ABSTRACT
Acute interstitial nephritis (AIN), defined by the presence of interstitial inflammation accompanied by tubulitis, is an often overlooked cause of acute kidney injury (AKI). It is now well established that severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection can cause a wide variety of kidney injuries, most commonly acute tubular injury and collapsing glomerulopathy. In comparison, AIN is rarely documented in association with SARS-CoV-2 both anecdotally and in larger series of autopsy or biopsy studies. In this issue of the Journal, León-Román describe five cases of AIN in patients with a history of coronavirus disease 2019 (COVID-19) and highlight AIN as a possibly under-reported or ignored facet of renal disease associated with SARS-CoV-2. They describe three scenarios in which AIN can be seen: (i) SARS-CoV-2 infection after diagnosis of AIN, (ii) AIN followed by SARS-CoV-2 infection in the same admission and (iii) Severe SARS-CoV-2 and AIN possibly associated with SARS-CoV-2 itself. Overall, AIN remains rare in SARS-CoV-2 and causality is difficult to ascertain. Interestingly, AIN is not only seen in association with the disease itself but also with SARS-CoV-2 vaccination. This scenario is equally rare and causality is no less difficult to prove. A history of preceding SARS-CoV-2 infection and vaccination should be actively sought when patients present with otherwise unexplained AIN.
Keywords: acute interstitial nephritis, severe acute respiratory syndrome coronavirus 2, vaccination
INTRODUCTION
Since the first descriptions of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causing coronavirus disease 2019 (COVID-19), nephrologists have discovered that several forms of renal disease may be linked to the virus [1]. Clinical reports on acute kidney injury (AKI) in patients with severe SARS-CoV-2 infection [2, 3] were rapidly followed by reports showing the virus in urine [4]. Viral presence in tubular cells [5] remains controversial but documented various glomerular lesions have been documented, most commonly collapsing glomerulopathy. More recently, SARS-CoV-2 vaccination has also received considerable attention with several reports describing new glomerular lesions after vaccination; these reports are noteworthy for the variety of glomerular pathology described, ranging from minimal change disease to IgA nephropathy and crescentic lesions with or without antineutrophil cytoplasmic antibodies (ANCA). It has not yet been established how SARS-CoV-2 vaccination can trigger so many different lesions, and causality remains difficult to ascertain. In comparison, acute interstitial nephritis (AIN) is rare both in the context of SARS-CoV-2 infection and vaccination. In this issue of the Journal León-Román et al. [6] highlight AIN as a poorly understood renal lesion in SARS-CoV-2 infection. In this editorial, we aim to put their findings into perspective. We discuss reports of AIN in patients with SARS-CoV-2 infection and vaccination, speculate about underlying mechanisms and also emphasize important caveats and areas of uncertainty.
AIN and SARS-CoV-2 infection
In this issue of the Journal, León-Román et al. [6] highlight AIN as an interesting and probably often overlooked, aspect of renal disease associated with SARS-CoV-2 infection. They describe five additional cases of AIN associated with SARS-CoV-2 infection [6] in different settings. Two of these cases occurred in patients diagnosed with AIN several years previously. In both cases, the cause of the AIN was secondary to immunological therapy for metastatic cancer. These patients then subsequently contracted SARS-CoV-2 and required hospital admission. Both cases had a good outcome and their clinical course is what one expects in similar immuno-suppressed patients acquiring COVID-19 infection. In two cases [6], the initial presenting feature was acute kidney injury (AKI) secondary to AIN caused by medications with a known associated with AIN- namely ciprofloxacin and proton pump inhibitors (PPIs), respectively. Both patients developed SARS-CoV-2 infection in the hospital within days of their initial hospital admission. One patient unfortunately died, whilst the other made a good recovery. The final case, and probably the most interesting of the five, is a patient who presented with severe SARS-CoV-2, required intensive care admission and developed an AKI requiring continuous veno-venous haemofiltration. A kidney biopsy showed AIN that was felt to be due to COVID-19, although the patient also had exposure to ceftriaxone, tobramycin and linezolid. This patient was treated with steroids and had a good outcome.
Acute and chronic tubulointerstitial nephritis is one of the most common, but often overlooked, causes of AKI. Since patients with AIN frequently present with nonspecific clinical features, kidney biopsy plays an important role in establishing the diagnosis. Pathologic diagnosis of AIN is characterized by the presence of interstitial inflammation associated with tubulitis. When a cause of AIN can be determined, medications are the most common aetiology followed by autoimmune diseases (such as Sjogren's syndrome, IgG4-related disease and sarcoidosis), infection and other rare entities such as Tubulointerstitial Nephritis and Uveitis (TINU) syndrome. Infectious aetiologies of AIN are relatively uncommon, but have been described in association with various bacterial, viral and fungal microorganisms including cases with granuloma formation [7].
Virus-associated AIN is most frequently seen in transplanted patients and is most often caused by BK polyomavirus, JC polyomavirus, cytomegalovirus [8] and adenovirus [9]. When compared with other cases of AIN in which histology is often nonspecific, virus-associated AIN is unique, because kidney biopsy can often establish specific aetiology by demonstration of viral infection in the kidney using immunohistochemical stains. Consequently, direct infection of the kidney, together with the cytotoxic T-cell response directed against the viral compound, likely plays a role in the pathogenesis of some virus-associated AIN.
