Abstract
Background
Messenger RNA (mRNA)–1273 vaccine demonstrated 93.2% efficacy against coronavirus disease 2019 (COVID-19) in the Coronavirus Efficacy (COVE) trial. The humoral immunogenicity results are now reported.
Methods
Participants received 2 mRNA-1273 (100 µg) or placebo injections, 28 days apart. Immune responses were evaluated in a prespecified, randomly selected per-protocol immunogenicity population (n = 272 placebo; n = 1185 mRNA-1273). Serum binding antibodies (bAbs) and neutralizing antibodies (nAbs) to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)–spike protein were assessed at days 1, 29, and 57 by baseline SARS-CoV-2–negative (n = 1197) and SARS-CoV-2–positive (n = 260) status, age, and sex.
Results
SARS-CoV-2–negative vaccinees had bAb geometric mean AU/mL levels of 35 753 at day 29 that increased to 316 448 at day 57 and nAb inhibitory dilution 50% titers of 55 at day 29 that rose to 1081 at day 57. In SARS-CoV-2–positive vacinees, the first mRNA-1273 injection elicited bAb and nAb levels that were 11-fold (410 049) and 27-fold (1479) higher than in SARS-CoV-2–negative vaccinees, respectively, and were comparable to levels after 2 injections in uninfected participants. Findings were generally consistent by age and sex.
Conclusions
mRNA-1273 elicited robust serologic immune responses across age, sex, and SARS-CoV-2 status, consistent with its high COVID-19 efficacy. Higher immune responses in those previously infected support a booster-type effect.
Clinical Trials Registration. NCT04470427.
Keywords: COVID-19, immunogenicity, mRNA-1273, SARS-CoV-2, pseudovirus neutralizing antibody assay, spike-binding antibody assay, COVE trial
mRNA-1273 elicited robust humoral immunogenicity in a subset of participants of the phase 3 COVE study across age, sex, and baseline SARS-CoV-2 status, consistent with its high COVID-19 efficacy. Higher immune responses in those previously infected with SARS-CoV-2 support a booster-type effect.
The messenger RNA (mRNA)–1273 vaccine was highly effective in preventing coronavirus disease 2019 (COVID-19) in the Coronavirus Efficacy (COVE) trial [1, 2]. Assessment of vaccine-induced immune responses is important to advance our understanding of how serologic responses contribute to protection against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, and to inform decisions on regulatory approvals including immunobridging between age groups, since the humoral immune responses are a correlate of efficacy against mild-to-moderate disease, and potential need for booster doses [3]. In clinical trials, mRNA-1273 vaccine induced anti–SARS-CoV-2 spike (S) binding antibody (bAb) levels and SARS-CoV-2 neutralizing antibody (nAb) titers in adults that were comparable to or higher than those in plasma from recovered COVID-19 patients, as well as generally consistent immune responses in older and younger participants including adolescents [4–6]. Additionally, mRNA-1273 vaccine elicits neutralizing activity against emerging SARS-CoV-2 variants and rapid rises in nAb titers following booster doses, indicative of immune memory [7–12].
Recently, an mRNA-1273 efficacy of 93.2% with an acceptable safety profile was reported at the completion of the blinded phase of the COVE trial [2]. Here we describe the humoral immunogenicity results of 2 doses of mRNA-1273 administered 28 days apart in trial participants who were SARS-CoV-2–negative (infection-naive) or SARS-CoV-2–positive (prior infection).
METHODS
Study Design
This currently ongoing randomized, stratified, observer-blind, placebo-controlled trial evaluated the efficacy, safety, and immunogenicity of mRNA-1273 SARS-CoV-2 vaccine compared with placebo in medically stable adults [1, 2]. The trial design, study outcomes, and assessments were previously described and are provided in the Supplementary Materials [1, 2].
The trial is conducted in accordance with the International Council for Technical Requirements for Registration of Pharmaceuticals for Human Use, Good Clinical Practice Guidance, and applicable government regulations. The central Institutional Review Board approved the protocol and consent forms. All participants provided written informed consent.
Participants, Randomization, and Masking
Eligible participants were adults aged ≥18 years with no known history of SARS-CoV-2 infection, whose locations or circumstances put them at appreciable risk for SARS-CoV-2 infection and/or risk of severe COVID-19 [1, 2]. Inclusion/exclusion criteria are provided in the Supplementary Materials.
Participants were randomized 1:1 to receive mRNA-1273 vaccine (100 µg) or placebo, stratified by age and severe COVID-19 risk criteria (18–64 years and not at risk; 18–64 years at risk; ≥65 years). Severe COVID-19 risk factors were defined according to the Centers for Disease Control and Prevention guidelines at the time of study design [13]. The investigators, study staff, participants, site monitors, sponsor personnel (or designees), and laboratory personnel were blinded to study treatment through blinded phase completion. The vaccine was administered by deltoid intramuscular injection using a 2-dose regimen given 28 days apart in 0.5 mL containing 100 µg of mRNA-1273 or saline placebo [1].
Outcomes
A secondary objective of the COVE trial was the immunogenicity of 2 injections of mRNA-1273 given 28 days apart, assessed in the immunogenicity subset, a stratified random sample of participants in the full analysis set (FAS) [1, 2]. Participants in the FAS with nonmissing baseline characteristics for the strata who received both planned injections (injection 2 received within 21–42 days post–injection 1), no major protocol deviations, and serum samples available at both days 1 (baseline) and 57 were eligible for inclusion in the prespecified random subset (Supplementary Table 1, Supplementary Figure 1, Supplementary Methods).
Immunogenicity was assessed in serum samples from trial participants using validated assays for the detection of bAb against the SARS-CoV-2–S-protein, including an electrochemiluminescence immunoassay (Meso-Scale Discovery multiplex assay [MSD]; Vaccine Immunology Program, Vaccine Research Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health) and an enzyme-linked immunosorbent assay (ELISA; PPD Laboratories). Neutralizing antibody titers were evaluated using a SARS-CoV-2–S protein (D614G) pseudotyped virus neutralization assay (PsVNA) in 293/ACE2 cells (Neutralizing Antibody Core Laboratory, Duke University). The S receptor-binding domain (RBD) and the SARS-CoV-2–nucleocapsid protein (NP) antibody assays (MSD), used as supportive analyses, were not yet validated at the time of the analysis. Human convalescent sera collected during March–December 2020 from symptomatic COVID-19 patients served as reference antibody levels (MSD assay and ELISA), and from asymptomatic, symptomatic, or hospitalized COVID-19 patients (PsVNA). Assay details are further described in the Supplementary Methods and Supplementary Table 2. The endpoints evaluated included quantified S-protein–specific bAb geometric mean (GM) levels on days 1 (baseline), 29, and 57 and GM fold rise (GMFR) relative to day 1 on days 29 and 57; and SARS-CoV-2–specific nAb GM titers (GMTs) on days 1, 29, and 57 and GMFR relative to day 1 on days 29 and 57. Participants provided nasopharyngeal swabs and blood samples before the first (day 1) and second (day 29) injections. Seroresponses (bAb and nAb) to mRNA-1273 vaccination were assessed at days 29 and 57.
