Abstract
Objective
The aim of this study was to evaluate the prognostic significance of the combination of the magnetic resonance spectroscopy (MRS) parameters and systemic immune-inflammation index (SII) in patients with brain metastases (BMs) from non-small cell lung cancer (NSCLC) treated with stereotactic radiotherapy.
Methods
A total of 118 NSCLC patients with BM who were treated with stereotactic radiotherapy were retrospectively enrolled in this study. All patients underwent MRS and blood samples test for SII analysis before the initiation of stereotactic radiotherapy. The correlation between the parameters of MRS and SII level was assessed using Spearman’s correlation coefficient. The cutoff values for the parameters of MRS, SII, and clinical laboratory variables were defined by the receiver operating characteristic (ROC) curve analysis to quantify these predictive values. The prognostic factors of overall survival (OS) and progression-free survival (PFS) curves were assessed using the Kaplan–Meier and Cox proportional hazards models.
Results
The median follow-up time was 25 months (range, 12–49 months). The optimal cutoff point for the choline/creatine (Cho/Cr) ratio and SII were 1.50 and 480, respectively. The Cho/Cr ratio was negatively correlated with SII (rs = 0.164, p = 0.075), but there was a trend. The C-SII score was established by combining the Cho/Cr ratio and SII. Patients with both an elevated Cho/Cr ratio (>1.50) and an elevated SII (>480) were given a C-SII score of 2, and patients with one or neither were given a C-SII score of 1 or 0, respectively. The Kaplan–Meier analysis showed that a C-SII score of 2 was significantly linked with poor OS and PFS (p < 0.001 and p < 0.001, respectively). In the Cox proportional hazards model, the C-SII score independently predicted OS [hazard ratio (HR), 1.749; 95% CI, 1.176–2.601; p = 0.006] and PFS (HR, 2.472; 95% CI, 1.624–3.763; p < 0.001).
Conclusion
The C-SII score was more accurate for predicting the clinical outcomes of NSCLC patients with BM who underwent stereotactic radiotherapy. The C-SII score, which was superior to either score alone, could be used to identify BM in NSCLC patients with poor outcomes.
Keywords: NSCLC, brain metastases, SII, MRS, prognosis
Introduction
Lung cancer is the most common cancer and the leading cause of cancer-related mortality in China (1). Non-small cell lung cancer (NSCLC) accounts for approximately 85% of all lung cancer cases, and NSCLC has a high predilection to metastasize to the brain (2). Brain metastasis (BM) occurs in approximately 20%–40% of patients with NSCLC and has become an important factor affecting the clinical outcomes of patients despite active treatments (3, 4). Radiation therapy includes stereotactic radiotherapy and whole-brain radiation treatment. Stereotactic radiotherapy has shown advantages over whole-brain radiotherapy as an effective treatment of choice for BM patients because of excellent local control and minimal toxicity (5–9). However, the patients’ median survival time is <12 months, and the quality of life of patients with BM is low, which poses a major challenge for NSCLC patients with BM (10). Reliable prognostic indicators must be identified to look for a better treatment choice and assess patient prognosis.
Consensus regarding abnormal metabolism in tumor tissue has been reached, and this study of metabolomics continues to provide new insight into the biological behavior of the tumor (11). Advanced magnetic resonance spectroscopy (MRS) technique can be applied to answer various clinical questions and evaluate cellular metabolic biochemical composition. The MRS technique provides principal metabolic biochemical composition such as N-acetyl-aspartate (NAA), choline (Cho), and creatine (Cr). Cho reflects synthesis and metabolism of cell membranes and turnover of the cell membrane during breakdown (12). Cr reflects the energy metabolism of the brain tissue cell, which are constant in normal brain tissue (13). NAA, a brain tissue neuronal marker, is reduced in the development of brain tumors (13). MRS parameters have widely been shown to be capable of identifying the biology of tumor metabolism and can monitor the treatment effect or tumor progression (14, 15). Our primary study has shown that the Cho/Cr ratio has been independently verified as a useful predictor in the prognosis of NSCLC patients (16). However, the occurrence of tumors is a comprehensive result of various systemic disorders, and its characteristics cannot be accurately predicted simply from one aspect. Cancer-related inflammation is a necessary component of the tumor microenvironment, and inflammatory cells may play a critical role in the development of malignancies (17). Furthermore, systemic immune-inflammation involves metabolic biochemical mechanisms where cancer cells express immune-inflammatory cytokines, potentially reflecting the biological activity of tumor cells. These theories continue to enhance our understanding of tumors and inflammation. In the clinics, systemic immune-inflammation index (SII), derived from neutrophil (N), lymphocyte (L), and platelet (P) in peripheral blood, has been deeply investigated in many malignancies. SII has become a reliable biomarker to reflect the immune and inflammation status of host and has been used as a prognostic index in multiple malignant cancers, including NSCLC (18–22). MRS detects the biological activity of cellular metabolism, while SII indicates immune-inflammation status, and both have been independently verified as useful predictors in the prognosis of NSCLC patients. However, clinical data focusing on the predictive value of combination of the MRS and SII for BM in NSCLC patients treated with stereotactic radiotherapy remain unknown.
This study was performed to explore the prognostic value of the C-SII score for BM in NSCLC patients treated with stereotactic radiotherapy and aimed to provide appropriate and individualized therapy in clinical treatment.
