the mean evaluation of all the treatments, entomopathogens induced more protection (86%) on Awash variety than they did (77%) on Venes variety (Table 2). In general, B. thuringeinsis-AAUF6 performed as good as the other entomopathogenic fungi (B. bassiana-AAUB28 and M. anisopliae-AAUM78) in protecting the two tomato varieties from leaf miner pest under greenhouse conditions. B. thuringeinsis-AAUF6 was better induced leaf protection than the commercial B. thuringeinsis showed that less performance on leaf protection compared to chemical insecticides [53].
3.2.2. Fruit protection
In this study, the findings showed significant (F 8, 57 = 12.23, p <0.002) variation among treatments to protect the fruits of both Awash and Venes varieties from insect damage (Table 3). Thus, B. bassiana-AAUB03 treated plants produced the largest (93%) number of marketable fruits (36 fruits/plant) on Awash variety with 13–40% increase in the number of marketable fruits over other entomopathogens and the standard insecticide treatments. In another study, B. bassiana was less effective (29%) on fruit protection from the T. absoluta as reported by Shahini et al. [47].
Table 3.
Effectiveness of treatments in reducing fruit damage of tomato by T. absoluta under greenhouse conditions.
| Treatments |
Awash variety |
Venes variety |
||||
|---|---|---|---|---|---|---|
| TNF | NMF | Protection (%) | TNF | NMF | Protection (%) | |
| B. bassiana-AAUB03 | 39.3 ± 2.4a | 36.7 ± 1.2a | 93.5b | 28.5 ± 1.4a | 23.9 ± 1.0a | 83.7a |
| B. bassiana-AAUB28 | 35.7 ± 2.7b | 25.2 ± 0.8d | 70.6ef | 22.4 ± 1.7c | 15.1 ± 0.8d | 67.4c |
| M. anisopliae-AAUM39 | 29.7 ± 1.9d | 22.8 ± 1.0ef | 76.9cd | 26.7 ± 1.4b | 16.2 ± 1.2cd | 60.5d |
| M. anisopliae-AAUM78 | 33.7 ± 2.7c | 31.8 ± 0.8b | 94.4b | 28.8 ± 1.8a | 24.2 ± 1.1a | 84.2a |
| B. thuringeinsis-AAUF6 | 30 ± 2.5d | 28.5 ± 0.8c | 95.7a | 29.3 ± 2.1a | 24.5 ± 0.8a | 83.7a |
| B. thuringeinsis-AAUMF9 | 29.3 ± 2.2de | 21.9 ± 1.1f | 75.4d | 27.6 ± 2.3a | 19.9 ± 1.0b | 72.1bc |
| Tutan36% SC (insecticide) | 29.3 ± 2.2de | 27.5 ± 0.8c | 94.2b | 23.9 ± 1.6c | 21.5 ± 1.0b | 90.0a |
| Untreated control | 16.7 ± 2.1 | 3.4 ± 1.1 | 20.4 ± 5.2g-j | 12.2 ± 1.4 | 2.74 ± 0.8 | 22.5 ± 6.3f-i |
TNF, mean total number of fruits; NMF, mean number of marketable fruits; same letters within the column indicate no significant differences between treatments (p <0.05).
The other entomopathogens induced the production of 70–95% of marketable fruits with less number of fruits (21–32 fruits/plant) compared to B. bassiana-AAUB03 treated plants (36 fruits/plant) on the Awash variety (Table 3). On the other hand, Venes variety showed significant variation (F 27, 43 = 6.23, p <0.001) in different treatments compared to Awash variety (Table 3). Thus, B. thuringeinsis-AAUF6, M. anisopliae-AAUM78 and B. bassiana-AAUB03 treated plants produced 24 marketable fruits/plant followed by insecticide and B. thuringeinsis-AAUMF9 that produced 22 and 20 marketable, respectively showing 38% increase compared to the least effective treatments, B. bassiana-AAUB28 and M. anisopliae-AAUM39. In general, the various treatments produced 60–84% of marketable fruits from the total count and displayed 5-9-fold higher number of fruits than the untreated control plants that were almost totally infected with the insect pest.
3.2.3. Fruit yield
The marketable fruit yield obtained from different treatments was significantly (P < 0.05) higher than the yield of untreated Awash tomato variety (Table 4). The data showed that B. bassiana-AAUB03 treatment induced the highest fruit yield (3.6 kg/plant) which was twice more than the ones treated with B. thuringeinsis-AAUMF9. However, the marketable fruit yield (3–3.2 kg/plant) from insecticide, Tutan 36%SC treated plant was not significantly different from plants treated with M. anisopliae-AAUM78, B. thuringeinsis-AAUF6.
Table 4.
