Abstract
Background
Patients with solid or hematological tumors or neurological and immune-inflammatory disorders are potentially fragile subjects at increased risk of experiencing severe coronavirus disease 2019 and an inadequate response to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccination.
Methods
We designed a prospective Italian multicenter study to assess humoral and T-cell responses to SARS-CoV-2 vaccination in patients (n = 378) with solid tumors (ST), hematological malignancies (HM), neurological disorders (ND), and immunorheumatological diseases (ID). A group of healthy controls was also included. We analyzed the immunogenicity of the primary vaccination schedule and booster dose.
Results
The overall seroconversion rate in patients after 2 doses was 62.1%. Significantly lower rates were observed in HM (52.4%) and ID (51.9%) than in ST (95.6%) and ND (70.7%); a lower median antibody level was detected in HM and ID versus ST and ND (P < .0001). Similar rates of patients with a positive SARS-CoV-2 T-cell response were found in all disease groups, with a higher level observed in ND. The booster dose improved the humoral response in all disease groups, although to a lesser extent in HM patients, whereas the T-cell response increased similarly in all groups. In the multivariable logistic model, independent predictors of seroconversion were disease subgroup, treatment type, and age. Ongoing treatment known to affect the immune system was associated with the worst humoral response to vaccination (P < .0001) but had no effect on T-cell responses.
Conclusions
Immunosuppressive treatment more than disease type per se is a risk factor for a low humoral response after vaccination. The booster dose can improve both humoral and T-cell responses.
Keywords: Fragile patients, SARS-CoV-2 mRNA vaccine, humoral immunity, T-cell immunity
Compared with healthy individuals, fragile patients have a lower rate of humoral and cellular response after primary vaccination schedule and booster dose. The type of immunosuppressive treatment has greater impact on humoral response than disease subtype, but it has a lower influence on T-cell response.
In immunocompromised patients, coronavirus disease 2019 (COVID-19) has been associated with an increased risk of hospitalization and death in comparison with the general population [1–4]. The messenger RNA (mRNA)-1273 (Moderna) and BNT162b2 (Pfizer BioNTech) vaccines have shown high efficacy in preventing COVID-19 in healthy individuals [5, 6]. However, patients with solid tumors (ST), hematological malignancies (HM), and immunorheumatological (ID) and neurological (ND) diseases were not included in pivotal trials. A poor humoral response after natural infection [7] or vaccination [8–16] was reported in patients with malignancies and/or diseases requiring immunosuppressive therapies. This impaired response varies according to the intensity of the immune suppressive treatment. Although data on seroconversion are available, the effectiveness of vaccination on the antigen-specific T-cell response as well as the effect of a booster dose in these fragile populations remains largely unknown [17, 18]. In addition, time-dependent waning of the vaccine-induced immune response [19] has been reported in healthy subjects, highlighting the potential need for a booster [20]. In September 2021, the Italian authorities approved the administration of an additional vaccine dose to fragile patients, including the 4 categories evaluated in the present study.
Thirteen Italian research hospitals conducted a prospective study (VAX4FRAIL) aimed at evaluating the efficacy and safety of mRNA-based vaccination in patients affected by HM, ST, ND, and ID [21]. Here, we present the results on the humoral and T-cell responses after complete mRNA-based vaccination and after the booster dose.
METHODS
Study Design
Between March and August 2021, 570 patients with a diagnosis of HM, ST, ND, or ID were included in the study. The study was approved by the Italian Medicines Agency and by the ethics committee (code 304, 2021). The control group consisted of 180 healthy healthcare workers (HCWs) matched for sex and age. Written informed consent was obtained from all study participants.
Other inclusion criteria were age ≥18 years, mRNA-based vaccination, and a life expectancy of at least 12 months at the time of vaccine administration. The main exclusion criterion was the presence of a previous laboratory-confirmed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection (serology and/or molecular test). Patients experiencing a molecularly confirmed SARS-CoV-2 breakthrough infection (reverse transcriptase-polymerase chain reaction assay) or seroconversion to anti-Nucleocapsid antibody during follow-up were also excluded (n = 16). Given the disease heterogeneity of the study population, patients were subdivided into 4 subgroups according to the expected immune impairment attributable to their immunosuppressive treatment (detailed in the Supplementary Materials).
In September 2021, the Italian authorities approved for immunocompromised patients an additional dose (booster) to be administered at least 28 days after the second dose. Because most patients in our study were vaccinated between March and April 2021 and the administration of the third dose started in September 2021, the median interval between the second and third dose was 5 months.
Laboratory Procedures
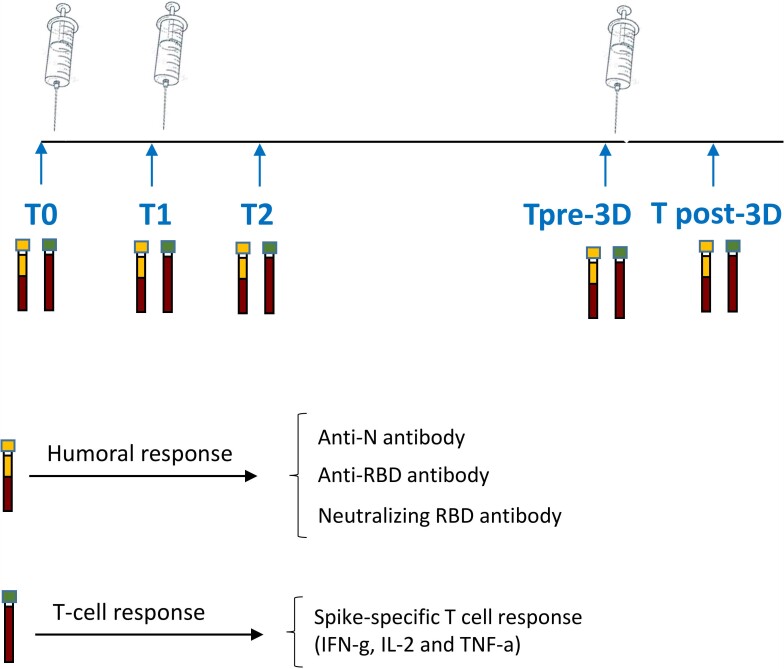
Anti-Spike SARS-CoV-2 antibodies and T-cell response were monitored at 5 time points (Figure 1): day of first dose administration (T0); day of second dose administration (T1); 5–7 weeks after T0 in patients receiving the Pfizer/BioNTech vaccine and 6–8 weeks after T0 in patients receiving the Moderna vaccine (T2); day of the booster dose (pre-3rd dose, T pre-3D); and 3 or 4 weeks after (T post-3D).
Figure 1.
Study design. Schematic representation of the timeline of immune monitoring of the clinical study. Abbreviations: RBD, receptor binding domain; T0, before vaccination; T1, after 3–4 weeks from T0; T2, 5–8 weeks from T0; Tpre-3D. 5 months from T2; Tpost-3D, 2-4 weeks from Tpre-3D.
The primary endpoint of the study was the seroconversion rate assessed at T2 in patients compared with HCWs. Secondary endpoints were the humoral and cellular responses at each time point in the fragile population compared with the HCW group. The immune response was evaluated also in disease- and treatment-specific subgroups. A final secondary endpoint was the neutralization activity of vaccine-induced anti-Spike antibodies. The humoral response was analyzed by quantifying anti-N-protein immunoglobulin G and anti-receptor binding domain (RBD) immunoglobulin G (Architect i2000sr, Abbott Diagnostics, Chicago, IL, USA). A neutralization assay was performed on anti-RBD-positive samples to evaluate the functional activity of vaccination-induced anti-Spike antibodies.
The T-cell response to vaccination was assessed through a standardized whole-blood assay as previously described [22] and detailed in the Supplementary Materials.
Statistical Methods
Quantitative variables were summarized as median and interquartile range (IQR), whereas categorical variables were reported as absolute count and percentage. Differences in seroconversion rates across subgroups were analyzed using the χ2 test, and from a multivariable logistic regression model we obtained the odds ratios with their 95% confidence intervals (CIs). The model outcome was the seroconversion status at T2 (yes vs no), and independent variables were identified on the basis of availability as required by the study protocol (disease, age, sex, comorbidities, and therapy) and used to adjust the vaccination effect on outcome. Current therapy was classified into 4 classes (no therapy and 3 groups) according to the presumed treatment-induced immunosuppression.
The Mann-Whitney test was used to assess differences in antibody titers and correlations between humoral and cellular immunity were evaluated with Spearman’s rho correlation coefficient.
The SPSS v.20.0 (IBM) statistical software was used for the analysis.
RESULTS
Patient Characteristics
Between March and August 2021, 570 patients and 180 HCWs were enrolled in the VAX4FRAIL study; 465 patients received the BNT162b2 and 105 the mRNA-1273 vaccine. One hundred and ninety-five of 570 patients were excluded because they did not meet the inclusion criteria or because the samples were not collected at all prespecified timepoints. Our analysis was therefore conducted on a final cohort of 375 patients; the median age was 59 years (range, 19–86) and 209 patients (55.7%) were women. One hundred patients (26.7%) had HM, 114 (30.4%) ST, 79 (21.0%) ID, and 82 (21.9%) ND (Table 1).
Table 1.
Demographic and Clinical Features of Enrolled Patients
| HM (n = 100) | ST (n = 114) | ID (n = 79) | ND (n = 82) | |
|---|---|---|---|---|
| Sex (no., %) | ||||
| Male | 54 (54.0%) | 48 (42.1%) | 32 (40.5) | 32 (39.0) |
| Female | 46 (46.0%) | 66 (57.9%) | 47 (59.5) | 50 (61.0) |
| Age (median, IQR) | 61 (52–69) | 62 (54–70) | 58 (48–64) | 55 (39–65) |
| Comorbidities (no., %)a | ||||
| Yes | 63 (63.6%) | 66 (62.3%) | 44 (55.7%) | 38 (46.3%) |
| Metabolic | 17 (17.0%) | 20 (17.5%) | 14 (17.7%) | 4 (4.9%) |
| Cardiological | 35 (35.0%) | 42 (36.8%) | 12 (15.2%) | 21 (25.6%) |
| Pneumological | 5 (5.0%) | 5 (4.4%) | 17 (21.5%) | 9 (11.0%) |
| Other | 39 (39.0%) | 39 (34.2%) | 28 (35.4%) | 24 (29.3%) |
Abbreviations: HM, hematological malignancies; ID, immunorheumatological diseases; ND, neurological disorders; ST, solid tumors.
