Abstract
Hematopoietic stem cell (HSC) transplantation is the only curative therapy for many diseases. HSCs from umbilical cord blood (UCB) source have many advantages over from bone marrow. However, limited HSC dose in a single CB unit restrict its widespread use. Over the past two decades, ex vivo HSC expansion with small molecules has been an effective approach for obtaining adequate HSCs. Till now, several small-molecule compounds have entered the phase I/II trials, showing safe and favorable pharmacological profiles. As HSC expansion has become a hot topic over recent years, many newly identified small molecules along with novel biological mechanisms for HSC expansion would help solve this challenging issue. Here, we will give an overview of HSC biology, discovery and medicinal chemistry development of small molecules, natural products targeting for HSC expansion, and their recent clinical progresses, as well as potential protein targets for HSC expansion.
Key words: HSC, Small molecule, Expansion, Clinical, Targets
Graphical abstract
Hematopoietic stem cell (HSC) could be ex vivo expanded by small molecules, which is a safe strategy for obtaining adequate HSCs, bringing hope for serious hematologic diseases.

1. Introduction
Hematopoietic stem cells (HSCs) are recognized by two exclusive characteristics of self-renewal and multilineage differentiation potential, which make HSC transplantation the only life-saving therapy for many hematologic diseases1. HSC transplantation (HSCT) has been widely applied to treat a broad spectrum of hematologic malignancies in clinic, as well as inherited metabolic diseases, autoimmune diseases, sickle cell disease and severe combined immunodeficiency (SCID)2,3. To date, although there have been many efficacious drugs to ameliorate hematologic diseases, HSCT is the most efficacious and the only curative therapy that could eradicate hematologic malignancy. There are four main sources for HSCs: bone marrow (BM), mobilized peripheral blood (mPB), placenta blood and human umbilical cord blood. Both bone marrow and mobilized peripheral blood are conventional and most common sources for HSCT therapy. Unfortunately, due to the lack of human leukocyte antigen (HLA)-matched donor, about 30% patients will not have an HLA-matched donor4. Furthermore, graft vs. host disease (GVHD) remains the most slippery issue that increases the transplant-related mortality of patients receiving mismatched unrelated BM donor.
In recent years, the utilization of UCB has developed rapidly and become an increasingly attractive source for HSC transplantation due to dramatic advantages such as high percentage of HSCs, easy availability, low risk of virus transmission and tolerance of partial HLA-mismatches5,6. More importantly, cord blood transplantation is associated with low incidence of chronic GVHD, which threatens long-term survival of patients5. However, the lower absolute number of HSCs in a single UCB unit are unmet for adult patients (only 5% CB units provides sufficient cell dose for adults), and restrict UCB to pediatric setting5,6. Low number of HSCs in a single UCB unit directly lead to delayed engraftment characterized by longer time to neutrophil and platelet recovery, which results in high risks of infection, transplantation failure and relapse6. Recent years, the use of two CB units has improved infusion amount of HSC. However, it shows similar outcomes to a single unit while is associated with a higher risk of GVHD7. Since ex vivo expansion of HSC is a promising approach to obtain adequate HSC numbers, great efforts have been made to explore feasible methods in clinic during the past two decades. This approach would enable clinical availability of small size and better HLA-matched CB unit.
Since the first UCB transplantation performed in 1988 in a child with Fanconi anemia6, extensive researches have focused on finding efficacious small molecules for ex vivo HSC expansion and great achievements have been made especially in the past decade. Cytokines or growth factors, which have been early intensively investigated to expand HSC, are now essential conditions for culture of HSC expansion. Several small molecules have completed phase I/II clinical trials and some are preparing for phase III trials. Just recently, data of a phase II/III trial of copper chelator tetraethylenepentamine (TEPA) on 101 patients at 25 sites has been reported8. To some extent, this is the first well-established small molecule which is also the first small molecule on clinical trial for HSC expansion9. Subsequently, more potent and efficacious small molecules SR1, nicotinamide and UM171 were reported in 2010, 2012 and 2014, respectively10, 11, 12. All of them have been proved safe and feasible in phase I/II trials5,13,14. These small molecules usher a new era for ex vivo HSC expansion.
Over the past few years, many novel and potent small molecules have been identified, each of which has individual targets and exclusive mode of action. Identification of novel and druggable targets such as YTHDF213 and MLLT314 not only unveils complex mechanisms of HSC regeneration but also moves forward further discovery of more efficacious small molecules for HSC ex vivo expansion.
In this review, we provide an overview of HSC biology and its signaling pathways, and outline small molecules for HSC expansion as well as their activities, mode of action, and clinical progresses. From pharmaceutical chemist's perspective, in order to clarify the medicinal chemistry involved in this field, binding mode, and structure‒activity relationship (SAR) studies of several molecules are overviewed, if provided in the literatures. Besides, potential targets for ex vivo HSC expansion are also described in the final part.
2. HSC signaling and niche biology
2.1. General characteristic of HSC
Among mammals, HSC emerges and generates in the yolk sac and aorta-gonad-mesonephros (AGM) region, respectively, and then migrates to the placenta, fetal liver and spleen, followed by residing in bone marrow eventually for life-long hematopoiesis15. After birth, HSC primarily lives in bone marrow but mobilizes into circulation in response to injury.
HSCs are capable of self-renewing to replenish themselves while giving rise to all mature blood cells and immune cells16. Long-term HSCs (LT-HSCs) commonly maintain a quiescent state for protecting from detrimental stress, while enter into cell cycle upon hematological stresses, holding a marked capacity for self-renewal and long-term hematopoiesis17. On the other hand, short-term HSCs (ST-HSCs), which are derived from LT-HSCs, feature limited self-renewal potential and could differentiate into multipotent progenitors (MPPs). MPPs give rise to lineage-committed progenitors such as common myeloid progenitors (CMPs) and common lymphoid progenitors (CLPs)1. These lineage-committed progenitors subsequently give rise to specific lineage progenitors which produce all mature blood and immune cells. Hematopoietic progenitors have limited self-renewal capacity and differential potential and fail in vitro within several weeks1.
CD34+ cells are most enriched for HSCs, but only share 0.5%–5% of blood cells in human16. Actually, HSC represents a heterogeneous pool comprising of a variety of subpopulations which differ in functional and molecular phenotype17. In terms of molecular phenotype, different HSC subpopulation constitutively expresses a set of specific surface antigen markers. For instance, human HSCs are usually defined as CD34+CD38−CD49f+CD90+CD45RA−LIN− cells, and CD34−CD150+CD48−CD117+SCA1+LIN− is commonly used to define mouse HSCs18. On the other hand, different HSC subpopulation shows distinct self-renewal and multipotency capacity.
A single long-term HSC could reconstitute the whole blood system of lethally irradiated mice (with hematopoiesis failure)19. Self-renewing activity of these bona fide HSCs also enable reconstitution in secondary recipients for life-long hematopoiesis. Human HSCs capable of repopulating in NOD-SCID or NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ (NSG) mice are termed NOD-SCID repopulating cells (SRCs), which are candidate HSCs10. In practice, activity of these long-term repopulating units is determined by competitive repopulating assay and serial transplantation assay, while absolute number is quantified by colony-forming unit (CFU) assay and limiting dilution assay (LDA)20.
2.2. HSC niche biology
HSC resides in unique bone marrow microenvironment (generally termed “niche”) for maintenance and hematopoiesis17,21,22. Niche for HSCs could be divided into endosteal or vascular niche23. LT-HSCs commonly locate adjacent to endosteum while those active ST-HSCs reside closely to sinusoids23.
Nowadays, by virtue of high-resolution imaging techniques and other advanced biotechnologies, spatial distribution of niche cell type and cell-to-cell interaction within niche could be visualized24. Endothelial cells and mesenchymal stromal cells (MSCs) maintain HSC via secretion of SCF (stem cell factor) and CXCL1219,23. Macrophages, osteoclasts and non-myelinating Schwann cells regulate HSCs through either direct or indirect effect17,21. Other cells, such as perivascular stromal cells and regulatory T cells, were also found to regulate HSCs17,21. Furthermore, sympathetic nerves are also known to regulate the HSC niche21. These niche cells and nerves work in concert to form a complex network to precisely regulate HSC cell fate decision through various extracellular cues such as cytokines and cell-to-cell interaction17,21. Furthermore, distinct niche exist for functionally and molecularly heterogeneous HSC pool21.
Briefly, HSCs live in a perivascular niche, where their maintenance, self-renewal and differentiation are strictly regulated.
2.3. Signaling pathways orchestrating HSC fate decision
In addition to extracellular cues, intracellular factors, such as metabolism, key proteins and many signaling pathways, are also essential for regulating HSC fate decision17. Two conserved signaling pathways, Wnt and Notch signaling, which are widely present in various tissues and involved in many diseases, play important roles for regulating HSC fate decision25, 26, 27, 28. Canonical Wnt pathway is mainly associated with self-renewal while noncanonical Wnt pathway regulates HSC quiescence26. Notch signaling plays a crucial role for HSC development during embryogenesis, and adult HSC differentiation toward T cells27,28. Apart from the two pathways, others such as TGF-β/Smad signaling are also involved in HSC fate regulation29. As many conserved pathways orchestrate to corporately form an intricate signal network for regulating HSC fate, the balance between HSC self-renewal and differentiation is highly ensured in a meticulous way. As a result, we will shortly describe basic transduction processes of Wnt and Notch pathways and discuss their involvement in HSC biology, and most importantly, in HSC expansion.
2.3.1. Wnt signaling
The Wnt signaling pathway is highly conserved during lengthy evolution throughout every stages and systems of all multicellular animals30. It plays an essential role in embryonic HSC development, adult HSCs self-renewal, lineages specification and maintenance26,31, 32, 33. A wide range of diseases (reviewed extensively by Nusse et al.25 and Clevers et al.34) including hematologic diseases have been found to be associated with this pathway. The Wnt cascade represents 19 distinct Wnt ligands and more than 10 Frizzled receptors plus several co-receptors, which partially account for its broad spectrum of biological effects among many different species25. Broadly speaking, Wnt signaling pathway is divided into canonical and noncanonical pathway. Canonical Wnt pathway (Fig. 1), in which β-catenin is a critical mediator, modulates much on HSC self-renewal26. In the absence of Wnt signal, glycogen synthase kinase 3β (GSK-3β), CK1 (casein kinase 1) plus several tumor suppressor factors like Axin (axis inhibition protein) and APC (adenomatous polyposis coli) corporately form a destruction complex to phosphorylate β-catenin and lead to its proteasomal degradation upon ubiquitination32. Conversely, when Wnt signaling is on (in the presence of Wnt ligand), inactivation of destruction complex contributes to dissociation, buildup and accumulation of β-catenin, which promotes its translocation to the nucleus32. Dephosphorylated β-catenin binds to T cell factor (TCF) and form transcription activator complex for promoting expression of Wnt target genes32. Given the roles of Wnt cascade for HSCs development and cell fate decision at multiple stages of all metazoan species, the ancient pathway has been of great importance for regulating hematopoiesis.
Figure 1.
Canonical Wnt/β-catenin signaling pathway. (a) In the absence of Wnt protein, CK1 and GSK3β, as well as the anchor proteins Axin and APC form a destruction complex. β-Catenin in the cytoplasm is phosphorylated by CK1 and GSK3β, followed by bound to β-transducin-repeat-containing protein (β-TrCP), leading to ubiquitylation and degradation by the proteasome. In the nucleus, TCF recruits transcriptional co-repressors such as Groucho/TLE (transducin-like enhancer) proteins to block Wnt target genes transcription. (b) After Wnt binds to co-receptor LRP5/6 and Frizzled receptor, CK1 and GSK3β phosphorylate LRP5/6. β-Catenin is released from destruction complex and translocated to the nucleus, which binds to TCF to activate transcription.
In 2003, Willert and colleagues35 demonstrated the active form of secreted Wnt3a protein enhanced mice BM HSCs self-renewal and led to subsequent ex vivo expansion. In consistence with this, loss of Wnt3a in BM niche compromised self-renewal capacity of LT-HSC36. Intriguingly, subsequent studies suggested that Wnt3a diminished long-term repopulating capacity by inducing human HSPC multilineage differentiation37, though others indicated that constitutive activation of canonical β-catenin signaling blocks multilineage differentiation and compromises hematopoietic reconstitution. Until later compelling evidence explained these paradoxical results that it was the different level of Wnt signaling that accounted for distinct HSCs self-renewal capacities in the past studies38. High levels of canonical Wnt signaling promoted differentiation and diminished HSCs self-renewal capacity while mild Wnt activation enhanced HSCs self-renewal capacity and promoted expansion. Of note, Reya et al.39 suggested a crosstalk between Wnt and Notch signaling that Wnt activation led to increased Notch1 expression, which revealed that both the two conserved signaling pathways worked cooperatively to regulate HSCs fate decision, especially self-renewal.
