Abstract
The main pathogenic enteric viruses able to persist in the environment, such as hepatitis A virus (HAV), Norwalk-like virus (NLV), enterovirus (EV), rotavirus (RV), and astrovirus (AV), were detected by reverse transcription-PCR and hybridization in shellfish during a 3-year study. Oyster samples (n = 108), occasionally containing bacteria, were less frequently contaminated, showing positivity for AV (17%), NLV (23%), EV (19%), and RV (27%), whereas mussel samples, collected in areas routinely impacted by human sewage, were more highly contaminated: AV (50%), HAV (13%), NLV (35%), EV (45%), and RV (52%). Sequences obtained from HAV and NLV amplicons showed a great variety of strains, especially for NLV (strains close to Mexico, Snow Mountain Agent, or Norwalk virus). Viral contamination was mainly observed during winter months, although there were some seasonal differences among the viruses. This first study of virus detection over a fairly long period of time suggests that routine analysis of shellfish by a molecular technique is feasible.
Sewage pollution can contaminate shellfish-growing waters with various enteric viruses of human origin (37). However, only Norwalk-like viruses (NLVs) and hepatitis A virus (HAV) have been clearly implicated in outbreaks linked to shellfish consumption (13, 34, 50). Although several studies have evaluated enteroviruses as indicators of viral contamination of shellfish, little is known about annual variation in shellfish contamination with these and other human enteric viruses over a period of several years or about the possible effect of seasonality on virus prevalence in shellfish.
Technological advances in molecular detection methods have led to the development of sensitive, specific assays (e.g., reverse transcription [RT]-PCR and hybridization) for the detection of viruses, including those that grow poorly or not at all in cell culture, such as NLV, HAV, and rotavirus (RV). Moreover, preliminary steps, such as concentration of viruses from the sample and nucleic acid purification (removal of inhibitors), are essential for final PCR accuracy and reproducibility. Different methods have been proposed for determining viral contamination based on whole shellfish (8, 29) or dissected tissue (4, 50) for all types of virus (4, 22, 24) or for specific viruses (9, 10, 27). The method based on dissected tissues (4) is considered to be specific, reliable, and reproducible (3), providing a nucleic acid extract that allows detection of most enteric viruses (33). This method was used here to assess the viral contamination of five shellfish beds subjected to varying levels of pollution over a 3-year period. The main pathogenic enteric viruses able to persist in the environment were searched for, especially those previously implicated in outbreaks, such as HAV or NLV, or those already detected in the environment, such as enterovirus (EV), RV, or astrovirus (AV).
MATERIALS AND METHODS
Shellfish sampling.
Oysters (Crassostrea gigas) and mussels (Mytilus galloprovincialis) were collected monthly from August 1995 to August 1998 in southern France. Each sample was composed of at least 20 oysters or 30 mussels. Five sites were selected for their levels of bacterial contamination. The two mussel beds (D and E) were frequently contaminated by fecal coliforms (FCs) and Salmonella, classified in European Community (EC) categories C and D (prohibited for human consumption) (Directive 91/492/EC). The three oyster beds (A, B, and C) were occasionally contaminated by FCs and did not correspond to commercial areas (EC category B; subjected to purification, relaying, or cooking by an approved method). The shellfish were kept at 4°C during shipment and arrived at the laboratory 1 day after collection.
Shellfish processing for virus concentration.
On arrival, the shellfish were shucked, and the stomach and digestive diverticula were removed by dissection, aliquoted under 1.5 g, and kept frozen until analysis. Oyster samples (suspected to be less contaminated) were always dissected before mussel samples, and care was taken to avoid contamination among samples during dissection. Dissected tissues were frozen (−20°C) in aliquots of 1.5 g until they were processed (three aliquots were prepared for each sample). For analysis, the tissues were thawed on ice and processed as described previously with one minor modification for tissue homogenization (4, 33).
Primers.
All primers and probes have already been published and used in different studies (Table 1). Primers for EV, HAV, and RV detection were already used by us in environmental studies (14, 30). For NLV detection, primer selection was based on studies performed on reference strains (1, 21, 30). For AV, the primers amplified a small portion (89 bp) of the well-conserved 3′ end (40), and the specificity was confirmed by an internal probe (36).
TABLE 1.
Primer sets and probes used for human enteric virus detection
| Virus | Primer | Probe | Sequence | Locationa | Fragment length (bp) | Reference |
|---|---|---|---|---|---|---|
| AV | Mon2 | GCTTCTGATTAAATCAATTTT | 6776–6797 | 40 | ||
| Mon1 | CGAGTAGGATCGAGGGTA | 6709–6727 | 89 | 40 | ||
| Ap | ACCATTTAAAATTGATTTAATC | 6767–6786 | 36 | |||
| EV | E1 | TCCGGCCCCTGAATGCGG | 446–463 | 7 | ||
| E2 | CACCGGATGGCCAATCCAAT | 623–642 | 196 | 7 | ||
| Ep | ACACGGACACCCAAAGTAGTCGGTTGG | 533–559 | 7 | |||
| Primer 2 | CAAGCACTTCTGTTTCCCCGG | 162–182 | 26 | |||
| Primer 3 | ATTGTCACCATAAGCAGCCA | 577–596 | 434 | 26 | ||
| HAV | H1 | GGAAATGTCTCAGGTACTTTCTTTG | 2389–2413 | 43 | ||
| H2 | GTTTTGCTCCTCTTTATCATGCTATG | 2167–2192 | 247 | 43 | ||
| Hp | TCAACAACAGTTTCTACAGA | 2233–2252 | 43 | |||
| NLV | P110 | GTAAAACGACGGCCAGACDATYC | 4865–4884 | 28 | ||
| NVP36 | CAGGAAACAGCTATGACATAAAAG | 4487–4501 | 397 | 25 | ||
| P69 | GGCCTGCCATCTGGATTGCC | 4733–4752 | 150 | 25 | ||
| NI | GAATTCCATCGCCCACTGGCT | 4768–4788 | 116 | 21 | ||
| SR48 | GTGAACAGCATAAATCACTGG | 4766–4786 | 118 | 1 | ||
| SR50 | GTGAACAGTATAAACCACTGG | 4766–4786 | 118 | 1 | ||
| SR52 | GTGAACAGTATAAACCATTGG | 4766–4786 | 118 | 1 | ||
| NVP116 | CTCTGTGCACTITCTGAAGT | 4796–4815 | 28 | |||
| NVP117 | ACCTTGTGTGCCATGTCTGA | 4793–4812 | 28 | |||
| NVP118 | CTATGTGCACTGTCAGGAGT | 4796–4815 | 28 | |||
| SR47 | ATGTCAGGGGACAGGTTTGT | 4817–4836 | 1 | |||
| SR61 | ATGTCGGGGCCTAGTCCTGT | 4817–4836 | 1 | |||
| RV | Beg9 | GGCTTTAAAAGAGAGAATTTCCGTCTGG | 1–28 | 19 | ||
| R4 | GATCCTGTTGGCCATCC | 376–392 | 392 | 17 | ||
| RFP5 | GTATGGTATTGAATATACCAC | 51–71 | 17 | |||
| G1 | GTCACCATCATTGATTGAGTACTT | 315–339 | 48 | |||
| G2 | TTCATCATCTGAAATCTCATTTTTA | 315–339 | 48 | |||
| G3 | TGAATTATCATTTATTTCTGTTGCT | 315–339 | 48 | |||
| G4 | TTCAGTGTCACTAATTTGAGTTGGA | 315–339 | 48 |
RT-PCR.