AKI is common amongst hospitalized patients with COVID-19 and a prevalence of up to 30% has been reported [10] although the exact figures remain disputed [11]. Although most do not undergo biopsy, COVID-19 is associated with a wide variety of kidney pathologies, most notably collapsing glomerulopathy in patients with high-risk APOL1 genotype and acute tubular injury in patients with severe COVID-19 [12–15]. However, only a few reports of AIN have been described in patients with COVID-19 [16–20].
The small number of cases of AIN following or during SARS-CoV-2 infection underscores the impression that AIN is an unusual complication. Furthermore, these reported cases highlight the difficulty and caution needed to establish COVID-19 as a possible cause or trigger of AIN from some other causes. For instance, a 12-year-old girl presented with a 3-week history of reduced appetite, weakness, weight loss and an AKI (creatinine 1.2 mg/dL) [20]. She was found to have SARS-CoV-2 IgG antibodies (titre 195 AU/mL, cut-off > 11.9 AU/mL) but tested negative for COVID-19 twice on routine swab testing, suggesting previous SARS-CoV-2 infection. She had tubular proteinuria. A kidney biopsy confirmed the presence of AIN with marked neutrophilic and lympho-plasma-cellular invasion of the interstitium. There were no immune deposits or virus-like particles seen on electron microscopy. She was treated with steroids and had complete resolution of kidney impairment [20]. In this case and in other reports, there was no convincing evidence of direct viral infection of the kidney parenchyma, in contrast to the typical cases of virus-associated AIN. Therefore, even in this previously healthy patient without exposure to medications, whether this patient's AIN is a direct result of SARS-CoV-2 infection or if the viral infection acted as a ‘second-hit’ to trigger AIN from other causes, such as TINU, one of the most common causes of AIN in the paediatric population, is not clear.
What does the report by León-Román et al. add to the literature? Their work reminds us to be mindful of AIN in patients with COVID-19 and highlights the existence of AIN and SARS-CoV-2 in different settings: (i) SARS-CoV-2 infection some years after initial diagnosis of AIN, (ii) AIN and SARS-CoV-2 infection in the same admission and (iii) severe SARS-CoV-2 and AIN possibly associated with SARS-CoV-2 itself. Furthermore, given the lack of convincing viral infection of the kidney in patients with AIN and COVID-19, their report highlights the need for nephrologists to be cautious when considering SARS-CoV-2 as a possible cause of AIN. In particular, nephrologists should carefully assess the timing of SARS-CoV-2 infection and onset of kidney injury, and exclude other causes of AIN before concluding that AIN is likely caused by SARS-CoV-2. In fact, as illustrated by León-Román et al. [6] in this issue, alternative causes for AIN are present in some cases, such as antibiotics administered for concurrent bacterial pneumonia or proton pump inhibitors (PPIs). Importantly, nephrologists should also be careful to exclude unusual causes of AIN in this setting, such as AIN caused by traditional or herbal medicines taken in an attempt to prevent SARS-CoV-2 [21].
It is also worth considering the possibility of whether any of the antiviral drugs used more recently in SARS-CoV-2 infection could potentially cause AIN, as other antivirals have been reported as possible triggers of AIN. Duque et al. described AIN associated with antiviral treatment for hepatitis C infection [22]. Famciclovir has also been reported in conjunction with AIN [23]. Whether or not antivirals used in the treatment of COVID-19 are capable of causing AIN is currently unknown although a case of AIN associated with lopinavir/ritonavir has been reported in a different context [24]. The topic of nephrotoxicity of antiviral drugs, in general, is reviewed elsewhere [25].
There are several possible reasons for the paucity of AIN reported in patients with COVID-19. One possibility is under-detection since most patients with AKI and COVID-19 do not undergo kidney biopsy. Alternatively, steroid therapy administered for COVID-19, especially in more severe diseases may result in recovery and under-reporting of AIN. Lastly, the paucity of AIN may be due to a lack of significant direct infection of kidney parenchyma, even among those with severe infection. This is in contrast to BK virus infection where high viral load consistently correlates with the presence of AIN. However, the possibility of the immune response against filtered viral proteins causing AIN in rare predisposed patients cannot entirely be ruled out.
Irrespective of the discussion of causality, AIN is often regarded as an under-recognized and thus under-reported disease [26]. Given that prompt treatment with either steroids or removal of the causative drug is likely to drastically improve outcomes for patients with AIN, it is important for clinicians to keep an open mind as to the cause of AKI in patients with COVID-19, particularly those that are not responding to conventional management. Where possible, correctly identifying the cause of AKI in patients with COVID-19 is important because it has been shown that the rate of kidney function decline post-discharge is significantly greater (−11.3mL/min/1.73m2 faster) in those patients with a diagnosis of COVID-19 compared with those without [27].