Patients and Methods
Study Design
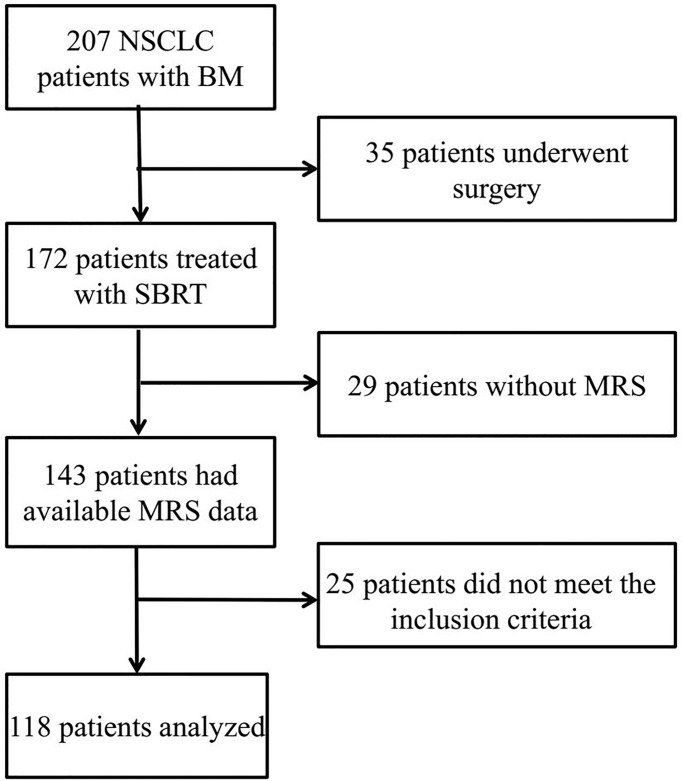
Between January 2014 and December 2017, the 118 patients with histologically confirmed NSCLC receiving stereotactic radiotherapy at The Affiliated Suzhou Hospital of Nanjing Medical University were retrospectively reviewed. Clinicopathological characteristics such as age, gender, Karnofsky Performance Status (KPS), patient histology, number of BMs, and TNM stage of patients were collected through their medical records. The primary lesions had been resected or stably controlled. The MRS metabolism composition and immune-inflammatory parameters were obtained from the peripheral blood and MRS, respectively. The inclusion criteria were as follows: i) age > 18 years, ii) biopsy histological confirmation of NSCLC, iii) Eastern Cooperative Oncology Group (ECOG) scores ranging from 0 to 2, iv) MRS imaging obtained before treatment with no contraindications, v) the number of BM ≤ 3, and vi) no hematological disorders. The specific patient flowchart is shown in Figure 1 .
Figure 1.
Patients’ screening process and results.
Radiation Treatment
Enhanced CT scans (Philips Brilliance Big Bore CT) imaging was acquired and fused with a T1-weighted post-gadolinium MRI scan within 7 days of CT localization. The gross tumor volume (GTV) is accurately delineated by the identification of fusion images on the planning system, or 18F-fluorodeoxyglucose (FDG) PET/CT. At the same time, we have mapped out the critical organ structures (organ at risk (OAR)), including the brain stem, left eye, right eye, left optic nerve, right optic nerve, lens, and optic chiasm. The range of stereotactic radiotherapy doses used was 40–60 Gy. All radiation plans were created with arcs of 6-MV photons ( Figure 2 ). Patients were given corresponding dehydration therapy to reduce brain edema during treatment.
Figure 2.
SBRT target area in a 58-year-old man with squamous cell lung cancer of the right frontal lobe: CT (A) and MRI (B). SBRT, stereotactic body radiotherapy.
Magnetic Resonance Spectroscopy Analysis
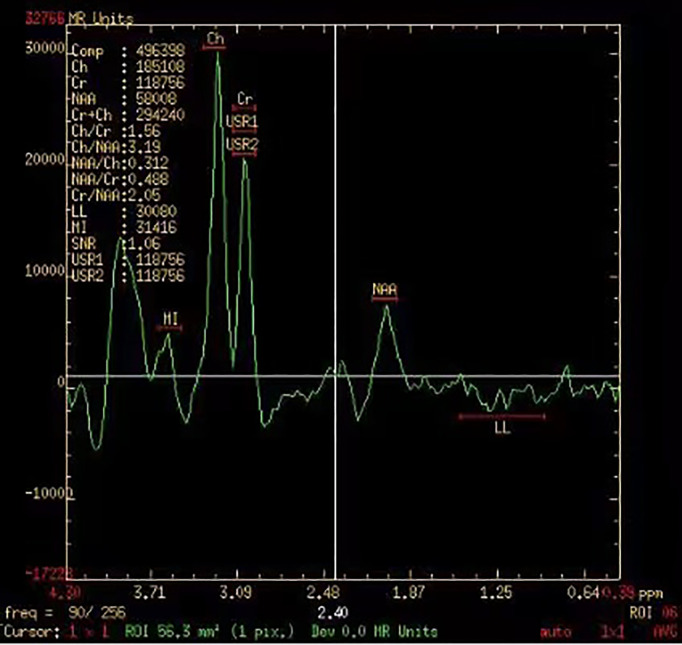
The brain scan sequence (MRI plain scanning, enhanced MRI scan, and MRS scan) was performed using a 3.0-T clinical scanner magnetic resonance machine (Philips Healthcare, Andover, MA, USA). We used point-resolved spectroscopic (PRESS) to perform multivoxel MRS sequence scanning and determined the region of interest (ROI) by three clinicians and radiologists. Normal brain tissue at the opposite side of the tumor lesion was selected as the reference area. 1H-MRS scanning parameters were as follows: repetition time (TR), 1,500 ms; echo time (TE), 135 ms; field of view (FOV), 230 mm × 230 mm; slice thickness, 5 mm; scan time, 350 s; and voxel size, 1 × 1 × 1 mm3 ( Figure 3 ). The MRS analysis software was used to automatically measure and calculate the relative metabolite intensities of the signals. MRS evaluation indicators included Cho, Cr, NAA, Cho/Cr ratio, and Cho/NAA.
Figure 3.
Magnetic resonance spectroscopy imaging of a 66-year-old women with NSCLC. NSCLC, non-small cell lung cancer.
Peripheral Blood Sample Analysis
We collected peripheral venous blood from the patients 7 days before stereotactic radiotherapy. We strictly followed the inclusion criteria and performed related index analyses. SII was calculated using the formula SII = P * N/L, which is based on platelet (P), neutrophil (N), and lymphocyte (L) count. The neutrophil-to-lymphocyte ratio (NLR) was defined as the absolute neutrophil count divided by the absolute lymphocyte count. The platelet-to-lymphocyte ratio (PLR) was defined as the absolute platelet count divided by the absolute lymphocyte count.
Definition of C-SII Score
Based on the optimal cutoff value, the Cho/Cr ratio and SII were split into low and high groups, respectively. In this study, we constructed a novel prognostic C-SII score system, which included both the Cho/Cr ratio and SII. We assigned a score of 0–2 to the Cho/Cr ratio (low and high) and SII (low and high). Patients were classified into three subgroups: low Cho/Cr ratio and low SII group (score = 0), low Cho/Cr ratio or low SII group (score = 1), and high Cho/Cr ratio and high SII group (score = 2).