RT-PCR was performed according to the instructions of the murine leukemia virus RT and Taq polymerase supplier (Perkin-Elmer Corp.). For RV amplification, RT-PCR was performed as previously described (14). PCR amplification was performed for 40 cycles (94°C for 30 s, 50°C for 30 s, and 72°C for 30 s) with final extension at 72°C for 7 min in a thermocycler (9600 or 2400 Cycler; Perkin-Elmer Corp.). The amplified products were detected by electrophoresis on a 9% polyacrylamide gel and stained with ethidium bromide (30).
Detection of inhibitory compounds.
Internal controls (IC) were used in RT-PCR to evaluate the presence of inhibitory compounds in enzymatic reactions. A single-stranded (ss) RNA IC was constructed from the EV genome (32), and a double-stranded (ds) RNA IC was constructed from the RV genome (14). Different primer sets and probes were used to avoid false-positive samples after dot blot hybridization (i.e., contamination by IC): ssRNA IC is amplified by primers 2 and 3 (26) but not by primer set E1-E2 (a primer set used preferentially for detection of EV contamination in shellfish samples), and dsRNA is not recognized by probe RFP5.
For inhibitor monitoring, 1 μl of a dilution of IC 10-fold higher than the limit detectable by RT-PCR was mixed with 1 μl of each nucleic acid extract and subjected to amplification. ssRNA and dsRNA IC were tested separately. When inhibitory compounds were present, an additional purification step was performed: nucleic acid extracts were filtered through a Sephadex G150 column or adsorbed onto granular cellulose as previously described (30). If inhibition persisted, a new extraction was done. When no inhibitors were detected, RT-PCR was performed in the absence of added IC to avoid false-negative results due to competition.
Hybridization.
For dot blot analysis, the PCR product was diluted in a buffer (10 mM Tris–HCl [pH 8.0], 1 mM EDTA [pH 8.0]), denatured for 5 min at 95°C, and chilled directly on ice. The PCR products were blotted onto a positively charged nylon membrane (Boehringer Mannheim) under vacuum and fixed for 5 min by UV cross-linking. Positive controls were introduced on each membrane to control hybridization. All probes were labeled with digoxigenin using the 3′ tailing kit (Boehringer Mannheim). After prehybridization for 30 min at 50°C, hybridization was performed at 50°C for 2 h (except for RV probes at 42°C). The hybridized probes were detected by chemiluminescence (Boehringer Mannheim) according to the manufacturer's protocol using a Bio-Rad multi-imager. A sample was considered positive if a positive signal was obtained after hybridization with or without previous observation of a band of the expected size on the gel.
Sequencing.
RT-PCR products were cloned into the pCRII vector in the TA cloning kit (Invitrogen, Leek, The Netherlands). After transformation into TA Cloning One shot-competent cells (Invitrogen), positive clones were identified by miniprep analysis. Sequencing was performed on at least three different randomly selected clones (ESGS, Paris, France) with an ABI 373A automated sequencer and a Taq DieDeoxy Terminator cycle-sequencing kit (Applied Biosystems).
Sequence analysis was done with the Distances and Growtree programs from the Genetics Computer Group suite of programs (INFOBIOGEN, Centre National de Recherche Scientifique, Paris, France).
Bacteriological analysis.
Shellfish were shucked, and the tissue and liquor were homogenized in a Waring blender with 1 volume of 10% (wt/vol) NaCl-water. FCs were determined by conductance measurement (15), and Salmonella was detected using the technique specified by the DGAL/SVHA/C8/V08-052 circular of September 1993 (enrichment in liquid medium and culture on Hektoen plates).
RESULTS
A total of 181 shellfish samples were collected during the study: 108 oyster samples from sites A, B, and C and 73 mussel samples from sites D and E. Three oyster samples (the November and December 1997 samples from site B and the August 1997 sample from site C) and one mussel sample (the May 1996 sample from site D) could not be collected as scheduled. Oyster samples were obtained from areas with a low level of fecal contamination, and only nine samples (8%) showed elevated FC counts (>300 FC/100 g). Three of these nine specimens had no detectable virus contamination. Mussels, however, were collected from an area known to be heavily impacted by human sewage, and 28 of the samples (38%) showed elevated FC counts. Five of these specimens contained no detectable enteric viruses. Fifty-nine oyster samples (55%) and 55 mussel samples (75%) contained one or more enteric viruses. Only 39 oyster samples (36%) and 12 mussel samples (16%) had no FCs and no enteric viruses.
Analysis of oyster samples.
Oysters were collected from three sites subjected to occasional bacterial contamination as confirmed by FC counts. Bacterial contamination above the European standard (>300 FC/100 g) was detected in nine samples, and Salmonella was detected in one sample (January 1996). Viral contamination was found mainly during the cold season (from October to March). Nine of the 11 oyster samples contaminated by more than three types of viruses were detected in December and January (Table 2).
