Abstract
Lipid nanoparticle (LNP)-based drug delivery systems have become the most clinically advanced non-viral delivery technology. LNPs can encapsulate and deliver a wide variety of bioactive agents, including the small molecule drugs, proteins and peptides, and nucleic acids. However, as the physicochemical properties of small- and macromolecular cargos can vary drastically, every LNP carrier system needs to be carefully tailored in order to deliver the cargo molecules in a safe and efficient manner. Our group applied the combinatorial library synthesis approach and in vitro and in vivo screening strategy for the development of LNP delivery systems for drug delivery. In this Review, we highlight our recent progress in the design, synthesis, characterization, evaluation, and optimization of combinatorial LNPs with novel structures and properties for the delivery of small- and macromolecular therapeutics both in vitro and in vivo. These delivery systems have enormous potentials for cancer therapy, antimicrobial applications, gene silencing, genome editing, and more. We also discuss the key challenges to the mechanistic study and clinical translation of new LNP-enabled therapeutics.
Key words: Lipid nanoparticle, Combinatorial library, Drug delivery, Cancer therapy, Protein delivery, Gene therapy
Graphical abstract
Lipid nanoparticles are a potent and versatile platform for the delivery of small molecule drugs, proteins, and nucleic acids, for a variety of therapeutic applications including genome editing, cancer therapy, and more.
1. Introduction
Lipid nanoparticles (LNPs) are a potent and versatile platform of intracellular delivery, and have been extensively explored in the delivery of nucleic acids, proteins, and small molecule drugs1, 2, 3, 4. Notable applications include the first-ever U.S. Food and Drug Administration (FDA)-approved nano-drug, Doxil®, which uses LNPs for the delivery of the anticancer drug doxorubicin, and Epaxal®, which uses LNPs for the delivery of hepatitis A protein antigens. Most recently, mRNA (messenger RNA) vaccines developed by Pfizer/BioNTech (BNT162b2 or Comirnaty®) and Moderna (mRNA-1273 or SpikeVax®) have contributed significantly to the fight of the COVID-19 pandemic through the use of LNP-based delivery systems5.
For intracellular delivery purposes, nanomaterials including the lipid nanoparticles, (naturally derived and fully synthetic) polymeric nanoparticles, and inorganic nanoparticles (e.g., gold nanoparticles, carbon- and silica-based nanoparticles, and iron oxide nanoparticles) have been intensively studied6, 7, 8, 9, 10, 11. Each delivery system has its own advantages and disadvantages. Generally speaking, the lipid-based delivery systems usually have simple formulation procedures and compositions, good biocompatibility, and high bioavailability. So far, the lipid-based drug formulations are the most common FDA-approved nano-drugs. Despite the great success that LNP delivery technology has achieved in both basic research and clinical translations, there have been sustained efforts focused on developing more efficient and specific LNP delivery systems to apply to new therapeutics to different organs and tissues12, 13, 14, 15. Considering that each type of cargo molecule (small molecule drugs and biologics) has its distinct physicochemical properties (e.g., hydrophobicity and hydrophilicity of small molecules; molecular weight, geometry, surface charge, and hydrodynamic size of biologics), the carrier LNP system needs to be carefully tailored for particular delivery applications16, 17, 18, 19. Currently, there is no widely accepted principle or guideline for the optimal design of LNP carrier materials, due to the lack of understanding surrounding the relationship between the chemical/supramolecular structure and function of LNP-based drug and gene delivery systems. Efforts have been focused on the structure–activity relationship of LNPs, but the progress has been slow. Hence, the trial-and-error screening method as an alternative strategy has been extensively adapted for the development of LNP delivery systems3,20,21.
The screening approach is reliant on a large group of structurally diverse lipid materials. The combinatorial library strategy was first developed in 2002 to construct the lipid materials in a time- and material-efficient manner22. The combinatorial library strategy allows researchers to easily synthesize more than hundreds of structurally diverse lipid molecules with a minimal reactants and simple synthetic reactions. For this purpose, the solid-phase synthesis, Michael addition, epoxide-amine ring-opening reaction, ‘click’ chemistry, and other approaches have been successfully employed by our group and others for the synthesis of combinatorial libraries of lipid materials23. These lipids have been utilized for the delivery of a variety of bioactive agents, such as anticancer drugs, antibiotics, enzymes, antibodies, mRNA, pDNA (plasmid DNA), siRNA (small/short interfering RNA), etc.24, 25, 26.
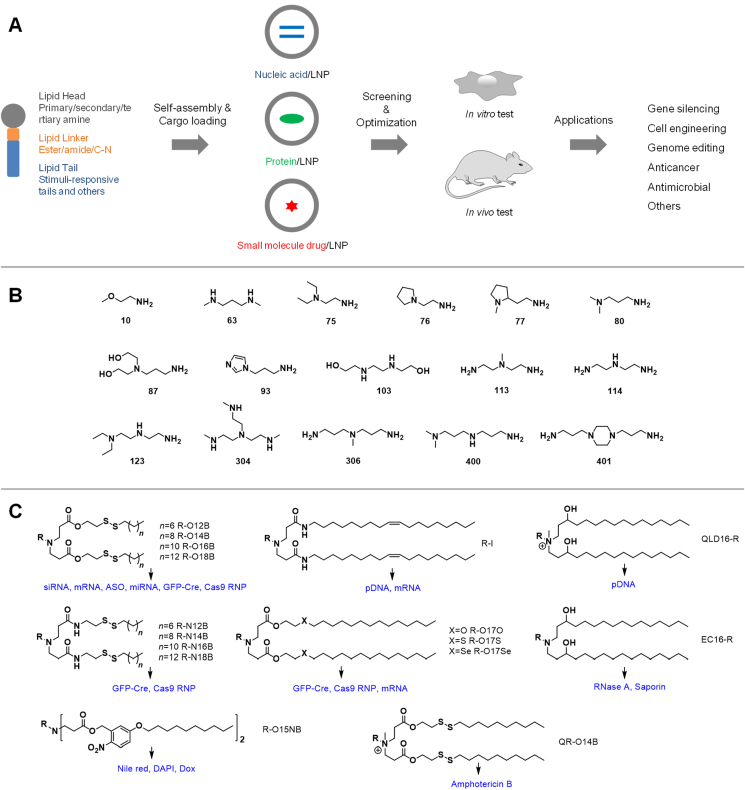
In this review, we highlight our advances in the development of new combinatorial lipids for drug delivery (Fig. 1). We adapted the Michael addition reaction to conjugate commercially-available amine-containing hydrophilic head groups with novel hydrophobic tail groups to synthesize new lipid molecules. We primarily employed the Michael addition because it offers certain advantages, such as solvent-free reaction condition, high conversion yield, easy purification of products, etc. The structures of lipid head (e.g., amine number, species of amines, number and hydrophobicity of the substitution groups), linker (e.g., ester, amide, and C‒N bond), and tail (e.g., tail number and length, heteroatoms, degradable bonds, etc.) groups can be altered to fine-tune the physicochemical properties of the lipid molecules. We then discuss our results regarding lipid nanoparticle fabrication, characterization, and their applications in delivering nucleic acids (siRNA, mRNA, ASO (antisense oligonucleotide), miRNA (microRNA), DNA), proteins, ribonucleoprotein (RNP) complexes, and small molecule drugs (anticancer drug, Dox and antifungal drug, Amphotericin B). These new delivery systems show great promise for use in cancer therapy, antimicrobial application, cell engineering, gene silencing, and genome editing. Lastly, we discuss the key challenges and opportunities in the mechanistic study and clinical translation of combinatorial LNP-enabled therapeutics.
Figure 1.
Schematic illustration of the development of LNP-based delivery systems for nucleic acids, proteins, and small molecule drugs. (A) Combinatorial lipid molecules containing hydrophilic head, linker, and hydrophobic tails were designed and synthesized at first. LNPs were fabricated through self-assembly procedures and cargo molecules (e.g., nucleic acids, proteins, and drugs) were encapsulated. The delivery systems are then tested in vitro and in vivo small animal models and the formulation are optimized through molecular and supramolecular engineering approaches. Our LNP drug delivery systems found wide applications in gene silencing, cell engineering, genome editing, and many others. (B) Representative chemical structures of amine-containing lipid head groups. (C) Representative structures of novel lipid molecules developed in our group for intracellular delivery of nucleic acids, proteins, and small molecule drugs.
2. LNPs for the delivery of nucleic acids
Gene therapy offers tremendous potential for the prevention and treatment of a variety of human diseases and disorders. However, the lack of safe and efficient delivery systems has been the most significant obstacle in the successful translation of these new DNA- and RNA-based therapeutics27,28. LNPs are the most advanced non-viral delivery carriers for gene delivery, as evidenced by the success of Onpattro® and the COVID-19 mRNA vaccines29,30. In this section, we discuss our efforts towards developing novel LNPs with new and enhanced properties for DNA and RNA delivery.
2.1. Bioreducible LNPs for siRNA delivery for gene silencing
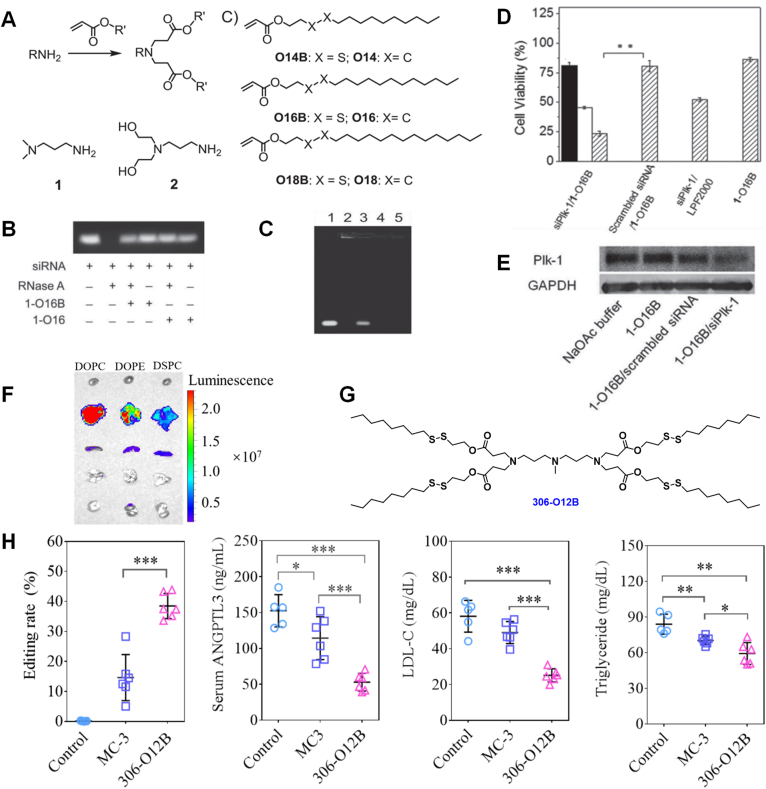
Strong interactions (e.g., through supramolecular interaction or covalent conjugation) are usually required for the delivery systems to efficiently complex with, protect, and deliver siRNA molecules into cytosol for inducing RNA interference. However, a strong binding between the cargo and carrier materials may hinder the successful release of the cargo. We exploited the possibility of using intracellular trigger-sensitive LNPs for siRNA delivery and release by incorporating a stimuli-responsive group into combinatorial lipid structures. In 2014, Wang et al.31 reported on a group of disulfide bond-containing LNPs for the intracellular delivery of siRNA for gene knockdown. This group of new lipid molecules, termed R-OnB (e.g., R-O16B; R stands for amine head number, O presents the acrylate linker bond, 16 is the tail length, and B means bioreducible), were synthesized through a one-step Michael addition reaction (Fig. 2A). LNPs were fabricated though lipid self-assembly in acidic sodium acetate buffer, and siRNA were loaded by taking advantage of the electrostatic interactions between positively charged cationic LNPs and negatively charged siRNA molecules. We demonstrated that the 1-O16B (also known as 80-O16B) LNPs can efficiently protect cargo siRNA from RNase degradation in vitro (Fig. 2B), and effectively release siRNA in the presence of GSH (glutathione; Fig. 2C). The free thiol-containing GSH was used to mimic the reducing intracellular environment that disrupts the disulfide bond in the 1-O16B lipid tails. The GSH-triggered LNP dissociation was also proved by TEM examination. We then observed that the R-OnB LNPs can efficiently mediate the endo/lysosome escape of siRNA and induce targeted gene knockdown by >70%. We further optimized the formulation (by adjusting N/P ratios) and delivery conditions (by varying exposure concentrations), and applied these improvements to deliver siRNA targeting polo-like kinase 1 (Plk1), which is a protein kinase that plays a crucial role in cell proliferation and cancer progression. We found that the 1-O16B LNP/siRNA system could efficiently knockdown the Plk1 expression in cell culture and significantly inhibit the growth of various cancer cell lines (HeLa, MDA-MB-231, and 4T1) in vitro (Fig. 2D and E). This study demonstrated that this group of disulfide-containing LNPs was promising carrier materials for the intracellular delivery of siRNA for potential cancer therapies. This study was significant because we firstly develop stimuli-responsive combinatorial lipids and demonstrated their advantages in in vitro siRNA delivery.