Statistical Analysis
At the final efficacy analysis at completion of the blinded phase (26 March 2021), immunogenicity of mRNA-1273 was evaluated in participants who were baseline SARS-CoV-2–negative (reverse-transcription polymerase chain reaction [RT-PCR] SARS-CoV-2 test and Roche Elecsys anti-CoV-2-NP) and positive (RT-PCR SARS-CoV-2 test and/or Roche Elecsys anti-CoV-2-NP) at day 1 (Supplementary Methods). Immunogenicity data from quantitative assays at prespecified timepoints (days 1, 29, and 57) for bAb to S protein, and PsVNA nAb inhibitory dilution 50% (ID50) and 80% (ID80) titers were analyzed. Exploratory analyses of bAb against RBD and NP (MSD) were also performed. Quantitative GM levels and GMT at each timepoint, and GMFR of bAb or nAb at each postbaseline timepoint over predose day 1 with corresponding 95% confidence intervals (CIs; t distribution of log-transformed values, back-transformed to original scale) are provided by treatment and baseline SARS-CoV-2 status. Descriptive summary statistics (median, minimum, and maximum) are provided. For the analysis, antibody values reported below the lower limit of quantitation (LLOQ) were replaced by 0.5 × LLOQ, and values above the upper limit of quantitation (ULOQ) were converted to the ULOQ if no actual value was reported. Actual values reported beyond the validated ULOQ were used in the analysis and summarized. Seroresponses in participants were defined as a ≥4-fold-increase in GM levels and GMT from baseline, ≥4 times the LLOQ for baseline antibody levels <LLOQ, or a 4 times or higher fold-rise for baseline antibody levels ≥LLOQ, and were also assessed using assay-specific definitions (Supplementary Methods). Qualitative data were summarized as frequencies of responses by treatment group and baseline SARS-CoV-2 status at each timepoint assessed.
RESULTS
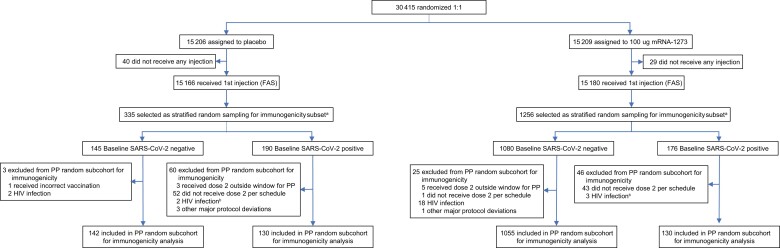
Among the 30 415 participants randomized in the COVE trial (27 July–23 October 2020), 1591 participants in the FAS with available immunogenicity samples (n = 335 placebo; n = 1256 mRNA-1273) were evaluated in the prespecified random immunogenicity subcohort (Figure 1, Supplementary Table 1, Supplementary Methods). Of these, 1457 participants (92%) were included in the per-protocol random subcohort for immunogenicity (272 [8%] placebo; 1185 [94%] mRNA-1273). A total of 63 (19%) participants in the placebo group and 71 (6%) in the mRNA-1273 group were excluded from the per-protocol random subcohort immunogenicity set, most commonly due to not receiving dose 2 per schedule or human immunodeficiency virus (HIV) infection. Of eligible participants within the immunogenicity set, 142 and 130 were SARS-CoV-2–negative and –positive at baseline (day 1), respectively, in the placebo group and 1055 and 130 in the mRNA-1273 group.
Figure 1.
Trial profile immunogenicity analysis population. The per-protocol subcohort for immunogenicity analysis consisted of participants in the full analysis set (FAS) who were sampled into the random subcohort and received both planned doses (ie, received assigned treatment) with dose 2 received within 21–41 days after dose 1, and no major protocol deviations that impacted critical or key data. aStratified random sampling criteria were FAS participants with nonmissing information on strata, based on per-protocol rules consistent with those used for efficacy and those with immunogenicity data at days 1, 29, and 57, all adjudicated coronavirus disease 2019 cases (Supplementary Table 1). Samples from people living with human immunodeficiency virus were excluded due to a known interference of antiretroviral medications with pseudovirus neutralizing antibody assay [41]. Data cutoff: 26 March 2021. Abbreviations: FAS, full analysis set; HIV, human immunodeficiency virus; mRNA, messenger RNA; PP, per-protocol; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Baseline characteristics of the immunogenicity analysis population were generally balanced by treatment group, as in the COVE population (Table 1 and Supplementary Table 3) [1, 2]. The mean age was 53.3 years (range, 18–87 years), 46% were female, 72% were white, and racial and ethnic representations were consistent with US demographics, including 18% black or African American and 32% Hispanic or Latino participants. Overall risk for severe COVID-19 was present in 37% of the population and was higher in the baseline SARS-CoV-2–negative (40%) than –positive (22%) groups.
Table 1.
Baseline Demographics and Characteristics of the Immunogenicity Subset
| Characteristic | Baseline SARS-CoV-2–negativea | Baseline SARS-CoV-2–positivea | Overall | |||
|---|---|---|---|---|---|---|
| Placebo (n = 142) |
mRNA-1273 (n = 1055) |
Placebo (n = 130) |
mRNA-1273 (n = 130) |
Placebo (n = 272) |
mRNA-1273 (n = 1185) |
|
| Age at screening, y, mean (range) | 53.6 (19–85) | 54.5 (18–87) | 49.6 (20–83) | 47.2 (20–84) | 51.7 (19–85) | 53.7 (18–87) |
| Age and health risk for severe COVID-19b | ||||||
| 18–64 y and not at risk | 49 (35) | 360 (34) | 80 (62) | 88 (68) | 129 (47) | 448 (38) |
| 18–64 y and at risk | 45 (32) | 340 (32) | 30 (23) | 19 (15) | 75 (28) | 359 (30) |