TABLE 2.
Detection of enteric viruses in the five shellfish-sampling points during the 3-year study
| Date | Presence of virusa
|
|||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Site A
|
Site B
|
Site C
|
Site D
|
Site E
|
||||||||||||||||||||||||||
| EV | HAV | NLV | AV | RV | FCsb | EV | HAV | NLV | AV | RV | FCs | EV | HAV | NLV | AV | RV | FCs | EV | HAV | NLV | AV | RV | FCs | EV | HAV | NLV | AV | RV | FCs | |
| 1995 | ||||||||||||||||||||||||||||||
| Aug | ND | ND | <30 | + | 140 | + | + | ND | ||||||||||||||||||||||
| Sep | + | 500 | 57 | <30 | + | ND | + | + | + | + | + | 3,400 | ||||||||||||||||||
| Oct | + | <30 | <30 | <30 | + | 78 | + | + | 300 | |||||||||||||||||||||
| Nov | + | 30 | + | 60 | + | <30 | + | + | + | 832 | + | + | ND | |||||||||||||||||
| Dec | + | + | + | 640 | + | <30 | 159 | + | + | + | + | + | 1,789 | + | + | + | + | ND | ||||||||||||
| 1996 | ||||||||||||||||||||||||||||||
| Jan | + | + | + | 2,970 | + | + | + | 300 | + | 51 | + | + | + | + | 2,600 | + | + | + | + | 3,380 | ||||||||||
| Feb | + | + | 213 | + | + | + | <30 | + | <30 | 733 | + | + | + | ND | ||||||||||||||||
| Mar | + | + | ND | + | + | + | ND | + | + | <30 | 7,800 | + | + | + | + | ND | ||||||||||||||
| Apr | ND | ND | <30 | + | + | + | <30 | + | + | 243 | ||||||||||||||||||||
| May | <30 | <30 | + | + | 2,970 | NS | NS | NS | NS | NS | NS | + | + | 128 | ||||||||||||||||
| Jun | <30 | + | 45 | <30 | + | + | + | 1,390 | + | + | <30 | |||||||||||||||||||
| Jul | + | + | ND | ND | 139 | 128 | ND | |||||||||||||||||||||||
| Aug | + | <30 | + | 49 | + | <30 | + | + | <30 | + | + | + | + | 128 | ||||||||||||||||
| Sep | ND | + | ND | + | 143 | <30 | + | + | ND | |||||||||||||||||||||
| Oct | <30 | ND | + | ND | + | + | + | 80 | + | + | 404 | |||||||||||||||||||
| Nov | + | + | 108 | + | 47 | + | <30 | + | + | + | + | 2,020 | + | + | + | + | 46 | |||||||||||||
| Dec | + | + | + | + | <30 | + | + | + | + | 341 | + | + | + | + | <30 | + | + | + | + | + | 875 | + | + | + | + | 760 | ||||
| 1997 | ||||||||||||||||||||||||||||||
| Jan | + | + | + | + | 937 | + | + | + | + | 31 | + | + | + | + | <30 | + | + | + | + | + | 1,100 | + | + | + | + | ND | ||||
| Feb | <30 | <30 | + | <30 | + | + | + | + | + | 48 | + | 520 | ||||||||||||||||||
| Mar | <30 | <30 | <30 | + | + | + | <30 | + | + | + | <30 | |||||||||||||||||||
| Apr | <30 | 232 | + | <30 | <30 | + | + | <30 | ||||||||||||||||||||||
| May | 2,970 | 130 | <30 | 139 | + | + | 645 | |||||||||||||||||||||||
| Jun | + | 95 | + | <30 | <30 | + | 500 | <30 | ||||||||||||||||||||||
| Jul | 53 | + | <30 | + | 115 | <30 | 3,870 | |||||||||||||||||||||||
| Aug | <30 | + | <30 | NS | NS | NS | NS | NS | NS | 387 | + | 83 | ||||||||||||||||||
| Sep | + | <30 | + | <30 | + | <30 | 1,200 | + | 341 | |||||||||||||||||||||
| Oct | + | 36 | + | <30 | + | <30 | 44 | + | + | 264 | ||||||||||||||||||||
| Nov | + | <30 | NS | NS | NS | NS | NS | NS | <30 | 44 | + | + | 3,350 | |||||||||||||||||
| Dec | + | <30 | NS | NS | NS | NS | NS | NS | + | <30 | + | + | + | 233 | + | + | + | 265 | ||||||||||||
| 1998 | ||||||||||||||||||||||||||||||
| Jan | + | + | 41 | + | 139 | + | <30 | + | + | 2,280 | + | + | 937 | |||||||||||||||||
| Feb | 47 | + | <30 | <30 | + | + | 568 | + | 387 | |||||||||||||||||||||
| Mar | + | 197 | <11 | + | <11 | + | + | ND | + | + | + | + | ND | |||||||||||||||||
| Apr | + | <30 | <30 | 3,380 | + | + | 265 | + | + | + | + | 341 | ||||||||||||||||||
| May | 5,670 | <30 | 300 | 261 | 3,380 | |||||||||||||||||||||||||
| Jun | + | <30 | <30 | <30 | + | 205 | + | <30 | ||||||||||||||||||||||
| Jul | <30 | 69 | <30 | 265 | 122 | |||||||||||||||||||||||||
| Aug | + | 53 | <30 | <30 | 568 | 53 | ||||||||||||||||||||||||
+, sample found positive by RT-PCR and hybridization for EV, HAV, NLV, AV, or RV.
FC concentration (most probable number/100 g of shellfish) detected in the shellfish sample. Underlined numbers indicate the presence of Salmonella; ND, not done; boldface numbers, above the acceptable European bacterial standard (forbidden for human consumption); all others are in accordance with the European bacterial standard for human consumption; NS, no sample.
For the three sites, AV was detected in 18 samples (17%), NLV was detected in 25 samples (23%), EV was detected in 21 samples (19%), and RV was detected in 30 samples (27%). HAV was never detected in oyster samples (Table 2).
