Abstract
Background
Although neutralizing antibodies to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) correlate with protection against coronavirus disease 2019 (COVID-19), little is known about the neutralizing and antibody-dependent cell-mediated cytotoxicity (ADCC) responses to COVID-19, multisystem inflammatory syndrome in children (MIS-C), and COVID-19 vaccination in children.
Methods
We enrolled children 0–21 years of age with a history of COVID-19 (n = 13), MIS-C (n = 13), or 2 doses of BNT162b2 vaccination (n = 14) into a phlebotomy protocol. We measured pseudovirus neutralizing and functional ADCC antibodies to SARS-CoV-2 variants, including Omicron (B.1.1.529).
Results
The primary BNT162b2 vaccination series elicited higher neutralizing and ADCC responses with greater breadth to SARS-CoV-2 variants than COVID-19 or MIS-C, although these were diminished against Omicron.
Conclusions
Serologic responses were significantly reduced against variants, particularly Omicron.
Keywords: ADCC, COVID, MIS-C, SARS-CoV-2, pediatric
Binding, neutralizing, and antibody-dependent cell-mediated cytotoxicity (ADCC) responses against SARS-CoV-2 variants including Omicron (B.1.1.529) were assessed in children with MIS-C, convalescing from COVID-19, and after BNT162b2 vaccination. Vaccination elicited greater and broader responses against variants than COVID-19 or MIS-C.
On November 26, 2021, the World Health Organization (WHO) declared severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) B.1.1.529 (Omicron) a variant of concern after its identification in South Africa [1]. Subsequently, Omicron became a predominant circulating strain in the United States [2] and was associated with increased hospitalizations in both children [3] and adults [4]. The Omicron variant’s rapid spread was attributed to both enhanced transmissibility and the ability to evade pre-existing infection- or vaccine-induced immunity. These characteristics were thought to be due to the large number of changes in the SARS-CoV-2 spike protein in comparison with wild-type, especially those localized to the receptor binding domain and N-terminal domain [5]. Although plasma binding and neutralizing antibody responses have been reported for cohorts of infected and vaccinated adults [6, 7], little is known about the vaccine-induced antibody responses to variants including Omicron in children. Data are also limited regarding nonneutralizing functional antibody responses to SARS-CoV-2, including antibody-dependent cell-mediated cytotoxicity (ADCC) antibodies, which have been found to correlate with protection against some viral infections [8, 9]. In this study, we measured immunoglobulin (Ig)G binding, pseudovirus neutralizing, and functional ADCC antibody responses to SARS-CoV-2 variants of concern, including the Omicron variant, in children after coronavirus disease 2019 (COVID-19), multisystem inflammatory syndrome in children (MIS-C), and BNT162b2 (Pfizer-BioNTech) vaccination to identify differences in functional antibody responses.
METHODS
Patient Enrollment
Pediatric participants from 0 to 21 years of age were enrolled into a phlebotomy protocol after informed consent and assent, as appropriate for age. Prospective and/or residual blood samples from the clinical laboratory at Children’s Healthcare of Atlanta were collected and processed as approved by Emory University Institutional Review Board. Samples were collected approximately 1 month after receipt of 2 doses of BNT162b2 vaccine administered according to Emergency Use Authorization (EUA) by the US Federal Drug Administration (FDA); or 1 month after laboratory-confirmed symptomatic COVID-19; or during acute hospitalization for MIS-C, as defined by the Centers for Disease Control and Prevention (CDC) case definition [10], because MIS-C typically follows SARS-CoV-2 infection by 2–6 weeks [11].
Binding Antibody Assays
Binding IgG antibodies to SARS-CoV-2 spike variants, including wild-type (Wuhan-hu-1), Alpha (B.1.1.7), Beta (B.1.351), Delta (B.1.617.2), Gamma (P.1), and Omicron (B.1.1.529; BA.1) were measured using a MesoScale Discovery V-PLEX SARS-CoV-2 panel per the manufacturer’s protocol. Titers were expressed as binding antibody units (BAU)/mL.