Statistical Analysis
The predictive value of the MRS and peripheral blood parameters was assessed by calculating the area under the ROC curve (AUC). A ROC curve was generated to obtain the Cho, Cr, NAA, Cho/Cr ratio, Cho/NAA, N, P, L, SII, NLR, and PLR cutoff values. Spearman’s coefficient test was performed to explore correlations between the Cho/Cr ratio and SII. Survival curves were analyzed to assess the survival time distribution by the Kaplan–Meier method and compared using the log-rank test to test the significance of overall survival (OS) and progression-free survival (PFS) among the different prognostic groups. Univariate and multivariate analysis logistic regression analyses were used to determine independent prognostic factors. Two-sided p-values <0.05 were considered statistically significant. All analyses were conducted by using SPSS v19.0 software (SPSS, Inc., Chicago, IL, USA) and GraphPad Prism 5 software (GraphPad, San Diego, CA, USA).
Results
Clinicopathological Characteristics of Patients
As shown in Table 1 , a total of 118 patients were enrolled in this retrospective study. Ages ranged from 52 to 71 years (median, 59 years). Most patients (54.2%) were female, and the majority (74.6%) has adenocarcinoma. The findings showed 72 (61.0%) with 1 BM and 88 (74.6) patients with neurologic symptoms. Regarding staging, 75 patients (63.6%) had T1–T2 stage, and 31.4% of patients had N2 and N3 stage tumors. After a median follow-up of 25 months (range 15–49 months), 69 of the 118 (58.5%) patients suffered recurrence and metastasis, and 66 (55.9%) patients died.
Table 1.
The clinical characteristics of all NSCLC patients with BM.
| Variables | Values |
|---|---|
| Gender | |
| Male Female |
54 (45.8) 64 (54.2) |
| Age (years) | |
| <60 ≥60 |
58 (49.2) 60 (50.8) |
| Smoke | |
| No Yes |
53 (44.9) 65 (55.1) |
| KPS | |
| 90-100 70-80 |
55 (46.6) 63 (53.4) |
| Patient histology | |
| SCC AD |
30 (25.4) 88 (74.6) |
| Number of BMs | |
| 1 2-3 |
72 (61.0) 46 39.0) |
| Maximum diameter | |
| < 2cm ≥2 cm |
62 (52.5) 56 (47.5) |
| Neurologic symptoms | |
| No Yes |
30 (25.4) 88 (74.6) |
| Tumor stage | |
| T1-T2 T3-T4 |
75 (63.6) 43 (36.4) |
| Node stage | |
| N0-N1 N2-N3 |
81 (68.6) 37 (31.4) |
| TNM stage | |
| I II-III |
61 (51.7) 57 (48.3) |
| CEA | |
| Normal Elevated |
70 (59.3) 48 (40.7) |
KPS, Karnofsky performance score; SCC, squamous cell carcinoma; AD, adenocarcinoma; BM, brain metastases; CEA, carcinoembryonic antigen.
Choline/Creatine Ratio and Systemic Immune-Inflammation Index
With Youden’s J-statistics establishing 1.50 (sensitivity, 78.3%; specificity, 61.2%) as optimal cutoff values for the Cho/Cr ratio to predict PFS, the ROC analysis calculated the AUC as 0.737. The same analysis showed that the optimal cutoff value was 480 (sensitivity, 69.6%; specificity, 75.5%) for SII with the largest AUC of 0.712 ( Figure 4 ). The Cho/Cr ratio and SII levels of pretreatment were 1.60 (0.83–5.68) and 481.71 (280.29–978.81), respectively. Table 2 presents the results of quantitative parameters of MRS and immune-inflammatory. We observed a negative correlation between the Cho/Cr ratio and SII, but there was a positive trend (p = 0.075; Spearman’s correlation coefficient, rs = 0.164) ( Figure 5 ).
Figure 4.
Receiver operating characteristic (ROC) curve analysis of (A) Cho/Cr and (B) SII. Cho, choline; Cr, creatine; SII, systemic immune-inflammation index.
Table 2.
A summary of MRS metabolic characteristics and inflammation index.
| Median (range) | |
|---|---|
| Signal | |
| Cho | 2.03 (0.93-3.37) |
| Cr | 1.28 (0.43-2.20) |
| NAA | 0.82 (0.21-1.72) |
| Cho/Cr | 1.60 (0.83-5.68) |
| Cho/NAA | 2.44 (0.89-14.38) |
| Peripheral blood inflammation index | |
| Neutrophil | 4.91 (2.36-9.88) |
| Lymphocyte | 1.93 (0.82-3.57) |
| Platelet | 188 (102-402) |
| SII | 481.71 (280.29-978.81) |
| NLR | 2.59 (1.37-5.97) |
| PLR | 99.21 (38.98-168.00) |
Cho, choline; Cr, creatine; NAA, N-acetyl-aspartate; NLR, Neutrophil-to-lymphocyte ratio; PLR, Platelet-to-lymphocyte ratio; SII, Systemic immune-inflammation index.
Figure 5.
Correlations between Cho/Cr and SII. Cho, choline; Cr, creatine; SII, systemic immune-inflammation index.
Survival Analysis
After a median follow-up of 25 months, OS (median OS 23 vs. 18 months) and PFS (median PFS 13 vs. 9 months) in the low Cho/Cr ratio group as compared with the high Cho/Cr ratio were significantly prolonged. In particular, patients with high SII showed a shorter OS (median OS 18 vs. 20 months) and PFS (median PFS 9 vs. 11.5 months) ( Figure 6 ). The median OS in patients with a C-SII score of 2 was significantly lower than the OS in patients with a C-SII score of 1 and a C-SII score of 0 (18 vs. 18 vs. 23 months; p < 0.001) ( Figure 7A ). The median PFS rates were 13, 9, and 9 months for patients with C-SII scores of 0, 1, and 2, respectively (p < 0.001) ( Figure 7B ).
Figure 6.
Kaplan–Meier survival curves of Cho/Cr and SII in NSCLC patients with brain metastases. (A) Overall survival curves of Cho/Cr. (B) Progression-free survival curves of Cho/Cr. (C) Overall survival curves of SII. (D) Progression-free survival curves of SII. Cho, choline; Cr, creatine; SII, systemic immune-inflammation index.
Figure 7.
Kaplan–Meier survival curves depicting outcomes of overall survival (A) and progression-free survival (B) according to the C-SII score.