The small number of cases of AIN following or during SARS-CoV-2 infection underscores the impression that AIN is an unusual complication given the paucity of AIN in small and large series of biopsy and autopsy renal tissue obtained from SARS-CoV-2 patients. It is also possible that subtle presentations of AIN occur more frequently but that such cases recover spontaneously or do not undergo kidney biopsy. It is also worth remembering that subtle urinary abnormalities are surprisingly common in SARS-CoV-2 infection when compared with the incidence of AKI and to histological data. George et al. showed that SARS-CoV-2 spike protein is detectable in as many as 25% of patients and that many of them have albuminuria although the long-term implications of such findings remain unclear [28]. It is possible that mild and subtle cases of AIN are overlooked not least because quite a few of these patients are too unwell to undergo renal biopsy. Other patients may not undergo biopsy due to coagulopathy or because clinicians feel a biopsy is not warranted where meaningful immunosuppressive treatment would not be feasible anyway. Equally, one could speculate that steroid treatment is now so commonplace, especially across the more severe end of the spectrum of SARS-CoV-2 that some milder cases of AIN may recover and escape detection following steroid treatment.
Whilst reports of AIN as a cause for AKI in patients diagnosed with COVID-19 are few, there is a growing body of evidence to suggest that more subtle forms of tubulointerstitial injury are an important part of the pathophysiological response. In a study performed in France, 71 patients with COVID-19 were admitted to intensive care over a month-long period [29]. Of the 71 patients, 57 developed AKI and of these 10 required renal replacement therapy (RRT). Proteinuria was assessed with both protein: creatinine ratio (uPCR) and albumin: creatinine ratio (uACR). The median uPCR was significantly raised at 82 mg/mmol whereas the median uACR was normal at 0.23 mg/mmol. This discrepancy suggests a predominant tubulointerstitial injury which could indicate acute tubular injury or interstitial nephritis, or both. Interestingly, there was a low incidence of glycosuria pointing away from renal Fanconi syndrome. Another study has demonstrated that SARS-CoV-2 causes a proximal tubular dysfunction characterized by low molecular weight proteinuria, hypophosphataemia, hypouricaemia and neutral aminoaciduria [30].
Finally, one might wonder whether AIN is ever associated with SARS-CoV-2 infection in transplant patients. Westhoff et al. report the interesting case of a kidney-pancreas transplant recipient with AIN and SARS-CoV-2 RNA in tubular cells [31]. Overall AIN is believed to be rare in renal transplant recipients (and likely difficult to distinguish from T-cell mediated rejection) presumably due to the effect of the maintenance immunosuppressive medication [32].
AIN and COVID vaccination
A broad variety of glomerular lesions have been reported in conjunction with SARS-CoV-2 vaccination [33, 34]. Contemporary series report minimal change disease, IgA nephropathy, crescentic glomerulonephritis and collapsing glomerulopathy [13]. It is difficult to understand how a single trigger i.e. SARS-CoV-2 vaccination (although through different vaccines) could cause such a variety of lesions. It is conceivable that these vaccines act as a ‘second hit’ on a background of genetic vulnerability. Similar models of pathogenesis are being discussed for other glomerular diseases such as IgA nephropathy [35]. In comparison, AIN following SARS-CoV-2 vaccination is rare [36]. Prior to the pandemic, AIN had already been described in conjunction with other vaccines [37] such as influenza [38].
In June 2021, de la Flor described the first case of AIN in conjunction with the Pfizer–BioNTech COVID-19 vaccine [39]. Soon after Czerlau et al. reported on a case series of five patients with AIN associated with SARS-CoV-2 mRNA vaccination [40]. Interestingly, one of their patients had a pre-existent glomerular disease and the authors emphasized that patients with pre-existent kidney diseases can be affected as well [40]. Early evaluation for AIN and consideration of renal biopsy is, therefore, warranted in such patients i.e. if renal function worsens after vaccination with mRNA-based SARS-CoV-2 vaccines [40]. They also speculated about the pathogenic role of lipid or polyethylene glycol lipid component of the vaccine [40].
Our own first case of AIN associated with preceding SARS-CoV-2 vaccination [41] was a man in his mid-forties who presented with AKI requiring renal replacement therapy (RRT) only weeks after his second SARS-CoV-2 vaccination (AstraZeneca). Somewhat unexpectedly, kidney biopsy showed florid AIN (Fig. 1) for which there was no other obvious alternative explanation, although we acknowledged the possibility that we had missed clues in the history despite comprehensive assessment [41]. He recovered renal function with steroid treatment.
FIGURE 1:
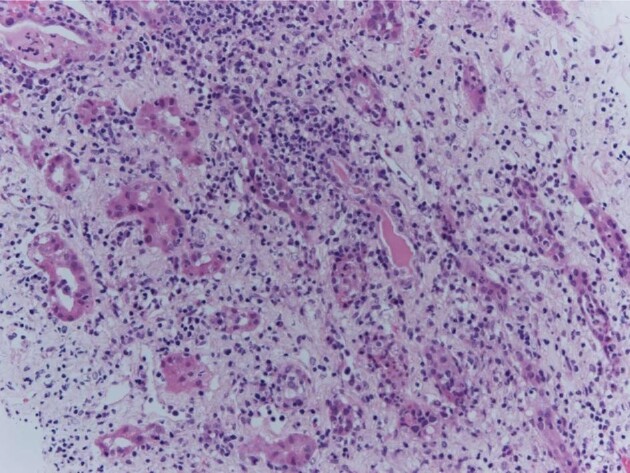
Renal biopsy showing AIN in conjunction with SARS-CoV-2 vaccination. Haematoxylin-Eosin (HE) stain, x 200. There is interstitial oedema with mixed inflammatory infiltrate composed of lymphocytes, plasma cells, scattered eosinophils and neutrophils. From [41], with permission.
