Abstract
Objectives
Autoantibody seroconversion has been extensively studied in the context of COVID-19 infection but data regarding post-vaccination autoantibody production is lacking. Here we aimed to determine the incidence of common autoantibody formation following mRNA COVID-19 vaccines in patients with inflammatory arthritis (IA) and in healthy controls.
Methods
Autoantibody seroconversion was measured by serum ELISA in a longitudinal cohort of IA participants and healthy controls before and after COVID-19 mRNA-based immunization.
Results
Overall, there was a significantly lower incidence of ANA seroconversion in participants who did not contract COVID-19 prior to vaccination compared with those who been previously infected (7.4% vs 24.1%, P = 0.014). Incidence of de novo anti-CCP seroconversion in all participants was low at 4.9%. Autoantibody levels were typically of low titre, transient, and not associated with increase in IA flares.
Conclusions
In both health and inflammatory arthritis, the risk of autoantibody seroconversion is lower following mRNA-based immunization than following natural SARS-CoV-2 infection. Importantly, seroconversion does not correlate with self-reported IA disease flare risk, further supporting the encouragement of mRNA-based COVID-19 immunization in the IA population.
Keywords: ANA, COVID-19 vaccines, COVID-19 infection, autoantibodies, inflammatory arthritis
Rheumatology key messages.
This study demonstrates a low incidence of autoantibody production after immunization with mRNA-based COVID-19 regimen.
Inflammatory arthritis (IA) disease flare rate was not increased after mRNA-based COVID-19 immunization.
COVID-19 immunization is unlikely to cause disease flare or lead to de novo serologic autoimmunity.
Introduction
Recent studies have shown elevated rates of autoantibody production in patients hospitalized with COVID-19 infection, including ANA and antibodies associated with antiphospholipid syndrome [1–3]. While some groups have noted an association between autoantibody production and worse prognosis in COVID-19 infection [4–7], the long-term clinical implications of these autoantibodies remain unknown. Reassuringly, transient production of autoantibodies such as ANA and rheumatoid factor have been found during several other (viral, bacterial and parasitic) infections, without subsequent development of autoimmune sequelae [8, 9]. To date, whether vaccination against SARS-CoV-2 with novel mRNA constructs may lead to an increase in autoantibody production (or clinically evident autoimmunity) is yet to be determined, although recent evidence suggests that mRNA vaccines have a good safety profile in rheumatoid arthritis patients [10]. Here, we describe the incidence of autoantibody seroconversion in a prospective cohort of patients with inflammatory arthritis (IA) and healthy controls after a two-dose mRNA COVID-19 vaccination regimen.
Methods
Participants
Established patients with IA [RA or spondyloarthritis (SpA), including PsA, n = 138] participating in the WARCOV/SAGA study at the New York University Langone Health system in New York City [11] who received either BNT162b2 mRNA or mRNA-1273 vaccination were assessed at baseline and at various time points after completing a two-dose regimen during the period from 23 December 2020 through 21 November 2021. IA diagnosis was determined by a referring rheumatologist based on ACR-EULAR 2010 RA classification criteria, CASPAR criteria (PsA) or ASAS criteria (SpA). Healthy subjects served as controls (n = 26). This study has been approved by NYU institutional review board (protocol 20–01078) and all participants have provided written informed consent.
Humoral immune response to mRNA COVID-19 vaccines
Humoral immune response was assessed by testing IgG antibodies against the spike protein of SARS-CoV-2. Quantification by ELISA [Anti-SARS-CoV-2 QuantiVac ELISA (IgG), Euroimmun (Lübeck, Germany)] was performed in our lab as described [12] according to manufacturers’ protocols, using a Spectramax Plus 384 spectrocytometer.