| ≥65 y | 48 (34) | 355 (34) | 20 (15) | 23 (18) | 68 (25) | 378 (32) |
| Sex | ||||||
| Male | 76 (54) | 560 (53) | 77 (59) | 70 (54) | 153 (56) | 630 (53) |
| Female | 66 (47) | 495 (47) | 53 (41) | 60 (46) | 119 (44) | 555 (47) |
| Ethnicity | ||||||
| Hispanic/Latino | 47 (33) | 334 (32) | 45 (35) | 41 (32) | 92 (34) | 375 (32) |
| Not Hispanic/Latino | 95 (67) | 717 (68) | 84 (65) | 88 (68) | 179 (66) | 805 (68) |
| Not reported/ unknown | 0 | 4 (0) | 1 (1) | 1 (1) | 1 (0) | 5 (0) |
| Race and ethnicity groupc | ||||||
| Minority | 71 (50) | 522 (50) | 74 (57) | 64 (49) | 145 (53) | 586 (50) |
| Nonminority | 71 (50) | 533 (51) | 56 (43) | 66 (51) | 127 (47) | 599 (51) |
| Race | ||||||
| White | 103 (73) | 767 (73) | 87 (67) | 89 (69) | 190 (70) | 856 (72) |
| Black/African American | 24 (17) | 188 (18) | 38 (29) | 29 (22) | 62 (23) | 217 (18) |
| Asian | 6 (4) | 26 (3) | 1 (1) | 6 (5) | 7 (3) | 32 (3) |
| American Indian/Alaska Native | 2 (1) | 17 (2) | 1 (1) | 0 | 3 (1) | 17 (1) |
| Native Hawaiian/other Pacific Islander | 0 | 5 (1) | 0 | 0 | 0 | 5 (0) |
| Multiracial | 4 (3) | 15 (1) | 0 | 1 (1) | 4 (2) | 16 (1) |
| Other | 3 (2) | 27 (3) | 2 (2) | 4 (3) | 5 (2) | 31 (3) |
| Not reported or unknown | 0 | 10 (1) | 1 (1) | 1 (1) | 1 (0) | 11 (1) |
| Baseline RT-PCR results | ||||||
| Negative | 142 (100) | 1055 (100) | 125 (96) | 129 (99) | 267 (98) | 1184 (100) |
| Positive | 0 | 0 | 3 (2) | 1 (1) | 3 (1) | 1 (0) |
| Missing | 0 | 0 | 2 (2) | 0 | 2 (1) | 0 |
| Baseline bAb anti–SARS-CoV-2d | ||||||
| Negative | 142 (100) | 1055 (100) | 0 | 1 (1) | 142 (52) | 1056 (89) |
| Positive | 0 | 0 | 130 (100) | 129 (99) | 130 (48) | 129 (11) |
| Risk for severe COVID-19 at screening | ||||||
| At risk | 58 (41) | 416 (39) | 34 (26) | 22 (17) | 92 (34) | 438 (37) |
| Not at risk | 84 (59) | 639 (61) | 96 (74) | 108 (83) | 180 (66) | 747 (63) |
| Risk factor for severe COVID-19 at screeninge | ||||||
| Chronic lung disease | 15 (11) | 83 (8) | 6 (5) | 3 (2) | 21 (8) | 86 (7) |
| Significant cardiac disease | 10 (7) | 79 (8) | 7 (5) | 6 (5) | 17 (6) | 85 (7) |
| Severe obesity | 20 (14) | 135 (13) | 12 (9) | 10 (8) | 32 (12) | 145 (12) |
| Diabetes | 26 (18) | 182 (17) | 15 (12) | 6 (5) | 41 (15) | 188 (16) |
| Liver disease | 1 (1) | 17 (2) | 0 | 0 | 1 (0) | 17 (1) |
| HIV | 0 | 0 | 0 | 0 | 0 | 0 |
| Body mass index, kg/m2 | ||||||
| No. | 142 | 1050 | 130 | 129 | 272 | 1179 |
| Mean (SD) | 31.4 (9) | 31.0 (8) | 30.6 (7) | 29.2 (7) | 31.0 (8) | 30.8 (8) |
Percentages based on immunogenicity per-protocol subset, randomly sampled from the full analysis set of the Coronavirus Efficacy (COVE) trial as detailed in the Supplementary Methods.
Abbreviations: bAb, binding antibody; COVID-19, coronavirus disease 2019; HIV, human immunodeficiency virus; IRT, interactive response technology; mRNA, messenger RNA; RT-PCR, reverse-transcription polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SD, standard deviation.
Baseline SARS-CoV-2 status was positive if there was immunologic or virologic evidence of prior COVID-19, defined as positive RT-PCR test, or bAb against SARS-CoV-2–nucleocapsid above limit of detection or lower limit of quantitation at day 1; negative was defined as negative RT-PCR test and negative bAb against SARS-CoV-2 assay result at day 1.
Based on stratification factor from IRT, participants who were <65 years old were categorized as at risk for severe COVID-19 illness if they had at least 1 of the risk factors specified in the study protocol at screening.
Minority includes black/African American, Hispanic/Latino, American Indian/Alaska Native, and Native Hawaiian/other Pacific Islander; nonminority includes all other races with observed race (Asian, multiracial, white, other) and observed ethnicity not Hispanic or Latino.
Elecsys nucleocapsid assay.
Participants could be under 1 or more categories and were counted once at each category. Data cutoff: 26 March 2021.
The validated assay methods used for the measurement of anti-S bAb levels were highly correlated with one another and with the nAb PsVNA in the baseline SARS-CoV-2–negative and –positive groups (r = 0.873–0.990; Supplementary Figure 2). Immunogenicity results (days 1, 29, and 57) are presented by SARS-CoV-2 baseline status, age, and sex.
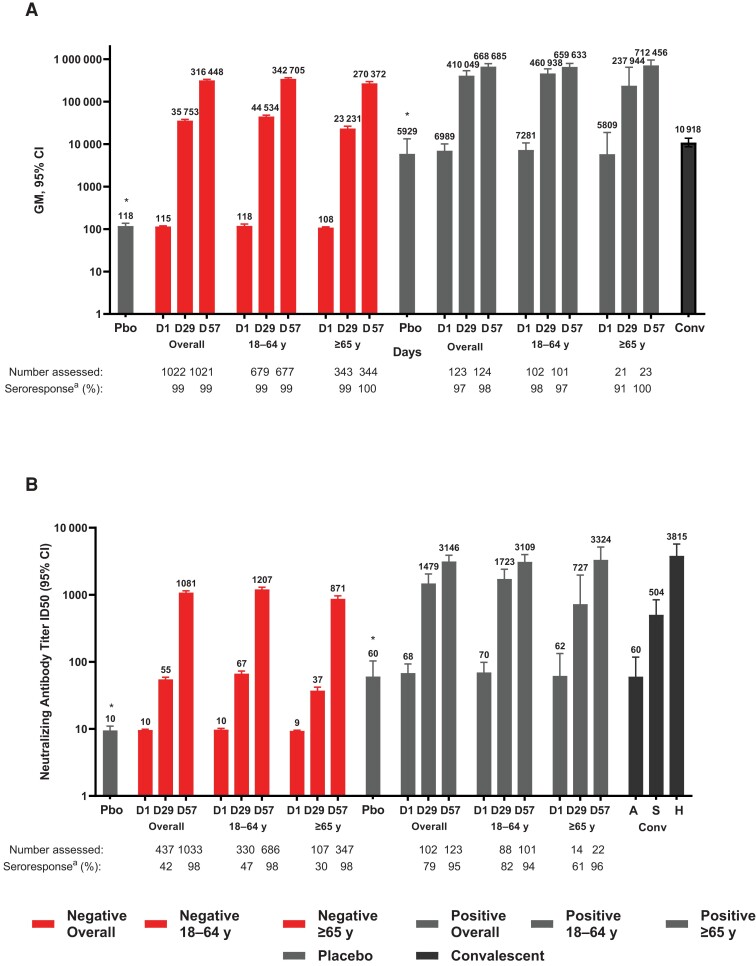
Day 1 bAb GM (AU/mL) levels were 62- and 61-fold higher in SARS-CoV-2–positive participants than those who were SARS-CoV-2–negative in the placebo and mRNA-1273 groups, respectively, by the MSD assay (Figure 2 and Table 2). Levels of bAb remained unchanged during days 1–57 in placebo recipients who were SARS-CoV-2-positive or -negative at baseline. Following mRNA-1273 vaccination in the SARS-CoV-2–negative group, bAb increased 310-fold at day 29 and 2737-fold at day 57 from day 1 GM levels. In SARS-CoV-2–positive mRNA-1273 recipients, bAb GM levels increased 60-fold at day 29 and 96-fold at day 57 and were 11- and 2-fold higher at days 29 and 57, respectively, compared with those baseline SARS-CoV-2–negative. By day 29, bAb seroresponse rates of >97% were observed, regardless of baseline SARS-CoV-2 status.
Figure 2.