As of late 2021, we reviewed 10 cases of AIN following SARS-CoV-2 vaccination which had been described in the literature [36] including one case with granulomatous lesions [17]. Since then, an additional case similar to our own first case was reported [42]. Lim et al. recently added two more cases, both with partial recovery after steroid treatment [43]. It is interesting to note that a case of relapse of IgG4-related interstitial disease following SARS-CoV-2 vaccination has also been reported [44]. More cases continue to emerge [45–47] although the overall number remains small, given how common SARS-CoV-2 vaccination is in the general population. Table 1 shows all cases of AIN in conjunction with SARS-CoV-2 vaccination as of the time of writing. So far as we can see, there does not seem to be a preponderance of any particular vaccine although mRNA-based vaccines seem to cause more allergic reactions overall [48].
Table 1.
Cases of acute interstitial nephritis following SARS-CoV-2 vaccination reported in the literature as of 4 May 2022
| Author/Country of case report | Age (yrs) | Sex | Time to presentation from day of vaccination | Significant co-morbidities | New onset or relapse | Vaccine brand | Vaccine dose | Baseline Creatinine (µmol/L) | Presentation Creatinine (µmol/L) | Kidney Biopsy | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Choi J H et al./South Korea [45] | 17 | M | 3 days | New- onset | Pfizer | Second | Not known | 265 | Interstitial infiltrates mainly mononuclear. Focal and moderate interstitial fibrosis and tubular atrophy in 20% of cortex. Negative IF. Findings consistent with AIN. | Supportive care | Discharged after 1 week | |
| Choi J H et al./South Korea [45] | 12 | M | 3 weeks | New- onset | Pfizer | Second | Not known | 199 | Tubules showed severe necrosis with heavy infiltration of neutrophils, eosino- phils and mononuclear cells in the interstitium. Slight foot process effacement. | Oral steroids on day 10 of hospitalization | Recovery of renal function | |
| Czerlau et al./Switzerland [40] | 55 | M | 4 days | Hypertension, prostate cancer treated with prostatectomy | New-onset | Pfizer | Second | 76.5 | 355 | Lymphocytes, plasma cells, macrophages, eosinophilic granulo-cytes and some neutrophilic granulo-cytes, tubulitis and interstitial oedema | Steroid treatment—dose and length of treatment not specified | Serum creatinine following treatment is 88 µmol/L |
| Czerlau et al./Switzerland [40] | 54 | M | 3 days | Myocardial infarction | New-onset | Moderna | Second | Not known | 268 | Lymphocytes, plasma cells, macrophages, and eosinophilic granulocytes, two granulomas, tubulitis + tubular destruction. Glomerular lesions in keeping with FSGS | Steroid treatment—dose and length of treatment not specified | Serum creatinine following treatment is 235 µmol/L |
| Czerlau et al./Switzerland [40] | 58 | M | ‘A few days’ | FSGS refractory to treatment, with multiple relapses | New-onset | Moderna | Second | 167 | 355 | Lymphocytes, plasma cells, macrophages, with tubulitis and interstitial oedema | Steroid treatment—dose and length of treatment not specified | Serum creatinine following treatment is 210 µmol/L |
| Czerlau et al./Switzerland [40] | 38 | F | 1 month | Ulcerative colitis—received ustekinumab previously for treatment | New-onset | Moderna | Second | 76 | 86 | Lymphocytes, plasma cells, macrophages, sporadic eosinophilic granulocytes and neutrophil granulo-cytes with tubulitis + interstitial oedema. EM shows mesangial IgA deposition. | Steroid treatment—dose and length of treatment not specified | Serum creatinine following treatment is 72 µmol/L |
| Czerlau et al./Switzerland [40] | 35 | F | Exact time not specified | Rheumatoid arthritis—on certolizumab treatment since 2016 | New-onset | Pfizer | Second | 49 | 100 | Lymphocytes, plasma cells, macrophages, sporadic eosinophilic granulocytes and neutrophil granulo- cytes with tubulitis + interstitial oedema. EM shows mesangial IgA deposition. | Steroid treatment—dose and length of treatment not specified | Serum creatinine following treatment is 90 µmol/L |
| De la Flor et al./Spain [39] | 78 | M | 3 weeks | Hypertension, type 2 diabetes mellitus | New-onset | Pfizer | First | 150 | 475 | Features of AIN along with glomerular sclerosis and other chronic changes | IV MP followed by oral steroids | Remained dialysis-dependent |
| Dheir H et al./Turkey [50] | 44 | F | 48 hours | New- onset | Pfizer | First | Not known | 186 | Tubulointerstitial inflammatory infiltration containing eosinophils and lymphocytes and interstitial oedema | Haemodialysis. Oral steroids 1 mg/kg | Complete recovery of renal function | |