Autoantibody assessment
ANA was assessed by ELISA (ANAscreen IgG ELISAs, Eagle Biosciences, Amherst, NH, USA) as a screening test. In those who were ANA positive, additional autoantibodies (dsDNA, Smith, RNP, SSA, SSB, Scl-70, CNP and Jo-1) were analysed via semiquantitative ELISA (ANA Pro ELISA Kit, Eagle Biosciences). Second generation anti-CCP autoantibody titres in all participants were assessed by quantitative ELISA (Anti-CCP ELISA Assay Kit, Eagle Biosciences). All ELISAs were performed in-house according to manufacturers’ protocols, using a Spectramax Plus 384 spectrocytometer.
Statistical analysis
For statistical analysis, autoantibody conversion was defined as having a positive autoantibody at any time point evaluated. When a baseline time-point was missing (i.e. either prior to having natural COVID-19 infection or prior to mRNA COVID-19 immunization), the autoantibody was imputed to be negative. We report the incidence proportion defined as case during this time period divided by population at start, unless otherwise specified. Group comparisons were based on Wilcoxon rank sum test and Fisher’s exact test for continuous and categorical variables, respectively, unless otherwise specified. The two-sided test was performed with significance level of 0.05.
Results
As part of the NYU WARCOV/SAGA study [11], we studied a total of 164 participants (n = 26 healthy controls; and n = 138 IA). Table 1 describes the clinical and demographic characteristics of the cohort. Healthy controls and IA participants did not differ significantly in age or gender. Overall, the cohort was predominantly Caucasian. Incidence of prior COVID infection (as defined by pre-vaccination positive COVID-19 PCR test and/or positive COVID-19 antibody test) was proportionally, albeit not statistically, lower in healthy controls than in IA (11.5% vs.18.8%, P = 0.575; Table 1). The majority of participants received the BNT162b2 mRNA vaccine compared with mRNA-1273 (69.5% vs 30.5%, P = <0.0001; Table 1), including all of the healthy controls who received BNT162b2. IA was classified as either RA (n = 68; 49.3%) or SpA (n = 70; 50.7%) (Table 1). ANA seroconversion rate after BNT162b2 vaccination was not statistically different between healthy controls and IA (7.7% vs 11.4%, P = 0.731; Table 1) and was not statistically different between IA that received BNT162b2 and IA that received mRNA-1273 (10.5% vs 10%; P > 0.999; Table 1).
Table 1.
Demographics, disease diagnosis and prior SARS-CoV-2 infection status in IA and healthy controls
| Characteristic | Healthy (n = 26) | IA (n = 138) | Total (n = 164) | P-value |
|---|---|---|---|---|
| Age, mean (SD) | 51.0 (13.2) | 52.6 (14.2) | 52.3 (14.0) | 0.433 |
| Female, n (%) | 15 (57.7%) | 103 (74.6%) | 118 (72.0%) | 0.096 |
| Race n (%) | 0.024 | |||
| Asian | 9 (34.6%) | 14 (10.1%) | 23 (14.0%) | 0.003 |
| American Indian/Native American | 0 (0.0%) | 1 (0.7%) | 1 (0.6%) | >0.999 |
| Black/African American | 1 (3.8%) | 15 (10.9%) | 16 (9.8%) | <0.0001 |
| Other | 0 (0.0%) | 8 (5.8%) | 8 (4.9%) | 0.358 |
| White/Caucasian | 16 (61.5%) | 100 (72.5%) | 116 (70.7%) | 0.347 |
| Hispanic ethnicity, n (%) | 1 (3.8%) | 27 (19.6%) | 28 (17.1%) | 0.051 |
| Diagnosis, n (%) | ||||
| Spondyloarthritis | — | 70 (50.7%) | 70 (42.7%) | |
| Rheumatoid arthritis | — | 68 (49.3%) | 68 (41.5%) | |
| Healthy | 26 (100.0%) | 0 (0.0%) | 26 (15.9%) | |
| Prior COVID-19 infection, n (%) | 3 (11.5%) | 26 (18.8%) | 29 (17.7%) | 0.575 |
| Type of vaccine, n (%) | <0.0001 | |||
| BNT162b2 mRNA (Pfizer) | 26 (100.0%) | 88 (63.8%) | 114 (69.5%) | |
| mRNA-1273 (Moderna) | — | 50 (36.2%) | 50 (30.5%) | |
| ANA conversion after vaccine, n (%) | ||||
| BNT162b2 mRNA (Pfizer) | 2 (7.7%) | 10 (11.4%) | 12 (10.5%) | 0.731 |
| mRNA-1273 (Moderna) | — | 5 (10%) | 5 (10%) |
IA: inflammatory arthritis.