Univariate and multivariate Cox regression analyses were performed to explore the factors affecting survival in this study. The univariate analysis revealed that age (p = 0.022), smoking status (p = 0.023), and KPS (p = 0.003) were significantly associated with OS; KPS (p = 0.014) and patient histology (p = 0.003) were significantly associated with PFS. In terms of MRS and peripheral blood parameters, PLR (p = 0.033, p = 0.006), SII (p = 0.001, p = 0.002), and Cho/Cr ratio (p = 0.007, p < 0.001) were positively associated with OS and PFS. We found no significant association of survival with the number of BMs, maximum diameter, neurologic symptoms, and TNM stage ( Tables 3 , 4 ).
Table 3.
Univariate analysis of OS and PFS based on clinical-pathological data.
| Variables | OS | PFS | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Gender | ||||||
| Male Female |
Reference 0.911 |
0.560-1.482 | 0.707 | Reference 0.929 |
0.577-1.495 | 0.761 |
| Age (years) | ||||||
| <60 ≥60 |
Reference 1.791 |
1.088-2.946 | 0.022 | Reference 1.476 |
0.909-2.386 | 0.115 |
| Smoke | ||||||
| No Yes |
Reference 1.847 |
1.089-3.133 | 0.023 | Reference 1.536 |
0.937-2.517 | 0.089 |
| KPS | ||||||
| 90-100 70-80 |
Reference 2.206 |
1.298-3.750 | 0.003 | Reference 1.874 |
1.133-3.099 | 0.014 |
| Patient histology | ||||||
| SCC AD |
Reference 1.294 |
0.717-2.337 | 0.392 | Reference 2.797 |
1.418-5.519 | 0.003 |
| Number of BMs | ||||||
| 1 2-3 |
Reference 0.828 |
0.501-1.371 | 0.464 | Reference 0.879 |
0.536-1.441 | 0.608 |
| Maximum diameter | ||||||
| < 2cm ≥2 cm |
Reference 0.976 |
0.602-1.582 | 0.920 | Reference 1.264 |
0.784-2.039 | 0.336 |
| Neurologic symptoms | ||||||
| No Yes |
Reference 0.733 |
0.449-1.196 | 0.213 | Reference 0.868 |
0.538-1.400 | 0.562 |
| Tumor stage | ||||||
| T1-T2 T3-T4 |
Reference 0.604 |
0.349-1.047 | 0.072 | Reference 0.834 |
0.501-1.388 | 0.484 |
| Node stage | ||||||
| N0-N1 N2-N3 |
Reference 0.782 |
0.449-1.362 | 0.385 | Reference 1.077 |
0.642-1.806 | 0.778 |
| TNM stage | ||||||
| I II-III |
Reference 1.048 |
0.639-1.718 | 0.852 | Reference 1.136 |
0.704-1.834 | 0.602 |
| CEA | ||||||
| Normal Elevated |
Reference 0.879 |
0.535-1.444 | 0.611 | Reference 1.158 |
0.717-1.870 | 0.548 |
OS, overall survival; PFS, progression-free survival; HR, hazard ratio; CI, confidence interval; KPS, Karnofsky performance score; SCC, squamous cell carcinoma; AD, adenocarcinoma; BM, brain metastases; CEA, carcinoembryonic antigen.
Table 4.
Univariate analysis of OS and PFS based on MRS metabolic characteristics and inflammation index.
| Variables | OS | PFS | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Cho | ||||||
| ≤2.50 >2.50 |
Reference 1.223 |
0.696-2.150 | 0.484 | Reference 1.551 |
0.902-2.665 | 0.113 |
| Cr | ||||||
| ≤0.50 >0.50 |
Reference 0.641 |
0.155-2.648 | 0.539 | Reference 0.501 |
0.121-2.074 | 0.341 |
| NAA | ||||||
| ≤1.50 >1.50 |
Reference 0.896 |
0.325-2.472 | 0.832 | Reference 1.188 |
0.477-2.960 | 0.712 |
| Cho/Cr | ||||||
| ≤1.50 >1.50 |
Reference 2.140 |
1.230-3.723 | 0.007 | Reference 2.863 |
1.586-5.169 | <0.001 |
| Cho/NAA | ||||||
| ≤3.50 >3.50 |
Reference 1.485 |
0.903-2.442 | 0.119 | Reference 1.619 |
0.995-2.633 | 0.052 |
| Neutrophil | ||||||
| ≤6.13 >6.13 |
Reference 1.682 |
0.993-2.849 | 0.053 | 1.581 | 0.935-2.671 | 0.087 |
| Lymphocyte | ||||||
| ≤2.70 >2.70 |
Reference 1.507 |
0.717-3.170 | 0.280 | Reference 1.441 |
0.727-2.858 | 0.296 |
| Platelet | ||||||
| ≤169 >169 |
Reference 1.336 |
0.794-2.246 | 0.275 | Reference 1.361 |
0.812-2.280 | 0.242 |
| SII | ||||||
| ≤480 >480 |
Reference 2.513 |
1.445-4.370 | 0.001 | Reference 2.297 |
1.370-3.851 | 0.002 |
| NLR | ||||||
| ≤2.50 >2.50 |
Reference 1.296 |
0.769-2.187 | 0.330 | Reference 1.182 |
0.724-1.929 | 0.503 |
| PLR | ||||||
| ≤91.50 >91.50 |
Reference 1.854 |
1.053-3.265 | 0.033 | Reference 2.211 |
1.261-3.876 | 0.006 |
OS, overall survival; PFS, progression-free survival; HR, hazard ratio; CI, confidence interval; Cho, choline; Cr, creatine; NAA, N-acetyl-aspartate; NLR: Neutrophil-to-lymphocyte ratio; PLR: Platelet-to-lymphocyte ratio; SII: Systemic immune-inflammation index.
To verify the predictive value of the C-SII score in NSCLC with BM, we performed multivariate Cox proportional hazards regression analysis including age, smoking status, KPS, patient histology, and C-SII score. The C-SII score is an independent prognostic factor for OS (hazard ratio (HR), 1.749; 95% CI, 1.176–2.601; p = 0.006) and PFS (HR, 2.472; 95% CI, 1.624–3.763; p < 0.001) ( Table 5 ).
Table 5.