The fruit weight and marketability of tomato per plant treated with entomopathogens under greenhouse conditions.
| Treatments |
Awash variety |
Venes variety |
||||
|---|---|---|---|---|---|---|
| TFW/P (Kg) | MFW/P (Kg) | MF (%) | TFW/P (Kg) | MFW/P (Kg) | MF (%) | |
| B. bassiana-AAUB03 | 3.8 ± 1.2a | 3.6 ± 0.9a | 94.7a | 3.4 ± 0.6a | 3.1 ± 0.9a | 91.2a |
| B. bassiana-AAUB28 | 3.3 ± 0.9a | 2.7 ± 1.2ab | 81.8b | 3 ± 0.3a | 2.4 ± 1.2ab | 80b |
| M. anisopliae-AAUM39 | 2.8 ± 1.0ab | 2.1 ± 0.1b | 75.0c | 2.9 ± 0.2ab | 2.1 ± 1.2b | 72.4c |
| M. anisopliae-AAUM78 | 3.6 ± 0.8a | 3.2 ± 2.3a | 88.9b | 3.7 ± 1.3a | 3.7 ± 1.2a | 89.2ab |
| B. thuringeinsis-AAUF6 | 3.5 ± 2.1a | 3.2 ± 0.9a | 91.4a | 3.8 ± 1.6a | 3.5 ± 1.0a | 92.1a |
| B. thuringeinsis AAUMF9 | 2.6 ± 0.7b | 1.9 ± 1.5c | 73.1c | 2.2 ± 0.8bc | 1.6 ± 1.3c | 72.7c |
| Tutan36%SC (insecticide) | 3.2 ± 0.8a | 3 ± 1.3a | 93.8a | 3 ± 1.2a | 2.8 ± 1.2a | 93.3a |
| Untreated control | 1.1 ± 2.7c | 0.28 ± 1.3e | 25.5efg | 1.3 ± 1.0d | 0.5 ± 0.8d-f | 38.5d-g |
TFW, total fruit weight per plant; MFW, marketable fruit weight per plant; MF, marketable fruits per plant; same letters within the column indicate no significant differences between treatments (p <0.05).
The fruit yield pattern was similar to the same treatments on Venes variety in that B. thuringeinsis-AAUF6 produced 3.5 kg/plant of marketable fruits, followed by M. anisopliae-AAUM78 (3.3 kg/plant), B. bassiana-AAUB03 (3.1 kg/plant) and Tutan36%SC (2.8 kg/plant) that were twice more protective than the least effective strain (B. thuringeinsis-AAUMF9). In all cases, marketable fruit yield obtained from the above mentioned isolates was more than 90% of the total yield. The rest of the isolates also exhibited 72–89% marketable fruit yield with both tomato varieties. This implies that most of the isolates performed as good as those treated with Coragen 200 SC (insecticides) that protected the tomato and produced up to 94% marketable fruit yield with local Coshoro variety in Ethiopia [49]. Buragohain et al. [50] in India also reported that commercially formulated B. bassiana (Green Beauveria® and BB Power®) and B. thuringeinsis (Green Larvicide® and Delfin® WG) showed more than 90% of marketable fruit yield of tomato by protecting them from T. absoluta.
3.3. Field study
3.3.1. Leaf protection
The three effective strains (B. bassiana-AAUB03, M. anisopliae-AAUM78, and B. thuringiensis-AAUF6) significantly reduced leaf infestation (LI) of Gellila tomato variety when applied in single or in combination with each other and Tutan36% SC (insecticide) under field conditions (Table 5). All treatments except M. anisopliae-AAUM78 (T2) and B. thuringeinsis-AAUF6 (T3) showed 91–95% leaf protection with significant (F 18, 67) = 13.12, p <0.001) variation on leaf infestation (LI) (Table 5). In fact, at the early stage of tomato plantation, the insect infestation rate was between, 12.1 and 39.8% (data not shown), that was reduced to 4.7–9.7%, due to repeated application of entomopathogens and increase in age of the plants (Table 5). Klieber and Reineke [51] also reported the same pattern as a function of time and duration of biopesticide application. Similarly, commercial B. bassiana (Beaucitech® WP) and Metarhizium strain (Metatech® WP) showed 90–98% and 88–95% leaf protection on Roma tomato variety, respectively [52].
Table 5.
The effect of entomopathogen treatments to reduce tomato (Gellila variety) leaf infestation from T. absoluta under field conditions.
| No | Treatment | Mean LI (%) | Protection (%) |
|---|---|---|---|
| T1 | B. bassiana-AAUB03 | 8.27 ± 0.9c | 91.73a |
| T2 | M. anisopliae-AAUM78 | 12.71 ± 0.8b | 87.29b |
| T3 | B. thuringeinsis-AAUF6 | 17.84 ± 1.0a | 82.16b |
| T4 | AAUF6 + AAUM78 | 8.59 ± 1.0c | 91.41a |
| T5 | AAUB03 + AAUM78 + AAUF6 | 4.68 ± 1.3e | 95.32a |
| T6 | AAUB03 + AAUM78 + 25% Tutan36%SC | 5.62 ± 1.5d | 94.38a |
| T7 | AAUF6 + 50% Tutan36%SC | 7.77 ± 0.7c | 92.23a |
| T8 | Tutan36%SC (positive control) | 6.12 ± 0.9d | 93.88a |
| T9 | Untreated control | 93.88 ± 1.0 | - |
LI, mean leaf infestation; same letters within the column indicate no significant differences between treatments (p <0.05).