Pseudovirus Neutralizing Antibody Assays
To perform pseudovirus neutralizing antibody assays, sera were serially diluted 3-fold with a starting dilution of 1:15. Diluted pseudoviruses (Supplementary Methods, Supplementary Figure 1) were added at a 1:1 volumetric ratio, and the plate was incubated for 1.5 hours at 37°C. Then, 100 µL serum-virus mixture was transferred to a 96-well plate containing 293T-ACE2 cells. After 48-hour incubation, the cells were lysed and relative luminescence units (RLUs) were read on a luminometer. The neutralizing antibody titer to each variant spike pseudovirus was expressed as the effective concentration at which 50% of the virus was neutralized (EC50) (Supplementary Figure 2). The lower limit of detection (LLOD) was defined as the starting dilution of 1:30.
Antibody-Dependent Cell-Mediated Cytotoxicity Assays
The development of SARS-CoV-2 variant spike protein ADCC target cell lines was performed as previously described for wild-type (Wuhan-hu-1) spike [12]. In brief, we generated stably transfected dual-reporter SARS-CoV-2 variant spike protein target cell lines with inducible expression of Alpha, Beta, Delta, Gamma, or Omicron spike proteins regulated by tetR in a T-Rex-293 cell line stably transfected with plasmids encoding enhanced green fluorescent protein and luciferase. We confirmed inducible surface expression of the variant spike proteins using flow cytometry and selected for high-expressing clones (Supplementary Figure 3).
To perform ADCC assays, sera were serially diluted 3-fold with a starting dilution of 1:30. The ADCC activity was measured using NK-CD16(+) effector cells with a luminescence readout. The percentage lysis of target cells was calculated for each well using the formula: ADCC (%) = [RLU*(no antibody) – RLU (with antibody)]/RLU (no antibody) × 100. The serum dilution at which ADCC crossed the 10% cutoff was considered the endpoint titer.
Statistical Analysis
Statistical analysis was performed in GraphPad Prism v7.0. Baseline demographic characteristics were analyzed using descriptive statistics. Antibody titers were presented as geometric mean titers (GMTs), and all undetectable titers were assigned a value of one half LLOD for a given assay. Log-transformed titers were compared using Student’s t tests. Linear regression analyses and Pearson correlation coefficients of log-transformed titers were also determined. P ≤ .05 were considered statistically significant.
RESULTS
Patient Enrollment
We collected blood samples from 13 children hospitalized with MIS-C, 13 children approximately 1-month posthospitalization for COVID-19, and 14 children approximately 1-month postvaccination with 2 doses of BNT162b2 administered according to the FDA EUA (Supplementary Table 1). Sample collection from children with convalescent COVID-19 was completed on May 10, 2021 and collection from children with MIS-C was completed on June 17, 2021 (collected before the surges of Delta and Omicron variants in the United States) [2]. None of these children had received COVID-19 vaccination before enrollment. The FDA issued an EUA for the Pfizer BNT162b2 vaccination for children 12 to 15 years of age on May 10, 2021. Sample collection from children postvaccination was completed on July 16, 2021. None of the vaccinated children had a history of prior SARS-CoV-2 infection.
For the 13 children with laboratory-confirmed COVID-19, samples were collected a median of 45 days (interquartile range [IQR], 34–68) pos-tonset of COVID-19 symptoms. Nine of the 13 were hospitalized for symptomatic COVID-19, and 1 was hospitalized for an alternative etiology (suicide attempt) and incidentally tested positive for SARS-CoV-2 with mild upper respiratory symptoms. The median duration of index hospitalization was 2 days (IQR, 1–6), with 8 (62%) who required intensive care, and 1 (8%) who was immunocompromised. All 13 children with MIS-C were hospitalized, and samples were collected a median of 5 days (IQR, 2–8) post-onset of MIS-C symptoms, which typically follows SARS-CoV-2 infection by 2–6 weeks. The median duration of index hospitalization for MIS-C was 5 days (IQR, 2–8), with 6 (46%) who required intensive care, and none who were immunocompromised. For the 14 vaccinated children, their postvaccination samples were collected a median of 34 days (IQR, 32–42) after the second dose of BNT162b2 vaccination, which was administered according to the FDA EUA.
