Abstract
The sensitivity and specificity of SARS-CoV-2 antigen tests have not been widely assessed in children. We evaluated children presenting to outpatient care with Quidel Sofia SARS-CoV-2 antigen test (Sofia-Ag-RDT) compared against Cepheid Xpert Xpress SARS-CoV-2/Flu/RSV reverse transcriptase-polymerase chain reaction test from November 2020 to April 2021. Sofia-Ag-RDT had the highest sensitivity in symptomatic (82%; 95% confidence interval, 68%-91%) children.
Keywords: antigen assay, COVID-19, pediatrics, rapid test, RT-PCR, SARS-CoV-2
Quidel Sofia SARS-CoV-2 antigen test has better diagnostic performance for SARS-CoV-2 infection in symptomatic children than in asymptomatic children.
An accurate diagnosis of SARS-CoV-2 infection is crucial. While traditional reverse transcriptase-polymerase chain reaction (RT-PCR)-based diagnostics are the gold standard, they pose challenges, including limited testing sites and availability, delayed turnaround time, and cost. Antigen testing may circumvent these challenges; however, it has not been widely evaluated for performance in children.
Symptoms of SARS-CoV-2 infection in children are clinically indistinguishable from other respiratory illnesses, including fever, cough, congestion, and/or rhinorrhea [1]. Pediatricians are responsible for reliable diagnosis and timely decisions regarding school participation. Accordingly, it is critical that accurate rapid tests become available, as children have an average of 13 acute respiratory infections during the first 2 years of life [2]. Furthermore, children under 5 years of age are vulnerable to infection, as they are not yet vaccine-eligible, and masking policies vary throughout the United States.
Multiple rapid diagnostic tests (RDTs) for the detection of SARS-CoV-2 operate under emergency use authorization (EUA) through the Food and Drug Administration (FDA). Previous studies evaluating SARS-CoV-2 antigen RDTs in symptomatic children report variable performance, including Abbott BinaxNOW with 85% sensitivity and 91%-100% specificity [3, 4] and Panbio-COVID-19 Ag Rapid Test with 62% sensitivity and 100% specificity in children under 12 years of age [5]. The Quidel Sofia SARS-CoV-2 antigen assay (Sofia-Ag-RDT) is a lateral flow assay that qualitatively detects SARS-CoV-2 nucleocapsid (N) antigen from nasopharyngeal or nasal swabs and is authorized for use via EUA in patients with ≤5 days of symptoms [6]. Performance data for Sofia-Ag-RDT provided in the instructions for use included only 28 individuals between 6 and 21 years and did not include any children under 5 years [6]. Previous studies with Sofia-Ag-RDT have reported sensitivity and specificity of 80% and 98.9%, respectively, in symptomatic adults [7] and 72.7% and 100%, respectively, in children [8]. However, the Sofia-Ag-RDT study in children noted that sensitivity decreased significantly after a person was symptomatic for >5 days, but analyzed symptomatic children as a single group, independent of days of symptoms. BinaxNOW, Panbio-COVID-19 Ag, and Sofia-Ag-RDT had inferior performance in asymptomatic patients, with sensitivities of 65.4% [4], 43% [5], and 41.2% [7], respectively. Because the Sofia platform was available in our outpatient offices, we sought to investigate if this assay could be effectively used by clinicians in symptomatic and asymptomatic children by comparing results with that of the Cepheid Xpert Xpress SARS-CoV-2/Flu/RSV RT-PCR test (Xpert-RT-PCR).
METHODS
Ethics
This study was submitted to and approved by the UPMC Quality Assurance Board as a Quality Improvement project. Parents verbally consented to test their child for this study. Patient data collected for this project were de-identified, obtained, and stored securely in a Health Insurance Portability and Accountability Act of 1996 (HIPAA)-compliant REDCap database (NIH/NCATS UL1 TR000445) [9].