Early studies suggested addition of Wnt3a or Wnt5a proteins promoted HSC proliferation and self-renewal in culture34,35. Recently, Janda et al.40 developed water-soluble surrogate Wnt agonists to activate canonical β-catenin signaling in vivo and displayed supportive effects in many organoid cultures in vitro comparably to Wnt3a, providing more benefits for studies on Wnt pathway and potential means for stem cells regeneration. Another Wnt protein Wnt9a was found to mediate early HSPC amplification41. All these evidence suggest an important role for Wnt signaling in HSC self-renewal and expansion, and it is possible to ex vivo expand HSCs through regulating Wnt pathway.
2.3.2. Notch signaling
As is the case with Wnt pathway, Notch signaling pathway is also highly conserved and occurs in numerous aspects of HSC biology, and its diverse biological effects depend on specific context and condition28. Notch pathway (Fig. 2) involves four receptors Notch1–4, five Notch ligands Delta (Delta1, Delta3, and Delta4) and Jagged (Jagged1 and Jagged2), target genes, and regulatory elements27. After Notch ligand binds to its receptor, the receptor undergoes two proteolytic cleavages, and then the Notch intracellular domain (ICD) is released, which recruits other components to form the transcriptional activation complex subsequent to translocation to the nucleus27,28,42. In cooperation with specific co-activators, the complex activates target genes transcription.
Figure 2.
Overview of Notch signaling pathway. After Notch receptor and its ligand interact at the cell-to-cell surface, Notch receptor is cleaved twice by ADAM metalloprotease and γ-secretase. Notch ICD translocates into the nucleus, and then binds to the DNA binding protein CSL and recruits several co-activators to initiate Notch target genes transcription.
Efforts in exploring effective protocols for ex vivo HSCs expansion employing Notch ligands have been made a lot despite the elusive functions of Notch signaling in adult HSCs. Jagged1, which is secreted by osteoblastic cells, occurred in a Notch1-dependent manner to support the expansion of HSCs both in vitro and in vivo43. Later, soluble Jagged1 was demonstrated to enable modest HSCs expansion in vitro and enhance long-term engraftment in NSG mice44,45. Negishi et al.46 developed a transgenic hJ1-NOG mice model, in which BM niche was engineered to express human Jagged1 and transplanted human CB HSCs showed 5-fold expansion compared to control.
In 2003, Varnum-Finney et al.47 found a combination of immobilized Delta1 and cytokines increased murine marrow precursor numbers by multi-log, and these expanded precursors were capable of short-term repopulation and long-term T-lymphoid reconstitution in mice. Subsequently, they further used this immobilized Delta1 to ex vivo expand human CD34+CD38− cord blood progenitors, which exhibited a more than 100-fold increase in HSPC numbers, higher SRC frequency and enhanced short-term repopulating capacities in a low Delta1 dose culture48, 49, 50. These preclinical results proved preliminary safety and feasibility of Notch ligand for HSC expansion.
They then enrolled 10 patients to conduct a phase I clinical trial on Delta1-expanded CB unit (Table 1)50. 7 out of 10 patients remained alive without related diseases and established complete engraftment derived from donor. No infusional toxicities or other safety concerns were observed in all patients. A rapid neutrophil engraftment (median time 16 days) was observed compared to double CB unit's control. Intriguingly, hints from previous studies in which Delta1 contributes more to short-term repopulation and myeloid lineages reconstitution implied that the expanded unit is responsible for short-term repopulation, i.e., short-term repopulating progenitor cells accounted for the rapid neutrophil engraftment. At six months after transplantation, donor hematopoiesis derived from Detla1-expanded unit is almost lost. Subsequent clinical observations indicated that the unmanipulated unit contributes mostly to long-term hematopoiesis while the expanded unit contributes to myeloid reconstitution51. Some researchers explained that immune cells present in the unmanipulated unit produce an immune rejection against the expanded unit, and the former becomes the “winning” unit50, 51, 52. Another rational explanation suggested that the portion of primitive HSC responsible for long-term repopulating depletes over the course of reconstitution50,52. Double armed clinical studies or studies on single expanded unit are still required to test whether this method expands bona fide HSC in cord blood unit and larger phase II/III studies are also required to evaluate whether rapid engraftment brings about improved overall survival of patients.
Table 1.
Detailed outcome of completed clinical trials involving manipulated UCB units.
| Small molecule | ClinicalTrials.gov identifier | Status | Expansion effecta | GVHD incidence | Survival | Ref. |
|---|---|---|---|---|---|---|
| SR1 | NCT01474681 | Phase I/II completed | 330-fold CD34+ cells 854-fold TNCs |
Grade III–IV aGVHD 29% (100 days) | OS 55% (12 months) TRM 45% (12 months) |
63 |
| UM171 | NCT02668315 | Phase I/II completed | 35.4-fold CD34+ cells | Grade II–IV aGVHD 64% (1 year) Grade III–IV aGVHD 10% (1 year) Chronic GVHD 17% (1 year) No moderate to severe chronic GVHD |
TRM 5% (1 year) OS 90% PFS 74% |
5 |
| NAM | NCT01221857 | Phase I completed | 486-fold TNCs 72-fold CD34+ cells |
Grade II aGVHD 45% No grades III/IV aGVHD Chronic GVHD 18% |
OS 82% (1 year) PFS 73% (1 year) |
52 |
| NAM | NCT01816230 | Phase I/II completed | 33-fold CD34+ cells | Grade II–IV aGVHD 44% (100 days) Grade III–IV aGVHD 11% (100 days) Chronic GVHD 40% (2 years) Moderate to severe chronic GVHD 10% (2 years) |
Nonrelapse mortality 24% (2 years) DFS 43% (2 years) OS 51% (2 years) Relapse 33% (2 years) |
64 |
| TEPA | ‒ | Phase I/II completed | 219-fold TNCs 6-fold CD34+ cells |
No grade III–IV aGVHD Grade II aGVHD 44% Chronic GVHD 50% |
OS 90% (100 days) 70% (180 days) 40% (>21 months) |
9 |
| TEPA | NCT00469729 | Phase II/III completed | 400-fold TNCs 77-fold CD34+ cells |
Grade III–IV aGVHD 19.4% Grade II–IV aGVHD 31.6% Chronic GVHD 18.4% |
OS 84.2% (100 days) | 8 |
| Notch-ligand | – | Phase I completed | 562-fold TNCs 164-fold CD34+ cells |
Grade II aGVHD 90% Grade III aGVHD 10% Chronic limited GVHD 30% |
OS 70% (354 days) | 50 |
| dm-PGE2 | NCT00890500 | Phase I completed | – | Grade I aGVHD 25% Skin-limited grade II aGVHD 16.7% Skin-limited cGVHD 16.7% |
PFS 61.7% at 1 year 31.3% at 2 years OS 75% (1 year) 38.9% (2 years) |
78 |
TNC, total nucleated cells; TRM, transplant-related mortality; OS, overall survival; PFS, progression-free survival; DFS, disease-free survival; aGVHD, acute GVHD; cGVHD, chronic GVHD.
These data is relative to input cells content.
3. Cytokine and growth factor
Due to low dose of HSCs in human cord blood, people have starved for obtaining sufficient HSCs to satisfy the unmet need. Initial attempts to ex vivo HSC expansion were inspired by diverse cytokines and growth factors. SCF is the first defined cytokine for promoting HSC/HPC expansion in vitro despite low efficiency alone. Subsequently, several potent cytokines and growth factors were identified, including thrombopoietin (TPO), interleukin-3/6/11 (IL-3/6/11), Fms-like tyrosine kinase 3 ligand (FLT-3L), and G-CSF (granulocyte colony-stimulating factor)53. Although these cytokines alone are inefficient to support HSC expansion in vitro, a combination of several cytokines appears to be effective. Combination of SCF, FLT-3L, IL-3/6 and TPO are widely used for HSC ex vivo expansion in culture53. However, basic expansion culture systems are incapable of maintaining sustaining HSC expansion and eventually lead to depletion of HSC pool size.
Angiopoietin-like proteins (Angptls) family contains eight members, which play different roles in lipid metabolism, angiogenesis inflammation, cancer cell motility and HSC expansion54. Angptl3 was found to support HSCs maintenance, self-renewal and expansion in bone marrow niche55. Zhang et al.56 suggested that Angptl2, 3, 5, and 7 induced expansions of long-term HSCs in synergy with SCF, TPO, FGF-1 and IGF-2, especially that Angptl2 and Angptl3 induce 24- and 30-fold net increase in LT-HSCs, respectively. Further studies by Akhter et al.57 suggested that Angptl1, 2, 3, 4, 6 and 7 supported mouse HSCs survival while caused a 3- to 4.5-fold net expansion compared to cocktail of SCF, TPO, FGF-1 and IGF-2.
Sonic hedgehog (Shh) protein is one of three members of Hedgehog (Hh) family in human. Addition of Shh induced human CD34+CD38− cells proliferation and doubled the CFU numbers after 7 days in vitro treatment58. Most importantly, Shh treated human primitive HSCs showed increased repopulating ability via inhibition of downstream BMP-4 expression58.
Pleiotrophin (PTN) is a heparin-binding growth factor secreted by bone marrow perivascular stromal cells and vascular endothelial cells (ECs)59. Secretion of pleiotrophin from distinct niche compartments regulates HSC maintenance and self-renewal via inhibition of protein tyrosine phosphatase-ζ (PTPζ)59, 60, 61, 62. PTN induced marked expansion of human CB HSCs and murine LT-HSCs in culture supplemented with other cytokines, and accelerated hematopoietic recovery of irradiated mice62.
4. Small molecules for ex vivo HSC expansion
Although a variety of cytokines are capable of expanding HSCs, these factors are insufficient to maintain long-term and robust HSC expansion. Commonly-used cytokine cocktail (SCF, TPO, IL3/6/11, and FLT-3L) only provided transient (1‒2 weeks) and limited HSC expansion. In a clinical trial, transient neutrophil recovery and lack of long-term engraftment in vivo was observed in Delta1-expanded CB graft. Thus, this strategy proved overall unsuccessful. Compared to cytokines or growth factors, small molecules have several advantages, such as definite effects, easily manipulated and low cost, which make them be widely investigated and used for ex vivo HSC expansion. Several small molecules proved safe and feasible in clinical trials. Recent years, many novel small molecules for HSC expansion have been reported. The development of small molecules is shown in Fig. 3. In this section, we will first review small molecules in clinical trials, and then outline novel small molecules according to their targets or properties. In order to clarify the medicinal chemistry involved in this field, binding mode and SAR studies of several molecules are also discussed in details. Small molecules in clinical trials, recent reported compounds, natural products and endogenous substances for ex vivo HSC expansion are summarized in Table 1, Table 2, Table 3, respectively.
Figure 3.
Chronological development of small molecules for ex vivo HSC expansion.
Table 2.
Summary of recent small molecules for HSC expansion.
| Compd. | Reported in | Target | Expansion effect | SRC expansion | Ref. |
|---|---|---|---|---|---|
| SW033291 | 2015 | 15-PGDH | ‒ | 2.4-fold | 79 |
| DJ001 | 2019 | PTPσ | 4.07-fold LSK | ‒ | 83 |
| C7 | 2018 | p38 | 3.7-fold CD34+38−45+45RA− | 2.9-fold | 87 |
| JNK-IN-8 | 2019 | JNK | 8-fold CD34+38−45RA−90+ | 3.88-fold | 91 |
| 005A | 2015 | p18 | aED50 5.21 nmol/L | 2.72-fold | 98 |
| CPI-203 | 2020 | BET | 5–10 folds HSCs | 5‒10-fold | 99 |
| GW9662 | 2018 | PPAR-γ | 7-fold CD34+CD38− | 5-fold | 101 |
| Alexidine | 2015 | Ptpmt1 | ‒ | ∼2-fold | 103 |
| OAC1 | 2016 | OCT4 | 2.2-fold CD34+CD38− 2.8-fold HSCs |
6.3-fold | 106 |
| NR101 | 2009 | TPO | 2.3-fold CD34+CD38− | 2.3-fold | 107 |
| Eltrombopag | 2018 | TPO | ‒ | 1.7-fold | 108 |
| BIO | 2011 | GSK-3 | 2-fold CFU activity | ‒ | 111 |
| CHIR99021 | 2005, 2012 | GSK-3 | 7-fold TNC | 5-fold | 109,112 |
| VPA | 2005 | HDAC | CD34+ cell numbers↑ | 6-fold | 121,122 |
| LMK235 | 2018 | HDAC5 | ‒ | 4.3-fold | 130 |
| 5azaD | 2006 | DNMT | 2.5-fold CD34+ 40-fold CD34+CD90+ |
‒ | 132 |
| zVADfmk/zLLYfmk | 2010 | Cysteine protease | 3-fold CD34+CD38− | – | 135 |
| DEAB | 2006 | ALDH | 11.6-foldb CD34+CD38− | 3.4-fold | 136 |
Determined as the ratio of CFU-nmEM colonies.
Relative to input, others without note are relative to control.
Table 3.