Multivariate Cox regression analysis of OS and PFS.
| Variables | OS | PFS | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Age (years) | ||||||
| <60 ≥60 |
Reference 1.749 |
1.028-2.978 | 0.039 | |||
| Smoke | ||||||
| No Yes |
Reference 1.106 |
0.618-1.980 | 0.735 | |||
| KPS | ||||||
| 90-100 70-80 |
Reference 1.515 |
0.848-2.706 | 0.161 | Reference 1.263 |
0.746-2.139 | 0.385 |
| Patient histology | ||||||
| SCC AD |
Reference 4.217 |
1.657-10.736 | 0.003 | |||
| PLR | ||||||
| ≤91.50 >91.50 |
Reference 1.209 |
0.654-2.237 | 0.545 | Reference 0.556 |
0.242-1.273 | 0.165 |
| C-SII score | ||||||
| 0 1-2 |
Reference 1.749 |
1.176-2.601 | 0.006 | Reference 2.472 |
1.624-3.763 | <0.001 |
BM, brain metastases; HR, hazard ratio; CI, confidence interval; OS, overall survival; PFS, progression-free survival; Cho, choline; Cr, creatine; NAA, N-acetyl-aspartate.
Discussion
A reliable prognostic prediction score system is crucial in risk stratification for patients and for adjusting appropriate treatment strategies for NSCLC with BM. In the present study, we investigated the utility of the Cho/Cr ratio and SII and C-SII scores on prognosis in NSCLC patients. The results showed that the C-SII score is an independent predictor of OS and PFS among NSCLC patients with BM.
In recent years, stereotactic radiotherapy is used to treat limited numbers of BM, since this therapy is less invasive than drugs and surgical resection and has better local control (23, 24). Therefore, the use of stereotactic radiotherapy is recommended to further control BM risk (25). At present, various blood indicators have been evaluated for their prognostic roles in NSCLC patients with BM, such as neuron‐specific enolase (NSE) (26), carcinoembryonic antigen (CEA) (27), and Lung-molGPA (28). However, there are no reliable predictors that can reflect different tumor biological behaviors. Hence, searching for accurate prognostic factors is of great clinical application value.
The occurrence of tumors is often accompanied by changes in metabolic biochemical composition. MRS is a non-invasive and sensitive imaging method that allows researchers to measure and visualize metabolic biochemical information from brain tumor tissues (29). Increasing evidence has indicated that MRS can identify tumor active regions and enhance more individualized response-based treatment in high-grade glioma (15). An ongoing effort at the Tehran University of Medical Sciences has shown that MRS parameters can improve the accuracy of predictive nomograms to assess the risk of biochemical recurrence after radical prostatectomy in prostate cancer (30). More comprehensive understanding of the biochemical composition changes in metabolites for tumors is urgently needed. The typical MRS metabolic abnormalities of BM often include increased Cho and decreased NAA and Cr. Minicozzi et al. observed in thirty-six head and neck cancer cases that the Cho/Cr ratio is significantly elevated in the group with poor response (14). Fink et al. found that multivoxel MRS Cho/Cr ratio peak-area shows a great advantage for distinguishing glioma recurrence (31). Negendank and colleagues conducted a co-operative study with 15 clinical research centers and confirmed that Cho was higher in glial tumors than in non-involved brain tissues (32). Dowling and coworkers revealed that Cho concentrations and NAA in tumor tissue were higher than normal values cancer (33). In this study, our result reported that the Cho/Cr ratio was an independent relevant factor for death and progression (p = 0.006 and p < 0.001). Whether Cho and Cr were not correlated with prognosis of NSCLC with BM is unknown.
Accumulating studies have substantiated that peripheral venous blood markers can reflect the condition of the host immune-inflammation status. Counts of the peripheral immune-inflammatory cells, such as platelet, lymphocytes, and neutrophils, have confirmed the reliable association link between inflammatory cells and prognosis in malignant tumors (34–37). SII is an integrated parameter, including platelets, neutrophils, and lymphocytes, and has been proved to be an independent predictor of malignant tumors (22, 38–43). The value of SII in predicting clinical outcomes for cancer patients may be associated with the function of platelets, neutrophils, and lymphocytes. Platelets release growth factors and pro-angiogenic protein and protect tumor cells from an immune response (44). Neutrophils can take part in various stages of growth and metastasis of tumors and generate immunosuppressive effects by producing and secreting cytokines, chemokines, and proteases (45). In contrast to neutrophils, lymphocytes exert an important antitumor immune response and have been proved to be related to systematic immune surveillance (46). In this study, the association between SII and clinical outcomes of patients in NSCLC with BM was evaluated. Our results indicated that SII was significantly associated with OS and PFS (p = 0.001 and p = 0.002).
Clinicians can evaluate clinical indicators such as tumor size, degree of tumor differentiation, or tumor location, but these evaluation criteria are based on individual subjective evaluation and judgment. The heterogeneity of individual tumors is largely reflected in the biological characteristics of tumors and the host immune-inflammatory state. Considering a problem from multiple angles can lead to breakthroughs. Recently, many scholars have realized that combining two peripheral blood indexes can be considered a useful independent prognostic marker of tumors. In the retrospective study initiated by Chen et al., their results revealed that the combination of circulating tumor cells with CEA has a better disease prediction than alone in NSCLC patients (47). Huang et al. showed that preoperative combined NLR and fibrinogen concentration can be used as independent prognostic indicators for OS (HR, 1.512; 95% CI, 1.283–1.783; p < 0.001) (48). Although Guo et al. (49) and Schernberg et al. (50) conducted related studies on PET/CT combined with blood inflammation indicators predicting prognosis and obtained positive results, clinical data focusing on the predictive value of the combination of the MRS and SII for BM in NSCLC patients treated with stereotactic radiotherapy have not been reported. We previously used MRS alone to evaluate the prognosis of patients with BM in NSCLC patients and revealed that a positive Cho/Cr ratio was an independent risk factor for OS (p = 0.009) and PFS (p = 0.006) (16). The Cho/Cr ratio or SII alone may not be sufficient to accurately reflect the tumor characteristics. Using the C-SII scores system may be a more accurate choice. In our study, our results revealed that patients with a C-SII score of 2 (Cho/Cr > 1.50 and SII > 480) have poorer clinical outcomes than patients with a C-SII score of 1 (Cho/Cr > 1.50 or SII > 480) or C-SII score of 0 (Cho/Cr ≤ 1.50 and SII ≤ 480). In the multivariate Cox regression analyses, study results demonstrated that the C-SII score independently predicted OS (HR, 1.749; 95% CI, 1.176–2.601; p = 0.006) and PFS (HR, 2.472; 95% CI, 1.624–3.763; p < 0.001).
