Abstract
Neurodegenerative diseases (NDDs) such as Alzheimer's disease (AD) and Parkinson's disease (PD) are a heterogeneous group of disorders characterized by progressive degeneration of neurons. NDDs threaten the lives of millions of people worldwide and regretfully remain incurable. It is well accepted that dysfunction of mitochondria underlies the pathogenesis of NDDs. Dysfunction of mitochondria results in energy depletion, oxidative stress, calcium overloading, caspases activation, which dominates the neuronal death of NDDs. Therefore, mitochondria are the preferred target for intervention of NDDs. So far various mitochondria-targeting drugs have been developed and delightfully some of them demonstrate promising outcome, though there are still some obstacles such as targeting specificity, delivery capacity hindering the drugs development. In present review, we will elaborately address 1) the strategy to design mitochondria targeting drugs, 2) the rescue mechanism of respective mitochondria targeting drugs, 3) how to evaluate the therapeutic effect. Hopefully this review will provide comprehensive knowledge for understanding how to develop more effective drugs for the treatment of NDDs.
KEY WORDS: Neurodegenerative diseases, Mitochondria, Targeting drug, Apoptosis, Reactive oxygen species, Adenosine triphosphate, Mitochondrial membrane potential, Evaluation
Graphical abstract
This review elaborately addresses the strategy to design mitochondria targeting drugs and its rescue mechanism on neurodegenerative diseases, and how to evaluate the therapeutic effect.
1. Introduction
Neurodegenerative diseases (NDDs) are a heterogeneous group of disorders characterized by progressive degeneration of neurons especially in central nervous system including highly prevalent Alzheimer's disease (AD) and Parkinson's disease (PD)1. Despite impressive efforts taken, NDDs remain incurable and no effective medication could delay or stop its progression. Ascending evidences suggest that dysfunction of mitochondria plays crucial role in the pathogenesis of NDDs2. Clinical trials based on mitochondria cascade related hypothesis account for 17% of all AD trials3. Mitochondria are organelles that generate basic energy, adenosine triphosphate (ATP), for most types of cells in our body. Besides maintaining energy homeostasis, mitochondria are also the central mediator of cellular signaling, cell survival4. Neurons are particularly energy demanding cells due to its unique structure and function5. Dysfunction of mitochondria leads to energy depletion, oxidative stress, calcium overloading, caspases activation, which confers the neuronal death of NDDs via apoptosis, pyroptosis or ferroptosis6. Therefore, to combat NDDs, mitochondria are the preferred target for intervention. So far various mitochondria-targeting drugs have been developed and applied and delightfully some of them demonstrate promising outcome. In present review, we will summarize and address 1) how to design mitochondria-targeted drugs, 2) what is the respective rescue mechanism of the drugs, 3) how to assess the therapeutic effect.
2. Strategy for mitochondria targeting
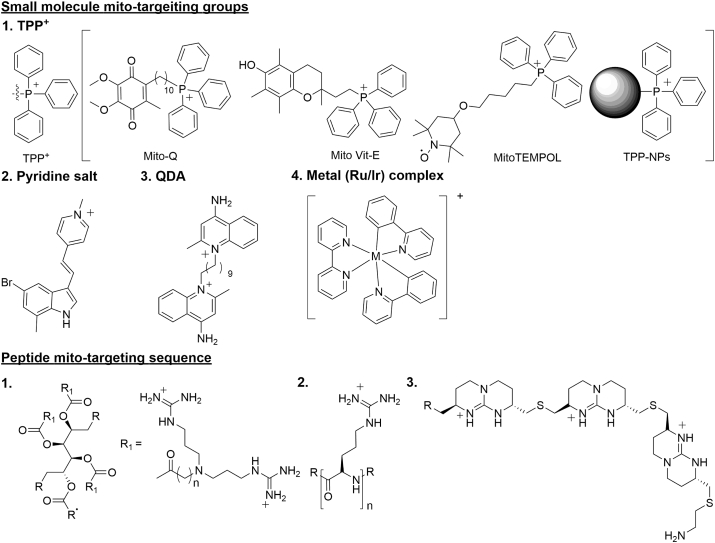
Mitochondria are the pivotal organelle of cells with unique structure formed by two lipid-bilayer membranes. Drugs with mitochondrial targeting ability could not only enhance the efficiency but also minimize side effects induced by distribution in the cytoplasm. Efficient and specific mitochondrial targeting is the precondition to develop drugs for mitochondrial related diseases such as PD, AD and cancer. To achieve mitochondria targeting, many strategies have been proposed and verified to be feasible (Fig. 1 and Table 17, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30).
Figure 1.
The structures of small molecules and peptides with mitochondria targeting ability.
Table 1.
The list of mitochondria targeting molecules and its targeting mechanism.
| Chemical nature | Group | Molecule | Targeting mechanism | Advantage/disadvantage | Ref. |
|---|---|---|---|---|---|
| Small molecule | TPP+ | Mito-Q; Mito-Vit-E; Mito-TEMPOL; TPP+ functionalized liposomes; Ceria nanoparticle; Silica nanoparticles | Positive charge and lipid solubility | Easy to synthesis/cytotoxicity for drug delivery | 7, 8, 9, 10, 11, 12, 13, 14, 15 |
| Small molecule | Pyridinium salt | 5BMF@HSA complex | Positive charge and lipophilic, HSA increasing water solubility | Easy to synthesis, tunable luminescence/cytotoxicity for drug delivery | 16 |
| Small molecule | DQA | QDAsomes | Amphipathic | Easy to synthesis, cytotoxicity for drug delivery/low endosomal escape ability and transfection efficiency | 17,18 |
| Small molecule | Ru/Ir | Ru/Ir complexes | Positive charge and lipophilic | Cytotoxicity for cancer therapy/multimodal therapy | 19,20 |
| Peptide | S-S peptides | SS-02, SS-31 | Positive charge and lipophilic | Hypotoxicity, antioxidant/complex | 21 |
| Peptide | MPP | MPP | Positive charge and lipophilic | Hypotoxicity, tunability, desirable pharmacokinetic profiles/complex synthesis | 22,23 |
| CPMs | CPM | CPM1, CPM2, CPM3 | Amphipathic | Efficient and universal delivery of cargos/complex synthesis | 24 |
| Mito-Porter | Octa arginine | (DF)-Mito-Porter, ASO-Mito-Porer, DOX-Mito-Porter | Lipid compositions promote its fusion with the mitochondrial membrane | Efficient and universal delivery/complex synthesis | 25, 26, 27, 28, 29 |
| Gramicidin S | Gramicidin S | XJB-5-131 | High affinity for the membrane | Antioxidant/complex synthesis | 30 |
2.1. Small molecule facilitated mitochondria targeting
2.1.1. Triphenyl phosphonium cation (TPP+)
There is a large transmembrane potential of 120–140 mV (negative inside) in mitochondria, which could be utilized to drive the positively charged molecules to mitochondria. TPP+, one kind of lipophilic cations, could easily pass-through lipid bilayers duo to the existing of the potential gradient. The uptake of lipophilic cations into mitochondria increases 10-fold for every 61.5 mV of membrane potential at 37 °C leading to 100–500-fold accumulation7. Many mitochondrial targeted drugs are developed with TPP+ assistance, including Mito-Q, Mito-Vit-E, 2,2,6,6-tetramethyl-4-[5-(triphenylphosphonio)pentoxy]piperidin-1-oxy bromide (Mito-TEMPOL). Mito-Q was prepared by conjugating ubiquinone to TPP+ moiety, which could be taken up rapidly by mitochondria due to the existence of Δψ8. Mito-Q has been widely used as antioxidants7 to preventing cell death. Mito-Vit-E was constructed by conjugating vitamin E and TPP+, and the latter drove the compound to mitochondria. Mito-Vit-E as antioxidant was also proved to invigorate mitochondrial function and prevented cell death9 Mito-Q and MitoVit-E, at low concentrations, showed higher efficiency in protecting cells against peroxide-induced oxidative damage and apoptosis, compared to the untargeted antioxidants (i.e., Vit-E and ubiquinone-10). In addition, the antioxidation ability of Mito-Q was affected by membrane potential, while MitoVit-E was not affected10. Mito-TEMPOL was a well-known mitochondria-targeted superoxide scavenger. Mito-TEMPOL treatment could attenuate ATP depletion mediated necrosis and apoptosis by preserving mitochondrial integrity and decreasing BAX translocation to mitochondria11.
Besides utilized in preparing compounds, TPP+ was also applied in the nanoparticles (NPs) construction, including functionalized liposomes, dendritic polymers, cilia, silica, which endowed the NPs with mitochondria targeting capacity. Following liposomal formation with conventional method, TPP+ was decorated on the surface of liposomes to get functionalized TPP+-liposomes. To reduce the cytotoxicity of liposomes, dimyristoyl triphenylphosphonium (DM-TPP) was admitted, which did not compromise its ability to target mitochondria12. Dendritic polymers were widely used in drug delivery due to its capacity of encapsulating drugs. Presence of TPP+ moiety on the surface of dendrimetric core enabled mitochondria targeting and rendered better drug delivery effectiveness13. Hyeon's group synthesized small and positively charged TPP+-ceria NPs capable of localizing to mitochondria in various cell lines. TPP+-ceria NPs were biocompatible and scavenged mitochondrial reactive oxygen species (ROS) efficiently to reduce oxidative stress in vitro and in vivo14. This kind NPs was single function and could just remove ROS, which greatly limited its application. TPP+ was further incorporated into universal and biodegradable silica nanoparticles (BS-NPs) to deliver functional proteins into the mitochondria, which showed higher efficiency in drug packaging, protection of protein activity and controlled release in mitochondria15.
