Abstract
Background
Paxlovid was granted an Emergency Use Authorization for the treatment of mild to moderate coronavirus disease 2019 (COVID-19), based on the interim analysis of the Evaluation of Protease Inhibition for COVID-19 in High-Risk Patients (EPIC-HR) trial. Paxlovid effectiveness needs to be assessed in a noncontrolled setting. In this study we used population-based real-world data to evaluate the effectiveness of Paxlovid.
Methods
The database of the largest healthcare provider in Israel was used to identify all adults aged 18 years or older with first-ever positive test for severe acute respiratory syndrome coronavirus 2 between January and February 2022, who were at high risk for severe COVID-19 and had no contraindications for Paxlovid use. Patients were included irrespective of their COVID-19 vaccination status. Cox hazard regression was used to estimate the 28-day hazard ratio (HR) for severe COVID-19 or mortality with Paxlovid examined as time-dependent variable.
Results
Overall, 180 351 eligible patients were included; of these, only 4737 (2.6%) were treated with Paxlovid, and 135 482 (75.1%) had adequate COVID-19 vaccination status. Both Paxlovid and adequate COVID-19 vaccination status were associated with significant decrease in the rate of severe COVID-19 or mortality with adjusted HRs of 0.54 (95% confidence interval [CI], .39–.75) and 0.20 (95% CI, .17–.22), respectively. Paxlovid appears to be more effective in older patients, immunosuppressed patients, and patients with underlying neurological or cardiovascular disease (interaction P < .05 for all). No significant interaction was detected between Paxlovid treatment and COVID-19 vaccination status.
Conclusions
This study suggests that in the era of Omicron and in real-life settings, Paxlovid is highly effective in reducing the risk of severe COVID-19 or mortality.
Keywords: COVID-19, nirmatrelvir/ritonavir, Paxlovid, SARS-CoV-2, vaccine
We assessed the real-world effectiveness of Paxlovid in high-risk COVID-19 patients. The study illustrates that Paxlovid is highly effective in real-world settings in the Omicron era and significantly reduced the risk of death and progression to severe COVID-19.
The coronavirus disease 2019 (COVID-19) pandemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has been one of the greatest threats to public health in the 21st century with >400 million identified cases and >5.9 million deaths reported worldwide (as of 22 February 2022) [1].
Multiple antivirals, monoclonal antibodies, and immunomodulatory drugs have been suggested as treatments for SARS-CoV-2 infection [2–4], but most of these measures have not effectively reduced the risk of progression to severe disease or are too expensive or logistically difficult to treat widely.
Paxlovid is a new oral antiviral drug, produced by Pfizer for use against COVID-19, given for 5 consecutive days to patients with mild to moderate disease. Paxlovid consists of nirmatrelvir, a novel SARS-CoV-2 main protease inhibitor targeting 3CLpro of SARS-CoV-2, plus ritonavir (for its action as an inhibitor of cytochrome P450 3A4 to decrease nirmatrelvir metabolism and increase its serum levels) [5, 6].
On 22 December 2021, the United States Food and Drug Administration (FDA) granted Emergency Use Authorization (EUA) for the unapproved product Paxlovid, for the treatment of mild to moderate COVID-19 in adults and pediatric patients (≥12 years of age weighing at least 40 kg) at increased risk of progression to severe COVID-19 [7]. The data supporting the EUA were based on the interim analysis of 1039 Paxlovid-treated patients and 1046 control patients receiving placebo, in the Evaluation of Protease Inhibition for Covid-19 in High-Risk Patients (EPIC-HR) trial (NCT04960202). EPIC-HR was a phase 2/3 randomized, double-blind, placebo-controlled study in nonhospitalized symptomatic adult patients with a first laboratory-confirmed diagnosis of SARS-CoV-2 infection, and at least 1 risk factor for progression to severe disease. The interim analysis showed reduced risk of hospitalization due to COVID-19 and reduced mortality in a 28-day follow-up, by 88%, in the Paxlovid group compared to the placebo group [8, 9]. It should be noted that the EPIC-HR trial was conducted in the pre-BA.1 (Omicron) era. Thus, the generalizability of the results to infection with the Omicron variant is limited.
We aimed to conduct a large retrospective cohort study of high-risk COVID-19 patients, identified between January and February 2022 in Israel, to examine the real-life effectiveness of Paxlovid in preventing progression to severe COVID-19 and mortality.
METHODS
Sources of Data
This study is based on data from 2 sources: the Clalit Health Services (CHS) database and the Israeli Ministry of Health (MOH) COVID-19 database. CHS provides inclusive healthcare for more than half of the Israeli population (∼4.7 million). CHS maintains a database that receives information from multiple sources including records of primary care physicians, community specialty clinics, hospitalizations, laboratories, and pharmacies. A registry of chronic diseases diagnoses is compiled from these data sources.
