Abstract
Metabolic activity was measured in the laboratory at temperatures between 5 and −20°C on the basis of incorporation of 14C-labeled acetate into lipids by samples of a natural population of bacteria from Siberian permafrost (permanently frozen soil). Incorporation followed a sigmoidal pattern similar to growth curves. At all temperatures, the log phase was followed, within 200 to 350 days, by a stationary phase, which was monitored until the 550th day of activity. The minimum doubling times ranged from 1 day (5°C) to 20 days (−10°C) to ca. 160 days (−20°C). The curves reached the stationary phase at different levels, depending on the incubation temperature. We suggest that the stationary phase, which is generally considered to be reached when the availability of nutrients becomes limiting, was brought on under our conditions by the formation of diffusion barriers in the thin layers of unfrozen water known to be present in permafrost soils, the thickness of which depends on temperature.
In numerous previously published articles the authors described microbial metabolic activity at subzero temperatures. In more recent reviews (6, 9, 14, 22), the authors agree that earlier reports of microbial activity (mostly bacterial activity) at temperatures below −12°C were unsubstantiated. Microbial growth or metabolic activity has been reported in permafrost bacteria at −10°C (11) and in the antarctic cryptoendolithic microbial community at temperatures between −5 and −10°C (7, 28), and the temperature limit of bacterial growth in frozen food is generally considered to be −8°C (9). In arctic and antarctic lichens, photosynthetic activity has been observed in a similar temperature range (12) and, more recently, at −17°C (23). However, no quantitative measurements of the dynamics of metabolic activity or of growth have been described. We attempted to quantify metabolic activity at subzero temperatures in the native bacterial population of Siberian permafrost by measuring the incorporation of sodium acetate into lipids over a 550-day period.
Significant numbers of viable bacteria (102 to 108 cells g−1) are known to be present in permafrost that is 1 to 3 million years old in the arctic (10, 21, 24, 29) and in permafrost that is probably older in Antarctica (32; E. I. Friedmann, A. D. Gilichinsky, G. S. Wilson, V. Ostroumov, E. A. Vorobyova, V. S. Soina, V. A. Shcherbakova, T. A. Vishnivetskaya, J. P. Chanton, R. O. Friedmann, C. P. McKay, and E. Rivkina, Abstr. 8th ISSOL Meet., 11th Int. Conf. Origin Life 1996, abstr. 60, 1996); all of the bacteria that have been characterized so far have been psychrotrophs (psychrotolerant mesophiles). The ratio of aerobic bacteria to anaerobic bacteria seems to vary according to the geological history. Comparative quantitative studies have not been performed. Permafrost sediments of alluvial, lake, and marine origin that formed under anoxic conditions contain high numbers of anaerobes (compared to total cell counts), like the samples studied by Rivkina et al. (21), whereas other samples, like the samples described by Shi et al. (24) or the sample used in the present study, seem to be dominated by aerobes. Although the exact number of anaerobes in our sample is not known, the anaerobes that were present, which were unable to metabolize under the conditions used in the experiment, obviously did not affect the results.
Permafrost soil is known to contain unfrozen water (5, 17), and the most biologically important feature of this water is that it makes mass transfer (of ions and liquid water) possible in permafrost (18). Mass exchange is greatest in microzones with low ice contents and smallest at sites where the ice content is high or in solid ice (18). Thus, although the physical structure of permafrost makes metabolic activity possible, it has often been assumed (29) that in permafrost (at temperatures around −10°C in Siberia and as low as −27°C in Antarctica) microorganisms are in a state of anabiosis (lack of metabolic activity, suspended animation). In the present study we attempted to quantify metabolic activity at temperatures down to −20°C, which approached the limit of measurability by methods that are presently available.