2.1.2. Pyridinium salt
Pyridinium salts are another type of commonly utilized mitochondrial targeting groups with positive charge and lipophilicity. Unlike TPP+, which is modified with long alkyl chain links, pyridine salts are often modified with ethylenic bonds to form large conjugated systems. The conjugated systems and electron-withdrawing ability of pyridine salt can produce or regulate molecular luminescence, which is applicable for the optical detection of mitochondria. However, lipophilicity of pyridine salts hinder its accessibility to tissues in vivo. To cope with this issue, Cheng's group developed human serum albumin facilitated pyridine salt complex which easily accessed to tumor tissue with mitochondria targeting ability and induced tumor cell apoptosis16, which achieved a breakthrough in pyridinium salt in vivo application. The cytotoxicity of the pyridine salt molecule was a defect of its mitochondrial detection, but it displayed advantages in tumor treatment.
2.1.3. DQA/DOA somes
Dequalinium (DQA) is a cationic bola amphiphilic composed of two quinaldinium rings linked by ten methylene groups which can self-assemble into liposome-like vesicles DQA-based liposomes (DQAsomes). DQA exhibited mitochondria-targeted ability and could induce ROS production by inhibiting ATP synthesis. DQA has been demonstrated to synergize the antitumor effects of tumor necrosis factor (TNF) against the growth of rat colon tumor isografts17. DQAsomes exhibited mitochondria-targeting ability and selectively delivered biologically drugs (e.g., DNA) to mitochondria of mammalian cells18. However, the low endosomal escape ability and transfection efficiency of DQAsomes limit its application in mitochondria-targeted delivery.
2.2. Metal complexes
Metal complexes were reported to be able to quickly penetrate the membrane and highly accumulate in mitochondria due to its high positive charge and good lipophilicity19. So far most of the metal complexes were prepared for anti-tumor. Hetero-binuclear iridium-platinum (Ir-Pt) and iridium-ruthenium (Ir-Ru) complex were found to induce cell death via mitochondrial DNA damage, metabolism alteration and mitochondrial superoxide accumulation19,20, which served as a dual function, mitochondria-targeting, photoactivated chemotherapy (PACT) and photodynamic therapy (PDT) agent. Noteworthy, these mitochondria-targeted complexes contain heavy metals, which makes it difficult to be used in vivo or in clinical trials duo to the toxicity of heave metals.
2.3. Mitochondria targeting peptides
Peptide is another tool used for facilitating mitochondria targeting. Peptide-based delivery scaffolds offer attractive features such as ease of synthesis, tunability, biocompatibility, and high uptake both in cell and in vivo. Owing to its advantages, peptides are highly adaptable for delivering chemically diverse cargo. Mitochondria targeting peptides normally are cationic charged and lipophilic, which allows them to harness the negative membrane potential of mitochondria, and favorably interact with hydrophobic membranes of mitochondria. Compared with TPP+ and pyridinium salt, peptides did not cause mitochondrial depolarization at millimole concentrations, whereas TPP+/pyridinium salt caused cytotoxicity at concentrations higher than 10 μmol/L. And peptides were more suitable for disease treatment and drug delivery in the living body for their biocompatibility. The structures of common peptides targeting mitochondria are showed in Fig. 1.
2.3.1. Szeto-Schiller (SS) peptides
The SS tetra peptides represent a series of mitochondria targeting peptides that feature a common structural motif of alternating aromatic and basic residues. The antioxidant properties of SS-02 and SS-31 likely originate from their dimethyl tyrosine (Dmt) residues. More specifically, tetra peptides SS-31 and SS-02 were found to be equally effective in scavenging H2O2 and inhibiting linoleic acid oxidation in vitro. The basic residues served for localization in the inner mitochondrial membrane, and the dimethyl tyrosine phenol moieties of SS-02 and SS-31 were likely responsible for chemically reducing reactive oxygen species and peroxide bonds. SS tetra peptides displayed anti-oxidation property. Peptides SS-02 and SS-31 were demonstrated to ameliorate apoptosis of t-BHP-treated cells. In an ex vivo reperfusion study of guinea pig heart, both SS-02 and SS-31 were able to prevent myocardial stunning and significantly improved contractile force21.
2.3.2. Mitochondria-penetrating peptides (MPPs)
The MPPs are cationic and lipophilic which facilitate its permeation into the hydrophobic mitochondrial membrane. Mitochondria targeting capacity of MPPs could be finely tuned by altering lipophilicity and charge22. MMPs did not show obvious cytotoxicity even at high concentrations. But MMPs could efficiently target mitochondria and facilitate robust delivery of bioactive compounds, such as drugs, antioxidants, and photosensitizers, to achieve the treatment of disease22,23. Numata's group reported that low concentrations of peptides were sufficient to deliver DNA into the mitochondria and rendered protein expression in a short incubation period23. The advantages of MPPs facilitated delivery included tunability, ease of synthesis, desirable pharmacokinetic profiles in vitro and in vivo.
2.4. Cell-penetrating motifs (CPMs)
CPMs are consisted with four guanidinium groups and one or two aromatic hydrophobic groups (naphthalene) assembled through a central scaffold (a benzene ring). CPMs showed high efficiency of mitochondria delivery and with minimal effect on the viability or the mitochondrial membrane potential of mammalian cells24. They efficiently and specifically delivered small molecules, peptides, and other cargoes into the mitochondrial matrix of mammalian cells with greatly enhanced anticancer activity.
2.5. Mito-Porter
Liposome-based carrier (Mito-Porters) was first reported in 2008 by Harashima's group. Mito-Porters carried with octa arginine modifications to facilitate entering cells via micropinocytosis and the lipid compositions promoted both its fusion with the mitochondrial membrane and the release of its cargo to the intra-mitochondrial compartment in living cells25. In 2012, Dual Function (DF)-Mito-Porter encapsulating DNase I was constructed, which effectively delivered the DNase I into the mitochondria and achieved selective mitochondrial genome edition26. Mito-Porters displayed delivery ability for different kinds of molecules, and was utilized for drug delivery in mitochondrial disease. In 2019, Harashima's group reported the efficient packaging of ASO in the Mito-Porter via a nanoparticle packaging method, which showed a 10-fold higher delivery efficiency than the conventional method27. In 2020, Harashima's group encapsulated DOX in the Mito-Porter, which led to enhanced DOX accumulate in tumor mitochondria28. Mito-Porter facilitated wild-type mitochondrial pre-tRNAPhe (pre-WT-tRNAPhe) transfection into disease associated mitochondrial cells. The mutation rate of tRNAPhe was decreased, and therapeutic effect sustained for more than one week29.
2.6. XJB gramicidin S (GS) analogs
XJB peptides are derived from membrane-active GS antibiotics which had a high affinity for the mitochondria membrane. The mitochondrial targeting portion of XJB-5-131 consists of the Leu–dPhe–Pro–Val–Orn fragment of GS and antioxidant 4-amino-TEMPO (4-AT). The nitrogen-oxide radicals in XJB-5-131 accepted an electron and converted them into hydroxylamine, which scavenged ROS. McMurray's group reported that XJB-5-131 eliminated mitochondria ROS30.
3. Rescue effect and mechanism of mitochondria targeting drugs for NDDs
Given the crucial role of mitochondria dysfunction in pathogenesis of NDDS, mitochondria targeting drugs especially attract the interest of clinician and researchers. So far, abundant kinds of drugs have been designed and prepared which displayed gratified effects. To better understand the rescue effect and mechanism of mitochondria targeting drugs, we classify them into four categories based on the biological nature of the drug targets: mitochondrial nucleic acid, mitochondrial protein, small molecule, and metal ions (Table 227,31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55). The pharmacological mechanisms of drugs are elaborately described.
Table 2.
The list of drugs targeting mitochondria for the therapeutics of NDDs.