We established a C-SII score system by combining the Cho/Cr ratio and SII in this study, and preliminary results showed that it was an accurate and reliable system for evaluating prognosis in NSCLC patients with BM. However, only 118 NSCLC with BM patients were assessed in this study because of the limited number of enrolled cases. In addition, incomplete clinical data and loss of follow-up were inevitable because of the long duration of this retrospective study. There is selection bias when clinicians and radiologists use MRS to determine the ROI. These limitations require further evaluation, and we need a validation cohort to further verify our conclusions in the future.
Conclusion
NSCLC patients with a high Cho/Cr ratio and high SII had significantly poor outcomes in the present study. The C-SII score system is a strongly unfavorable survival index in assessing risk stratification for NSCLC patients with BM, which suggests that clinicians should adjust the treatment strategy and generalize clinical application.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics Statement
This study was approved by the ethics committee of the Rizhao Center Hospital and The Affiliated Suzhou Hospital of Nanjing Medical University. This study was conducted in strict accordance with the national institutes of health guidelines. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
DG, JL, YL, QC, YZ, XG, SJ, and SZ prepared and reviewed the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by a grant (GSWS2020067) from the Gusu Health Talent Program.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer Statistics in China, 2015. CA Cancer J Clin (2016) 66(2):115–32. doi: 10.3322/caac.21338 [DOI] [PubMed] [Google Scholar]
- 2. Pugh TJ, Gaspar LE. Prophylactic Cranial Irradiation for Patients With Lung Cancer. Clin Lung Cancer (2007) 8(6):365–8. doi: 10.3816/CLC.2007.n.016 [DOI] [PubMed] [Google Scholar]
- 3. Zakaria R, Das K, Bhojak M, Radon M, Walker C, Jenkinson MD. The Role of Magnetic Resonance Imaging in the Management of Brain Metastases: Diagnosis to Prognosis. Cancer Imaging (2014) 14:8. doi: 10.1186/1470-7330-14-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wolf A, Kvint S, Chachoua A, Pavlick A, Wilson M, Donahue B, et al. Toward the Complete Control of Brain Metastases Using Surveillance Screening and Stereotactic Radiosurgery. J Neurosurg (2018) 128(1):23–31. doi: 10.3171/2016.10.JNS161036 [DOI] [PubMed] [Google Scholar]
- 5. Ostrom QT, Wright CH, Barnholtz-Sloan JS. Brain Metastases: Epidemiology. Handb Clin Neurol (2018) 149:27–42. doi: 10.1016/B978-0-12-811161-1.00002-5 [DOI] [PubMed] [Google Scholar]
- 6. Sallabanda M, Garcia-Berrocal MI, Romero J, Garcia-Jarabo V, Exposito MJ, Rincon DF, et al. Brain Metastases Treated With Radiosurgery or Hypofractionated Stereotactic Radiotherapy: Outcomes and Predictors of Survival. Clin Transl Oncol (2020) 22(10):1809–17. doi: 10.1007/s12094-020-02321-x [DOI] [PubMed] [Google Scholar]
- 7. Nieder C, Mehta MP, Geinitz H, Grosu AL. Prognostic and Predictive Factors in Patients With Brain Metastases From Solid Tumors: A Review of Published Nomograms. Crit Rev Oncol Hematol (2018) 126:13–8. doi: 10.1016/j.critrevonc.2018.03.018 [DOI] [PubMed] [Google Scholar]
- 8. Brown PD, Jaeckle K, Ballman KV, Farace E, Cerhan JH, Anderson SK, et al. Effect of Radiosurgery Alone vs Radiosurgery With Whole Brain Radiation Therapy on Cognitive Function in Patients With 1 to 3 Brain Metastases: A Randomized Clinical Trial. JAMA (2016) 316(4):401–9. doi: 10.1001/jama.2016.9839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yamamoto M, Serizawa T, Shuto T, Akabane A, Higuchi Y, Kawagishi J, et al. Stereotactic Radiosurgery for Patients With Multiple Brain Metastases (JLGK0901): A Multi-Institutional Prospective Observational Study. Lancet Oncol (2014) 15(4):387–95. doi: 10.1016/S1470-2045(14)70061-0 [DOI] [PubMed] [Google Scholar]
- 10. Chen AM, Jahan TM, Jablons DM, Garcia J, Larson DA. Risk of Cerebral Metastases and Neurological Death After Pathological Complete Response to Neoadjuvant Therapy for Locally Advanced Nonsmall-Cell Lung Cancer: Clinical Implications for the Subsequent Management of the Brain. Cancer (2007) 109(8):1668–75. doi: 10.1002/cncr.22565 [DOI] [PubMed] [Google Scholar]
- 11. Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg Effect: The Metabolic Requirements of Cell Proliferation. Science (2009) 324(5930):1029–33. doi: 10.1126/science.1160809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Miller BL, Chang L, Booth R, Ernst T, Cornford M, Nikas D, et al. In Vivo 1H MRS Choline: Correlation With In Vitro Chemistry/Histology. Life Sci (1996) 58(22):1929–35. doi: 10.1016/0024-3205(96)00182-8 [DOI] [PubMed] [Google Scholar]
- 13. Maheshwari SR, Fatterpekar GM, Castillo M, Mukherji SK. Proton MR Spectroscopy of the Brain. Semin Ultrasound CT MR (2000) 21(6):434–51. doi: 10.1016/S0887-2171(00)90036-2 [DOI] [PubMed] [Google Scholar]
- 14. Minicozzi A, Mosconi E, Cordiano C, Rubello D, Marzola P, Ferretti A, et al. Proton Magnetic Resonance Spectroscopy: Ex Vivo Study to Investigate its Prognostic Role in Colorectal Cancer. BioMed Pharmacother (2013) 67(7):593–7. doi: 10.1016/j.biopha.2013.05.002 [DOI] [PubMed] [Google Scholar]