Summary of natural products and endogenous substances for ex vivo HSC expansion.
| Compd. | Target/signaling | Expansion effect | Ref. |
|---|---|---|---|
| Rapamycin | mTOR | ‒ | 112 |
| Garcinol/isogarcinol | HATs | 4.5-/7.4-fold HSCs 2.5-fold SRCs (garcinol) |
118 |
| TSA | HDAC | 2.5-fold CD34+ 40-fold CD34+CD90+ |
132 |
| EchA | ROS inhibition PI3K/AKT activation |
1.33-fold CD34+ 1.7-fold CFUs |
137 |
| ASPP049 | Hippo signaling | ∼1.5-fold CD34+CD38− ∼2-fold hCD45+ |
143 |
| Chrysin | p19, p57, MNDA, NR4A2, HOXB4 | ∼1.5-fold HSCs 2.7-fold SRCs |
145 |
| Resveratol | ‒ | 1.84-fold CD34+/CD133+ | 138, 139, 140 |
| CAPE | HIF-1α, SDF-1α and VEGF-A | 1.47-fold CFUs 1.77-fold SRCs |
141, 142 |
| 5-HT | Apoptosis inhibition | 1.73-fold CFU-F 12.2-fold CD34+ |
146 |
| α-Tocopherol | ERK1/2 | 2-fold LSKs | 147 |
| ATRA | RA receptor | 4-fold CFUs | 148,149 |
4.1. Small molecules in clinical trials
4.1.1. SR1
A breakthrough occurred in 2010 that Boitano et al.10 reported a promising purine derivative SR1 (1, Fig. 4) screened from a 100,000 compounds library to effectively expand HSC. In cooperation with cytokines combination, SR1 treatment led to a 50-fold expansion of CB CD34+ cells, a 65-fold increase in CFUs and a 17-fold increase in SRC numbers. Mechanistically, SR1 binds to and directly inhibits aryl hydrocarbon receptor (AHR) in human and monkey, with a high inhibitory activity (IC50) of 127 nmol/L, regardless of no activity on murine HSC. More significantly, a concentration of SR1 of 120 nmol/L resulted in a 50% increase in percent human CB CD34+ cells compared to cytokines alone cultures.
Figure 4.
SAR studies of SR1.
A phase I/II clinical study (NCT01474681, Table 1) on SR1-expanded UCB unit combination with an unmanipulated unit have obtained encouraging outcomes including significant CD34+ cells expansion, enhanced engraftment and rapid hematopoietic recovery63. In this study, SR1-treated UCB units underwent marked median expansion of CD34+ cells at 330-fold over initial numbers. Compared to Notch-mediated expansion (Table 1) in which long-term engraftment in almost all settings was lastly derived from the unmanipulated UCB unit50, SR1-expanded unit was responsible for durable and robust engraftment, similarly to that of nicotinamide52,63. In addition, a median time of 49 days to platelet recovery in SR1-group was much shorter than 89 days in control. Interestingly, 5 of 17 patients showed an exclusive mixed myeloid chimerism from both units and were observed with a particularly short median time at 7 days to neutrophil recovery, while this demonstrated no responsible for platelet recovery. However, high incidence of transplant-related mortality of 45%, and undesirable overall survival of 45% were observed. In addition, grade 3–4 acute GVHD remained a high percentage of 29%.
Recently, two phase II trials (NCT03674411 and NCT03406962) have enrolled participants to further evaluate SR1-expanded CB unit on hematologic malignancies or inherited metabolic diseases. It is encouraging that in 2018 FDA granted regenerative medicine advanced therapy (RMAT) designation to the SR1-expanded HSCs product (termed MGTA-456) for inherited metabolic disorders treatment.
Of note, the author provided a SAR study on the C2, C6, and N9 position of purine ring of SR1 (Fig. 4). SAR studies suggested that 4-hydroxylphenthylamino moiety at C6 position is not essential and replacement with indole-3-ethylamino group results in 6-fold increase in expansion EC50 value of 0.02 μmol/L (3, Fig. 5). With respect to arylalkylamino group, the distance between nitrogen and aromatic ring is essential for ex vivo expansion activity; ethyl chain length is desirable and anilines results in loss of activity. Benzothiophene group is most preferred among a series of aryl/heteroaryl such as benzene, pyrimidine, furan and thiophene. Activity of these compounds could be modulated by replacement of iso-propyl with other aliphatic chain; 2-butyl outcompetes 3-butyl and iso-propyl, and it leads to a 4-fold increase in activity (EC50 0.03 μmol/L) relative to iso-propyl, as exemplified by compound 2 (Fig. 5).
Figure 5.
Structures and activities of compounds 1‒3.
4.1.2. UM171
In 2014, Fares et al.12 identified a potent small molecule for expanding hCD34+ mPB cells, UM729 (4, Fig. 6), from 5280 compounds. After systematic structural optimization by SAR of more than 300 analogs, UM171 (5, Fig. 6) was confirmed as the top candidate possessing a strong expanding activity of 17–19 nmol/L which was over 10-fold than that of UM72912. Both UM171 and UM729 could enhance repopulating capacity of macaque CD34+ cells by three folds compared to control. The numbers of LT-HSCs in fed-batch culture (established medium delivery system) supplemented with UM171 increased by over 13-fold. Furthermore, transplantation assays showed that UM171 maintained long-term repopulating capacity of LT-HSCs and therefore enabled persistent engraftment in secondary recipients for over 18 weeks. Mechanism studies suggested that UM171 did not inhibit AHR while repressed gene expression involved in erythroid and megakaryocytic differentiation, and highly up-regulated TMEM183A expression as well as transcripts of some surface molecules.
Figure 6.
Discovery and structural optimization of UM171.
A phase I/II trial (NCT02668315, Table 1) was conducted between Feb 2016 and Nov 2018 aimed to evaluate safety and feasibility of single UM171-expanded CB unit on 27 patients suffered from hematologic malignancies5. In the phase II cohort, 22 patients received single expanded CB unit despite slow neutrophil engraftment at a median time of 18 days relative to that of nicotinamide64 and SR163. UM171 outcompeted both two compounds with respect to low incidence of grade 3–4 acute GVHD (10%) and no moderate to severe chronic GVHD as well as low transplant-related mortality (5%, in 1 year). Furthermore, overall survival and progression-free survival was remarkable, 90% and 74%, respectively. Taken together, UM171 expanded single CB unit appears to be safe and feasible because of low transplant-related mortality and low incidence of GVHD in addition to no graft failure. A phase III trial is needed to further compare UM171 and standard condition (two unmanipulated CB units).
In addition, they also initiated two phase II clinical trials to further evaluate this HSC product in terms of transplant-related mortality and relapse-free survival on leukemia or myelodysplasia patients (NCT03913026 and NCT04103879). Furthermore, a clinical study (NCT03441958) has been set up to evaluate the safety and efficacy on multiple myeloma.
Recently, Feng et al.65 designed and synthesized two series of pyrimidoindole analogs in which the tetrazol group substitutes in C6 or C7 to discuss the SAR of UM171 (Fig. 6). These analogs were tested to increase the percentage of CD34+CD38− cells which are enriched for HSCs. They focused on assessing the effect of tetrazol group at C6 or C7 position and effect of substituents at C4 position on HSC activity. SAR studies revealed that substitution site of tetrazol group affected activity profoundly (Fig. 6). Same substitution at C6 or C7 position (6 and 7) shows completely distinct activity. 6 shows an EC50 value of 473.43 nmol/L while 7 has no activity. Although both 8 and 9 bear a same tetrahydroisoquinolino ethylamino and share a same EC50 value of 30 nmol/L, the different site of tetrazol group results in about three-fold difference in expanding CD34+CD38− cells. The multi-lineage potential of 8-treated HSC was maintained with a 1.4-fold increase in CFUs. 8 is a promising agent, but further studies are needed to evaluate whether 8-expanded HSCs maintain long-term repopulating capacity in animal model.
4.1.3. Nicotinamide
As a form of vitamin B-3, nicotinamide (NAM, 10, Fig. 7) is essential for human living and have diverse biological effects. Peled et al.11 observed its unique effect on HSCs expansion. NAM treatment resulted in an 80-fold increase in CB CD34+ cells, along with a robust expansion of CD34+CD38− subpopulation. Transplantation assays suggested several folds increase in NOD/SCID repopulating cells after NAM treatment and improvement of engraftment potential of NAM-treated HSCs. NAM appeared to attenuate differentiation and enhance self-renewal capacity. Furthermore, NAM improved homing efficiency of CD34+ cells dependent on CXCR4 to SDF-1 (stromal cell derived factor-1, also known as chemokine CXCL12, regulator for HSC homing) by 6 folds. Mechanistically, NAM selectively inhibited SIRT1 (Sirtuin1, class III NAD-dependent histone deacetylase) to exert the expansion effect. A specific SIRT1 inhibitor EX-527 (11, Fig. 7) also has similar expansion effect with NAM11.
Figure 7.
Structures of compounds 10–14.
As inspired by remarked preclinical outcomes, there has been a class of clinical studies going on. Gamida Cell first conducted a phase I trial (NCT01221857, Table 1) which enrolled 12 patients in Duke University School of Medicine during Dec 2010 and Aug 2012 to preliminarily assess the engraftment of NAM-expanded CB HSC product, NiCord52. This novel protocol provided long-term engraftment in 8 of 10 patients for more than 2 years. Not only NAM-treated CB units achieved rapid hematopoietic recovery that median time to neutrophil and platelet engraftment was at 13 and 33 days compared to 25 and 37 days in historical control, respectively, but also showed high overall survival of 82% and progression-free survival of 73%. Among eight patients with partial or complete myeloid chimerism derived from NAM-expanded unit, six of which achieved stable NAM-derived T-cell engraftment. Coinfusion of an uncultured T cell fraction could possibly account for the predominance of the NAM-expanded unit. Also of note, there were no cases of acute grade 3–4 GVHD but two developed chronic GVHD.
Once the safety and efficacy of NAM were justified (NiCord-provided hematopoiesis has sustained for more than 7 years), they further dissected whether single CB unit expanded with NAM could achieve successful engraftment (NCT01816230, Table 1)64. As a result, 36 patients were enrolled to receive single expanded unit at 11 places. In this cohort, hematopoietic recovery appeared to be fast with median time to neutrophil at 13 days (21 days for control, 146 patients), accounting for less hospital stay and decreased incidence of infection. Most significantly, this NAM-based therapy demonstrated low incidences of severe acute or chronic GVHD, and high disease-free survival of 43% and low nonrelapse mortality of 24% despite possibly higher risk of relapse in 2 years (Table 1).
Previously, NiCord have been received orphan drug designation for AML treatment by European Medicines Agency's (EMA's) Committee and for treatment of several hematologic malignancies by FDA. Furthermore, FDA granted breakthrough therapy designation for NiCord in blood cancer. Recently, EMA have extended orphan drug designation for NiCord to treatment for hematopoietic stem cell transplantation. In the future, a phase III trial (NCT02730299) for NiCord will be conducted on 120 patients with hematologic malignancies on multicenter.
4.1.4. TEPA
Early studies have indicated that cord blood-derived HSC differentiation was inhibited and proliferation was enhanced in the context of low copper condition by addition of copper chelator, tetraethylenepentamine (TEPA, 12, Fig. 7) while differentiation was accelerated under high copper condition66,67. TEPA resulted in a stable and robust increase in CB-derived CD34+ cells and its primitive progenitor subpopulation, which showed enhanced repopulating capacity in NOD/SCID mice68,69.
In 2008, Gamida Cell reported a phase I/II trial (Table 1) to evaluate StemEx (an expanded CD133+ fraction from single CB unit along with the unmanipulated fraction) transplantation on 10 patients with hematologic malignancies9. Despite a limited CD34+ cell expansion (6-fold average) and low TNCs (total nucleated cells) content after TEPA expansion, high overall survival of 90% in 100 days was seen in nine patients and no cases of grades 3–4 acute GVHD occurred. However, slow neutrophil and platelet recovery (median time 30 and 48 days, respectively) was observed.
Base on the feasibility of StemEx, 101 patients were enrolled at 25 sites, and 295 patients from international registries received double unmanipulated CB units transplantation as a comparator (NCT00469729, Table 1)8. Regarding hematopoietic recovery, median time to neutrophil and platelet of TEPA group was 21 and 54 days, comparing 28 and 108 days in control, respectively. Although the 100-day overall survival of the TEPA group (84.2%) is superior to that of control group (74.6%), the 180-day overall survival is similar. The primary cause for the ascending late infection and mortality is reduced immune cells infusion. Also, the incidence of acute and chronic GVHD is similar between two groups.
4.1.5. PGE2
Early in 40 years ago, accumulated evidence revealed PGE2 (13, Fig. 7) has a stimulative effect on HSCs and mature blood cells despite many contradictory observations70. Until 2007, a stable PGE2 derivative 16,16-dimethyl-PGE2 (dmPGE2, 14, Fig. 7) was found by North et al.71 to increase CFUs and SRC frequency in murine which was transplanted with dmPGE2-treated BM cells. Hoggatt et al.72 reported that short ex vivo exposure of murine HSCs to dmPGE2 promotes their homing, survival and proliferation, with an enhanced long-term repopulating capacity.