| No. | Drug | Application | Target | Mechanism | Ref. |
|---|---|---|---|---|---|
| 1 | MtZFNs | Zebra fishes Hek 293T cells |
mtDNA | Bind and cleave the mutated DNA and retains the wild type mtDNA | 31 |
| 2 | Mito TALENs | iPSC cells | mtDNA | Deletion sites for selective cleavage of mtDNA | 32 |
| 3 | Mito CRISPR/Cas9 | HEK-292T cells | mtDNA | Mitochondrial specific CAS 9 editing mtDNA m.3243A>G gene sites or knock in homologous recombination strategy to repair mutated mtDNA | 33,34 |
| 4 | DdCBE | HEK293T cells | mtDNA | Specifically catalyzes the transformation of CG base pairs of mtDNA into AT | 35 |
| 5 | Mito-Porter | Hela cells G625A cells |
mtRNA | Knockdown the mRNA encoded complex Ⅱ or introduce mito codon regulation system | 27 |
| 6 | ZFNs | iPSC cells | SNCA gene | Reprogram A53T mutation in iPSCs | 36 |
| 7 | TALENs | iPSC cells | CAG gene | Replace pathogenic CAG repeats with normal repeats gene sequences through homologous recombination | 37 |
| 8 | CRISPR/KamiCas9 | HD mice model | hHTT-82Q gene | sgHTT1 targeting HTT gene with the aim of permanently blocking leading to HTT silencing | 38 |
| 9 | Bip-V5 | Rat 6-OHDA PD model | BAX/BAK | Inhibit BCL-2 | 39 |
| 10 | MANF | Rat 6-OHDA PD model | BAX | Inhibit BAX activation | 40 |
| 11 | KuA | Mice MPP+ model | BAX/BCL-2 | Reduce BAX/BCL-2 ratio | 41 |
| 12 | VBIT-3, VBIT-4 | MEF cells | VDAC1 | Inhibition of VDAC1 oligomerization | 42 |
| 13 | VDAC1 N-terminal peptides | NSC-34 cells | VDAC1 | Interact with VDAC1, reduce channel conductance | 43 |
| 14 | Nobiletin | Rat model | Complex Ⅰ | Restored the activity of complex Ⅰ | 44 |
| 15 | NaHS | Animal model | ETC | Mediated oxygen consumption rate | 45 |
| 16 | Compound A | Rat PD model | Complex Ⅱ | Blocked BIM-induced apoptosis after BAX is activated on the mitochondria | 46 |
| 17 | rT1 | COS-7 cells | ATP synthase | Through TOM20 targets mitochondrial membrane ATP synthase | 47 |
| 18 | Nicotinamide riboside/NR | A-T fibroblasts, Atm–/– mice | NAD+ | Boosted NAD+ levels by promoting mitophagy in a PINK1-dependent manner | 48 |
| 19 | Rapamycin | SCIRI mice model | mTOR | Promoted mitophagy and attenuating SCIRI-induced apoptosis | 49 |
| 20 | Urolithin A | C. elegans AD models | PINK1 | Stimulated mitophagy, suppressed of neuroinflammation | 50 |
| 21 | Rilmenidine | ALS mice | SOD1 | Induced autophagy, promoted autophagic clearance of mutant SOD1 and efficient mitophagy | 51 |
| 22 | Mito Q | Rat LTP model | ROS | Mitochondria-targeted antioxidants | 52 |
| 23 | Mito-TEMPO | Primary microglia | ROS | Inhibit the increase of TNF-α, IL-1β, IL-6 and iNOS | 53 |
| 24 | SS peptides | Caco2 cells | ROS | Targeted delivery of antioxidants to the inner mitochondrial membrane | 54 |
| 25 | Pyridoxine | PC12 cells | GSH | Alleviated the significant decrease of GSH to maintain the activity of complex Ⅰ | 55 |
3.1. Drugs targeting mitochondria nucleic acid
Mitochondria, different from other organelles, have its own DNA, mitochondria DNA (mtDNA). mtDNA encodes 22 tRNAs, 2 rRNAs and 13 mRNAs which dominate the translation of electron transport chain proteins, mitochondrial inner and outer membrane proteins56. Mutation of mtDNA is implicated in the pathogenesis of NDDs. Therefore, drugs targeting mtDNA repairing or editing are developed and applied for the treatment of NDDs.
Gammage et al.31 used the zinc finger nuclei (ZFNs) structure to knockout mtDNA with disease associated mutation. Transcription activator-like factor nucleases (TALENs) were also reported to edit mtDNA and effectively ameliorated phenotypes of disease32. Both ZFNs and TALENs can recognize and eliminate the mutated sequence of mtDNA by nuclease. Regretfully it can only delete aberrant mtDNA, but it cannot correct or insert deficient mtDNA sequence. In 2012, one novel gene editing technique, CRISPR/Cas9 was developed, which provided alternative way for mtDNA editing especially when combined with mitochondria targeting strategy. Jo et al.33 constructed mitochondrial targeting sequence (MTS) conjugated Cas9 (MTS-Cas9) which realized mtDNA edition and interrupted mitochondria protein expression. In 2019, Bian et al.34 constructed a mito-CRISPR/Cas9 system which inserted a single stranded homologous DNA into mtDNA, which for the first time achieved mutated mtDNA reparation by homologous recombination strategy. In 2020, Mok et al.35 constructed DddA-derived cytosine-based editor (DdCBE) by introducing a mitochondrion targeting internal toxin DddA. DdCBE penetrated into inner membrane of mitochondria, and catalyzed the mtDNA CG to AT conversion. Kawamura et al.27 constructed a Mito-Porter to silence the target mtRNA which rendered knockdown of complex Ⅱ RNA. To date, a number of systems for gene editing have been developed and achieved therapeutic effect in hereditary NDDs. For example, ISoldner et al.36 used ZFNs to repair A53T mutation of SNCA gene in patient-specific induced pluripotent stem cells (iPSC) in familial Parkinson's disease with SNCA gene mutation. An et al.37 used TALENs technology to replace pathogenic CAG repeats with normal repeats gene sequences through homologous recombination in iPSC. Merienne et al.38 used KamiCas9 self-inactivating CRISPR/Cas9 system to inactivate mutant huntingtin in Huntington's Disease (HD) mice model. Regretfully, those gene editing strategy had not been endowed with mitochondria targeting ability.
Though mtDNA editing techniques have been proved to be feasible for intervention of mitochondria genes. There were still gaps in applying them into NDDs treatment, indicating more efforts are needed to broaden it in the future. The mitochondrial genome-targeted edition represents one promising therapeutic candidate for treatment of mitochondrial related diseases including NDDs.
3.2. Drugs targeting mitochondria proteins
Mitochondria proteins, including respiratory chain complexes I to IV, ATP synthase, mitochondria membrane protein, are the executors of mitochondria function. Dysfunction of those protein is closely related to the pathogenesis of NDDs. Some proteins, such as PARKIN, PINK1 though not mitochondria derived ones, dysfunction of which are also implicated in NDDs. Drugs targeting those proteins exhibit promising therapeutic effects for NDDs.
3.2.1. Drugs targeting mitochondria membrane proteins (MMPs)
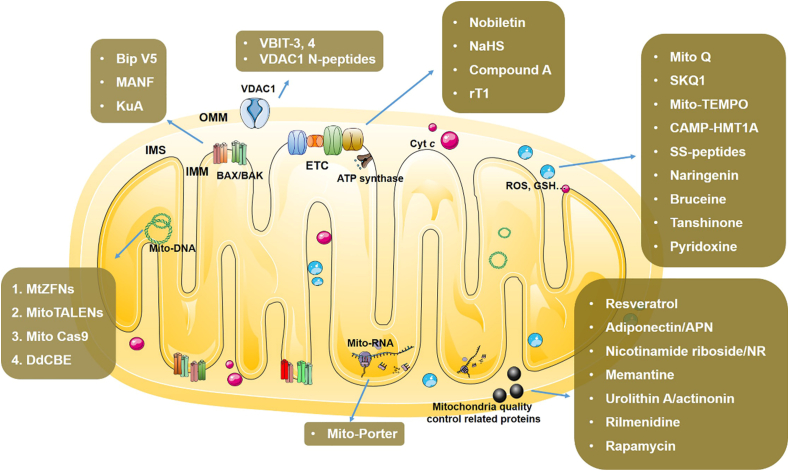
MMPs played crucial roles in maintaining the integrity and permeability of mitochondria and disruption of which often leads to neurons apoptosis and consequent NDDs6. BCL-2 family proteins, including BCL-2, MCL-1, BAX, BIM, BAD, were typical MMPs mediating the integrity of mitochondria outer membrane. BCL-2 and MCL-1 represented anti-apoptotic proteins, whereas BAX, BIM, BAD represented pro-apoptotic proteins, and imbalance of them could result in cell apoptosis37. Analogues of BCL-2/MCL-1/BCL-xL or inhibitors of BAX/BAD/BIM are widely applied in the treatment of NDDs.
Lopez et al.39 designed a BAX-inhibiting peptide V5 (Bip-V5) which displayed anti-apoptotic effect in rat 6-OHDA induced PD model. Mesencephalic astrocyte-derived neurotrophic factor (MANF) was proved to be an inhibitor of proapoptotic BAX protecting neurons in rat 6-OHDA model40. In addition, kukoamine A (KuA) also reduced the BAX/BCL-2 ratio, thereby inhibiting the apoptosis of neurons in MPP+ induced animal models41. Voltage dependent anion channel 1 (VDAC1) is a mitochondria outer membrane protein and overexpression of VDAC1 enhances mitochondria permeability and subsequent cell apoptosis. VDAC1 inhibitors, VBIT-4, were reported to prevent the cell apoptosis via decreasing cellular ROS and Ca2+42. Shteinfer-Kuzmine et al.43 found that inhibitory peptide of VDAC1 could ameliorate phenotype of Amyotrophic Lateral Sclerosis (ALS).
3.2.2. Drug targeting electron transport chain (ETC) proteins
ETC proteins are key mediator of oxidative phosphorylation and energy conversion in mitochondria, which are classified as complexes I, II, III and IV. Complexes I and II catalyze the transfer of electrons from nicotinamide adenine dinucleotide (NADH) and flavine adenine dinucleotide (FADH2) to ubiquinone (UQ), complexes III transfer electrons from ubiquinone to cytochrome c (Cyt c), and complexes IV transfer electrons from Cyt c to O2. Dysfunction of ETC protein would compromise mitochondrial oxidative phosphorylation and lead to NDDs57. Hence, compounds that could destroy ETC were widely utilized to create in vitro or in vivo NDDs models. Whereas, drugs that could protect the ETC exhibited rescue effects on NDDs. Amarsanaa et al.44 reported that nobiletin restored the activity of rat brain neurons by restoring complex I activity. Kumar et al.45 proposed that NaHS could increase the mitochondrial complex I, II and IV mediated oxygen consumption rates in hyperhomocysteinemia (HHCY) animal model. Jiang et al.46 designed and synthesized a small molecule compound A which targeted the succinate dehydrogenase subunit B (SDHB) of complex II, and prevented the death of dopaminergic neurons in rat PD model. Kam et al.47 extracted a cysteine rich peptide, rT1, from hibiscus syriacus, and proved that rT1 could prompt ATP synthase and cell survival by targeting ETC.