The COVID-19 database is maintained by the Israeli MOH and contains data on vaccination, SARS-CoV-2 polymerase chain reaction (PCR) and antigen test results, and COVID-19 hospitalizations. The collected data are transferred daily to the healthcare providers. Several high-quality studies related to COVID-19 have been conducted based on integrated data from these 2 databases [10, 11]. A detailed description of the databases is provided in the Supplementary Appendix.
Study Population
In this retrospective cohort study, we used the computerized database of CHS to identify all adults aged 18 years or older with first-ever positive test for SARS-CoV-2 (including PCR or antigen tests), performed between 1 January and 28 February 2022. To minimize confounding by indication, inclusion in this study was limited to patients, who were potentially candidates for Paxlovid treatment, with at least 1 comorbidity or condition associated with high risk for severe COVID-19, as in the EPIC-HR trial, including age ≥60 years, body mass index (BMI) ≥30 kg/m2, diabetes, hypertension, cardiovascular disease, chronic liver disease, chronic lung disease, chronic kidney disease, neurological disease, immunosuppression, and malignancy [12]. Unlike the EPIC-HR trial that excluded patients who have received any dose of a SARS-CoV-2 vaccine, in this study patients were eligible to be included irrespective of their SARS-CoV-2 vaccination status.
Exclusion criteria were use of medications that were contraindicated for use with Paxlovid (Supplementary Table 1), estimated glomerular filtration rate <30 mL/minute/1.73 m2, dialysis, weight <40 kg, or pregnancy [6]. We also excluded patients treated with molnupiravir and patients who received Paxlovid >5 days following their positive SARS-CoV-2 test date.
In addition, to minimize selection bias, we excluded patients whose first positive SARS-CoV-2 test was performed during hospitalization or at the same day of admission. These patients are likely to have more advanced illness and unlikely to be treated with Paxlovid. Hence, their inclusion might selectively inflate outcome occurrence only in patients untreated with Paxlovid, thus favoring Paxlovid treatment.
Study Outcome and Follow-up
Study outcome was defined as the composite of severe COVID-19 or COVID-19–specific mortality, based on data extracted from the COVID-19 database. COVID-19 severity was determined according to the Israeli MOH guidelines, which are in accordance with the World Health Organization definitions [13]. Specifically, individuals who had oxygen saturation <94% on room air at sea level, a ratio of arterial partial pressure of oxygen to fraction of inspired oxygen <300 mm Hg, or a respiratory rate >30 breaths/minute were considered as patients with severe illness.
Follow-up started at the date of SARS-CoV-2 test, and patients were followed from the start dates until the first occurrence of severe COVID-19, death, follow-up of 28 days, or end of follow-up on 10 March 2022, whichever came first.
Study Variables
For each patient, we extracted sociodemographic data including age, sex, population sector (general Jewish, ultra-Orthodox Jewish, Arab), and socioeconomic status (SES) (low, middle, high), which was based on the SES scores of the clinic neighborhood as defined by the Israeli Central Bureau of Statistics. In addition, we extracted COVID-19 vaccination dates and data on comorbidities and conditions associated with high risk for severe COVID-19, including BMI, diabetes, hypertension, cardiovascular disease, chronic lung disease, chronic liver disease, chronic kidney disease, neurological disorders, and immunosuppression [12]. As SES, population sector, and BMI had some missing values, we used these variables as categorical variables that included a separate category for missing values.
COVID-19 vaccination status at study entry was classified into 2 categories: adequate vs nonadequate vaccination status (Supplementary Appendix).
Data Analysis
Continuous variables were summarized with mean and standard deviation (SD), and categorical variables were summarized with count and proportion. Multivariable logistic regression models, using backward selection, were used to identify risk factors independently associated with starting Paxlovid treatment within 5 days of positive test for SARS-CoV-2.
Multivariable Cox proportional hazard regression models were used to assess the association between Paxlovid use and the composite of severe COVID-19 or mortality, and to estimate the hazard ratios (HRs) and 95% confidence intervals (CIs). Paxlovid was modeled as a time-dependent variable, allowing subjects to transfer from one exposure group to another during follow-up. The multivariable Cox regression models were adjusted to baseline covariates selected by the method of backward selection from the following: age, population sector, SES, BMI, diabetes, hypertension, cardiovascular disease, chronic liver disease, chronic lung disease, chronic kidney disease, neurological disease, malignancy in the prior year, immunosuppression, and COVID-19 vaccination status. An interaction was also examined between Paxlovid and variables that remained in the final multivariable model.