Single-virus-type contamination was detected in 41 samples: 5 AV, 9 EV, 13 NLV, and 14 RV (Table 2). This single-virus-type contamination was detected year-round, but six samples contaminated by only NLV were detected during November. Six samples contaminated by EV (among nine positive samples) were detected from August to October. Bacterial contamination was lower than the European standard for 38 samples contaminated by viruses.
Analysis of mussel samples.
Mussels were collected in areas subjected to sewage discharge and were prohibited for collection and human consumption. Several positive samples were detected in these two sites.
For site D, 13 samples were negative and 23 were positive for viral contamination. AV was detected in 17 samples, NLV was detected in 9 samples, EV was detected in 14 samples, RV was detected in 16 samples, and HAV was detected in 6 samples (Table 2). At this site, HAV was detected only in November, December, or January. Thirty-three percent of the samples (12 of 36) were contaminated by at least three types of viruses, and 9 of these samples were detected during cold months (October to February). Single-virus-type contamination was detected in six samples: two by RV (August 1995 and June 1998), two by AV (September 1995 and June 1997), one by EV (October 1995), and one by NLV (August 1997; FCs and Salmonella were also present).
For site E, only four samples were negative for all viruses searched for. Thirty-five percent of the samples (13 of 37) were contaminated by at least three types of virus, mainly between November and April (11 of 13). NLV was detected as a single viral contaminant twice (February 1997 and June 1998) in association with bacterial contamination. RV was detected alone in three samples (August 1997 and February and May 1998), two of which were also contaminated by FCs. AV was detected alone in the July 1997 sample with no bacteria. For this site, AV was detected in 20 samples, NLV was detected in 17 samples, EV was detected in 19 samples, RV was detected in 22 samples, and HAV was detected in 4 samples. HAV was detected in September 1995 and in March and April 1998 and was always associated with another viral contaminant and bacteria.
Seasonal variations.
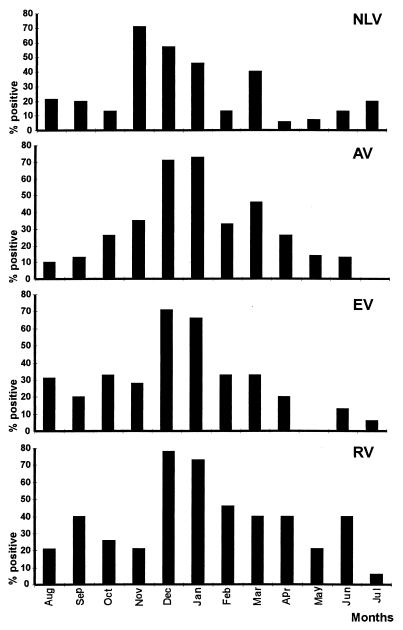
Results obtained for the five sampling points during the 3-year study were pooled by month to evaluate the seasonal distribution. Viral contamination was detected mainly during cold months (November to March) (Fig. 1). However, a different pattern was seen with each virus. NLV was low in spring but increased slightly during the summer and was detected most frequently in November. AV was very low during the summer (no detection at all in July) and increased during the winter. EV followed the same pattern as AV, although an increase was also observed during late summer (mainly August). RV showed no seasonal distribution and varied from one month to another. There were not enough positive HAV samples to study seasonal variation.
FIG. 1.
Seasonal variations observed during the 3-year study. The results are expressed as the percentage of positive samples for each virus per month for the five sampling points over the 3 years.
Sequence analysis. (i) NLV.
Among the 51 positive samples for NLV, 23 sequences were obtained from 21 samples (11 from oyster samples and 10 from mussel samples). Sequences were obtained from 10 amplicons amplified by P110/P36, 6 amplified by P110/NI, 6 amplified by P110/SR, and 1 amplified by P110/P69. Both genogroup 1 and 2 NLVs were detected at all five points during the 3-year period (Fig. 2). For example, in January 1996 a strain closely related to Desert Shield virus was found in a mussel sample (site E) and a strain similar to Lordsdale virus was found in an oyster sample (site B). Some strains detected in 1995, 1996, and 1997 in oyster and mussel samples seemed to be very similar. The same sequence was found at one sampling point (site D) in November 1996 and at sites D and E 5 months later (March 1997). Two mussel samples from site B were contaminated by two different strains from genogroup 1 in October 1995 and December 1996.
FIG. 2.
Phylogenetic analysis of NLV sequences obtained from shellfish samples during the 3-year study. The phylogenetic analysis was performed on 80-base nucleotide sequences from the RNA polymerase region with a GROWTREE phylogram using uncorrected distances. The sequences are designated by the letter of the sampling site (A to E) and by the month and year of sample collection. For example C-0697 is the sequence obtained from the oyster sample from site C collected in June 1997. The GenBank accession numbers of the reference strains are as follows: Toronto virus, U02030; Mexico virus, U22498; Snow Mountain Agent (SMA), L23831; Lordsdale virus, X86557; Hawaii virus, U07611; Southampton virus, L07418; desert shield virus (DSV), U04469; and Norwalk virus, M87661. The scale represents the number of nucleotide substitutions per nucleotide site.
(ii) HAV.
Seven samples were sequenced among the 10 positive samples, including four sequences obtained from 6 positive site D samples and three sequences from 4 positive site E samples. Analysis of the 150 bases of the N-terminal VP1 capsid protein region revealed that all sequences obtained showed 90.6 to 96% identity with the sequence of the HM175 reference strain and thus belonged to subgenotype IB (Fig. 3). The two sequences detected in site E in January and March 1998 were identical. However, the two sequences detected in site D in November and December 1995 showed a difference of 3 bases (both samples were sequenced twice in both directions to confirm these differences).
FIG. 3.
Aligment of the nucleotide sequences obtained from the HAV amplicons of the N-terminal VP1 capsid protein region of the positive samples with the published sequence HM175. The percentage at the end of each sequence represents identity with the reference strain HM175.