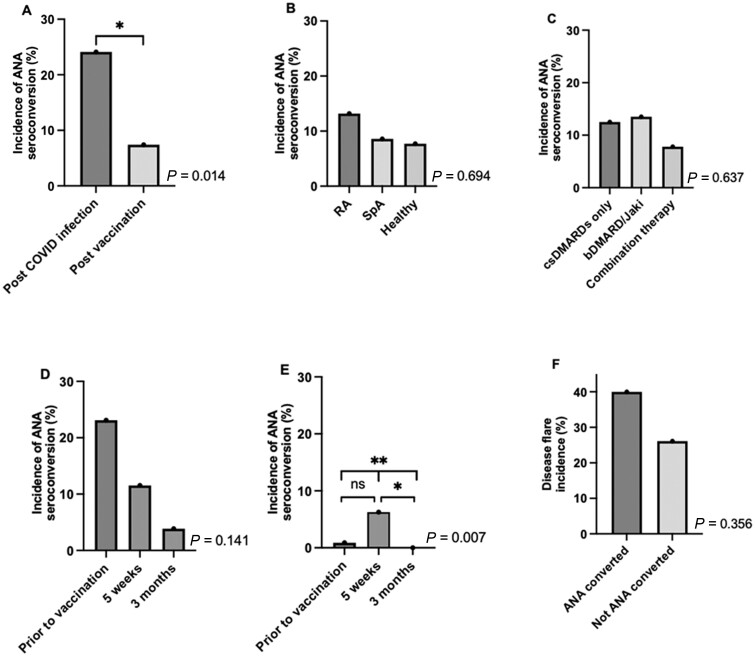
During the follow-up period, 17 of 164 (10.4%) participants developed a positive ANA. The majority of those who had seroconversion of their ANA belonged to the IA group (n = 15/17; 88.2%; Supplementary Table S1, available at Rheumatology online). There was no difference in age, sex, race, ethnicity or disease status between those who converted and those who did not. The incidence of ANA seroconversion in all participants was significantly higher in those who had been previously infected with COVID-19 followed by immunization compared with those who had only been exposed to mRNA-based vaccination [24.1% (n = 7/29) vs 7.4% (n = 10/135), P = 0.014] (Fig. 1A). The incidence of ANA seroconversion in RA participants was numerically (but not significantly) higher than in participants with SpA or healthy controls, (13.2% vs 8.6% vs 7.7% respectively, P = 0.593) (Fig. 1B).
Fig. 1.
Incidence of ANA seroconversion and disease flare
ANA seroconversion incidence in: (A) previously infected healthy control and IA participants compared to those who had not been exposed to natural infection; (B) RA, SpA and healthy controls; and (C) IAs on chronic treatment with csDMARDs, bDMARD/Jaki or combination therapy. ANA seroconversion incidence over time in: (D) previously COVID-19 infected IA participants; and (E) IA participants who hadn’t been exposed to COVID-19 natural infection. ANA incidence is significantly different across all three time-points (P = 0.007) and significantly different between 5-week and 3-month time-points (P = 0.014). (F) Self-reported IA flare incidence in those that ANA seroconverted compared to those that did not. Time-points in (D, E) as follows: prior to vaccination; 5 weeks post first vaccination dose; 3 months post first vaccination dose. Autoantibody conversion status was missing for 14% of participants at 3-month time-point. Status was imputed based on immediately prior or 6-month visit. P-values for overall homogeneity across groups were performed by Fisher’s exact test and reported for (A)–(F). *P < 0.05; **P < 0.01.