| Fenoglio R et al./Italy [51] | F | 78 | 52 days | Not stated | New-onset | Pfizer | First | Not stated | Not stated | Severe interstitial infiltration by mononuclear cells and polymorphonuclear leucocytes | Dialysis. Oral steroids | Dialysis discontinuation after 2 months |
| Fenoglio R et al./Italy [51] | F | 57 | 82 days | Not stated | New-onset | Pfizer | Second | Not stated | Not stated | Severe interstitial infiltration by mono-nuclear cells and polymorphonuclear leukocytes. | Oral steroids | |
| Fenoglio R et al./Italy [51] | F | 65 | 24 days | Not stated | New-onset | Oxford-AstraZeneca | Second | Not stated | Not stated | Severe interstitial infiltration by mononuclear cells and polymorphonuclear leucocytes | Dialysis Oral steroids | |
| Jongvilaikasem P and Rianthavorn P/Thailand [52] | 14 | M | 5 days | New onset | Pfizer | First | Not known | 177 | Normal glomeruli with foot process effacement on EM. tubular injury and interstitial infiltrate | IV MP followed by oral steroids. Haemodialysis for 3 weeks | Improved creatinine to 47 µmol/L | |
| Liew et al./United Kingdom [41] | 53 | M | 3 days | Hypertension | New-onset | Oxford-AstraZeneca | Second | Not known | 1034 | Morphologically normal glomeruli with interstitial oedema and infiltrate of lymphocytes, plasma cells and neutrophils with tubulitis | Oral steroid treatment | Improvement of renal function. Dialysis-independent following discharge |
| Mira F S et al./Portugal [53] | 45 | F | 8 days | Total thyroidectomy secondary to multinodular goitre | New-onset | Pfizer | Second | 75 | 1626 | Mild interstitial infiltrate with oedema and acute tubular necrosis. 20% IFTA |
Haemodialysis. MTP 500 mg daily for 3 days, followed by 50 mg prednisolone |
Improvement of renal function- creatinine 168 µmol/L 4 days post discharge. |
| Rieckmann S et al./Germany [54] | 63 | M | 3 weeks | New-onset | Pfizer | First | Normal range (not specified) | 1679 | Acute tubular necrosis, interstitial oedema and lymphoplasma-cellular interstitial infiltration with few eosinophil granulocytes |
RRT on intensive care unit. Oral steroids 250 mg for 3 days then reduced to 80 mg daily. |
Haemodialysis discontinued after 2 weeks. | |
| Rieckmann S et al./Germany [54] | 18 | M | 6 weeks | New- onset | Pfizer | Second | Not known | 150 |
Lymphoplasma-cellular infiltration + eosinophil granulocytes and diffuse acute tubular necrosis. Mesangial IgA |
Oral steroids 50 mg per day. | Complete recovery of renal function within 2 weeks. | |
| Rieckmann S et al./Germany [54] | 25 | F | 3 weeks | New-onset | Pfizer | Third | Not known | 1034 | Severe, locally destructive interstitial nephritis with prominent diffuse acute tubular necrosis and slight eosinophilia | Oral steroids 250 mg per day for 3 days then reduced to 80 mg daily | Recovery of renal function within days. | |
| Unver et al./Turkey [55] | 67 | F | 3 weeks | Type 2 diabetes mellitus. Recent new-onset minimal change disease following first dose of CoronaVac | New-onset | CoronaVac | Second | Not known (serum creatinine was 53 µmol/L) | 371 | Degeneration of proximal tubular cells and interstitial inflammation. Proteinaceous material was detected in many tubule lumens. | Pulsed IV MP followed by oral steroids. Patient was then commenced on cyclosporine treatment | Ongoing treatment. Proteinuria of 3g/day still apparent from last follow-up |
| Wu et al./United Kingdom [36] | 69 | F | 5 days | Rheumatoid arthritis, Sjøgren's syndrome, hypertension, hypothyroidism and anxiety | New-onset | Oxford-AstraZeneca | First | 85 | 245 | Florid interstitial infiltrate with prominent eosinophils, with no glomerular abnormalities and no chronic interstitial damage | Commenced on oral steroids. Discontinuation of regular medications such as ramipril, lansoprazole, methotrexate and paroxetine | Improved serum creatinine to 130 µmol/L and resolved peripheral eosinophilia |
| Wu et al./United Kingdom [36] | 60 | F | 2 weeks | Hypertension | New-onset | Oxford-AstraZeneca | Second | 59 | 754 | Widespread interstitial infiltrates in keeping with AIN | Single dose IV pulsed MP followed by oral steroids. | Full clinical recovery. Serum creatinine was 216 µmol/L in last follow-up review |
AIN, acute interstitial nephritis; EM, electron microscopy; FSGS, focal segmental glomerulosclerosis; IF, immunofluorescence; IFTA, interstitial fibrosis and tubular atrophy; M, male; MCD, minimal change disease; MP, methylprednisolone; IgA, immunoglobulin A; IV, intravenous.