Comorbidities include metabolic, cardiological, pneumological, and other relevant diseases.
Impact of Different Diseases on Humoral Response to Vaccination
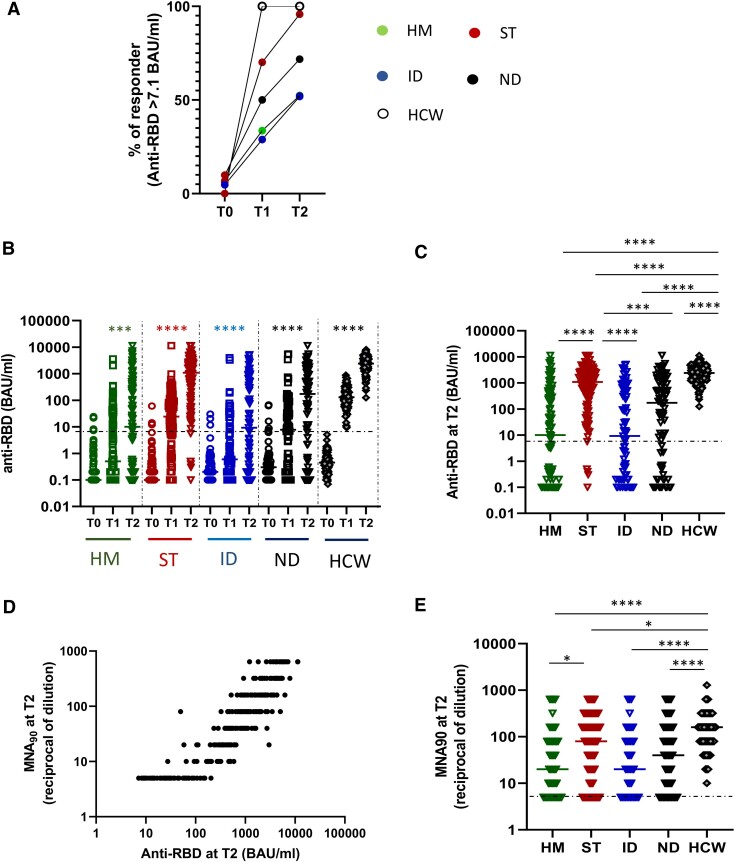
Overall, a significantly lower proportion of patients (69.1%; 95% CI, 64.4–73.7) compared with HCWs (100%, P < .00001) seroconverted after the second dose (T2). Similarly, we reported a lower median titer of anti-RBD antibodies at T2 in patients compared with HCWs (patient median: 172.8 [IQR: 0.7–1387.0] vs HCW median: 2405 [IQR: 1343.0–3848.0], P < .0001).
The disease groups showed different humoral response kinetics, leading to a different frequency of responder patients as described in Figure 2A. Specifically, patients affected by HM and ID had a significantly lower seroconversion rate at T2 (52.4%; 95% CI, 42.2–61.8 and 51.9%; 95% CI, 39.6–61.6, respectively) compared with ST and ND patients (95.6%; 95% CI, 92.4–98.8 and 70.7%; 95% CI, 60.9–80.6, respectively, P < .00001) and HCWs (100%).
Figure 2.
Impact of different diseases on humoral response. A, The percentage of patients (HM, green dot; ST, red dots; ID, blue dots; ND, black dots; HCW, white dots) presenting a positive anti-RBD response (>7.1 BAU/mL) at each time point (T0, T1, and T2) is shown. B, Kinetics of humoral immune response before and after vaccination in HM (green dots), ST (red dots), ID (blue dots), ND (black dots), and HCW (gray dots). SARS-CoV-2 specific anti-RBD Abs were measured in sera samples at each time point. Anti-RBD-immunoglobulin G are expressed as BAU/mL and values >7.1 BAU/mL are considered positive. Differences were evaluated by Friedman paired test. ****P < .0001. C, The level of anti-RBD antibodies at T2 was compared among groups and was expressed as BAU/mL. Differences were evaluated by Kruskal-Wallis test. *P < .05; **P < .01; ***P < .001; ****P < .0001. HM: median = 10.0 BAU/mL (IQR 0.1–392.5 BAU/mL); ST: 1094.6 BAU/mL (IQR 265.1–2697.9 BAU/mL); ID: 9.2 BAU/mL (IQR 0.2–503.8 BAU/mL); NT: 172.9 BAU/mL (IQR 1.7–1457.8 BAU/mL) and HCW: 2405.0 BAU/mL (IQR 1343–3848 BAU/mL, respectively). D, The correlation between the levels of anti-RBD and neutralization titer at T2 for all fragile patients are shown. Each black dot represents one sample. Spearman test: rho = 0.9202, P < .0001. E, The levels of neutralizing antibody at T2 were quantified by microneutralization assay (MNA90) in all groups and were expressed as reciprocal of dilution. Differences were evaluated by Kruskal-Wallis test. *P < .05; ****P < .0001. HM: median = 20 reciprocal of dilution (IQR 5–80); ST: 80 reciprocal of dilution (IQR 20–240); ID: 20 reciprocal of dilution (IQR 5–80); NT: 40 reciprocal of dilution (IQR 8.75–160) and HCW: 160 (IQR 80–320). Abbreviations: Abs, antibodies; HCW, health care workers; HM, hematological malignancies; ID, immune-rheumatological diseases; IQR, interquartile range; ND, neurological disorders; RBD, receptor binding domain; SARS-CoV-2, severe acute respiratory syndrome 2 virus; ST, solid tumors; T0, before vaccination; T1, after 3–4 weeks from T0; T2, 5–8 weeks from T0.
In each patient group, vaccination stimulated a humoral response with a significant increase in anti-RBD antibodies (P < .0001 for each group, Figure 2B). However, a lower titer at T2 was observed in the HM and ID groups compared with ST, ND, and HCWs (P < .001, Figure 2C). We therefore checked the neutralizing activity against SARS-CoV-2 infectivity in a BSL-3 facility. This assay was performed on anti-RBD–positive samples. The percentage of patients showing neutralizing activity at T2 was 73% (HM), 80.7% (ST), 69.2% (ID), and 74.9% (ND) vs 100% in HCWs. A positive correlation between anti-RBD titer and neutralization was observed (rho = 0.92, P < .0001, Figure 2D). All patients had a significantly lower titer of neutralizing antibodies than HCWs, and the values in HM patients were lower than in ST patients (P < .0001; Figure 2E).
HM patients treated with B-cell–depleting therapies had the lowest seroconversion rate (0%) and the lowest median antibody titer (0.01 BAU/mL, IQR 0.01–0.04). In the ID subgroups, the lowest antibody levels were detected in patients with antineutrophil cytoplasmic antibody-associated vasculitis or interstitial lung disease undergoing treatment with anti-CD20 monoclonal antibodies with or without corticosteroids (25.0%; 95% CI, 11.6–38.4 and median = 0.02 BAU/mL, IQR 0.01–0.06). Among ND patients, the lowest humoral response rate was documented in individuals with multiple sclerosis receiving anti-CD20 monoclonal antibodies, with a seroconversion rate of 39.4% (95% CI, 22.7–51.1) and a median antibody titer of 0.03 U/mL (IQR 0.01–0.10).
Disease Subgroups and T-cell Response to Vaccination
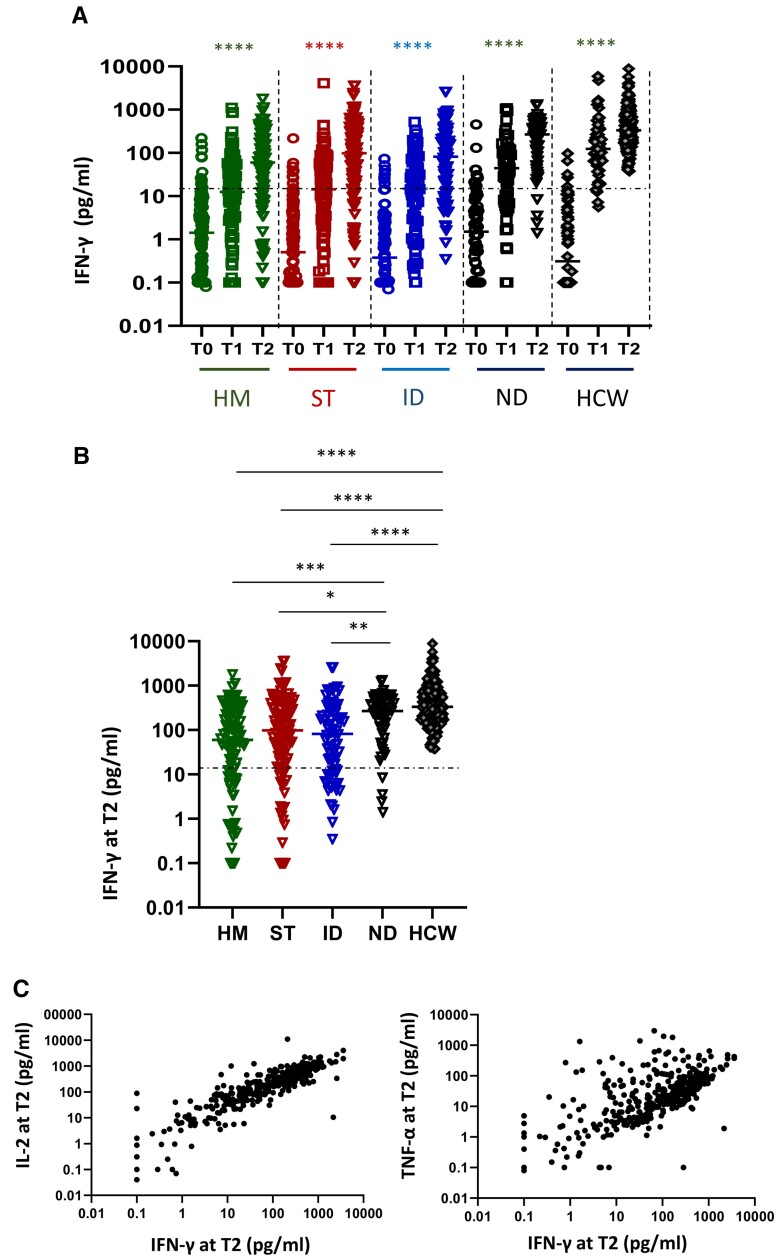
A lower frequency of spike-specific T-cell responses (defined as interferon-γ [IFN-γ] levels ≥12 pg/mL) was detected in patients compared with HCWs (80.0%; 95% CI, 75.9–84.0 vs 100%, P < .001). A T-cell response was observed in 218 (84.2%) patients also having antibodies and in 82 (70.7%) who did not seroconvert (P = .003). Only 34 (9.1%) patients were negative for both types of immune response.