Sites, Sample Collection, and Testing
Between November 2020 and April 2021, we collected samples from 273 symptomatic and 138 asymptomatic children presenting to Children’s Community Pediatrics or UPMC Children’s Hospital of Pittsburgh Express Care. We sequentially collected one nasal swab from each naris. One swab was tested on the in-office Sofia-Ag-RDT (Quidel; San Diego, CA) using the direct swab method at the time of collection as per the manufacturer’s instructions [6]. Testing was completed, and results were interpreted and recorded by trained staff at the collection site per The Clinical Laboratory Improvement Amendments of 1988 (CLIA’ 88) for waived laboratory testing by trained personnel. The other swab was placed in 3 mL viral transport media and sent via courier to the clinical microbiology lab at UPMC Children’s Hospital of Pittsburgh for testing by Xpert-RT-PCR (Cepheid; Sunnyvale, CA) as per the manufacturer’s instructions [10] in a moderate-high-complexity laboratory as per CLIAʹ 88.
Data Collection and Analysis
Children were considered symptomatic if they presented with symptoms that are listed in Table 1. For symptomatic children, if available, children were grouped into ≤3 and 4-5 days from the onset of symptoms until testing was collected, with the first day of symptoms considered “Day 1.” Tests from 21 children were excluded, as they had symptoms for >5 days at the time of collection. Asymptomatic children did not have any symptoms of illness and had not recently recovered from illness. They were tested because of known exposure to COVID-19 or because they required tests for return to school or sports. Positive exposure was documented by the clinician and defined as at least 15 minutes of un-masked contact within 6 feet with an individual positive for SARS-CoV-2. We compared Sofia-Ag-RDT results with those from Xpert-RT-PCR as the reference standard and also calculated sensitivity, specificity, and positive and negative predictive values (PPVs and NPVs) using contingency tables. Cycle threshold (CT) values from Xpert-RT-PCR were collected and analyzed for positive specimens.
Table 1.
Selected Demographic and Clinical Characteristics of Symptomatic and Asymptomatic Children
| Xpert-RT-PCR | Sofia-Ag-RDT | Total | |||
|---|---|---|---|---|---|
| Positive | Negative | Positive | Negative | ||
| Symptomatic children—No. | 44 | 208 | 38 | 211 | 252 |
| Age (mean years, range) | 7.1 (0-16) | 5.5 (0.2-16) | 6.8 (0-16) | 5.5 (0.2-16) | 5.8 (0-16) |
| Sex (no. of children, %) | |||||
| Male | 19 (43) | 110 (53) | 16 (42) | 111 (53) | 129 (51) |
| Symptoms (no. of children, %) | |||||
| Fever | 20 (45) | 82 (39) | 20 (52) | 80 (38) | 102 (40) |
| Cough | 18 (41) | 101 (49) | 15 (39) | 101 (48) | 119 (47) |
| Congestion | 24 (55) | 136 (65) | 23 (60) | 135 (64) | 160 (63) |
| Headache | 14 (32) | 37 (18) | 12 (32) | 38 (18) | 51 (20) |
| Sore throat | 10 (23) | 49 (24) | 9 (24) | 49 (23) | 59 (23) |
| Loss of taste | 3 (7) | 2 (1) | 2 (5) | 3 (1) | 5 (2) |
| Abdominal pain | 1 (2) | 6 (3) | 0 (0) | 7 (3) | 7 (3) |
| Nausea | 2 (5) | 9 (4) | 1 (3) | 10 (5) | 11 (4) |
| Vomiting | 2 (5) | 9 (4) | 2 (5) | 9 (4) | 11 (4) |
| Diarrhea | 5 (11) | 9 (4) | 3 (8) | 10 (5) | 14 (6) |
| Myalgia | 2 (5) | 6 (3) | 2 (5) | 5 (2) | 8 (3) |
| Fatigue | 2 (5) | 9 (4) | 2 (5) | 9 (4) | 11 (4) |
| No. of symptoms (mean, range) | 2.4 (1-7) | 2.2 (1-5) | 2.5 (1-7) | 2.2 (1-5) | 2.3 (1-7) |
| No. of days of symptoms before test (mean, range) | 2.6 (1-5) | 2.6 (1-5) | 2.7 (1-5) | 2.6 (1-5) | 2.6 (1-5) |
| Children with known SARS-CoV-2 exposure (No., %) | 33 (75) | 49 (24) | 27 (71) | 54 (26) | 82 (33) |
| Avg. CT (mean, range) | 22.2 (12.5-38.4) | — | 21.2 (12.5-38.4) | 25.9 (15.1-33.5) | 22.2 (12.5-38.4) |
| Asymptomatic children | 14 | 124 | 10 | 128 | 138 |
| Age (mean years, range) | 6.6 (2-11) | 7.4 (0-15) | 6.7 (2-11) | 7.3 (0-15) | 7.3 (0-15) |
| Sex (no. of children, %) | |||||
| Male | 10 (71) | 67 (54) | 6 (60) | 71 (56) | 77 (56) |
| Reason for testing (no. of children, %) | |||||
| Exposure | 12 (86) | 104 (84) | 9 (90) | 107 (84) | 116 (84) |
| No. of days post-exposure to test (mean, range) | 4.1 (1-9) | 4.9 (1-26) | 4.4 (1-12) | 4.9 (1-26) | 4.8 (1-26) |
| Avg. CT (mean, range) | 26.7 (18.4-36.4) |
— | 22.3 (18.4-31.0) |
33.8 (28.3-38.1) |
26.7 (18.4-38.1) |
Abbreviations: CT, cycle threshold; RDT, rapid diagnostic tests; RT-PCR, reverse transcriptase-polymerase chain reaction.