Furthermore, dmPGE2 accelerated hematopoietic recovery and had hematopoiesis-protective effects in sublethally irradiated murine and zebrafish73,74. PGE2 could activate Wnt signaling pathway to promote HSC self-renewal75. HSCs express four G-protein coupled E-prostanoid receptors, EP1–4, each of which distinguishes in mode of actions and biological outcomes76. In addition, the PGE2/Wnt interaction is conserved in vertebrate for regeneration and recovery, and binding of PGE2 to EP4 regulates the stability of β-catenin via cAMP/PKA signaling and subsequently activates Wnt pathway74, 75, 76.
Preclinical studies in which dmPGE2-expanded UCB unit enhanced engraftment in NOD/SCID mice, showed safety and potency of dmPGE2 for hUCB transplantation77. All these evidence provided safety, efficacy and definite molecule mechanisms of dmPGE2, and paved the way to phase I clinical trial (NCT00890500, Table 1). A rapid neutrophil recovery (median time 17.5 vs. 21 days in control) and preferential long-term engraftment (10 of 12 treated patients) was observed in patients who were infused a dmPGE2-UCB unit along with an unmanipulated CB unit compared to double unmanipulated UCB units78.
4.2. Recent advances of novel small molecules
4.2.1. 15-PGDH inhibitor
As is previously described, PGE2 promotes HSC homing and expansion and enhances hematopoiesis after irradiation71,77. A clinical trial has proved that PGE2 or dmPGE2 enhanced CB units engraftment in patients78. Inhibiting prostaglandin-degrading enzyme 15-PGDH enhances PGE2 levels in vivo, and favors tissue repair and hematopoietic recovery79,80. Either 15-PGDH knockout or pharmacologic inhibition of 15-PGDH resulted in increase in numbers of HSC and mature blood cell79. As a result, 15-PGDH inhibitors are promising agents for ex vivo HSC expansion. Zhang et al.79 screened from 230,000 compounds and identified a potent 15-PGDH inhibitor SW033291 (15, Fig. 8) with a dissociation constant Ki = 0.1 nmol/L and inducing 2-fold increase in PGE2 levels in vivo. Bone marrow cells ex vivo treated with 15 were significantly enhanced with repopulating capacity. Also, 15 benefited irradiated mice hematopoietic recovery after allogeneic transplantation. Due to poor solubility of 15, they did further medicinal chemistry work to improve its druglike properties81. Through a systematic SAR studies, they obtained four sulfoxides with better potency profile both in vitro and in cells but also improved solubility. Hydrochloride salt of (+)-16 (R-enantiomer of 16), was proved over 10,000-fold more soluble than racemic 15 (Fig. 8).
Figure 8.
Structures, biological activities, solubility, and metabolism profile of compounds 15‒19.
SAR studies suggested that sulfoxide moiety is essential for enzyme inhibition activity and cellular activity (Fig. 9). Conversion of sulfoxide to sulfide or sulfone led to loss of substantial activity and 10-fold less active than 15, respectively. In addition, replacement of sulfoxide with carbonyls attenuated potency. Putative inhibitor-enzyme binding model suggested that sulfoxide mimics the charge distribution and the transition state of 15-hydroxyl of PGE2, and therefore blocks key Tyr151 of 15-PGDH for degrading PGE2. This possibly explained R-enantiomer of sulfoxides significantly outperformed S-enantiomer in terms of in vitro enzyme inhibition and metabolism stability in vivo as exemplified below by (+)-16, (+)-17 and (+)-19. Size and properties of sulfoxide side chain dramatically affected inhibition activity and activity in cells. Introducing ether group to alkyl side chain retained activity while improved 10-fold solubility. The phenyl group in pyridine ring was dispensable for enzyme inhibition and allowed for replacement with heterocyclic rings, but important for maintaining activity in cells. Introducing dimethylimidazolyl to replace phenyl moiety yielded 16 whose hydrochloride salt is 10,000-fold more soluble in water than 16. S-Enantiomer of 16 almost lost activity both in vitro and in vivo. (+)-16 has marked metabolism dynamics profile in human liver with a desirable half-time of over 240 min without any toxicity. Substitution on phenyl ring maintained activity and changed molecular polarity with an improvement of solubility, such as 19 which features an ethylene glycol moiety and led to 10-fold solubility improvement and longer half-time in mouse liver while as active as 15. Thienyl group in 15 is not essential and is of well tolerance for heteroaryls, such as thiazolyl and oxazolyl, despite slightly decreased activities. Furthermore, in addition to thienopyridine, thienopyrimidine as central ring also maintained activity, such as 17 or 18 (Fig. 8). All four optimized sulfoxides doubled PGE2 levels in bone marrow of mouse at 3 h and were more metabolically stable.
Figure 9.
SAR summary of 15-PGDH inhibitors.
(+)-16 possesses 10,000-fold solubility and better in vivo activity relative to 15 by replacement of phenyl with a 1,3-dimethylimidazolyl and substitution of thiophene with thiazole. In consistence with 15, (+)-16 was demonstrated to enhance HSC homing and engraftment, and accelerated hematopoietic recovery in animal HSCT model, without any adverse effects80. Thus, 15-PGDH inhibitors may also serve as therapeutic agents for ameliorating engraftment after HSCT.
4.2.2. PTPσ inhibitor
Protein tyrosine phosphatase (PTP) family comprises of several therapeutic members, whereas progresses in druggable small molecules targeting on PTPs are lagging behind. In 2015, Quarmyne et al.82 found that PTPσ was expressed by murine and human HSC and loss of PTPσ resulted in enhanced long-term repopulating capacity. HSCs derived from Ptprs (encode PTPσ) -knockout mice were capable of long-term repopulating, and negative selection of receptor PTPσ (PTPσ−) CB cells comparably resulted in increased repopulating capacity comparing with those of PTPσ+. Recently, Zhang et al.83 screened from 80,000 compounds according to similar structure features of a known PTPσ inhibitor with IC50 of 2.3 μmol/L (Fig. 10). Two 2-arylamino-1-arylpropenone inhibitors were identified, one of which compound DJ001 (20) showed an IC50 of 1.5 μmol/L and had strong ex vivo HSC expansion activity. They further synthesized more than 50 analogs to provide 22, while 22 is less active than 20 (Fig. 10). 20 suppressed HSCs apoptosis, enhanced HSCs survival and proliferation, and promoted hematologic recovery in irradiated mice83. It is worth noting that 20 promoted irradiated human HSCs self-renewal and hematopoietic reconstitution. Mechanistic studies discovered that 20 improves hematopoietic recovery via activation of RAC1 and downstream signaling cascade activation (e.g., BCL-XL, CDK2, and ERK1/2) in response to selective and allosteric binding to PTPσ.
Figure 10.
Discovery of PTPσ inhibitor DJ001.
4.2.3. Small molecule regulators of MAPK signaling
Simply speaking, three primary classes of kinase ERKs, JNKs and p38MAPKs constitute mitogen activated protein kinases (MAPKs) family84. For HSC expansion, there have been many studies focusing on small molecules targeting MAPKs signaling, and great achievement has been achieved. All these studies suggested that targeting MAPKs may be a promising strategy for promoting HSC expansion.
4.2.3.1. p38 inhibitor
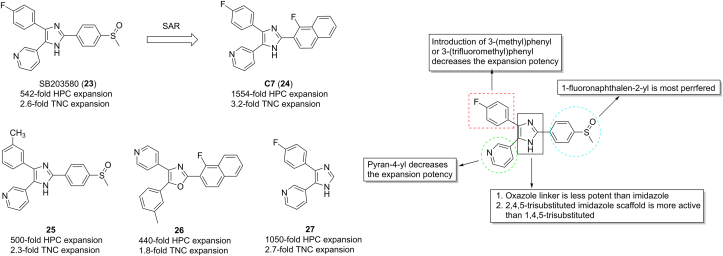
p38 signaling is involved in erythroid development and negatively regulates myeloid differentiation in normal conditions. Activation of p38 compromises HSC self-renewal capacity and affects quiescence state maintenance. Pharmacologic inhibition of p38 could enhance multilineage hematopoiesis recovery in myelodysplastic syndromes (MDSs)85. Inhibition of p38 by SB203580 (23) resulted in both hUCB CD133+ HSPCs and mouse Lin−Sca-1+c-Kit+ (LSK, enriched for HSCs) cells expansion and enhanced self-renewal and repopulating capacity (Fig. 11)86. Recently, Bari et al.87 established ∼50 azole molecules by SAR studies based on 23. Replacement of 4-methylsulfinylphenyl by 1-fluoronaphthalen-2-yl at C3 of core imidazole ring of 23 provided compound C7 (24, Fig. 11), which shows an over 1500-fold expansion of HSPC of unfractionated CB-MNC (mononucleated cell), 2.9-fold higher than that of 23. An over 1.7-fold HSPC expansion occurred in 24-treatment group relative to 23 despite similar repopulating capacity. Interestingly, 23 in combination with UM171 robustly promoted CD34+ HSPC expansion with at least 6-fold higher than 24 alone.
Figure 11.
Structures of 23‒27 and SAR summary of 2,4,5-trisubstituted imidazole p38 inhibitors.
SAR studies suggested that replacement of 4-fluorophenyl on imidazole ring with 3-(methyl)phenyl or 3-(trifluoromethyl)phenyl decreased activity, as exemplified by 25 (Fig. 11). In terms of imidazole core, oxazole almost lost activity, such as 26. In addition, 2,4,5-trisubstituted imidazole was better than 1,4,5-trisubstituted imidazole. At C5 of imidazole ring, pyran-4-yl was less active than pridine-3-yl. Conversion of sulfoxide phenyl with 1-fluoronaphthalen-2-yl provided 24, which is significant more active than 23. Removal of phenyl methyl sulfoxide yielded 27, which was about 2-fold more active in HSC expansion than 23.
4.2.3.2. JNK inhibitor
Of the mitogen activated protein kinases (MAPKs) family, the c-Jun N-terminal kinases (JNKs) consist of several exclusive members JNK1/2/384. JNK signaling over-activation is commonly present in cancer, inflammation and neurodegenerative diseases, and JNK pathway plays an important role for the normal hematopoiesis and development of leukemia88,89. Recently, investigators from Peking University used a potent JNKs inhibitor JNK-IN-8 (28, Fig. 15) to effectively expand human CB HSPC and its primitive subpopulations90,91. 28-treated HSCs resulted in a remarkable 3.88-fold increase in SRC frequency in primary recipients and could reconstitute secondary recipients for over 21 weeks. Early in 2011, Zhang et al.90 demonstrated that 28 is a potent selective JNKs covalent inhibitor. Among several families of kinase, 28 specifically inhibited JNKs of CMCG family. The introduction of flag methyl in 28 increased substantially selectivity over JNKs relative to its analog without methyl whose co-crystal structure with JNK3 (PDB 3V6S) suggested that acrylamide warhead (reactive groups for covalently targeting conserved residues) was covalently bound to the conserved Cys154 and the aminopyrimidine motif formed hydrogen bonds with the kinase hinge residue Met149. This “methyl effect” has been early observed with imatinib92.
Figure 15.
Structures of 28 and 38‒42.
4.2.4. p18 inhibitor
As a member of INK4 CKI (cyclin-dependent-kinase inhibitor) family, p18 is responsible for regulating cell cycle via inhibition of downstream CDK4/6 signaling. Many studies have demonstrated deletion of p18 resulted in enhanced self-renewal capacity and robust ex vivo expansion of HSC without depletion in a long period93, 94, 95, 96. Others also indicated a special role for p18 in preserving HSC quiescence status97. Therefore, Gao et al.95 performed an in silico virtual screening for p18 inhibitors based on computational predication of binding pocket of p18/CDK6 complex crystal structure (PDB 1G3N) and further confirmed by in vitro and in vivo assays (Fig. 12). Compound P18IN011 and P18IN003 (29 and 37, Fig. 13) were identified as hit compounds from library. Strong hydrogen bonds and hydrophobic interactions were observed between 29 and specific residues in interface of binding site of p18 and CDK6 by docking studies, suggesting 29 likely blocks or interferes the inhibition of p18 towards CDK6. Both 29 and 37 could support ex vivo expansion of functional mouse HSCs with ED50 of 3.61 and 6.12 μmol/L, respectively, and enhanced engraftment capacity of treated-HSC in primary and secondary recipients. They also found that these p18 inhibitors did not influence the cell cycle status and apoptosis but promoted self-renewal division.
Figure 12.
Identification and optimization of the first specific p18 inhibitor for HSC expansion.
Figure 13.
Structures and activities of 29‒37, and SAR summary of p18 inhibitors.