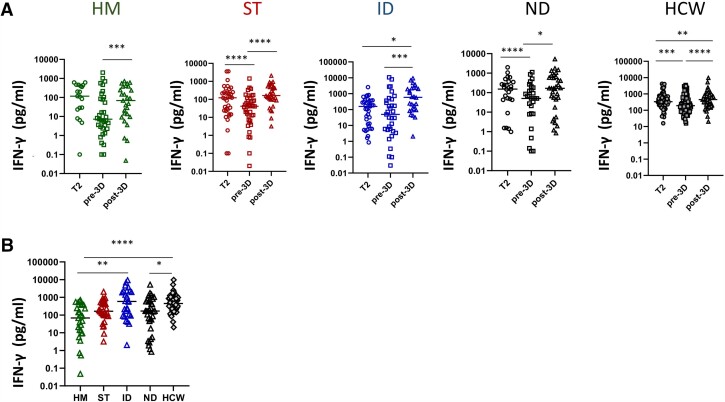
The level of T-cell response significantly increased over time in patient groups as well as in HCWs (P < .0001, Figure 3A). Nevertheless, HM, ST, and ID patients had significantly lower IFN-γ production at T2 than ND patients and HCWs (P < .05, Figure 3B). The T-cell response in the ND group was similar to that of HCWs. Among fragile patients, IFN-γ values were directly correlated with interleukin-2 and tumor necrosis factor-α levels (rho = 0.87 and rho = 0.63, P < .0001 for both), suggesting a coordinated T-cell response to vaccination (Figure 3C). A significant correlation between anti-RBD and T-cell response was observed in HCWs (P = .0016, r = 0.2334) but not in fragile patients (P = .1429, r = 0.1406).
Figure 3.
Impact of different diseases on T-cell response. A, Kinetic of T-cell response before and after vaccination in HM (green dots), ST (red dots), ID (blue dots), ND (black dots), and HCW (gray dots). Spike-specific T-cell response was measured after stimulation of whole blood with specific peptides at each time point. T-cell response was expressed as pg/mL of IFN-γ and values >12 pg/mL are considered positive. Differences were evaluated by Friedman paired test. ****P < .0001. B, The level of T cell response at T2 was compared among groups and was expressed as pg/mL of IFN-γ. Differences were evaluated by Kruskal-Wallis test. *P < .05; **P < .01; ***P < .001; ****P < .0001. HM: median = 60.2 pg/mL (IQR 9.4–247.2 pg/mL); ST: 98.6 pg/mL (IQR 18.9–335.1 pg/mL); ID: 81.8 pg/mL (IQR 12.1–284.1 pg/mL); NT: 268.5 pg/mL (IQR 107.6–505.5 pg/mL) and HCW: 331.9 pg/mL (IQR 189.9–765.0 pg/mL, respectively). C: The correlation between the levels of IFN-γ and interleukin-2 or IFN-γ and tumor necrosis factor-α at T2 for all fragile patients are shown. Each black dot represents one sample. Spearman test: rho = 0.8739 and .6368, P < .0001. Abbreviations: HCW, health care workers; HM, hematological malignancies; ID, immune-rheumatological diseases; ND, neurological disorders; ST, solid tumors; T0, before vaccination; T1, after 3–4 weeks from T0; T2, 5–8 weeks from T0.
Booster Dose Effect on B-cell and T-cell Response
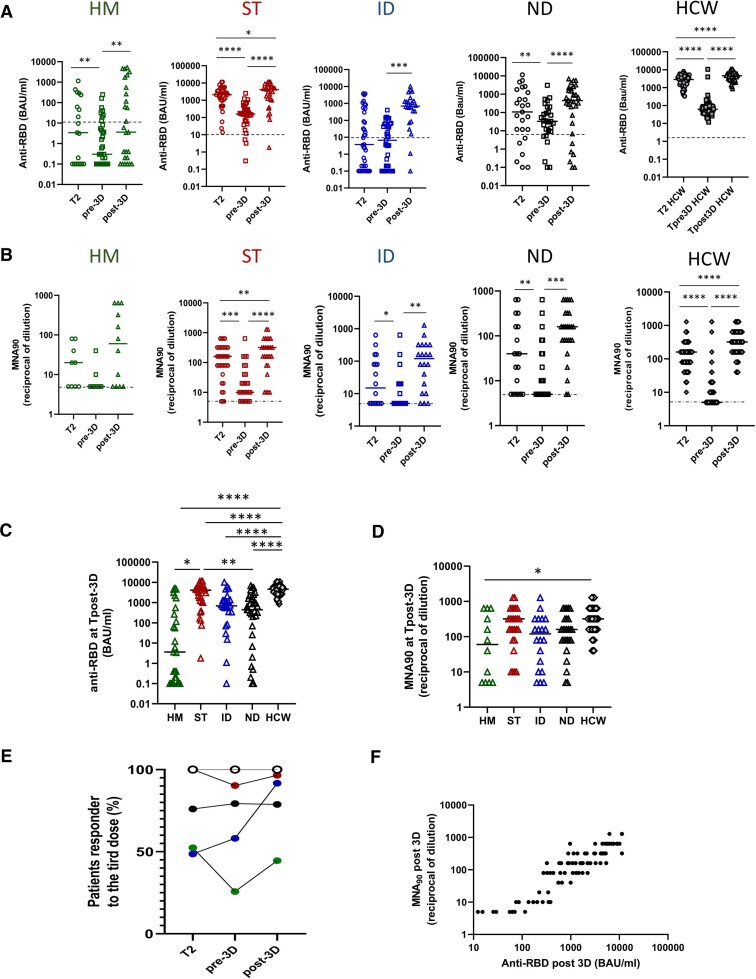
The median interval between the second and third vaccine dose was 5 months in patients and 8 months in HCWs. Samples before (T pre-3D) and after (T post-3D), the third dose was collected in a cohort of 120 patients (HM = 19, ST = 37, ID = 37, and ND = 27). The median humoral and T-cell response levels before and after the booster dose were compared with those of 67 HCWs.
The antibody level decreased after the second dose of vaccine in patient groups as well as in HCWs (P < .001) except for ID patients, in whom the level was maintained (Figure 4A). HM patients had the lowest antibody titer, and a large proportion became seronegative (74.3%). The median fold decrease in anti-RBD antibodies between T2 and T pre-3D was 3.1, 6.6, 1, and 1.4 for HM, ST, ID, and ND, respectively. Comparison between groups highlighted the lowest anti-RBD titer in HM versus all other groups (HM: 0.3 BAU/mL [IQR 0.1–8.1]; ST: 158.1 BAU/mL [IQR 58.0–444.6]; ID: 6.6 BAU/mL [IQR: 0.2–59.9]; ND: 32.6 BAU/mL [IQR: 9.3–151.9], P < .0001). The neutralization test performed on anti-RBD-positive samples also confirmed a significant reduction of protective antibodies in all groups (Figure 4B).
Figure 4.