Figure 2.
Disulfide bond-containing bioreducible LNPs for the delivery of siRNA and mRNA. (A) Chemical structures of amine head groups (1 and 2) and tail groups (bioreducible O14B, O16B, and O18B; non-reducible O14, O16, and O18), and the synthetic route employed for the synthesis of lipids (e.g., R-O14B). (B) Gel electrophoresis analysis showed 1-O16B and 1-O16 LNPs protected cargo siRNA from RNase A degradation. (C) GSH-triggered siRNA release from bioreducible 1-O16B LNPs. Lane 1, siRNA; 2, siRNA/1-O16B; 3, siRNA/1-O16B treated with 5 mmol/L GSH; 4, siRNA/1-O16; 5, siRNA/1-O16 treated with 5 mmol/L GSH. (D) Cell viability assay of MDA-MB-231 cells treated with siPlk-1/LNPs (1-O16B, 1-O16, and Lipofectamine 2000). Black bar, 16 nmol/L; blank bar, 32 nmol/L; shadow bar, 50 nmol/L. Data is presented as mean ± SD, n = 3, ∗∗P < 0.05. (E) Plk-1 protein expression in MDA-MB-231 cells after transfection with siPlk-1 or scrambled siRNA complexed with 1-O16B LNPs. Reproduced with permission from Ref. 31. Copyright © 2014 John Wiley & Sons Inc. (F) Biodistribution profiles of fLuc mRNA/306-O12B LNPs formulated with cholesterol, DMG-PEG, and different phospholipid (DSPC, DOPE, or DOPC), determined by the In vivo Imaging System. (G) Chemical structure of 306-O12B lipid. (H) Next generation sequencing analysis of the indels in liver and serum analyses of ANGPTL3 protein, triglyceride, and LDL-C level of mice on Day 7 post administrated with Cas9 mRNA and sgAngptl3 loaded 306-O12B LNPs. The total RNA dose is 3.0 mg/kg body weight. MC-3 was used as a positive control. n = 5 or 6, ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001. Reproduced with permission from Ref. 36. Copyright © 2021 National Academy of Sciences USA.
In our following studies, we tested the disulfide-containing 8B-3 LNPs (also known as 306-O18B-3; amine head 306 conjugated with three O18B tails) for in vivo siRNA delivery. These LNPs were applied to an NOD scid gamma (NSG) JJN3 intraperitoneal mouse model. 8B-3 LNPs encapsulated with siRNA targeting the IGKC gene significantly reduced circulating Kappa light chains in the NSG JJN3 mice after three daily IP injections32,33.
Overall, our findings indicate that these newly developed bioreducible LNPs are safe and efficient delivery carriers for siRNA molecules. Targeted gene knockdown can be achieved both in vitro and in vivo. Further investigation is underway in order to optimize new applications for disulfide-containing LNPs in siRNA-enabled cancer therapies and for the treatment of hematologic disorders34,35.
2.2. Biodegradable LNPs for mRNA delivery for genome editing and cell engineering
In addition to siRNA delivery, the disulfide-containing LNPs were also employed for mRNA delivery. In 2019, we reported on using R-O16B and R-N16B (bioreducible lipids with an amide bond linker) LNPs for the encapsulation and delivery of CRISPR/Cas9 mRNA and gRNA37. A small group of bioreducible LNPs was screened in vitro for mRNA delivery, and BAMEA-O16B (also known as 113-O16B) was identified to be able to deliver mRNA safely and efficiently in cultured mammalian cells. Using a GFP reporter system, it was found that the BAMEA-O16B LNPs complexed with Cas9 mRNA and sgRNA induced 90% gene knockout in HEK cells. Furthermore, the Cas9 mRNA and sgRNA targeting the PCSK9 (proprotein convertase subtilisin/kexin type 9) gene were loaded into BAMEA-O16B LNPs and administered to C57BL/6 mouse through intravenous injection. The bioreducible LNPs successfully mediated PCSK9 gene knockout in the hepatocytes as evidenced by ca. 80% serum PCSK9 protein down-regulation. More importantly, the biochemical tests (changes in serum levels of aspartate transaminase (AST), alanine aminotransferase (ALT), and total bilirubin) and histological examination (haematoxylin and eosin (H&E) staining) revealed that the LNP-based CRISPR/Cas9 genome editing system did not significantly induce damage to the liver tissue. As PCSK9 is a validated therapeutic target for lipid metabolism modulation, our approach of using non-viral, biodegradable LNPs for the systemic delivery of CRISPR/Cas9 mRNA for PCSK9 knockout offers a safe and efficient approach for lowering the serum levels of lipids and potentially reducing the overall risk of cardiovascular diseases.
In 2021, we expanded the library of disulfide-containing lipids by conjugating a series of multiple amine-containing heads with bioreducible tails of different tail lengths (e.g., O10B, O12B, O14B and O16B)36. 306-O12B LNP was able to efficiently deliver mRNA in vivo after systemic administration (intravenous injection) using a bioluminescence-based screening approach (Fig. 2F and G). We then optimized the LNP formulations by altering the chemistry of helper lipids, the molar ratios of active and helper lipids, and the lipid/mRNA ratios. As a result, a formulation of 306-O12B/cholesterol/DSPC/DMG-PEG = 50/38.5/10/1.5 (molar ratio) and lipid/mRNA = 7.5/1 was identified to be the best formulation for delivering mRNA into the mouse liver after systemic injection. Cas9 mRNA and sgRNA targeting the Angptl3 gene were encapsulated by the optimized LNPs and injected to C57BL/6 mice. Angptl3 gene knockout in the liver was demonstrated by the T7E1 assay and next-generation sequencing (NGS; editing rate was ca. 38.5%). Furthermore, serum analyses revealed that the serum ANGPTL3 protein, LDL-C, and triglyceride levels had 65.2%, 56.8%, and 29.4% reductions after the treatment (Fig. 2H). More importantly, it was found that our 306-O12B LNP formulation performed better than the gold standard, MC3 LNP, through a head-to-head comparison study. This study was significant because it firstly showed that LNP-mediated CRISRP/Cas9 mRNA delivery can induce sufficient and long-term hepatic Angptl3 knockout in mouse model without evident side-effects. We expect that our bioreducible LNPs will advance the systemic delivery of CRISPR/Cas9 genome editing therapeutics for the treatment of hyperlipidemia and other types of liver-associated diseases and disorders38,39.
The bioreducible LNPs can also be used for ex vivo applications. In a following study, we encapsulated the Cas9 mRNA and sgRNA targeting the neuron restrictive silencing factor (NRSF) into 400-O16B LNPs, among other LNPs40. The bioreducible LNPs successfully delivered the bioactive molecules to cultured human mesenchymal stem cells (hMSCs) and induced Nrsf gene knockout. The neural-like differentiation of hMSCs was indicated by the cellular expression of synaptophysin (SYP), brain-derived neurotrophic factors (BDNF), neuron-specific enolase (NSE), and neuron-specific growth-associated proteins (SCG10).
To further expand the library of biodegradable lipids, we further incorporated novel head groups (imidazole and its analogs)41 and tails (cholesteryl-based and cyclic benzylidene acetal-containing tails)42,43 into the lipid molecule design. Our studies found that these newly developed lipid materials are safe and efficient carriers for the intracellular delivery of mRNA, both in vitro and in vivo in mouse models. More importantly, several LNP platforms were identified to be able to deliver CRISPR/Cas9 mRNA and gRNA components to certain tissues (e.g., spleen, lung, skeletal muscle and brain) and induce genome editing events after systemic or local administrations. Further investigation is underway to find other applications of our bioreducible LNP delivery systems for cell engineering and regenerative medicine.
2.3. Bioreducible LNPs for ASO delivery to inhibit gene translation
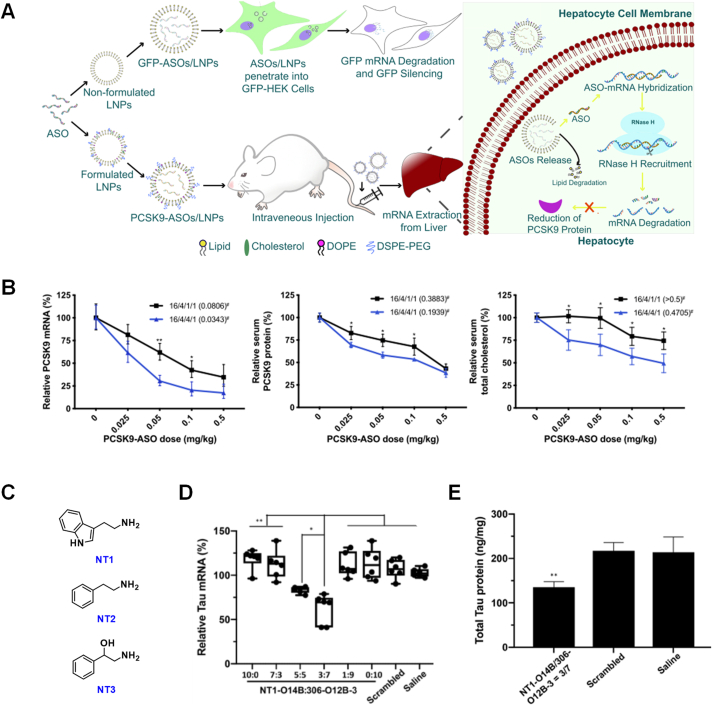
ASOs are single-stranded chemically modified nucleic acids (typically 18–25 bases in length) that are used to bind with target RNA sequences for RNA degradation and manipulation44. Our bioreducible LNPs have been successfully employed for the delivery of ASOs in vivo in mice to inhibit certain gene translations. Our bioreducible LNPs have been shown to successfully deliver the CRISPR/Cas9 system to mouse livers to induce PCSK9 gene knockout for managing hyperlipidemia37. In another study, we demonstrated that the disulfide-containing LNPs (e.g., 306-O12B-3) were able to deliver ASO molecules targeting the PCSK9 mRNA and induce mRNA and protein knockdown through systemic administration (Fig. 3A)45. The inhibition of PCSK9 mRNA translation in the liver also resulted in a significant reduction of total serum cholesterol levels in treated mice (Fig. 3B). In addition, the bioreducible LNPs showed excellent biocompatibility both in vitro in cell culture and in vivo in mouse model. In comparison with the genome editing-mediated gene knockout, the ASO-enabled gene knockdown provides an alternative transient approach for managing serum lipid levels.
Figure 3.
Bioreducible LNPs and NT LNPs for the delivery of ASOs for targeted gene silencing. (A) Schematic illustration of bioreducible LNP-mediated ASO delivery for the silencing of Gfp (in vitro in cell culture) and Pcsk9 (in vivo in mouse) genes. (B) 306-O12B-3 LNP-mediated PCSK9 ASO delivery induced successful reductions in PCSK9 mRNA and protein levels in the liver tissue, and serum cholesterol levels. Two ASO/LNP formulations (306-O12B-3/cholesterol/DOPE/DSPE-PEG2000 at the ratio of 16/4/1/1 or 16/4/4/1, weight ratios) and four ASO doses (0.025, 0.05, 0.1, and 0.5 mg/kg body weight) were examined. n = 3, ∗P < 0.05, ∗∗P < 0.01. Reproduced with permission from Ref. 45 under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC). Copyright © 2020 American Society of Gene & Cell Therapy. (C) Chemical structures of neurotransmitters (NT1, NT2, and NT3) used in our study for the synthesis of NT lipids. (D and E) NT LNP-mediated Tau ASO delivery induced successful (D) tau mRNA and (E) total tau protein reductions in the brain tissue in vivo in C57BL/6 mice after systemic administration. The mice received either saline or ASO in lipid formulations via tail vein injection at 1 mg ASO/kg body weight on days 0, 4, 8, 12, and 16. On Day 20, mice were sacrificed, and brain tissue was collected and analyzed. Data is represented as box and whisker plots with individual points overlaid, error bars represent maximum and minimum values and the boxed line represents the median. n = 6, ∗P < 0.05, ∗∗P < 0.01. Reproduced with permission from Ref. 46 under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC). Copyright © 2020 American Association for the Advancement of Science.