Binding and Pseudovirus Neutralizing Antibody Responses
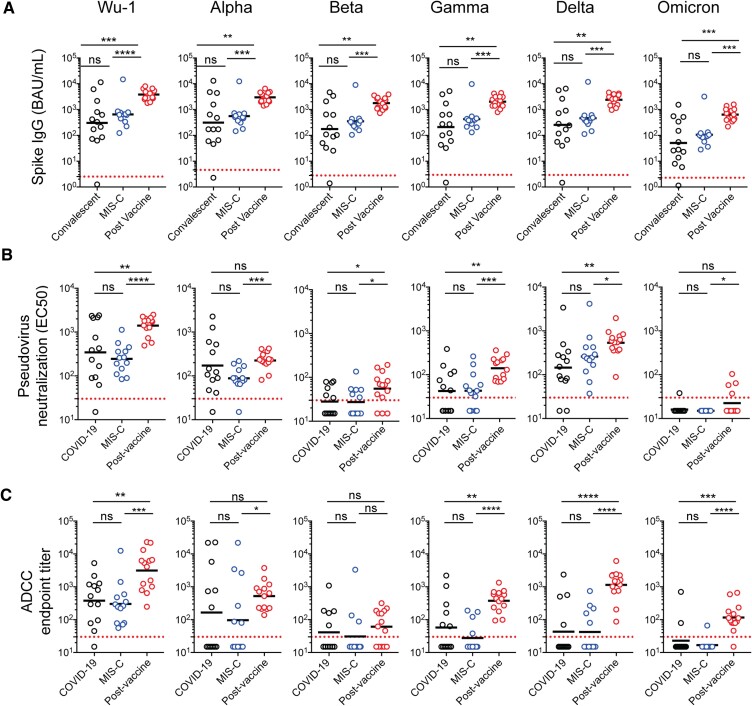
Binding IgG antibodies to all SARS-CoV-2 spike variants tested were significantly higher in children post-BNT162b2 vaccination than in children with MIS-C or convalescent COVID-19 (P < .05 for all comparisons) (Figure 1A). In contrast, there were no significant differences in binding IgG titers between the MIS-C and convalescent COVID-19 cohorts.
Figure 1.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variant spike binding immunoglobulin G (IgG), pseudovirus neutralizing, and antibody-dependent cell-mediated cytotoxicity (ADCC) antibody responses in children. A, Binding IgG titers to SARS-CoV-2 variant spike proteins were measured using a MesoScale Discovery V-PLEX assay and reported in World Health Organization/NIBSC units (binding antibody units [BAU]/mL). B, Pseudovirus neutralizing antibody titers to the SARS-CoV-2 spike variants were expressed as the estimated concentration at which 50% pseudoviruses were neutralized (EC50). C, The ADCC antibody responses to SARS-CoV-2 variants were expressed as end-point titers. One child in the multisystem inflammatory syndrome in children (MIS-C) cohort had insufficient sample volume for binding IgG analysis. Each dot represents 1 tested specimen. The dashed line represents the lower limits of detection for each respective variant and assay. Unpaired, 2-tailed t tests were performed for statistical comparisons. *, P < .05; **, P < .01; ***, P < .001; ****, P < .0001. NS, not significant.
After BNT162b2 vaccination, pseudovirus neutralizing antibody responses to the SARS-CoV-2 wild-type Wuhan (GMT 1402; 95% confidence interval [CI], 1041–1889), Beta (GMT 55; 95% CI, 34–91), Gamma (GMT 141; 95% CI, 101–197), and Delta (GMT 554; 95% CI, 372–824) variants were significantly higher than those observed in children with acute MIS-C and convalescent COVID-19 (P < .05 for all comparisons) (Figure 1B). Although postvaccination neutralizing titers to the Alpha (GMT 226; 95% CI, 172–295) and Omicron (GMT 22; 95% CI, 15–33) variants were significantly higher than observed during acute MIS-C (P < .05), they were not significantly different from titers in those convalescing from COVID-19. The Alpha variant became predominant during the period of specimen collection from convalescent COVID-19 and acute MIS-C patients, which may explain these observations [2]. Neutralizing activity was least against the Omicron variant in all 3 groups. There were no significant differences in neutralizing antibody titers between the MIS-C and convalescent COVID-19 cohorts.