Kinetic of humoral response after 2 and 3 doses of vaccine in diseases groups. A, The level of the anti-RBD antibody was compared in HM (green dots), ST (red dots), ID (blue dots), ND (black dots), and HCW (gray dots) at 3 different time points: after 2 doses (T2) and before (pre-3D) and after (post-3D) the booster dose. Differences were evaluated by Wilcoxon paired test. HM: median T2: 3.4 BAU/mL (IQR 0.1-238.8 BAU/mL); median pre-3D: 0.3 BAU/mL (IQR 0.1-8.1 BAU/mL); median post-3D: 3.6 BAU/mL (IQR 0.1-555.6 BAU/mL). **P < .001. ST: median T2: 2089 BAU/mL (IQR 956.7–3652.0 BAU/mL); median pre-3D: 158.1 BAU/mL (IQR 58.0–444.6 BAU/mL); median post-3D: 4093 BAU/mL (IQR 1051.0–5769.0 BAU/mL). *P < .05; ****P < .0001. ID: median T2: 3.7 BAU/mL (IQR 0.15–400.4 BAU/mL); median pre-3D: 6.6 BAU/mL (IQR 0.2–59.9 BAU/mL); median post-3D: 694.0 BAU/mL (IQR 150.0–1356.0 BAU/mL). ***P < .001. ND: median T2: 107.0 BAU/mL (IQR 7.8–1510.0 BAU/mL); median pre-3D: 32.6 BAU/mL (IQR 9.3–151.9 BAU/mL); median post-3D: 443.0 BAU/mL (IQR 48.0–1770.0 BAU/mL). **P < .01, ****P < .0001. HCW: median T2: 2646.0 BAU/mL (IQR 1529.0–3958.0 BAU/mL); median pre-3D: 60.20 BAU/mL (IQR 39.3–93.5BAU/mL); median post-3D: 4608.0 BAU/mL (IQR 3302.0-6030.0 BAU/mL). ****P < .0001. B, The level of the neutralizing antibody was compared in HM (green dots), ST (red dots), ID (blue dots), ND (black dots), and HCW (gray dots) at 3 different time points: after 2 doses (T2) and before (pre-3D) and after (post-3D) the booster dose. Differences were evaluated by Wilcoxon paired test. HM: median T2: 20 reciprocal of dilution (IQR 5–60); median pre-3D: 5 reciprocal of dilution (IQR 5–7.5); median post-3D: 60 reciprocal of dilution (IQR 5–560). ST: median T2: 160 reciprocal of dilution (IQR 80–320); median pre-3D: 10 reciprocal of dilution (IQR 6.2–40.0); median post-3D: 320 reciprocal of dilution (IQR 80–640). **P < .01, ***P < .001, ****P < .0001. ID: median T2: 15 reciprocal of dilution (IQR 5–100); median pre-3D: 5 reciprocal of dilution (IQR 5–20); median post-3D: 120 reciprocal of dilution (IQR 12.5–320). *P < .05, **P < .01. ND: median T2: 40 reciprocal of dilution (IQR 5–160); median pre-3D: 5 reciprocal of dilution (IQR 5–40); median post-3D: 160 reciprocal of dilution (IQR 80–320). **P < .01, ***P < .001. HCW: median T2: 160 reciprocal of dilution (IQR 80–160); median pre-3D: 5 reciprocal of dilution (IQR 5–10); median post-3D: 320 reciprocal of dilution (IQR 160-640). **** P < .0001. C, The level of anti-RBD antibodies at Tpost-3D was compared among groups and was expressed as BAU/mL. Differences were evaluated by Kruskal-Wallis test. *P < .05; **P < .01; ****P < .0001. HM: median = 3.6 BAU/mL (IQR .1-555.6 BAU/mL); ST: 4093.0 BAU/mL (IQR 1051.0–5769.0 BAU/mL); ID: 694.0 BAU/mL (IQR 150.0–1356.0 BAU/mL); ND: 443 BAU/mL (IQR 48.1–1770.0 BAU/mL) and HCW: 4608.0 BAU/mL (IQR 3302.0–6030.0 BAU/mL, respectively). D, The level of neutralizing antibodies at Tpost-3D was compared among groups and was expressed as reciprocal of dilution. Differences were evaluated by Kruskal-Wallis test. *P < .05. HM: median = 60 reciprocal of dilution (IQR 5–560); ST: 320 reciprocal of dilution (IQR 80–640); ID: 120 reciprocal of dilution (IQR 12.5–320); ND: 160 reciprocal of dilution (IQR 180–320) and HCW: 320 reciprocal of dilution (IQR 160–640, respectively). E, The percentage of patients (HM, green dot; ST, red dots; ID, blue dots; ND, black dots; HCW, white dots) presenting a positive anti-RBD response (>7.1 BAU/mL) at T2, before (pre-3D) and after (post-3D) the booster dose is shown. F, The correlation between the levels of anti-RBD and neutralization titer at T post-3D for all fragile patients is shown. Each black dot represents 1 sample. Spearman test: rho: .8965, P < .0001). Abbreviations: HCW, health care workers; HM, hematological malignancies; ID, immune-rheumatological diseases; IQR, interquartile range; ND, neurological disorders; ST, solid tumors; T2: 5–8 weeks from T0; Tpre-3D, 5 months from T2; Tpost-3D, 2–4 weeks from Tpre-3D.
After the third dose, the prevalence of anti-SARS-CoV-2 antibodies in the entire population showed a slight, nonsignificant increase from 67.0% (95% CI, 57.4–76.7) to 81.5% (95% CI, 73.0–89.9). In particular, 33% of patients not responding to the first 2 doses seroconverted after the third dose. The third dose was effective in increasing the anti-RBD titer in all groups, although with different strengths (Figure 4A). In HM patients, the seroconversion rate was persistently low (44.5%), whereas in ID patients it increased markedly, reaching 90% (Figure 4E). Similar results were seen in the evaluation of neutralization (Figure 5B). Specifically, the neutralizing titers after the third dose showed a positive correlation with anti-RBD data (rho = 0.8965, P < .0001, Figure 4F) and a significant improvement in all groups (P < .01) except for HM (Figure 4B). Compared with HCWs, all patient groups showed lower anti-RBD titers (Figure 4C). The neutralization titers of patients were comparable to those of HCWs in all groups except HM.
Figure 5.
Kinetics of T-cell response after 2 and 3 doses of vaccine in diseases groups. A, S-specific T-cell response expressed as pg/mL of IFN-γ was quantified before and after the booster dose in HM, ST, ID, ND and HCW. Differences were evaluated by Wilcoxon paired test. HM: median T2: 116.5 pg/mL (IQR 23.2–436.6 pg/mL); median pre-3D: 7.24 pg/mL (IQR 2.3–94.6 pg/mL); median post-3D: 68.5 pg/mL (IQR 9.5–378.3 pg/mL). *** P < .001. ST: median T2: 122.8 pg/mL (IQR 23.0–250.8 pg/mL); median pre-3D: 41.3 pg/mL (IQR 10.0–104.7 pg/mL); median post-3D: 163.1 pg/mL (IQR 95.2–522.7 pg/mL). ****P < .0001. ID: median T2: 158.45 pg/mL (IQR 10.6–354.8 pg/mL); median pre-3D: 49.4 pg/mL (IQR 4.8–402.3 pg/mL); median post-3D: 590.2 pg/mL (IQR 92.4–2223.0 pg/mL). *P < .05, ***P < .001. ND: median T2: 152.8 pg/mL (IQR 42.9–358.6 pg/mL); median pre-3D: 50.6 pg/mL (IQR 8.3-170.3 pg/mL); median post-3D: 167.5 pg/ml (IQR 31.6-624.5 pg/mL). *P < .05, ***P < .001. HCW: median T2: 335.9 pg/mL (IQR 199.0–679.0 pg/mL); median pre-3D: 190.8 pg/mL (IQR 88.7–437.2 pg/ml); median post-3D: 448.9 pg/mL (IQR 197.3–862.2 pg/mL). **P < .01, ***P < .001, ****P < .0001. B, S-specific T-cell response at Tpost-3D was compared among groups and was expressed as pg/mL. Differences were evaluated by Kruskal-Wallis test. *P < .05; **P < .01; ****P < .0001. HM: median = 68.6 pg/mL (IQR 9.5–378.3 pg/mL); ST: 163.1 pg/mL (IQR 95.2–522.7 pg/mL); ID: 590.2 pg/mL (IQR 92.4–2223.0 pg/mL); ND: 167.5 pg/mL (IQR 31.6–624.5 pg/mL) and HCW: 448.9 pg/mL (IQR 197.3–852.2 pg/mL). Abbreviations: HCW, health care workers; HM, hematological malignancies; ID, immune-rheumatological diseases; IQR. interquartile range; ND, neurological disorders; ST, solid tumors; T2, 5–8 weeks from T0; Tpre-3D, 5 months from T2; Tpost-3D, 2–4 weeks from Tpre-3D.
The T-cell response after the first 2 doses decreased over time in all groups, but more significantly in ST and ND (P < .01, Figure 5A). The median fold decrease in T-cell response (level of IFN-γ) between T2 and T pre-3D was 1.9, 2.1, 0.6, and 2.5 for HM, ST, ID, and ND, respectively. Nevertheless, the median T-cell response level before the booster dose was not different across groups (P = .2366). The third dose was able to improve the T-cell response in all groups (P < .0001). A significantly lower T-cell response was observed in HM and ND patients, whereas ST and ID patients showed an activity similar to that of HCWs (Figure 5B). Of note, in patients receiving the third dose the percentage of double-negatives, defined as individuals failing to develop both B-cell and T-cell responses to vaccination, decreased from 7.8% to 1.3%.
Impact of Different Treatments on Response to Vaccination
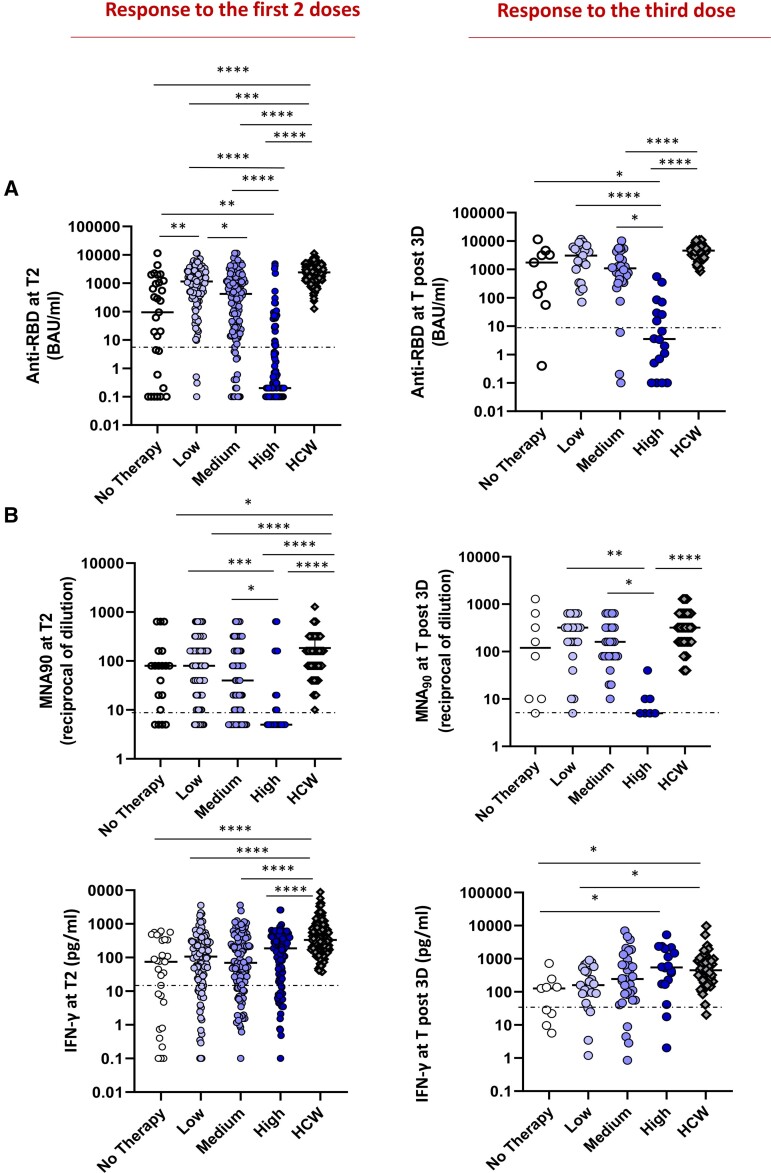
Humoral and T-cell responses were then evaluated in patients according to the received or ongoing treatment and its presumed immunosuppression (Table 2). The treatments of the high-risk group (ie, those with predicted high immunosuppressive activity) were associated with a markedly lower humoral response to the first 2 doses (22.9%; 95% CI, 15.0–30.8) than in the intermediate- and low-risk groups (84.2%; 95% CI, 77.6–90.7, P < .0001, and 97.3%; 95% CI, 94.3–100, P < .0001, respectively). Similar results were obtained for the response to the third dose: the high-risk treatment group showed a lower humoral response (41.2%) than the intermediate- and low-risk groups (90.0%, P = .0003 and 100%, P < .0001).