DISCUSSION
The purpose of this study was to evaluate the feasibility of monitoring shellfish viral contamination and obtaining viral-contamination data over a period of several years for the main pathogenic enteric viruses able to persist in the environment. Different sensitive methods are now available to evaluate viral contamination in shellfish (10, 11, 27, 28, 50). The method used in this study was based on dissected-tissue analysis (4, 27), which offers several advantages: (i) previous studies have shown that most viruses are concentrated in these tissues (44, 46), (ii) dissected hepato-pancreas tissue has fewer inhibitors than whole tissue, and (iii) the results obtained are more representative of the global contamination of shellfish samples (1.5 g of pancreatic tissue represents about 5 oysters and 10 mussels). Another important point is that the sensitivity threshold is sufficient to detect positive results after gel electrophoresis (data not shown), thus allowing PCR product sequencing.
Several measures were taken in order to obtain reliable results. First, all shellfish extractions were performed by the same person. Second, RT-PCR master mix was prepared for 50 reactions, controlled with a viral or IC RNA, and then used for shellfish analysis without a positive control (to avoid cross-contamination) but with a negative control. Third, no more than 10 samples were analyzed at the same time to avoid cross-contamination and excessive handling time between the first and last reaction tubes. Fourth, each extract was controlled for inhibitors by prior amplification with an ssIC and a dsIC to avoid false-negative results. Fifth, all RT-PCR products were hybridized under stringent conditions to confirm the specificity of the amplification and to enhance sensitivity. Finally, positive samples for NLV and HAV were amplified a second time prior to cloning and sequencing to confirm the first result and to be sure that the PCR product had not been contaminated during PCR analysis (gel and dot blot).
Since the publication of studies dealing with EVs in the 1960s and 1970s (38), reports of viral contamination in natural shellfish have been infrequent, and little is known about the occurrence of viruses in shellfish beds. Based on our previous report (30), two types of sites were selected: those occasionally contaminated with bacteria (the three oyster beds; purification is needed before consumption according to the EC regulation) and those under continuous sewage contamination and prohibited from production for human consumption (the two wild-mussel beds, as this site was too contaminated for oyster growth). This selection was made to evaluate whether a difference in bacterial contamination (occasionally or routinely) could be responsible for a difference in viral contamination of shellfish. The results showed good correlation between bacterial and viral contamination in sites heavily impacted by human sewage but no correlation in sites occasionally contaminated by sewage discharge, which raises a question as to the choice of bacterial indicator in such sites. Moreover, the data obtained from these two different sites and species of shellfish vary and thus require separate comment.
In polluted sites, more than 75% of shellfish were contaminated by at least one type of virus. It is surprising that RV was detected in site D almost year-round in 1997, since this virus causes seasonal peaks of gastroenteritis during the winter in France (12). Recently, Green and Lewis (20), who used primers amplifying a part of gene 6, also detected RV year-round in sewage and shellfish. One possible explanation for these observations could be virus shedding by the population all year long. However, as viruses are degraded more rapidly at warm temperatures and in sunlight (38), high concentrations need to be excreted during the summer to contaminate shellfish, which is not in accordance with clinical data. Another explanation could be that the primers used amplified animal strains; this was shown to be possible by restriction fragment length polymorphism in sewage (14). This potential shellfish contamination by animal strains needs to be considered, since reassortment has been reported for RV strains (45).
The other notable point about mussel samples concerned HAV detection. The level of contamination (13%) was about the same as that found by Lee et al. (27) (12%) and Chung et al. (8) (15%), whereas other authors have reported detection in sediment or water but not in shellfish (20, 42). At site D, HAV was found regularly each winter during the 3-year study. In this area of France, many inhabitants have come from North Africa, where HAV is endemic, and often return for vacation during August. In terms of incubation time (6 to 8 weeks), this virus input could be attributed to the infection of children during August and the development of subclinical illness in October or November. The strains detected are closely related to sequences reported in a molecular study performed on South African strains (51). In our study, only strains belonging to subgenotype I were detected, whereas in western France, both subgenotypes 1A and B have been reported (2).
Viral contamination was less frequent in oyster samples. First, 45% of the samples were not contaminated and 38% were contaminated by only one type of virus, whereas only 13% of mussel samples showed a single type of contamination. Second, most contaminated samples were detected during the cold months, and very few were positive during spring or summer, except for six samples positive for EV during the summer of 1996. Data obtained from the French EV monitoring network (managed by the National Center for Enteroviruses and Hepatitis A, Lyon, France) showed an increase of EV infection in France during 1996, which could account for the contamination observed in the environment.
Contamination was less frequent in oyster than in mussel samples: 3-fold less for AV (17 versus 51%), 2.5-fold less for EV (19 versus 45%), and 2-fold less for RV (27 versus 52%). However, NLV showed a different pattern, with detection in 23% of oyster samples and in 35% of mussels (only a 1.5-fold difference). No explanation can be given for this difference, and more data are needed to confirm this observation. In England, NLV contamination was found in 27% of samples collected at a highly polluted field site (28) and in 56% of samples obtained from a commercial producer before depuration (23). NLV and RV were detected as single contaminants in 13 and 13.9%, respectively, of oyster samples, with no bacterial contamination (except for one NLV-positive sample). This may be an explanation for NLV outbreaks linked to consumption of shellfish meeting bacterial criteria. On the other hand, RV has never been clearly incriminated in outbreaks linked to shellfish consumption. Other factors, such as immunity and asymptomatic illness, need to be taken into account.
AV was detected alone in only 4.6% of oyster samples, and its occurrence rate among all samples was quite high (30% of oyster and mussel samples). It is surprising that so many positive samples were detected, since epidemiological data indicate a low frequency of gastroenteritis due to AV. The primers used have been proved specific for serotypes 1 to 4 (36), and they were very sensitive in our study, in which many samples gave positive signals on gel that were all confirmed by hybridization (data not shown). However, as they amplified a conserved region, it was impossible to differentiate the serotypes. As AV is very resistant, it can persist in marine waters for extended periods of time (6), which may account for the high contamination observed. A clear seasonal pattern was apparent, with no virus in summer and a contamination peak in winter concomitant with gastroenteritis in the population. This absence of AV during the summer has also been reported for sewage and environmental samples (16).