Spike binding and neutralizing antibody titers by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) baseline status and age. Geometric mean (GM) concentrations of SARS-CoV-2–specific spike (S) binding antibodies assessed by Meso-Scale Discovery Multiplex assay (MSD) (A) and neutralizing antibody GM titers by pseudovirus neutralizing antibody assay (PsVNA) ID50 (inhibitory dilution 50%, defined as the serum dilution at which SARS-CoV-2 infection is reduced by 50% in PsVNA) (B) at the corresponding visit days (days 1, 29, and 57) and baseline SARS-CoV-2 status and age groups. The lower limit of quantitation (LLOQ) and upper limit of quantitation (ULOQ) were, respectively, 200 and 1 128 439 AU/mL for MSD and 19 and 4404 GM ID50 for PsVNA assays. Antibody values <LLOQ were replaced by 0.5 × LLOQ and those >ULOQ were converted to the ULOQ if actual values were not reported and where available are reported. The 95% confidence intervals (CIs) were calculated based on the t-distribution of the log-transformed values for GM levels and GM ID50 titers, then back-transformed to the original scale for presentation. *Responses in participants who received placebo (Pbo) averaged across days, study vaccine, and age group for SARS-CoV-2–negative and –positive cohorts. aSeroresponses at participant levels defined as a ≥4-fold increase in GM levels and titers from baseline ≥4 × LLOQ for those with baseline antibody levels <LLOQ, or a 4 times or higher-level ratio in participants with baseline antibody level ≥LLOQ. Human convalescent sera (Conv) collected from coronavirus disease 2019 patients tested by MSD (n = 84) and PsVNA (n = 165; 34 asymptomatic [A], 71 symptomatic [S], and 60 hospitalized [H]) assays served as reference control titers. For conversion of binding antibody AU/mL to binding antibody units, multiply AU units by 0.009 for MSD spike (S2-P) protein, and for conversion to international units by 0.242 for PsVNA ID50 titers [3]. Data cutoff: 26 March 2021.
Table 2.
Binding Antibody Levels and Neutralizing Antibody Titers by Severe Acute Respiratory Syndrome Coronavirus 2 Status
| SARS-CoV-2 Status | Spike-Binding Antibody | Neutralizing Antibody (ID50) | ||
|---|---|---|---|---|
| SARS-CoV-2–negative | Placebo (N = 142) |
mRNA-1273 (N = 1055) |
Placebo (N = 142) |
mRNA-1273 (N = 1055) |
| Baseline No.a | 139 | 1046 | 142 | 1052 |
| GM level/titerb | 115 | 115 | 9 | 10 |
| (95% CI)c | (105–125) | (111–119) | (NE–NE) | (9–10) |
| Day 29 No.a | 139 | 1040 | 142 | 1053 |
| GM level/titerb | 117 | 35 753 | 10 | 55 |
| (95% CI)c | (106–130) | (33 376–38 299) | (9–10) | (51–59) |
| GMFRd | 1 | 310 | 1 | 6 |
| (95% CI)c | (1–1) | (288–333) | (1–1) | (5–6) |
| Seroreponsee | ||||
| no./N1 (%)f | 1/137 (1) | 1022/1033 (99) | 1/142 (1) | 437/1052 (42) |
| (95% CI)g | (0–4) | (98–100) | (0–4) | (39–45) |
| Day 57 No.a | 141 | 1035 | 142 | 1053 |
| GM level/titerb | 125 | 316 448 | 10 | 1081 |
| (95% CI)c | (106–147) | (300 071–333 719) | (9–11) | (1020–1146) |
| GMFRd | 1 | 2737 | 1 | 112 |
| (95% CI)c | (1–1) | (2572–2913) | (1–1) | (105–120) |
| Seroreponsee | ||||
| no./N1 (%)f | 2/139 (1) | 1021/1027 (99) | 2/142 (1) | 1033/1050 (98) |
| (95% CI)g | (0–5) | (99–100) | (0–5) | (97–99) |
| SARS-CoV-2–positive | Placebo (N = 130) |
mRNA-1273 (N = 130) |
Placebo (N = 130) |
mRNA-1273 (N = 130) |
| Baseline No.a | 128 | 127 | 129 | 130 |
| GM level/titerb | 7127 | 6989 | 83 | 68 |
| (95% CI)c | (4899–10 368) | (4832–10 109) | (59–115) | (50–93) |
| Day 29 No.a | 127 | 130 | 129 | 130 |
| GM level/titerb | 5672 | 410 049 | 53 | 1479 |
| (95% CI)c | (3955–8135) | (313 904–535 643) | (40–71) | (1070–2045) |
| GMFRd | 1 | 60 | 1 | 22 |
| (95% CI)c | (1–1) | (46–80) | (1–1) | (16–29) |
| Seroreponsee | ||||
| no./N1 (%)f | 2/126 (2) | 123/127 (97) | 0/128 (0) | 102/130 (79) |
| (95% CI)g | (0–6) | (92–99) | (0–3) | (70–85) |
| Day 57 No.a | 128 | 130 | 130 | 130 |
| GM level/titerb | 5186 | 668 685 | 48 | 3146 |
| (95% CI)c | (3609–7451) | (570 884–783 242) | (35–64) | (2540–3897) |
| GMFRd | 1 | 96 | 1 | 46 |
| (95% CI)c | (1–1) | (69–134) | (1–1) | (34–63) |
| Seroreponsee | ||||
| no./N1 (%)f | 3/127 (2) | 124/127 (98) | 1/129 (1) | 123/130 (95) |
| (95% CI)g | (1–7) | (93–100) | (0–4) | (89–98) |
Spike-binding antibody (MSD) and PsVNA values reported as below the lower limit of quantitation (LLOQ) are replaced by 0.5 × LLOQ. Values greater than the upper limit of quantitation (ULOQ) were replaced by the ULOQ if actual values were not available, and where available are reported. LLOQ = 200 and ULOQ = 1 128 439 for MSD S2-P, and LLOQ = 19 and ULOQ = 4404) for PsVNA ID50.
Abbreviations: CI, confidence interval; GM, geometric mean; GMFR, geometric mean fold rise; ID50, pseudovirus neutralizing titer (inhibitory dilution 50%); mRNA, messenger RNA; MSD, Meso-Scale Discovery multiplex assay; NE, not estimated; PsVNA, pseudovirus neutralizing antibody assay; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Number of participants with nonmissing data at the corresponding timepoint.
GM level for MSD and GM titer for PsVNA assays.
95% CI calculated based on the t-distribution of the log-transformed values or the difference in the log-transformed values for GM value and GMFR, respectively, then back-transformed to the original scale for presentation.
GMFR of S protein–specific binding antibody relative to day 1 on days 29 and 57, and GMFR of SARS-CoV-2–specific neutralizing antibody relative to day 1 on days 29 and 5.
Seroresponses at participant levels defined as a ≥4-fold increase in GM levels and titers from baseline ≥4 × LLOQ for those with baseline antibody levels <LLOQ, or a 4 times or higher-level ratio in participants with baseline antibody level ≥LLOQ.
Number of participants meeting the criterion at the time point; percentages are based on N1, the number of participants with nonmissing data at baseline and the corresponding time point.
95% CI is calculated using the Clopper-Pearson method. For conversion of binding antibody AU/mL to binding antibody units, multiply AU units by 0.009 for MSD spike (S2-P) protein and for conversion to international units by 0.242 for PsVNA ID50 titers [3]. Data cutoff: 26 March 2021.