Statistical analyses were performed using IBM SPSS Statistics version 28.0 (IBM, New York, New York), and SAS version 9.3 software (SAS Institute, Cary, North Carolina).
Ethical Considerations
This study was approved by the institutional review board of Lady Davis Medical Canter and the data utilization committee of CHS. Owing to the retrospective nature of the study, a waiver of informed consent was granted by the institutional reviewed board.
RESULTS
Study Population Characteristics
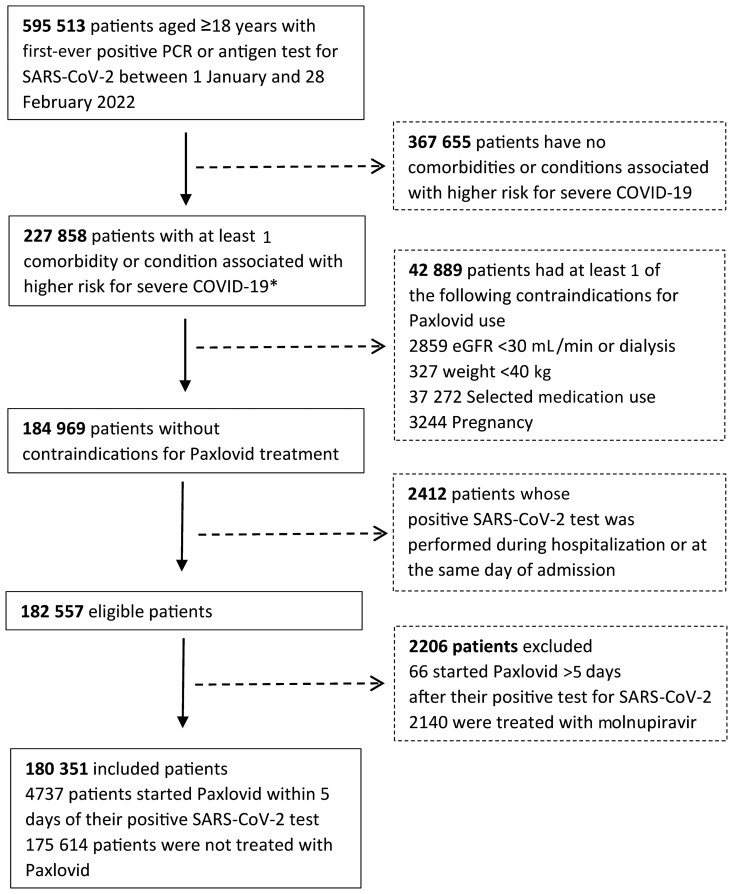
Overall, 180 351 patients were included and were eligible for receiving Paxlovid in CHS, during January–February 2022. A flowchart depicting study population selection is shown in Figure 1. Overall, 4737 received Paxlovid; of these, 3361 (71.0%) received Paxlovid within 3 days of a positive test for SARS-CoV-2 infection (Supplementary Figure 1). The mean age of eligible patients was 54.2 (SD, 16.9), 73 959 (41.0%) were males, and 135 482 (75.1%) had adequate COVID-19 vaccination status. Patients treated with Paxlovid were older, more likely to be male, more likely to belong to the Jewish population sector and to higher socioeconomic class, and in general more likely to have higher frequency of underlying comorbidities. The baseline sociodemographic and clinical characteristics of eligible patients are shown in Table 1.
Figure 1.
Flowchart for selection of study population. *Any of the following: age ≥60 years, body mass index ≥30 kg/m2, diabetes, hypertension, cardiovascular disease, chronic liver disease, chronic lung disease, chronic kidney disease, neurological disease, immunosuppression, and malignancy. Abbreviations: COVID-19, coronavirus disease 2019; eGFR, estimated glomerular filtration rate; PCR, polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Table 1.