The analysis of environmental samples involves problems with inhibitors, low virus concentrations, and sequence variation. As the concentration-extraction procedure is not virus specific, the nucleic acid of several viruses can be extracted at the same time. RT-PCR must be performed under stringent conditions and confirmed by hybridization. It is very difficult to sequence amplicons, since most PCR signals are very weak. This is particularly important for NLV detection, as strain diversity is very large. Broadly reactive primers are required to detect all these strains, as well as an annealing temperature sufficient to ensure specificity. In the absence of a unique primer set consensus sequence for the detection of these strains, one degenerate primer was used for RT and six primers were used for PCR in four sets. These results confirm our previous report (31), indicating the value of these different primer sets, since each primer set was useful for obtaining a sequence. During the 3-year period of the study, a very large diversity of strains was found among NLVs. Strains close to prototype strains (Mexico, Snow Mountain Agent, or Norwalk) were detected throughout the study and at all the sampling sites. The same sequence was found at intervals of several months, and 11 samples were contaminated by a closely related sequence. As the sequence compared was short (80 bp) and the region was variable (polymerase), no conclusions could be drawn about strain identity. In England, a strain was found repeatedly over a 4-month period (23). Moreover, two of our samples were contaminated by two sequences. This cocontamination has already been observed in shellfish implicated in an outbreak (50) or collected in a commercial area (23). Although little is currently known about NLV circulation in France, a study of acute gastroenteritis in children showed that Bristol- and Toronto-like strains were detected most frequently (5). Epidemiological studies characterizing strains circulating in different countries (35, 41, 52) have shown the predominance of one strain over several years (35), with outbreaks of the same strains at the same time at geographically distant points (41). Contaminated shellfish, and more generally a polluted environment, can play an important role either as reservoirs for some virus strains that cause outbreaks at intervals of several months or as tools to disseminate some strains via commercial exchange.
To our knowledge no data about viral contamination over a 3-year period has been previously reported. The results obtained in our study were difficult to interpret, and the time period was too short to allow a clear demonstration of seasonality or a relationship between fecal indicators and the different viruses screened. In fact, the main purpose of the study was to consider the feasibility of evaluating shellfish viral contamination on a routine basis. Several environmental (rain, temperature, sewage treatment plant efficiency, etc.) or population (epidemic or endemic viral circulation, etc.) factors that could help in interpreting data are currently under investigation. A first approach has shown that winter gastroenteritis outbreaks have an impact on the environment and can be responsible for the increased viral contamination observed in that season (39).
The mean number of positive samples found in this study is in accordance with data in the literature indicating that no real difference exists in this respect among countries with shellfish-growing areas. Even though technical improvements are still needed for RT-PCR (47), quantification (18, 32), and PCR significance (49), current methods allow the detection of viruses in the environment. These data will be helpful in enabling epidemiologists to understand virus circulation and allowing producers to commercialize “virus-safe shellfish.” These methods will also be of particular importance for improved shellfish depuration (46).
ACKNOWLEDGMENTS
We are grateful to J. C. Sauvagnardes and collaborators for sampling and bacteriological analysis and to H. Kopecka (Institut Pasteur, France), D. Hervio-Heath (IFREMER), and M. K. Estes (Baylor College of Medicine, Houston, Tex.) for their helpful advice and comments on the manuscript. We are indebted to R. Atmar (Baylor College of Medicine) for a critical review of the manuscript.
Financial support was received from the French Ministry of Environment (Contrat INSERM 1996 no. EN96C11).
REFERENCES
- 1.Ando T, Jin Q, Gentsch J R, Monroe S, Noel J, Dowell S F, Cicirello H G, Khon M A, Glass R I. Epidemiologic applications of novel molecular methods to detect and differentiate small round structured viruses (Norwalk-like viruses) J Med Virol. 1995;47:145–152. doi: 10.1002/jmv.1890470207. [DOI] [PubMed] [Google Scholar]
- 2.Apaire-Marchais V, Robertson B H, Aubineau-Ferre V, Le Roux M G, Leveque F, Schawartzbrod L, Billaudel S. Direct sequencing of hepatitis A virus strains isolated during an epidemic in France. Appl Environ Microbiol. 1995;61:3977–3980. doi: 10.1128/aem.61.11.3977-3980.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atmar R L, Neil F H, Woodley C M, Manger R, Fout G S, Burkhardt W, Leja L, McGovern E R, Le Guyader F, Metcalf T G, Estes M K. Collaborative evaluation of a method for the detection of Norwalk virus in shellfish tissues by PCR. Appl Environ Microbiol. 1996;62:254–258. doi: 10.1128/aem.62.1.254-258.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Atmar R L, Neill F H, Romalde J L, Le Guyader F, Woodley C M, Metcalf T G, Estes M K. Detection of Norwalk virus and hepatitis A virus in shellfish tissues with the PCR. Appl Environ Microbiol. 1995;61:3014–3018. doi: 10.1128/aem.61.8.3014-3018.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bon F, Fascia P, Dauvergne M, Tenenbaum D, Planson H, Petion A M, Pothier P, Kohli E. Prevalence of group A rotavirus, human calcivirus, astrovirus, and adenovirus type 40 and 41 infections among children with acute gastroenteritis in Dijon, France. J Clin Microbiol. 1999;37:3055–3058. doi: 10.1128/jcm.37.9.3055-3058.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bosch A, Pinto R, Villena C, Abad F X. Persistence of human astroviruses in fresh and marine water. Water Sci Tech. 1997;35:243–247. [Google Scholar]