Levels of bAb were 2-fold higher in SARS-CoV-2–negative mRNA-1273 recipients 18–64 years old than those aged ≥65 years at day 29 and similar at day 57 (Figure 2 and Supplementary Table 4). In SARS-CoV-2–positive mRNA-1273–vaccinated participants, bAb levels on day 29 were also 2-fold higher in younger than older adults and comparable at day 57 for the 2 age groups. The bAb levels were higher in younger (10-fold at day 29) and older (2- and 3-fold at day 57) SARS-CoV-2–positive than –negative participants. Seroresponse rates remained high (>91%–100%) across both age groups on days 29 and 57. Generally similar bAb levels were seen in female and male SARS-CoV-2–negative participants at days 29 and 57; however, bAb levels were 2-fold higher for SARS-CoV-2–positive males than females at day 29 and similar at day 57. Seroresponse rates were comparable irrespective of sex and previous infection (Supplementary Figure 3 and Supplementary Table 5).
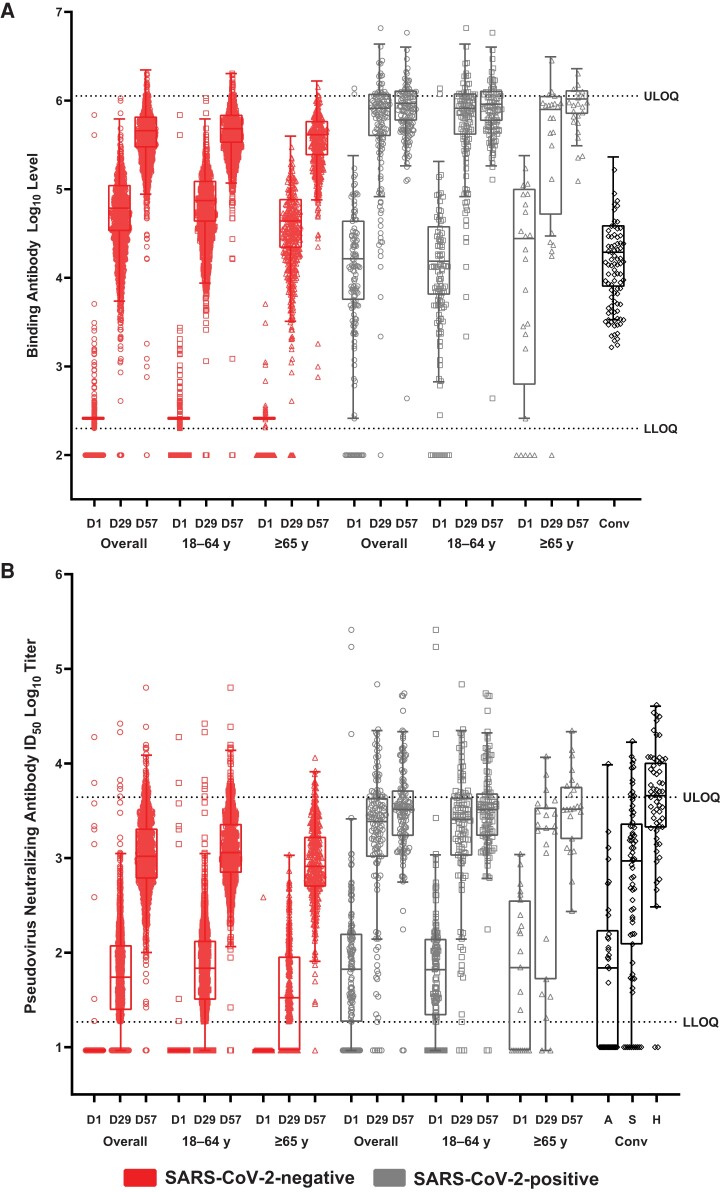
Overall, bAb GM levels at days 29 and 57 were higher in SARS-CoV-2–negative (3.3- to 29.0-fold) and in SARS-CoV-2–positive (38- to 61-fold) participants than bAb GM levels in convalescent sera and were also higher at days 29 (2- to 31-fold) and 57 (22- to 65-fold) across SARS-CoV-2–negative and –positive age groups, respectively (Figure 2). Findings were consistent by sex groups (Supplementary Figure S3). The distributions of bAb responses showed comparable patterns and median magnitudes of bAb values that exceeded those of convalescent COVID-19 patient sera, regardless of SARS-CoV-2 status and age (Figure 3).
Figure 3.
Distribution of binding and neutralizing antibody responses by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) status and age. Reciprocal endpoint geometric mean (GM) spike (S) binding antibody log10 levels by Meso-Scale Discovery Multiplex assay (MSD; A), and neutralizing antibody titers by S-pseudovirus neutralizing antibody assay (PsVNA) (inhibitory dilution 50%, defined as the serum dilution at which SARS-CoV-2 infection is reduced by 50% in PsVNA; ID50 log10) (B) at the corresponding visit days (days 1, 29, and 57), and baseline SARS-CoV-2 status and age groups. The lower limit of quantitation (LLOQ) and upper limit of quantitation (ULOQ), respectively, were log10 2.3 and 6.1 for MSD and log10 1.3 and 3.6 for PsVNA ID50 assays. Antibody values reported as below the LLOQ were replaced by 0.5 × LLOQ. Values greater than the ULOQ are replaced by the ULOQ if actual values were not available and where available are reported. Human convalescent sera (Conv) collected from coronavirus disease 2019 patients tested by MSD (n = 84) and PsVNA (n = 165; 34 asymptomatic [A], 71 symptomatic [S], and 60 hospitalized [H]) assays served as reference control titers. Boxes and horizontal bars denote interquartile (IQR) ranges and median endpoint titers; whisker endpoints are the maximum and minimum values below or above the median ± 1.5 times the IQR. Data cutoff: 26 March 2021.
Results for ELISA were similar to those of the MSD assay across age, sex, and SARS-CoV-2 status groups (Supplementary Figure 4 and Supplementary Tables 5 and 6). In an exploratory analysis, bAb GM levels specific to SARS-CoV-2-NP in the mRNA-1273 group were comparable to placebo in SARS-CoV-2–negative and –positive participants during days 1–57, and were 10- to 19-fold higher in the SARS-CoV-2–positive group (Supplementary Table 7). Exploratory results for SARS-CoV-2–specific bAb to the RBD were similar to those observed for full-length S-protein, regardless of age and SARS-CoV-2 status (Supplementary Figure 5 and Supplementary Table 8).
The nAb ID50 GMTs were 9- and 7-fold higher at baseline in SARS-CoV-2–positive compared with SARS-CoV-2–negative participants in the placebo and mRNA-1273 groups, respectively; titers remained unchanged over time in both placebo groups (Figure 2 and Table 2). In SARS-CoV-2–negative participants, nAb GMT ID50 increased 6-fold on day 29 and 108-fold on day 57 compared to baseline (Figure 2 and Table 2). In SARS-CoV-2–positive participants, GMT increases from baseline on day 29 (22-fold) and on day 57 (46-fold) were 27-fold higher at day 29 and 3-fold higher at day 57 than those of SARS-CoV-2–negative participants. Seroresponses were 42% in SARS-CoV-2–negative and 79% in SARS-CoV-2–positive mRNA recipients at day 29, and by day 57 were >95%, regardless of SARS-CoV-2 status.