Most cases reported so far recover to some degree with steroid treatment but it is important to appreciate that AIN associated with SARS-CoV-2 vaccination is not always benign. The case described by De la Flor et al. remained dialysis-dependent despite biopsy and steroid treatment [39].
All cases described in the literature have to be viewed with a degree of caution when it comes to causality. As part of the assessment, strong temporal association, lack of chronic lesions, absence of concomitant diseases and medication linked to AIN should all be considered [41]. It is quite possible that patients were too ill to remember alternative causes of AIN, such as the use of non-steroidal anti-inflammatory drugs (NSAIDs) or PPIs prior to the onset of kidney disease. Alternatively, they may have forgotten to mention the use of over-the-counter medication or they may be too embarrassed to report this. It is also the case that SARS-CoV-2 vaccination is such a common event in the general population that it is difficult to find any patient presenting with new-onset renal disease who has not had some form of SARS-CoV-2 vaccination in the recent past. We have ourselves encountered the occasional patient with pre-existing kidney disease who experienced worsening kidney function in conjunction with SARS-CoV-2 vaccination but where a biopsy was considered inappropriate or too risky and causality, therefore, remains difficult to assess. Again, it is difficult to completely exclude that these patients could have used NSAIDs or felt unwell overall with some degree of dehydration after vaccination. We emphasize the role of detailed history taking, as well as the fact, that all patients with otherwise unexplained AIN should be asked about prior SARS-CoV-2 vaccination.
CONCLUSION
We now have a much greater understanding of the types of renal injury caused by both SARS-CoV-2 infection, and also vaccination, than we did at the onset of the pandemic. AIN, however, has received much less attention than glomerular disease and many questions remain unanswered. The link between SARS-CoV-2 infection and vaccination and AIN remains incompletely understood. In patients with AIN in conjunction with SARS-CoV-2 infection, there is a lack of robust evidence that the virus is incorporated into kidney tissue when techniques such as immunohistochemistry and in-situ hybridization are used. This is in contrast to other viral-associated AIN such as the BK virus. Secondly, AIN is rarely seen in biopsy and autopsy series of patients with SARS-CoV-2 infection. Finally, it is noted that there are potentially other causes for AIN in many of the reported cases of AIN in the context of SARS-CoV-2 infection (such as antibiotics, PPIs or NSAIDs) and, to a lesser degree, in patients where AIN occurs after SARS-CoV-2 vaccination. Causality is, therefore, difficult to prove in many, if not most, cases. It is also worth keeping a sense of perspective: as of April 2022, the WHO reports a total of 509 531 232 cases of COVID-19 worldwide with 11 438 720 838 vaccine doses administered [49]. In the general population, preceding SARS-CoV-2 infection and vaccination are common in any patient's history. On the other hand, it seems possible that AIN is under-diagnosed, under-reported or both, in conjunction with COVID-19 and COVID vaccination. This could apply where clinicians assume that both the disease and vaccination are so ubiquitous that taking a detailed vaccination and COVID history isn't worth their while. We should use the work by León-Román [6] as a useful reminder that there may be more to renal disease associated with SARS-CoV-2 infection and vaccination than just glomerular disease. To ensure we are not missing AINything, we recommend vigilance and careful history taking, particularly in patients presenting with otherwise unexplained AIN.
Contributor Information
Joshua Storrar, Department of Nephrology, Northern Care Alliance NHS Foundation Trust, Salford Royal Hospital, Salford, UK.
Satoru Kudose, Department of Pathology and Cell Biology, Columbia University Irving Medical Center, NY, NY, USA.
Alexander Woywodt, Department of Nephrology, Lancashire Teaching Hospitals NHS Foundation Trust, Preston, UK.
CONFLICT OF INTEREST STATEMENT
A.W. is member of the CKJ editorial board.
