Abstract
We evaluated the neutralization efficiency against SARS-CoV-2 Omicron variant in maternal and cord blood sera after antenatal BNT162b2 vaccination. Neutralizing antibodies against Omicron were lacking at the time of delivery after 2-dose vaccination. A third booster dose was essential in building neutralizing antibody capacity against Omicron among mothers and neonates.
Keywords: COVID-19, Omicron, pregnancy, SARS-CoV-2, vaccination
Coronavirus disease 2019 during pregnancy can result in severe disease and adverse maternal and perinatal outcomes [1]. Messenger RNA (mRNA)–based vaccines were shown to be effective in preventing severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)–related maternal illness and provide first-line neonatal protection [1, 2]. The evolution of SARS-CoV-2 variants of concern (VOCs), including the recent emergence of the Omicron variant, has raised concerns regarding vaccine efficacy, given its ability to cause reinfection and escape from vaccine-induced humoral immunity [3, 4]. Whether the antibody response elicited by mRNA vaccines will effectively neutralize infection with emerging VOCs in the setting of pregnancy remains unclear.
Here, we examined the neutralizing capacity of maternal and neonatal sera against the Omicron and Delta VOCs following antenatal mRNA vaccination.
METHODS
Study Population
A prospective study following women admitted for delivery was performed during February 2021–November 2021 at Hadassah Medical Center, a tertiary care university-affiliated hospital in Jerusalem, Israel, with more than 10 000 deliveries annually. Women who received the SARS-CoV-2 BNT162b2 mRNA (Pfizer-BioNTech) vaccine during pregnancy were eligible for this study. Parturients who delivered prematurely (<37 weeks of gestation), multifetal gestations, those vaccinated at later than 36 weeks of gestation, and those who did not complete the 2-dose vaccine series prior to delivery were excluded. All women who completed the 2-dose vaccine series within the recommended time frame were included. Women with a prior history of SARS-CoV-2 infection were ineligible for this study. All participants were tested and found to be negative for nucleocapsid immunoglobulin G (IgG). Demographic and clinical data were collected at the time of enrollment. The Hadassah Medical Center Institutional Review Board approved this study.
Laboratory Methods
Following delivery, maternal and cord blood sera were collected for antibody measurement. Spike protein receptor-binding domain (RBD)–specific IgG levels (Architect SARS-CoV-2 IgG II Quant assay, Abbott Diagnostics, Chicago) were evaluated in maternal and cord/neonatal blood sera. The nucleocapsid IgG assay (Architect SARS-CoV-2 IgG II Quant assay, Abbott Diagnostics, Chicago) was also performed on maternal blood sera to exclude prior infection. Standard assay controls (negative, low-positive, and positive; supplied by the manufacturer and used in accordance with the manufacturer’s specifications), along with internal quality controls (prepared at the Hadassah Clinical Virology Laboratory from pooled sera and established for statistically based control limits in accordance with the laboratory’s ISO 15189 accreditation requirements), were included in each assay. The controls’ values were monitored using the Westgard rules for the specified ranges to control for the system and assay performances.
Neutralizing efficiency against wild-type SARS-CoV-2 and the Delta and Omicron variants was defined using the SARS-CoV-2 virus microneutralization assay as previously described [5], with minor modifications. Briefly, serial 2-fold dilutions of heat-inactivated serum samples (starting from 1:10; diluted in Dulbecco’s modified Eagle’s medium in a total volume of 50 μL) were incubated with an equal volume of viral solution containing 100 tissue culture infectious doses of SARS-CoV-2 isolates (wild-type USA-WA1/2020 [NR-52281; obtained from BEI Resources], Delta [hCoV-19/Israel/CVL-12804/2021], and Omicron [hCoV19/Israel/CVL-n49814/2021] variants) for 1 hour in a 96-well plate (at 37°C in humidified atmosphere with 5% carbon dioxide). The serum–virus mixtures (100 µL; 8 replicates of each serum dilution) were then added to a 96-well plate that contained a semiconfluent Vero E6 cell monolayer (American Type Culture Collection CRL-1586; maintained as described in [6]). Following 3 days of incubation (at 37°C in a humidified atmosphere with 5% carbon dioxide), the cells in each well were scored for viral cytopathic effect (CPE). The neutralization titer (NT50) was defined as the reciprocal of the highest serum dilution that protected 50% of culture wells from CPE. An NT50 of 10 served as the detection limit. Positive and negative serum controls, cell control, and a viral back-titration control were included in each assay.
Statistical Analyses
Significance between groups was assessed using the χ2 test and Fisher exact test for categorical variables, while the Mann–Whitney U test was used for continuous variables. Correlations were reported using the Spearman test with the correspondent ρs and P values. The data were analyzed using Software Package for Statistics and Simulation (IBM SPSS version 24, IBM Corp, Armonk, NY).