Table 2.
Patients Were Grouped into 4 Different Subgroups According to Expected Immune Impairment Attributable to Their Immunosuppressive Treatment
| HM (n = 100) | ST (n = 114) | ID (n = 79) | ND (n = 82) | |
|---|---|---|---|---|
| No therapy | 20 (20.0%) | 5 (4.6%) | 0 (0%) | 4 (4.9%) |
| Low riska | 30 (30.0%) | 57 (52.3%) | 1 (1.3%) | 24 (29.3%) |
| Medium riskb | 15 (15.0%) | 47 (43.1%) | 37 (46.8%) | 21 (25.6%) |
| High riskc | 35 (35.0%) | 0 (0%) | 41 (51.9%) | 33 (40.2%) |
Abbreviations: HM, hematological malignancies; ID, immunorheumatological diseases; ND, neurological disorders; ST, solid tumors.
Low risk: anti-CD30 MoAb, checkpoint inhibitors MoAb; target therapies; hypomethylation agents, or corticosteroids.
Medium risk: chemotherapy (ongoing or in the last 6 months); Bruton’s tyrosine kinase inhibitors; BCL-2 inhibitors; anti-CD38 MoAb without immunomodulatory drugs (IMIDs); immunosuppressive agents like methotrexate, mycophenolate mofetil, azathioprine, and cyclosporine.
High risk: Anti-B-cell therapy (ongoing or in the last 12 months): anti-CD20 monoclonal antibody (MoAb) or anti-CD19 chimeric antigen receptor T-cell therapies); allogeneic HSCT.
A lower anti-RBD titer to the first 2 doses and the booster dose was also observed with high-risk treatments compared with the other groups (P < .0001, Figure 6A). The neutralization assay performed on anti-RBD-positive patients showed lower activity in the high-risk group, highlighting a heavily dampened humoral response (Figure 6B). Accordingly, in the multivariable logistic model (Table 3), the independent predictors of seroconversion were disease subgroup, treatment subgroup, and age. Compared with HCWs, the anti-RBD and neutralization titers were lower in patient groups at T2 (P < .0001), whereas after the third dose the significant reduction with respect to HCWs was limited to patients receiving high- and intermediate-risk treatments (P < .0001). In striking contrast, the T-cell response was similar in all groups after 2 and 3 vaccine doses, regardless of the treatment type (Figure 6C), but lower than in HCWs (P < .0001 and P < .05, respectively).
Figure 6.
Impact of different therapy on immune response. Independently from the diseases, the patients were divided on the basis of therapy in 4 groups: untreated (white dots) or treated by therapy with a low (light violet dots), medium (dark violet dots), and high (blue dots) impact on the immune system. A group of HCW was added as a control. The immunogenicity of 2 or 3 doses of vaccine was compared among groups. A, The levels of anti-RBD in the 4 patient groups and in HCW are shown. Differences were evaluated by Kruskal-Wallis test. **P < .01; ***P < .001; ****P < .0001. Response after 2 doses: no therapy: median = 94.2 BAU/mL (IQR .4–1133.0 BAU/mL); low impact: 1144.0 BAU/mL (IQR 368.1–11,360.0 BAU/mL); medium impact: 420.3 BAU/mL (IQR 17.4–1563.0 BAU/mL); high impact: 0.2 (IQR 0.1–6.4 BAU/mL); HCW: 2405.0 BAU/mL (IQR 1343–3848 BAU/mL). Response after the third dose: no therapy: median = 1748 BAU/mL (IQR 95.4–3917.0 BAU/mL); low impact: 3044 BAU/mL (IQR 998.8–6175.0 BAU/mL); medium impact: 1088.0 BAU/mL (IQR 380.1–2536.0 BAU/mL); high impact: 3.5 (IQR .4–39.6 BAU/mL); HCW: post-3D: 4608.0 BAU/mL (IQR 3302.0–6030.0 BAU/mL). B, The level of neutralizing antibodies in the 4 groups is shown. Differences were evaluated by Kruskal-Wallis test. *P < .05; ****P < .0001. Response after 2 doses: no therapy: median = 80 reciprocal of dilution (IQR 10–160); low impact: 80 reciprocal of dilution (IQR 20–160); medium impact: 40 reciprocal of dilution (IQR 6.2–160.0); high impact: 5 reciprocal of dilution (IQR 5–20); HCW: 160 (IQR 80–320). Response after the third dose: no therapy: median = 120 reciprocal of dilution (IQR 10–560); low impact: 320 reciprocal of dilution (IQR 140–400); medium impact: 160 reciprocal of dilution (IQR 80–320); high impact: 5 reciprocal of dilution (IQR 5–10); HCW: 320 reciprocal of dilution (IQR 160–640). C: The T-cell response, analyzed by quantifying IFN-γ in the 4 groups is shown. Differences were evaluated by Kruskal-Wallis test. Response after 2 doses: no therapy: median = 74.9 pg/mL (IQR 2.7–338.4 pg/mL); low impact: 105.9 pg/mL (IQR 26.3–291.9 pg/mL); medium impact: 69.2 pg/mL (IQR 18.1–297.3 pg/mL); high impact: 186.3 pg/mL (IQR 22.7–390.0); HCW: 331.9 pg/mL (IQR 189.9–765.0 pg/mL). Response after the third dose: no therapy: median = 127.2 pg/mL (IQR 15.8–192.0 pg/mL); low impact: 158.9 pg/mL (IQR 87.2–536.4 pg/mL); medium impact: 243.4 pg/mL (IQR 69.3–799.7 pg/mL); high impact: 545.3 pg/mL (IQR 171.9–2049.0). HCW: 448.9 pg/mL (IQR 197.3–852.2 pg/mL). Abbreviations: HCW, health care workers; IQR, interquartile range; RBD, receptor binding domain; T2, 5–8 weeks from T0; Tpost-3D, 2–4 weeks from Tpre-3D.
Table 3.
Factors Associated With the Humoral Response at T2 (Multivariable Logistic Model)
| OR (95% CI) | P Value | |
|---|---|---|
| Sex | ||
| Male | .97 (0.51–1.84) | 0.93 |
| Female | 1 | |
| Age, y | .97 (.95–.99) | .026 |
| Comorbidities | ||
| Yes | .90 (.45–1.78) | .76 |
| No | 1 | |
| Subgroups | ||
| Hematological | 1 | .001 |
| Solid tumors | 8.09 (2.38–27.53) | .068 |
| Immunorheumatological | 2.36 (.94–5.91) | .006 |
| Neurological | 3.72 (1.45–9.54) | |
| Current therapy (impact on immune system) | ||
| No therapy | 1 | <.0001 |
| Low | 16.75 (3.85–72.85) | 0.23 |
| Medium | 1.98 (0.65–6.07) | <.0001 |
| High | 0.112 (0.04–0.35) | |
An OR >1 means a positive association between that characteristic and humoral response. P values lower than .05 are indicated in bold.
Abbreviations: CI, confidence interval; OR, odds ratio.
DISCUSSION
In this prospective multicenter trial, we found a suboptimal immune response induced by BNT16b2 and mRNA-1273 vaccines in fragile patients. HM and ID patients showed the lowest prevalence of anti-SARS-CoV-2 antibodies, similarly to other published studies [8, 9, 23]. This finding is largely due to the detrimental effect of anti-B-cell therapies. Our results highlight that treatment-defined subgroups were more capable than disease-defined ones of predicting the humoral response. A model combining disease types with treatment-induced immunosuppression will be more informative for health authorities.
The negative effect of B-cell–depleting therapies, resulting mostly from anti-CD20 monoclonal antibodies, lasted up to 12 months after the end of treatment, in line with other recent reports [17, 24, 25]. This can be explained by the prolonged half-life of these drugs and by the subsequent long-lasting B-cell depletion [26, 27]. As in other recent studies, we observed a high seroconversion rate among ST patients, probably because of using treatments with low lympholytic activity [13, 28]. However, the antibody titers were lower than those of HCWs, suggesting an impaired immune response.
Although the precise definition of all factors responsible for protection against COVID-19 remains to be determined, the relationship between in vitro neutralization levels and protection against symptomatic COVID-19 has been widely described [29]. Interestingly, we reported not only reduced antibody levels, but also a reduced neutralizing activity.
In contrast to the humoral response, less is known about the protection induced by the T-cell response. Several groups reported a role of T cells in protecting against severe COVID-19 [30–32], also in HM patients [33]. In a recent study, we evaluated the cellular response in 99 hematological patients after 2 doses of mRNA vaccines and a specific T-cell response was detected in 86% of them. Of note, 74% of seronegative patients had a T-cell response, but both cellular and humoral responses were absent in 13.1% [17]. Our study confirms the T cell–mediated response rate after 2 doses of vaccine. In addition, we were able to demonstrate the lack of association between humoral and cellular responses and the substantial stability of the T-cell response independent of treatment.
Our study was conducted when the Omicron variant was not yet prevalent. However, considering that the protection against Omicron achieved after the third vaccine dose in healthy subjects is also dependent on the T-cell activity directed against invariant epitopes of the spike protein [34], we can hypothesize a positive role of the cellular response also among our patients.
The scientific community concurs about the need for a booster dose, given the rapid spread of delta and omicron variants in addition to the waning immunity provided by the primary vaccination [35, 36]. The greatest benefit from a booster dose is postulated in immunocompromised patients, and several recent studies have reported an improved humoral response [20, 37–39]. At 4 weeks after the booster dose, we saw an increase in humoral response and neutralizing antibodies, but the seroconversion rate and antibody titers were lower in HM than other diseases, highlighting the peculiar immune impairment of these patients. By contrast, ID patients showed an excellent response to the third dose, reaching a >90% seroconversion rate and an anti-RBD titer higher than after the first 2 doses. ID patients also showed an increase in antibody levels over time after the 2 doses rather than a decrease, suggesting that this population requires more time to reach a strong B-cell response, which can be further improved by a booster dose.