3.2.3. Drugs targeting mitochondria quality control related proteins
Mitochondria maintain the dynamic balance between biogenesis and clearance, which is important for cell survival. Mitochondrial biogenesis is a process of new mitochondria producing which is mainly regulated by PGC-1α and its downstream NRF1/2. The damaged or dysfunctional mitochondria are degraded by autophagy, termed as mitophagy, and deficiency of mitophagy is thought to be one causative factors of NDDs. So far PINK1/PARKIN mediated mitophagy was well defined. As a kinase, the activation of PINK1 causes the phosphorylation of ubiquitin, further leads to the activation of parkin, so that mitochondrial proteins are ubiquitinated, and finally cause mitochondrial degradation. Triggering mitochondria biogenesis or prompting mitophagy is one therapeutic strategy. Curcumin or pigment epithelium-derived factor (PEDF) rescued PD models in vitro and in vivo via stimulating mitochondria biogenesis58. Adiponectin (APN), a multi-functional adipokine which sensitizes the insulin signals, was proved to stimulate mitochondria biogenesis in AD59. Yang et al.48 showed that nicotinamide riboside (NR) prevented neurodegeneration by promoting mitophagy in PINK1-dependent manner. Memantine was found to enhance mitophagy in pluripotent stem cell derived neurons from PD patients. Li et al.49 demonstrated that rapamycin, a pharmacological inhibitor of mTOR, attenuated spinal cord injury by promoting mitophagy. Urolithin A or actinonin treatment stimulated mitophagy and reversed memory impairment in AD models50. Rilmenidine, an anti-hypertensive agent, administration promoted mitophagy in mutant SOD1 induced ALS mice51.
3.3. Drugs targeting redox of mitochondria
Mitochondria provide most of the energy to maintain the cells survival and function. Producing together with ETC, the majority of mitochondria ROS takes part in various cellular physiological processes, such as stimulating lipid peroxidation, stimulating cellular calcium signal and regulating mitochondrial calcium uptake60. The healthy cells have its own antioxidant system, including superoxide dismutase, glutathione peroxidase and peroxidase, to keep the redox balance. However, when genetic or environmental factors lead to excessive ROS generation or deficient antioxidant, the redox balance will be broken and diseases such as NDDs occur. So, reducing ROS or increasing antioxidant represent one way for conquering NDDs.
A variety of antioxidants targeting ROS have been reported for the treatment of NDDs. Mito-Q was synthesized by covalently linking ubiquinone to mitochondria targeting moiety TPP+, and the later one facilitated its penetration to mitochondria. Mito-Q displayed rescue effect in AD, PD and Huntingtin diseases models by scavenging ROS61. SkQ1 is also a quinone based antioxidant with mitochondria targeting features, and its scavenging rate of lipid peroxidation was four times that of Mito-Q and showed neuroprotective effects52. Mito-TEMPOL is another widely used mitochondrial targeted antioxidant. It cannot only scavenge ROS, but also oxidative iron. Kang et al.53 computationally designed and prepared peptide-based antioxidant, CAMP-HMT1A by fusing protein human metallothionein 1A (HMT1A) with a cell penetrating artificial mitochondrial targeting peptide (CAMP). CAMP-HMT1A alleviated mitochondria damage and movement impairment of PD model. SS-31 is also one antioxidant peptides which can target the delivery of antioxidants to the mitochondrial inner membrane, the site of ROS production54. Natural products, such as naringenin, bruceine, and tanshinone, conferred neuroprotection in NDDs models by stimulating glutathione (GSH) production. Pyridoxine, also known as vitamin B6, was found to promote GSH biosynthesis and alleviate manifestation of MPTP induced PD mice55 (see Fig. 2).
Figure 2.
NDDs drugs with specific mitochondrial targets.
4. Evaluation the rescue effect of mitochondria targeting drugs for NDDs
After administration of the drugs, its rescue effects on NDDs need to be evaluated according to outcome of models or patients. Given that there is no one standard program applicable to assess all the subjects, we will address the evaluation from three aspects: molecular level, cellular level and organism level (Table 3).
Table 3.
Table of assessment.
| Level | Target | Assay |
|---|---|---|
| Molecular level | ATP | Luminescent ATP detection assay kit; the O2 consumption rate (OCR); the extracellular acidification rate (ECAR); cell titer Glo luminescent reagent; luciferin–luciferase reaction; fluorescence probes |
| Free radicals | Specific fluorescent probes (DPPH, DCFH-DA, H2DCFDA, DHR, DCF); flow cytometry analysis; ESR; confocal microscope; spectrophotometry; chemiluminescence | |
| GSH | GSH assay kit; spectrophotometry | |
| SOD | Western blot; MitoSOX (specific fluorescent probe); immunoblotting | |
| LDH | LDH assay kit | |
| CCO | Spectrophotometer; Western blot; quantitative cytochrome oxidase (CCO); histochemistry | |
| MMP | Tetramethylrhodamine methyl ester (TMRM); 123-rhodamine; flow cytometry analysis; rhodamine 800; JC-1/JC-10 | |
| mtDNA | Western blot; DNA polymerase γ; mtDNA single-stranded DNA-binding protein (SSBP1); twinkle | |
| Mitochondrial complex | Spectrophotometer; immunohistochemistry; spectrophotometric enzyme assays; microplate assay kit; BN-PAGE | |
| Cellular level | Cell viability and cell morphology | CCK8; MTT; TUNEL assay; AnnexinV-FITC/PI staining; Co-IP; flow cytometry; immunofluorescence; Hoechst and rhodamin staining; Golgi staining; silver staining; Nissl's staining; virus-based neural tracer technology; LFB and Masson staining |
| Mitochondrial morphology | Transmission electron microscopy (TEM); Mito trackers; JC-1; DRP1 or MFN2 immunofluorescent imaging; immunofluorescent staining of TOM20/TIM23 | |
| Axons, dendrites and synapses | Immunofluorescence analysis; TEM; MAP-2 or β-tubulin staining; Golgi's staining; rhodamine-phalloidin; Nlgn1 or Nrxn3 staining | |
| Electrophysiological Feature | Whole-cell patch-clamp; brain slices patch-clamp; multichannel electrophysiological | |
| Organism level | Memory assessment | Y-maze test; Barnes; Morris water maze; visual water task; water maze reversal; NOL test; NOR test; long-term potentiation (LTP) |
| Locomotor assays | Open field test; pole test; beam hang test; catalepsy measurement; passive avoidance test; rotarod task; forced swimming test; gait analysis; balance beam test; climb test; curling; BBB scale | |
| Brain imaging | CT; FMRI; DTI |
4.1. Molecular level evaluation
When drugs exert its therapeutic effects on NDDs, it would inevitably lead to the change of mitochondria related molecules, such as proteins, mtDNA, ATP, radicals, mitochondrial membrane potential (MMP).
4.1.1. Adenosine triphosphate (ATP)
The fundamental role of mitochondria is to produce ATP. So, ATP is the key parameter to assess the effect of mitochondria targeting drugs. ATP level could be directly assessed by ATP fluorescence detection kit with the help of microplate reader62. Cell Titer Glo Luminescent reagent can detect intracellular ATP concentration by luciferin-luciferase reaction63. ATP level in different cellular compartments could be monitored with the fluorescence resonance energy transfer (FRET)-based genetically encoded indicators, such as AT1.03 composed of the epsilon subunit of the bacterial FoF1-ATP synthase64. Extracellular and intracellular ATP could be visualized by the single-wave length genetically encoded fluorescent sensors (iATPSnFRs). iATPSnFR1.0 responded to relevant ATP with enhanced fluorescence of specific cell types and sub-cellular compartments65. Wu et al.66 reported a new series of genetically encoded G protein-coupled receptor activation-based (GRAB) sensors for monitoring the ATP in targeted cells, called GRABATP1.0. GRABATP1.0 realized ATP detection with high sensitivity, selectivity, and spatiotemporal resolution. Recently, an aptamer-based fluorescence ATP probe was reported, which could sensitively detect ATP in cells as well as cardio-tissue67. ATP could also be indirectly determined by measuring the oxygen consumption rate (OCR)68 and the extracellular acidification rate (ECAR)69.
4.1.2. Free radicals
Free radicals, partially generated from mitochondria, are endowed with strong oxidation and high chemical reaction activity. Free radicals could be detected by spectrophotometry. Moreover, fluorescent probes such as DCFH-DA70,71, H2DCFDA72,73, DHR74, DCF75 could be used to detect ROS and reactive nitrogen species. With the help of flow cytometry or confocal microscopy, free radicals could also be quantified. ROS production was also determined by Amplex Red horseradish peroxidase and Electron Paramagnetic Resonance (EPR) spectroscopy70,71. GSH was one of the most abundant antioxidants in cells, and was essential to maintain redox balance of mitochondria. The content of GSH was determined by GSH kit, which indirectly reflected the ROS level72.
4.1.3. Oxidoreductases
Oxidoreductases such as superoxide dismutase (SOD), lactate dehydrogenase (LDH), cytochrome oxidases (CCO) are the enzymes that catalyze the redox reaction. Activity or level of SOD/LDH reflects the mitochondria function as well as the effect of drugs. SOD could be detected by Western blot62 or fluorescent probes such as MitoSox59,72. LDH levels can be determined directly using the LDH assay kit and CCO could be detected by Western blot or histochemistry staining62.