MATERIALS AND METHODS
Permafrost sediment samples were obtained by drilling in the Kolyma-Indigirka Lowland in northeast Siberia (69°29′N, 156°59′E) in August 1991. A short summary of the microbiology and geology of permafrost and of pertinent literature, as well as methods of drilling, sample handling, and storage, has been published elsewhere (24). In short, permafrost cores were obtained with a drill that operates without drilling fluid, which would contaminate biological samples. The surfaces of the 20- to 30-cm-long cores were cleaned on site by shaving with an alcohol-sterilized knife and then split into ca. 5-cm-long segments, which were either used immediately for microbiological studies or placed in metal containers and kept frozen during all phases of transportation and storage. For microbiological studies in the laboratory, core segments were split aseptically, and samples were taken from the freshly broken center of each core. In tests to determine the possibility of contamination during the drilling process, the drilling barrel was seeded with a pure culture of Serratia marcescens for 2 h before drilling. In a separate test, drilled frozen core segments were seeded for several hours to several months at −10°C with a culture S. marcescens. In tests in which the isolation technique described above was used, S. marcescens was found only on the surfaces of the frozen cores, never inside the cores. The native temperature of the permafrost soil was −10°C, the ice content was 23.0%, the organic C content was 1.15 to 1.29%, and the freezing point was −0.8 ± 0.18°C. In our experiments, we used portions of core 1/91 (drilled in the immediate vicinity of well 6/90 described by Shi et al. [24]) that were from a depth of 8.5 to 25.0 m and were between ca. 2 and 3 million years old. The homogenized soil contained 1.1 × 108 cells g−1 as determined by direct visual microscopic counting and 2 × 105 cells g−1 as determined by visual plate counting.
About 3 kg of permafrost soil was aseptically mixed in a mechanical mixer at about 0°C, and 50-g aliquots were distributed into 100-ml jars. Fifty microliters of 14C-labeled sodium acetate (25 μCi of activity) was injected into each jar. Immediately after injection, the samples were cooled to −20°C and kept at this temperature overnight. The next day, samples were immersed in thermostatically controlled water bath incubators at 5.0, 0.0, −1.5, −5.0, −10.0, −15.0, and −20.0°C. The radioactivity at the beginning of incubation and the radioactivity of a sample treated with 5 ml of 37% formaldehyde prior to acetate labeling and incubated at room temperature for 270 days were both 9.8 cpm, and this value was considered the baseline for subsequent measurements. Most measured values were based on the total extracted lipids of single 50-g samples; the only exceptions were the formaldehyde-killed background value and the values obtained at −1.5°C, each of which represented the average of the values obtained with three 50-g samples. The error of mean in the averages was 40 to 50%. After incubation, lipids were extracted by a method modified from the methods of Bligh and Dyer (4) and White et al. (31). Each sample was transferred to a 500-ml flask containing 50 ml of chloroform, 50 ml of methanol, and 27 ml of distilled water, shaken, and allowed to stand for 2 h; then the sample was filtered and transferred to a 300-ml separatory funnel. The single phase was broken by the addition of 50 ml of chloroform and 50 ml of distilled water and vigorous shaking. After each preparation stood overnight, the chloroform phase was collected and dried under a stream of N2. To remove water-soluble contaminants, as described by Kates (13), we redissolved the dry lipid faction in 2.5 ml of chloroform, transferred it to a 15-ml centrifuge tube containing 2.5 ml of methanol and 2.2 ml of distilled water, mixed it by vortexing, and centrifuged it briefly. After the upper layer was removed with a Pasteur pipette, the chloroform layer was dried under N2.
Activity was counted with a high-sensitivity liquid scintillation analyzer (Packard Tri-Carb model 1050). To ensure that low values were meaningful, we used low-level counting region optimization (19) to improve the limit of detection, and each sample was counted 10 times for 120 min to yield a limit of error (2 ς) of less than 5%.
The organic C content was determined by the wet oxidation method (25). We determined the freezing point with 10 replicates by monitoring the output of differential thermocouples during cooling and registering exotherms.
For plate counting, bacteria were separated from soil by sonication in a 0.1% pyrophosphate solution (3) and stained with propidium iodide for fluorescence microscopy (30) or plated onto oligotrophic PYGV medium (26) and incubated at room temperature.
The unfrozen water content was measured by adiabatic and differential calorimetry (2).
RESULTS
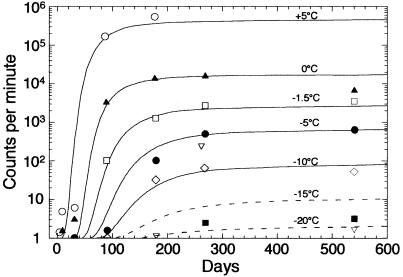
The data in Table 1 show a consistent and expected pattern. After a relatively brief lag time, the number of counts increased markedly and then leveled off to a constant value. Very low counts, only several counts per minute above the baseline, were observed in samples incubated at −15.0 and −20.0°C.