The findings showed that the single treatment of B. bassiana-AAUB03 (T1) maintained its leaf protection (91%) as good as the combined treatments (T4, T5, T6 and T7) (Table 5). Allegrucci et al [20] corroborated that B. bassiana is efficient in its endophytic colonization to induced direct (86%) and indirect (73%) leaf damage protection from leaf miner. The IPM treatments with consortia of entomopathogens and reduced dose of the chemical insecticide was equally effective as full dose insecticide (positive control) treated plants (65%) in the control of T. absoluta (Table5). This may be associated with the ability of the entomopathogens to produce different kinds of secondary metabolites that synergistically induce systemic and repellant responses against pests [19, 53].
Furthermore, combined treatments of the entomopathogenic fungi and quarter reduced concentration of Tutan36% SC was the second most effective treatment in reducing leaf infestation. This suggests that reduction in pest infestation can be enhanced due to chemical interference on the physiology of the insect and increase their susceptibility to entomopathogens [47, 54].
3.3.2. Fruit protection
The total and marketable fruit production was significantly (F 23, 82 = 6.15, p <0.001) different among the treatments in the field conditions (Table 6). Tomato plants treated with B. bassiana-AAUB03 (T1) produced a large number of total (80.3 fruits/plant) fruits per plant and 69.3 fruits/plant with 86.3% marketable fruits. Whereas, combined treatments of entomopathogens with quarter-reduced concentration of Tutan 36%SC (insecticide) (T6) and full dose (T7) of insecticide was scored equal number of total fruits (79 fruits/plant) and 72 fruits/plant with 92.1% of marketability. These treatments showed 15–18 fruits/plant marketable fruit production increment compared to the least effective entomopathogen (M. anisopliae-AAUM78) (T2) that scored 45 fruits/plant with 74% of marketability.
Table 6.
Efficacy of entomopathogens in reducing fruit damage of tomato (Gellila variety) from T. absoluta under field conditions.
| No | Treatment | Total fruits/plant | Marketable fruit/plant | Protection (%) |
|---|---|---|---|---|
| T1 | B. bassiana-AAUB03 | 80.3 ± 0.9a | 69.3 ± 0.9a | 86.3b |
| T2 | M. anisopliae-AAUM78 | 64.3 ± 1.2d | 54.0 ± 1.2c | 74.0c |
| T3 | B. thuringeinsis-AAUF6 | 76.3 ± 1.0b | 65.3 ± 1.0b | 75.6c |
| T4 | AAUF6 + AAUM78 | 71.3 ± 1.2b | 61.7 ± 2.1b | 86.5b |
| T5 | AAUB03 + AAUM78 + AAUF6 | 67.0 ± 1.1d | 61.0 ± 1.9bc | 91.0a |
| T6 | AAUB03 + AAUM78 + 25% Tutan36%SC | 78.7 ± 0.6a | 72.5 ± 0.2a | 92.1a |
| T7 | AAUF6 + 50% Tutan36%SC | 65.0 ± 1.4d | 56.7 ± 1.3c | 87.2b |
| T8 | Tutan36%SC (positive control) | 79.3 ± 0.9a | 72.3 ± 1.0a | 91.2a |
| T9 | Untreated control | 40.0 ± 1.5efg | 12.0 ± 1.4d-f | 30b-g |
Same letters within the column indicate no significant differences between treatments (p <0.05).
The tomato plants treated with B. thuringeinsis-AAUF6 (T3) showed considerable (75.3 fruits/plant) number of total fruits (65.3 fruits/plant) with 75.6% marketability followed by combined treatment of B. thuringeinsis-AAUF6 + M. anisopliae-AAUM78 (T4) that showed 71.3 fruits/plant with 86.5% marketable fruits (Table 7). However, the consortium of all entomopathogens (T5) was effective in fruit protection (91%) but relatively less effective on fruit number production. In general, marketable fruit production in all the treatments was highly significant (p <0.05) compared to untreated (T9) control showing 5-folds of improvement by protecting plants from the insect pest.
Table 7.
Marketable fruits from tomato (Gellila variety) plants treaded with entomopathogens under field conditions.
| No | Treatments | Total and Marketable fruit weights |
||
|---|---|---|---|---|
| TFW/P (kg) | MFW/P (kg) | MF (%) | ||
| T1 | B. bassiana-AAUB03 | 11.21 ± 1.0a | 9.67 ± 1.1a | 86.3b |
| T2 | M. anisopliae-AAUM78 | 8.73 ± 0.9b | 7.33 ± 1.0c | 78.9c |
| T3 | B. thuringiensis-AAUF6 | 10.75 ± 0.7a | 8.4 ± 0.7b | 78.1c |
| T4 | AAUF6 + AAUM78 | 10 ± 0.8ab | 8.75 ± 0.9b | 87.5b |
| T5 | AAUB03 + AAUM78 + AAUF6 | 10.42 ± 1.0a | 9.72 ± 1.0a | 93.3a |
| T6 | AAUB03 + AAUM78 + 25% Tutan36%SC | 11.64 ± 0.9a | 10.92 ± 1.0a | 93.8a |
| T7 | AAUF6 + 50% Tutan36%SC | 10.53 ± 0.8ab | 9.18 ± 0.9a | 87.2b |
| T8 | Tutan36%SC (insecticide) | 11.33 ± 0.3a | 9.81 ± 1.0a | 86.6b |
| T9 | Untreated control | 5.25 ± 0.6c-e | 1.58 ± 0.7d-f | 30.1c-f |
TFW, total fruit weight per plants; MFW, marketable fruit weight per plant; MF, marketable fruits per plant; same letters within the column indicate no significant differences between treatments (p <0.05).