4.1.4. Mitochondrial membrane potential (MMP, ΔΨm)
MMP is an important indicator of mitochondrial function and integrity. Physiological MMP (ΔΨm 120–140 mV) allowed routine ATP production and loss of MMP led to apoptosis by efflux of macromolecules like Cyt c and caspase-9/caspase-3 cascade activation76. MitoTracker (Invitrogen), tetramethylrhodamine methyl ester (TMRM)77, and other dyes can be used to assess MMP by fluorescence spectrophotometry78. Noteworthy, some dyes such as rhodamine B methyl ester (RhB-ME) was thermochromic, which might interfere the detection of MMP. The temperature-insensitive dyes such as rhodamine 800 were preferred79. In addition, fluorescent probes such as JC-1/JC-10 can also assess MMP by bioimaging or flow cytometry72.
4.1.5. Mitochondria DNA (mtDNA)
Mutation or deletion of mtDNA contributes the pathogenesis of NDDs. mtDNA sequence and copy numbers can be checked by quantitative RT-PCR. Moreover, there are enzymes involved in mitochondrial DNA replication, such as DNA polymerase γ, mtDNA single-stranded DNA-binding protein 1 (SSBP1) and TWINKLE. These enzymes can be determined by Western blot to reflect the ability of mtDNA replication62.
4.1.6. Mitochondrial complex
Mitochondria complexes are the electron carrier in charge of electron transfer during mitochondrial oxidative phosphorylation. The expression level of the mitochondrial respiratory chain complexes could be detected by the immunohistochemical analysis. The activity of the mitochondrial respiratory chain complexes could be examined by specific kit, and spectrophotometric assays80. Besides the classical spectrophotometric analysis, the blue native polyacrylamide gel electrophoresis (BN-PAGE) was also a useful tool for visualizing the activities of mitochondrial phosphorylation enzymes81.
4.2. Cellular level evaluation
Besides biomolecules, the structure of mitochondria, the cell viability and morphology can also reflect the effects of mitochondria targeting drugs.
4.2.1. Cell viability and the cell morphology
Progressive neurodegeneration is the feature of NDDs. The neurons viability assessment tells the effectiveness of mitochondria drugs, which can be detected by CCK8 or MTT assay59. Meanwhile, Annexin V-fluorescein isothiocyanate (Annexin V-FITC)/propidium iodide (PI) double-staining can be adopted to determine the cell viability via flow cytometry or fluorescent microscope. Annexin-V positive and PI negative cells were defined as early necrotic cells while Annexin-V negative and PI positive cells were regarded as late necrotic cells82. TUNEL assay also detects apoptotic neurons83. Cytoplasm atrophy and nucleus condensation are typical morphology of neurons under apoptosis. Rhodamin and Hoechst 33258 staining, haemotoxylin and eosin (H&E) staining, immunostaining for neuronal markers84, rhodamine-phalloidin staining85, Golgi staining86, silver staining87, Nissl staining88, Luxol fast blue (LFB) staining and Masson staining171, and Virus-based neural tracer technology89 are used to display cell morphology more intuitively.
4.2.2. Mitochondrial morphology
Mitochondria are highly dynamic organelles that constantly undergo fission and fuse to ensure mitochondria quantity control, which is regulated by proteins, such as DRP1 and MFN2. If this process goes wrong, cells may subject to apoptosis as happened in NDDs. The morphology mitochondria can be observed using a transmission electron microscope (TEM). There are many mito-trackers developed so far, which can label mitochondria specifically and confer the morphology visible under microscope. DRP1 or MFN2 immunofluorescent imaging can also be used to observe the dynamic change of mitochondria. Swelling is one morphological manifestation of mitochondria suffered in NDDs, which was checked by immunofluorescent staining of TOM20/TIM2390.
4.2.3. Axons, dendrites and synapses
Neurons have some specific structures, such as axons, dendrites and synapses, to maintain their physiological functions. Synapses lose and axon degeneration often occurs in neurons of NDDs, and neurite deficit impairs neural connectivity and brain function. MAP-2 or β-tubulin are often utilized to label the axons so as to determine their structures and integrity in NDDs. Golgi's staining is commonly used to demonstrate the entire neuronal morphology including its axon, dendrite, and synaptic spine. Monomeric fluorescent protein co-transfected with specific dendritic spine protein is utilized to label the dendritic spine of dissociated neurons. Immunofluorescent assay of rhodamine-phalloidin can be applied to detect the neurite length91. Synapses stained with postsynaptic neuroligin-1 (Nlgn1) or presynaptic neurexin-3 (Nrxn3) can be observed under fluorescent microscope or transmission electron microscope.
4.2.4. Electrophysiological feature
In NDDs, the electrophysiological features of neuron are often altered. So recording and analyzing the electrophysiological activity of neurons provides one window to assess the therapeutic effect of drugs on NDDs. Various techniques are developed to record the electrophysiological features in vitro and in vivo. Whole-cell patch-clamp recording can observe the electrophysiological properties of single neuron, which help to determine the function and classification of neurons92. The patch-clamp recorded action potentials of brain slices representing the plasticity of neurons in learning and memory. Local field potentials (LFPs) recorded from the brain reflected the changes of membrane potential derived from population of neuronal somata located nearby the recording electrode93. Multichannel electrophysiological recording technique can be used in vivo to monitoring the activity of different neuron populations in real-time with drug administration.
4.3. Organism level
After administration of mitochondria drugs, subjects, including animal or patients, with NDDs will demonstrate manifestations in organism level which can be used to assess the effect of drugs. Mental, memorial, locomotorial indicators are often adopted.
4.3.1. Memory assessment
Y maze test, Morris water maze, visual water task, water maze reversal, the novel object recognition test, and the novel object location test can be used to test the learning and memory abilities subjects. If the mitochondria targeting drugs display rescue effect, those parameters reflecting learning and memory will be improved. The long-term potentiation (LTP) is a long-lasting strengthening of the response of a postsynaptic nerve cell to stimulation across the synapse that occurs with repeated stimulation and is thought to be related to learning and long-term memory. It is a persistent enhancement in signal transduction between two neurons, which is widely accepted as one of the main molecular mechanisms underlying learning and memory. So LTP assay showed the effect of drugs mechanically.
4.3.2. Locomotor assays
Open field test, pole test, beam hang test, step-by-step passive avoidance test, step-down passive avoidance test, rotarod, forced swimming test, gait analysis for stride length, balance beam and climb test are often applied to determine the capability of subjects in sensing of balance, motor coordination, muscle tone and depressive symptoms94. Curling was once used to monitor motility defects of Caenorhabditis elegans with PD95. The Basso, Beattie and Bresnahan locomotor rating scale is currently used to evaluate the functional recovery of locomotor capacity in rats83. Locomotor assays are essential to assess the effect of mitochondria targeting drugs for NDDs.
4.3.3. Brain imaging
The rescue effect of mitochondria targeting drugs to NDD can also be validated with brain imaging, such as computed tomography (CT), functional magnetic resonance imaging (fMRI), and diffusion tensor imaging (DTI). Those imaging techniques provide solid evidence to show the structure, and function recovery after treatment.
5. Perspective
Mitochondria-targeting drugs have brought hope for effective treatment of NDDs. But there is still a big gap between what are achieved and expected. Novel therapeutic methods are required to better tackle NDDs. Firstly, better understanding the mechanisms underlying NDDs pathogenesis is needed, so as to identify targets of intervention. Secondly, response triggered release of mitochondria targeting drugs is one trend of drug development for improving its specificity and reducing undesired effect96. Thirdly, mitochondria transplantation represents alternative strategy for therapeutic of NDDs97, though the way to harvest functional mitochondria remains to be settled. PROteolysis TArgeting Chimeras (PROTACs), one technique recently designed for specific targeting protein degradation, attracted increasing attention in drug development98. Given the characterized protein aggregation in NDDs, PROTACs combined with mitochondria targeting strategy is worthy of expectation for treatment of NDDs. In the future, great efforts need to be taken to combine advantages of different fields such materials, chemistry, and biology and propose better solution strategy of drug development toward NDDs.
Acknowledgments
This study was supported by the National Key R&D Program of China (2020YFA0709900), the National Natural Science Foundation of China (22077101), China-Sweden Joint Mobility Project (51811530018, China) and Postdoctoral Research Funding Schemes of Jiangsu Province (2021, China).
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Contributor Information
Li Lu, Email: luli@sxmu.edu.cn.
Chengwu Zhang, Email: chengwu_zhang@sxmu.edu.cn.
Lin Li, Email: iamlli@njtech.edu.cn.
Author contributions
Lin Li, Chengwu Zhang and Li Lu proposed the conception for the review. Jiajia Xu, Wei Du and Yunhe Zhao wrote the manuscript and prepared the tables and figures. Kahleong Lim provide critical suggestion on manuscript preparation. All authors read and approved the final manuscript.