TABLE 1.
Net counts for 14C-labeled acetate incorporation into lipidsa
| Temp (°C) | cpm after incubation for:
|
||||||
|---|---|---|---|---|---|---|---|
| 1 Day | 7 Days | 30 Days | 90 Days | 180 Days | 270 Days | 550 Days | |
| 5 | 0 | 4.67 | 7.03 | 163,415 | 526,695 | 22,000 | 7,702 |
| 0 | 1.74 | 3.13 | 3,500 | 13,277 | 15,000 | 6,301 | |
| −1.5 | 0 | 95.17 | 700 | 1,200 | 1,245 | ||
| −5 | 1.68 | 74 | 400 | 472 | |||
| −10 | 34.9 | 63.7 | 50 | ||||
| −15 | 5.0 | 2.4 | 2.8 | ||||
| −20 | 1.7 | 2.0 | |||||
For all data, the limit of error (2ς) was less than 5%.
To analyze the data, we fitted a simple sigmoidal curve to each set of measured values for temperatures between 5.0 and −10.0°C (Fig. 1) by using the following expression:
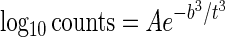 |
1 |
where A and b are the constants that specify each curve and t is time (in days). Although equation 1 is purely empirical, A and b have physical significance; A is the asymptotic value reached for long times, and b is a parameter that determines the maximum rate of nutrient incorporation during the rapid rise just after the lag period ends. The values of A and b were determined for each curve for temperatures from 5 to −10°C. For temperatures of 5, 0, −1.5, −5, −10, −15, and −20°C, the values for A were 5.6, 4.2, 3.4, 2.8, 1.9, 1.02, and 0.3, respectively, and the values for b were 24, 53, 76, 102, 127, 158, and 186 days, respectively. The doubling time scale (T) is given by the maximum value of (ln 2)/[d (ln C)/dt] where C is the counts, as follows:
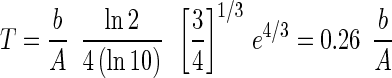 |
2 |
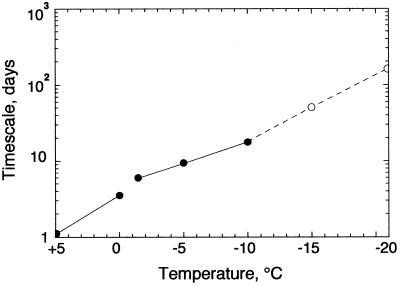
The curves for −15 and −20°C were extrapolated from the equation because measured values near the limit of detection were not sufficiently accurate. As Fig. 1 shows, simple sigmoidal curves represented reasonably well the data for temperatures between 0 and −10°C and were consistent with the trend of the data for −15 and −20°C. As determined from the sigmoidal equation, the maximum incorporation rate, the steepest part of the sigmoidal curve, occurred when the log counts equaled one-quarter of the final asymptotic value. This maximum incorporation rate could be expressed as a minimum doubling time, the length of time that it would take for the counts to increase by a factor of two at the maximum rate of incorporation. The doubling times determined in this way are shown in Fig. 2. As expected, the doubling time increased smoothly as the temperature decreased; it was about 1 day at 5°C and 3 days at 0°C. At these temperatures no ice was present; freezing occurred at temperatures between 0 and −1.5°C, and the slight discontinuity in the incorporation rate at this point is evident in both Fig. 1 and Fig. 2. At −1.5°C, the doubling time increased to about 6 days, and at −10°C the doubling time increased to 20 days. Our values indicate that the doubling time at −15°C was more than 40 days and the doubling time at −20°C was about 160 days.
FIG. 1.
Incorporation of 14C-labeled acetate by the native bacterial population in Siberian permafrost over a 550-day period. Because of the very low counts, we calculated the curves for −15 and −20°C by using equation 1. For all data the limit of error (2ς) was less than 5%.
FIG. 2.
Minimum doubling times of the bacterial population in permafrost at different temperatures. Symbols: ●, data calculated from data in Fig. 1; ○, data calculated by means of equation 1.
At 5°C the measured values began to decrease after 180 days, and at 0°C the measured values began to decrease after 270 days (data not shown), apparently because of exhaustion of the label. At temperatures between 0°C and −10.0°C, the stationary phase was reached in 200 to 350 days. It is most probably this stationary phase that represents the long-term equilibrium state in permafrost.