Antibody-Dependent Cell-Mediated Cytotoxicity Antibody Responses
Similar findings were observed for functional ADCC responses (Figure 1C). Postvaccination ADCC antibody titers to the SARS-CoV-2 wild-type (GMT 3136; 95% CI, 1406–6996), Gamma (GMT 376; 95% CI, 240–588), Delta (GMT 1145; 95% CI, 616–2126), and Omicron (GMT 117; 95% CI, 67–203) variants were significantly higher than MIS-C and convalescent COVID-19 titers (P < .05 for all comparisons). Although postvaccination ADCC titers to the Alpha (GMT 522; 95% CI, 299–912) variant were significantly higher than those with MIS-C (GMT 96; 95% CI, 21–452), they did not differ significantly from convalescent COVID-19 titers (GMT 165; 95% CI, 27–989). There were also no significant differences in ADCC titers to the Beta variant among postvaccine (GMT 61; 95% CI, 21–122), MIS-C (GMT 31; 95% CI, 12–81), and convalescent COVID-19 (GMT 41; 95% CI, 17–99). Overall, both neutralizing and ADCC antibody titers were highest to the wild-type strain and least to the Omicron variant.
Correlation Between Binding, Pseudovirus Neutralizing, and Antibody-Dependent Cell-Mediated Cytotoxicity Antibodies to Severe Acute Respiratory Syndrome Coronavirus 2 Variants
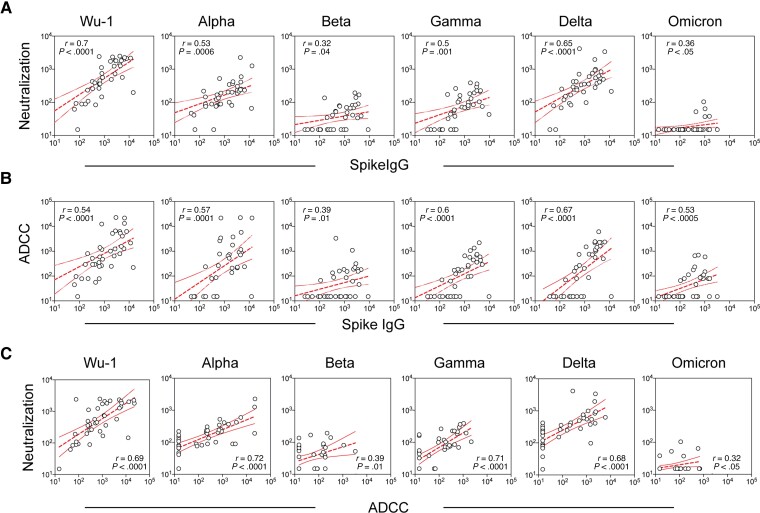
Correlations between SARS-CoV-2 spike IgG binding, pseudovirus neutralizing, and ADCC antibody responses for the respective variants were determined by linear regression analyses of the log-transformed titers and Pearson correlation coefficients (Figure 2). Binding IgG titers correlated with (1) neutralizing and ADCC titers for the wild-type (Wu-1) and (2) all variants tested (Alpha, Beta, Gamma, Delta, and Omicron). Likewise, neutralizing and ADCC titers correlated for all variants. However, correlation was weakest for Beta (r = 0.39, P < .01) and Omicron (r = 0.32, P < .05) variants, which overall had the lowest titers. The ratio of neutralizing to ADCC GMTs also differed among the variants (Supplementary Table 2).
Figure 2.