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Mukherjee A., Becerra Calixto A.D., Chavez M., Delgado J.P., Soto C. Mitochondrial transplant to replenish damaged mitochondria: a novel therapeutic strategy for neurodegenerative diseases?. Prog Mol Biol Transl Sci. 2021;177:49–63. doi: 10.1016/bs.pmbts.2020.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Roca-Portoles A., Tait S.W.G. Mitochondrial quality control: from molecule to organelle. Cell Mol Life Sci. 2021;78:3853–3866. doi: 10.1007/s00018-021-03775-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu P.P., Xie Y., Meng X.Y., Kang J.S. History and progress of hypotheses and clinical trials for Alzheimer's disease. Signal Transduct Target Ther. 2019;4:29. doi: 10.1038/s41392-019-0063-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goyal S., Chaturvedi R.K. Mitochondrial protein import dysfunction in pathogenesis of neurodegenerative diseases. Mol Neurobiol. 2021;58:1418–1437. doi: 10.1007/s12035-020-02200-0. [DOI] [PubMed] [Google Scholar]
- 5.Wang X.L., Feng S.T., Wang Z.Z., Chen N.H., Zhang Y. Role of mitophagy in mitochondrial quality control: mechanisms and potential implications for neurodegenerative diseases. Pharmacol Res. 2021;165:105433. doi: 10.1016/j.phrs.2021.105433. [DOI] [PubMed] [Google Scholar]
- 6.Bock F.J., Tait S.W.G. Mitochondria as multifaceted regulators of cell death. Nat Rev Mol Cell Biol. 2020;21:85–100. doi: 10.1038/s41580-019-0173-8. [DOI] [PubMed] [Google Scholar]
- 7.Murphy M.P. Targeting lipophilic cations to mitochondria. Biochim Biophys Acta. 2008;1777:1028–1031. doi: 10.1016/j.bbabio.2008.03.029. [DOI] [PubMed] [Google Scholar]
- 8.Solesio M.E., Prime T.A., Logan A., Murphy M.P., Del Mar Arroyo-Jimenez M., Jordan J., et al. The mitochondria-targeted anti-oxidant MitoQ reduces aspects of mitochondrial fission in the 6-OHDA cell model of Parkinson's disease. Biochim Biophys Acta. 2013;1832:174–182. doi: 10.1016/j.bbadis.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 9.Mao G., Kraus G.A., Kim I., Spurlock M.E., Bailey T.B., Zhang Q., et al. A mitochondria-targeted vitamin E derivative decreases hepatic oxidative stress and inhibits fat deposition in mice. J Nutr. 2010;140:1425–1431. doi: 10.3945/jn.110.121715. [DOI] [PubMed] [Google Scholar]
- 10.Jauslin M.L., Meier T., Smith R.A.J., Murphy P.M. Mitochondria-targeted antioxidants protect friedreich ataxia fibroblasts from endogenous oxidative stress more effectively than untargeted antioxidants. FASEB J. 2003;17:1972–1974. doi: 10.1096/fj.03-0240fje. [DOI] [PubMed] [Google Scholar]
- 11.Zhelev Z., Bakalova R., Aoki I., Lazarova D., Saga T. Imaging of superoxide generation in the dopaminergic area of the brain in Parkinson's disease, using mito-TEMPO. ACS Chem Neurosci. 2013;4:1439–1445. doi: 10.1021/cn400159h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paleos C.M., Tsiourvas D., Sideratou Z. Triphenylphosphonium decorated liposomes and dendritic polymers: prospective second generation drug delivery systems for targeting mitochondria. Mol Pharm. 2016;13:2233–2241. doi: 10.1021/acs.molpharmaceut.6b00237. [DOI] [PubMed] [Google Scholar]
- 13.Rothbard J.B., Jessop T.C., Wender P.A. Adaptive translocation: the role of hydrogen bonding and membrane potential in the uptake of guanidinium-rich transporters into cells. Adv Drug Deliv Rev. 2005;57:495–504. doi: 10.1016/j.addr.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 14.Kwon H.J., Kim D., Seo K., Kim Y.G., Han S.I., Kang T., et al. Ceria nanoparticle systems for selective scavenging of mitochondrial, intracellular, and extracellular reactive oxygen species in Parkinson's disease. Angew Chem Int Ed. 2018;57:9408–9412. doi: 10.1002/anie.201805052. [DOI] [PubMed] [Google Scholar]
- 15.Yuan P., Mao X., Wu X., Liew S.S., Li L., Yao S.Q. Mitochondria-targeting, intracellular delivery of native proteins using biodegradable silica nanoparticles. Angew Chem Int Ed. 2019;58:7657–7661. doi: 10.1002/anie.201901699. [DOI] [PubMed] [Google Scholar]
- 16.Qian K., Chen H., Qu C., Qi J., Du B., Ko T., et al. Mitochondria-targeted delocalized lipophilic cation complexed with human serum albumin for tumor cell imaging and treatment. Nanomedicine. 2020;23:102087. doi: 10.1016/j.nano.2019.102087. [DOI] [PubMed] [Google Scholar]
- 17.Weissig V. From serendipity to mitochondria-targeted nanocarriers. Pharm Res. 2011;28:2657–2668. doi: 10.1007/s11095-011-0556-9. [DOI] [PubMed] [Google Scholar]
- 18.D'Souza G.G., Boddapati S.V., Weissig V. Mitochondrial leader sequence—plasmid DNA conjugates delivered into mammalian cells by DQAsomes co-localize with mitochondria. Mitochondrion. 2005;5:352–358. doi: 10.1016/j.mito.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 19.Ouyang C., Chen L., Rees T.W., Chen Y., Liu J., Ji L., et al. A mitochondria-targeting hetero-binuclear Ir(III)–Pt(II) complex induces necrosis in cisplatin-resistant tumor cells. Chem Commun. 2018;54:6268–6271. doi: 10.1039/c8cc02795a. [DOI] [PubMed] [Google Scholar]
- 20.Zhang C., Guan R., Liao X., Ouyang C., Rees T.W., Liu J., et al. A mitochondria-targeting dinuclear Ir–Ru complex as a synergistic photoactivated chemotherapy and photodynamic therapy agent against cisplatin-resistant tumour cells. Chem Commun. 2019;55:12547–12550. doi: 10.1039/c9cc05998a. [DOI] [PubMed] [Google Scholar]
- 21.Hoye A.T., Davoren J.E., Wipf A.P. Targeting mitochondria. Acc Chem Res. 2008;41:87–97. doi: 10.1021/ar700135m. [DOI] [PubMed] [Google Scholar]
- 22.Horton K.L., Stewart K.M., Fonseca S.B., Guo Q., Kelley S.O. Mitochondria-penetrating peptides. Chem Biol. 2008;15:375–382. doi: 10.1016/j.chembiol.2008.03.015. [DOI] [PubMed] [Google Scholar]
- 23.Chuah J.A., Yoshizumi T., Kodama Y., Numata K. Gene introduction into the mitochondria of Arabidopsis thaliana via peptide-based carriers. Sci Rep. 2015;5:7751. doi: 10.1038/srep07751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Appiah Kubi G., Qian Z., Amiar S., Sahni A., Stahelin R.V., Pei D. Non-peptidic cell-penetrating motifs for mitochondrion-specific cargo delivery. Angew Chem Int Ed. 2018;57:17183–17188. doi: 10.1002/anie.201811940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamada Y., Akita H., Kamiya H., Kogure K., Yamamoto T., Shinohara Y., et al. MITO-Porter: a liposome-based carrier system for delivery of macromolecules into mitochondria via membrane fusion. Biochim Biophys Acta. 2008;1778:423–432. doi: 10.1016/j.bbamem.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 26.Yamada Y., Harashima H. Delivery of bioactive molecules to the mitochondrial genome using a membrane-fusing, liposome-based carrier, DF-MITO-Porter. Biomaterials. 2012;33:1589–1595. doi: 10.1016/j.biomaterials.2011.10.082. [DOI] [PubMed] [Google Scholar]
- 27.Kawamura E., Hibino M., Harashima H., Yamada Y. Targeted mitochondrial delivery of antisense RNA-containing nanoparticles by a MITO-Porter for safe and efficient mitochondrial gene silencing. Mitochondrion. 2019;49:178–188. doi: 10.1016/j.mito.2019.08.004. [DOI] [PubMed] [Google Scholar]
- 28.Yamada Y., Munechika R., Satrialdi, Kubota F., Sato Y., Sakurai Y., et al. Mitochondrial delivery of an anticancer drug via systemic administration using a mitochondrial delivery system that inhibits the growth of drug-resistant cancer engrafted on mice. J Pharm Sci. 2020;109:2493–2500. doi: 10.1016/j.xphs.2020.04.020. [DOI] [PubMed] [Google Scholar]
- 29.Kawamura E., Maruyama M., Abe J., Sudo A., Takeda A., Takada S., et al. Validation of gene therapy for mutant mitochondria by delivering mitochondrial RNA using a MITO-Porter. Mol Ther Nucleic Acids. 2020;20:687–698. doi: 10.1016/j.omtn.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xun Z., Rivera-Sanchez S., Ayala-Pena S., Lim J., Budworth H., Skoda E.M., et al. Targeting of XJB-5-131 to mitochondria suppresses oxidative DNA damage and motor decline in a mouse model of Huntington's disease. Cell Rep. 2012;2:1137–1142. doi: 10.1016/j.celrep.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gammage P.A., Rorbach J., Vincent A.I., Rebar E.J., Minczuk M. Mitochondrially targeted ZFNs for selective degradation of pathogenic mitochondrial genomes bearing large-scale deletions or point mutations. EMBO Mol Med. 2014;6:458–466. doi: 10.1002/emmm.201303672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang Y., Wu H., Kang X., Liang Y., Lan T., Li T., et al. Targeted elimination of mutant mitochondrial DNA in MELAS-iPSCs by mitoTALENs. Protein Cell. 2018;9:283–297. doi: 10.1007/s13238-017-0499-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jo A., Ham S., Lee G.H., Lee Y.I., Kim S., Lee Y.S., et al. Efficient mitochondrial genome editing by CRISPR/Cas9. Biomed Res Int. 2015;215:305716. doi: 10.1155/2015/305716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bian W.P., Chen Y.L., Luo J.J., Wang C., Xie S.L., Pei D.S. Knock-in strategy for editing human and zebrafish mitochondrial DNA using Mito-CRISPR/Cas9 system. ACS Synth Biol. 2019;8:621–632. doi: 10.1021/acssynbio.8b00411. [DOI] [PubMed] [Google Scholar]