In 2020, we reported on the development of a library of neurotransmitter-derived lipids (also known as NT lipids) in order to deliver ASOs and other types of therapeutic agents to the brain and explored their applications to penetrate the blood‒brain barrier (BBB) to deliver various cargo molecules into the mouse brain tissue after systemic administrations (Fig. 3C)46. We used a mixed formulation of bioreducible lipids 306-O12B-3 and NT1-O14B to encapsulate and deliver ASOs targeting the Tau mRNA. The tau-ASO mediated tau protein reduction showed great promise in the treatment of Alzheimer's disease, seizures, etc., after local administrations47,48. The mice that received five doses of ASO/LNP (1 mg/kg of ASO) showed up to 50% tau mRNA reduction (Fig. 3D) and significant tau protein knockdown in the brain tissue (Fig. 3E). In this study, we showed that by simply engineering the molecular structure of lipids and incorporating novel chemical moieties can induce hugh impact on the biological property of lipid nanoparticles. Overcoming the BBB through a non-invasive pathway and efficiently delivering therapeutics to the brain tissue has been a great challenge, and we provided a simple and effective strategy to overcome this challenge. Our study opens new possibilities of using BBB-penetrating LNPs to delivery ASOs and potentially other nucleic acids to the brain tissue for the treatment of various neurological disorders49, 50, 51.
2.4. Bioreducible LNPs for miRNA delivery for cell differentiation and tissue regeneration
miRNA functions in cell differentiation, which determines the fate of stem cells. Our bioreducible LNPs have demonstrated to be effective for miRNA delivery in the context of cell engineering and tissue regeneration. In one study, we used the disulfide-containing bioreducible LNPs to deliver miRNA to hMSCs to induce neuronal differentiation52. A small group of LNPs were fabricated and complexed with fluorescent dye-labeled miRNA. After incubating the miRNA/LNP with cultured hMSCs, two LNPs (87-O16B and 306-O16B-3) were identified to be efficient for miRNA delivery. By examining the 306-headed LNPs, it was found that shortening or elongating the alkyl linear lipid tail reduced miRNA delivery efficacy. miRNA-9, which can promote neuronal differentiation in stem cells, was then delivered to hMSCs. Both the change in cell morphology (elongation and dendrite formation) and neuronal marker gene upregulation (microtubule-associated protein 2 (MAP2) and neuron-specific enolase (NSE)) were successfully induced. These findings show that our bioreducible LNPs can be utilized for the neuronal differentiation of hMSCs through either CRISPR/Cas9 mRNA delivery40 or miRNA delivery52. Future studies will focus on investigating the long-term effects induced by these two different strategies and explore their applications in nerve tissue regeneration.
Previous research illustrated that the miRNA-335-5p can promote osteogenic differentiation. We applied these findings to use bioreducible LNPs (e.g., L8, also known as 80-O16B) for the delivery of miRNA-335-5p53,54. In one study, we showed that delivering miRNA-335-5p using bioreducible LNPs successfully induced osteogenic gene expression (i.e., BSP, Osx, Runx2, and Satb2) in vitro in C3H10T1/2 cells and bone marrow stromal cells (BMSCs). Significant DKK1 down-regulation was also observed in the treated cells, which was consistent with previous findings55. Furthermore, the in vivo studies using mouse models with critical-sized defects on the calvarial bone showed that our miRNA delivery systems can significantly promote bone healing either after direct local administration of LNP systems or local application of LNP-transfected BMSCs. We expect that with further formulation optimization, our bioreducible LNP-mediated miRNA delivery systems can be used to induce osteogenic differentiation and calvarial bone regeneration56.
2.5. Unsaturated LNPs and quaternized LNPs for DNA delivery
Since most of the previously reported combinatorial lipid molecules have saturated alkyl tails, we explored the synthesis and application of combinatorial lipids with unsaturated tails for the delivery of nucleic acids57. Previous studies indicated that lipids with lower saturation levels might correlate with higher gene transfection efficacy, as unsaturated lipid tails increase the fluidity of lipid membranes, which can potentially increase the efficacies of cell internalization and endo/lysosome escape58. In one study, we firstly introduced the unsaturated tail structure to the combinatorial lipid design in order to further improve the delivery performance. Two groups of lipid molecules with an unsaturated oleyl acrylamide tail and a saturated n-octadecyl acrylamide tail were synthesized. In vitro delivery of DNA and mRNA encoding GFP revealed that the unsaturated LNPs outperformed the saturated LNPs, as none of the saturated LNPs induced GFP expression, while unsaturated LNPs induced up to 80% delivery. Through this structure‒activity study, it was found that the saturated LNPs formed large, highly compact structures while encapsulating nucleic acid molecules, while the unsaturated LNPs formed loosely packed nanocomplexes that could readily release the cargo molecules upon the supramolecular-driven electrostatic competition bindings. With further formulation optimization by varying the lipid/gene ratio, we demonstrated that our optimized unsaturated LNPs are versatile transfection reagents that can efficiently deliver both DNA and mRNA into cancerous (e.g., HeLa, HepG2, MCF-7, and MDA-MB-231) and non-cancerous (NIH-3T3 and BJ) cell lines. Our study shows the critical role that the hydrophobic tail structure plays in determining the physicochemical properties of combinatorial LNPs and their intracellular gene delivery performance. This study examined the intracellular DNA and mRNA delivery performance of unsaturated combinatorial lipids in vitro in cell culture. It is unclear at this stage if this group of new lipids can induce efficient gene delivery in vivo in small animal models, which merits further investigation.
In another study, we constructed a small library of lipids containing ester, amide, and C–N linker bonds59. In vitro screening in HeLa cells using the plasmid DNA encoding β-gal revealed that 14N-87 (also known as 87-N14) had the highest intracellular DNA delivery efficacy. We noticed that contrary to the 14N-87, the ester linker-containing 14O-87 (also known as 87-O14) LNPs were unable to deliver DNA after being stored for longer than 2 h. The FT-IR analysis indicated that the ester bonds in the 14O-87 molecules were degraded or hydrolyzed during storage. These results suggest that the stability of lipid linker group needs to be carefully examined when developing LNP carriers for DNA and other types of nucleic acids. The chemical structure change of carrier lipid molecules can probably result in changes in the supramolecular structure of lipids nanoparticles and DNA/LNP nanocomplexes, which could affect the interactions between the delivery system and the biological system. The ideal LNP drug formulations should stay stable during storage and transportation, and should be able to transfect target cells safely and efficiently. Considering that the C–N linker has a better stability profile than the ester linker, we tried to employ the lipid with C–N linkers for DNA delivery and achieved satisfactory results60. However, it should be noted that, combinatorial lipids with the ester linkers might show better biodegradability and biocompatibility comparing with the lipids with C–N linkers. A head-to-head comparison of the metabolism and degradation profiles of these lipids will provide more information. In another study, inspired by previously reported lipid structures with permanent positive charge that were successful for DNA delivery61, we developed a group of quaternized lipids through a two-step conjugation reaction. The quaternized lipids have permanent positive charges in the head group and the chargeability does not rely on the pH values, which may contribute to forming more robust and stable gene delivery systems. An in vitro screening approach helped us identify several LNP formulations that had better plasmid DNA delivery efficacies than Lipofectamine 2000. We found that the DNA encapsulation capability and delivery efficacy of the quaternized LNPs were reliant on the perfect balance of the lipid tail length, active-to-helper lipids ratio, and the lipids-to-DNA ratio. Further studies are needed to illustrate the delivery efficacy and potential toxicity of the quaternized lipids in vivo in mall animal models. Overall, we demonstrated that tailoring the head, linker, and tail structures of combinatorial lipid molecules improved various physicochemical properties (e.g., hydrodynamic size, tightness, and storage stability) and biological effects (e.g., transfection efficacy and cytotoxicity). Future directions include further optimizing these LNP formulations, incorporating DNA cargos with therapeutic effects, and exploring their applications in the treatment of cancer, infectious diseases, cardiovascular diseases, neurological disorders, etc.62.
2.6. LNPs for the delivery of mRNA vaccines and LNPs for extrahepatic delivery
Generally speaking, the lipid nanoparticle-based drug and gene delivery systems have been the most common FDA-approved nanomedicine. Onpattro® and the two COVID-19 mRNA vaccines developed by Moderna and Pfizer/BioNTech are the most recent approved lipid nano-drugs. Even though the FDA-approved LNP-based gene therapeutics does not have a long history, DNA and RNA-based vaccines and other therapeutics have been studied by researchers for decades. Over the years, LNPs showed superior properties as carrier systems for the therapeutic RNA molecules. Several LNPs based on DLin-DMA, DLin-KC2-DMA, and DLin-MC3-DMA have been especially reported to be highly potent for RNA delivery63, 64, 65, 66. The DLin-MC3-DMA is the active lipid used in Onpattro® for siRNA delivery to hepatic cells. So far, several LNP systems have been developed that can deliver mRNA and other types of nucleic acids into hepatocytes with high efficacy and specificity. Efforts are being made to develop new LNP systems that can target extrahepatic sites, such as the lung, spleen, brain, and others67, 68, 69, 70. Specifically, Siegwart et al.14,71,72 found that by incorporating lipid excipients into LNPs, the biodistribution profiles can be hugely affected and liver, lung, and spleen can be targeted with relative high specificity. With further development, these new systems can unleash the great potential of LNPs for treatment of human diseases and conditions that affect liver and other major organs. As to the LNP-based mRNA vaccines, SM-102 and ALC-0315 have been adopted by Moderna and Pfizer/BioNTech for fabricating mRNA-1273 and BNT162b25. Besides the COVID-19 vaccines, other types of mRNA/LNP vaccines are under intensive investigation for fighting against the HIV, Zika, influenza, and many others63,65,73, 74, 75, 76.
3. LNPs for the delivery of proteins
Protein and peptide-based therapeutics have been heavily researched in the last few decades. In comparison with traditional small molecule drugs, protein therapeutics can be more specific with better biocompatibility profiles. More importantly, proteins are capable of interfering with certain biological pathways and interacting with proteins and other biomolecules that are ‘undruggable’ by small molecule drugs77. Protein therapeutics have been intensively explored as new modalities for the treatment of cancer, diabetes, infectious diseases, respiratory diseases, etc. However, many of the currently used protein therapeutics (e.g., enzymes, antibodies, and hormones) target secreted or extracellular domains78,79. Developing the delivery of proteins to target intracellular domains can open up protein-based therapeutics to more applications. In our studies, combinatorial LNPs were successfully employed to load and deliver a variety of cell-impermeable proteins for genome engineering, cancer therapy, and more.