DISCUSSION
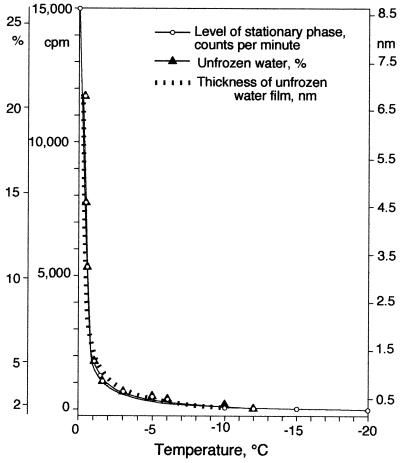
All of the incorporation curves in Fig. 1 resemble typical growth curves in shape, and they can be considered indicators and, we suggest, indirect measurements of growth. The curves flatten out at different levels of radioactivity (Fig. 1 and 3), and these levels seem to be correlated with temperature. This pattern is highly unusual, as flattening of growth curves is usually due to limitation by an external factor, generally exhaustion of nutrients. The same should apply to incorporation curves. To interpret the different levels of flattening in the curves, we must consider the physical structure of permafrost. The permafrost sediment in our samples was a nonsaline loamy soil. The content of particles that were less than 10 μm in diameter was 32% by weight. In the frozen state, this soil contains 5 to 6% unfrozen water by weight at −1.5°C, 2.0 to 3.0% unfrozen water by weight at −10°C, and 1 to 2% unfrozen water by weight at temperatures between −15 and −20°C, as shown in Fig. 3. The water layer thickness curve is based on the measurements of Nersesova and Tsytovich (17), the first of their kind, which were obtained with kaolinite. Subsequently, other workers (1, 2) repeated the experiment of these workers with different soils and obtained virtually identical results. In frozen soils, both soil particles and bacterial cells are covered by thin films of water; nutrients reach the cells and waste products are eliminated by diffusion through the narrow channels of unfrozen water. In permafrost, therefore, access to nutrients and the ability to eliminate waste materials are limited by the thickness of the unfrozen films of water, which in turn depends on temperature. The thickness of the films decreases from about 15 nm at −1.5°C to about 5 nm at −10°C (Fig. 3). Ultimately, the slow buildup of diffusion gradients progressively slows and perhaps stops the movement of both nutrients and waste materials.
FIG. 3.
Levels at which bacterial growth reaches a stationary phase (Fig. 1), measured amounts of unfrozen water, and calculated thicknesses of unfrozen water films in permafrost (18) plotted versus temperature. The similarity of the curves suggests that the stationary phase is reached as a result of a diffusion barrier in the thin film of water, the thickness of which depends on temperature.
It is significant that in the temperature range used in our study the shapes of the curves for the levels of stationary phases, the amounts of unfrozen water in permafrost, and the thicknesses of the unfrozen water layers were remarkably similar (Fig. 3). We suggest that the temperature dependence of the levels of stationary phases is due to the decrease in the thickness of the liquid water layer in permafrost. Thus, it is not the absolute exhaustion of nutrients but the inaccessibility of nutrients due to a diffusion barrier formed at different levels depending on the temperature that limits metabolic activity and results in the asymptotic behavior of the radioactive counts (flattening of the curves) with time.
We can interpret our results as follows. The thawing and mixing of the soil at the beginning of the experiment destroyed the physical structure of the permafrost, including the osmotic gradients in the water films. The rapid freezing of the samples at −20°C at the beginning of the experiment resulted in the formation of a permanent ice structure within a relatively short time, probably minutes (5), so the channels to sources of nutrients were fully open. Metabolic activity (nutrient uptake) in the log phase and calculated doubling times reflect the physiological growth potential under optimal conditions (e.g., laboratory conditions), whereas in nature (i.e., under stable permafrost conditions) the bacterial population is in a stationary phase; microbial life in nature is rarely in the log phase of growth (16). Thus, we concluded that measurable metabolic activity of permafrost bacteria is possible at temperatures down to at least −20°C, but in the stationary phase, which is reached less than 1 year after freezing, the level of activity, if any, is not measurable with our present methods.
The ecological significance of the metabolic state of permafrost bacteria becomes evident when we consider that permafrost underlies about 20% of the Earth's land surface, including 85% of Alaska, 55% of Russia and Canada, and probably all of Antarctica (20). This considerable mass of frozen soil, which is up to several hundred meters deep, harbors very large numbers of viable bacteria. Our results probably apply to bacteria living within the entire temperature range of permafrost on Earth.