Statistical Considerations
We used t-tests for comparison of CT values and considered P < .05 statically significant. We calculated 95% confidence intervals (CIs) for all sensitivities, specificities, PPVs, and NPVs. Values were calculated using GraphPad Prism 9.3.1 (GraphPad Software, San Diego, CA). Based on community prevalence, we assumed that 25% of children would be positive for SARS-CoV-2. If we expected a sensitivity of 85% for Sofia-Ag-RDT, we would need 245 patients to produce CIs of +/− 10% for test sensitivity [11]. Our study was sufficiently powered for the testing of symptomatic children but underpowered for the testing of asymptomatic children and for days post-symptom onset.
RESULTS
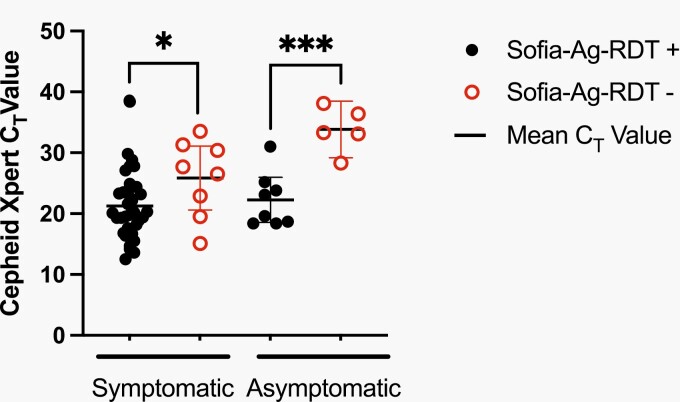
Demographic and clinical characteristics are presented in Table 1. Symptomatic children most frequently presented with congestion (63%), cough (47%), or fever (40%). For the symptomatic group, SARS-CoV-2 prevalence was 17.4% via RT-PCR. We found 8 false-negative (FN), 2 false-positive, and 236 concordant results using Sofia-Ag-RDT (Table 1). Three Sofia-Ag-RDT tests resulted in “indeterminate”; all had negative Xpert-RT-PCR results. The 8 FN results had a mean CT value of 25.9 (range: 15.1-33.5), significantly higher than the mean CT value for concordant samples (21.2, range: 12.5-38.4; P = .04; Figure 1). The overall sensitivity and specificity of Sofia-Ag-RDT were 82% (CI, 68%-91%) and 99% (CI, 97%-100%), respectively (Table 2). Sensitivity was highest among symptomatic children tested during the first 3 days of illness (84%; CI, 68%-93%) (Table 2) but trended down as the days from symptom onset increased (70%; CI, 40%-89% at day 4-5). PPV for symptomatic children was 95% (CI, 83%-99%) with NPV of 96% (CI, 93%-98%) (Table 2). Performance was relatively unchanged among age groups or the number of presenting symptoms (Table 2).
Figure 1.
Xpert-RT-PCR cycle threshold (Ct) value for symptomatic and asymptomatic children. Ct values as determined via Xpert-RT-PCR are shown grouped by symptomatic and asymptomatic children. Positive Sofia-Ag-RDT results are denoted in closed circles, whereas negative Sofia-Ag-RDT results are denoted in open circles. The line denotes the mean value. *P < .05; ***P < .001. Abbreviations: RDT, rapid diagnostic tests; RT-PCR, reverse transcriptase-polymerase chain reaction.