3.1. Bioreducible LNPs and chalcogen-containing LNPs for the delivery of genome editing proteins
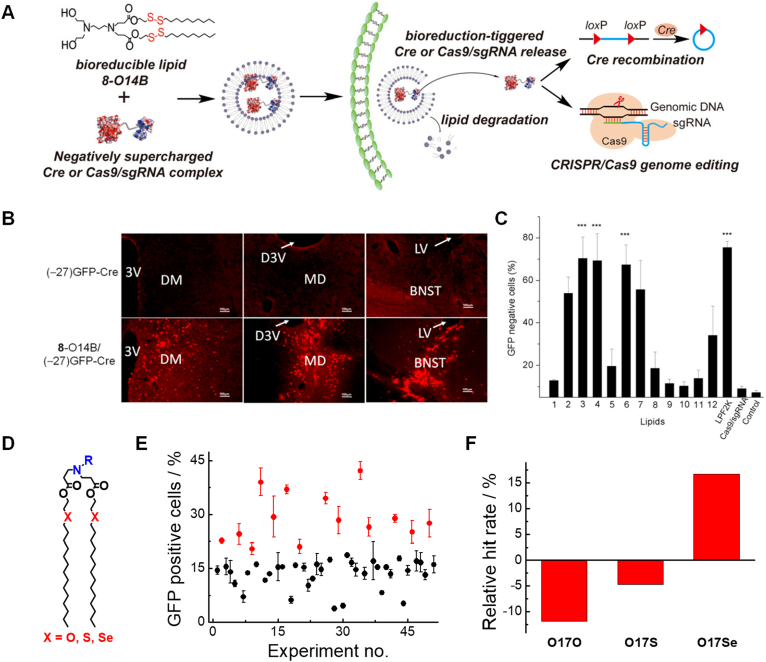
Recently developed genome editing technologies, such as the CRISPR/Cas9 (clustered regularly interspaced short palindromic repeat associated protein 9) platform, have enabled specific gene knockout, knock-in, correction, and epigenome modification in a highly convenient and precise manner80. However, in order to exert their functions in target mammalian cells, genome editing components need to be delivered into the cell nucleus. For this purpose, both the physical methods and carrier-mediated delivery systems have been developed for genome-editing protein delivery81, 82, 83, 84, 85, 86. In 2016, our group first explored the application of combinatorial bioreducible LNPs for the delivery of genome editing proteins in vitro and in vivo in mouse model (Fig. 4A)87. A small library of disulfide-containing LNPs was synthesized, and an in vitro screening was conducted using negatively super-charged Cre recombinase (GFP-Cre) as the cargo and engineered DsRed-HeLa cell line as the reporter. The bioreducible LNPs were used in this study as we expected them to be capable of complexing with negatively-charged proteins (Cre recombinase and Cas9 RNP complex) through electrostatic interactions and releasing the cargo into cytoplasm triggered by GSH-induced lipid cleavage and nanoparticle degradation. The 8-O14B (also known as 87-O14B) LNP showed the highest delivery efficacy and gene recombination efficacy. In addition, many of our bioreducible LNPs induced higher efficacies than Lipofectamine 2000. We then demonstrated that the 8-O14B LNPs efficiently mediated endosome/lysosome escape, and evenly distributed the cargo proteins in the cell nucleus. Notably, it was found that the gene recombination efficacy was reliant on the concentration of delivered proteins, the tail length of 87-haeded bioreducible lipids, and the charge density of engineered Cre recombinase. To further test if this system can mediate protein delivery in vivo and enable genome engineering, the GFP-Cre protein loaded 8-O14B LNPs were injected into the brain of the Rosa26tdTomato mouse. TdTomato red fluorescent protein expressions were identified in multiple brain regions (e.g., dorsomedial hypothalamic nucleus (DM), mediodorsal thalamic nucleus (MD), and bed nucleus of the stria terminalis (BNST)) six days after administration (Fig. 4B). This is one of the earliest studies to successfully apply LNP delivery systems to in vivo genome engineering. Furthermore, we demonstrated that the bioreducible LNPs could also deliver the Cas9/gRNA ribonucleoprotein (RNP) complex into mammalian cells (Fig. 4C)87. Using a GFP-HEK reporter cell line, it was found that multiple bioreducible LNPs (e.g., 3-O14B) mediated ∼70% GFP knockout, but 8-O14B was inefficient in delivering Cas9 RNP system. This indicates that the structure of the cargo molecule (e.g., molecular weight, hydrodynamic size, geometry, and surface charge) can significantly affect the physicochemical properties of LNP-based delivery systems, and the LNPs need to be carefully tailored to the different types of cargo molecules and applications.
Figure 4.
Bioreducible LNPs and chalcogen-containing LNPs for the delivery of genome engineering proteins. (A) Schematic illustration of the disulfide-containing 8-O14B LNP-mediated GFP-Cre and CRISPR/Cas9 RNP delivery. (B) 8-O14B LNP-enabled Cre-mediated gene recombination in the brain tissue of Rosa26tdTomato mouse model. (C) Bioreducible LNPs can deliver Cas9/sgRNA RNP targeting the GFP gene and induce gene knockout in cultured GFP-HEK cell line. Reproduced with permission from Ref. 87. Copyright © 2016 National Academy of Sciences USA. (D) Chemical structures of chalcogen-containing lipids, R-O17O, R-O17S, and R-O17Se. (E) Chalcogen LNP-mediated GFP-Cre delivery to DsRed-HeLa cell line. Percentage of GFP positive cells shown for 51 LNPs tested. Data points marked in red for LNPs induced high level of transfection (>20%). (F) The tail structure (O17O, O17S and O17Se)-influenced GFP-Cre protein transfection activity. The relative hit rates for officious LNPs of lipids with O17O, O17S and O17Se tails are −11.9%, −4.7% and 16.7%, respectively, relative to the initial chalcogen-containing library. Reproduced with permission from Ref. 88. Copyright © 2018 Elsevier.
After we demonstrated that the bioreducible LNPs can deliver Cas9 RNP through local administration, we further tested if these LNPs were capable of mediating RNP delivery through systemic administration route. Comparing with the local delivery, systemic delivery presents more physical and biochemical challenges to the delivery system. The biodistribution of bioreducible LNPs loaded with Cas9 RNP after intravenous injection was studied in Balb/c mouse89. In vivo and ex vivo imaging showed that Cas9/sgRNA-encapsulated disulfide- and amide-containing 103-N16B LNPs accumulated primarily in the liver, 30 min after tail vein injection. Our ongoing research focuses on formulation optimization to further enhance the delivery efficacy and minimize the liver toxicity of the RNP-LNP system. We expect by designing and incorporating gRNAs that target functional genes in the liver tissue, these bioreducible LNPs can be used for creating new therapeutics for liver-associated diseases or disorders through liver-specific genome editing90.
In addition to modifying the disulfide-containing bioreducible LNPs (e.g., tuning the alkyl chain length of OnB tails and incorporating non-linear cholesteryl moiety into the tail structure), we tried to introduce new building blocks into combinatorial lipid synthesis to investigate if new and better delivery performance could be achieved. In one study, we integrated ether, thiol ether, and selenide ether moieties into lipid hydrophobic tails (termed as R-O17O, R-O17S, and R-O17Se) and investigated how the hetero atom affects the intracellular delivery efficacy of genome engineering proteins (Fig. 4D)88. Using the GFP-Cre recombinase and DsRed-HeLa cell line, we found that the combinatorial lipids with O17Se tails outperformed the O17O and O17S lipids (Fig. 4E and F). These results revealed that even the one-atom difference in lipid tail structure can have huge impact on the intracellular delivery performance of lipid nanoparticles. Further investigation revealed that the lipids with high apparent pKa (i.e., pKa >5.1 in TNS (2-(p-toluidinynaphthalene-6-sulphonic acid)) assay) and high phospholipid membrane disruption capabilities (indicated as OD405 > 0.2 in hemolysis assay) are more likely to induce success intracellular protein delivery, and many of the O17Se LNPs possess these two characteristics. However, the underlying mechanism for this phenomenon is unclear at this stage, which merits further investigation. After the in vitro studies, three LNPs (e.g., 76-O17O, 76-O17S and 76-O17Se) were injected to Ai14 mice through the tail vein. Successful gene recombination and reporter fluorescent protein expression were observed in the lungs of mice injected with O17S and O17Se tailed LNPs. We also demonstrated that several chalcogen-containing LNPs can deliver Cas9/sgRNA RNP efficiently into cultured mammalian cell lines without inducing significant cytotoxicity. Furthermore, the new combinatorial LNPs developed by our group have demonstrated effective delivery of genome editing proteins in engineered tissue models and in small animal models, and their applications in oral protein delivery, cancer therapy, and treatment of hereditary hearing disorders were expanded upon91, 92, 93.
3.2. LNPs for intracellular delivery of cytotoxic proteins and more
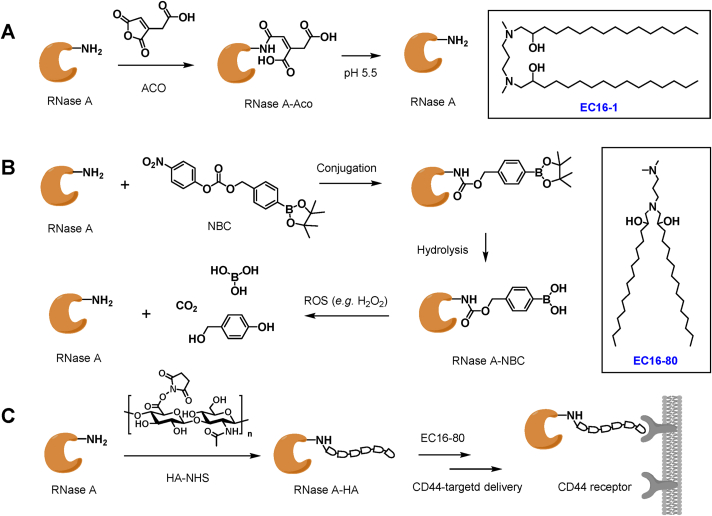
Besides genome engineering, we also reported on using combinatorial LNPs for intracellular protein delivery used in cancer therapies94, 95, 96, 97. Most of the combinatorial lipids were developed for gene delivery, and only a few had been successfully demonstrated to be effective for intracellular delivery of therapeutic proteins. In one study, we fabricated a group of C–N linker-containing combinatorial lipids by conjugating amine heads with epoxide-containing tail groups through a one-step ring-opening reaction94. The combinatorial lipids with C–N linkers can be readily synthesized by using commercially-available amine heads and epoxide tails. Comparing with the aforementioned disulfide-containing bioreducible lipids, lipids with C–N linkers do not have the stimuli-responsive feature. However, the C–N linker might provide better stability than the ester linker in the R-OnB bioreducible lipids. The nomenclature of the lipid molecules with C–N linker was EC16-1 (also known as 63-EC16). The EC16-tailed LNPs were employed for the delivery of two cytotoxic proteins, RNase A and saporin. Both RNase A and saporin can induce severe toxicity once internalized into the cells, which are being explored as new generation protein-based anticancer drugs. However, naked RNase A and saporin cannot bypass the phospholipid bilayer cell membrane efficiently. In this study, we intended to use combinatorial LNPs as the carriers for the intracellular delivery of cytotoxic proteins. In our initial tests, it was discovered that these C–N linked LNPs were unable to deliver the proteins that induce cell inhibition, and we speculated that it may be due to the weak association of the protein/LNP complex. We then modified the RNase A and saporin with a pH-responsive small molecule ligand, ACO, by conjugating the free lysine residue on proteins with cis-aconitic anhydride (Fig. 5A). The ACO modification caged primary amine groups into the protein and introduced extra negative charges so that the protein can bind with the cationic EC16-tailed LNPs more strongly through electrostatic interactions. More importantly, the ACO ligand can be cleaved under low pH (e.g., in late endosome), so the activity of the modified proteins can be, therefore, restored. We demonstrated that the EC-16 LNPs efficiently delivered Aco-modified proteins into various cancerous cell lines in vitro and induced significant cytotoxicity. Subsequently, in vivo protein delivery was tested by using saporin loaded EC16-1 LNPs and 4T1 tumor-bearing mouse models. The LNPs delivered saporin protein into tumor tissues after systemic administration and greatly inhibited tumor growth, which was most likely due to the enhanced permeability and retention (EPR) effects. In comparison, no tumor inhibition was observed from mice treated with free saporin. Similarly, we demonstrated that this approach could be used for constructing the reactive oxygen species (ROS)-responsive phenylboronic ester (NBC)-modified protein (Fig. 5B) and CD44-targeting hyaluronic acid (HA)-modified protein for cancer cell inhibition (Fig. 5C)95,98. Overall, our strategy of integrating combinatorial cationic LNP delivery systems with chemically modified reversible protein derivatives provides a safe and highly effective approach for overcoming the innate drawbacks of protein therapeutics and constructing novel protein-based drug formulations for potential cancer therapy. However, further investigation is needed to illustrate the distribution and metabolism of carrier LNPs and cargo proteins after administrations, the short- and long-term toxicity induced by the delivery system, and the anticancer efficacy under various treatment conditions.
Figure 5.
Schematic illustrations of chemical modification methods for protein delivery. (A) Preparation of NBC-modified protein and ROS-triggered cleavage of NBC moiety. (B) Synthesis of ACO-modified protein and low pH-triggered ACO detachment. (C) Conjugation of HA to protein for CD44-targeted protein delivery. EC16-80 and EC16-1 are the two representative lipids employed for protein delivery.
Intracellular protein delivery mediated by combinatorial LNPs has great potential for developing new therapeutic modalities. This can be seen by the LNP-mediated non-surgical method that we developed for fertility reduction in rat models99. In this study, saporin loaded LNPs were modified with anti-Mullerian hormone receptor type II antibodies to target the gonadal cells. After intravenous administration, the targeted protein/LNP system negatively impacted reproduction in rats, including sperm production, estrous cyclicity and testicular and ovarian morphology, without causing any significant side-effects. In comparison with the traditional surgical approach, our LNP-enabled non-surgical approach can be developed into a safe and convenient alternative strategy for controlling the overproduction of pet and wildlife. This combinatorial LNP-based intracellular protein delivery system has also been used in other applications, such as the pre-fused proteolysis-targeting chimaera (PROTAC)-directed protein degradation and cancer immunotherapy100,101.