3.3.3. Fruit yields
The marketable fruit yield (kg/plant) of the single and combined entomopathogens treated Gellila variety showed considerable variations (Table 7). Thus, almost all of the strains applied in consortia and combined with insecticide showed the highest percentage of marketable fruit (87.2–93.8%) yields compared to positive control (86.6%). All entomopathogens combined treatments (T5) and the two entomopathogenic fungi (B. bassiana-AAUB03 + M. anisopliae-AAUM78) combined with quarter-reduced concentration of insecticide (T6) showed marketable fruit yield of 10.9 and 9.7 kg/plant, respectively. Fruits obtained from the plants treated with consortia of entomopathogens showed a 25% significant (P < 0.05) difference between the most and the least effective treatments with 40–55% yield increase compared to the untreated control.
It is interesting to note that there was no significant (P < 0.05) variation in marketable fruits yield between the plants treated with B. bassiana-AAUB03, combination of B. thuringeinsis-AAUF6 and half concentration of Tutan36% SC and the full dose of Tutan36%SC treated plants. The finding showed that 9.7, 9.2 and 9.8 kg/plant with 86.3, 87.2 and 86.6% of fruit marketability, respectively (Table 7). Similarly, the Coshoro variety tomato treated with B. bassiana was produced 98.2% of marketable fruit yield in Ethiopia [49]. However, the second least effective entomopathogen, B. thuringeinsis-AAUF6 showed more than 2-folds of fruit yields (8.4 kg/plant) compared to 3.2 kg/plant of marketable fruit yields scored by tomato plants treated with commercial B. thuringeinsis (Costar®) under field conditions [55].
All these effective treatments showed 20–33% marketable yield increment compared to plants treated with the least effective strain (M. anisopliae-AAUM78). Shibru and Getu [49] have reported 78.5% of marketable fruit yield from the tomato treated with M. anisopliae, which is comparable with the result (78.9%) obtained from our M. anisopliae-AAUM78. Thus, treatments with their own effectiveness in control of T. absoluta enhanced the production of marketable yield compared to untreated plants. This suggests that all of the treatment options are effective to control T. absoluta and produce tomato on sustainable basis.
3.4. Effect of entomopathogens on the growth of tomato plants
Interestingly, none of the applied entomopathogens caused any negative effect on the growth of tomato, rather they showed better growth performance and productivity in both greenhouse and field conditions (Table 8). All the tomato varieties (Awash, Venes and Gellila) treated with entomopathogens showed 40–83 cm of shoot length and 12–45 number of shoot branches (Table 8). It is almost equal or better compared to tomato treated with Tutan36%SC. In fact, B. bassiana showed plant growth promotion and increased height, number of leaves, grain weight, crop yield, and germination on the corn crop besides reducing larvae of Rachiplusia un [56].
Table 8.
The effect of entomopathogens on the growth of tomato varieties under greenhouses and field conditions.
| Treatments |
Results from greenhouse condition |
|||
|---|---|---|---|---|
|
Awash variety |
Venes variety |
|||
| SL (cm) | PB (n) | SL (cm) | PB (n) | |
| AAUB03 | 48 ± 1.24b | 15 ± 1.44a | 43 ± 3.07c | 16 ± 1.58b |
| AAUB28 | 52 ± 1.89a | 12 ± 1.44c | 47 ± 1.94b | 16 ± 1.44b |
| AAUM39 | 53 ± 2.36a | 16 ± 1.00a | 40 ± 2.42d | 15 ± 1.23b |
| AAUM78 | 43 ± 1.44c | 14 ± 1.00b | 44 ± 2.30c | 16 ± 1.00b |
| AAUF6 | 50 ± 2.04ab | 14 ± 1.23b | 48 ± 1.44ab | 18 ± 1.00a |
| AAUMF9 | 51 ± 1.62a | 15 ± 1.23a | 51 ± 2.24a | 12 ± 1.41d |
| Tutan36%SC (Positive control) | 49 ± 2.47ab | 15 ± 1.44a | 49 ± 1.00ab | 14 ± 1.23c |
| Without any (Negative control) | 43 ± 2.12c | 8 ± 1.23d | 48 ± 3.56ab | 10 ± 1.44e |
| Results from field condition | Gellila variety | |
|---|---|---|
| AAUB03 | 79.45 ± 2.08ab | 35.8 ± 1.45c |
| AAUM78 | 79.07 ± 1.54ab | 37 ± 2.72c |
| AAUF6 | 79.82 ± 2.45ab | 39.7 ± 1.34b |
| AAUF6 + AAUM78 | 81.64 ± 1.98a | 44.93 ± 0.94a |
| AAUB03 + AAUM78 + AAUF6 | 83.84 ± 3.12a | 45.11 ± 2.54a |
| AAUB03 + AAUM78 + 25% Tutan36%SC | 79.25 ± 2.09ab | 38.73 ± 3.21b |
| AAUF6 + 50% Tutan36%SC | 77.32 ± 2.06b | 36.52 ± 4.67c |
| Tutan36%SC (Positive control) | 71.05 ± 2.45c | 33.18 ± 4.23c |
| Without any (Negative control) | 67.3 ± 4.12d | 26.41 ± 1.43e |
SL, mean shoot length; PB, mean number of branches per plant; same letters within the column indicate no significant differences between treatments (p <0.05).