- 15. Quon H, Brunet B, Alexander A, Murtha A, Abdulkarim B, Fulton D, et al. Changes in Serial Magnetic Resonance Spectroscopy Predict Outcome in High-Grade Glioma During and After Postoperative Radiotherapy. Anticancer Res (2011) 31(10):3559–65. doi: 10.1245/s10434-011-2001-z [DOI] [PubMed] [Google Scholar]
- 16. Jia C, Li Z, Guo D, Zhang Z, Yu J, Jiang G, et al. Brain Metastases of Non-Small Cell Lung Cancer: Magnetic Resonance Spectroscopy for Clinical Outcome Assessment in Patients With Stereotactic Radiotherapy. Onco Targets Ther (2020) 13:13087–96. doi: 10.2147/OTT.S286893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Grivennikov SI, Greten FR, Karin M. Immunity, Inflammation, and Cancer. Cell (2010) 140(6):883–99. doi: 10.1016/j.cell.2010.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Aziz MH, Sideras K, Aziz NA, Mauff K, Haen R, Roos D, et al. The Systemic-Immune-Inflammation Index Independently Predicts Survival and Recurrence in Resectable Pancreatic Cancer and Its Prognostic Value Depends on Bilirubin Levels: A Retrospective Multicenter Cohort Study. Ann Surg (2019) 270(1):139–46. doi: 10.1097/SLA.0000000000002660 [DOI] [PubMed] [Google Scholar]
- 19. Gao Y, Zhang H, Li Y, Wang D, Ma Y, Chen Q. Preoperative Increased Systemic Immune-Inflammation Index Predicts Poor Prognosis in Patients With Operable non-Small Cell Lung Cancer. Clinica chimica acta; Int J Clin Chem (2018) 484:272–7. doi: 10.1016/j.cca.2018.05.059 [DOI] [PubMed] [Google Scholar]
- 20. Huang L, Liu S, Lei Y, Wang K, Xu M, Chen Y, et al. Systemic Immune-Inflammation Index, Thymidine Phosphorylase and Survival of Localized Gastric Cancer Patients After Curative Resection. Oncotarget (2016) 7(28):44185–93. doi: 10.18632/oncotarget.9923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nie D, Gong H, Mao X, Li Z. Systemic Immune-Inflammation Index Predicts Prognosis in Patients With Epithelial Ovarian Cancer: A Retrospective Study. Gynecol Oncol (2019) 152(2):259–64. doi: 10.1016/j.ygyno.2018.11.034 [DOI] [PubMed] [Google Scholar]
- 22. Yang R, Chang Q, Meng X, Gao N, Wang W. Prognostic Value of Systemic Immune-Inflammation Index in Cancer: A Meta-Analysis. J Cancer (2018) 9(18):3295–302. doi: 10.7150/jca.25691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. O'Neill BP, Iturria NJ, Link MJ, Pollock BE, Ballman KV, O'Fallon JR. A Comparison of Surgical Resection and Stereotactic Radiosurgery in the Treatment of Solitary Brain Metastases. Int J Radiat Oncol Biol Phys (2003) 55(5):1169–76. doi: 10.1016/S0360-3016(02)04379-1 [DOI] [PubMed] [Google Scholar]
- 24. Rades D, Veninga T, Hornung D, Wittkugel O, Schild SE, Gliemroth J. Single Brain Metastasis: Whole-Brain Irradiation Plus Either Radiosurgery or Neurosurgical Resection. Cancer (2012) 118(4):1138–44. doi: 10.1002/cncr.26379 [DOI] [PubMed] [Google Scholar]
- 25. Rades D, Blanck O, Khoa MT, Vant P, Hung NQ. Validation of a Survival Score for Patients Receiving Radiosurgery or Fractionated Stereotactic Radiotherapy for 1 to 3 Brain Metastases. In Vivo (2018) 32(2):381–4. doi: 10.21873/invivo.11249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chen Y, Peng W, Huang Y, Chen J, Su G, Jiang C, et al. Significance of Serum Neuron-Specific Enolase Before Treatment in Predicting Brain Metastases and Prognosis of Advanced non-Small Cell Lung Cancer. Zhonghua zhong liu za zhi [Chinese J Oncology] (2015) 37(7):508–11. [PubMed] [Google Scholar]
- 27. Arrieta O, Saavedra-Perez D, Kuri R, Aviles-Salas A, Martinez L, Mendoza-Posada D, et al. Brain Metastasis Development and Poor Survival Associated With Carcinoembryonic Antigen (CEA) Level in Advanced non-Small Cell Lung Cancer: A Prospective Analysis. BMC Cancer (2009) 9:119. doi: 10.1186/1471-2407-9-119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sperduto PW, Yang TJ, Beal K, Pan H, Brown PD, Bangdiwala A, et al. Estimating Survival in Patients With Lung Cancer and Brain Metastases: An Update of the Graded Prognostic Assessment for Lung Cancer Using Molecular Markers (Lung-molGPA). JAMA Oncol (2017) 3(6):827–31. doi: 10.1001/jamaoncol.2016.3834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McIntyre DJ, Madhu B, Lee SH, Griffiths JR. Magnetic Resonance Spectroscopy of Cancer Metabolism and Response to Therapy. Radiat Res (2012) 177(4):398–435. doi: 10.1667/RR2903.1 [DOI] [PubMed] [Google Scholar]
- 30. Parizi MK, Razi A, Alizadeh S, Kasaeian A. The Role of Magnetic Resonance Spectroscopy Imaging Parameters to Predict Early Biochemical Recurrence After Radical Prostatectomy. Prague Med Rep (2019) 120(2-3):74–83. doi: 10.14712/23362936.2019.12 [DOI] [PubMed] [Google Scholar]
- 31. Fink JR, Carr RB, Matsusue E, Iyer RS, Rockhill JK, Haynor DR, et al. Comparison of 3 Tesla Proton MR Spectroscopy, MR Perfusion and MR Diffusion for Distinguishing Glioma Recurrence From Posttreatment Effects. J Magn Reson Imaging (2012) 35(1):56–63. doi: 10.1002/jmri.22801 [DOI] [PubMed] [Google Scholar]
- 32. Negendank WG, Sauter R, Brown TR, Evelhoch JL, Falini A, Gotsis ED, et al. Proton Magnetic Resonance Spectroscopy in Patients With Glial Tumors: A Multicenter Study. J Neurosurg (1996) 84(3):449–58. doi: 10.3171/jns.1996.84.3.0449 [DOI] [PubMed] [Google Scholar]