Baseline Sociodemographic and Clinical Characteristics (N = 180 351)
| Variable | All Patients (N = 180 351) |
Patients Treated With Paxlovid (n = 4737) | Patients Not Treated With Paxlovid (n = 175 614) |
|---|---|---|---|
| Age, y, mean ± SD | 54.2 ± 16.9 | 68.5 ± 12.5 | 53.9 ± 16.8 |
| Male sex | 73 959 (41.0) | 1992 (42.1) | 71 967 (41.0) |
| Population sectora | |||
| Arab | 33 058 (18.3) | 300 (6.3) | 32 758 (18.7) |
| Ultra-Orthodox Jewish | 7031 (3.9) | 202 (4.3) | 6835 (3.9) |
| General Jewish | 139 698 (77.5) | 4234 (89.4) | 135 464 (77.1) |
| SESa | |||
| Low | 63 738 (35.3) | 1120 (23.6) | 62 618 (35.7) |
| Middle | 77 018 (42.7) | 2090 (44.1) | 74 928 (42.7) |
| High | 38 539 (21.4) | 1517 (32.0) | 37 022 (21.1) |
| Comorbidities | |||
| BMIa ≥30 kg/m2 | 99 876 (55.4) | 1938 (40.9) | 97 938 (55.8) |
| Diabetes | 27 673 (20.9) | 1826 (38.5) | 35 847 (20.4) |
| Hypertension | 54 692 (30.3) | 2447 (51.7) | 52 245 (29.7) |
| Cardiovascular disease | 24 999 (13.9) | 1506 (31.8) | 23 493 (13.4) |
| Chronic liver disease | 1802 (1.0) | 69 (1.5) | 1733 (1.0) |
| Chronic lung disease | 6728 (3.7) | 499 (10.5) | 6229 (3.5) |
| Chronic kidney disease | 4896 (2.7) | 231 (4.9) | 4665 (2.7) |
| Neurological disease | 11 291 (6.3) | 327 (6.9) | 10 964 (6.2) |
| Malignancy in the prior year | 1702 (0.94) | 178 (3.8) | 1524 (0.88) |
| Immunosuppression | 1530 (0.85) | 316 (6.7) | 1214 (0.69) |
| Adequate COVID-19 vaccination | 135 482 (75.1) | 3686 (77.8) | 131 796 (75.0) |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: BMI, body mass index; COVID-19, coronavirus disease 2019; SD, standard deviation; SES, socioeconomic status.
The following variables have missing values: population sector, 558 (0.31%); SES, 1056 (0.58%); and BMI, 1368 (0.76%).
Predictors of Paxlovid Treatment
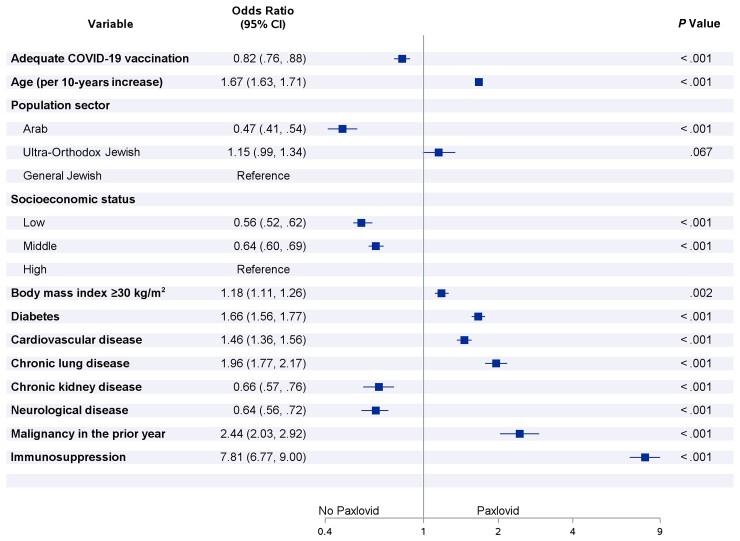
Multivariable logistic regression models showed that low-middle SES was associated with lower use of Paxlovid, compared to high SES. Belonging to the Arab population sector was also associated with lower use of Paxlovid, compared to the general Jewish sector. Patients with adequate COVID-19 vaccination status were less likely to be treated with Paxlovid in comparison to patients with inadequate COVID-19 vaccination status (Figure 2). Older age, obesity, diabetes, cardiovascular disease, chronic lung disease, malignancy, and immunosuppression were all independent predictor of higher use of Paxlovid, whereas chronic kidney disease and neurological disease were associated with lower likelihood of Paxlovid use (Figure 2).
Figure 2.
Multivariable odds ratios for risk factors associated with starting Paxlovid treatment within 5 days after positive test for severe acute respiratory syndrome coronavirus 2 (n = 211 279). *The following variables were included in the multivariable logistic regression model, using backward selection: age, sex, population sector, socioeconomic status, body mass index, diabetes, hypertension, cardiovascular disease, chronic liver disease, chronic lung disease, chronic kidney disease, neurological disease, malignancy in the prior year, immunosuppression, and adequate coronavirus disease 2019 vaccination. Abbreviations: CI, confidence interval; COVID-19, coronavirus disease 2019.