The entomopathogens might be fully colonize plants and induce growth besides insect damage reduction or entomopathogens could also antagonize certain pathogens and favor the growth of plants [56, 57]. In addition, Metarhizium spp. produced plant growth hormones (auxin and cytokinins), the cellular division stimulator [24] and nutrient bioavailability modifier, siderophore [58, 59] that augment growth of plants. Furthermore, endophytic microbes induce adaptation of plants to environmental stresses and improve metabolic pathways through interlinking each other to exchange compounds that improve efficient functioning [60], produce antibiotics and plant growth enhancing substances [61].
4. Conclusion
In conclusion, single and consortium application of B. bassiana, M. anisopliae and B. thuringeinsis against T. absoluta showed considerable leaf and fruit damage reductions. B. bassiana-AAUB03, M. anisopliae-AAUM78 and B. thuringiensis-AAUF6 are the most effective strains in T. absoluta management. Their consortia and combination with quarter or half dose of Tutan36% SC showed stronger activity in reduction of T. absoluta infestation. Besides to pest control activity, entomopathogens improved crop productivity without any effect on the growth of the plant in both greenhouse and field conditions. Therefore, application of entomopathogens in single, consortium and combining with reduced concentration of recommended rate of insecticide could be utilized in IPM strategy.
Declarations
Author contribution statement
Birhan Aynalem: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Diriba Muleta; Mulissa Jida; Fekadu Shemekite; Fassil Aseffa: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data included in article/supp. material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Duressa F.T. Newly emerging insect pests and diseases as a challenge for growth and development of Ethiopia: the case of Western Oromiya. J. Agri. Sci. Food Res. 2017;9:1–6. [Google Scholar]
- 2.Tonnang H.E.Z., Mohamed S.F., Khamis F., Ekesi S. Identification and risk assessment for worldwide invasion and spread of Tuta absoluta with a focus on Sub-Saharan Africa: implications for phytosanitary measures and management. PLoS One. 2015;10:1–19. doi: 10.1371/journal.pone.0135283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Santana P.A., Kumar L., Da-Silva R.S., Picanço M.C. Global geographic distribution of Tuta absoluta as affected by climate change. J. Pest. Sci. 2019;92:1373–1385. [Google Scholar]
- 4.Desneux N., Luna G.M., Guillemaud T., Urbaneja A. The invasive South American tomato pinworm, Tuta absoluta, continues to spread in Afro-Eurasia and beyond: the new threat to tomato world production. J. Pest. Sci. 2011;84:403–408. [Google Scholar]
- 5.El-Ghany N.M.A., Faucheux M.J. Sensory structures on the larval antennae and mouthparts of Tuta absoluta (Meyrick) (Lepidoptera: gelechiidae) Zool. Anz. 2021;294:28–38. [Google Scholar]
- 6.Silva A.G., Queiroz A.E., Arcanjo P.L., Lopes C.M., Araújo A.T., et al. Biological performance and oviposition preference of tomato pinworm Tuta absoluta when offered a range of Solanaceous host plants. Sci. Rep. 2021;11:1153. doi: 10.1038/s41598-020-80434-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Han P., Bayram Y., Shaltiel-Harpaz I., Sohrabi F., Saji A., et al. Tuta absoluta continues to disperse in Asia: damage, ongoing management and future challenges. J. Pest. Sci. 2018;23:23–38. [Google Scholar]
- 8.Ciceoi R., Iordăchescu M., Udriște A.A., Bădulescu L.A. Insights on solanaceous resistance against tomato leaf miner (Tuta absoluta), with emphasis on chemical compounds useful in integrated pest management. Rev. Not. Bot. Hort. Agrobo. 2021;49:12543. [Google Scholar]
- 9.Nderitu P.W., Jonsson M., Arunga E., Otieno M., Muturi J.J., et al. Combining host plant resistance, selective insecticides, and biological control agents for integrated management of Tuta absoluta. Adv. Agricult. 2020;2020:1–8. [Google Scholar]
- 10.Braham M., Hajji L. In: Management of Tuta Absoluta (Lepidoptera, Gelechiidae) with Insecticides on Tomatoes, Insecticides - Pest Engineering. Farzana Perveen Dr., editor. InTech; 2012. pp. 1–24. [Google Scholar]
- 11.Mergia M., Weldemariam E.D., Eklo O.M., Yimer G.T. Knowledge, attitude, and practice of farmers on pesticide use and their impacts on the environment and human health from small scale vegetable farming along the littoral of Lake Ziway, Ethiopia. Res. Square. 2021:1–23. [Google Scholar]