- 7.Chapman N M, Tracy S, Gauntt C J, Fortmueller U. Molecular detection and identification of enteroviruses using enzymatic amplification and nucleic acid hybridization. J Clin Microbiol. 1990;28:843–850. doi: 10.1128/jcm.28.5.843-850.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chung H, Jaykus L A, Sobsey M D. Detection of human enteric viruses in oysters by in vivo and in vitro amplification of nucleic acids. Appl Environ Microbiol. 1996;62:3772–3778. doi: 10.1128/aem.62.10.3772-3778.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Croci L, De Medici G, Morace G, Fiore A, Scalfaro C, Beneduce F, Toti L. Detection of hepatitis A virus in shellfish by nested reverse transcription-PCR. Int J Food Microbiol. 1999;48:67–71. doi: 10.1016/s0168-1605(99)00028-8. [DOI] [PubMed] [Google Scholar]
- 10.Cromeans T L, Nainan O V, Margolis H S. Detection of hepatitis A virus RNA in oyster meat. Appl Environ Microbiol. 1997;63:2460–2463. doi: 10.1128/aem.63.6.2460-2463.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Medici D, Beneduce F, Fiore A, Scalfaro C, Croci L. Application of reverse transcriptase nested PCR for detection of poliovirus in mussels. Int J Food Microbiol. 1998;40:51–56. doi: 10.1016/s0168-1605(98)00015-4. [DOI] [PubMed] [Google Scholar]
- 12.Desenclos J C, Rebiere L, Letrillard L, Flahaut A, Hubert B. Diarrhoea-related morbidity and rotavirus infection in France. Acta Paediatr Suppl. 1999;426:42–47. doi: 10.1111/j.1651-2227.1999.tb14325.x. [DOI] [PubMed] [Google Scholar]
- 13.Desenclos J C, Klontz K C, Wilder M H, Naiman O V, Margolis H S, Gunn R A. A multistate outbreak of hepatitis A caused by the consumption of raw oysters. Am J Public Health. 1991;81:1268–1272. doi: 10.2105/ajph.81.10.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dubois E, Le Guyader F, Haugarreau L, Kopecka H, Cormier M, Pommepuy M. Molecular epidemiological survey of rotaviruses in sewage by reverse transcriptase seminested PCR and restriction fragment length polymorphism assay. Appl Environ Microbiol. 1997;63:1794–1800. doi: 10.1128/aem.63.5.1794-1800.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dupont J, Menard D, Herve C, Chevallier F, Belliaef B, Minier B. Rapid estimation of Escherichia coli in live marine bivalve shellfish using automated conductance measurement. J Appl Bacteriol. 1996;80:81–90. doi: 10.1111/j.1365-2672.1996.tb03193.x. [DOI] [PubMed] [Google Scholar]
- 16.Egglestone S I, Caul E O, Vipond L B, Darville J M. Absence of human astrovirus RNA in sewage and environmental samples. J Appl Microbiol. 1999;86:709–714. doi: 10.1046/j.1365-2672.1999.00717.x. [DOI] [PubMed] [Google Scholar]
- 17.Flores J, Sears J, Schael I P, White L, Garcia D, Lanata C, Kapikian A Z. Identification of human rotavirus serotype by hybridization to polymerase chain reaction-generated probes derived from a hyperdivergent region of the gene encoding outer capsid protein VP7. J Virol. 1990;64:4021–4024. doi: 10.1128/jvi.64.8.4021-4024.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goswani B B, Koch W H, Cebula T A. Competitor template RNA for detection and quantitation of hepatitis A virus by PCR. BioTechniques. 1994;16:114–121. [PubMed] [Google Scholar]
- 19.Gouvea V, Glass R I, Woods P, Taniguchi K, Clark H F, Forrester B, Fang Z. Polymerase chain reaction amplification and typing rotavirus nucleic acid from stool specimens. J Clin Microbiol. 1990;28:276–282. doi: 10.1128/jcm.28.2.276-282.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Green D H, Lewis G D. Comparative detection of enteric viruses in wastewaters, sediments and oysters by reverse transcription-PCR and cell culture. Water Res. 1999;33:1195–1200. [Google Scholar]
- 21.Green J, Gallimore C I, Norcott J P, Lewis D, Brown D W G. Broadly reactive reverse transcriptase polymerase chain reaction for the diagnosis of SRSV associated gastroenteritis. J Med Virol. 1995;47:392–398. doi: 10.1002/jmv.1890470416. [DOI] [PubMed] [Google Scholar]
- 22.Green J, Henshilwood K, Gallimore C I, Brown D W G, Lees D N. A nested reverse transcriptase PCR assay for detection of small round structured viruses in environmentally contaminated molluscan shellfish. Appl Environ Microbiol. 1998;64:858–863. doi: 10.1128/aem.64.3.858-863.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hensilwood K, Green J, Lees D N. Monitoring the marine environment for small round structured viruses (SRSVS): a new approach to combating the transmission of these viruses by molluscan shellfish. Water Sci Tech. 1998;38:51–56. [Google Scholar]
- 24.Jaykus L A, De Leon R, Sobsey M D. A virion concentration method for detection of human enteric viruses in oysters by PCR and oligoprobe hybridization. Appl Environ Microbiol. 1996;62:2074–2080. doi: 10.1128/aem.62.6.2074-2080.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang X, Wang J, Graham D Y, Estes M K. Detection of Norwalk virus in stool by polymerase chain reaction. J Clin Microbiol. 1992;30:2529–2534. doi: 10.1128/jcm.30.10.2529-2534.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kopecka H, Dubrou S, Prevot J, Marechal J, Lopez-Pila J M. Detection of naturally occurring enteroviruses in waters by reverse transcription, polymerase chain reaction and hybridization. Appl Environ Microbiol. 1993;59:1213–1219. doi: 10.1128/aem.59.4.1213-1219.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee T, Yam W C, Tam T Y, Ho B S W, Ng M H, Broom M J. Occurrence of hepatitis A virus in green-lipped mussels (Perna viridis) Water Res. 1999;33:885–889. [Google Scholar]