In SARS-CoV–negative and –positive participants, nAb ID50 GMTs were 2-fold higher on day 29 and comparable at day 57 in those 18–64 vs ≥65 years of age (Figure 2 and Supplementary Table 4). The nAb GMTs in SARS-CoV-2–positive participants were higher at days 29 (26- and 20-fold) and 57 (3- and 4-fold) in younger vs older SARS-2-CoV–negative participants, respectively. Seroresponses were also lower in older (30% and 61%) than younger (47% and 82%) adults on day 29 in the SARS-CoV–negative and –positive groups, respectively, and by day 57 were >94%, regardless of baseline SARS-CoV-2 status and age. Among SARS-CoV-2–negative females and males, nAb titers were generally comparable at days 29 and 57; however, SARS-CoV-2–positive males had 2-fold higher nAb titers than females at day 29 and were similar at day 57 (Supplementary Figure 3 and Supplementary Table 5). Seroresponse rates were generally comparable between males and females regardless of SARS-CoV-2 status.
Neutralizing ID50 GMTs in SARS-CoV-2–negative participants were similar at day 29 and higher at day 57 (18-fold) compared with those of convalescent sera from asymptomatic patients, and lower at day 29 but higher (2-fold) at day 57 compared with symptomatic patient sera (Figure 2 and Supplementary Table 4). Among SARS-CoV–positive participants, nAb GMTs were higher than titers of sera from asymptomatic (25- and 52-fold) and symptomatic (3- and 6-fold) patients at both days. Compared with serum titers of hospitalized patients, nAb titers of SARS-CoV-2–negative participants were lower at both days, whereas those of SARS-CoV-2–positive participants were lower at day 29 and similar at day 57. In younger SARS-CoV-2–negative adults, nAb titers were similar to those of convalescent asymptomatic sera and lower in those older at day 29, but were higher in both age groups at day 57. In comparison with titers of symptomatic patient sera, nAb titers were lower at day 29 and higher at day 57 irrespective of age. Among SARS-CoV-2–positive participants, nAb GMTs were higher at both days compared with convalescent sera from asymptomatic and symptomatic patients in both age groups. Across age groups, nAb GMTs were lower than those of convalescent sera from hospitalized patients regardless of SARS-CoV-2 status at days 29 and 57, and were more similar in those SARS-CoV-2–positive at day 57. The distributions of nAb titers showed similar patterns and magnitudes of response in comparison with those of convalescent sera, across baseline SARS-CoV-2 status and age groups (Figure 3). Overall, comparable results for convalescent sera were seen at PsVNA ID80 titers and for nAb titers in females and males (Supplementary Figures 3 and 6).
Seroresponses evaluated by assay-specific definitions for bAb and nAb were consistent with those defined as a ≥4-fold increase from baseline for all 3 assays (Supplementary Table 9).
DISCUSSION
This immunogenicity analysis of the COVE study demonstrated robust SARS-CoV-2-S bAb and nAb responses following a 2-dose schedule of mRNA-1273 vaccine in a relatively large sample size of participants, consistent with its high efficacy in the trial [1, 2]. High levels of both bAb and nAb were observed following the first dose and increased further after a second dose, regardless of baseline SARS-CoV-2 infection status, and were as much as an order of magnitude higher in individuals with evidence of prior infection than in those infection naive after the first injection, suggesting an immune-enhancing effect. These immunologic findings were generally similar across age and sex groups.
Vaccine studies have generally evaluated bAb and nAb responses as correlates of protection or surrogate markers against infection for many viral diseases; however, such a relationship is just coming into view for COVID-19 [3, 14]. Several lines of evidence support that antibody responses elicited by SARS-CoV-2 infection or vaccines targeting the S-protein will contribute to a protective effect [15–20], including recent studies suggesting that neutralization titer may be an important predictor of vaccine efficacy [3, 14, 17]. In an analysis of the COVE study, estimated vaccine efficacies in mRNA-1273 recipients with day 57 nAb ID50 titers of 10–1000 IU/mL were 78%–96%, and in those with S-bAb levels of 33–4000 BAU/mL were 85%–94%, and comparable to the 93.2% efficacy in the trial [3]. The levels of nAb and bAb observed in our analysis at day 57 among baseline SARS-CoV-2–negative (261 IU/mL and 2789 BAU/mL) and –positive (761 IU/mL and 6123 BAU/mL) participants, respectively, using the same laboratory assays, approximate those reported protective levels. Additionally, in the COVE analysis, bAb results for RBD and S-protein assays were comparable and shown to be tightly correlated using the same MSD platform [3], as observed in our exploratory analysis.
Neutralizing assays are the gold standard for measurement of functional antiviral antibodies associated with protection against infection, while bAb assays detect all antibodies generated in response to the antigen of interest [21]. The concordance reported for bAb in relation to nAb titers and demonstrated relationships with vaccine efficacy against mild COVID-19, as well as the lesser variability of bAb assays, high-throughput capability, and ease of standardization, indicate the potential value of validated bAb assays as measures of protective immunity [3, 17, 22]. Moreover, the robust bAb increases observed at day 29 compared with those of nAb (GMFR, 66 vs 2) suggest that bAb could be used as an early, sensitive predictor of protective immunity. Additionally, in a post hoc analysis of HIV participants excluded from the immunogenicity set, due to the presence of antiretroviral therapy in HIV patient sera known to cause false-positive results in the PsVNA [41], bAb titers generally increased through day 57 following mRNA-1273 vaccination as seen in the immunogenicity set, indicating that bAb assays can be used to assess immune responses in this population (Supplementary Figure 7) [3].
In the absence of threshold antibody titers required for protection against COVID-19, immune responses following vaccination have been compared with those of convalescent patient sera [17]. In one study of COVID-19 vaccine clinical trials, a 50% protective neutralization level against detectable SARS-CoV-2 infection was equivalent to 20.2% (95% confidence interval [CI], 14.4%–28.4%) of mean convalescent levels (estimated ∼54 IU/mL [95% CI, 30–96 IU/mL]), and was significantly lower for severe infection (3% of mean convalescent level [95% CI, 0.7%–13%]) [17]. The median magnitudes of bAb and nAb values in our study were generally similar to or higher than those of convalescent sera from COVID-19 asymptomatic and symptomatic patients and lower than or comparable to sera from hospitalized patients, regardless of age, sex, and SARS-CoV-2 status, consistent with the clinical benefit seen in the COVE and effectiveness studies [1, 2, 23]. These results also indicate that vaccination may provide benefit in previously infected individuals [24, 25].
Immunological memory following SARS-CoV-2 infection has been broadly characterized [26–28]; however, phase 3 clinical trials of COVID-19 vaccines have focused on the measurement of serologic responses, and the contributions of memory responses to long-term protection is less known [29]. A key finding in our study was that bAb levels and nAb titers after the first injection were higher in SARS-CoV-2–positive than SARS-CoV-2–negative participants and comparable to those after the second injection in baseline-negative participants. These observations support an immune-booster effect in persons previously infected with SARS-CoV-2 [26], consistent with studies showing that vaccinating previously SARS-CoV-2–infected individuals is associated with increased antibody responses and disease protection after 1 vaccine dose [29–33]. Neutralizing titers are also enhanced against variant viruses 1 month after a third mRNA-1273 dose compared with a second injection in the primary vaccination series [7–11]. Given the robust response to 1 dose of mRNA-1273 observed in previously infected persons, it is plausible that a 1-dose regimen may be sufficient to achieve protection from COVID-19 outcomes in these persons. However, additional studies are needed to better understand the relationships between the durability of protection and humoral and cell-mediated immunogenicity for future planning of COVD-19 vaccines, including evaluation of booster doses and multivalent vaccines.