Correlation between severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variant spike binding immunoglobulin G (IgG), pseudovirus neutralizing, and antibody-dependent cell-mediated cytotoxicity (ADCC) antibody responses in children. Correlations of log-transformed antibody titers in all 3 combined cohorts (n = 39 children) were determined by linear regression and Pearson correlation coefficient. Correlations were made between A, SARS-CoV-2 variant spike binding IgG versus pseudovirus neutralizing titers; B, SARS-CoV-2 variant spike binding IgG versus ADCC endpoint titers; and C, pseudovirus neutralizing versus ADCC endpoint titers. Each dot represents a unique patient. Dashed lines are the linear regression lines with 95% confidence intervals. The Pearson correlation coefficients (r) and P values are indicated in the plots.
DISCUSSION
We measured the neutralizing and ADCC antibody responses to SARS-CoV-2 variants among children with MIS-C, convalescent COVID-19, or postvaccination. Overall, the primary BNT162b2 vaccination series elicited higher binding, neutralizing, and ADCC antibody responses with greater breadth to SARS-CoV-2 variants than did COVID-19 or MIS-C. Antibody titers were highest for the wild-type (Wuhan) strain and least for the Omicron variant. Despite the breadth of binding antibody responses elicited by primary vaccination series and infection, the magnitude of neutralizing and ADCC responses were significantly reduced against SARS-CoV-2 variants. This reduction may have contributed to the increased frequency of breakthrough infections and hospitalizations observed in children and adolescents during the period of Omicron predominance [3].
Although the role of SARS-CoV-2 neutralizing antibodies in protection against COVID-19 has been established, less is known about the role of ADCC and other functional antibodies. ADCC is an Fc-mediated process whereby effector immune cells, most commonly natural killer cells, bind to the Fc region of antibody-bound antigen expressed on infected target cells. The effector cells then undergo degranulation, releasing perforin and granzyme, and lyse infected cells. Antibody-dependent cell-mediated cytotoxicity has been found to be an important component of immunity against human immunodeficiency virus-1 [8], herpes simplex virus [9], and potentially other viral infections [13, 14]. We and others have previously identified ADCC antibodies to SARS-CoV-2 spike protein in adults after COVID-19 [12, 15]. In the current study, we applied the same methodology to develop ADCC assays for SARS-CoV-2 variant spike proteins. We found that ADCC titers correlated with neutralizing antibody titers, although the correlation differed among the variants and was weakest for Beta and Omicron. It is interesting to note that although neutralizing antibodies to Omicron were largely absent, ADCC antibodies to Omicron were detected in the majority of vaccinated children, suggesting a potential role for these functional antibodies in SARS-CoV-2 variant protection. Understanding the epitope specificity of ADCC antibodies and their relative contribution SARS-CoV-2 protective immunity could improve our understanding of SARS-CoV-2 breakthrough infections and facilitate rational vaccine design in the future.
Limitations of our study include the small sample size and single-center analysis. The timing of sample collection, both chronological and postexposure to vaccine or infection, may have impacted antibody titers. The majority of samples were collected during circulation of D614G and Alpha variants, although genotyping information of the SARS-CoV-2 viruses infecting these children was not available. Some differences in the baseline demographic characteristics of the groups were identified (including age, race, ethnicity), although these generally reflected the affected population seen at our center. The majority of participants with COVID-19 were hospitalized, which likely corresponded with increased disease severity and greater antibody responses in comparison to most pediatric infections. Prevaccination titers were not available for the vaccinated participants, nor were longitudinal or postbooster samples. Furthermore, the ADCC target cell line expressed only the variant spike proteins, and thus this may have led to an underestimation of plasma ADCC titers attributable to nonspike antigens among previously infected participants.
CONCLUSIONS
In conclusion, the primary BNT162b2 vaccination series elicited higher neutralizing and ADCC antibody responses with greater breadth to SARS-CoV-2 variants than did COVID-19 or MIS-C. The ADCC titers correlated with neutralizing antibody titers, although the correlations differed among SARS-CoV-2 variants. Future studies are needed to elucidate the epitope specificity and role of functional ADCC in protection against COVID-19.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank the Hope Clinic and Emory Children’s Center Vaccine Research Clinic (ECC-VRC) laboratories for processing clinical samples. We also thank the participants and their families for participating in this study.
Financial support. This work was funded by the Center for Childhood Infections and Vaccines (CCIV) at Emory University and Children’s Healthcare of Atlanta.