- 12.Srinivasan R., Sevgan S., Ekesi S., Tamò M. Biopesticide based sustainable pest management for safer production of vegetable legumes and brassicas in Asia and Africa. Pest Manag. Sci. 2019;75:2446–2454. doi: 10.1002/ps.5480. [DOI] [PubMed] [Google Scholar]
- 13.Scheepmakera J.W.A., Butt T.M. Natural and released inoculum levels of entomopathogenic fungal biocontrol agents in soil in relation to risk assessment and in accordancewith EU regulations. Biocontrol Sci. Technol. 2010;20:503–552. [Google Scholar]
- 14.Salama H., Saker M., Salama M., El-Banna A., Ragaie M., et al. Bacillus thuringiensis isolates from Egyptian soils and their potential activity against lepidopterous insects. Arch. Phytopathol. Plant Protect. 2012;45:1–13. [Google Scholar]
- 15.El-Ghany N.M.A., El-Ghany E.M.A., Salama H.S. Efficiency of new B. thuringiensis isolates from Egypt against the pink bollworm Pectinophora gossypiella (Saunders) Biopest. Int. 2015;11:12–19. [Google Scholar]
- 16.El-Ghany NMA A.S., Abdel-Razek, Ebadah I.M.A., Mahmoud Y.A. Evaluation of some microbial agents, natural and chemical compounds for controlling tomato leaf miner, Tuta absoluta (Meyrick) (Lepidoptera: gelechiidae) J. Plant Protect. Res. 2015;56:372–379. [Google Scholar]
- 17.Klieber J., Reineke A. The entomopathogen Beauveria bassiana has epiphytic and endophytic activity against the tomato leaf miner Tuta absoluta. J. Appl. Entomol. 2016;140:580–589. [Google Scholar]
- 18.Biswas C., Dey P., Satpathy S., Satya P., Mahapatra S.B. Endophytic colonization of white jute (Corchorus capsularis) plants by different Beauveria bassiana strains for managing stem weevil (Apion corchori) Phytoparasitica. 2013;41:17–21. [Google Scholar]
- 19.Russo M.L., Scorsetti A.C., Vianna M.F., Cabello M., Ferreri N., et al. Endophytic effects of Beauveria bassiana on corn (Zea mays) and its herbivore. Rachiplusia nu (Lepidoptera: Noctuidae) Insects. 2019;10:110–119. doi: 10.3390/insects10040110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Allegrucci N., Velázquez M.S., Russo M.L., Pérez E.M., Scorsetti C.A. Endophytic colonisation of tomato by the entomopathogenic fungus Beauveria bassiana: the use of different inoculation techniques and their effects on the tomato leaf miner Tuta absoluta (Lepidoptera: gelechiidae) J. Plant Protect. Res. 2017;57:206–213. [Google Scholar]
- 21.Pourtaghi E., Talaei-Hassanloui R., Nasibi F., Fotouhifar K. Endophytic colonization of tomato by Beauveria bassiana for control of the greenhouse whitefly, Trialeurodes vaporariorum (Hemiptera: aleyrodidae) Acta Biol. 2020;14:149–160. [Google Scholar]
- 22.Gunatilaka A.A. Natural products from plant-associated microorganisms: distribution, structural diversity, bioactivity, and implications of their occurrence. J. Nat. Prod. 2006;69:509–526. doi: 10.1021/np058128n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ownley H.B., Gwinn D.K., Vega E.F. Endophytic fungal entomopathogens with activity against plant pathogens: ecology and evolution. BioControl. 2010;55:113–128. [Google Scholar]
- 24.Liao X., Lovett B., Fang W., Leger J.R.S. Metarhizium robertsii produces indole-3-acetic acid, which promotes root growth in Arabidopsis and enhances virulence to insects. Microbiology. 2017;163:980–991. doi: 10.1099/mic.0.000494. [DOI] [PubMed] [Google Scholar]
- 25.Batta Y.A. Recent advances in formulation and application of entomopathogenic fungi for biocontrol of stored-grain insects. Biocontrol Sci. Technol. 2016;26:1171–1183. [Google Scholar]
- 26.Ibrahim L., Dakache C., Kreidy M.E., Dagher R., Ezzeddine N., et al. Environmentally sustainable production of Metrhizium anisopliae and Beauveria bassiana for control of Tuta absoluta. Int. J. Agri. Biosyst. Eng. 2017;2:1–12. [Google Scholar]
- 27.Shiberu T., Getu E. Entomopathogenic effect of Beauveria bassiana (Bals.) and Metarrhizium anisopliae (Metschn.) on Tuta absoluta (Meyrick) (Lepidoptera: gelechiidae) larvae under laboratory and glasshouse conditions in Ethiopia. J. Plant Pathol. Microbiol. 2017;8:411–414. [Google Scholar]
- 28.Aynalem B., Muleta D., Venegas J., Assefa F. Morphological, molecular, and pathogenicity characteristics of the native isolates of Metarhizium anisopliae against the tomato leaf miner, Tuta absoluta (Meyrick 1917) (Lepidoptera: gelechiidae) in Ethiopia. Egypt J. Biol. Pest Contr. 2020;30:1–11. [Google Scholar]