REFERENCES
- 1. Ng JH, Bijol V, Sparks MAet al. Pathophysiology and pathology of acute kidney injury in patients with COVID-19. Adv Chronic Kidney Dis 2020; 27: 365–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Larsen CP, Bourne TD, Wilson JDet al. Collapsing glomerulopathy in a patient with COVID-19. Kidney Int Rep 2020; 5: 935–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Peleg Y, Kudose S, D'Agati Vet al. Acute kidney injury due to collapsing glomerulopathy following COVID-19 infection. Kidney Int Rep 2020; 5: 940–945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sun J, Zhu A, Li Het al. Isolation of infectious SARS-CoV-2 from urine of a COVID-19 patient. Emerg Microbes & Infect 2020; 9: 991–993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Diao B, Wang C, Wang Ret al. Human kidney is a target for novel severe acute respiratory syndrome coronavirus 2 infection. Nature Communications 2021; 12: 2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. León-Román J, Agraz I, Vergara Aet al. COVID-19 infection and renal injury: where is the place for acute interstitial nephritis disease? Clin Kidney J 2022; doi: 10.1093/ckj/sfac079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Aleckovic-Halilovic M, Nel D, Woywodt A. Granulomatous interstitial nephritis: a chameleon in a globalized world. Clin Kidney J 2015; 8: 511–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wong KM, Chan YH, Chan SKet al. Cytomegalovirus-induced tubulointerstitial nephritis in a renal allograft treated by foscarnet therapy. Am J Nephrol 2000; 20: 222–224 [DOI] [PubMed] [Google Scholar]
- 9. Seralathan G, Kurien AA. Adenovirus interstitial nephritis: an unusual cause for early graft dysfunction. Indian J Nephrol 2018; 28: 385–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Adamczak M, Surma S, Wiecek A. Acute kidney injury in patients with COVID-19: epidemiology, pathogenesis and treatment. Adv Clin Exp Med 2022; 31: 317–326 [DOI] [PubMed] [Google Scholar]
- 11. Rubin S, Orieux A, Prevel Ret al. Characterisation of acute kidney injury in critically ill patients with severe coronavirus disease-2019 (COVID-19). medRxiv 2020; 13: 354–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ferlicot S, Jamme M, Gaillard Fet al. The spectrum of kidney biopsies in hospitalized patients with COVID-19, acute kidney injury, and/or proteinuria. Nephrol Dial Transplant 2021; 36: 1253–1262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jeyalan V, Storrar J, Wu HHLet al. Native and transplant kidney histopathological manifestations in association with COVID-19 infection: a systematic review. World J Transplant 2021; 11: 480–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kudose S, Batal I, Santoriello Det al. Kidney biopsy findings in patients with COVID-19. J Am Soc Nephrol 2020; 31: 1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nasr SH, Alexander MP, Cornell LDet al. Kidney biopsy findings in patients with COVID-19, kidney injury, and proteinuria. Am J Kidney Dis 2021; 77: 465–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Golmai P, Larsen CP, DeVita MVet al. Histopathologic and ultrastructural findings in postmortem kidney biopsy material in 12 patients with AKI and COVID-19. J Am Soc Nephrol 2020; 31: 1944–1947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Szajek K, Kajdi ME, Luyckx VAet al. Granulomatous interstitial nephritis in a patient with SARS-CoV-2 infection. BMC Nephrology 2021; 22: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kudose S, Santoriello D, Bomback ASet al. Longitudinal outcomes of COVID-19-Associated collapsing glomerulopathy and other podocytopathies. J Am Soc Nephrol 2021; 32: 2958–2969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. May RM, Cassol C, Hannoudi Aet al. A multi-center retrospective cohort study defines the spectrum of kidney pathology in Coronavirus 2019 Disease (COVID-19). Kidney Int 2021; 100: 1303–1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Serafinelli J, Mastrangelo A, Morello Wet al. Kidney involvement and histological findings in two pediatric COVID-19 patients. Pediatr Nephrol 2021; 36: 3789–3793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Parthasarathy R, Mathew M, Koshy Pet al. Traditional medicines prescribed for prevention of COVID-19: use with caution. Nephrology 2021; 26: 961–964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Duque JC, Dejman A, Venkat Vet al. Acute interstitial nephritis following treatment with direct-acting antiviral agents in hepatitis C virus-infected patients: a case series. Clin Nephrol 2021; 95: 22–27 [DOI] [PubMed] [Google Scholar]
- 23. Crouch A, Le M, Rogers Cet al. Evaluation of low dose famciclovir as herpes simplex virus and varicella zoster virus prophylaxis in cytomegalovirus low-risk solid organ transplant recipients. Transpl Infect Dis 2021; 23: e13711. [DOI] [PubMed] [Google Scholar]
- 24. Chughlay MF, Njuguna C, Cohen Ket al. Acute interstitial nephritis caused by lopinavir/ritonavir in a surgeon receiving antiretroviral postexposure prophylaxis. AIDS 2015; 29: 503–504 [DOI] [PubMed] [Google Scholar]
- 25. Leowattana W. Antiviral drugs and acute kidney injury (AKI). Infect Disord Drug Targets 2019; 19: 375–382 [DOI] [PubMed] [Google Scholar]