While comparable immunogenicity following mRNA-1273 vaccination has been previously reported in young and old adults [5, 6] and in adolescents [4], this phase 3 study demonstrates robust bAb and nAb responses regardless of age and SARS-CoV-2 baseline status in a large sample size that is representative of US ethnic and racial demographics. In the present study, regardless of age, bAb and nAb values were higher in baseline SARS-CoV-2–positive than –negative participants, as seen in the overall population, and were lower in older than younger adults who were SARS-CoV-2–baseline negative following both doses and baseline–positive after 1 dose; nonetheless, seroresponse rates were high (94%–100%) across both age groups at day 57. Our study indicated that serologic responses were generally comparable in infection-naive males and females at both days 29 and 57, consistent with efficacy findings in the trial [1, 2]; however, immune responses were numerically higher in SARS-CoV-2–positive males than females at day 29 but were similar at day 57 with little difference in seroresponse rates, regardless of sex. While females have been found to have more effective immunologic responses to a number of viruses following natural infection, studies of convalescent sera from COVID-19 patients have shown that male sex is associated with higher antibody titers than females, possibly due to increased risks of disease severity and outcomes in males, and enhanced immune responses to higher viral loads [34–36].
This study has some limitations. A serologic threshold of protective titer has not yet been established for SARS-CoV-2 infection; hence, the differences observed in comparison to convalescent sera are of unknown significance, and interpretation of the results must consider differences in COVID-19 convalescent titers attributed to various factors (patient age, disease severity, time since disease onset). Cellular immune responses in addition to humoral responses likely contribute to protection against COVID-19 and were not evaluated in the study; however, T-cell–mediated immunity following mRNA-1273 vaccination has been reported [5, 16, 22, 37, 38]. While immunogenicity data through 57 days following vaccination are presented, the long-term kinetics and durability of the response will be measured in the ongoing study. Additionally, antibody levels were measured against Wuhan 1 (D614G) and do not reflect immunogenicity against other variants; however, the effectiveness of mRNA-1273 against emerging variants over time has been demonstrated [7–11]. This study evaluated immunogenicity after 2 doses of mRNA-1273 separated by 28 days, and longer vaccination intervals, which can result in higher antibody titers can be evaluated in future studies [39]. Strong correlations between the bAb and nAb assays used in the immunogenicity assessment were observed and although nAb titers were evaluated by a pseudovirus assay, prior data suggest the assay is a relevant surrogate for live-virus neutralization [3, 40]. The consistency observed for assay-specific defined seroresponses vs those assessed as ≥4-fold increases from baseline in this study warrants additional investigation.
Overall, mRNA-1273 vaccination in the COVE trial elicited robust immunogenicity after the first and second injections, regardless of age, sex, and baseline SARS-CoV-2 status. The ongoing trial will provide additional data assessing the durability of the immune response and long-term efficacy of the vaccine.
Supplementary Material
Contributor Information
Hana M El Sahly, Molecular Virology and Microbiology, Baylor College of Medicine, Houston, Texas, USA.
Lindsey R Baden, Division of Infectious Diseases, Brigham and Women’s Hospital, Boston, Massachusetts, USA.
Brandon Essink, Meridian Clinical Research, Omaha, Nebraska, USA.
David Montefiori, Immune Assay Team, Department of Surgery, Duke University Medical Center, Durham, North Carolina, USA.
Adrian McDermont, Vaccine Research Center, National Institutes of Health, National Institute of Allergy and Infectious Diseases, Bethesda, Maryland, USA.
Richard Rupp, Sealy Institute for Vaccine Sciences, University of Texas Medical Branch, Galveston, Texas, USA.
Michael Lewis, Department of Pathology, Veterans Affairs Greater Los Angeles Healthcare, Los Angeles, California, USA.
Shobha Swaminathan, Department of Medicine, Rutgers, New Jersey Medical School, Newark, New Jersey, USA.
Carl Griffin, Lynn Health Science Institute, Oklahoma City, Oklahoma, USA.
Veronica Fragoso, Texas Center for Drug Development, DM Clinical Research, Houston, Texas, USA.
Vicki E Miller, Texas Center for Drug Development, DM Clinical Research, Tomball, Texas, USA.
Bethany Girard, Infectious Disease Development, Moderna, Inc., Cambridge, Massachusetts, USA.
Yamuna D Paila, Infectious Disease Development, Moderna, Inc., Cambridge, Massachusetts, USA.
Weiping Deng, Infectious Disease Development, Moderna, Inc., Cambridge, Massachusetts, USA.
Joanne E Tomassini, Infectious Disease Development, Moderna, Inc., Cambridge, Massachusetts, USA.
Robert Paris, Infectious Disease Development, Moderna, Inc., Cambridge, Massachusetts, USA.
Florian Schödel, Infectious Disease Development, Moderna, Inc., Cambridge, Massachusetts, USA.
Rituparna Das, Infectious Disease Development, Moderna, Inc., Cambridge, Massachusetts, USA.
Allison August, Infectious Disease Development, Moderna, Inc., Cambridge, Massachusetts, USA.
Brett Leav, Infectious Disease Development, Moderna, Inc., Cambridge, Massachusetts, USA.
Jacqueline M Miller, Infectious Disease Development, Moderna, Inc., Cambridge, Massachusetts, USA.
Honghong Zhou, Infectious Disease Development, Moderna, Inc., Cambridge, Massachusetts, USA.
Rolando Pajon, Infectious Disease Development, Moderna, Inc., Cambridge, Massachusetts, USA.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Author contributions. L. R. B., H. M. E., B. E., R. P., D. M., H. Z., and B. L. contributed to the design of the study and oversight. L. R. B., H. M. E., R. R., S. S., C. G., V. F., V. E. M., R. P., B. G., and Y. D. P. contributed to data collection. R. J. P., B. G., Y. D. P., D. M., and A. M. were responsible for immunogenicity assays. H. Z., W. D., R. P., J. E. T., A. A., H. M. E., L. R. B., B. E., B. L., D. M., and A. M. contributed to data analysis and/or interpretation of the data. All authors critically reviewed and provided input to manuscript drafts and approved the final version for submission to the journal.
Acknowledgments. We thank the Vaccine Research Center, National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH), for MSD Multiplex analysis; the Immune Assay Team at Duke University Medical Center, Durham, North Carolina, for PsVNA analysis; and Frank J. Dutko, a Moderna consultant, for editorial and figure development support.
Data sharing. As the trial is ongoing, access to patient-level data and supporting clinical documents with qualified external researchers may be available upon request and subject to review once the trial is complete.
Financial support. This work was supported by the Biomedical Advanced Research and Development Authority (contract number 75A50120C00034) and the National Institute of Allergy and Infectious Diseases (NIAID). The NIAID provided grant funding to the HIV Vaccine Trials Network (HVTN) Leadership and Operations Center (grant number UM1 AI 68614HVTN), the Statistics and Data Management Center (grant number UM1 AI 68635), the HVTN Laboratory Center (grant number UM1 AI 68618), the HIV Prevention Trials Network Leadership and Operations Center (grant number UM1 AI 68619), the AIDS Clinical Trials Group Leadership and Operations Center (grant number UM1 AI 68636), and the Infectious Diseases Clinical Research Consortium leadership group 5 (grant number UM1 AI148684-03). Funding to pay the Open Access publication charges for this article was provided by Moderna, Inc.