- 29.Aynalem B., Muleta D., Venegas J., Assefa F. Molecular phylogeny and pathogenicity of indigenous Beauveria bassiana against the tomato leaf miner, Tuta absoluta Meyrick 1917 (Lepidoptera: gelechiidae), in Ethiopia. J. Genetic Eng. Biotechnol. 2021;19:1–15. doi: 10.1186/s43141-021-00227-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aynalem B., Muleta D., Venegas J., Assefa F. Isolation, molecular characterization and pathogenicity of native Bacillus thuringiensis, from Ethiopia, against the tomato leafminer, Tuta absoluta: detection of a new high lethal phylogenetic group. Microbiol. Res. 2021;250:1–10. doi: 10.1016/j.micres.2021.126802. [DOI] [PubMed] [Google Scholar]
- 31.Sora A.S. Review on productivity of released tomato (Solanum Lycopersicum Mill.) varieties in different parts of Ethiopia. J. Hortic. Sci. 2018;1:102–114. [Google Scholar]
- 32.Roussos S., Bagnis C., Besnard O., Sigoilloe C., Duponnois R., et al. In: Coffee Biotechnology and Quality. Sera T., et al., editors. Kluwer Academie Publishers; 2000. Use of solid state fermentation for the production of fungal biopesticides spores for insect control; pp. 277–286. Printed in the Netherlands. [Google Scholar]
- 33.Gouli V., Gouli S., Brownbridge M., Skinner M., Parker L.B. The University of Vermont, Entomology Research Laboratory; Burlington, VT, USA: 2005. Manual for Mass Production of Entomopathogenic Fungi in Developing Countries with Particular Reference to Beauveria Bassiana and Metarhizium Anisopliae. [Google Scholar]
- 34.Habtegebriel B., Getu E., Dawd M., Seyoum E., Atnafu G., et al. Molecular characterization and evaluation of indigenous entomopathogenic fungal isolates against Sorghum Chafer, Pachnoda interrupta (Olivier) in Ethiopia. J. Entomol. Nematol. 2016;8:34–45. [Google Scholar]
- 35.Lobo K.S., Soares-da-Silva J., Silva M.C., Tadei W.P., Polanczyk R.A., et al. Isolation and molecular characterization of Bacillus thuringiensis found in soils of the Cerrado region of Brazil, and their toxicity to Aedesae gypti larvae. Rev. Bras. Entomol. 2018;62:5–12. [Google Scholar]
- 36.Alatar A.A., Faisal M., Abdel-Salam E.M., Canto T., Saquib Q., et al. Efficient and reproducible in vitro regeneration of Solanum lycopersicum and assessment genetic uniformity using flow cytometry and SPAR methods. Saudi J. Biol. Sci. 2017;24:1430–1436. doi: 10.1016/j.sjbs.2017.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin J.L., Luther C.G., Hanson P. AVRDC – The World Vegetable Center publication; 2015. Raising Healthy Tomato Seedlings; pp. 15–795. AVRDC – The World Vegetable Center publication. [Google Scholar]
- 38.Pham T.A., Kim J.J., Kim K. Optimization of solid-state fermentation for improved conidia production of Beauveria bassiana as a mycoinsecticide. MYCOBIOLOGY. 2010;38:137–143. doi: 10.4489/MYCO.2010.38.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qiu L., Li J., Li Z., Wang J. Production and characterization of biocontrol fertilizer from brewer’s spent grain via solid-state fermentation. Sci. Rep. 2019;9:1–9. doi: 10.1038/s41598-018-36949-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jaronski S.T. Mass Production of Beneficial Organisms. Elsevier; 2014. Mass production of entomopathogenic fungi: state of the art; pp. 357–413. [Google Scholar]
- 41.Roshandel S., Askary H., Hassanlouei R.T., Allahyari H. The effects of natural substrates on the sporulation and viability of conidia and blastospores of Metarhizium anisopliae. Biocontrol Plant Protect. 2016;4:94–111. [Google Scholar]
- 42.Cruz-Quiroz R., Robledo-Padilla F., Aguilar C., Roussos S. Forced aeration influence on the production of spores by Trichoderma strains. Waste Biomass Valorizat. 2017;8:2263–2270. Springer. [Google Scholar]
- 43.El-Ghany M.N.A., Abdel-Razek S.A., Djelouah K., Moussa A. Efficacy of bio-rational insecticides against Tuta absoluta (Meyrick) (Lepidoptera Gelechiidae) on tomatoes. Biosci. Res. 2018;15:28–40. [Google Scholar]
- 44.Qayyum A.M., Wakil W., Arif J.M., Sahi T.S., Dunlap A.C. Infection of Helicoverpa armigera by endophytic Beauveria bassiana colonizing tomato plants. Biol. Control. 2015;90:200–207. [Google Scholar]