Based on previous identification of p18 as lead compounds, they further used medicinal chemistry methodology to obtain optimized p18 inhibitors through a virtual screening, SAR and validation process98. Structural modification yielded three series compounds with four representative compounds possessing ED50 values less than about 10 nmol/L. SAR studies suggested that benzothiazine moiety is not the key group and lactone ring appears more potent especially bearing iso-propyl group (30‒32, Fig. 13). Sulfonyl is essential for H-bonding interaction and phenyl provides suitable distance between adjacent groups, which is important for extensive H-bonding interaction and hydrophobic interactions in conserved pocket of p18/CDK6. Also, replacement of acetamide group by other groups such as methyl group (33, Fig. 13) brings about 5-fold increase in activity relative to 31, indicating acetamide group is not crucial. Although benzoic acid 34 exhibits similar activity with 31, its sodium salt 35 shows strongest HSC expansion activity with an ED50 value of 5.21 nmol/L, which is over 690-fold active than 29 and has better solubility properties. Interestingly, activity of these compounds is very sensitive to configuration when replacement of benzothiazine ring with amino acid such as tyrosine (36, Fig. 13) showed that R-enantiomer outcompeted S- over 1000-fold. Compound 35 could robustly ex vivo expand human CB HSC and enhance about three times repopulating capacity. Furthermore, these expanded LT-HSCs enabled stable engraftment in SCID mice for at least 4 months without any cytotoxicity and leukemia potential96,98.
4.2.5. BET inhibitor
Bromodomain-containing proteins (BCPs) are able to regulate transcriptional process via recognition of epigenetic modification. Recently, Hua et al.99 screened 10 small molecules targeting BCPs, with a specific bromodomain and extra-terminal motif (BET) domain inhibitor CPI-203 (38, Fig. 15) being identified to expand human CB HSC and promote long-term repopulating capacity in vivo. It is interesting that CPI-203 also promotes megakaryocyte expansion, as previously reported.
4.2.6. Small molecule regulators of energy metabolism
As a member of nuclear hormone receptors, peroxisome proliferator-activated receptor γ (PPAR-γ) is an active regulator in glycometabolism and is involved in many cancers and metabolic diseases100. Recently, a study by Guo and co-workers101 revealed that PPAR-γ inhibition, by its antagonist GW9662 (39, Fig. 15), resulted in robust ex vivo expansion in CB HSPCs. These expanded HSPCs could repopulate primary and secondary NSG mice recipients and exhibited a 5-fold higher frequency of SRC relative to control. In addition, FBP-1 expression was also decreased in CB HSCs in the presence of 39, along with a host of differentiation-related genes. Also, glycolysis activity was enhanced following 39 treatment and expansion effect was abrogated by removal of glucose. These results suggested elevated glycolysis level via PPAR-γ–FBP-1 axis promoted HSC expansion and inhibited differentiation. Of note, another potent PPAR-γ antagonist T0070907 (40) and a specific FBP-1 inhibitor MB05032 (41) also promoted ex vivo expansion of CB HSC (Fig. 15)101.
An X-ray cocrystal structure of 39 in complex with PPAR-γ ligand binding domain (PDB 3B0R) suggested a covalent binding mode in which the phenyl of 39 is covalently bound to the Cys285 through a SNAr displacement (Fig. 14)102. In addition to covalent interaction, the amide group of the compound forms three hydrogen bonds with Cys285, Ser289 and Tyr327. The nitro group also interacts with two histones through hydrogen bonds. These extensive hydrogen bonds help the compound recognize and locate the polar pocket to specifically form covalent interaction with Cys285.
Figure 14.
X-ray cocrystal structure of compound 39 covalently bound to the PPAR-γ ligand binding domain (PDB 3B0R). The compound (yellow sticks) is covalently attached to Cys285 of PPAR-γ protein in a SNAr manner. Extensive hydrogen bonds (yellow dashed line, distance < 3.5 Å) are observed between the compound and several residues (cyan sticks).
Liu et al.103 reported that a selective mitochondrial phosphatase Ptpmt1 inhibitor alexidine dihydrochloride (42, Fig. 15) increased functional HSC numbers and promoted HSC long-term reconstitution capacity via activation of AMPK signaling. Furthermore, metformin was also reported to maintain HSC through similar mechanism with 42.
4.2.7. OCT4 agonist
Transcriptional factor octamer binding protein 4 (OCT4) belongs to the POU (PIT/OCT/UNC) homeodomain protein family. OCT4 is responsible for maintaining self-renewal and pluripotency of human embryonic stem cells (ESCs) via a modulation circuitry involving SOX2 and NANOG104. Also of note, in some conditions, OCT4 allows the direct conversion from fibroblast to hematopoietic progenitors105. In 2016, researchers used a known OCT4 agonist OAC1 (43, Fig. 16) to support robust ex vivo expansion of human CB CD34+ cells through downstream HOXB4 suppression106. A 2.8-fold increase in number of the most primitive HSCs was observed. Treatment of 43 also resulted in a 6.3-fold increase in SRC numbers which could long-term repopulate in NSG mice and secondary recipients without any leukemia potential.
Figure 16.
Structures of 43‒47.
4.2.8. c-MPL agonist
Thrombopoietin (TPO) receptor c-MPL plays an important role in HSC self-renewal and expansion. Nashino et al.107 reported a small molecule c-MPL agonist NR101 (44, Fig. 16) which induced more than two folds expansion of CD34+ subpopulation and about two-fold increase in SRC numbers in culture. NR-101 resulted in sustaining activation STAT5 and accumulation of HIF-1α protein.
Eltrombopag (45, Fig. 16) is a second generation marketed small molecule for treatment of chronic immune thrombocytopenia (ITP) and severe aplastic anemia (SAA) via binding and activating c-MPL108. A recent study by Kao and coworkers108 gave insight into novel mechanism that eltrombopag enhanced hematopoiesis in a TPO-independent fashion but rather mediated by its iron chelation effect. In their study, eltrombopag reduced chelatable iron concentration in HSC, associated with decreased cellular reactive oxygen species (ROS) levels, subsequently to elicit relevant genomic profile alteration such as energy metabolism. They found HIF-1α was also increased in the context of low chelatable iron, which is an important regulator for HSC maintenance and self-renewal. Interestingly, the cation chelation effect for eltrombopag is similar to that of TEPA, which has been on clinical trials as an effective copper chelator for enhancing HSC expansion as previously described.
4.2.9. GSK-3 inhibitor
GSK-3 is responsible for phosphorylation of a variety of protein, and therefore plays a pivotal role for signaling transduction in many pathways critical for HSC fate determination, such as Wnt, Notch and Hedgehog signaling109. Direct inhibition on GSK-3 elicits complicated downstream molecule events and positively regulates HSC self-renewal or maintenance109. Great efforts have been made to explore potent GSK-3 inhibitors and cooperation with other agents for valid HSC expansion in culture. Interestingly, prior to identification as a GSK-3 inhibitor, lithium has been used to ameliorate hematopoiesis and enables increase in CD34+ cells and platelets in patients' peripheral blood110. Strategies of targeting GSK-3 using small molecules have been effective and been explored a lot.
Ko et al.111 reported that treatment with BIO (46, Fig. 16) prolonged cell cycle and increased numbers of human CB HSC. Up-regulated CDK inhibitor p57 and down-regulated cyclinD1 may account for delayed cell cycle upon treatment with 46. Although pharmacological inhibition on GSK-3 favored embryonic stem cells maintenance via activation of Wnt signaling, 46 did not modulate Wnt pathway but rather regulated Notch and Angpt1/Tie2 to promote HSC expansion and repopulating capacity in NSG mice.
Trowbridge et al.109 reported that in vivo administration of a GSK-3 inhibitor CHIR99021 (47, Fig. 16) enhanced hematopoietic recovery in NSG mice, as a result, indicating a role for CHIR99021 in HSC maintenance and expansion. They employed combination of PI3K/AKT signaling agonist insulin and 47 to pharmacologically repress differentiation and promote self-renewal, yielding 100-fold transplantable HSCs. Later in 2012, Huang et al.112 followed reporting another combination of mTOR inhibitor rapamycin (54, Fig. 18) and 47, which supported maintenance of functional LT-HSCs and improved their repopulating ability in NSG mice. Arrest on cell cycle status without affecting apoptosis or death accounted for this expansion. Similarly, Li and colleagues113 reported using a combination of 47 and a p38 inhibitor SB203580 could promote human UCB HSCs expansion in vitro.
Figure 18.
Structures of natural products and endogenous substances for HSC expansion.
4.2.10. Epigenetic modulators
Manipulation on histone protein or genomic DNA by small molecules is capable of regulating chromatin status, which is critical for DNA accessibility for replicate and transcription114,115. Modification by either de/methylation or de/acetylation greatly affects the interaction between adjacent nucleosome units by alteration of charge property of residues. Genomic alteration by epigenetic modification plays an important role for HSC cell fate decision116,117. All these modifications are “writed” or “erased” by corresponding enzymes. Regulating these enzymes with small molecules for HSC expansion has been really remarkable these years.
4.2.10.1. Histone acetyltransferases (HATs) inhibitor
Nishino et al.118 screened from 92 natural products and first found garcinol and isogarcinol (55 and 56, respectively, Fig. 18) were capable of expanding HSC. Both compounds are non-selective histone acetyltransferases (HATs) inhibitors. Garcinol enabled over 4.5-fold expansion of human CB HSC, and a 7.4-fold expansion was observed with isogarcinol treatment. In addition, garcinol increased SRC numbers in NSG mice by 2.5-fold. Gene expression profile showed enhanced expression of hepatic leukemia factor (HLF) and reduced p53 level, both of which are important for self-renewal119,120, despite no changes in well-established self-renewal genes, such as HOXB4, BMI1, GATA2, Notch1, p21, p27, MYC, EGR, and EVI-1.
4.2.10.2. Histone deacetylase (HDAC) inhibitor
As an HDAC inhibitor, valproic acid (VPA, 48, Fig. 17) has been widely used for treatment of epilepsy and other neurologic diseases. In 2005, two research groups both reported that VPA induced HSC proliferation and self-renewal and delayed cell cycle without inducing apoptosis, more importantly, maintained HSC both in short-term and long-term121,122. Hoffman's team demonstrated that HSC expansion induced by VPA treatment was accompanied by phenotypic transformation and cellular reprogramming of HSC123, 124, 125, 126. VPA treatment promoted HSC multi-lineage reconstitution in serial transplantation in NSG mice126, 127, 128. VPA promoted HOXB4 and AC133 expression and histone acetylation level on their regulatory sites121,122. In addition, combination of VPA and GSK-3 inhibitor lithium preserved HSC stemness, repressed differentiation and enhanced hematopoietic potential129.
Figure 17.
Structures of 48‒53.
Recently, Huang et al.130 used a selective HDAC5 inhibitor LMK235 (49, Fig. 17) to promote human CB HSC homing. HDAC5 inhibition promoted p65 acetylation to result in CXCR4 upregulation in HSC surface, favoring HSC homing as well as engraftment to bone marrow niche via SDF-1/CXCR4 axis.
4.2.10.3. DNA methyltransferase inhibitor
Early studies have indicated combination of 5azaD (50, a DNA methyltransferase inhibitor, Fig. 17) and trichostatin A (TSA, 57, a histone deacetylase inhibitor, Fig. 18) altered HSC fate determination in vitro131. In 2006, Araki et al.132 confirmed the role of 50 and 57 for enhancing CB HSC repopulating capacity in NSG mice. Later in 2007, they suggested that combination of treatment of 50 and 57 led to slow rate of cell division and cell cycle of human HSC, and these compounds preserved HSC hematopoietic potential in immunodeficient mice even if undergoing serial cell division133,134. Self-renewal implicated genes such as HOXB4, BMI1, Notch1 and GATA2, and cell cycle inhibitors p21 and p27, were all found to up-regulated in cells upon 50/57 treatment.
4.2.11. Cysteine protease inhibitor
Over the course of HSC expansion in culture, apoptosis is an important cause for HSC pool depletion which leads to subsequent expansion failure. Sangeetha and coworkers135 employed two cysteine protease inhibitors zVADfmk and zLLYfmk (51 and 52, Fig. 17) to suppress HSC apoptosis and observed increased CB CD34+ cell numbers and enhanced their engraftment activity. This anti-apoptosis effect was possibly mediated by bcl-2, caspase-3 and Notch signaling.
4.2.12. Aldehyde dehydrogenase (ALDH) inhibitor
Aldehyde dehydrogenase (ALDH) is responsible for converting retinol (vitamin A) to retinoic acid and is highly enriched in HSCs. Chute et al.136 demonstrated that ALDH plays an important role for HSC differentiation and an ALDH inhibitor DEAB (53, Fig. 17) induced robust ex vivo expansion of CB HSCs and 3.4-fold increase in SRC numbers relative to uncultured counterparts. 53 delayed HSC differentiation and promoted self-renewal via inhibiting retinoic acid production. Consequently, modulation on retinoid signaling may be a promising strategy for HSC expansion.