Table 2.
Sofia Performance in Symptomatic and Asymptomatic Children According to Duration of Symptoms Before Test, Age, and Exposure to SARS-CoV-2
| Sensitivity | Specificity | Positive Predictive Value, % (CI) | Negative Predictive Value, % (CI) | Percent Agreement, % | |
|---|---|---|---|---|---|
| Symptomatic children (no., %) | |||||
| Days of symptoms before test | |||||
| ≤3 days | 27/32 (84; CI, 68-93) | 154/155 (99; CI, 96-100) |
96 (82-100) | 97 (93-99) | 181/187 (97) |
| 4-5 days | 7/10 (70; CI, 40-89) | 39/40 (98; CI, 87-100) |
88 (53-99) | 93 (81-98) | 46/50 (92) |
| Alla | 36/44 (82; CI, 68-91) | 203/205 (99; CI, 97-100) |
95 (83-99) | 96 (93-98) | 239/249 (96) |
| Age | |||||
| 0-5 years | 17/21 (81; CI, 60-92) | 114/116 (98; CI, 94-100) |
90 (67-98) | 97 (92-99) | 131/137 (96) |
| 6-10 years | 4/5 (80; CI, 38-99) | 57/57 (100; CI, 94-100) |
100 (51-100) | 98 (91-100) | 61/62 (99) |
| 11-16 years | 15/18 (83; CI, 61-94) | 32/32 (100; CI, 89-100) |
100 (80-100) | 91 (78-97) | 47/50 (95) |
| Asymptomatic children (no., %) | |||||
| All children | 9/14 (64; CI, 39-84) | 123/124 (99; CI, 96-100) |
90 (60-100) | 96 (91-98) | 132/138 (96) |
| Children with known exposure | 8/12 (67; CI, 39-86) | 103/104 (99; CI, 95-100) |
89 (57-99) | 96 (91-99) | 111/116 (96) |
Abbreviation: CI, confidence interval.
Group inclusive of above as well as 12 additional children who did not have the exact number of symptom days reported.
For asymptomatic children, we found 5 FN, 1 false-positive, and 132 concordant results using Sofia-Ag-RDT. The mean CT value for FN samples was 33.8 (range: 28.3-38.1; Figure 1). This was significantly higher than the mean CT value of samples positive by both methods (22.3, range: 18.4-31.0; P < .001; Figure 1). The overall SARS-CoV-2 prevalence via RT-PCR among both total and exposed asymptomatic children was 10%. The overall sensitivity was poor (64%; CI, 39%-84%), but specificity remained high (99%; CI, 96%-100%) (Table 2). The sensitivity of Sofia-Ag-RDT was not enhanced for children with a known COVID-19 exposure (67%; CI, 39%-86%) (Table 2). In asymptomatic children, the NPV was 96% (CI, 91%-99%) and the PPV was 90% (CI, 60%-100%). In exposed asymptomatic children, the PPV was 89% (CI, 57%-99%) (Table 2).
DISCUSSION
Our study evaluated Sofia-Ag-RDT for the diagnosis of SARS-CoV-2 infection in children. Overall, Sofia-Ag-RDT had 82% (CI, 68%-91%) sensitivity in symptomatic children but only 64% (CI, 39%-84%) sensitivity in asymptomatic children when compared with Xpert-RT-PCR, even in the setting of exposure. Sensitivity among symptomatic children was best when the testing was done within 3 days of symptom onset but trended downward when individuals were tested later in the course of illness. This is slightly improved sensitivity for symptomatic patients in the first 5 days of illness when compared with previous studies [8]. Accordingly, nucleic acid amplification test (NAAT) confirmation of Sofia-Ag-RDT-negative results in symptomatic children who present with 4-5 days of symptoms should be highly recommended based on the study results in conjunction with the recommendations by Centers for Disease Control and Prevention (CDC) and The Infectious Diseases Society of America (IDSA) to perform NAAT confirmation on all symptomatic patients with negative antigen tests and strong clinical suspicion for COVID-19, regardless of days of symptoms [12, 13].