- 45.Tadele S., Emana G. Entomopathogenic effect of Beauveria bassiana (Bals.) and Metarrhizium anisopliae (Metschn.) on Tuta absoluta (Meyrick) (Lepidoptera: gelechiidae) larvae under laboratory and glasshouse conditions in Ethiopia. J. Plant Pathol. Microbiol. 2017;8:411–415. [Google Scholar]
- 46.Hosseinzadeh A., Aramideh S., Ghassemi-Kahrizeh A. Efficacy of bio-insecticides on Tuta absoluta (Meyrick) (Lep.: gelechiidae) in laboratory and field conditions. IGR J. 2019;21:164–170. [Google Scholar]
- 47.Shahini S., Berxolli A., Kokojka F. Effectiveness of bio-insecticides and mass trapping based on population fluctuations for controlling Tuta absoluta under greenhouse conditions in Albania. Heliyon. 2020;6 doi: 10.1016/j.heliyon.2020.e05753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gharekhani G.H., Salek-Ebrahimi H. Archives Phytopathol Plant Protect; 2013. Evaluating the Damage of Tuta Absoluta (Meyrick) (Lepidoptera: Gelechiidae) on Some Cultivars of Tomato under Greenhouse Condition; pp. 1–10. [Google Scholar]
- 49.Shiberu T., Getu E. Evaluation of biopesticides on integrated management of tomato leaf miner, Tuta absoluta (Meyrick) (Gelechiidae: Lepidoptera) on tomato crops in Western Shewa of central Ethiopia. Entomol., Ornithol. Herpetol. 2018;7:1–8. [Google Scholar]
- 50.Buragohain P., Saikia D.K., Sotelo-Cardona P., Srinivasan R. Evaluation of bio-pesticides against the South American tomato leaf miner, Tuta absoluta Meyrick (Lepidoptera: gelechiidae) in India. Horticulturae. 2021;7:1–15. [Google Scholar]
- 51.Klieber J., Reineke A. The entomopathogenic Beauveria bassiana has epiphytic and endophytic activity against the tomato leaf miner Tuta absoluta. J. Appl. Entomol. 2016;140:580–589. [Google Scholar]
- 52.Ndereyimana A., Nyalala S., Murerwa P., Gaidashova S. Field efficacy of entomopathogens and plant extracts on Tuta absoluta Meyrick (Lepidoptera: gelechiidae) infesting tomato in Rwanda. Crop Protect. 2020;134 [Google Scholar]
- 53.Castillo D.L., Sword A.G. The endophytic fungal entomopathogens Beauveria bassiana and Purpureocillium lilacinum enhance the growth of cultivated cotton (Gossypium hirsutum) and negatively affect survival of the cotton bollworm (Helicoverpa zea) Biol. Control. 2015;89:53–60. [Google Scholar]
- 54.Jallow A.F.M., Dahab A.A., Albaho S.M., Devi Y.V. Efficacy of some biorational insecticides against Tuta absoluta (Meyrick) (Lepidoptera: gelechiidae) under laboratory and greenhouse conditions in Kuwait. J. Appl. Entomol. 2018:1–9. [Google Scholar]
- 55.Gonza´lez-Cabrera J., Molla´ O., Monto´n H., Urbaneja A. Efficacy of Bacillus thuringiensis (Berliner) in controlling the tomato borer, Tuta absoluta (Meyrick) (Lepidoptera: gelechiidae) BioControl. 2011;56:71–80. [Google Scholar]
- 56.Russo M.L., Scorsetti A.C., Vianna M.F., Cabello M., Ferreri N., et al. Endophytic effects of Beauveria bassiana on corn (Zea mays) and its herbivore, Rachiplusia nu (Lepidoptera: noctuidae) Insects. 2019;10:110–119. doi: 10.3390/insects10040110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gurulingappa P., Sword A.G., Murdoch G., McGee A.P. Colonization of crop plants by fungal entomopathogens and their effects on two insect pests when in planta. Biol. Control. 2010;55:34–41. [Google Scholar]
- 58.Joseph E., Cario S., Simon A., Worle M., Mazzeo R., et al. Protection of metal artifacts with the formation of metal-oxalates complexes by Beauveria bassiana. Front. Microbiol. 2012;2:270. doi: 10.3389/fmicb.2011.00270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jirakkakul J., Cheevadhanarak S., Punya J., Chutrakul C., Senachak J., et al. Tenellin acts as an iron chelator to prevent iron-generated reactive oxygen species toxicity in the entomopathogenic fungus Beauveria bassiana. FEMS Microbiol. Lett. 2015;362:1–8. doi: 10.1093/femsle/fnu032. [DOI] [PubMed] [Google Scholar]
- 60.Mishra A., Singh S.P., Mahfooz S., Bhattacharya A., Mishra N., et al. Bacterial endophytes modulates the withanolide biosynthetic pathway and physiological performance in Withania somnifera under biotic stress. Microbiol. Res. 2018;213:17–28. doi: 10.1016/j.micres.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 61.Lodewyck C., Vangronsveld J., Porteous F., Moore B.R.E., Taghavi S., et al. Endophytic bacteria and their potential applications. Crit. Rev. Plant Sci. 2002;21:583–606. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data included in article/supp. material/referenced in article.