A significant increase in the T-cell immune response after the third dose was observed in all disease groups. In contrast, Shroff et al reported no T-cell improvement early after the booster dose in patients with cancer [37]. This discrepancy with respect to our data could be due to the different timing of the analyses (2–4 weeks vs 1 week), suggesting the need for a longer time (at least 2 weeks) to see the positive effect on the T-cell response in fragile patients. However, given the still unknown protective effect of T-cell immunity, we might consider all patients who fail to develop a detectable humoral response after 3 doses of vaccine eligible for prophylaxis with passive immunization with anti-spike monoclonal antibodies. On the other hand, our results showed that the rate of seroconversion increased after the third dose, so we cannot rule out a potential benefit from a fourth dose in patients who have already completed their treatments. A potential limitation of our study is the lack of measurement of neutralization titers against the emerging omicron variant. However, it must be taken into account that in healthy subjects, a significant increase in the neutralizing response against this variant has already been demonstrated after the third dose [40, 41].
In conclusion, we found a lower seroconversion prevalence among immunosuppressed patients compared with HCWs. The lowest humoral response was reported in patients treated with anti–B-cell therapies. The T-cell response showed more encouraging results, suggesting a possible benefit of vaccination because of cellular immunity, particularly in light of the observation that T-cell epitopes are shared among wild-type and omicron variants [42]. Finally, the data on the third dose indicate a potential benefit of the booster and identify HM patients as the most fragile group.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. G. A., Al. Ma., and M. C. conceived the study; C. A., P. C., C. S., P. L. Z., R. M. , F. T. , F. C. , A. U., N. S., G. C., A. M., and F. L. designed the study; P. C., M. T. L. S., G. P., F. B., S. D., and N. S. managed the patients; D. G. performed the statistical analysis; V. S. monitored the study; C. A., V. B., A. S., G. M., and V. M. performed the immunological analysis; C. A., M. R., D. F., P. C., and F. B. analyzed the immunological data; P. C., C. A., and M. R. drafted the manuscript; all authors reviewed and agreed on the final version.
Acknowledgments. We are indebted to Research Director Dr. Giuseppe Ippolito, and the Deputy General Director for Health Research and Innovation, Dr. Gaetano Guglielmi, of the Italian Ministry of Health for their support to this study. We would also like to thank the patients who were enrolled in the VAX4FRAIL study and their families, the research nurses, the administrative staff, and all those who will be actively involved in their continuous care, study data collection and analysis, and ultimately in the scientific production that will result from this research.
Financial support . This work was supported by the Italian Ministry of Health within Ricerca Corrente 2021-Special Projects-VAX4FRAIL.
The VAX4FRAIL Study Group:
Principal investigators (alphabetical order): Giovanni Apolone (Fondazione IRCCS Istituto Nazionale dei Tumori di Milano); Alberto Mantovani (IRCCS Istituto Clinico Humanitas, Milano).
Scientific coordinators: Massimo Costantini (Fondazione IRCCS Istituto Nazionale dei Tumori di Milano), Nicola Silvestris (Università degli Studi di Messina).
Steering committee (alphabetical order): Chiara Agrati (IRCCS Istituto per le Malattie Infettive Lazzaro Spallanzani, Roma); Giovanni Apolone (Fondazione IRCCS Istituto Nazionale dei Tumori di Milano); Fabio Ciceri (IRCCS Ospedale San Raffaele, Milano); Gennaro Ciliberto (IRCCS Istituto Nazionale Tumori Regina Elena, Roma); Massimo Costantini (Fondazione IRCCS Istituto Nazionale dei Tumori di Milano); Franco Locatelli (Università La Sapienza, Roma); Alberto Mantovani (IRCCS Istituto Clinico Humanitas, Milano); Fausto Baldanti (Fondazione IRCCS Policlinico San Matteo di Pavia); Aldo Morrone (Istituto Dermatologico San Gallicano IRCCS, Roma); Angelo Paradiso (IRCCS Istituto Tumori “Giovanni Paolo II”, Bari); Carlo Salvarani (Azienda USL-IRCCS Reggio Emilia); Nicola Silvestris (Università degli Studi di Messina); Fabrizio Tagliavini (Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano); Antonio Uccelli (Ospedale Policlinico San Martino IRCCS, Genova); Pier Luigi Zinzani (IRCCS Azienda Ospedaliero-Universitaria di Bologna).
Disease Groups
Hematological malignancies Referent: Paolo Corradini (Fondazione IRCCS Istituto Nazionale dei Tumori, Milano);
Solid tumors Referent: Gennaro Ciliberto (IRCCS Istituto Nazionale Tumori Regina Elena, Roma);
Immunorheumatological diseases Referent: Carlo Salvarani (Azienda USL IRCCS Reggio Emilia);
NEurological diseases: Referent: Antonio Uccelli (Ospedale Policlinico San Martino IRCCS, Genova); Renato Mantegazza (Fondazione I.R.C.C.S Istituto Neurologico Carlo Besta (INCB), Milano).
Immunological Group
Referents: Chiara Agrati (IRCCS Istituto per le Malattie Infettive Lazzaro Spallanzani, Roma); Maria Rescigno (IRCCS Istituto Clinico Humanitas, Milano); Daniela Fenoglio (Ospedale Policlinico San Martino IRCCS, Genova);
Participants: Roberta Mortarini (Fondazione IRCCS Istituto Nazionale dei Tumori di Milano); Cristina Tresoldi (IRCCS Ospedale San Raffaele, Milano); Laura Conti, Chiara Mandoj (IRCCS Istituto Nazionale Tumori Regina Elena, Roma); Michela Lizier (IRCCS Humanitas Research Hospital, Rozzano, Milan); Stefania Croci (Azienda USL IRCCS Reggio Emilia); Fausto Baldanti (Fondazione IRCCS Policlinico San Matteo di Pavia); Vito Garrisi (IRCCS Istituto Tumori “Giovanni Paolo II”, Bari); Fulvio Baggi (Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano); Tiziana Lazzarotto, Francesca Bonifazi (IRCCS Azienda Ospedaliero-Universitaria di Bologna); Fulvia Pimpinelli (Istituto Dermatologico San Gallicano IRCCS, Roma); Concetta Quintarelli (IRCCS Ospedale Pediatrico Bambino Gesù, Roma); Rita Carsetti (IRCCS Ospedale Pediatrico Bambino Gesù, Roma).
INMI centralized laboratory (INMI Lazzaro Spallanzani – IRCCS, Roma) (alphabetical order)
Enrico Girardi (Scientific Director), Aurora Bettini; Veronica Bordoni; Concetta Castilletti; Eleonora Cimini; Rita Casetti; Francesca Colavita; Flavia Cristofanelli; Massimo Francalancia; Simona Gili; Delia Goletti; Giulia Gramigna; Germana Grassi; Daniele Lapa; Sara Leone; Davide Mariotti; Giulia Matusali; Silvia Meschi; Stefania Notari; Enzo Puro; Marika Rubino; Alessandra Sacchi; Eleonora Tartaglia
Clinical Task Force
Paolo Corradini, Silvia Damian, Vincenzo Marasco, Filippo de Braud (Fondazione IRCCS Istituto Nazionale dei Tumori di Milano); Maria Teresa Lupo Stanghellini, Lorenzo Dagna, Francesca Ogliari, Massimo Filippi, Alessandro Bruno, Gloria Catalano, Rosamaria Nitti (IRCCS Ospedale San Raffaele, Milano); Andrea Mengarelli, Francesco Marchesi, Giancarlo Paoletti e Gabriele Minuti, Elena Papa (IRCCS Istituto Nazionale Tumori Regina Elena, Roma); Elena Azzolini, Luca Germagnoli, Carlo Selmi, Maria De Santis, Carmelo Carlo-Stella, Alexia Bertuzzi, Francesca Motta, Angela Ceribelli, Chiara Miggiano, Giulia Fornasa (IRCCS Humanitas Research Hospital, Rozzano, Milan); Fausto Baldanti, Sara Monti, Carlo Maurizio Montecucco (Fondazione IRCCS Policlinico San Matteo di Pavia); Aldo Morrone, Dario Graceffa (Istituto Dermatologico San Gallicano IRCCS, Roma); Maria Grazia Catanoso, Monica Guberti, Carmine Pinto, Francesco Merli, Franco Valzania (Azienda USL-IRCCS Reggio Emilia); Rosa Divella, Antonio Tufaro, Vito Garrisi, Sabina Delcuratolo, Mariana Miano (IRCCS Istituto Tumori “Giovanni Paolo II,” Bari; Carlo Antozzi, Silvia Bonanno Rita Frangiamore, Lorenzo Maggi (Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano); Antonio Uccelli, Paolo Pronzato, Matilde Inglese, Carlo Genova, Caterina Lapucci, Alice Laroni, Ilaria Poirè (Ospedale Policlinico San Martino IRCCS, Genova); Marco Fusconi, Vittorio Stefoni, Maria Abbondanza Pantaleo (IRCCS Azienda Ospedaliero-Universitaria di Bologna).
Statistical Committee
Diana Giannarelli (IRCCS Istituto Nazionale Tumori Regina Elena, Roma).
e-CRF and Monitoring Referent
Valentina Sinno, Serena Di Cosimo (Fondazione IRCCS Istituto Nazionale dei Tumori di Milano).
Project Managers of the Study
Referents: Elena Turola, Azienda USL-IRCCS di Reggio Emilia.
Participants: Iolanda Pulice, Roberta Mennitto Fondazione IRCCS Istituto Nazionale dei Tumori, Milano); Stefania Trinca (IRCCS Ospedale San Raffaele, Milano); Giulia Piaggio (IRCCS Istituto Nazionale Tumori Regina Elena, Roma); Chiara Pozzi (IRCCS Humanitas Research Hospital, Rozzano, Milan); Irene Cassaniti (Fondazione IRCCS Policlinico San Matteo, Pavia); Alessandro Barberini (Istituto Dermatologico San Gallicano IRCCS, Roma); Arianna Belvedere (Azienda USL-IRCCS Reggio Emilia); Sabina Delcuratolo (IRCCS Istituto Tumori “Giovanni Paolo II,” Bari); Rinaldi Elena, Federica Bortone (Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano); Maria Giovanna Dal Bello (Ospedale Policlinico San Martino IRCCS, Genova); Silvia Corazza (IRCCS Azienda Ospedaliero-Universitaria, Bologna).