The average CT value of Sofia-Ag-RDT-negative, Xpert-RT-PCR-positive patients was significantly greater (25.9) than those with concordant tests (21.2), albeit with overlapping CI; however, many of the FN Sofia-Ag-RDT patients had CT values that could be consistent with infectious virus (<34) [14]. It is possible that their RDT specimen was inadequate, or inhibitors were present, but investigation of this was beyond this study’s scope. The most likely explanation for these discordant results is due to inherent differences in limits of detection between assay methodologies. The average CT value of Sofia-Ag-RDT-negative, Xpert-RT-PCR-positive children was significantly higher (33.8) than that of concordant asymptomatic children (22.3), suggesting that those missed by the RDT may have recently recovered from an asymptomatic infection and were less likely to be infectious at the time of testing, with most CT values > 33 [15]. However, interpretation of CT values as an indicator of previous disease or infectivity must be done with caution, as high CT values (>33) could also represent detection of infection very early in the disease course, when viral loads have yet to peak.
Limitations of our study include that we only evaluated 1 RDT, while 43 RDTs have received FDA EUA [16]. Most have not been validated in children and may exhibit differing performance. The small group size in the asymptomatic arm limited power; however, sensitivity in our study is similar to other RDTs in asymptomatic patients, including Sofia-Ag-RDT in adults [7]. This study was conducted before the emergence of Delta (March 2021) and Omicron (December 2021) variants in the United States.
Sofia-Ag-RDT is a lower-cost option for the rapid diagnosis of SARS-CoV-2 in symptomatic children. However, in instances where Sofia-Ag-RDT has inadequate performance, such as in asymptomatic children or in symptomatic children who test negative despite clinical suspicion of COVID-19, reflex or confirmation RT-PCR testing should be considered.
Notes
Authors’ contributions. M. C. F. performed retrospective chart review and data analysis and is the lead writer of the manuscript. T. J. F. performed a retrospective chart review and data analysis. J. I. provided administrative support and point of care testing resources oversight. J. R. provided administrative support and laboratory testing oversight. K. H. provided administrative support and point of care testing oversight. A. H. assisted in the study design, provided instrumentation for antigen testing, assisted with data gathering, and contributed to manuscript edits. A. W. assisted in administrative support, study design, and execution, and also contributed to manuscript edits. S. L. M. developed the study design; assisted in execution, oversight, and data analysis; and made significant contributions to the manuscript. All authors read and approved the final manuscript.
Financial support. Financial support was provided by Cepheid. In-kind support was provided by UPMC through its UPMC Children’s Community Pediatrics (CCP), University of Pittsburgh Physicians (UPP), and Laboratory Service Center divisions. M. C. F. is funded by the Pediatric Infectious Diseases Society/St. Jude Award for Clinical and Translational Research.
Ethics and consent to participate. This study was submitted to and deemed a Quality Improvement initiative and approved by the UPMC Quality Assurance Board; thus, our study does not include factors requiring either verbal or written patient consent. Patient data collected for this project were de-identified, obtained, and stored securely in a REDCap database (NIH/NCATS UL1 TR000445) via HIPAA-compliant procedures.
Consent for publication . Not applicable.
Potential conflicts of interest. S. L. M. reports grants and nonfinancial support from Cepheid for conducting this quality improvement project; after completion of this work, S. L. M. is now employed by Cepheid. The other authors have no conflicts of interest to report.
All authors have submitted the ICMJE Form for Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Acknowledgments. We would like to thank the UPMC Children’s Community Pediatrics practices and the UPMC Children’s Hospital of Pittsburgh Clinical Microbiology Laboratory for their support of this study.
Contributor Information
Megan Culler Freeman, Department of Pediatrics, Division of Infectious Diseases, University of Pittsburgh, Pittsburgh, Pennsylvania, USA.
Tanner J Freeman, Department of Pathology, University of Pittsburgh, Pittsburgh, Pennsylvania, USA.
Jennifer Iagnemma, Children’s Community Pediatrics, Pittsburgh, Pennsylvania, USA.
Jayne Rasmussen, Clinical Microbiology Laboratory, UPMC Children’s Hospital of Pittsburgh, Pittsburgh, Pennsylvania, USA.
Kelly Heidenreich, Children’s Community Pediatrics, Pittsburgh, Pennsylvania, USA.
Alan Wells, Department of Pathology, University of Pittsburgh, Pittsburgh, Pennsylvania, USA.