4. LNPs for the delivery of small molecule drugs
One of the greatest challenges in administering traditional small molecule chemotherapeutic drugs is overcoming the offsite interactions formed between the target cell and the drug administration site. Non-specific interactions between the administered drug and biomolecules can potentially induce unwanted toxicity or side-effects. Nanoparticle-based delivery systems have been extensively adapted to address this challenge102,103. Enormous evidence showed that nano-drug delivery systems can help solubilize hydrophobic drugs, protect cargo drug molecules from unfavorable hydrolysis and degradation, prolong half-life time in blood circulation, target specific organs and tissues, and control drug release rate, among other purposes. For these reasons, LNPs, polymers, and inorganic nanomaterials have been employed as nanocarriers for the delivery of small molecule drugs23,104, 105, 106. These delivery systems have demonstrated to be advantageous in comparison with free drugs, and have been used for the treatment of cancer, infectious diseases, inflammation diseases, neurological disorders, cardiovascular diseases, and many others107, 108, 109, 110. Our studies have shown that our combinatorial LNPs could be useful in delivering small molecule anticancer drugs in vitro and antimicrobials in vivo in mouse model.
4.1. Photo-responsive LNPs for anticancer drug delivery
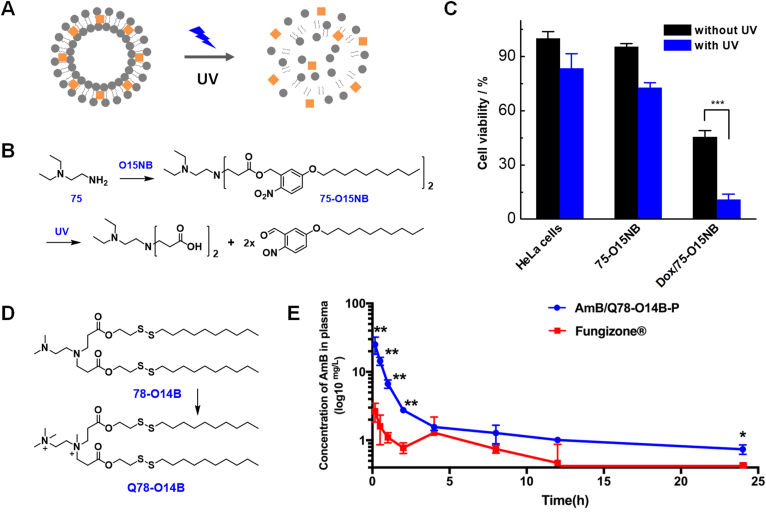
Since our previously developed bioreducible LNPs can respond to the intracellular reducing environment, which is an internal trigger, we further explored if the combinatorial LNPs can be sensitive to external triggers. External trigger-responsive LNP systems can potentially be used to realize the spatiotemporal-controlled drug delivery and release, which can greatly facilitate the development of precision nanomedicine. For developing external or physical stimuli responsive drug delivery systems, a wide range of chemical groups that are sensitive to light irradiation, electric field, high temperature, etc. have been incorporated into the carrier material design113, 114, 115. In this study, by integrating the o-nitrobenzyl ester bond into the hydrophobic tail, we synthesized a combinatorial library of photo-responsive lipids, termed as R-O15NB (Fig. 6A and B)111. Using DLS and TEM, we observed that upon 30 min of 365 nm UV light irradiation, the hydrodynamic sizes of all R-O15NB LNPs increased, and small spherical structures transitioned to large, irregular aggregates. The change in molecular structure during photo irradiation was monitored by UV–Vis absorption spectrum. All of the studied R-O15NB LNPs showed gradual decrease at 325 nm (characteristic absorption peak of intact o-nitrobenzyl derivative ester linkage) and increase at 420 nm (characteristic absorption peak of byproduct o-nitrobenzaldehyde derivatives) in absorption values. The formation of o-nitrobenzaldehyde byproducts was also evidenced by color change (from colorless at the beginning to yellowish after irradiation) of the LNP aqueous solutions. The photo-triggered cargo release behavior of R-O15NB LNPs was studied by using the polarity-sensitive fluorescent reporter Nile red-encapsulated LNPs. Around 80% of encapsulated Nile red molecules was released after ca. 12 min of photo irradiation. Subsequently, we demonstrated that the R-O15NB can deliver small molecule anticancer drug Dox into cultured HeLa cells, and photo irradiation enhanced the intracellular cargo release (Fig. 6C). As a proof-of-concept, we applied physical stimuli-responsive combinatorial lipid materials for controlled drug release. However, the applications of UV light-responsive drug delivery systems are largely restricted by the photo-toxicity and tissue penetration limitation of the UV light. Future studies will focus on developing visible and near infrared light-degradable LNPs to explore their applications in spatiotemporal controlled anticancer drug delivery116, 117, 118, 119, 120. In order to achieve this, new moieties that can be cleaved/degraded in the presence of long wavelength light irradiation need to be incorporated into the combinatorial lipid molecule design, and their properties including the drug encapsulation, delivery, and triggered degradation and release will be investigated.
Figure 6.
Photo-responsive LNPs for anticancer drug delivery and quaternized bioreducible LNPs for antifungal drug delivery. (A) Schematic illustration of photo-responsive LNPs for cargo encapsulation and photo irradiation-triggered release. (B) Synthetic route employed for the synthesis of 75-O15NB lipid and UV light induced lipid degradation reaction. (C) Cell viability analysis of Dox/75-O15NB treated and UV light irradiated HeLa cells. Two-tailed students' t-test, ∗∗∗P < 0.001. Reproduced with permission from Ref. 111. Copyright © 2019 American Chemistry Society. (D) Chemical structures of bioreducible 78-O14B lipid and quaternized Q78-O14B lipid. (E) Plasma concentrations of AmB after intravenous injection of AmB/Q78-O14B-P and Fungizone® at a dose of 2 mg AmB/kg body weight into SD rats. n = 3, ∗P < 0.05 and ∗∗P < 0.001. Reproduced with permission from Ref. 112. Copyright © 2020 American Chemistry Society.
4.2. Quaternized bioreducible LNPs for antifungal drug delivery
Many antimicrobial agents suffer from low solubility and unfavorable pharmacodynamics. For example, the antifungal drug Amphotericin B (AmB) has high activity and antifungal spectrum, but its application is largely limited by its poor solubility and in vivo toxicity. In 2020, we reported on the development of bioreducible LNP-based AmB formulations and compared new formulations with the FDA-approved Fungizone®112. We first tried to use the disulfide-containing bioreducible R-O14B LNPs (e.g., 78-O14B) to encapsulate AmB. The bioreducible LNPs were used here to reduce or diminish the potential toxicity induced by the carrier materials because of their biodegradability. The hydrophobic drug molecules, AmB were expected to be encapsulated into the hydrophobic domains of R-O14B LNPs. However, the AmB-LNPs turned out to be unstable, as aggregation and precipitation occurred after one week of storage. To increase the stability of AmB/LNP systems, DSPE-PEG2000 was added as a helper lipid and the amine head group was quaternized through reacting with methyl iodine (e.g., Q78-O14B; Fig. 6D). Both the PEG-lipid and lipid quaternization strategy were employed as we intended to increase the hydrophilicity of the AmB nano-drug formulations and decrease the particle aggregation through steric hindrance and surface charge repulsion. These optimized formulations showed excellent storage stabilities as no significant change in hydrodynamic sizes was recorded for two weeks after preparation. In vitro antifungal activity tests against the Candida albicans revealed that our quaternized bioreducible LNP-based AmB formulations had better antifungal activities comparing with free AmB and Fungizone®. Moreover, the new AmB/LNP formulations showed significantly lower hemolytic activity and cytotoxicity in the in vitro mammalian cell culture study. In addition, we demonstrated that the quaternized bioreducible AmB/LNP formulation had longer blood circulation time (Fig. 6E), higher drug retention in certain organs, and reduced nephrotoxicity and hepatotoxicity in vivo, in comparison with Fungizone®. Through this study, we provide a safe and effective AmB drug formulation with relative low fabrication costs. There is ongoing research examining the AmB/LNP system in vivo in small animal models with fungal infections.
5. Challenges and future studies
In addition to other studies, the studies published by our laboratory have demonstrated that the combinatorial library of LNPs can be used for the delivery of nucleic acids, proteins, and small molecule drugs, for a variety of applications in gene silencing, genome editing, cell engineering and tissue regeneration, cancer therapy, antimicrobial applications, and many others38,85,121, 122, 123. Nonetheless, challenges still exist in the successful clinical translation of these new drug formulations.
First, the structure–activity relationship of combinatorial LNP-based drug delivery systems needs to be further illustrated. In the studies of our laboratory and others, many effective LNPs were identified through in vitro and in vivo screening methods. Currently, there is no widely accepted design principle for LNP drug delivery systems. Even though we and others have shown that by altering the lipid chemistry and LNP formulation composition, certain properties (e.g., increase cell and tissue specificity, augmented intracellular transfection efficacy, triggered degradability, improved biocompatibility and reduced toxicity, increased membrane fluidity, and enhanced storage stability) could be introduced to the LNP system, a thorough lipid structure‒activity study is needed to fully understand how the LNP systems interact with biological systems both in cell culture and in vivo31,36,37,42,112. It is critical to understand how the combinatorial lipids with different head, linker, and tail groups and LNPs with different size, morphology, surface chemistry, and charge properties result in differences in cell transfection efficacy, cytotoxicity, and in vivo biodistribution profiles124, 125, 126, 127, 128. A mechanistic study will eventually enable researchers to fine-tune the physicochemical and biological properties of LNPs to achieve new and better drug delivery results. This will eventually help unleash the potential of LNPs for enabling a wide range of new therapies.
Second, the storage stabilities of LNP-based drug formulations need to be carefully examined. Successful translations to clinical settings require the LNP-based drug formulations to be stable during storage and transportation129,130. We showed that by increasing the hydrophilicity of bioreducible lipid head groups, the stability of LNP formulations loaded with the hydrophobic drug AmB was greatly improved112. However, the long-term stability, as well as the stability under different storage conditions (e.g., temperature, pH, ion strength and lyophilization) of quaternized AmB/LNPs needs further investigation. Moreover, the stability of LNP-mediated nucleic acid and protein delivery systems should also be investigated. For this purpose, the characterization of the changes in chemical structure of carrier lipid materials and cargo molecules as well as the supramolecular structure of cargo-loaded LNP systems will help us understand the physical and/or chemical alterations that occur during storage. This knowledge will eventually contribute to fabricating new LNP drug formulations with improved stability that can be applicable in clinical settings. It will greatly benefit the development of mRNA vaccines and other gene therapies as stable LNP formulations will help decrease the economic and logistic burdens of our society when public health crises are dealt with.
Third, a better understanding of the in vivo toxicity and biodegradation of combinatorial LNPs needs to be acquired. In our studies, we showed that the systemic administration of certain types of bioreducible LNPs (e.g., R-O16B and R-OCholB) did not induce significant hepatotoxicity or nephrotoxicity under the tested conditions (through standard biochemical analysis)36,38,42. Our future studies will employ both biochemical tests and histological analyses to examine damages/changes induced by combinatorial LNPs in multiple organs and tissues after systemic and local administrations. Furthermore, it is currently unknown whether our synthetic combinatorial LNPs can trigger an innate or adaptive immune response. Potential interactions between the immune system and drug delivery systems may positively or negatively impact the therapeutic effects depending on the biomedical applications of the LNP systems101,131, 132, 133. The immunogenicity of combinatorial LNPs has been overlooked in previous studies and deserves thorough investigation. In addition, the in vivo metabolism and biodegradation of combinatorial LNPs is not yet fully understood. Even though we have shown that some of the stimuli-responsive LNPs could be degraded in the presence of certain biochemical or physical triggers (e.g., GSH, low pH, and photo irradiation) in vitro, their in vivo degradation and metabolism profiles need to be further illustrated31,42,43,111.
Lastly, much work is needed in regards to the validation and optimization of combinatorial LNP-based drug delivery systems in small and large animal models. Our previous work primarily focused on the development of lipid materials and the examination in vitro in cultured cell lines and in vivo in small animal models23,38,85. So far, we have achieved successful long-term liver genome editing36,37, transient gene silencing33,45,46, tumor suppression94,101, and tissue regeneration53,54 in mouse models. To facilitate the clinical translation of these promising systems, the top performing LNP systems identified from our previous studies will be further tested in large animal models and non-human primates. These studies will provide critical information about the safety and efficacy of our new LNP drug delivery systems, which will greatly facilitate the formulation optimization and their clinical evaluation processes.
Overall, the synthetic combinatorial LNP system provides a potent and versatile platform for the delivery of a wide variety of therapeutic agents to enable new and better therapies. The successful clinical translation of LNP-based drug formulations is hugely reliant on our understanding of how drug-complexed LNP systems interact with the biological systems at the molecular, cellular, tissue, and whole organism levels. Combinatorial LNP-based drug delivery systems hold great promise in developing new drugs against cancer, metabolic diseases, neurological diseases, cardiovascular diseases, and many others, but it requires the close collaboration of material scientists, biologists, clinicians, and policy makers to fully realize their potential to improve human health.