RESULTS
Maternal and cord-blood sera were prospectively obtained from mother/newborn dyads following term delivery, after antenatal 2-dose BNT162b2 mRNA vaccination. Initiation of the primary vaccine dose was in the first (n = 19), second (n = 21), or third trimester (n = 13) of pregnancy, with the second vaccine dose administered 21 days later. We also included an additional group of parturients (n = 16) who further received a third booster dose during the third trimester. Patients’ characteristics are provided in Supplementary Table 1.
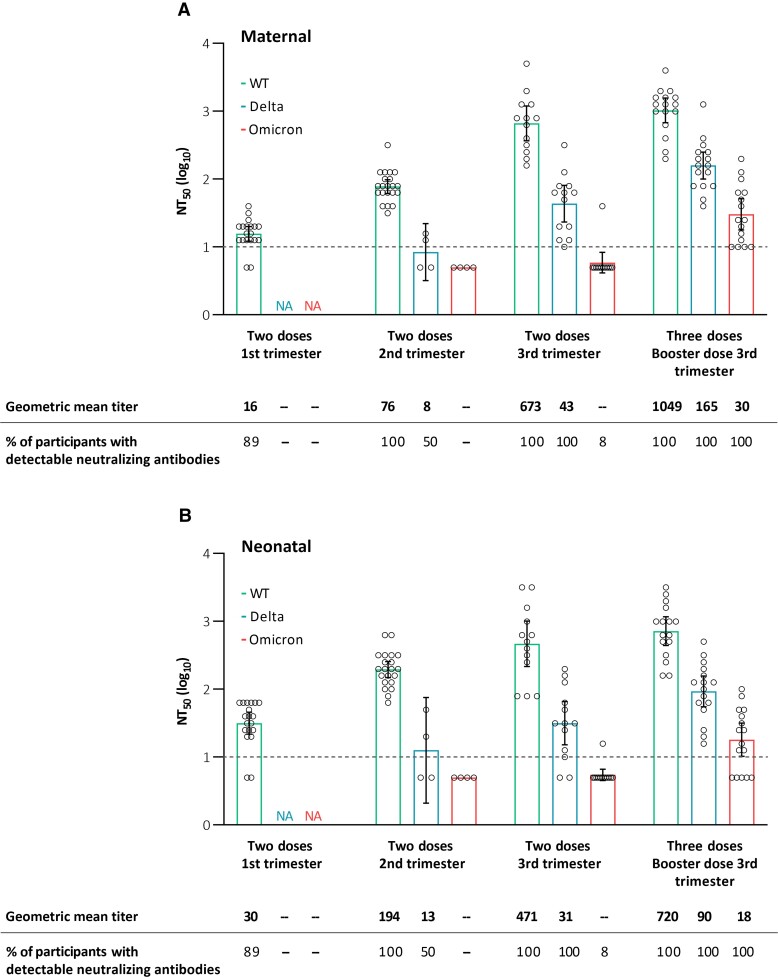
In those who completed the 2-dose vaccine series in the first and second trimesters, no neutralizing antibodies against the Delta and Omicron variants were detected in maternal and neonatal sera at the time of delivery, whereas maternal and neonatal neutralizing antibodies against the wild-type virus were detectable following all 2-dose timing regimens across the different trimesters (with titers gradually increasing following first-, second-, and third-trimester vaccination; Figure 1). Third-trimester 2-dose vaccination resulted in no detectable neutralizing antibodies against the Omicron variant, with moderate neutralization efficiency against the Delta variant (with an approximately 15-fold drop compared with wild-type virus neutralization). Importantly, antenatal receipt of the 3 vaccine doses was associated with detectable maternal and neonatal neutralizing antibodies against the Omicron variant (in all tested participants) and with enhanced maternal and neonatal neutralization efficiency against the wild-type virus and Delta variant (Figure 1). This finding is consistent with the recently demonstrated effect of a third mRNA vaccine dose among nonpregnant individuals [3, 7]. Following booster dose administration, there was still an approximately 34-fold drop in Omicron compared with wild-type virus neutralization potency, with an intermediate cross-neutralization potency (approximately 6- to 8-fold drop) shown for the Delta variant. Aligning with the relative decline in variant-specific neutralization potency, the correlation between neutralizing and binding anti-RBD antibody levels (measured by enzyme-linked immunosorbent assay) varied for the different variants and was high for the wild-type (ρs= .93; P < .001), moderate for the Delta (ρs= .67; P < .001), and weak for the Omicron variants (ρs= .51; P = .006; Supplementary Figure 1). Hence, the commonly used serological assays (based on wild-type virus sequence) may poorly correlate with functional antibody-based immunity against new VOCs, calling for the development of variant-adapted assays.
Figure 1.
Neutralizing antibody titers in maternal (A) and neonatal (B) sera against WT severe acute respiratory syndrome coronavirus 2 and the Delta and Omicron variants in those who completed the 2-dose vaccine series in the first, second, and third trimesters of pregnancy and those who additionally received a third booster dose. Neutralizing efficiency is reflected by NT50 values, measured in live virus microneutralization assay (see Methods section). The I bars represent 95% confidence intervals, and the circles represent the values in individual participants. The dashed line indicates the lower limit of detection of the assay (10). Samples with values below the lower limit of detection were assigned a value of 5. Abbreviations: NA, not available; NT50, neutralization titer; WT, wild type.