Effectiveness of Paxlovid
Overall, 942 events occurred in all 180 351 included patients, reflecting a crude incidence rate of 5.6 per 1000 person-months. The crude incidence rate was 3.4 per 1000 person-months in vaccinated patients and 13.4 per 1000 person-months in unvaccinated patients. A total of 39 events occurred in the 4737 patients treated with Paxlovid, reflecting a crude incidence rate of 10.4 per 1000 person-months, whereas 903 events occurred in the 175 614 patients not treated with Paxlovid, reflecting a crude incidence rate of 5.6 per 1000 person-months. The higher crude incidence rate in treated patients is likely attributed to older age and higher frequency of underlying comorbidities (Table 1). Indeed, Paxlovid was independently associated with a significantly decreased risk for the composite of severe COVID-19 or mortality, in a multivariable Cox regression models, with an HR of .54 (95% CI, .39–.75). Adequate COVID-19 vaccination status was also associated with significantly decreased risk for the composite of severe COVID-19 or mortality (HR, 0.20 [95% CI, .17–.22]) (Table 2).
Table 2.
Multivariable Cox Regression Analysis of Time to the Composite of Severe Coronavirus Disease 2019 or Mortality, Using Paxlovid Treatment as Time-Dependent Variable (N = 180 351)
| Variable | HR (95% CI) | P Value |
|---|---|---|
| Paxlovid | 0.54 (.39–.75) | <.001 |
| Adequate COVID-19 vaccination | 0.20 (.17–.22) | <.001 |
| Age (HR for each 10-year increase) | 2.28 (2.16–2.41) | <.001 |
| Male sex | 1.74 (1.52–1.99) | <.001 |
| Population sector | ||
| Arab | 1.36 (1.12–1.64) | .002 |
| Ultra-Orthodox Jewish | 0.82 (.59–1.15) | .260 |
| General Jewish | Reference | |
| Socioeconomic status | ||
| Low | 1.37 (1.10–1.70) | .005 |
| Middle | 1.20 (.99–1.47) | .068 |
| High | Reference | |
| Comorbidities | ||
| Diabetes | 1.32 (1.15–1.51) | <.001 |
| Cardiovascular disease | 1.70 (1.47–1.97) | <.001 |
| Chronic lung disease | 2.20 (1.85–2.61) | <.001 |
| Chronic kidney disease | 1.63 (1.36–1.94) | <.001 |
| Neurological disease | 2.28 (1.95–2.67) | <.001 |
| Malignancy in the prior year | 2.15 (1.54–3.00) | <.001 |
| Immunosuppression | 6.43 (4.95–8.34) | <.001 |
The following variables were included in the multivariable Cox regression model, using backward selection: age, sex, population sector, socioeconomic status, body mass index, diabetes, hypertension, cardiovascular disease, chronic liver disease, chronic lung disease, chronic kidney disease, neurological disease, malignancy in the prior year, immunosuppression, and adequate COVID-19 vaccination.
Abbreviations: CI, confidence interval; COVID-19, coronavirus disease 2019; HR, hazard ratio.
Age, male sex, low SES, belonging to the Arab population sector, diabetes, cardiovascular disease, chronic lung disease, chronic kidney disease, neurological disease, malignancy, and immunosuppression were retained in the Cox multivariable model and were all associated with increased risk for the composite of severe COVID-19 or mortality. Of them, immunosuppression, neurological disease, and malignancy had the strongest association, with HRs of 6.43 (95% CI, 4.95–8.34), 2.28 (95% CI, 1.95–2.67), and 2.15 (95% CI, 1.54–3.00), respectively. Age was also a strong risk factor with an HR of 2.28 (95% CI, 2.16–2.41) for each 10-year increase (Table 2).
A sensitivity analysis, restricted to patients diagnosed with COVID-19 after mid-January, when the Omicron variant was the prevailing circulating variant, included 146 228 eligible patients, of whom 3761 were treated with Paxlovid. This analysis shows that Paxlovid was associated with greater decrease in the composite of severe COVID-19 and mortality (HR, 0.43 [95% CI, .85–.64]) (Supplementary Table 2).