Acknowledgments
This study was supported by the National Institutes of Health (NIH) Grants R01 EB027170-04 and UG3 TR002636-01, USA.
Author contributions
Yamin Li and Qiaobing Xu conceived the idea. Yamin Li performed the major literature search and wrote the first draft. All the authors substantially contributed to their individual area of expertise, discussed, reviewed, and edited the manuscript before submission. All authors approved the final manuscript.
Conflicts of interest
The authors declare no conflicts of interest.
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences
References
- 1.van der Meel R., Chen S., Zaifman J., Kulkarni J.A., Zhang X.R.S., Tam Y.K., et al. Modular lipid nanoparticle platform technology for siRNA and lipophilic prodrug delivery. Small. 2021;17 doi: 10.1002/smll.202103025. [DOI] [PubMed] [Google Scholar]
- 2.Swingle K.L., Hamilton A.G., Mitchell M.J. Lipid nanoparticle-mediated delivery of mRNA therapeutics and vaccines. Trends Mol Med. 2021;27:616–617. doi: 10.1016/j.molmed.2021.03.003. [DOI] [PubMed] [Google Scholar]
- 3.Battaglia L., Gallarate M. Lipid nanoparticles: state of the art, new preparation methods and challenges in drug delivery. Expert Opin Drug Deliv. 2012;9:497–508. doi: 10.1517/17425247.2012.673278. [DOI] [PubMed] [Google Scholar]
- 4.Zhang Y., Sun C., Wang C., Jankovic K.E., Dong Y. Lipids and lipid derivatives for RNA delivery. Chem Rev. 2021;121:12181–12277. doi: 10.1021/acs.chemrev.1c00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schoenmaker L., Witzigmann D., Kulkarni J.A., Verbeke R., Kersten G., Jiskoot W., et al. mRNA-lipid nanoparticle COVID-19 vaccines: structure and stability. Int J Pharm. 2021;601:120586. doi: 10.1016/j.ijpharm.2021.120586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shen W., Wang R., Fan Q., Gao X., Wang H., Shen Y., et al. Natural polyphenol inspired polycatechols for efficient siRNA delivery. CCS Chemistry. 2020;2:146–157. [Google Scholar]
- 7.Xu W., Luo F.Q., Tong Q.S., Li J.X., Miao W.M., Zhang Y., et al. An intracellular pH-actuated polymer for robust cytosolic protein delivery. CCS Chemistry. 2021;3:431–442. [Google Scholar]
- 8.Xu Y., Mo J., Wei W., Zhao J. Oxidized black phosphorus nanosheets as an inorganic antiresorptive agent. CCS Chem. 2021;3:1105–1115. [Google Scholar]
- 9.Fattal E., Fay F. Nanomedicine-based delivery strategies for nucleic acid gene inhibitors in inflammatory diseases. Adv Drug Deliv Rev. 2021;175:113809. doi: 10.1016/j.addr.2021.05.019. [DOI] [PubMed] [Google Scholar]
- 10.Luo L., Qi Y., Zhong H., Jiang S., Zhang H., Cai H., et al. GSH-sensitive polymeric prodrug: synthesis and loading with photosensitizers as nanoscale chemo-photodynamic anti-cancer nanomedicine. Acta Pharm Sin B. 2022;12:424–436. doi: 10.1016/j.apsb.2021.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luo X.M., Yan C., Feng Y.M. Nanomedicine for the treatment of diabetes-associated cardiovascular diseases and fibrosis. Adv Drug Deliv Rev. 2021;172:234–248. doi: 10.1016/j.addr.2021.01.004. [DOI] [PubMed] [Google Scholar]
- 12.Ding F., Zhang H., Cui J., Li Q., Yang C. Boosting ionizable lipid nanoparticle-mediated in vivo mRNA delivery through optimization of lipid amine-head groups. Biomater Sci. 2021;9:7534–7546. doi: 10.1039/d1bm00866h. [DOI] [PubMed] [Google Scholar]
- 13.Guo S., Li K., Hu B., Li C., Zhang M., Hussain A., et al. Membrane-destabilizing ionizable lipid empowered imaging-guided siRNA delivery and cancer treatment. Explorations. 2021;1:35–49. doi: 10.1002/EXP.20210008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu S., Cheng Q., Wei T., Yu X., Johnson L.T., Farbiak L., et al. Membrane-destabilizing ionizable phospholipids for organ-selective mRNA delivery and CRISPR-Cas gene editing. Nat Mater. 2021;20:701–710. doi: 10.1038/s41563-020-00886-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patel P., Ibrahim N.M., Cheng K. The importance of apparent pKa in the development of nanoparticles encapsulating siRNA and mRNA. Trends Pharmacol Sci. 2021;42:448–460. doi: 10.1016/j.tips.2021.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pattipeiluhu R., Arias-Alpizar G., Basha G., Chan K.Y.T., Bussmann J., Sharp T.H., et al. Anionic lipid nanoparticles preferentially deliver mRNA to the hepatic reticuloendothelial system. Adv Mater. 2022;34 doi: 10.1002/adma.202201095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Basso J., Mendes M., Cova T., Sousa J., Pais A., Fortuna A., et al. A stepwise framework for the systematic development of lipid nanoparticles. Biomolecules. 2022;12:223. doi: 10.3390/biom12020223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou J.E., Sun L., Liu L., Jia Y., Han Y., Shao J., et al. Hepatic macrophage targeted siRNA lipid nanoparticles treat non-alcoholic steatohepatitis. J Control Release. 2022;343:175–186. doi: 10.1016/j.jconrel.2022.01.038. [DOI] [PubMed] [Google Scholar]
- 19.Han J.P., Kim M., Choi B.S., Lee J.H., Lee G.S., Jeong M., et al. In vivo delivery of CRISPR-Cas9 using lipid nanoparticles enables antithrombin gene editing for sustainable hemophilia A and B therapy. Sci Adv. 2022;8 doi: 10.1126/sciadv.abj6901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guimaraes P.P.G., Zhang R., Spektor R., Tan M., Chung A., Billingsley M.M., et al. Ionizable lipid nanoparticles encapsulating barcoded mRNA for accelerated in vivo delivery screening. J Contr Release. 2019;316:404–417. doi: 10.1016/j.jconrel.2019.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramishetti S., Hazan-Halevy I., Palakuri R., Chatterjee S., Naidu Gonna S., Dammes N., et al. A combinatorial library of lipid nanoparticles for RNA delivery to leukocytes. Adv Mater. 2020;32 doi: 10.1002/adma.201906128. [DOI] [PubMed] [Google Scholar]
- 22.Lenssen K., Jantscheff P., von Kiedrowski G., Massing U. Combinatorial synthesis of new cationic lipids and high-throughput screening of their transfection properties. ChemBioChem. 2002;3:852–858. doi: 10.1002/1439-7633(20020902)3:9<852::AID-CBIC852>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 23.Altinoglu S., Wang M., Xu Q.B. Combinatorial library strategies for synthesis of cationic lipid-like nanoparticles and their potential medical applications. Nanomedicine. 2015;10:643–657. doi: 10.2217/nnm.14.192. [DOI] [PubMed] [Google Scholar]
- 24.Farokhzad O.C., Langer R. Nanomedicine: developing smarter therapeutic and diagnostic modalities. Adv Drug Deliv Rev. 2006;58:1456–1459. doi: 10.1016/j.addr.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 25.Yu H.J., De Geest B.G. Nanomedicine and cancer immunotherapy. Acta Pharmacol Sin. 2020;41:879–880. doi: 10.1038/s41401-020-0426-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yaghmur A., Mu H. Recent advances in drug delivery applications of cubosomes, hexosomes, and solid lipid nanoparticles. Acta Pharm Sin B. 2021;11:871–885. doi: 10.1016/j.apsb.2021.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buck J., Grossen P., Cullis P.R., Huwyler J., Witzigmann D. Lipid-based DNA therapeutics: hallmarks of non-viral gene delivery. ACS Nano. 2019;13:3754–3782. doi: 10.1021/acsnano.8b07858. [DOI] [PubMed] [Google Scholar]
- 28.Kaczmarek J.C., Kowalski P.S., Anderson D.G. Advances in the delivery of RNA therapeutics: from concept to clinical reality. Genome Med. 2017;9:60. doi: 10.1186/s13073-017-0450-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Akinc A., Maier M.A., Manoharan M., Fitzgerald K., Jayaraman M., Barros S., et al. The Onpattro story and the clinical translation of nanomedicines containing nucleic acid-based drugs. Nat Biotechnol. 2019;14:1084–1087. doi: 10.1038/s41565-019-0591-y. [DOI] [PubMed] [Google Scholar]
- 30.Wang Z.J., Schmidt F., Weisblum Y., Muecksch F., Barnes C.O., Finkin S., et al. mRNA vaccine-elicited antibodies to SARS-CoV-2 and circulating variants. Nature. 2021;592:616–622. doi: 10.1038/s41586-021-03324-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang M., Alberti K., Varone A., Pouli D., Georgakoudi I., Xu Q.B. Enhanced intracellular siRNA delivery using bioreducible lipid-like nanoparticles. Adv Healthc Mater. 2014;3:1398–1403. doi: 10.1002/adhm.201400039. [DOI] [PubMed] [Google Scholar]
- 32.Ma X., Zhou P., Kugelmass A., Toskic D., Warner M., Lee L.X., et al. Effective lipidoid nanoparticle delivery in vivo of siRNA targeting kappa light chain production in a murine xenograft model. Blood. 2018;132:3208. [Google Scholar]
- 33.Ma X., Zhou P., Kugelmass A., Toskic D., Warner M., Lee L., et al. A novel xenograft mouse model for testing approaches targeting human kappa light-chain diseases. Gene Ther. 2019;26:187–197. doi: 10.1038/s41434-019-0070-y. [DOI] [PubMed] [Google Scholar]
- 34.Chalbatani G.M., Dana H., Gharagouzloo E., Grijalvo S., Eritja R., Logsdon C.D., et al. Small interfering RNAs (siRNAs) in cancer therapy: a nano-based approach. Int J Nanomed. 2019;14:3111–3128. doi: 10.2147/IJN.S200253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu B., Zhong L.P., Weng Y.H., Peng L., Huang Y.Y., Zhao Y.X., et al. Therapeutic siRNA: state of the art. Signal Transduct Tar. 2020;5:101. doi: 10.1038/s41392-020-0207-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qiu M., Glass Z., Chen J.J., Haas M., Jin X., Zhao X.W., et al. Lipid nanoparticle-mediated codelivery of Cas9 mRNA and single-guide RNA achieves liver-specific in vivo genome editing of Angptl3. Proc Natl Acad Sci U S A. 2021;118 doi: 10.1073/pnas.2020401118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu J., Chang J., Jiang Y., Meng X.D., Sun T.M., Mao L.Q., et al. Fast and efficient CRISPR/Cas9 genome editing in vivo enabled by bioreducible lipid and messenger RNA nanoparticles. Adv Mater. 2019;31:1902575. doi: 10.1002/adma.201902575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qiu M., Li Y.M., Bloomer H., Xu Q.B. Developing biodegradable lipid nanoparticles for intracellular mRNA delivery and genome editing. Accounts Chem Res. 2021;54:4001–4011. doi: 10.1021/acs.accounts.1c00500. [DOI] [PubMed] [Google Scholar]
- 39.Doudna J.A. The promise and challenge of therapeutic genome editing. Nature. 2020;578:229–236. doi: 10.1038/s41586-020-1978-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao X.W., Glass Z., Chen J.J., Yang L., Kaplan D.L., Xu Q.B. mRNA delivery using bioreducible lipidoid nanoparticles facilitates neural differentiation of human mesenchymal stem cells. Adv Healthc Mater. 2021;10:2000938. doi: 10.1002/adhm.202000938. [DOI] [PubMed] [Google Scholar]
- 41.Zhao X.W., Chen J.J., Qiu M., Li Y.M., Glass Z., Xu Q.B. Imidazole-based synthetic lipidoids for in vivo mRNA delivery into primary T lymphocytes. Angew Chem Int Ed. 2020;59:20083–20089. doi: 10.1002/anie.202008082. [DOI] [PubMed] [Google Scholar]