- 35.Mok B.Y., de Moraes M.H., Zeng J., Bosch D.E., Kotrys A.V., Raguram A., et al. A bacterial cytidine deaminase toxin enables CRISPR-free mitochondrial base editing. Nature. 2020;583:631–637. doi: 10.1038/s41586-020-2477-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.ISoldner F., Laganière J., Cheng A.W., Hockemeyer D., Gao Q., Alagappan R., et al. Generation of isogenic pluripotent stem cells differing exclusively at two early onset Parkinson point mutations. Cell. 2011;146:318–331. doi: 10.1016/j.cell.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.An M.C., Zhang N., Scott G., Montoro D., Wittkop T., Mooney S., et al. Genetic correction of Huntington's disease phenotypes in induced pluripotent stem cells. Cell Stem Cell. 2012;11:253–263. doi: 10.1016/j.stem.2012.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Merienne N., Vachey G., Longprez L., Meunier C., Zimmer V., Perriard G., et al. The self-inactivating KamiCas9 system for the editing of CNS disease genes. Cell Rep. 2017;20:2980–2991. doi: 10.1016/j.celrep.2017.08.075. [DOI] [PubMed] [Google Scholar]
- 39.Lopez J., Bessou M., Riley J.S., Giampazolias E., Todt F., Rochegüe T., et al. Mito-priming as a method to engineer Bcl-2 addiction. Nat Commun. 2016;7:10538. doi: 10.1038/ncomms10538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hellman M., Arumäe U., Yu L.Y. Mesencephalic astrocyte-derived neurotrophic factor (MANF) has a unique mechanism to rescue apoptotic neurons. J Biol Chem. 2011;286:2675–2680. doi: 10.1074/jbc.M110.146738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hu X., Song Q., Li X. Neuroprotective effects of kukoamine A on neurotoxin-induced Parkinson's model through apoptosis inhibition and autophagy enhancement. Neuropharmacology. 2017;117:352–363. doi: 10.1016/j.neuropharm.2017.02.022. [DOI] [PubMed] [Google Scholar]
- 42.Garner T.P., Amgalan D., Reyna D.E., Li S., Kitsis R.N., Gavathiotis E. Small-molecule allosteric inhibitors of BAX. Nat Chem Biol. 2019;15:322–330. doi: 10.1038/s41589-018-0223-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shteinfer-Kuzmine A., Argueti S., Gupta R. A VDAC1-derived N-terminal peptide inhibits mutant SOD1–VDAC1 interactions and toxicity in the SOD1 model of ALS. Front Cell Neurosci. 2019;13:346. doi: 10.3389/fncel.2019.00346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Amarsanaa K., Kim H.J., Ko E.A., Jo J., Jung S.C. Nobiletin exhibits neuroprotective effects against mitochondrial complex I inhibition via regulating apoptotic signaling. Exp Neurobiol. 2021;30:73–86. doi: 10.5607/en20051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kumar M., Sandhir R. Hydrogen sulfide attenuates hyperhomocysteinemia-induced mitochondrial dysfunctions in brain. Mitochondrion. 2020;50:158–169. doi: 10.1016/j.mito.2019.11.004. [DOI] [PubMed] [Google Scholar]
- 46.Jiang X., Li L., Ying Z., Pan C., Huang S., Li L., et al. A small molecule that protects the integrity of the electron transfer chain blocks the mitochondrial apoptotic pathway. Mol Cell. 2016;63:229–239. doi: 10.1016/j.molcel.2016.06.016. [DOI] [PubMed] [Google Scholar]
- 47.Kam A., Loo S., Dutta B., Sze S.K., Tam J.P. Plant-derived mitochondria-targeting cysteine-rich peptide modulates cellular bioenergetics. J Biol Chem. 2019;294:4000–4011. doi: 10.1074/jbc.RA118.006693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang B., Dan X., Hou Y., Lee J.H., Wechter N., Krishnamurthy S., et al. NAD+ supplementation prevents STING-induced senescence in ataxia telangiectasia by improving mitophagy. Aging Cell. 2021;20:e13329. doi: 10.1111/acel.13329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li Q., Gao S., Kang Z., Zhang M., Zhao X., Zhai Y., et al. Rapamycin enhances mitophagy and attenuates apoptosis after spinal ischemia–reperfusion injury. Front Neurosci. 2018;12:865. doi: 10.3389/fnins.2018.00865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fang E.F., Hou Y., Palikaras K., Adriaanse B.A., Kerr J.S., Yang B., et al. Mitophagy inhibits amyloid-β and tau pathology and reverses cognitive deficits in models of Alzheimer's disease. Nat Neurosci. 2019;22:401–412. doi: 10.1038/s41593-018-0332-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Perera N.D., Sheean R.K., Lau C.L., Shin Y.S., Beart P.M., Horne M.K., et al. Rilmenidine promotes MTOR-independent autophagy in the mutant SOD1 mouse model of amyotrophic lateral sclerosis without slowing disease progression. Autophagy. 2018;14:534–551. doi: 10.1080/15548627.2017.1385674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Genrikhs E.E., Stelmashook E.V., Popova O.V., Kapay N.A., Korshunova G.A., Sumbatyan N.V., et al. Mitochondria-targeted antioxidant SkQT1 decreases trauma-induced neurological deficit in rat and prevents amyloid-β-induced impairment of long-term potentiation in rat hippocampal slices. J Drug Target. 2015;23:347–352. doi: 10.3109/1061186X.2014.997736. [DOI] [PubMed] [Google Scholar]
- 53.Kang Y.C., Son M., Kang S., Im S., Piao Y., Lim K.S., et al. Cell-penetrating artificial mitochondria-targeting peptide-conjugated metallothionein 1A alleviates mitochondrial damage in Parkinson's disease models. Exp Mol Med. 2018;50:1–13. doi: 10.1038/s12276-018-0124-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Oliver D.M.A., Reddy P.H. Small molecules as therapeutic drugs for Alzheimer's disease. Mol Cell Neurosci. 2019;96:47–62. doi: 10.1016/j.mcn.2019.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wei Y., Lu M., Mei M., Wang H., Han Z., Chen M., et al. Pyridoxine induces glutathione synthesis via PKM2-mediated Nrf2 transactivation and confers neuroprotection. Nat Commun. 2020;11:941. doi: 10.1038/s41467-020-14788-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Anderson S., Bankier A.T., Barrell B.G., de Bruijn M.H., Coulson A.R., Drouin J., et al. Sequence and organization of the human mitochondrial genome. Nature. 1981;290:457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- 57.Chakravorty A., Jetto C.T., Manjithaya R. Dysfunctional mitochondria and mitophagy as drivers of Alzheimer's disease pathogenesis. Front Aging Neurosci. 2019;11:311. doi: 10.3389/fnagi.2019.00311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim J.Y., Park S., Park S.H., Lee D., Kim G.H., Noh J.E., et al. Overexpression of pigment epithelium-derived factor in placenta-derived mesenchymal stem cells promotes mitochondrial biogenesis in retinal cells. Lab Invest. 2021;101:51–69. doi: 10.1038/s41374-020-0470-z. [DOI] [PubMed] [Google Scholar]
- 59.Waragai M., Ho G., Takamatsu Y., Wada R., Sugama S., Takenouchi T., Masliah E., Hashimoto M. Adiponectin paradox in Alzheimer’s disease; relevance to amyloidogenic evolvability?. Front Endocrinol (Lausanne) 2020;11:108. doi: 10.3389/fendo.2020.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Angelova P.R., Abramov A.Y. Functional role of mitochondrial reactive oxygen species in physiology. Free Radic Biol Med. 2016;100:81–85. doi: 10.1016/j.freeradbiomed.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 61.Young M.L., Franklin J.L. The mitochondria-targeted antioxidant MitoQ inhibits memory loss, neuropathology, and extends lifespan in aged 3xTg-AD mice. Mol Cell Neurosci. 2019;101:103409. doi: 10.1016/j.mcn.2019.103409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hu H., Li M. Mitochondria-targeted antioxidant mitotempo protects mitochondrial function against amyloid beta toxicity in primary cultured mouse neurons. Biochem Biophys Res Commun. 2016;478:174–180. doi: 10.1016/j.bbrc.2016.07.071. [DOI] [PubMed] [Google Scholar]
- 63.Geng J. Andrographolide alleviates parkinsonism in MPTP-PD mice via targeting mitochondrial fission mediated by dynamin-related protein 1. Br J Pharmacol. 2019;176:4574–4591. doi: 10.1111/bph.14823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Imamura H., Nhat K.P., Togawa H., Saito K., Iino R., Kato-Yamada Y., et al. Visualization of ATP levels inside single living cells with fluorescence resonance energy transfer-based genetically encoded indicators. Proc Natl Acad Sci U S A. 2009;106:15651–15656. doi: 10.1073/pnas.0904764106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lobas M.A., Tao R., Nagai J., Kronschläger M.T., Borden P.M., Marvin J.S., et al. A genetically encoded single-wavelength sensor for imaging cytosolic and cell surface ATP. Nat Commun. 2019;10:711. doi: 10.1038/s41467-019-08441-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wu Z., He K., Chen Y., Li H., Pan S., Li B., et al. A sensitive GRAB sensor for detecting extracellular ATP in vitro and in vivo. Neuron. 2021;S0896:6273. doi: 10.1016/j.neuron.2021.11.027. [DOI] [PubMed] [Google Scholar]