It is interesting to compare our results for permafrost with another case of microbial activity that occurs both at subzero temperatures and on geological time scales, namely, the cryptoendolithic microbial community in the Antarctic desert. In contrast to permafrost, where the temperature is stable for up to millions of years, the environment inside porous rocks of the Antarctic desert is thermally highly unstable, and during the growth season (summer) the temperature oscillates across the freezing point (7, 8, 15). In this environment, during the approximately 104-year-long growth cycle, growth is continuous (an extended log phase) and ends abruptly when the carrying capacity of the porous rock substrate is reached, which results in exfoliation of the rock crust and loss (or death) of the organisms (27). Microbial growth patterns on geological time scales can therefore be very different depending on the conditions in the environment.
ACKNOWLEDGMENTS
This work was supported by NASA grant NAGW-4044 and NSF grant OPP-9420227 to E.I.F. and by Russian Academy of Sciences grants 98-04-48357 and 96-05-65226 E.M.R. and D.A.G., respectively.
We thank L. X. Finegold (Drexel University, Philadelphia, Pa.), K. H. Nealson (NASA Jet Propulsion Laboratory, Pasadena, Calif.), and G. Stotzky (New York University, New York, N.Y.) for critical reading of the manuscript and A. B. Thistle (Florida State University) for critical editing of the manuscript.
REFERENCES
- 1.Anderson D A. Ice nucleation and the substrate-ice interface. Nature. 1967;216:563–566. [Google Scholar]
- 2.Anderson D M, Morgenstern N R. Permafrost: Second International Conference. Washington, D.C.: National Academy of Sciences; 1973. Physics, chemistry, and mechanics of frozen ground: a review; pp. 257–288. [Google Scholar]
- 3.Balkwill D L, Rucinsky T E, Casida L E. Release of microorganisms from soil with respect to transmission electron microscopy viewing and plate counts. Antonie Leeuwenhoek. 1977;43:73–87. doi: 10.1007/BF02316212. [DOI] [PubMed] [Google Scholar]
- 4.Bligh E G, Dyer W J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 5.Ershov E D. General geocryology. Cambridge, United Kingdom: Cambridge University Press; 1998. [Google Scholar]
- 6.Finegold L. Molecular and biophysical aspects of adaptation of life to temperatures below the freezing point. Adv Space Res. 1996;18(12):87–95. [Google Scholar]
- 7.Friedmann E I, Kappen L, Meyer M A, Nienow J A. Long-term productivity in the cryptoendolithic microbial community of the Ross Desert, Antarctica. Microb Ecol. 1993;25:51–69. doi: 10.1007/BF00182129. [DOI] [PubMed] [Google Scholar]
- 8.Friedmann E I, McKay C P, Nienow J A. The cryptoendolithic microbial environment in the Ross Desert of Antarctica: satellite-transmitted continuous nanoclimate data, 1984 to 1986. Polar Biol. 1987;7:273–287. doi: 10.1007/BF00443945. [DOI] [PubMed] [Google Scholar]
- 9.Geiges O. Microbial processes in frozen food. Adv Space Res. 1996;18(12):109–118. [Google Scholar]
- 10.Gilichinsky D, Wagener S. Microbial life in permafrost: a historical review. Permafrost Periglacial Processes. 1995;6:243–250. [Google Scholar]
- 11.Gilichinsky D A, Wagener S, Vishnevetskaya T A. Permafrost microbiology. Permafrost Periglacial Processes. 1995;6:281–291. [Google Scholar]
- 12.Kappen L B, Schroeter B, Scheidegger C, Sommerkorn M, Hestmark G. Cold resistance and metabolic activity of lichens below 0°C. Adv Space Res. 1996;18(12):119–128. [Google Scholar]
- 13.Kates M, editor. Laboratory techniques in biochemistry and molecular biology. Vol. 3 1986. , part ii. Techniques of lipidology. Elsevier, Amsterdam, The Netherlands. [Google Scholar]