Alejandro Hoberman, Department of Pediatrics, University of Pittsburgh, P, ittsburgh, P, ennsylvania, USA .
Stephanie L Mitchell, Department of Pathology, University of Pittsburgh, Pittsburgh, Pennsylvania, USA.
Data Availability
Data are available upon request.
References
- 1. Dong Y, Mo X, Hu Y, et al. Epidemiology of COVID-19 among children in China. Pediatrics 2020; 145(6):e20200702. doi: 10.1542/peds.2020-0702. Epub 2020 Mar 16. PMID: 32179660. [DOI] [PubMed] [Google Scholar]
- 2. Sarna M, Ware RS, Sloots TP, Nissen MD, Grimwood K, Lambert SB. The burden of community-managed acute respiratory infections in the first 2-years of life. Pediatr Pulm 2016; 51:1336–46. [Google Scholar]
- 3. Shaikh N, Friedlander EJ, Tate PJ, et al. Performance of a rapid SARS-CoV-2 antigen detection assay in symptomatic children. Pediatrics 2021; 148(3):e2021050832. doi: 10.1542/peds.2021-050832. Epub 2021 May 26. PMID: 34039718. [DOI] [PubMed] [Google Scholar]
- 4. Pollock NR, Jacobs JR, Tran K, et al. Performance and implementation evaluation of the Abbott BinaxNOW rapid antigen test in a high-throughput drive-through community testing site in Massachusetts. J Clin Microbiol 2021; 59(5):e00083–21. doi: 10.1128/JCM.00083-21. PMID: 33622768; PMCID: PMC8091851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. LʹHuillier AG, Lacour M, Sadiku D, et al. Diagnostic accuracy of SARS-CoV-2 rapid antigen detection testing in symptomatic and asymptomatic children in the clinical setting. J Clin Microbiol 2021; 59:e00991–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Quidel Corporation. Sofia SARS Antigen FIA Package Insert. Published online 2020. Accessed September 2, 2021. https://www.fda.gov/media/137885/download
- 7. Pray IW, Ford L, Cole D, et al. Performance of an antigen-based test for asymptomatic and symptomatic SARS-CoV-2 testing at two university campuses—Wisconsin, September–October 2020. Mmwr Morbidity Mortal Wkly Rep 2021; 69:1642–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Beck ET, Paar W, Fojut L, Serwe J, Jahnke RR. Comparison of the Quidel Sofia SARS FIA test to the Hologic Aptima SARS-CoV-2 TMA test for diagnosis of COVID-19 in symptomatic outpatients. J Clin Microbiol 2021; 59:e02727–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cepheid. Xpert Xpress SARS-CoV2/Flu/RSV Instructions for Use. Published online 2020. Accessed September 2, 2021. https://www.fda.gov/media/142437/download
- 11. Buderer NMF. Statistical methodology: I. Incorporating the prevalence of disease into the sample size calculation for sensitivity and specificity. Acad Emerg Med 1996; 3:895–900. [DOI] [PubMed] [Google Scholar]
- 12. Hanson KE, Altayar O, Caliendo AM, et al. The Infectious Diseases Society of America guidelines on the diagnosis of coronavirus disease 2019 (COVID-19): antigen testing [published online ahead of print 2021]. Clin Infect Dis ciab557. https://academic.oup.com/cid/advance-article/doi/10.1093/cid/ciab557/6308396 [DOI] [PubMed] [Google Scholar]
- 13. CDC. Guidance for Antigen Testing for SARS-CoV-2 for Healthcare Providers Testing Individuals in the Community. Published 2022. Accessed March 9, 2022. https://www.cdc.gov/coronavirus/2019-ncov/lab/resources/antigen-tests-guidelines.html
- 14. Tom MR, Mina MJ. To interpret the SARS-CoV-2 test, consider the cycle threshold value. Clin Infect Dis 2020; 71:2252–2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Salvatore PP, Dawson P, Wadhwa A. et al. Epidemiological correlates of PCR cycle threshold values in the detection of SARS-CoV-2. Clin Infect Dis 2020; 72:e761–e767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Food and Drug Administration. In Vitro Diagnostic EUAs-Antigen Diagnostic Tests for SARS-CoV-2. Published May 12, 2021. Accessed January 04, 2022. https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/in-vitro-diagnostics-euas-antigen-diagnostic-tests-sars-cov-2
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon request.