- 26. Ng JH, Zaidan M, Jhaveri KDet al. Acute tubulointerstitial nephritis and COVID-19. Clin Kidney J 2021; 14: 2151–2157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nugent J, Aklilu A, Yamamoto Yet al. Assessment of acute kidney injury and longitudinal kidney function after hospital discharge among patients with and without COVID-19. JAMA Network Open 2021; 4: e211095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. George S, Pal AC, Gagnon Jet al. Evidence for SARS-CoV-2 spike protein in the urine of COVID-19 patients. Kidney360 2021; 2: 924–936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rubin S, Orieux A, Prevel Ret al. Characterization of acute kidney injury in critically ill patients with severe coronavirus disease 2019. Clin Kidney J 2020; 13: 354–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Werion A, Belkhir L, Perrot Met al. SARS-CoV-2 causes a specific dysfunction of the kidney proximal tubule. Kidney Int 2020; 98: 1296–1307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Westhoff TH, Seibert FS, Bauer Fet al. Allograft infiltration and meningoencephalitis by SARS-CoV-2 in a pancreas-kidney transplant recipient. Am J Transplant 2020; 20: 3216–3220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Josephson MA, Chiu MY, Woodle ES.et al. Drug-induced acute interstitial nephritis in renal allografts: histopathologic features and clinical course in six patients. Am J Kidney Dis 1999; 34: 540–548 [DOI] [PubMed] [Google Scholar]
- 33. Tan HZ, Tan RY, Choo JCJet al. Is COVID-19 vaccination unmasking glomerulonephritis? Kidney Int 2021; 100: 469–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bomback AS, Kudose S, D'Agati VD. De novo and relapsing glomerular diseases after COVID-19 vaccination: what do we know so far? Am J Kidney Dis 2021; 78: 477–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hu X, Feng J, Zhou Qet al. Respiratory syncytial virus exacerbates kidney damages in IgA nephropathy mice via the C5a-C5aR1 axis orchestrating Th17 cell responses. Front Cell Infect Microbiol 2019; 9: 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wu HHL, Li JWC, Bow Aet al. Acute interstitial nephritis following SARS-CoV-2 vaccination. Clin Kidney J 2022; 15: 576–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Patel C, Shah HH. Vaccine-associated kidney diseases: a narrative review of the literature. Saudi J Kidney Dis Transpl 2019; 30: 1002–1009 [DOI] [PubMed] [Google Scholar]
- 38. Sokoda T, Sawai T, Iwai Met al. A case of acute tubulointerstitial nephritis possibly associated with influenza HA vaccine. Nihon Shoni Jinzobyo Gakkai Zasshi 2007; 20: 55–59 [Google Scholar]
- 39. de la Flor J, Linares T, Alonso-Riaño Met al. A case of acute interstitial nephritis following the Pfizer-BioNTech COVID-19 vaccine. Nefrologia (Engl Ed) 2021; doi: 10.1016/j.nefro.2021.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Czerlau C, Bocchi F, Saganas Cet al. Acute interstitial nephritis after messenger RNA-based vaccination. Clin Kidney J 2022; 15: 174–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Liew SK, Nair B, So Bet al. Acute interstitial nephritis following SARS-CoV-2 virus vaccination. Clin Nephrol 2022; 97: 242–245 [DOI] [PubMed] [Google Scholar]
- 42. Mira FS, Costa Carvalho J, de Almeida PAet al. A case of acute interstitial nephritis after two doses of the BNT162b2 SARS-CoV-2 vaccine. Int J Nephrol Renovasc Dis 2021; 14: 421–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lim JH, Kim MS, Kim YJet al. New-onset kidney diseases after COVID-19 vaccination: a case series. Vaccines (Basel) 2022; 10: 302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Masset C, Kervella D, Kandel-Aznar Cet al. Relapse of IgG4-related nephritis following mRNA COVID-19 vaccine. Kidney Int 2021; 100: 465–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Choi JH, Kang KS, Han KH. Two adolescent cases of acute tubulointerstitial nephritis after second dose of COVID-19 mRNA vaccine. Hum Vaccin Immunother 2022; 1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rieckmann S, Seibert FS, Hogeweg Met al. Acute interstitial nephritis after vaccination with BNT162b2. J Nephrol 2022; 35: 779–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Schaubschlager T, Rajora N, Diep Set al. De novo or recurrent glomerulonephritis and acute tubulointerstitial nephritis after COVID-19 vaccination: a report of six cases from a single center. Clin Nephrol 2022; 97: 289–297 [DOI] [PubMed] [Google Scholar]
- 48. Chen M, Yuan Y, Zhou Yet al. Safety of SARS-CoV-2 vaccines: a systematic review and meta-analysis of randomized controlled trials. Infect Dis Poverty 2021; 10: 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. N.N. WHO COVID dashboard . https://covid19.who.int/Last (29 April 2022, date last accessed)
- 50. Dheir H, Sipahi S, Cakar GCet al. Acute tubulointerstitial nephritis after COVID-19 m-RNA BNT162b2 vaccine. Eur Rev Med Pharmacol Sci 2021; 25: 6171–6173 [DOI] [PubMed] [Google Scholar]
- 51. Fenoglio R, Lalloni S, Marchisio Met al. New onset biopsy-proven nephropathies after COVID vaccination. Am J Nephrol 2022; 53: 325–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Jongvilaikasem P, Rianthavorn P. Minimal change disease and acute interstitial nephritis following SARS-CoV-2 BNT162b2 vaccination. Pediatr Nephrol 2022; 1–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mira FS, Costa Carvalho J, de Almeida PA.et al. A case of acute interstitial nephritis after two doses of the BNT162b2 SARS-CoV-2 vaccine. Int J Nephrol Renovasc Dis 2021; 14: 421–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rieckmann S, Seibert FS, Hogeweg Met al. Acute interstitial nephritis after vaccination with BNT162b2. J Nephrol 2022; 35: 779–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Unver S, Haholu A, Yildirim S. Nephrotic syndrome and acute kidney injury following CoronaVac anti-SARS-CoV-2 vaccine. Clin Kidney J 2021; 14: 2608–2611 [DOI] [PMC free article] [PubMed] [Google Scholar]