We next focussed on ANA seroconversion in participants with IA. ANA seroconversion rate was similar across medication classes [conventional-synthetic DMARDs (csDMARDs), biologic DMARDs (bDMARD)/Janus kinase inhibitors (JAKi) or both] (Fig. 1C). Of IA participants who had previous COVID-19 infection, 23.1% (n = 6) had positive ANAs prior to vaccination, which decreased to 3.9% (n = 1) by 3 months (Fig. 1D). ANA seroconversion in COVID-19 naive IA patients peaked by five weeks post vaccination (6.3%, n = 7) and ANA positivity completely resolved by 3 months post vaccination (Fig. 1E). In fact, ANA antibodies were no longer present in all but one IA participant 3 months following vaccination. For those who seroconverted, ANA levels were generally of low titre; additionally, there was no difference in spike IgG titres between those who seroconverted and those who did not at any time-point (data not shown). Self-reported disease flare incidence was higher but not statistically different in IAs that ANA seroconverted compared with those who did not [40.0% (n = 6/15) vs 26.1% (n = 30/115), P = 0.356] (Fig. 1F). Overall, self-reported disease flare incidence after vaccination was 27.7% and did not differ between RA and SpA (data not shown).
For all participants that ANA seroconverted at any time-point, we further evaluated antibodies to dsDNA, Sm, RNP, SSA, SSB, Scl-70, CNP and Jo-1 from pre-immunization and up to 3 months post immunization. Of the 17 participants that ANA seroconverted, five (29.4%) did not produce any other autoantibodies tested, five (29.4%) participants produced one additional autoantibody and seven (41.2%) produced at least two (and up to eight) autoantibodies at some time-point after ANA seroconversion (Supplementary Fig. S1, available at Rheumatology online).
We also determined the incidence of anti-CCP antibody seroconversion in healthy control and IA participants after COVID-19 infection and/or immunization. Six (4.9%) participants demonstrated anti-CCP seroconversion at any time point after infection or immunization. There was a trend towards higher incidence in healthy controls compared with IA (11.5% vs 3.1%, respectively, P = 0.110) (Supplementary Table S2, available at Rheumatology online). By 3 months post-COVID-19 mRNA vaccination, all participants became anti-CCP negative, suggesting transience of this autoantibody as well (Supplemental Fig. S2, available at Rheumatology online). Notably, none of the 17 ANA seroconverters became anti-CCP positive during follow-up, suggesting a lack of correlation between anti-CCP and ANA seropositivity (data not shown).
Discussion
Similar to previously published data observing a high occurrence of autoantibody production in hospitalized COVID-19 infected patients [1–3], in our cohort the incidence of ANA seroconversion in those who were previously infected with COVID-19 was also elevated (24.1%). In fact, Chang et al. found that up to 49% of hospitalized patients had at least one autoantibody as assayed by 53-plex COVID-19 autoantigen array [3]. Conversely, the incidence of ANA seroconversion in the WARCOV/SAGA cohort following mRNA COVID-19 vaccination was relatively low (7.4%), suggesting a lower likelihood of autoantibody production after immunization compared with natural infection.
Importantly, ANA seroconversion in the NYU WARCOV/SAGA IA participants was transient following either natural infection and/or vaccination. However, Bhadelia et al. reported that autoantibodies can persist for up to 7 months after even mild infection, raising a question as to whether they could contribute to development of future autoimmunity [13]. Reassuringly, in our cohort, there was no significant difference in self-reported flares after immunization between those that ANA seroconverted and those that did not. However, the overall flare incidence was ∼26%; higher than that reported by Connolly et al. who observed flares (requiring treatment) in 11% of their rheumatic and musculoskeletal disease (RMD) cohort [14]. This discrepancy may be related to the relatively small sample size in both studies, the self-reporting nature of disease flare and the inclusion of events that did not require additional treatment in our cohort. Although there is conflicting data regarding whether COVID-19 infection can lead to the development of new onset RMD [15–18], our study results do not support the notion that ANA production is associated with disease flares in patients with IA.