References
- 1. Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 2021; 384:403–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. El Sahly HM, Baden LR, Essink B, et al. Efficacy of the mRNA-1273 SARS-CoV-2 vaccine at completion of blinded phase. N Engl J Med 2021; 385:1774–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gilbert PB, Montefiori DC, McDermott AB, et al. Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy clinical trial. Science 2022; 375:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ali K, Berman G, Zhou H, et al. Evaluation of mRNA-1273 SARS-CoV-2 vaccine in adolescents. N Engl J Med 2021; 385:2241–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Anderson EJ, Rouphael NG, Widge AT, et al. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med 2020; 383:2427–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chu L, McPhee R, Huang W, et al. A preliminary report of a randomized controlled phase 2 trial of the safety and immunogenicity of mRNA-1273 SARS-CoV-2 vaccine. Vaccine 2021; 39:2791–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wu K, Werner AP, Koch M, et al. Serum neutralizing activity elicited by mRNA-1273 vaccine. N Engl J Med 2021; 384:1468–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Choi A, Koch M, Wu K, et al. Safety and immunogenicity of SARS-CoV-2 variant mRNA vaccine boosters in healthy adults: an interim analysis. Nat Med 2021; 27:2025–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pegu A, O’Connell SE, Schmidt SD, et al. Durability of mRNA-1273 vaccine-induced antibodies against SARS-CoV-2 variants. Science 2021; 373:1372–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pajon R, Doria-Rose NA, Shen X, et al. SARS-CoV-2 Omicron variant neutralization after mRNA-1273 booster vaccination. N Engl J Med 2022; 386:1088–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Baden LR, El Sahly HM, Essink B, et al. Phase 3 trial of mRNA-1273 during the Delta-variant surge. N Engl J Med 2021; 385:2485–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Girard B, Tomassini JE, Deng W, et al. mRNA-1273 vaccine-elicited neutralization of SARS-CoV-2 Omicron in adolescents and children. medRxiv [Preprint]. Posted online 25 January 2022. 10.1101/2022.01.24.22269666. [DOI] [Google Scholar]
- 13. Centers for Disease Control and Prevention . Coronavirus disease 2019 (COVID 19).https://www.cdc.gov/coronavirus/2019-ncov/science/science-briefs/underlying-evidence-table.html. Accessed 5 March 2022.
- 14. Feng S, Phillips DJ, White T, et al. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat Med 2021; 27:2032–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chandrashekar A, Liu J, Martinot AJ, et al. SARS-CoV-2 infection protects against rechallenge in rhesus macaques. Science 2020; 369:812–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Corbett KS, Nason MC, Flach B, et al. Immune correlates of protection by mRNA-1273 vaccine against SARS-CoV-2 in nonhuman primates. Science 2021; 373:eabj0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med 2021; 27:1205–11. [DOI] [PubMed] [Google Scholar]
- 18. O’Brien MP, Forleo-Neto E, Musser BJ, et al. Subcutaneous REGEN-COV antibody combination to prevent Covid-19. New Engl J Med 2021; 385:1184–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McMahan K, Yu J, Mercado NB, et al. Correlates of protection against SARS-CoV-2 in rhesus macaques. Nature 2021; 590:630–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Simonovich VA, Burgos Pratx LD, Scibona P, et al. A randomized trial of convalescent plasma in Covid-19 severe pneumonia. New Engl J Med 2020; 384:619–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kellam P, Barclay W. The dynamics of humoral immune responses following SARS-CoV-2 infection and the potential for reinfection. J Gen Virol 2020; 101:791–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Plotkin SA. Updates on immunologic correlates of vaccine-induced protection. Vaccine 2020; 38:2250–7. [DOI] [PubMed] [Google Scholar]
- 23. Pawlowski C, Lenehan P, Puranik A, et al. FDA-authorized mRNA COVID-19 vaccines are effective per real-world evidence synthesized across a multi-state health system. Med (N Y) 2021; 2:979–92.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Steensels D, Pierlet N, Penders J, Mesotten D, Heylen L. Comparison of SARS-CoV-2 antibody response following vaccination with BNT162b2 and mRNA-1273. JAMA 2021; 326:1533–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Spicer KB, Glick C, Cavanaugh AM, Thoroughman D. Protective immunity after natural infection with severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2)—Kentucky, USA, 2020. Int J Infect Dis 2022; 114:21–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dan JM, Mateus J, Kato Y, et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science 2021; 371:eabf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hartley GE, Edwards ESJ, Aui PM, et al. Rapid generation of durable B cell memory to SARS-CoV-2 spike and nucleocapsid proteins in COVID-19 and convalescence. Sci Immunol 2020; 5:eabf8891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sette A, Crotty S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell 2021; 184:861–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Goel RR, Apostolidis SA, Painter MM, et al. Distinct antibody and memory B cell responses in SARS-CoV-2 naive and recovered individuals following mRNA vaccination. Sci Immunol 2021; 6:eabi6950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Samanovic MI, Cornelius AR, Gray-Gaillard SL, et al. Robust immune responses after one dose of BNT162b2 mRNA vaccine dose in SARS-CoV-2 experienced individuals. Sci Transl Med 2022; 14:eabi8961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jabal KA, Ben-Amram H, Beiruti K, et al. Impact of age, ethnicity, sex and prior infection status on immunogenicity following a single dose of the BNT162b2 mRNA COVID-19 vaccine: real-world evidence from healthcare workers, Israel, December 2020 to January 2021. Euro Surveill 2021; 26:2100096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chu L, Montefiori D, Huang W, et al. Immune memory response after a booster injection of mRNA-1273 for severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). medRxiv [Preprint]. Posted online 1 October 2021. 10.1101/2021.09.29.21264089. [DOI] [Google Scholar]
- 33. Leon TM, Dorabawila V, Nelson L, et al. COVID-19 cases and hospitalizations by COVID-19 vaccination status and previous COVID-19 diagnosis—California and New York, May–November 2021. MMWR Morb Mortal Wkly Rep 2022; 71:125–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Robbiani DF, Gaebler C, Muecksch F, et al. Convergent antibody responses to SARS-CoV-2 in convalescent individuals. Nature 2020; 584:437–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Klein SL, Pekosz A, Park H-S, et al. Sex, age, and hospitalization drive antibody responses in a COVID-19 convalescent plasma donor population. J Clin Invest 2020; 130:6141–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Takahashi T, Iwasaki A. Sex differences in immune responses. Science 2021; 371:347–8. [DOI] [PubMed] [Google Scholar]
- 37. Woldemeskel BA, Garliss CC, Blankson JN. SARS-CoV-2 mRNA vaccines induce broad CD4+ T cell responses that recognize SARS-CoV-2 variants and HCoV-NL63. J Clin Invest 2021; 131:e149335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sahin U, Muik A, Vogler I, et al. BNT162b2 vaccine induces neutralizing antibodies and poly-specific T cells in humans. Nature 2021; 595:572–7. [DOI] [PubMed] [Google Scholar]
- 39. Grunau B, Asamoah-Boaheng M, Lavoie PM, et al. A higher antibody response is generated with a 6- to 7-week (vs standard) severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine dosing interval. Clin Infect Dis 2022; 75:e888–e891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sholukh AM, Fiore-Gartland A, Ford ES, et al. Evaluation of cell-based and surrogate SARS-CoV-2 neutralization assays. J Clin Microbiol 2021; 59:e0052721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Huang D, Tran JT, Peng L, et al. A rapid assay for SARS-CoV-2 neutralizing antibodies that is insensitive to antiretroviral drugs. J Immunol 2021; 207:344–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.