DISCUSSION
We evaluated the neutralization efficiency of antenatal BNT162b2 vaccination against the Omicron and Delta VOCs among pregnant women and their neonates. We demonstrated that neutralizing antibodies against the Omicron variant were lacking in maternal and neonatal sera after 2-dose vaccination. A booster dose was shown to be essential for building neutralizing antibody capacity against the Omicron variant in mothers and neonates at the time of delivery and for bolstering the neutralization of the Delta variant. These data support the importance of a third booster dose in the setting of pregnancy for enhanced defense of mothers and neonates against breakthrough infections by emerging VOCs and can guide vaccine prioritization and timing strategies in the pregnant population. Potential caveats of the current study include its relatively small sample size and differences in vaccination-to-delivery interval among participants, precluding kinetic analyses. In addition, while the presence of nucleocapsid IgG was excluded in all study participants, as the levels of this antibody wane throughout time, the potential occurrence of prior remote infection remains possible. Finally, the durability of the impact of the third booster dose and its ability to confer maternal and neonatal protection are yet to be determined.
Supplementary Material
Contributor Information
Amihai Rottenstreich, Department of Obstetrics and Gynecology, Hadassah-Hebrew University Medical Center and Faculty of Medicine, Hebrew University of Jerusalem, Jerusalem, Israel.
Olesya Vorontsov, Clinical Virology Unit, Department of Clinical Microbiology and Infectious Diseases, Hadassah-Hebrew University Medical Center, Jerusalem, Israel.
Or Alfi, Clinical Virology Unit, Department of Clinical Microbiology and Infectious Diseases, Hadassah-Hebrew University Medical Center, Jerusalem, Israel.
Gila Zarbiv, Department of Obstetrics and Gynecology, Hadassah-Hebrew University Medical Center and Faculty of Medicine, Hebrew University of Jerusalem, Jerusalem, Israel.
Esther Oiknine-Djian, Clinical Virology Unit, Department of Clinical Microbiology and Infectious Diseases, Hadassah-Hebrew University Medical Center, Jerusalem, Israel.
Roy Zigron, Department of Obstetrics and Gynecology, Hadassah-Hebrew University Medical Center and Faculty of Medicine, Hebrew University of Jerusalem, Jerusalem, Israel.
Geffen Kleinstern, School of Public Health, University of Haifa, Haifa, Israel.
Shay Porat, Department of Obstetrics and Gynecology, Hadassah-Hebrew University Medical Center and Faculty of Medicine, Hebrew University of Jerusalem, Jerusalem, Israel.
Dana G Wolf, Clinical Virology Unit, Department of Clinical Microbiology and Infectious Diseases, Hadassah-Hebrew University Medical Center, Jerusalem, Israel.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. A. R. had full access to all data for the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: S. P., A. R., and D. G. W. Acquisition, analysis, or interpretation of data: All authors. Drafting of the manuscript: A. R., R. Z., G. Z., S. P., and D. G. W. Laboratory analyses: E. O.-D., O. V., O. A., M. M., and D. G. W. Statistical analysis: A. R. and G. K. All authors read and approved the final manuscript.
Data sharing. Individual-level data for this article will not be made publicly available. Requests for sharing of deidentified individual-level participant data for scientific research can be directed to the corresponding author. All proposals will be subject to scientific review and institutional review board approval at Hadassah Medical Center.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Morgan JA, Biggio Jr J, Martin JK, et al. Maternal outcomes after severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in vaccinated compared with unvaccinated pregnant patients. Obstet Gynecol 2022; 139:107–9. [DOI] [PubMed] [Google Scholar]
- 2. Rottenstreich A, Zarbiv G, Oiknine-Djian E, et al. Timing of SARS-CoV-2 vaccination during the third trimester of pregnancy and transplacental antibody transfer: a prospective cohort study. Clin Microbiol Infect 2022; 28:419–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Muik A, Lui BG, Wallisch AK, et al. Neutralization of SARS-CoV-2 Omicron by BNT162b2 mRNA vaccine-elicited human sera. Science 2022; 375:678–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cele S, Jackson L, Khoury DS, et al. Omicron extensively but incompletely escapes Pfizer BNT162b2 neutralization. Nature 2022; 602:654–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Percivalle E, Cambiè G, Cassaniti I, et al. Prevalence of SARS-CoV-2 specific neutralising antibodies in blood donors from the Lodi Red Zone in Lombardy, Italy, as at 06 April 2020. Euro Surveill 2020; 25:2001031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Alfi O, Yakirevitch A, Wald O, et al. Human nasal and lung tissues infected ex vivo with SARS-CoV-2 provide insights into differential tissue-specific and virus-specific innate immune responses in the upper and lower respiratory tract. J Virol 2021; 95:e0013021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nemet I, Kliker L, Lustig Y, et al. Third BNT162b2 vaccination neutralization of SARS-CoV-2 Omicron infection. N Engl J Med 2022; 386:492–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.