Potential conflicts of interest. E. J. A. has consulted for Pfizer, Sanofi Pasteur, Janssen, GSK, and Medscape, and his institution receives funds to conduct clinical research unrelated to this manuscript from MedImmune, Regeneron, PaxVax, Pfizer, GSK, Merck, Sanonfi-Pasteur, Janssen, and Micron. He also serves on data and safety monitoring boards for Kentucky BioProcessing, Inc. and Sanofi-Pasteur. His institution has also received funding from the National Institutes of Health (NIH) to conduct clinical trials of Moderna and Janssen coronavirus disease 2019 (COVID-19) vaccines. C.A.R.’s institution has received funding to conduct clinical research unrelated to this manuscript from BioFire Inc., GSK, MedImmune, Micron, Merck, Novavax, PaxVax, Regeneron, Pfizer, and Sanofi-Pasteur. She is coinventor of patented respiratory syncytial virus (RSV) vaccine technology unrelated to this manuscript, which has been licensed to Meissa Vaccines, Inc. Her institution has received funding from NIH to conduct clinical trials of Moderna and Janssen COVID-19 vaccines. L. J. A. has done paid consultancies on RSV vaccines for Bavarian Nordic, ClearPath Vaccines Company, Janssen, Pfizer, and ADVI; his laboratory is currently receiving funding through Emory University from Pfizer for laboratory studies for RSV surveillance studies in adults, Sciogen for animal studies of RSV vaccines, and Advaccine Pharmaceuticals, Ltd for RSV neutralizing antibody studies; he is a coinventor on several Centers for Disease Control and Prevention patents on the RSV G protein and its CX3C chemokine motif relative to immune therapy and vaccine development; and he is coinventor on a patent filing for use of RSV platform virus-like particles with the F and G proteins for vaccines. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Supplementary Material
Contributor Information
Christina A Rostad, Division of Infectious Diseases, Department of Pediatrics, Emory University School of Medicine, Atlanta, Georgia, USA; Center for Childhood Infections and Vaccines, Children’s Healthcare of Atlanta, Atlanta, Georgia, USA.
Xuemin Chen, Division of Infectious Diseases, Department of Pediatrics, Emory University School of Medicine, Atlanta, Georgia, USA; Center for Childhood Infections and Vaccines, Children’s Healthcare of Atlanta, Atlanta, Georgia, USA.
He-ying Sun, Division of Infectious Diseases, Department of Pediatrics, Emory University School of Medicine, Atlanta, Georgia, USA; Center for Childhood Infections and Vaccines, Children’s Healthcare of Atlanta, Atlanta, Georgia, USA.
Laila Hussaini, Division of Infectious Diseases, Department of Pediatrics, Emory University School of Medicine, Atlanta, Georgia, USA; Center for Childhood Infections and Vaccines, Children’s Healthcare of Atlanta, Atlanta, Georgia, USA.
Austin Lu, Division of Infectious Diseases, Department of Pediatrics, Emory University School of Medicine, Atlanta, Georgia, USA; Center for Childhood Infections and Vaccines, Children’s Healthcare of Atlanta, Atlanta, Georgia, USA.
Maria A Perez, Division of Infectious Diseases, Department of Pediatrics, Emory University School of Medicine, Atlanta, Georgia, USA; Center for Childhood Infections and Vaccines, Children’s Healthcare of Atlanta, Atlanta, Georgia, USA.
Hui Mien Hsiao, Division of Infectious Diseases, Department of Pediatrics, Emory University School of Medicine, Atlanta, Georgia, USA; Center for Childhood Infections and Vaccines, Children’s Healthcare of Atlanta, Atlanta, Georgia, USA.
Larry J Anderson, Division of Infectious Diseases, Department of Pediatrics, Emory University School of Medicine, Atlanta, Georgia, USA; Center for Childhood Infections and Vaccines, Children’s Healthcare of Atlanta, Atlanta, Georgia, USA.
Evan J Anderson, Division of Infectious Diseases, Department of Pediatrics, Emory University School of Medicine, Atlanta, Georgia, USA; Center for Childhood Infections and Vaccines, Children’s Healthcare of Atlanta, Atlanta, Georgia, USA; Department of Medicine, Emory University School of Medicine, Atlanta, Georgia, USA.