4.3. Natural products and endogenous substances
4.3.1. Natural products
Naturally occurring products are an important source for exploring novel agents for ex vivo HSC expansion. While natural active products are to be blamed for over-extensive biological activities and elusive molecular mechanisms, novel molecular scaffolds and exclusive effect from natural products have been at times to give researchers insight into drug discovery. In addition to HATs inhibitor garcinol and HDAC inhibitor trichostatin A described above, echinochrome A (Ech A, 58, Fig. 18) has recently been reported for ex vivo HSC expansion137. In this study, 58 induced 1.33-fold expansion of CD34+ cells and 1.7-fold CFUs increase in culture over control via Src/Lyn activation, ROS inhibition and activation of p110δ in PI3K/AKT. Resveratrol (59, Fig. 18) is a natural polyphenol and has broad-spectrum biological functions and health benefits. Apart from promoting hematopoiesis in vivo138,139, resveratrol also improved ex vivo expansion of CB HSC and enhanced engraftment of expanded HSCs in primary and secondary NSG mice recipients140.
Similar to Ech A, cafeic acid phenethly ester (CAPE, 60, Fig. 18) also acts as an antioxidant, derived from propolis. 60 promoted ex vivo CB-HSCs expansion, homing and engraftment in vivo, mediated by up-regulation of HIF-1α and vascular endothelial growth factor-A (VEGF-A)141,142.
Recently, researchers from Thailand reported a diarylheptanoid compound ASPP 049 (61) isolated from Curcuma comosa with ex vivo expansion activity and repopulating capacity in vivo143. RNA-seq analysis of expanded HSCs suggested Hippo pathway was most affected.
Chrysin (5,7-dihydroxyflavone, 62, Fig. 18) is a naturally occurring flavone, mainly found in passion flowers, honey, and propolis. It acts as an antioxidant to exert anti-tumor effects in several cancers, with several signaling been affected, such as MAPK and PI3K/AKT144. Screened from 85 antioxidant small molecules, chrysin was identified to expand HSC effectively and promote rapid engraftment in NOG mice, which might be attributed to its antioxidant-effect145. All discussed natural products are summarized in Table 3.
4.3.2. Endogenous substances
5-hydroxytryptamine (5-HT, 63, Fig. 18), also termed serotonin, is an endogenous neurotransmitter widely involved in nerve conduction. Yang et al.146 reported 5-HT enabled CB HSCs expansion in culture and increase in CFUs. More importantly, long-term repopulating capacity of these expanded HSCs was augmented. Mechanistic studies suggested 5-HT inhibits apoptosis and protects from mitochondria damage.
α-Tocopherol (64, Fig. 18) is one of vitamin E compound family members. Nogueira-Pedro et al.147 reported its special role in enhancing murine HSC activity in vivo, however, accompanied by ERK1/2 mediated differentiation. α-Tocopherol treatment induced 2-fold expansion in murine LSK cells.
As a derivative of vitamin A, all-trans retinoic acid (ATRA, 65, Fig. 18) is an endogenous agonist of RA receptor. Purton et al.148,149 reported ATRA enhanced mouse HSC repopulating and self-renewal ability involving Notch1 and HOXB4 activation, whereas contradicting effect occurred when ATRA treatment could reverse ALDH inhibition-induced enhancement of HSC self-renewal capacity136.
5. Potential targets for HSC ex vivo expansion
5.1. MLLT3
Ubiquitous histone modification process plays an essential role for DNA transcription, repair and replication by means of manipulation of DNA activity state150. MLLT3, also known as AF9, recognizes and binds to histone H3K9 acetylation site in transcription start sites via its YEATS domain, and recruits DOT1L to methylate H3K79 lysine for regulating target gene expression (Fig. 19A)151,152. A recent study indicated that MLLT3 is enriched in human HSC and is responsible for human HSC maintenance and repopulating capacity through promoting transcription of self-renewal related genes14. MLLT3-overexpression (MLLT3-OE) significantly enhanced CB HSC ex vivo expansion in either stromal stem cell co-culture condition or culture supplemented with SR1 and UM171. Limited dilution assay exhibited a 12.5-fold increase in transplantable HSC of MLLT3-OE HSCs relative to that of uncultured HSCs, and 5.2-fold higher than control vector. Sustaining MLLT3 overexpression in CB HSC in culture promoted stable multilineage reconstitution in primary and secondary recipients. Moreover, MLLT3-OE did not result in any lineage differentiation bias, aberrant proliferation or apoptosis, suggesting its well safety profile.
Figure 19.
(A) Recognition mode of AF9 YEATS domain to H3K9 peptide (green sticks) (PDB 4TMP). (B) Protein surface of YTHDF2 in complex with m6A mononucleotide and close up view of interaction between m6A mononucleotide and residues of YTHDF2 protein (PDB 4RDN). H-bonds are depicted in red dashed line, and key amino acid residues are labeled in black text.
Targeting this essential self-renewal regulator with specific small molecules in culture conditions may give hope for stable ex vivo HSC expansion.
5.2. Musashi-2
RNA binding protein-mediated post-transcription modification occurred in many aspects during the course of HSC self-renewal and differentiation, affecting a range of relevant mRNA translation153. RNA binding protein Musashi-2 has been implicated as an oncogenic factor in many types of cancer, for example, involvement of Musashi-2 in AML patients was usually associated with poor prognosis154. In normal HSCs, Musashi-2 is an important regulator for HSC maintenance and self-renewal155,156. Musashi-2 deficient mice were accompanied by hematopoiesis defect, and deletion of Musashi-2 in HSC led to loss of repopulating capacity155,156.
In 2016, Rentas and colleagues157 identified the role for Musashi-2 in HSC self-renewal, whose overexpression resulted in 17- and 23-fold increase in ST-HSC and LT-HSC, respectively. Musashi-2 overexpression promoted human CB HSPCs self-renewal and their engraftment in NSG mice, in contrast to Musashi-2 knockdown. Mechanistic studies suggested that Musashi-2 regulated post-transcriptional processes via inhibition of AHR signaling for promoting HSC self-renewal and ex vivo expansion, similar to SR1.
In addition to targeting on upstream signaling for Musashi-2 overexpression, another potential strategy is to activate RNA binding pocket or allosteric site with small molecule. There have been reported small molecules for targeting its RNA binding pocket158,159, which could provide some insight into developing potential Musashi-2 agonist.
5.3. YTHDF2
In all eukaryotes, N6-methyladenosine (m6A) is the most abundant epigenetic modification occurring in numerous mRNAs160. As a post-transcriptional modification way, m6A is associated with HSC specification in embryogenesis161, differentiation162, as well as leukemogenesis163. m6A regulates mRNA translation and decay by virtue of “writer” METTL3/METTL14, “eraser” FTO/ALKBH5 and “reader” YTHDF1–3164. Among m6A readers, YTHDF2 could recognize many m6A tagged mRNAs by its conserved aromatic cage165,166 and degrade them through subsequent recruitment of deadenylase complex167. Recent studies disclosed that depletion of YTHDF2 stabilized those m6A-marked mRNAs encoding for transcription factors which are critical for self-renewal in mouse HSPCs and human UCB HSCs, and as a result promoted ex vivo expansion of HSCs without any noticeable lineage bias or leukemic potential13,168. In addition, researchers from the University of Edinburgh indicated that YTHDF2 is dispensable for normal HSC activity but required for leukemic stem cells in AML169. These investigations demonstrate that YTHDF2 would be a promising target for obtaining sufficient transplantable HSCs in vitro, whereas the first YTHDF2 small molecule inhibitor has not yet been identified.
Crystal structure of YTHDF2 in complex m6A mononucleotide may give some insight into design of its inhibitors (Fig. 19B). YTHDF2 recognizes m6A through a conserved aromatic cage which is formed by three aromatic amino acid residues Trp432, Trp486 and Trp491. Three hydrogen bonds are formed between adenosine ring moiety and Tyr418, Asp422 and Cys433 residues. π–π stacking also generates between the adenosine ring and adjacent aromatic residues. To inhibit YTHDF2, heterocycle bearing sufficient H-bond donors is required for molecule–protein interaction. As the aromatic pocket is small and hydrophobic, substitutions on heterocycle are expected to small and nonpolar.
5.4. RET
The neurotrophic factor receptor RET was reported to express in HSC surface and mediate HSC survival, normal differentiation potential and repopulating capacity170. Activation of RET with glial derived neurotrophic factor (GDNF) family ligands (GFLs), which are secreted in HSC niche, promotes HSC expansion in vitro and transplantation efficiency in vivo170,171. Moreover, upon RET activation, a lot of signaling pathways, such as p38-MAPK, CREB, AKT, ERK1/2, NFκB and p53, were driven to collectively improve HSC expansion and repopulating activity. These findings may inspire the exploration of RET small-molecule agonists for human HSC ex vivo expansion and cord blood transplantation.
6. Summary and perspectives
As an alternative source of HSC, cord blood has several advantages such as easy availability, tolerance of partial HLA-mismatch and low incidence of chronic GVHD5. However, low stem cell dose limits the use of CB unit for transplantation to pediatric patients6. Ex vivo HSC expansion is an effective method for obtaining sufficient HSCs. Currently, although extensive efforts have been devoted to every approach, stable HSC expansion in vitro have been yet unattainable. The biggest hurdle laid ahead ex vivo HSC expansion is to balance self-renewal and differentiation in culture. Little is known about the definite molecule mechanisms of HSC self-renewal and why they fail in vitro. The answer for these queries may be found where HSC lives. Briefly, HSC lives in a bone marrow microenvironment supported by various cells. Diverse niche cell types and growth factors are involved in regulating HSC self-renewal and differentiation17,21,22. Stable and sustaining ex vivo HSC expansion may be available when all niche components and their molecular mechanism are fully elucidated.
In fact, the balance between self-renewal and differentiation in vivo is strictly controlled by delicate extracellular molecule cues and complex intracellular signaling pathways such as two conserved Wnt and Notch signaling26,27. Rapid self-renewal or inadequate differentiation pose risks of leukemic transformation, while inadequate self-renewal or rapid differentiation lead to HSC pool exhaustion53. Early to about twenty years ago, genetic manipulation of HSC such as HOXB4 gene led to hundred folds increase in self-renewal capacity172. Nevertheless, it is risky for patients to receive these HSCs which may lead to leukemia. As a result, transient and removable activation by extrinsic agents is desirable for ex vivo HSC expansion53. Early attempts begin with identification of a variety of cytokines or growth factors and cocktails of them. Combination of SCF, TPO, FLT-3L and IL-6 is a commonly used cytokine cocktail for HSC expansion culture. In addition to these classic cytokines, more developmental growth factors were identified for HSC expansion such as FGFs, IGFs, IGFBPs, Angptls, Shh, BMP-4, Wnt proteins and Notch ligands53. All of them support considerable HSC expansion while maintain long-term repopulating capacity. In 2010, Delaney et al.50 reported clinical outcomes of a phase I trial of Notch ligand-expanded CB unit. Rapid neutrophil recovery (main indicator for graft engraftment) was observed with a median time of 16 days. However, attempts to explore cytokines or growth factors for HSC expansion are overall unsuccessful because inevitable differentiation occurs commonly after transient expansion, which suggests additional agents are necessary53.
Compared to cytokines, small molecules have significant advantages such as having definite targets and effects, easily manipulated in vitro and low cost, which make them valuable candidate agents for clinical therapy in the future. As a result, people turn their sights to promising small molecules and great achievements have been made. Since the first small molecule TEPA was evaluated preclinically and in a phase I clinical trial during the first decade of this century, the last decade have seen rapid discovery and development of more potent and efficacious small molecules, such as SR1, PGE2, nicotinamide and UM171. All these small molecules significantly expand HSC numbers in CB unit and provide rapid hematopoietic recovery in patients compared to those unmanipulated units, with median time to neutrophil recovery generally shorter than 15 days and without any serious adverse events. However, rapid engraftment appears to not benefit disease-free survival, possibly expanded HSCs losing long-term reconstitution capacity in vivo.
Early attempts and exploration for small molecules such as GSK-3 inhibitors, epigenetic modulators laid out the foundation for finding more potent small molecules and novel targets109,111,132. Some endogenous substances such as 5-HT, α-tocopherol, and retinoic acid were all reported having HSC expansion activity146,147,149. In addition, many natural products such as garcinol, echinochrome A, and resveratrol were used to expand HSC and pleasant results were obtained118,137,139.
However, lineage commitment at the cost of HSC depletion commonly occurs when targeting on single signaling or target alone. Thus, regulation of single signaling or target may be not sufficient for multi-dimensional regulation of cellular processes to obtain stable ex vivo HSC expansion, because complex molecule signaling orchestrates HSC fate decision. For example, HSCs expanded by VPA underwent phenotypic transformation and cellular reprogramming involving mitochondrial remodeling and p53 activation123. As an integration of complex cellular events is required for ex vivo HSC expansion, it is possibly that the optimal condition for HSC expansion is combination of several small molecules. A cocktail of SR1, CAY10433 and VPA was reported to support ∼20-fold expansion of CD34+ cell and retain multilineage potential of these HSC in vivo173. Similar result was also observed in a cocktail of CHIR-99021, forskolin and OAC1174. A combination of GSK-3β inhibitor CHIR99021 and a p38 inhibitor SB203580 was also used to robustly expand HSC in vitro113.