Further, we did not find an association between strength of humoral response (i.e. spike IgG titers) in response to vaccine and/or natural infection and ANA seroconversion. Similarly, there was no significant difference in the incidence of ANA seroconversion between RA and SpA patients. Of those that ANA seroconverted, 70.6% produced at least one other autoantibody at the same or a later time-point. Interestingly, we observed that healthy controls may have a higher incidence of anti-CCP conversion compared with IA, although this observation is likely due to small sample size and the elimination from our analysis of IA with positive CCP at baseline.
Limitations of our study include a relatively small sample size (particularly for the healthy control group), a 12.8% attrition rate by three months after immunization, the use of semiquantitative ELISA for ANA measurement and the imputation of negative ANA status prior to SARS-CoV-2 infection in participants for whom serum samples were not available prior to infection. Additionally, robust assessment of disease activity scores was hindered by the protocol’s optional nature of physical exam at each time-point.
In summary, in a prospective cohort of patients with IA and healthy controls we observed a low and transient incidence of autoantibody seroconversion after COVID-19 mRNA vaccination. ANA seropositivity incidence was significantly higher after natural SARS-CoV-2 infection than following COVID-19 vaccination, providing further confirmation that mRNA-based immunization is safe for patients with IA. Larger studies are needed to validate these observations, especially in more diverse populations (both geographically as well as with other rheumatic diagnoses).
Supplementary Material
Acknowledgements
We would like to thank our patients and their families for participating in this study. We are grateful to Luz Alvarado, Rhina Medina, and Jyoti Patel for coordinating and laboratory efforts.
Funding: This work is supported by National Institute of Health/National Institute of Arthritis and Musculoskeletal and Skin Disease (R01AR074500 to J.U.S., T32-AR-069515 to J.U.S., R.H.H., R.B.B.); Rheumatology Research Foundation (Scientist Development Award to R.H.H.); Further invaluable funding during COVID-19 pandemic was provided to J.U.S. by Bloomberg Philanthropies; Pfizer COVID-19 Competitive Grant Program; The Beatrice Snyder Foundation.
Disclosure statement: J.U.S. declares that he has served as a consultant for Janssen, Novartis, Pfizer, Sanofi, BMS, Amgen, UCB and Abbvie and has received funding for investigator-initiated studies from Janssen and Pfizer. R.H.H. declares consulting fees from Janssen. G.S. declares consulting fees from AbbVie. S.R. declares consulting fees from Amgen, Janssen, Novartis and Pfizer. P.I. declares consulting fees from GSK and Janssen.
Data availability statement
The data underlying this article are available in the article and in its online supplementary material.
Supplementary data
Supplementary data are available at Rheumatology online.
Contributor Information
Rebecca B Blank, Division of Rheumatology.
Rebecca H Haberman, Division of Rheumatology.
Kun Qian, Division of Biostatistics, Department of Population Health.
Marie Samanovic, NYU Langone Vaccine Center, NYU School of Medicine, New York, NY, USA.
Rochelle Castillo, Division of Rheumatology.
Anthony Jimenez Hernandez, Division of Rheumatology.
Parvathy Vasudevapillai Girija, Division of Rheumatology.
Sydney Catron, Division of Rheumatology.
Zakwan Uddin, Division of Rheumatology.
Paula Rackoff, Division of Rheumatology.
Gary Solomon, Division of Rheumatology.
Natalie Azar, Division of Rheumatology.
Pamela Rosenthal, Division of Rheumatology.
Peter Izmirly, Division of Rheumatology.
Jonathan Samuels, Division of Rheumatology.
Brian Golden, Division of Rheumatology.
Soumya Reddy, Division of Rheumatology.
Mark J Mulligan, NYU Langone Vaccine Center, NYU School of Medicine, New York, NY, USA.
Jiyuan Hu, Division of Biostatistics, Department of Population Health.
Jose U Scher, Division of Rheumatology.