- 14.Mazur P. Limits of life at low temperatures and at reduced water contents and water activities. Origins Life. 1980;10:137–159. doi: 10.1007/BF00928665. [DOI] [PubMed] [Google Scholar]
- 15.McKay C P, Nienow J A, Meyer M A, Friedmann E I. Continuous nanoclimate data (1985–1988) from the Ross Desert (McMurdo Dry Valleys) cryptoendolithic microbial ecosystem. Antarct Res Ser. 1993;61:201–207. [Google Scholar]
- 16.Morita R Y. Bacteria in oligotrophic environments. New York, N.Y: Chapman & Hall; 1997. [Google Scholar]
- 17.Nersesova Z A, Tsytovich N A. Permafrost: Proceedings of International Conference. Washington, D.C.: National Academy of Sciences; 1966. Unfrozen water in frozen soils; pp. 230–234. [Google Scholar]
- 18.Ostroumov V E, Siegert C. Exobiological aspects of mass transfer in microzones of permafrost deposits. Adv Space Res. 1996;18(12):79–86. [Google Scholar]
- 19.Passo C J, Cordon T. Environmental liquid scintillation spectrometry. Meriden, Conn: Packard Instrument Company; 1944. [Google Scholar]
- 20.Péwé T L. The new encyclopaedia Britannica, macropaedia. Vol. 20. Chicago, Ill: Encyclopaedia Britannica; 1998. Ice and ice formations; pp. 737–759. [Google Scholar]
- 21.Rivkina E, Gilichinsky D, Wagener S, Tiedje J, McGrath J. Biogeochemical activity of anaerobic microorganisms from buried permafrost sediments. Geomicrobiol J. 1998;15:187–193. [Google Scholar]
- 22.Russell N J. Cold adaptation of microorganisms. Phil Trans R Soc London B Biol Sci. 1990;326:595–611. doi: 10.1098/rstb.1990.0034. [DOI] [PubMed] [Google Scholar]
- 23.Schroeter B, Green T G A, Kappen L, Seppelt R A. Carbon dioxide exchange at subzero temperatures: field measurements on Umbilicaria aprina in Antarctica. Crypt Bot. 1994;4:233–241. [Google Scholar]
- 24.Shi T, Reeves R H, Gilichinsky D A, Friedmann E I. Characterization of viable bacteria from Siberian permafrost by 16S rDNA sequencing. Microb Ecol. 1997;33:169–179. doi: 10.1007/s002489900019. [DOI] [PubMed] [Google Scholar]
- 25.Snyder J D, Trofymow J A. A rapid accurate wet oxidation diffusion procedure for determining organic carbon in plant and soil samples. Commun Soil Sci Plant Anal. 1984;15:587–597. [Google Scholar]
- 26.Staley J T. Prosthecomicrobium and Ancalomicrobium: new prosthecate freshwater bacteria. J Bacteriol. 1968;95:1921–1942. doi: 10.1128/jb.95.5.1921-1942.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun H J, Friedmann E I. Growth on geological time scales in the Antarctic cryptoendolithic microbial community. Geomicrobiol J. 1999;16:193–202. [Google Scholar]
- 28.Vestal J R. Carbon metabolism of the cryptoendolithic microbiota from the Antarctic desert. Appl Environ Microbiol. 1988;54:960–965. doi: 10.1128/aem.54.4.960-965.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vorobyova E, Soina V, Gorlenko M, Minkovskaya N, Zalinova N, Mamukelashvili A, Gilichinsky D, Rivkina E, Vishnivetskaya T. The deep cold biosphere: facts and hypothesis. FEMS Microbiol Rev. 1997;20:277–290. [Google Scholar]
- 30.Wan C P, Sigh R V, Lau B H S. A simple fluorometric assay for the determination of cell numbers. J Immunol Methods. 1994;173:265–272. doi: 10.1016/0022-1759(94)90305-0. [DOI] [PubMed] [Google Scholar]
- 31.White D C, Bobbie J R, Morrison S J, Oosterhof D K, Taylor C W, Meeter D A. Determination of microbial activity of estuarine detritus by relative rates of lipid biosynthesis. Limnol Oceanogr. 1977;22:1089–1099. [Google Scholar]
- 32.Wilson G S, Braddock P, Forman S L, Friedmann E I, Rivkina E M, Chanton J P, Gilichinsky D A, Fyodorov-Davidov D G, Ostroumov V E, Sorokovikov V, Wizevich M C. Coring for microbial records of Antarctic climate. Antarct J US 1996 Review. 1998;31(2):83–86. [Google Scholar]