Supplementary Material
Contributor Information
Paolo Corradini, Department of Oncology and Hematology, Fondazione IRCCS Istituto Nazionale dei Tumori di, Milano, Italy; School of Medicine, University of Milan, Milan, Italy.
Chiara Agrati, Cellular Immunology Laboratory, National Institute for Infectious Diseases L Spallanzani – IRCCS, Rome, Italy.
Giovanni Apolone, Fondazione IRCCS Istituto Nazionale dei Tumori di, Milano, Italy.
Alberto Mantovani, Humanitas Scientific Directorate, IRCCS Humanitas Research Hospital, Rozzano, Milan, Italy; Department of Biomedical Sciences, Humanitas University, Pieve Emanuele, Milan, Italy; William Harvey Research Institute, Queen Mary University, London, United Kingdom.
Diana Giannarelli, Biostatistical Unit, Istituto Nazionale Tumori Regina Elena IRCCS - IFO, Rome, Italy.
Vincenzo Marasco, Department of Oncology and Hematology, Fondazione IRCCS Istituto Nazionale dei Tumori di, Milano, Italy.
Veronica Bordoni, Cellular Immunology Laboratory, National Institute for Infectious Diseases L Spallanzani – IRCCS, Rome, Italy.
Alessandra Sacchi, Cellular Immunology Laboratory, National Institute for Infectious Diseases L Spallanzani – IRCCS, Rome, Italy.
Giulia Matusali, Virology Laboratory, National Institute for Infectious Diseases L Spallanzani – IRCCS, Rome, Italy.
Carlo Salvarani, Unità di Reumatologia, Azienda USL-IRCCS, Reggio Emilia, Italy; Unità di Reumatologia, Università degli Studi di Modena e Reggio Emilia, Modena.
Pier Luigi Zinzani, IRCCS Azienda Ospedaliero-Universitaria di Bologna, Istituto di Ematologia “Seràgnoli”, Bologna, Italy; Dipartimento di Medicina Specialistica, Diagnostica e Sperimentale, Università di Bologna, Bologna, Italy.
Renato Mantegazza, Neuromuscular Diseases and Neuroimmunology Unit, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano, Italy.
Fabrizio Tagliavini, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milan, Italy.
Maria Teresa Lupo-Stanghellini, Hematology and Bone Marrow Transplantation Unit, IRCCS San Raffaele Scientific Institute, Milano, Italy.
Fabio Ciceri, IRCSS San Raffaele Scientific Institute, Milano, Italy.
Silvia Damian, Department of Oncology and Hematology, Fondazione IRCCS Istituto Nazionale dei Tumori di, Milano, Italy.
Antonio Uccelli, Department of Neuroscience, Rehabilitation, Ophthalmology, Genetics, Maternal and Child Health (DINOGMI) University of Genoa, Genoa, Italy; IRCCS Ospedale Policlinico San Martino, Genoa, Italy.
Daniela Fenoglio, IRCCS Ospedale Policlinico San Martino, Genoa, Italy; Centre of Excellence for Biomedical Research and Department of Internal Medicine, University of Genoa, Genoa, Italy.
Nicola Silvestris, Medical Oncology Department, IRCCS Istituto Tumori “Giovanni Paolo II”, Bari, Italy; Department of Biomedical Sciences and Human Oncology, University of Bari “Aldo Moro”, Bari, Italy.
Fausto Baldanti, Molecular Virology Unit, Fondazione IRCCS Policlinico San Matteo, Pavia, Italy; Department of Clinical, Surgical, Diagnostics and Pediatric Sciences, University of Pavia, Pavia, Italy.
Giulia Piaggio, SAFU Unit IRCCS Regina Elena, National Cancer Institute, Istituti Fisioterapici Ospitalieri (IFO), Rome, Italy.
Gennaro Ciliberto, National Cancer Institute, Istituti Fisioterapici Ospitalieri (IFO), Rome, Italy.
Aldo Morrone, San Gallicano Dermatological Institute IRCCS, Rome, Italy.
Franco Locatelli, Department of Pediatric Hematology and Oncology and Cell and Gene Therapy, IRCCS Ospedale Pediatrico Bambino Gesù, Rome, Italy; Department of Gynecology-Obstetrics and Pediatrics, University ‘La Sapienza’, Roma, Italy.
Valentina Sinno, Department of Oncology and Hematology, Fondazione IRCCS Istituto Nazionale dei Tumori di, Milano, Italy.
Maria Rescigno, Department of Biomedical Sciences, Humanitas University, Pieve Emanuele, Milan, Italy; Mucosal Immunology and Microbiota Unit, IRCCS Humanitas Research Hospital, Rozzano, Milano, Italy.
Massimo Costantini, Azienda Unità Sanitaria Locale (USL)-IRCCS Reggio Emilia, Reggio Emilia, Italy.
VAX4FRAIL Study Group:
Giovanni Apolone, Alberto Mantovani, Massimo Costantini, Nicola Silvestris, Chiara Agrati, Giovanni Apolone, Fabio Ciceri, Gennaro Ciliberto, Massimo Costantini, Franco Locatelli, Alberto Mantovani, Fausto Baldanti, Aldo Morrone, Angelo Paradiso, Carlo Salvarani, Nicola Silvestris, Fabrizio Tagliavini, Antonio Uccelli, Pier Luigi Zinzani, Paolo Corradini, Gennaro Ciliberto, Carlo Salvarani, Antonio Uccelli, Renato Mantegazza, Chiara Agrati, Maria Rescigno, Daniela Fenoglio, Roberta Mortarini, Cristina Tresoldi, Laura Conti, Chiara Mandoj, Michela Lizier, Stefania Croci, Fausto Baldanti, Vito Garrisi, Fulvio Baggi, Tiziana Lazzarotto, Francesca Bonifazi, Fulvia Pimpinelli, Concetta Quintarelli, Rita Carsetti, Enrico Girardi, Aurora Bettini, Veronica Bordoni, Concetta Castilletti, Eleonora Cimini, Rita Casetti, Francesca Colavita, Flavia Cristofanelli, Massimo Francalancia, Simona Gili, Delia Goletti, Giulia Gramigna, Germana Grassi, Daniele Lapa, Sara Leone, Davide Mariotti, Giulia Matusali, Silvia Meschi, Stefania Notari, Enzo Puro, Marika Rubino, Alessandra Sacchi, Eleonora Tartaglia, Paolo Corradini, Silvia Damian, Vincenzo Marasco, Filippo de Braud, Maria Teresa Lupo Stanghellini, Lorenzo Dagna, Francesca Ogliari, Massimo Filippi, Alessandro Bruno, Gloria Catalano, Rosamaria Nitti, Andrea Mengarelli, Francesco Marchesi, Giancarlo Paoletti e Gabriele Minuti, Elena Papa, Elena Azzolini, Luca Germagnoli, Carlo Selmi, Maria De Santis, Carmelo Carlo-Stella, Alexia Bertuzzi, Francesca Motta, Angela Ceribelli, Chiara Miggiano, Giulia Fornasa, Fausto Baldanti, Sara Monti, Carlo Maurizio Montecucco, Aldo Morrone, Dario Graceffa, Maria Grazia Catanoso, Monica Guberti, Carmine Pinto, Francesco Merli, Franco Valzania, Rosa Divella, Antonio Tufaro, Vito Garrisi, Sabina Delcuratolo, Mariana Miano, Antonio Uccelli, Paolo Pronzato, Matilde Inglese, Carlo Genova, Caterina Lapucci, Alice Laroni, Ilaria Poirè, Marco Fusconi, Vittorio Stefoni, Maria Abbondanza Pantaleo, Diana Giannarelli, Valentina Sinno, Serena Di Cosimo, Elena Turola, Iolanda Pulice, Stefania Trinca, Giulia Piaggio, Chiara Pozzi, Irene Cassaniti, Alessandro Barberini, Arianna Belvedere, Sabina Delcuratolo, Rinaldi Elena, Federica Bortone, Maria Giovanna Dal Bello, and Silvia Corazza
References
- 1. Passamonti F, Cattaneo C, Arcaini L, et al. Clinical characteristics and risk factors associated with COVID-19 severity in patients with haematological malignancies in Italy: a retrospective, multicentre, cohort study. Lancet Haematol 2020; 7:e737–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Salvarani C, Bajocchi G, Mancuso P, et al. Susceptibility and severity of COVID-19 in patients treated with bDMARDS and tsDMARDs: a population-based study. Ann Rheum Dis 2020; 79:986.2–988. Available at:https://ard.bmj.com/lookup/doi/10.1136/annrheumdis-2020-217903. [DOI] [PubMed] [Google Scholar]
- 3. Saini KS, Tagliamento M, Lambertini M, et al. Mortality in patients with cancer and coronavirus disease 2019: a systematic review and pooled analysis of 52 studies. Eur J Cancer 2020; 139:43–50. Available at:https://linkinghub.elsevier.com/retrieve/pii/S0959804920304627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Laroni A, Schiavetti I, Sormani MP, Uccelli A. COVID-19 in patients with multiple sclerosis undergoing disease-modifying treatments. Mult Scler J 2020; 27:2126–36. [DOI] [PubMed] [Google Scholar]
- 5. El Sahly HM, Baden LR, Essink B, et al. Efficacy of the mRNA-1273 SARS-CoV-2 vaccine at completion of blinded phase. N Engl J Med 2021; 385:1774–85. Available at:https://www.nejm.org/doi/10.1056/NEJMoa2113017. Accessed 29 December 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med 2020; 383:2603–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Passamonti F, Romano A, Salvini M, et al. COVID-19 elicits an impaired antibody response against SARS-CoV-2 in patients with haematological malignancies. Br J Haematol 2021; 195:371–7. Available at:https://onlinelibrary.wiley.com/doi/10.1111/bjh.17704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Perry C, Luttwak E, Balaban R, et al. Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with B-cell non-Hodgkin lymphoma. Blood Adv 2021; 5:3053–61. Available at:https://ashpublications.org/bloodadvances/article/5/16/3053/476534/Efficacy-of-the-BNT162b2-mRNA-COVID-19-vaccine-in. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pimpinelli F, Marchesi F, Piaggio G, et al. Fifth-week immunogenicity and safety of anti-SARS-CoV-2 BNT162b2 vaccine in patients with multiple myeloma and myeloproliferative malignancies on active treatment: preliminary data from a single institution. J Hematol Oncol 2021; 14:81. Available at:https://jhoonline.biomedcentral.com/articles/10.1186/s13045-021-01090-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mrak D, Tobudic S, Koblischke M, et al. SARS-CoV-2 vaccination in rituximab-treated patients: B cells promote humoral immune responses in the presence of T-cell-mediated immunity. Ann Rheum Dis 2021; 80:1345–50. Available at:https://pubmed.ncbi.nlm.nih.gov/34285048/. Accessed 8 December 2021. [DOI] [PubMed] [Google Scholar]