References
- 1. Woodruff MC, Ramonell RP, Lee FEH, Sanz I.. Clinically identifiable autoreactivity is common in severe SARS-CoV-2 infection. medRxiv 2020;https://doi.org/10.1101/2020.10.21.20216192; preprint: not peer reviewed. [Google Scholar]
- 2. Zuo Y, Estes SK, Ali RA. et al. Prothrombotic autoantibodies in serum from patients hospitalized with COVID-19. Sci Transl Med 2020;12:eabd3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chang SE, Feng A, Meng W. et al. New-onset IgG autoantibodies in hospitalized patients with COVID-19. Nat Commun 2021;12:5417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pascolini S, Vannini A, Deleonardi G. et al. COVID-19 and immunological dysregulation: can autoantibodies be useful? Clin Transl Sci 2021;14:502–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bertin D, Brodovitch A, Beziane A. et al. Anticardiolipin IgG autoantibody level is an independent risk factor for COVID-19 severity. Arthritis Rheumatol 2020;72:1953–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Trahtemberg U, Rottapel R, Santos CC. D. et al. Anti-cardiolipin and other anti-phospholipid antibodies in critically ill COVID-19 positive and negative patients. Ann Rheum Dis 2021;80:1236–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gomes C, Zuniga M, Crotty KA. et al. Autoimmune anti-DNA and anti-phosphatidylserine antibodies predict development of severe COVID-19. Life Sci Alliance 2021;4:e202101180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Saad MA, Alfishawy M, Nassar M. et al. COVID-19 and autoimmune diseases: a systematic review of reported cases. Curr Rheumatol Rev 2020;17:193–204. [DOI] [PubMed] [Google Scholar]
- 9. Rivera-Correa J, Rodriguez A.. Divergent roles of antiself antibodies during infection. Trends Immunol 2018;39:515–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Farroni C, Picchianti-Diamanti A, Aiello A. et al. Kinetics of the B- and T-cell immune responses after 6 months from SARS-CoV-2 mRNA vaccination in patients with rheumatoid arthritis. Front Immunol 2022;13:846753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Haberman R, Axelrad J, Chen A. et al. Covid-19 in immune-mediated inflammatory diseases - case series from New York. N Engl J Med 2020;383:85–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Haberman RH, Herati RS, Simon D. et al. Methotrexate hampers immunogenicity to BNT162B2 mRNA covid-19 vaccine in immune-mediated inflammatory disease. Ann Rheum Dis 2020;80:1339–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bhadelia N, Belkina AC, Olson A. et al. Distinct Autoimmune Antibody Signatures Between Hospitalized Acute COVID-19 Patients, SARS-CoV-2 Convalescent Individuals, and Unexposed Pre-Pandemic Controls. medRxiv. 2021; 10.1101/2021.01.21.21249176; preprint: not peer reviewed. [DOI]
- 14. Connolly CM, Ruddy JA, Boyarsky BJ. et al. Disease flare and reactogenicity in patients with rheumatic and musculoskeletal diseases following two-dose SARS–CoV-2 messenger RNA vaccination. Arthritis Rheumatol 2022;74:28–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mantovani Cardoso E, Hundal J, Feterman D, Magaldi J.. Concomitant new diagnosis of systemic lupus erythematosus and COVID-19 with possible antiphospholipid syndrome. Just a coincidence? A case report and review of intertwining pathophysiology. Clin Rheumatol 2020;39:2811–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hsu TYT, D’Silva KM, Patel NJ. et al. Incident systemic rheumatic disease following COVID-19. Lancet Rheumatol 2021: e402–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zamani B, Moeini Taba SM, Shayestehpour M.. Systemic lupus erythematosus manifestation following COVID-19: a case report. J Med Case Rep 2021;15:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Borghi MO, Beltagy A, Garrafa E. et al. Anti-phospholipid antibodies in COVID-19 are different from those detectable in the anti-phospholipid syndrome. Front Immunol 2020;11:584241. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its online supplementary material.