- 42.Li Y.M., Jarvis R., Zhu K.X., Glass Z., Ogurlu R., Gao P.Y., et al. Protein and mRNA delivery enabled by cholesteryl-based biodegradable lipidoid nanoparticles. Angew Chem Int Ed. 2020;59:14957–14964. doi: 10.1002/anie.202004994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li Y.M., Li R., Chakraborty A., Ogurlu R., Zhao X.W., Chen J.J., et al. Combinatorial library of cyclic benzylidene acetal-containing pH-responsive lipidoid nanoparticles for intracellular mRNA delivery. Bioconjugate Chem. 2020;31:1835–1843. doi: 10.1021/acs.bioconjchem.0c00295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gagliardi M., Ashizawa A.T. The challenges and strategies of antisense oligonucleotide drug delivery. Biomedicines. 2021;9:433. doi: 10.3390/biomedicines9040433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang L., Ma F.H., Liu F., Chen J.J., Zhao X.W., Xu Q.B. Efficient delivery of antisense oligonucleotides using bioreducible lipid nanoparticles in vitro and in vivo. Mol Ther Nucleic Acids. 2020;19:1357–1367. doi: 10.1016/j.omtn.2020.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ma F.H., Yang L., Sun Z.R., Chen J.J., Rui X.H., Glass Z., et al. Neurotransmitter-derived lipidoids (NT-lipidoids) for enhanced brain delivery through intravenous injection. Sci Adv. 2020;6 doi: 10.1126/sciadv.abb4429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Devos S.L., Goncharoff D.K., Chen G., Kebodeaux C.S., Yamada K., Stewart F.R., et al. Antisense reduction of tau in adult mice protects against seizures. J Neurosci. 2013;33:12887–12897. doi: 10.1523/JNEUROSCI.2107-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.DeVos S.L., Miller R.L., Schoch K.M., Holmes B.B., Kebodeaux C.S., Wegener A.J., et al. Tau reduction prevents neuronal loss and reverses pathological tau deposition and seeding in mice with tauopathy. Sci Transl Med. 2017;9 doi: 10.1126/scitranslmed.aag0481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Feng J., Lepetre-Mouelhi S., Gautier A., Mura S., Cailleau C., Coudore F., et al. A new painkiller nanomedicine to bypass the blood–brain barrier and the use of morphine. Sci Adv. 2019;5 doi: 10.1126/sciadv.aau5148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou Y., Zhu F., Liu Y., Zheng M., Wang Y., Zhang D., et al. Blood–brain barrier-penetrating siRNA nanomedicine for Alzheimer’s disease therapy. Sci Adv. 2020 doi: 10.1126/sciadv.abc7031. 6:eabc7031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Santos T., Boto C., Saraiva C.M., Bernardino L., Ferreira L. Nanomedicine approaches to modulate neural stem cells in brain repair. Trends Biotechnol. 2016;34:437–439. doi: 10.1016/j.tibtech.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 52.Takeda Y.S., Wang M., Deng P., Xu Q.B. Synthetic bioreducible lipid-based nanoparticles for miRNA delivery to mesenchymal stem cells to induce neuronal differentiation. Bioeng Transl Med. 2016;1:160–167. doi: 10.1002/btm2.10021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sui L., Wang M., Han Q.Q., Yu L.M., Zhang L., Zheng L.L., et al. A novel lipidoid-microRNA formulation promotes calvarial bone regeneration. Biomaterials. 2018;177:88–97. doi: 10.1016/j.biomaterials.2018.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang Q., Wang X.Y., Valverde P., Murray D., Dard M.M., Van Dyke T., et al. Osteogenic effects of microRNA-335-5p/lipidoid nanoparticles coated on titanium surface. Arch Oral Biol. 2021;129:105207. doi: 10.1016/j.archoralbio.2021.105207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang J., Tu Q.S., Bonewald L.F., He X., Stein G., Lian J., et al. Effects of miR-335-5p in modulating osteogenic differentiation by specifically downregulating Wnt antagonist DKK1. J Bone Miner Res. 2011;26:1953–1963. doi: 10.1002/jbmr.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Leng Q.P., Chen L.N., Lv Y.G. RNA-based scaffolds for bone regeneration: application and mechanisms of mRNA, miRNA and siRNA. Theranostics. 2020;10:3190–3205. doi: 10.7150/thno.42640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang M., Sun S., Alberti K.A., Xu Q.B. A combinatorial library of unsaturated lipidoids for efficient intracellular gene delivery. ACS Synth Biol. 2012;1:403–407. doi: 10.1021/sb300023h. [DOI] [PubMed] [Google Scholar]
- 58.Zhi D.F., Zhang S.B., Wang B., Zhao Y.N., Yang B.L., Yu S.J. Transfection efficiency of cationic lipids with different hydrophobic domains in gene delivery. Bioconjugate Chem. 2010;21:563–577. doi: 10.1021/bc900393r. [DOI] [PubMed] [Google Scholar]
- 59.Sun S., Wang M., Knupp S.A., Soto-Feliciano Y., Hu X., Kaplan D.L., et al. Combinatorial library of lipidoids for in vitro DNA delivery. Bioconjugate Chem. 2012;23:135–140. doi: 10.1021/bc200572w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sun S., Wang M., Alberti K.A., Choy A., Xu Q.B. DOPE facilitates quaternized lipidoids (QLDs) for in vitro DNA delivery. Nanomedicine. 2013;9:849–854. doi: 10.1016/j.nano.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 61.Torchilin V.P. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov. 2005;4:145–160. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- 62.Patil S.D., Rhodes D.G., Burgess D.J. DNA-based therapeutics and DNA delivery systems: a comprehensive review. AAPS J. 2005;7:E61–E77. doi: 10.1208/aapsj070109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Buschmann M.D., Carrasco M.J., Alishetty S., Paige M., Alameh M.G., Weissman D. Nanomaterial delivery systems for mRNA vaccines. Vaccines. 2021;9:65. doi: 10.3390/vaccines9010065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jayaraman M., Ansell S.M., Mui B.L., Tam Y.K., Chen J., Du X., et al. Maximizing the potency of siRNA lipid nanoparticles for hepatic gene silencing in vivo. Angew Chem Int Ed. 2012;51:8529–8533. doi: 10.1002/anie.201203263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pilkington E.H., Suys E.J.A., Trevaskis N.L., Wheatley A.K., Zukancic D., Algarni A., et al. From influenza to COVID-19: lipid nanoparticle mRNA vaccines at the frontiers of infectious diseases. Acta Biomater. 2021;131:16–40. doi: 10.1016/j.actbio.2021.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Semple S.C., Akinc A., Chen J., Sandhu A.P., Mui B.L., Cho C.K., et al. Rational design of cationic lipids for siRNA delivery. Nat Biotechnol. 2010;28:172–176. doi: 10.1038/nbt.1602. [DOI] [PubMed] [Google Scholar]
- 67.Evers M.J.W., Du W., Yang Q., Kooijmans S.A.A., Vink A., van Steenbergen M., et al. Delivery of modified mRNA to damaged myocardium by systemic administration of lipid nanoparticles. J Contr Release. 2022;343:207–216. doi: 10.1016/j.jconrel.2022.01.027. [DOI] [PubMed] [Google Scholar]
- 68.Hagino Y., Khalil I.A., Kimura S., Kusumoto K., Harashima H. GALA-modified lipid nanoparticles for the targeted delivery of plasmid DNA to the lungs. Mol Pharm. 2021;18:878–888. doi: 10.1021/acs.molpharmaceut.0c00854. [DOI] [PubMed] [Google Scholar]
- 69.Shankar R., Joshi M., Pathak K. Lipid nanoparticles: a novel approach for brain targeting. Pharm Nanotechnol. 2018;6:81–93. doi: 10.2174/2211738506666180611100416. [DOI] [PubMed] [Google Scholar]
- 70.Zhang R., El-Mayta R., Murdoch T.J., Warzecha C.C., Billingsley M.M., Shepherd S.J., et al. Helper lipid structure influences protein adsorption and delivery of lipid nanoparticles to spleen and liver. Biomater Sci. 2021;9:1449–1463. doi: 10.1039/d0bm01609h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cheng Q., Wei T., Farbiak L., Johnson L.T., Dilliard S.A., Siegwart D.J. Selective organ targeting (SORT) nanoparticles for tissue-specific mRNA delivery and CRISPR-Cas gene editing. Nat Nanotechnol. 2020;15:313–320. doi: 10.1038/s41565-020-0669-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wei T., Cheng Q., Min Y.L., Olson E.N., Siegwart D.J. Systemic nanoparticle delivery of CRISPR-Cas9 ribonucleoproteins for effective tissue specific genome editing. Nat Commun. 2020;11:3232. doi: 10.1038/s41467-020-17029-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bahl K., Senn J.J., Yuzhakov O., Bulychev A., Brito L.A., Hassett K.J., et al. Preclinical and clinical demonstration of immunogenicity by mRNA vaccines against H10N8 and H7N9 influenza viruses. Mol Ther. 2017;25:1316–1327. doi: 10.1016/j.ymthe.2017.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li Y., Li C., Jiang T., Zhou L., Zhang P., Kwok R.T.K., et al. Aptamer-Anchored rubrene-loaded organic nanoprobes for cancer cell targeting and imaging. CCS Chem. 2019;1:251–260. [Google Scholar]
- 75.Yang X., Chang J., Jiang Y., Xu Q., Wang M., Mao L. In vivo activation of pro-protein therapeutics via chemically engineered enzyme cascade reaction. CCS Chem. 2021;3:780–790. [Google Scholar]
- 76.Zhao Z., Wang Y., Qiu L., Fu T., Yang Y., Peng R., et al. New insights from chemical biology: molecular basis of transmission, diagnosis, and therapy of SARS-CoV-2. CCS Chem. 2021;3:1501–1528. [Google Scholar]
- 77.Akbaba H., Erel-Akbaba G., Senturk S. Recruitment of solid lipid nanoparticles for the delivery of CRISPR/Cas9: primary evaluation of anticancer gene editing. Nanomedicine. 2021;16:963–978. doi: 10.2217/nnm-2020-0412. [DOI] [PubMed] [Google Scholar]
- 78.Usmani S.S., Bedi G., Samuel J.S., Singh S., Kalra S., Kumar P., et al. THPdb: database of FDA-approved peptide and protein therapeutics. PLoS One. 2017;12 doi: 10.1371/journal.pone.0181748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li Y.M., Li P.X., Li R., Xu Q.B. Intracellular antibody delivery mediated by lipids, polymers, and inorganic nanomaterials for therapeutic applications. Adv Ther. 2020;3:2000178. [Google Scholar]
- 80.Anzalone A.V., Koblan L.W., Liu D.R. Genome editing with CRISPR-Cas nucleases, base editors, transposases and prime editors. Nat Biotechnol. 2020;38:824–844. doi: 10.1038/s41587-020-0561-9. [DOI] [PubMed] [Google Scholar]
- 81.Glass Z., Li Y.M., Xu Q.B. Nanoparticles for CRISPR-Cas9 delivery. Nat Biomed Eng. 2017;1:854–855. doi: 10.1038/s41551-017-0158-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang M., Glass Z.A., Xu Q. Non-viral delivery of genome-editing nucleases for gene therapy. Gene Ther. 2017;24:144–150. doi: 10.1038/gt.2016.72. [DOI] [PubMed] [Google Scholar]
- 83.Glass Z., Lee M., Li Y.M., Xu Q.B. Engineering the delivery system for CRISPR-based genome editing. Trends Biotechnol. 2018;36:173–185. doi: 10.1016/j.tibtech.2017.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li Y.M., Glass Z., Xu Q.B. Rescued from the fate of neurological disorder. Nat Biomed Eng. 2018;2:469–470. doi: 10.1038/s41551-018-0267-1. [DOI] [PubMed] [Google Scholar]
- 85.Chang J., Chen X.H., Glass Z., Gao F., Mao L.Q., Wang M., et al. Integrating combinatorial lipid nanoparticle and chemically modified protein for intracellular delivery and genome editing. Accounts Chem Res. 2019;52:665–675. doi: 10.1021/acs.accounts.8b00493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li Y.M., Glass Z., Huang M.Q., Chen Z.Y., Xu Q.B. Ex vivo cell-based CRISPR/Cas9 genome editing for therapeutic applications. Biomaterials. 2020;234:119711. doi: 10.1016/j.biomaterials.2019.119711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang M., Zuris J.A., Meng F.T., Rees H., Sun S., Deng P., et al. Efficient delivery of genome-editing proteins using bioreducible lipid nanoparticles. Proc Natl Acad Sci U S A. 2016;113:2868–2873. doi: 10.1073/pnas.1520244113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li Y.M., Yang T., Yu Y.J., Shi N., Yang L., Glass Z., et al. Combinatorial library of chalcogen-containing lipidoids for intracellular delivery of genome-editing proteins. Biomaterials. 2018;178:652–662. doi: 10.1016/j.biomaterials.2018.03.011. [DOI] [PubMed] [Google Scholar]