- 33. Dowling C, Bollen AW, Noworolski SM, McDermott MW, Barbaro NM, Day MR, et al. Preoperative Proton MR Spectroscopic Imaging of Brain Tumors: Correlation With Histopathologic Analysis of Resection Specimens. AJNR Am J Neuroradiol (2001) 22(4):604–12. doi: 10.1002/glia.440020403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Grenader T, Nash S, Plotkin Y, Furuse J, Mizuno N, Okusaka T, et al. Derived Neutrophil Lymphocyte Ratio may Predict Benefit From Cisplatin in the Advanced Biliary Cancer: The ABC-02 and BT-22 Studies. Ann Oncol (2015) 26(9):1910–6. doi: 10.1093/annonc/mdv253 [DOI] [PubMed] [Google Scholar]
- 35. Kao SC, Pavlakis N, Harvie R, Vardy JL, Boyer MJ, van Zandwijk N, et al. High Blood Neutrophil-to-Lymphocyte Ratio Is an Indicator of Poor Prognosis in Malignant Mesothelioma Patients Undergoing Systemic Therapy. Clin Cancer Res (2010) 16(23):5805–13. doi: 10.1158/1078-0432.CCR-10-2245 [DOI] [PubMed] [Google Scholar]
- 36. Templeton AJ, McNamara MG, Seruga B, Vera-Badillo FE, Aneja P, Ocana A, et al. Prognostic Role of Neutrophil-to-Lymphocyte Ratio in Solid Tumors: A Systematic Review and Meta-Analysis. J Natl Cancer Inst (2014) 106(6):dju124. doi: 10.1093/jnci/dju124 [DOI] [PubMed] [Google Scholar]
- 37. Yao M, Liu Y, Jin H, Liu X, Lv K, Wei H, et al. Prognostic Value of Preoperative Inflammatory Markers in Chinese Patients With Breast Cancer. Onco Targets Ther (2014) 7:1743–52. doi: 10.2147/OTT.S69657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hong X, Cui B, Wang M, Yang Z, Wang L, Xu Q. Systemic Immune-Inflammation Index, Based on Platelet Counts and Neutrophil-Lymphocyte Ratio, Is Useful for Predicting Prognosis in Small Cell Lung Cancer. Tohoku J Exp Med (2015) 236(4):297–304. doi: 10.1620/tjem.236.297 [DOI] [PubMed] [Google Scholar]
- 39. Tong YS, Tan J, Zhou XL, Song YQ, Song YJ. Systemic Immune-Inflammation Index Predicting Chemoradiation Resistance and Poor Outcome in Patients With Stage III non-Small Cell Lung Cancer. J Trans Med (2017) 15(1):221. doi: 10.1186/s12967-017-1326-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yucel S, Bilgin B. The Prognostic Values of Systemic Immune-Inflammation Index and Derived Neutrophil-Lymphocyte Ratio in EGFR-Mutant Advanced non-Small Cell Lung Cancer. J Oncol Pharm Pract (2021) 27(1):71–7. doi: 10.1177/1078155220913106 [DOI] [PubMed] [Google Scholar]
- 41. Bilgetekin I, Basal FB. Systemic Immune-Inflammation Index is the Best Prognostic Factor in Patients With Advanced Stage Adenocarcinoma of the Lung Treated With Pemetrexed. J Coll Physicians Surg Pak (2020) 30(9):933–9. doi: 10.29271/jcpsp.2020.09.933 [DOI] [PubMed] [Google Scholar]
- 42. Shinko D, Diakos CI, Clarke SJ, Charles KA. Cancer-Related Systemic Inflammation: The Challenges and Therapeutic Opportunities for Personalized Medicine. Clin Pharmacol Ther (2017) 102(4):599–610. doi: 10.1002/cpt.789 [DOI] [PubMed] [Google Scholar]
- 43. Tomita M, Ayabe T, Maeda R, Nakamura K. Systemic Immune-Inflammation Index Predicts Survival of Patients After Curative Resection for Non-Small Cell Lung Cancer. In Vivo (2018) 32(3):663–7. doi: 10.21873/invivo.11291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Franco AT, Corken A, Ware J. Platelets at the Interface of Thrombosis, Inflammation, and Cancer. Blood (2015) 126(5):582–8. doi: 10.1182/blood-2014-08-531582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Coffelt SB, Wellenstein MD, de Visser KE. Neutrophils in Cancer: Neutral No More. Nat Rev Cancer (2016) 16(7):431–46. doi: 10.1038/nrc.2016.52 [DOI] [PubMed] [Google Scholar]
- 46. Al-Shibli KI, Donnem T, Al-Saad S, Persson M, Bremnes RM, Busund LT. Prognostic Effect of Epithelial and Stromal Lymphocyte Infiltration in non-Small Cell Lung Cancer. Clin Cancer Res (2008) 14(16):5220–7. doi: 10.1158/1078-0432.CCR-08-0133 [DOI] [PubMed] [Google Scholar]
- 47. Chen X, Wang X, He H, Liu Z, Hu JF, Li W. Combination of Circulating Tumor Cells With Serum Carcinoembryonic Antigen Enhances Clinical Prediction of non-Small Cell Lung Cancer. PloS One (2015) 10(5):e0126276. doi: 10.1371/journal.pone.0126276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Huang W, Wang S, Zhang H, Zhang B, Wang C. Prognostic Significance of Combined Fibrinogen Concentration and Neutrophil-to-Lymphocyte Ratio in Patients With Resectable Non-Small Cell Lung Cancer. Cancer Biol Med (2018) 15(1):88–96. doi: 10.20892/j.issn.2095-3941.2017.0124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Guo D, Jin F, Jing W, Li M, Chen D, Zou B, et al. Incorporation of the SUVmax Measured From FDG PET and Neutrophil-To-Lymphocyte Ratio Improves Prediction of Clinical Outcomes in Patients With Locally Advanced Non-Small-Cell Lung Cancer. Clin Lung Cancer (2019) 20(6):412–9. doi: 10.1016/j.cllc.2019.06.008 [DOI] [PubMed] [Google Scholar]
- 50. Schernberg A, Reuze S, Orlhac F, Buvat I, Dercle L, Sun R, et al. A Score Combining Baseline Neutrophilia and Primary Tumor SUVpeak Measured From FDG PET is Associated With Outcome in Locally Advanced Cervical Cancer. Eur J Nucl Med Mol Imaging (2018) 45(2):187–95. doi: 10.1007/s00259-017-3824-z [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.