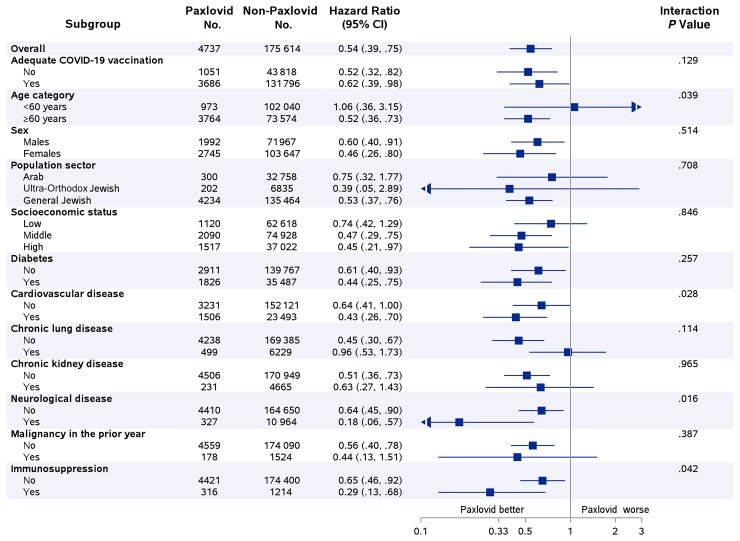
An interaction was examined between Paxlovid and all variables that were retained in the multivariable Cox regression model (Figure 3). This analysis suggests that Paxlovid appears to be more effective in older patients, patients with cardiovascular disease, patients with neurological disease, and in immunosuppressed patients (P value for interaction < .05 for all). The magnitude of Paxlovid effectiveness appears to be unrelated to COVID-19 vaccination status (P value for interaction = .129) (Figure 3).
Figure 3.
The effectiveness of Paxlovid in reducing the risk of severe coronavirus disease 2019 or mortality by subgroups of selected sociodemographic and clinical variables. *Selection of examined subgroups was based on variables that were retained in the multivariable Cox hazard regression analysis of the overall effect (Table 2). Abbreviations: CI, confidence interval; COVID-19, coronavirus disease 2019.
DISCUSSION
In this real-world study, we show that treatment with Paxlovid in the first 5 days of SARS-CoV-2 infection is associated with markedly reduced risk of progression to severe COVID-19 or mortality, regardless of vaccination status for SARS-CoV-2. Notably, this study was conducted in Israel when Omicron was the dominant variant, and shows high effectiveness of Paxlovid against infection with the Omicron variant. In addition, this study confirms that having adequate vaccination status against SARS-CoV-2 remains the most effective treatment in preventing severe illness.
Although the magnitude of the effect is smaller, the direction of the results is in line with the results of interim analysis of the EPIC-HR trial, a phase 2/3 randomized, double-blind, placebo-controlled study that provided the evidence upon which the FDA based its decision to grant Paxlovid an EUA for the treatment of mild to moderate COVID-19 [5–7].
The magnitude of risk reduction was larger in the EPIC-HR trial (88%) than in the current study (46%). The lower risk reduction we have observed in the real-world setting might be explained by several differences between the studies, including those related to the virus, study design, and settings. In particular, in the EPIC-HR trial the B.1.617.2 (Delta) strain was the dominant variant (98%), including clades 21J, 21A, and 21I [5, 7], while the current study was conducted when Paxlovid was first introduced in Israel on January 2022 simultaneously with the beginning of the fifth COVID-19 wave in the country with a predominant circulation of the BA.1 variant, Omicron (approximately 95%) [14]. It has been described that Omicron causes lower rates of severe cases [15]. As for study design differences, we included patients with laboratory confirmation of SARS-CoV-2 infection, as we had no data on COVID-19–related symptoms. In the EPIC-HR trial, only symptomatic adults were included, prone to more severe disease [5]. Moreover, Paxlovid might have been administered earlier in the trial compared to patients in this real-life cohort, as treatment in the trial was given up to 5 days from symptoms onset, while in the current study patients were included up to 5 days from SARS-CoV-2 laboratory confirmation; assuming that symptoms usually precede laboratory confirmation, higher effectiveness of earlier treatment is expected in the trial. In addition, we followed an intention-to-treat approach, and as such the current results are likely an underestimation of the real effect of treatment, provided that adherence in the real-world setting might be lower than in a clinical trial.
Importantly also, the EPIC-HR trial included only patients who were unvaccinated for COVID-19 while in the current study both vaccinated and unvaccinated patients were included. However, a subgroup analysis in our study showed that the magnitude of the effectiveness of Paxlovid is similar in vaccinated and unvaccinated patients. Moreover, our study showed that COVID-19 vaccine is independently associated with a significant decrease in the risk of severe COVID-19 and mortality with an estimated relative risk reduction of 80%. These findings are in line with previous studies, and strongly suggest that COVID-19 vaccine remains the most important medical intervention available to lower the risk of complications and death in patients with COVID-19 [16–18].