As is so successful that small molecules have made great landmark for HSC expansion, however, currently almost all clinical trials restricted to evaluate two CB units (one expanded, the other unmanipulated). Therefore, in some context, effect of small molecules may be elusive, possibly due to the unmanipulated CB unit. In fact, in many cases, long-term engraftment derived from the expanded unit completely lost after several months. There have been two reasonable explanations50, 51, 52: (1) HSCs in the expanded unit are only responsible for short-term repopulation, and long-term HSCs gradually deplete in vivo. (2) Immune cells present in the unmanipulated unit produce an immune rejection against the expanded unit, and the former becomes the winning unit. Moreover, in some studies63,78, small molecules appeared not to produce significant positive outcomes in terms of overall survival and disease-free overall survival, despite rapid neutrophil recovery or low incidence of GVHD. Clinical studies based on single expanded CB unit have proceeded slow and these outcomes are not desirable8,9,64. We suppose these issues will be handled well as we get a comprehensive knowledge about HSC in the future. Perhaps, the true answer for ex vivo HSC expansion is not small molecules alone, but a combination of multiple approaches which need multidisciplinary struggle.
Applications of new technologies and methodologies have allowed for high efficiency in discovery of novel small molecules and targets. Additionally, the discovery and development of small molecules for HSC expansion really requires an integration of interdisciplinary cooperation, such as chemistry, biology and computer science. In recent years, many novel and potent small molecules have been identified by means of new technologies, such as high throughput screening (HTS), computer-aided drug design (CADD) and artificial intelligence (AI). Furthermore, prompt developments in synthetic chemistry have benefited and allowed for large-scale preparation of desired compound for further animal experiment and clinical trials, so did combinatorial chemistry enable rapid and convenient establishment of a huge library of small molecules for HTS. Traditional medicinal chemistry strategies involving SAR studies and rational drug design provide optimization strategy for lead compound to obtain better potency and druggable properties. In terms of HSC expansion, as exemplified by eltrombopag, drug repurposing strategy enables the availability of clinical approved drug for new diseases or therapies with significantly reduced time-waste and cost. Covalent inhibitors such as JNK-IN-8 and GW9662 were reported to support HSC expansion via covalent interaction with targets. p18 inhibitors were identified by in silico screening based on known p18/CDK6 complex crystal structure and optimized by SAR studies95,98. Solubility of 15-PGDH inhibitor SW033291 was improved by 10,000-fold through SAR studies79,81. In addition, several novel and safe targets for ex vivo HSC expansion have been reported recently, such as YTHDF2 and MLLT313,14.
The field of HSC expansion has enjoyed a banner decade, with a number of potent small molecules and several novel biological targets for ex vivo expansion of HSC intensively developed. It is conceivable that, as a branch of regenerative medicine, sufficient HSCs expanded by small molecules or other approaches will be clinical availability for treatments of human diseases in the near future.
Acknowledgments
This study was supported by National Natural Science Foundation of China (82073701, 31900687), Natural Science Foundation of Jiangsu Province (SBK2019040713, China) and the Project Program of State Key Laboratory of Natural Medicines, China Pharmaceutical University (SKLNMZZ202013, China). This study was also supported by Jiangsu Key Laboratory of Drug Design and Optimization, China Pharmaceutical University (No. 2020KFKT-5, China), and “Double First-Class” University Project (CPU2018GF04, China).
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Author contributions
Conception and design: Jiaxing Li, Xiao Wang, Peng Yang. Acquisition, analysis and integration of data: Jiaxing Li, Xiao Wang, Jiayu Ding, Wenjian Min, Yasheng Zhu, Wenbing Kuang, Kai Yuan, Chengliang Sun, Peng Yang. Writing-review and editing: Jiaxing Li, Xiao Wang, Peng Yang. Administrative, technical, or material support: Xiao Wang, Peng Yang.
Conflicts of interest
The authors declare no competing financial interests.
References
- 1.Laurenti E., Gottgens B. From haematopoietic stem cells to complex differentiation landscapes. Nature. 2018;553:418–426. doi: 10.1038/nature25022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Booth C., Gaspar H.B., Thrasher A.J. Treating immunodeficiency through HSC gene therapy. Trends Mol Med. 2016;22:317–327. doi: 10.1016/j.molmed.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Riviere I., Dunbar C.E., Sadelain M. Hematopoietic stem cell engineering at a crossroads. Blood. 2012;119:1107–1116. doi: 10.1182/blood-2011-09-349993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gragert L., Eapen M., Williams E., Freeman J., Spellman S., Baitty R., et al. HLA match likelihoods for hematopoietic stem-cell grafts in the U.S. registry. N Engl J Med. 2014;371:339–348. doi: 10.1056/NEJMsa1311707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen S., Roy J., Lachance S., Delisle J.S., Marinier A., Busque L., et al. Hematopoietic stem cell transplantation using single UM171-expanded cord blood: a single-arm, phase 1–2 safety and feasibility study. Lancet Haematol. 2020;7:134–145. doi: 10.1016/S2352-3026(19)30202-9. [DOI] [PubMed] [Google Scholar]
- 6.Ballen K.K., Gluckman E., Broxmeyer H.E. Umbilical cord blood transplantation: the first 25 years and beyond. Blood. 2013;122:491–498. doi: 10.1182/blood-2013-02-453175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wagner J.E., Jr., Eapen M., Carter S., Wang Y., Schultz K.R., Wall D.A., et al. One-unit versus two-unit cord-blood transplantation for hematologic cancers. N Engl J Med. 2014;371:1685–1694. doi: 10.1056/NEJMoa1405584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stiff P.J., Montesinos P., Peled T., Landau E., Goudsmid N.R., Mandel J., et al. Cohort-controlled comparison of umbilical cord blood transplantation using carlecortemcel-L, a single progenitor-enriched cord blood, to double cord blood unit transplantation. Biol Blood Marrow Transplant. 2018;24:1463–1470. doi: 10.1016/j.bbmt.2018.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Lima M., McMannis J., Gee A., Komanduri K., Couriel D., Andersson B.S., et al. Transplantation of ex vivo expanded cord blood cells using the copper chelator tetraethylenepentamine: a phase I/II clinical trial. Bone Marrow Transplant. 2008;41:771–778. doi: 10.1038/sj.bmt.1705979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boitano A.E., Wang J., Romeo R., Bouchez L.C., Parker A.E., Sutton S.E., et al. Aryl hydrocarbon receptor antagonists promote the expansion of human hematopoietic stem cells. Science. 2010;329:1345–1348. doi: 10.1126/science.1191536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peled T., Shoham H., Aschengrau D., Yackoubov D., Frei G., Rosenheimer G.N., et al. Nicotinamide, a SIRT1 inhibitor, inhibits differentiation and facilitates expansion of hematopoietic progenitor cells with enhanced bone marrow homing and engraftment. Exp Hematol. 2012;40:342–355. doi: 10.1016/j.exphem.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 12.Fares I., Chagraoui J., Gareau Y., Gingras S., Ruel R., Mayotte N., et al. Cord blood expansion. Pyrimidoindole derivatives are agonists of human hematopoietic stem cell self-renewal. Science. 2014;345:1509–1512. doi: 10.1126/science.1256337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Z., Qian P., Shao W., Shi H., He X.C., Gogol M., et al. Suppression of m6A reader Ythdf2 promotes hematopoietic stem cell expansion. Cell Res. 2018;28:904–917. doi: 10.1038/s41422-018-0072-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Calvanese V., Nguyen A.T., Bolan T.J., Vavilina A., Su T., Lee L.K., et al. MLLT3 governs human haematopoietic stem-cell self-renewal and engraftment. Nature. 2019;576:281–286. doi: 10.1038/s41586-019-1790-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mikkola H.K., Orkin S.H. The journey of developing hematopoietic stem cells. Development. 2006;133:3733–3744. doi: 10.1242/dev.02568. [DOI] [PubMed] [Google Scholar]
- 16.Seita J., Weissman I.L. Hematopoietic stem cell: self-renewal versus differentiation. Wiley Interdiscip Rev Syst Biol Med. 2010;2:640–653. doi: 10.1002/wsbm.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pinho S., Frenette P.S. Haematopoietic stem cell activity and interactions with the niche. Nat Rev Mol Cell Biol. 2019;20:303–320. doi: 10.1038/s41580-019-0103-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilkinson A.C., Igarashi K.J., Nakauchi H. Haematopoietic stem cell self-renewal in vivo and ex vivo. Nat Rev Genet. 2020;21:541–554. doi: 10.1038/s41576-020-0241-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamamoto R., Morita Y., Ooehara J., Hamanaka S., Onodera M., Rudolph K.L., et al. Clonal analysis unveils self-renewing lineage-restricted progenitors generated directly from hematopoietic stem cells. Cell. 2013;154:1112–1126. doi: 10.1016/j.cell.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 20.Eaves C.J. Hematopoietic stem cells: concepts, definitions, and the new reality. Blood. 2015;125:2605–2613. doi: 10.1182/blood-2014-12-570200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morrison S.J., Scadden D.T. The bone marrow niche for haematopoietic stem cells. Nature. 2014;505:327–334. doi: 10.1038/nature12984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boulais P.E., Frenette P.S. Making sense of hematopoietic stem cell niches. Blood. 2015;125:2621–2629. doi: 10.1182/blood-2014-09-570192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumar S., Geiger H. HSC niche biology and HSC expansion ex vivo. Trends Mol Med. 2017;23:799–819. doi: 10.1016/j.molmed.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coutu D.L., Kokkaliaris K.D., Kunz L., Schroeder T. Three-dimensional map of nonhematopoietic bone and bone-marrow cells and molecules. Nat Biotechnol. 2017;35:1202–1210. doi: 10.1038/nbt.4006. [DOI] [PubMed] [Google Scholar]
- 25.Nusse R., Clevers H. Wnt/β-catenin signaling, disease, and emerging therapeutic modalities. Cell. 2017;169:985–999. doi: 10.1016/j.cell.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 26.Staal F.J., Chhatta A., Mikkers H. Caught in a Wnt storm: complexities of Wnt signaling in hematopoiesis. Exp Hematol. 2016;44:451–457. doi: 10.1016/j.exphem.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 27.Pajcini K.V., Speck N.A., Pear W.S. Notch signaling in mammalian hematopoietic stem cells. Leukemia. 2011;25:1525–1532. doi: 10.1038/leu.2011.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Siebel C., Lendahl U. Notch signaling in development, tissue homeostasis, and disease. Physiol Rev. 2017;97:1235–1294. doi: 10.1152/physrev.00005.2017. [DOI] [PubMed] [Google Scholar]
- 29.Blank U., Karlsson G., Karlsson S. Signaling pathways governing stem-cell fate. Blood. 2008;111:492–503. doi: 10.1182/blood-2007-07-075168. [DOI] [PubMed] [Google Scholar]
- 30.Loh K.M., van Amerongen R., Nusse R. Generating cellular diversity and spatial form: Wnt signaling and the evolution of multicellular animals. Dev Cell. 2016;38:643–655. doi: 10.1016/j.devcel.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 31.Richter J., Traver D., Willert K. The role of Wnt signaling in hematopoietic stem cell development. Crit Rev Biochem Mol Biol. 2017;52:414–424. doi: 10.1080/10409238.2017.1325828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Staal F.J., Luis T.C., Tiemessen M.M. WNT signalling in the immune system: WNT is spreading its wings. Nat Rev Immunol. 2008;8:581–593. doi: 10.1038/nri2360. [DOI] [PubMed] [Google Scholar]
- 33.Sugimura R., He X.C., Venkatraman A., Arai F., Box A., Semerad C., et al. Noncanonical Wnt signaling maintains hematopoietic stem cells in the niche. Cell. 2012;150:351–365. doi: 10.1016/j.cell.2012.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clevers H., Nusse R. Wnt/β-catenin signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 35.Willert K., Brown J.D., Danenberg E., Duncan A.W., Weissman I.L., Reya T., et al. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature. 2003;423:448–452. doi: 10.1038/nature01611. [DOI] [PubMed] [Google Scholar]
- 36.Luis T.C., Weerkamp F., Naber B.A., Baert M.R., de Haas E.F., Nikolic T., et al. Wnt3a deficiency irreversibly impairs hematopoietic stem cell self-renewal and leads to defects in progenitor cell differentiation. Blood. 2009;113:546–554. doi: 10.1182/blood-2008-06-163774. [DOI] [PubMed] [Google Scholar]