- 67.Liu W., Zhu X., Mozneb M., Nagahara L., Hu T.Y., Li C.Z. Lighting up ATP in cells and tissues using a simple aptamer-based fluorescent probe. Mikrochim Acta. 2021;188:352. doi: 10.1007/s00604-021-05012-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang X., Liu J., Wang J., Han L., Ma S., Zhao M., Xi G. Adenosine triphosphate and zinc(II) ions responsive pyrene based turn-on fluorescent probe and its application in live cell imaging. J Photochem Photobiol B. 2021;223:112279. doi: 10.1016/j.jphotobiol.2021.112279. [DOI] [PubMed] [Google Scholar]
- 69.Kim J.D., Yoon N.A., Jin S., Diano S. Microglial UCP2 mediates inflammation and obesity induced by high-fat feeding. Cell Metab. 2019;30:952–962. doi: 10.1016/j.cmet.2019.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Marano S., Minnelli C., Ripani L., Marcaccio M., Laudadio E., Mobbili G., et al. Insights into the antioxidant mechanism of newly synthesized benzoxazinic nitrones: in vitro and in silico studies with DPPH model radical. Antioxidants (Basel) 2021;10:1224. doi: 10.3390/antiox10081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lohan S.B., Ivanov D., Schüler N., Berger B., Zastrow L., Lademann J., et al. Switching from healthy to unhealthy oxidative stress – does the radical type can be used as an indicator?. Free Radic Biol Med. 2021;162:401–411. doi: 10.1016/j.freeradbiomed.2020.10.319. [DOI] [PubMed] [Google Scholar]
- 72.Uppakara K., Jamornwan S., Duan L.X., Yue K.R., Sunrat C., Dent E.W., et al. Novel α-lipoic acid/3-n-butylphthalide conjugate enhances protective effects against oxidative stress and 6-OHDA induced neuronal damage. ACS Chem Neurosci. 2020;11:1634–1642. doi: 10.1021/acschemneuro.0c00105. [DOI] [PubMed] [Google Scholar]
- 73.Kang S., Piao Y., Kang Y.C., Lim S., Pak Y.K. Qi-activating quercetin alleviates mitochondrial dysfunction and neuroinflammation in vivo and in vitro. Arch Pharm Res. 2020;43:553–566. doi: 10.1007/s12272-020-01238-x. [DOI] [PubMed] [Google Scholar]
- 74.Zakaria A., Hamdi N., Abdel-Kader R.M. Methylene blue improves brain mitochondrial ABAD functions and decreases Aβ in a neuroinflammatory Alzheimer's disease mouse model. Mol Neurobiol. 2016;53:1220–1228. doi: 10.1007/s12035-014-9088-8. [DOI] [PubMed] [Google Scholar]
- 75.Dragicevic N., Copes N., O'Neal-Moffitt G., Jin J., Buzzeo R., Mamcarz M., et al. Melatonin treatment restores mitochondrial function in Alzheimer's mice: a mitochondrial protective role of melatonin membrane receptor signaling. J Pineal Res. 2011;51:75–86. doi: 10.1111/j.1600-079X.2011.00864.x. [DOI] [PubMed] [Google Scholar]
- 76.Watts L.T. Stimulating mitochondria to protect the brain following traumatic brain injury. Neural Regen Res. 2016;11:1403–1404. doi: 10.4103/1673-5374.191205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu Z.D., Zhang S., Hao J.J., Xie T.R., Kang J.S. Cellular model of neuronal atrophy induced by DYNC1I1 deficiency reveals protective roles of RAS–RAF–MEK signaling. Protein Cell. 2016;7:638–650. doi: 10.1007/s13238-016-0301-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Maliyakkal N., Appadath Beeran A., Udupa N. Nanoparticles of cisplatin augment drug accumulations and inhibit multidrug resistance transporters in human glioblastoma cells. Saudi Pharm J. 2021;29:857–873. doi: 10.1016/j.jsps.2021.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xie T.R., Liu C.F., Kang J.S. Dye-based mito-thermometry and its application in thermogenesis of brown adipocytes. Biophys Rep. 2017;3:85–91. doi: 10.1007/s41048-017-0039-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hoes M.F., Grote B.N., Kijlstra J.D., Kuipers J., Swinkels D.W., Giepmans B.N.G., et al. Iron deficiency impairs contractility of human cardiomyocytes through decreased mitochondrial function. Eur J Heart Fail. 2018;20:910–919. doi: 10.1002/ejhf.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zerbetto E., Vergani L., Dabbeni-Sala F. Quantification of muscle mitochondrial oxidative phosphorylation enzymes via histochemical staining of blue native polyacrylamide gels. Electrophoresis. 1997;18:2059–2064. doi: 10.1002/elps.1150181131. [DOI] [PubMed] [Google Scholar]
- 82.Han Y., Chu X., Cui L., Fu S., Gao C., Li Y., Sun B. Neuronal mitochondria-targeted therapy for Alzheimer's disease by systemic delivery of resveratrol using dual-modified novel biomimetic nanosystems. Drug Deliv. 2020;27:502–518. doi: 10.1080/10717544.2020.1745328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li H., Wang C., He T., Zhao T., Chen Y.Y., Shen Y.L., et al. Mitochondrial transfer from bone marrow mesenchymal stem cells to motor neurons in spinal cord injury rats via gap junction. Theranostics. 2019 17;9:2017–2035. doi: 10.7150/thno.29400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Völker J., Engert J., Völker C., Bieniussa L., Schendzielorz P., Hagen R., et al. Isolation and characterization of neural stem cells from the rat inferior colliculus. Stem Cells Int. 2019;2019:5831240. doi: 10.1155/2019/5831240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xiang Y., Niu Y., Xie Y., Chen S., Zhu F., Shen W., Zeng L.H. Inhibition of RhoA/Rho kinase signaling pathway by fasudil protects against kainic acid-induced neurite injury. Brain Behav. 2021;11:e2266. doi: 10.1002/brb3.2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pedrazzoli M., Medelin M., Marchiotto F., Cisterna B., Malatesta M., Buffelli M. An improved and simplified protocol to combine Golgi-Cox staining with immunofluorescence and transmission electron microscopy techniques. Neurochem Int. 2021;142:104922. doi: 10.1016/j.neuint.2020.104922. [DOI] [PubMed] [Google Scholar]
- 87.Lavenir I., Passarella D., Masuda M., Curry A., Holton J.L., Ghetti B., et al. Silver staining (Campbell-Switzer) of neuronal α-synuclein assemblies induced by multiple system atrophy and Parkinson's disease brain extracts in transgenic mice. Acta Neuropathol Commun. 2019;7:148. doi: 10.1186/s40478-019-0804-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Magnain C., Augustinack J.C., Tirrell L., Fogarty M., Frosch M.P., Boas D., et al. Colocalization of neurons in optical coherence microscopy and Nissl-stained histology in Brodmann's area 32 and area 21. Brain Struct Funct. 2019;224:351–362. doi: 10.1007/s00429-018-1777-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chen Y.C., Ma N.X., Pei Z.F., Wu Z., Do-Monte F.H., Keefe S., et al. A neuroD1 AAV-Based gene therapy for functional brain repair after ischemic injury through in vivo astrocyte-to-neuron conversion. Mol Ther. 2020;28:217–234. doi: 10.1016/j.ymthe.2019.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ng C.H., Guan M.S., Koh C., Ouyang X., Yu F., Tan E.K., et al. AMP kinase activation mitigates dopaminergic dysfunction and mitochondrial abnormalities in Drosophila models of Parkinson’s disease. J Neurosci. 2012;32:14311–14317. doi: 10.1523/JNEUROSCI.0499-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mor D.E., Sohrabi S., Kaletsky R., Keyes W., Tartici A., Kalia V., et al. Metformin rescues Parkinson's disease phenotypes caused by hyperactive mitochondria. Proc Natl Acad Sci U S A. 2020;117:26438–26447. doi: 10.1073/pnas.2009838117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cadwell C.R., Palasantza A., Jiang X., Berens P., Deng Q., Yilmaz M., et al. Electrophysiological, transcriptomic and morphologic profiling of single neurons using Patch-seq. Nat Biotechnol. 2016;34:199–203. doi: 10.1038/nbt.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pasquereau B., Tremblay L., Turner R.S. Local field potentials reflect dopaminergic and non-dopaminergic activities within the primate midbrain. Neuroscience. 2019;399:167–183. doi: 10.1016/j.neuroscience.2018.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hang L., Thundyil J., Goh G.W.Y., Lim K.L. AMP kinase activation is selectively disrupted in the ventral midbrain of mice deficient in Parkin or PINK1 expression. Neuromolecular Med. 2019;21:25–32. doi: 10.1007/s12017-018-8517-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hautbergue G.M., Castelli L.M., Ferraiuolo L., Sanchez A., Cooper J., Higginbottom A., et al. SRSF1-dependent nuclear export inhibition of C9ORF72 repeat transcripts prevents neurodegeneration and associated motor deficits. Nat Commun. 2017;8:16063. doi: 10.1038/ncomms16063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gao F., Xiong Z. Reactive oxygen species responsive polymers for drug delivery systems. Front Chem. 2021;9:649048. doi: 10.3389/fchem.2021.649048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Norat P., Soldozy S., Sokolowski J.D., Gorick C.M., Kumar J.S., Chae Y., et al. Mitochondrial dysfunction in neurological disorders: exploring mitochondrial transplantation. NPJ Regen Med. 2020;5:22. doi: 10.1038/s41536-020-00107-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lu B., Ye J.P. Commentary: PROTACs make undruggable targets druggable: challenge and opportunity. Acta Pharm Sin B. 2021;11:3335–3336. doi: 10.1016/j.apsb.2021.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]