- 28.Lees D N, Henshilwood K, Gallimore C I, Brown D W G. Detection of small round structured viruses in shellfish by reverse transcription-PCR. Appl Environ Microbiol. 1995;61:4418–4424. doi: 10.1128/aem.61.12.4418-4424.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lees D N, Henshilwood K, Dore W J. Development of a method for detection of enteroviruses in shellfish by PCR with poliovirus as a model. Appl Environ Microbiol. 1994;60:2999–3005. doi: 10.1128/aem.60.8.2999-3005.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Le Guyader F, Dubois E, Menard D, Pommepuy M. Detection of hepatitis A virus, rotavirus, and enterovirus in naturally contaminated shellfish and sediment by reverse transcription-seminested PCR. Appl Environ Microbiol. 1994;60:3665–3671. doi: 10.1128/aem.60.10.3665-3671.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Le Guyader F, Estes M K, Hardy M E, Neill F H, Green J, Brown D W G, Atmar R L. Evaluation of a degenerate primer for the PCR detection of human caliciviruses. Arch Virol. 1996;141:2225–2235. doi: 10.1007/BF01718228. [DOI] [PubMed] [Google Scholar]
- 32.Le Guyader F, Menard D, Dubois E, Haugarreau L, Kopecka H, Pommepuy M. Use of an RT-PCR internal control to evaluate viral removal. Water Sci Tech. 1997;35:461–465. [Google Scholar]
- 33.Le Guyader F, Miossec L, Haugarreau L, Dubois E, Kopecka H, Pommepuy M. RT-PCR evaluation of viral contamination in five shellfish beds over a 21-month period. Water Sci Tech. 1998;38:45–50. [Google Scholar]
- 34.Le Guyader F, Neill F H, Estes M K, Monroe S S, Ando T, Atmar R L. Detection and analysis of a small round structured virus strain in oysters implicated in an outbreak of acute gastroenteritis. Appl Environ Microbiol. 1996;62:4268–4272. doi: 10.1128/aem.62.11.4268-4272.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maguire A J, Green J, Brown D W G, Desselberger U, Gray J J. Molecular epidemiology of outbreaks of gastroenteritis associated with small round structured viruses in East Anglia, United Kingdom, during the 1996–1997 season. J Clin Microbiol. 1999;37:81–89. doi: 10.1128/jcm.37.1.81-89.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marx F E, Taylor M B, Grabow W O K. A comparison of two sets of primers for the RT-PCR detection of astrovirus in environmental samples. Water S A. 1997;23:257–262. [Google Scholar]
- 37.Metcalf T G. Viruses in shellfish growing waters. Environ Int. 1982;7:21–27. [Google Scholar]
- 38.Metcalf T G, Melnick J L, Estes M K. Environmental microbiology: from detection of virus in sewage and water by isolation to identification by molecular biology—a trip of over 50 years. Annu Rev Microbiol. 1995;49:461–487. doi: 10.1146/annurev.mi.49.100195.002333. [DOI] [PubMed] [Google Scholar]
- 39.Miossec L, Le Guyader F, Haugarreau L, Comps M A, Pommepuy M. Possible relationship between a winter epidemic of acute gastroenteritis in France and viral contamination of shellfish. J Shellfish Res. 1998;17:1661–1664. [Google Scholar]
- 40.Mitchell D K, Monroe S S, Jiang X, Matson D O, Glass R I, Pickering L K. Virologic features of an astrovirus diarrhea outbreak in a day care center revealed by reverse transcriptase polymerase chain reaction. J Infect Dis. 1995;172:1437–1444. doi: 10.1093/infdis/172.6.1437. [DOI] [PubMed] [Google Scholar]
- 41.Noel J S, Fankhauser R L, Ando T, Monroe S S, Glass R I. Identification of a distinct common strain of ‘Norwalk-like viruses’ having a global distribution. J Infect Dis. 1999;179:1334–1344. doi: 10.1086/314783. [DOI] [PubMed] [Google Scholar]
- 42.Pina S, Puig M, Lucena F, Jofre J, Girones R. Viral pollution in the environment and in shellfish: human adenovirus detection by PCR as an index of human viruses. Appl Environ Microbiol. 1998;64:3376–3382. doi: 10.1128/aem.64.9.3376-3382.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roberston B H, Brown V K, Khanna B. Altered hepatitis A VP1 protein resulting from cell culture propagation of virus. Virus Res. 1989;13:207–212. doi: 10.1016/0168-1702(89)90016-6. [DOI] [PubMed] [Google Scholar]
- 44.Romalde J L, Estes M K, Szucs G, Atmar R L, Woodley C M, Metcalf T G. In situ detection of hepatitis A virus in cell cultures and shellfish tissues. Appl Environ Microbiol. 1994;60:1921–1926. doi: 10.1128/aem.60.6.1921-1926.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Santos N, Lima R C C, Nozawa C M, Linhares R E, Gouvea V. Detection of porcine rotavirus type G9 and a mixture of types G1 and G5 associated with Wa-like VP4 specificity: evidence for natural human-porcine genetic reassortment. J Clin Microbiol. 1999;37:2734–2736. doi: 10.1128/jcm.37.8.2734-2736.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schwab K J, Neill F H, Estes M K, Metcalf T G, Atmar R L. Distribution of Norwalk virus within shellfish following bioaccumulation and subsequent depuration by detection using RT-PCR. J Food Prot. 1998;61:1674–1680. doi: 10.4315/0362-028x-61.12.1674. [DOI] [PubMed] [Google Scholar]
- 47.Schwab K J, Neill F H, Estes M K, Atmar R L. Improvements in the RT-PCR detection of enteric viruses in environmental samples. Water Sci Tech. 1998;38:83–86. [Google Scholar]
- 48.Sethabutr O, Unicomb L E, Holmes I H, Taylor D N, Bishop R F, Echevirria P. Serotyping of human group A rotavirus with oligonucleotide probes. J Infect Dis. 1990;162:368–372. doi: 10.1093/infdis/162.2.368. [DOI] [PubMed] [Google Scholar]
- 49.Sobsey M D, Batigelli D A, Shin G A, Newland S. RT-PCR amplification detects inactivated viruses in water and wastewater. Water Sci Tech. 1998;38:91–94. [Google Scholar]
- 50.Sugieda M, Nakajima K, Nakajima S. Outbreaks of Norwalk-like virus associated gastroenteritis traced to shellfish: coexistence of two genotypes in one specimen. Epidemiol Infect. 1996;116:339–346. doi: 10.1017/s0950268800052663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Taylor M B. Molecular epidemiology of South African strains of hepatitis A virus: 1982–1996. J Med Virol. 1997;51:273–279. doi: 10.1002/(sici)1096-9071(199704)51:4<273::aid-jmv3>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 52.Vinje J, Altena S A, Koopmans M P G. The incidence and genetic variability of small round-structured viruses in outbreaks of gastroenteritis in the Netherlands. J Infect Dis. 1997;176:1374–1378. doi: 10.1086/517325. [DOI] [PubMed] [Google Scholar]