It should be noted that the number of eligible patients identified in our study (n = 180 351) was much higher than the number of patients who were actually treated with Paxlovid (n = 4737). This difference might be explained by several factors. First, eligibility in the current study was based on at least 1 risk factor for severe COVID-19 as in the EPIC-HR trial [5], whereas in CHS, the eligibility for treatment was centrally determined by CHS and appears to have followed a more stringent criteria due to fear of adverse events and drug interactions. However, the effect of this selection process is likely to bias the results toward the null as treated patients are expected to have more risk factors for severe illness, which is also suggested from our data. In addition, relying on the date of SARS-CoV-2 test in our study to identify eligible patients, and not on the start of symptoms, might have inflated the number of identified potentially eligible patients. Indeed, it is likely that many of identified patients were in reality not eligible to receive Paxlovid because their symptoms started >5 days before the SARS-CoV-2 test. In addition, the untreated group likely includes asymptomatic patients who are less likely to develop the study outcome, hence biasing the results toward the null. It is also worth noting that data on the characteristics and the proportion of eligible patients that refused the medication were unavailable.
This study has other limitations. As with any retrospective cohort study that is based on data from clinical and administrative databases, a possible limitation may be related to the quality of the data. Despite that, information about study outcomes and the administration of COVID-19 vaccines, collected prospectively as part of the Israeli MOH COVID-19 database, are considered complete. Information about Paxlovid is also closely monitored by CHS and is considered complete. In addition, this retrospective cohort study is observational in nature; thus, even with adjustment for a large number of confounders associated with high risk for severe COVID-19, residual confounding remains of concern. Furthermore, relying on the date of SARS-CoV-2 test in our study, to identify eligible patients, and not on the start of symptoms might have introduced selection bias by allowing differentially patients with delayed diagnosis and more advanced disease to be included more in the untreated group, hence favoring Paxlovid treatment. However, we tried to minimize this bias by excluding patients whose SARS-CoV-2 test was performed during hospitalization or at the day of admission, as these patients are more likely to have advanced illness at the time of diagnosis.
Interestingly, our study also shows differences within population sectors and SES status with lower tendency to receive Paxlovid and a higher risk of severe COVID-19 or mortality in the Arab minority. Explanations for the health gaps include socioeconomic differences and differences in social structure including community support, access to healthcare, medical awareness, and underlying comorbidities.
Despite the shown effectiveness, it should be noted that Paxlovid is not recommended in several medical conditions and has complex drug-drug interactions, due to the ritonavir component of the combination. Therefore, clinicians should carefully review the patient’s concomitant medications and evaluate potential drug-drug interactions. This may limit the use of Paxlovid in high-risk patients, for whom other alternative antivirals might be appropriate [19–21].
In summary, this study suggests that treatment with Paxlovid is associated with prominent reduction of severe COVID-19 and mortality in real life and in the era of Omicron, especially in older patients. This study further confirms that COVID-19 vaccine remains the most effective intervention to prevent disease progression and death among COVID-19 patients.
Supplementary Material
Contributor Information
Ronza Najjar-Debbiny, Infection Control and Prevention Unit, Lady Davis Carmel Medical Center, Haifa, Israel; Ruth and Bruce Rappaport Faculty of Medicine, Technion-Israel Institute of Technology, Haifa, Israel.
Naomi Gronich, Ruth and Bruce Rappaport Faculty of Medicine, Technion-Israel Institute of Technology, Haifa, Israel; Department of Community Medicine and Epidemiology, Lady Davis Carmel Medical Center, Haifa, Israel.
Gabriel Weber, Ruth and Bruce Rappaport Faculty of Medicine, Technion-Israel Institute of Technology, Haifa, Israel; Infectious Diseases Unit, Lady Davis Carmel Medical Center, Haifa, Israel.
Johad Khoury, Pulmonology Division, Lady Davis Carmel Medical Center, Haifa, Israel; Pulmonology, Critical Care and Sleep Medicine, Yale School of Medicine, New Haven, Connecticut, USA.
Maisam Amar, Infectious Diseases Unit, Lady Davis Carmel Medical Center, Haifa, Israel; Internal Medicine C, Lady Davis Carmel Medical Center, Haifa, Israel.
Nili Stein, Department of Community Medicine and Epidemiology, Lady Davis Carmel Medical Center, Haifa, Israel; Statistical Unit, Lady Davis Carmel Medical Center, Haifa, Israel.
Lee Hilary Goldstein, Ruth and Bruce Rappaport Faculty of Medicine, Technion-Israel Institute of Technology, Haifa, Israel; Internal Medicine C, Emek Medical Center, Afula, Israel; Clinical Pharmacology Unit, Emek Medical Center, Afula, Israel.
Walid Saliba, Ruth and Bruce Rappaport Faculty of Medicine, Technion-Israel Institute of Technology, Haifa, Israel; Department of Community Medicine and Epidemiology, Lady Davis Carmel Medical Center, Haifa, Israel; Translational Epidemiology Unit and Research Authority, Lady Davis Carmel Medical Center, Haifa, Israel.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. R. N.-D., N. G., and W. S. conceived the study and edited the final manuscript. W. S. and N. S. conducted the analysis. All authors contributed to study design, revised the manuscript for important intellectual content, were responsible for the decision to submit for publication, and approved the final submitted version of the manuscript. All authors had full access to the deidentified data in the study. R. N.-D., N. S., and W. S. accessed and verified the data underlying the study and take responsibility for the data.