- 11. Delvino P, Bartoletti A, Cassaniti I, et al. Impact of immunosuppressive treatment on the immunogenicity of mRNA COVID-19 vaccine in vulnerable patients with giant cell arteritis. Rheumatology 2021; 61:870–2. Available at:https://pubmed.ncbi.nlm.nih.gov/34664631/. Accessed 29 December 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Picchianti-Diamanti A, Aiello A, Laganà B, et al. Immunosuppressive therapies differently modulate humoral- and T-cell-specific responses to COVID-19 mRNA vaccine in rheumatoid arthritis patients. Front Immunol 2021; 12:3572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Monin L, Laing AG, Muñoz-Ruiz M, et al. Safety and immunogenicity of one versus two doses of the COVID-19 vaccine BNT162b2 for patients with cancer: interim analysis of a prospective observational study. Lancet Oncol 2021; 22:765–78. Available at:http://www.ncbi.nlm.nih.gov/pubmed/33930323%0Ahttp://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC8078907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Apostolidis SA, Kakara M, Painter MM, et al. Cellular and humoral immune responses following SARS-CoV-2 mRNA vaccination in patients with multiple sclerosis on anti-CD20 therapy. Nat Med 2021; 27:1990–2001. Available at:https://www.nature.com/articles/s41591-021-01507-2. Accessed 12 December 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tortorella C, Aiello A, Gasperini C, et al. Humoral- and T-cell–specific immune responses to SARS-CoV-2 mRNA vaccination in patients with MS using different disease-modifying therapies. Neurology 2022; 98:e541–54. Available at:https://n.neurology.org/content/early/2021/11/22/WNL.0000000000013108. Accessed 29 December 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cassaniti I, Bergami F, Arena F, et al. Immune response to BNT162b2 in solid organ transplant recipients: negative impact of mycophenolate and high responsiveness of SARS-CoV-2 recovered subjects against delta variant. Microorg 2021; 9:2622. Available at:https://www.mdpi.com/2076-2607/9/12/2622/htm. Accessed 29 December 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Marasco V, Carniti C, Guidetti A, et al. T-cell immune response after mRNA SARS-CoV-2 vaccines is frequently detected also in the absence of seroconversion in patients with lymphoid malignancies. Br J Haematol 2022; 196:548–58. Available at:https://onlinelibrary.wiley.com/doi/10.1111/bjh.17877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cassaniti I, Bergami F, Percivalle E, et al. Humoral and cell-mediated response against SARS-CoV-2 variants elicited by mRNA vaccine BNT162b2 in healthcare workers: a longitudinal observational study. Clin Microbiol Infect 2021; 28:301.e1–8. Available at:http://www.clinicalmicrobiologyandinfection.com/article/S1198743X2100536X/fulltext. Accessed 29 December 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mizrahi B, Lotan R, Kalkstein N, et al. Correlation of SARS-CoV-2-breakthrough infections to time-from-vaccine. Nat Commun 2021; 12:6379. Available at:https://www.nature.com/articles/s41467-021-26672-3. Accessed 13 November 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kamar N, Abravanel F, Marion O, Couat C, Izopet J, Del Bello A. Three doses of an mRNA COVID-19 vaccine in solid-organ transplant recipients. N Engl J Med 2021; 385:661–2. Available at:http://www.nejm.org/doi/10.1056/NEJMc2108861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Agrati C, Di Cosimo S, Fenoglio D, et al. COVID-19 vaccination in fragile patients: current evidence and an harmonized transdisease trial. Front Immunol 2021; 12:704110. Available at:https://www.frontiersin.org/articles/10.3389/fimmu.2021.704110/full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Agrati C, Castilletti C, Goletti D, et al. Coordinate induction of humoral and spike specific T-cell response in a cohort of Italian health care workers receiving BNT162b2 mRNA vaccine. Microorganisms 2021; 9:1315. Available at:https://www.mdpi.com/2076-2607/9/6/1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Furer V, Eviatar T, Zisman D, et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Ann Rheum Dis 2021; 80:1330–38. Available at:https://pubmed.ncbi.nlm.nih.gov/34127481/. Accessed 14 November 2021. [DOI] [PubMed] [Google Scholar]
- 24. Ghione P, Gu JJ, Attwood K, et al. Impaired humoral responses to COVID-19 vaccination in patients with lymphoma receiving B-cell-directed therapies. Blood 2021; 138:811–4. Available at:https://pubmed.ncbi.nlm.nih.gov/34189565/. Accessed 8 December 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sormani MP, Inglese M, Schiavetti I, et al. Effect of SARS-CoV-2 mRNA vaccination in MS patients treated with disease modifying therapies. EBioMedicine 2021; 72:103581. Available at:http://www.thelancet.com/article/S2352396421003741/fulltext. Accessed 8 December 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li J, Levi M, Charoin J-E, et al. Rituximab exhibits a long half-life based on a population pharmacokinetic analysis in non-Hodgkin’s lymphoma (NHL) patients. Blood 2007; 110:2371. Available at:https://ashpublications.org/blood/article/110/11/2371/57202/Rituximab-Exhibits-a-Long-HalfLife-Based-on-a.17515402 [Google Scholar]
- 27. Salles G, Barrett M, Foà R, et al. Rituximab in B-cell hematologic malignancies: A review of 20 years of clinical experience. Adv Ther 2017; 34:2232–73. Available at:http://link.springer.com/10.1007/s12325-017-0612-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Goshen-Lago T, Waldhorn I, Holland R, et al. Serologic status and toxic effects of the SARS-CoV-2 BNT162b2 vaccine in patients undergoing treatment for cancer. JAMA Oncol 2021; 7:1507–13. Available at:https://pubmed.ncbi.nlm.nih.gov/34236381/. Accessed 14 November 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med 2021; 27:1205–11. Accessed 13 November 2021. [DOI] [PubMed] [Google Scholar]
- 30. Shrotri M, van Schalkwyk MCI, Post N, et al. T cell response to SARS-CoV-2 infection in humans: A systematic review. PLoS One 2021; 16:e0245532. Available at:https://pubmed.ncbi.nlm.nih.gov/33493185/. Accessed 13 November 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chen Z, John Wherry E. T cell responses in patients with COVID-19. Nat Rev Immunol 2020; 20:529–36. Available at:http://www.nature.com/articles/s41577-020-0402-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Le Bert N, Clapham HE, Tan AT, et al. Highly functional virus-specific cellular immune response in asymptomatic SARS-CoV-2 infection. J Exp Med 2021; 218:e20202617. Available at:https://rupress.org/jem/article/218/5/e20202617/211835/Highly-functional-virus-specific-cellular-immune. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bange EM, Han NA, Wileyto P, et al. CD8+ T cells contribute to survival in patients with COVID-19 and hematologic cancer. Nat Med 2021; 27:1280–9. Available at:http://www.nature.com/articles/s41591-021-01386-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Skelly DT, Harding AC, Gilbert-Jaramillo J, et al. Two doses of SARS-CoV-2 vaccination induce robust immune responses to emerging SARS-CoV-2 variants of concern. Nat Commun 2021; 12:5061. Available at:https://www.nature.com/articles/s41467-021-25167-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Krause PR, Fleming TR, Peto R, et al. Considerations in boosting COVID-19 vaccine immune responses. Lancet 2021; 398:1377–80. Available at:http://www.thelancet.com/article/S0140673621020468/fulltext. Accessed 13 November 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Juno JA, Wheatley AK. Boosting immunity to COVID-19 vaccines. Nat Med 2021; 27:1874–75. Available at:https://www.nature.com/articles/s41591-021-01560-x. Accessed 13 November 2021. [DOI] [PubMed] [Google Scholar]
- 37. Shroff RT, Chalasani P, Wei R, et al. Immune responses to two and three doses of the BNT162b2 mRNA vaccine in adults with solid tumors. Nat Med 2021; 27:2002–11. Accessed 13 November 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Felten R, Gallais F, Schleiss C, et al. Cellular and humoral immunity after the third dose of SARS-CoV-2 vaccine in patients treated with rituximab. Lancet Rheumatol 2022; 4:e13–6. Available at:http://www.thelancet.com/article/S2665991321003519/fulltext. Accessed 12 December 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ligumsky H, Dor H, Etan T, et al. Immunogenicity and safety of BNT162b2 mRNA vaccine booster in actively treated patients with cancer. Lancet Oncol 2022; 23:193–5. Available at:http://www.thelancet.com/article/S1470204521007154/fulltext. Accessed 29 December 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Garcia-Beltran WF, St Denis KJ, Hoelzemer A, et al. mRNA-based COVID-19 vaccine boosters induce neutralizing immunity against SARS-CoV-2 omicron variant. Cell 2022; 185:457–66.e4. Available at:https://www.medrxiv.org/content/10.1101/2021.12.14.21267755v1. Accessed 27 December 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dejnirattisai W, Shaw RH, Supasa P, et al. Reduced neutralisation of SARS-CoV-2 omicron B.1.1.529 variant by post-immunisation serum. Lancet 2022; 399:234–6. Available at:http://www.thelancet.com/article/S0140673621028440/fulltext. Accessed 29 December 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ledford H. How severe are omicron infections? Nature 2021; 600:577–8. Available at:https://www.nature.com/articles/d41586-021-03794-8. Accessed 29 December 2021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.