- 89.Li Y.M., Bolinger J., Yu Y.J., Glass Z., Shi N., Yang L., et al. Intracellular delivery and biodistribution study of CRISPR/Cas9 ribonucleoprotein loaded bioreducible lipidoid nanoparticles. Biomater Sci. 2019;7:596–606. doi: 10.1039/c8bm00637g. [DOI] [PubMed] [Google Scholar]
- 90.Cox D.B.T., Platt R.J., Zhang F. Therapeutic genome editing: prospects and challenges. Nat Med. 2015;21:121–131. doi: 10.1038/nm.3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gao X., Tao Y., Lamas V., Huang M.Q., Yeh W.H., Pan B.F., et al. Treatment of autosomal dominant hearing loss by in vivo delivery of genome editing agents. Nature. 2018;553:217–221. doi: 10.1038/nature25164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ho T.C., Kim H.S., Chen Y.M., Li Y.M., LaMere M.W., Chen C., et al. Scaffold-mediated CRISPR-Cas9 delivery system for acute myeloid leukemia therapy. Sci Adv. 2021;7 doi: 10.1126/sciadv.abg3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yang T., Han H.B., Chen Y., Yang L., Parker R., Li Y.M., et al. Study the lipidoid nanoparticle mediated genome editing protein delivery using 3D intestinal tissue model. Bioact Mater. 2021;6:3671–3677. doi: 10.1016/j.bioactmat.2021.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang M., Alberti K., Sun S., Arellano C.L., Xu Q.B. Combinatorially designed lipid-like nanoparticles for intracellular delivery of cytotoxic protein for cancer therapy. Angew Chem Int Ed. 2014;53:2893–2898. doi: 10.1002/anie.201311245. [DOI] [PubMed] [Google Scholar]
- 95.Wang M., Sun S., Neufeld C.I., Perez-Ramirez B., Xu Q.B. Reactive oxygen species-responsive protein modification and its intracellular delivery for targeted cancer therapy. Angew Chem Int Ed. 2014;53:13444–13448. doi: 10.1002/anie.201407234. [DOI] [PubMed] [Google Scholar]
- 96.Altinoglu S.A., Wang M., Li K.Q., Li Y.Y., Xu Q.B. Intracellular delivery of the PTEN protein using cationic lipidoids for cancer therapy. Biomater Sci. 2016;4:1773–1780. doi: 10.1039/c6bm00580b. [DOI] [PubMed] [Google Scholar]
- 97.Zhang G.N., Gupta P., Wang M., Barbuti A.M., Ashby C.R., Zhang Y.K., et al. Lipid-saporin nanoparticles for the intracellular delivery of cytotoxic protein to overcome ABC transporter-mediated multidrug resistance in vitro and in vivo. Cancers. 2020;12:498. doi: 10.3390/cancers12020498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang X.Y., Li Y.M., Li Q.S., Neufeld C.I., Pouli D., Sun S., et al. Hyaluronic acid modification of RNase A and its intracellular delivery using lipid-like nanoparticles. J Control Release. 2017;263:39–45. doi: 10.1016/j.jconrel.2017.01.037. [DOI] [PubMed] [Google Scholar]
- 99.Meadows K.L., Li Y., Xu Q., Ayres S. A novel non-surgical method to reduce fertility using the rat as a model. Clin Theriogenol. 2019;11:31–41. [Google Scholar]
- 100.Chen J.J., Qiu M., Ma F.H., Yang L., Glass Z., Xu Q.B. Enhanced protein degradation by intracellular delivery of pre-fused PROTACs using lipid-like nanoparticles. J Control Release. 2021;330:1244–1249. doi: 10.1016/j.jconrel.2020.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chen J.J., Qiu M., Ye Z.F., Nyalile T., Li Y.M., Glass Z., et al. In situ cancer vaccination using lipidoid nanoparticles. Sci Adv. 2021;7 doi: 10.1126/sciadv.abf1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bamrungsap S., Zhao Z.L., Chen T., Wang L., Li C.M., Fu T., et al. Nanotechnology in therapeutics: a focus on nanoparticles as a drug delivery system. Nanomedicine. 2012;7:1253–1271. doi: 10.2217/nnm.12.87. [DOI] [PubMed] [Google Scholar]
- 103.Couvreur P. Nanoparticles in drug delivery: past, present and future. Adv Drug Deliv Rev. 2013;65:21–23. doi: 10.1016/j.addr.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 104.Paranjpe M., Muller-Goymann C.C. Nanoparticle-mediated pulmonary drug delivery: a review. Int J Mol Sci. 2014;15:5852–5873. doi: 10.3390/ijms15045852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Matoba T., Egashira K. Nanoparticle-mediated drug delivery system for cardiovascular disease. Int Heart J. 2014;55:281–286. doi: 10.1536/ihj.14-150. [DOI] [PubMed] [Google Scholar]
- 106.Riehemann K., Schneider S.W., Luger T.A., Godin B., Ferrari M., Fuchs H. Nanomedicine-challenge and perspectives. Angew Chem Int Ed. 2009;48:872–897. doi: 10.1002/anie.200802585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Amiri M., Jafari S., Kurd M., Mohamadpour H., Khayati M., Ghobadinezhad F., et al. Engineered solid lipid nanoparticles and nanostructured lipid carriers as new generations of blood–brain barrier transmitters. ACS Chem Neurosci. 2021;12:4475–4490. doi: 10.1021/acschemneuro.1c00540. [DOI] [PubMed] [Google Scholar]
- 108.Okur N.U., Siafaka P.I., Gokce E.H. Challenges in oral drug delivery and applications of lipid nanoparticles as potent oral drug carriers for managing cardiovascular risk factors. Curr Pharm Biotechnol. 2021;22:892–905. doi: 10.2174/1389201021666200804155535. [DOI] [PubMed] [Google Scholar]
- 109.Qin X., He L., Fan D., Liang W., Wang Q., Fang J. Targeting the resolution pathway of inflammation using Ac2-26 peptide-loaded PEGylated lipid nanoparticles for the remission of rheumatoid arthritis. Asian J Pharm Sci. 2021;16:483–493. doi: 10.1016/j.ajps.2021.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Singh M.S., Ramishetti S., Landesman-Milo D., Goldsmith M., Chatterjee S., Palakuri R., et al. Therapeutic gene silencing using targeted lipid nanoparticles in metastatic ovarian cancer. Small. 2021;17 doi: 10.1002/smll.202100287. [DOI] [PubMed] [Google Scholar]
- 111.Li Y.M., Chakraborty A., Chen J.J., Xu Q.B. Combinatorial library of light-cleavable lipidoid nanoparticles for intracellular drug delivery. ACS Biomater Sci Eng. 2019;5:2391–2398. doi: 10.1021/acsbiomaterials.9b00445. [DOI] [PubMed] [Google Scholar]
- 112.Liu F., Yang L., Li Y.M., Junier A., Ma F.H., Chen J.J., et al. In vitro and in vivo study of amphotericin B formulation with quaternized bioreducible lipidoids. ACS Biomater Sci Eng. 2020;6:1064–1073. doi: 10.1021/acsbiomaterials.9b01722. [DOI] [PubMed] [Google Scholar]
- 113.Oliva N., Almquist B.D. Spatiotemporal delivery of bioactive molecules for wound healing using stimuli-responsive biomaterials. Adv Drug Deliv Rev. 2020;161–162:22–41. doi: 10.1016/j.addr.2020.07.021. [DOI] [PubMed] [Google Scholar]
- 114.Steele T.W.J., Klok H.A. Stimuli-sensitive and -responsive polymer biomaterials. Biomacromolecules. 2018;19:1375–1377. doi: 10.1021/acs.biomac.8b00145. [DOI] [PubMed] [Google Scholar]
- 115.Wang Y., Byrne J.D., Napier M.E., DeSimone J.M. Engineering nanomedicines using stimuli-responsive biomaterials. Adv Drug Deliv Rev. 2012;64:1021–1030. doi: 10.1016/j.addr.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Katz J.S., Burdick J.A. Light-responsive biomaterials: development and applications. Macromol Biosci. 2010;10:339–348. doi: 10.1002/mabi.200900297. [DOI] [PubMed] [Google Scholar]
- 117.Li Y.M., Qian Y.F., Liu T., Zhang G.Y., Liu S.Y. Light-triggered concomitant enhancement of magnetic resonance imaging contrast performance and drug release rate of functionalized amphiphilic diblock copolymer micelles. Biomacromolecules. 2012;13:3877–3886. doi: 10.1021/bm301425j. [DOI] [PubMed] [Google Scholar]
- 118.Mura S., Nicolas J., Couvreur P. Stimuli-responsive nanocarriers for drug delivery. Nat Mater. 2013;12:991–1003. doi: 10.1038/nmat3776. [DOI] [PubMed] [Google Scholar]
- 119.Theato P., Sumerlin B.S., O'Reilly R.K., Epps T.H. Stimuli responsive materials. Chem Soc Rev. 2013;42:7055–7056. doi: 10.1039/c3cs90057f. [DOI] [PubMed] [Google Scholar]
- 120.Qiao Y.T., Wan J.Q., Zhou L.Q., Ma W., Yang Y.Y., Luo W.X., et al. Stimuli-responsive nanotherapeutics for precision drug delivery and cancer therapy. Wires Nanomed Nanobi. 2019;11:e1527. doi: 10.1002/wnan.1527. [DOI] [PubMed] [Google Scholar]
- 121.Dong Y., Siegwart D.J., Anderson D.G. Strategies, design, and chemistry in siRNA delivery systems. Adv Drug Deliv Rev. 2019;144:133–147. doi: 10.1016/j.addr.2019.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Melamed J.R., Hajj K.A., Chaudhary N., Strelkova D., Arral M.L., Pardi N., et al. Lipid nanoparticle chemistry determines how nucleoside base modifications alter mRNA delivery. J Control Release. 2021;341:206–214. doi: 10.1016/j.jconrel.2021.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhao W., Hou X., Vick O.G., Dong Y. RNA delivery biomaterials for the treatment of genetic and rare diseases. Biomaterials. 2019;217:119291. doi: 10.1016/j.biomaterials.2019.119291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Suzuki Y., Ishihara H. Structure, activity and uptake mechanism of siRNA-lipid nanoparticles with an asymmetric ionizable lipid. Int J Pharm. 2016;510:350–358. doi: 10.1016/j.ijpharm.2016.06.124. [DOI] [PubMed] [Google Scholar]
- 125.Gindy M.E., DiFelice K., Kumar V., Prud'homme R.K., Celano R., Haas R.M., et al. Mechanism of macromolecular structure evolution in self-assembled lipid nanoparticles for siRNA delivery. Langmuir. 2014;30:4613–4622. doi: 10.1021/la500630h. [DOI] [PubMed] [Google Scholar]
- 126.Whitehead K.A., Dorkin J.R., Vegas A.J., Chang P.H., Veiseh O., Matthews J., et al. Degradable lipid nanoparticles with predictable in vivo siRNA delivery activity. Nat Commun. 2014;5:4277. doi: 10.1038/ncomms5277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wang Y., Wang J., Zhu D., Wang Y., Qing G., Zhang Y., et al. Effect of physicochemical properties on in vivo fate of nanoparticle-based cancer immunotherapies. Acta Pharm Sin B. 2021;11:886–902. doi: 10.1016/j.apsb.2021.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Huang Y., Yu Q., Chen Z., Wu W., Zhu Q., Lu Y. In vitro and in vivo correlation for lipid-based formulations: current status and future perspectives. Acta Pharm Sin B. 2021;11:2469–2487. doi: 10.1016/j.apsb.2021.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ball R.L., Bajaj P., Whitehead K.A. Achieving long-term stability of lipid nanoparticles: examining the effect of pH, temperature, and lyophilization. Int J Nanomed. 2017;12:305–315. doi: 10.2147/IJN.S123062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhao P., Hou X., Yan J., Du S., Xue Y., Li W., et al. Long-term storage of lipid-like nanoparticles for mRNA delivery. Bioact Mater. 2020;5:358–363. doi: 10.1016/j.bioactmat.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhang H., You X., Wang X., Cui L., Wang Z., Xu F., et al. Delivery of mRNA vaccine with a lipid-like material potentiates antitumor efficacy through Toll-like receptor 4 signaling. Proc Natl Acad Sci U S A. 2021;118 doi: 10.1073/pnas.2005191118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lonez C., Vandenbranden M., Ruysschaert J.M. Cationic lipids activate in-tracellular signaling pathways. Adv Drug Deliv Rev. 2012;64:1749–1758. doi: 10.1016/j.addr.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 133.Viana I.M.O., Roussel S., Defrene J., Lima E.M., Barabe F., Bertrand N. Innate and adaptive immune responses toward nanomedicines. Acta Pharm Sin B. 2021;11:852–870. doi: 10.1016/j.apsb.2021.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]