Data availability. Individual-level data are not publicly available due to legal restrictions. All data relevant to this analysis were presented in the article.
Potential conflicts of interest. The authors: No potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. World Health Organization (WHO) . WHO coronavirus (COVID-19) dashboard. 2022. Available at: https://covid19.who.int/. Accessed 30 April 2022.
- 2. Drożdżal S, Rosik J, Lechowicz K et al. An update on drugs with therapeutic potential for SARS-CoV-2 (COVID-19) treatment. Drug Resist Updat 2021; 59:100794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Parums DV. Editorial: current status of oral antiviral drug treatments for SARS-CoV-2 infection in nonhospitalized patients. Med Sci Monit 2022; 28:e935952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Weinreich DM, Sivapalasingam S, Norton T, et al. REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19. N Engl J Med 2021; 384:238–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hammond J, Leister-Tebbe H, Gardner A, et al. EPIC-HR Investigators. Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19. N Engl J Med 2022; 386:1397–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. US Food and Drug Administration . Fact sheet for healthcare providers: emergency authorization for Paxlovid. 2022. Available at: https://www.fda.gov/media/155050/download. Accessed 30 April 2022.
- 7. US Food and Drug Administration . Emergency use authorization 105. 2021. Available at: https://www.fda.gov/media/155049/download. Accessed 30 April 2022.
- 8. Pfizer Inc . Pfizer announces additional phase 2/3 study results confirming robust efficacy of novel COVID-19 oral antiviral treatment candidate in reducing risk of hospitalization or death. 2021. Available at: https://www.pfizer.com/news/press-release/press-release-detail/pfizer-announces-additional-phase-23-study-results. Accessed 30 April 2022.
- 9. Mahase E. Covid-19: Pfizer's Paxlovid is 89% effective in patients at risk of serious illness, company reports. BMJ 2021; 375:n2713. [DOI] [PubMed] [Google Scholar]
- 10. Barda N, Dagan N, Ben-Shlomo Y, et al. Safety of the BNT162b2 mRNA Covid-19 vaccine in a nationwide setting. N Engl J Med 2021; 385:1078–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Arbel R, Hammerman A, Sergienko R, et al. BNT162b2 vaccine booster and mortality due to Covid-19. N Engl J Med 2021; 385:2413–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Centers for Disease Control and Prevention . Underlying medical conditions associated with higher risk for severe COVID-19: information for healthcare professionals. 2022. Available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/underlyingconditions.html. Accessed 30 April 2022. [PubMed]
- 13. National Institute of Health , COVID-19 treatment guidelines 2021. Available at: https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/, Accessed 14 June 2022.
- 14. Our World in Data . SARS-CoV-2 variants in analyzed sequences, Israel. Available at: https://ourworldindata.org/grapher/covid-variants-area?country=∼ISR. Accessed 30 April 2022.
- 15. Madhi SA, Kwatra G, Myers JE, et al. Population immunity and Covid-19 severity with Omicron variant in South Africa. N Engl J Med 2022; 386:1314–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rosenberg ES, Holtgrave DR, Dorabawila V, et al. New COVID-19 cases and hospitalizations among adults, by vaccination status—New York, May 3–July 25, 2021. MMWR Morb Mortal Wkly Rep 2021; 70:1150–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tenforde MW, Self WH, Naioti EA, et al. Sustained effectiveness of Pfizer-BioNTech and Moderna vaccines against COVID-19 associated hospitalizations among adults—United States, March–July 2021. MMWR Morb Mortal Wkly Rep 2021; 70:1156–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bajema KL, Dahl RM, Evener SL, et al. Comparative effectiveness and antibody responses to Moderna and Pfizer-BioNTech COVID-19 vaccines among hospitalized veterans—five Veterans Affairs Medical Centers, United States, February 1–September 30, 2021. MMWR Morb Mortal Wkly Rep 2021; 70:1700–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bernal A J, da Silva MM G, Musungaie DB, et al. Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients. N Engl J Med 2022; 386:509–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Singh AK, Singh A, Singh R, Misra A. Molnupiravir in COVID-19: a systematic review of literature. Diabetes Metab Syndr 2021; 15:102329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wen W, Chen C, Tang J, et al. Efficacy and safety of three new oral antiviral treatment (molnupiravir, fluvoxamine and Paxlovid) for COVID-19: a meta-analysis. Ann Med 2022; 54:516–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.