References
- 1. World Health Organization . Classification of omicron (B.1.1.529): SARS-CoV-2 variant of concern. Available at: https://www.who.int/news/item/26-11-2021-classification-of-omicron-(b.1.1.529)-sars-cov-2-variant-of-concern. Accessed 7 March 2022.
- 2. Lambrou AS, Shirk P, Steele MK, et al. Genomic surveillance for SARS-CoV-2 variants: predominance of the delta (B.1.617.2) and omicron (B.1.1.529) variants — United States, June 2021–January 2022. MMWR Morb Mortal Wkly Rep 2022; 71:206–11. 10.15585/mmwr.mm7106a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Marks KJ, Whitaker M, Anglin O, et al. Hospitalizations of children and adolescents with laboratory-confirmed COVID-19 — COVID-NET, 14 states, July 2021–January 2022. MMWR Morb Mortal Wkly Rep 2022; 71:271–8. 10.15585/mmwr.mm7107e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Taylor CA, Whitaker M, Anglin O, et al. COVID-19–associated hospitalizations among adults during SARS-CoV-2 delta and omicron variant predominance, by race/ethnicity and vaccination status — COVID-NET, 14 states, July 2021–January 2022. MMWR Morb Mortal Wkly Rep 2022; 71:466–73. 10.15585/mmwr.mm7112e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yu J, Collier AY, Rowe M, et al. Neutralization of the SARS-CoV-2 omicron BA.1 and BA.2 variants. N Engl J Med 2022; 386:1579–80. 10.1056/NEJMc2201849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schmidt F, Muecksch F, Weisblum Y, et al. Plasma neutralization of the SARS-CoV-2 omicron variant. N Engl J Med 2022; 386:599–601. 10.1056/NEJMc2119641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nemet I, Kliker L, Lustig Y, et al. Third BNT162b2 vaccination neutralization of SARS-CoV-2 omicron infection. N Engl J Med 2022; 386:492–4. 10.1056/NEJMc2119358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Haynes BF, Gilbert PB, McElrath MJ, et al. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med 2012; 366:1275–86. 10.1056/NEJMoa1113425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kohl S. Role of antibody-dependent cellular cytotoxicity in neonatal infection with herpes simplex virus. Rev Infect Dis 1991; 13(Suppl 11):S950–2. 10.1093/clind/13.supplement_11.s950. [DOI] [PubMed] [Google Scholar]
- 10. CDC Health Alert Network . Multisystem Inflammatory Syndrome in Children (MIS-C) associated with Coronavirus Disease 2019 (COVID-19). Available at: https://emergency.cdc.gov/han/2020/han00432.asp. Accessed 7 June 2022 .
- 11. Godfred-Cato S, Bryant B, Leung J, et al. COVID-19–associated multisystem inflammatory syndrome in children — United States, March–July 2020. MMWR Morb Mortal Wkly Rep 2020; 69:1074–80. 10.15585/mmwr.mm6932e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen X, Rostad CA, Anderson LJ, et al. The development and kinetics of functional antibody-dependent cell-mediated cytotoxicity (ADCC) to SARS-CoV-2 spike protein. Virology 2021; 559:1–9. 10.1016/j.virol.2021.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. van Erp EA, Luytjes W, Ferwerda G, et al. Fc-mediated antibody effector functions during respiratory syncytial virus infection and disease. Front Immunol 2019; 10:548. 10.3389/fimmu.2019.00548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen X, Anderson LJ, Rostad CA, et al. Development and optimization of a Zika virus antibody-dependent cell-mediated cytotoxicity (ADCC) assay. J Immunol Methods 2021; 488:112900. 10.1016/j.jim.2020.112900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tso FY, Lidenge SJ, Poppe LK, et al. Presence of antibody-dependent cellular cytotoxicity (ADCC) against SARS-CoV-2 in COVID-19 plasma. PLoS One 2021; 16:e0247640. 10.1371/journal.pone.0247640. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.