- 37.Duinhouwer L.E., Tuysuz N., Rombouts E.W., Ter Borg M.N., Mastrobattista E., Spanholtz J., et al. Wnt3a protein reduces growth factor-driven expansion of human hematopoietic stem and progenitor cells in serum-free cultures. PLoS One. 2015;10 doi: 10.1371/journal.pone.0119086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luis T.C., Naber B.A., Roozen P.P., Brugman M.H., de Haas E.F., Ghazvini M., et al. Canonical wnt signaling regulates hematopoiesis in a dosage-dependent fashion. Cell Stem Cell. 2011;9:345–356. doi: 10.1016/j.stem.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 39.Reya T., Duncan A.W., Ailles L., Domen J., Scherer D.C., Willert K., et al. A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature. 2003;423:409–414. doi: 10.1038/nature01593. [DOI] [PubMed] [Google Scholar]
- 40.Janda C.Y., Dang L.T., You C., Chang J., de Lau W., Zhong Z.A., et al. Surrogate Wnt agonists that phenocopy canonical Wnt and β-catenin signalling. Nature. 2017;545:234–237. doi: 10.1038/nature22306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grainger S., Richter J., Palazon R.E., Pouget C., Lonquich B., Wirth S., et al. Wnt9a is required for the aortic amplification of nascent hematopoietic stem cells. Cell Rep. 2016;17:1595–1606. doi: 10.1016/j.celrep.2016.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bray S.J. Notch signalling: a simple pathway becomes complex. Nat Rev Mol Cell Biol. 2006;7:678–689. doi: 10.1038/nrm2009. [DOI] [PubMed] [Google Scholar]
- 43.Calvi L.M., Adams G.B., Weibrecht K.W., Weber J.M., Olson D.P., Knight M.C., et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- 44.Butler J.M., Nolan D.J., Vertes E.L., Varnum-Finney B., Kobayashi H., Hooper A.T., et al. Endothelial cells are essential for the self-renewal and repopulation of Notch-dependent hematopoietic stem cells. Cell Stem Cell. 2010;6:251–264. doi: 10.1016/j.stem.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Poulos M.G., Guo P., Kofler N.M., Pinho S., Gutkin M.C., Tikhonova A., et al. Endothelial Jagged-1 is necessary for homeostatic and regenerative hematopoiesis. Cell Rep. 2013;4:1022–1034. doi: 10.1016/j.celrep.2013.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Negishi N., Suzuki D., Ito R., Irie N., Matsuo K., Yahata T., et al. Effective expansion of engrafted human hematopoietic stem cells in bone marrow of mice expressing human Jagged1. Exp Hematol. 2014;42:487–494. doi: 10.1016/j.exphem.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 47.Varnum-Finney B., Brashem-Stein C., Bernstein I.D. Combined effects of Notch signaling and cytokines induce a multiple log increase in precursors with lymphoid and myeloid reconstituting ability. Blood. 2003;101:1784–1789. doi: 10.1182/blood-2002-06-1862. [DOI] [PubMed] [Google Scholar]
- 48.Delaney C., Varnum-Finney B., Aoyama K., Brashem-Stein C., Bernstein I.D. Dose-dependent effects of the Notch ligand Delta1 on ex vivo differentiation and in vivo marrow repopulating ability of cord blood cells. Blood. 2005;106:2693–2699. doi: 10.1182/blood-2005-03-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ohishi K., Varnum-Finney B., Bernstein I.D. Delta-1 enhances marrow and thymus repopulating ability of human CD34+CD38– cord blood cells. J Clin Invest. 2002;110:1165–1174. doi: 10.1172/JCI16167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Delaney C., Heimfeld S., Brashem-Stein C., Voorhies H., Manger R.L., Bernstein I.D. Notch-mediated expansion of human cord blood progenitor cells capable of rapid myeloid reconstitution. Nat Med. 2010;16:232–236. doi: 10.1038/nm.2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baron F., Nagler A. Novel strategies for improving hematopoietic reconstruction after allogeneic hematopoietic stem cell transplantation or intensive chemotherapy. Expert Opin Biol Ther. 2017;17:163–174. doi: 10.1080/14712598.2017.1269167. [DOI] [PubMed] [Google Scholar]
- 52.Horwitz M.E., Chao N.J., Rizzieri D.A., Long G.D., Sullivan K.M., Gasparetto C., et al. Umbilical cord blood expansion with nicotinamide provides long-term multilineage engraftment. J Clin Invest. 2014;124:3121–3128. doi: 10.1172/JCI74556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Walasek M.A., van Os R., de Haan G. Hematopoietic stem cell expansion: challenges and opportunities. Ann N Y Acad Sci. 2012;1266:138–150. doi: 10.1111/j.1749-6632.2012.06549.x. [DOI] [PubMed] [Google Scholar]
- 54.Santulli G. Angiopoietin-like proteins: a comprehensive look. Front Endocrinol (Lausanne) 2014;5:4. doi: 10.3389/fendo.2014.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zheng J., Huynh H., Umikawa M., Silvany R., Zhang C.C. Angiopoietin-like protein 3 supports the activity of hematopoietic stem cells in the bone marrow niche. Blood. 2011;117:470–479. doi: 10.1182/blood-2010-06-291716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang C.C., Kaba M., Ge G., Xie K., Tong W., Hug C., et al. Angiopoietin-like proteins stimulate ex vivo expansion of hematopoietic stem cells. Nat Med. 2006;12:240–245. doi: 10.1038/nm1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Akhter S., Rahman M.M., Lee H.S., Kim H.J., Hong S.T. Dynamic roles of angiopoietin-like proteins 1, 2, 3, 4, 6 and 7 in the survival and enhancement of ex vivo expansion of bone-marrow hematopoietic stem cells. Protein Cell. 2013;4:220–230. doi: 10.1007/s13238-013-2066-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bhardwaj G., Murdoch B., Wu D., Baker D.P., Williams K.P., Chadwick K., et al. Sonic hedgehog induces the proliferation of primitive human hematopoietic cells via BMP regulation. Nat Immunol. 2001;2:172–180. doi: 10.1038/84282. [DOI] [PubMed] [Google Scholar]
- 59.Himburg H.A., Termini C.M., Schlussel L., Kan J., Li M., Zhao L., et al. Distinct bone marrow sources of pleiotrophin control hematopoietic stem cell maintenance and regeneration. Cell Stem Cell. 2018;23:370–381. doi: 10.1016/j.stem.2018.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Himburg H.A., Yan X., Doan P.L., Quarmyne M., Micewicz E., McBride W., et al. Pleiotrophin mediates hematopoietic regeneration via activation of RAS. J Clin Invest. 2014;124:4753–4758. doi: 10.1172/JCI76838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Himburg H.A., Harris J.R., Ito T., Daher P., Russell J.L., Quarmyne M., et al. Pleiotrophin regulates the retention and self-renewal of hematopoietic stem cells in the bone marrow vascular niche. Cell Rep. 2012;2:964–975. doi: 10.1016/j.celrep.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Himburg H.A., Muramoto G.G., Daher P., Meadows S.K., Russell J.L., Doan P., et al. Pleiotrophin regulates the expansion and regeneration of hematopoietic stem cells. Nat Med. 2010;16:475–482. doi: 10.1038/nm.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wagner J.E., Jr., Brunstein C.G., Boitano A.E., DeFor T.E., McKenna D., Sumstad D., et al. Phase I/II trial of StemRegenin-1 expanded umbilical cord blood hematopoietic stem cells supports testing as a stand-alone graft. Cell Stem Cell. 2016;18:144–155. doi: 10.1016/j.stem.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Horwitz M.E., Wease S., Blackwell B., Valcarcel D., Frassoni F., Boelens J.J., et al. Phase I/II study of stem-cell transplantation using a single cord blood unit expanded ex vivo with nicotinamide. J Clin Oncol. 2019;37:367–374. doi: 10.1200/JCO.18.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Feng Y., Xie X.Y., Yang Y.Q., Sun Y.T., Ma W.H., Zhou P.J., et al. Synthesis and evaluation of pyrimidoindole analogs in umbilical cord blood ex vivo expansion. Eur J Med Chem. 2019;174:181–197. doi: 10.1016/j.ejmech.2019.04.042. [DOI] [PubMed] [Google Scholar]
- 66.Peled T., Landau E., Prus E., Treves A.J., Nagler A., Fibach E. Cellular copper content modulates differentiation and self-renewal in cultures of cord blood-derived CD34+ cells. Br J Haematol. 2002;116:655–661. doi: 10.1046/j.0007-1048.2001.03316.x. [DOI] [PubMed] [Google Scholar]
- 67.Peled T., Glukhman E., Hasson N., Adi S., Assor H., Yudin D., et al. Chelatable cellular copper modulates differentiation and self-renewal of cord blood-derived hematopoietic progenitor cells. Exp Hematol. 2005;33:1092–1100. doi: 10.1016/j.exphem.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 68.Peled T., Landau E., Mandel J., Glukhman E., Goudsmid N.R., Nagler A., et al. Linear polyamine copper chelator tetraethylenepentamine augments long-term ex vivo expansion of cord blood-derived CD34+ cells and increases their engraftment potential in NOD/SCID mice. Exp Hematol. 2004;32:547–555. doi: 10.1016/j.exphem.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 69.Peled T., Mandel J., Goudsmid R.N., Landor C., Hasson N., Harati D., et al. Pre-clinical development of cord blood-derived progenitor cell graft expanded ex vivo with cytokines and the polyamine copper chelator tetraethylenepentamine. Cytotherapy. 2004;6:344–355. doi: 10.1080/14653240410004916. [DOI] [PubMed] [Google Scholar]
- 70.Feher I., Gidali J. Prostaglandin E2 as stimulator of haemopoietic stem cell proliferation. Nature. 1974;247:550–551. doi: 10.1038/247550a0. [DOI] [PubMed] [Google Scholar]
- 71.North T.E., Goessling W., Walkley C.R., Lengerke C., Kopani K.R., Lord A.M., et al. Prostaglandin E2 regulates vertebrate haematopoietic stem cell homeostasis. Nature. 2007;447:1007–1011. doi: 10.1038/nature05883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hoggatt J., Singh P., Sampath J., Pelus L.M. Prostaglandin E2 enhances hematopoietic stem cell homing, survival, and proliferation. Blood. 2009;113:5444–5455. doi: 10.1182/blood-2009-01-201335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hoggatt J., Singh P., Stilger K.N., Plett P.A., Sampson C.H., Chua H.L., et al. Recovery from hematopoietic injury by modulating prostaglandin E2 signaling post-irradiation. Blood Cells Mol Dis. 2013;50:147–153. doi: 10.1016/j.bcmd.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ikushima Y.M., Arai F., Hosokawa K., Toyama H., Takubo K., Furuyashiki T., et al. Prostaglandin E2 regulates murine hematopoietic stem/progenitor cells directly via EP4 receptor and indirectly through mesenchymal progenitor cells. Blood. 2013;121:1995–2007. doi: 10.1182/blood-2012-06-437889. [DOI] [PubMed] [Google Scholar]
- 75.Goessling W., North T.E., Loewer S., Lord A.M., Lee S., Stoick-Cooper C.L., et al. Genetic interaction of PGE2 and Wnt signaling regulates developmental specification of stem cells and regeneration. Cell. 2009;136:1136–1147. doi: 10.1016/j.cell.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hagedorn E.J., Durand E.M., Fast E.M., Zon L.I. Getting more for your marrow: boosting hematopoietic stem cell numbers with PGE2. Exp Cell Res. 2014;329:220–226. doi: 10.1016/j.yexcr.2014.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Goessling W., Allen R.S., Guan X., Jin P., Uchida N., Dovey M., et al. Prostaglandin E2 enhances human cord blood stem cell xenotransplants and shows long-term safety in preclinical nonhuman primate transplant models. Cell Stem Cell. 2011;8:445–458. doi: 10.1016/j.stem.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cutler C., Multani P., Robbins D., Kim H.T., Le T., Hoggatt J., et al. Prostaglandin-modulated umbilical cord blood hematopoietic stem cell transplantation. Blood. 2013;122:3074–3081. doi: 10.1182/blood-2013-05-503177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang Y., Desai A., Yang S.Y., Bae K.B., Antczak M.I., Fink S.P., et al. Inhibition of the prostaglandin-degrading enzyme 15-PGDH potentiates tissue regeneration. Science. 2015;348:aaa2340. doi: 10.1126/science.aaa2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Desai A., Zhang Y., Park Y., Dawson D.M., Larusch G.A., Kasturi L., et al. A second-generation 15-PGDH inhibitor promotes bone marrow transplant recovery independently of age, transplant dose and granulocyte colony-stimulating factor support. Haematologica. 2018;103:1054–1064. doi: 10.3324/haematol.2017.178376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Antczak M.I., Zhang Y., Wang C., Doran J., Naidoo J., Voruganti S., et al. Inhibitors of 15-prostaglandin dehydrogenase to potentiate tissue repair. J Med Chem. 2017;60:3979–4001. doi: 10.1021/acs.jmedchem.7b00271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Quarmyne M., Doan P.L., Himburg H.A., Yan X., Nakamura M., Zhao L., et al. Protein tyrosine phosphatase-σ regulates hematopoietic stem cell-repopulating capacity. J Clin Invest. 2015;125:177–182. doi: 10.1172/JCI77866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang Y., Roos M., Himburg H., Termini C.M., Quarmyne M., Li M., et al. PTPσ inhibitors promote hematopoietic stem cell regeneration. Nat Commun. 2019;10:3667. doi: 10.1038/s41467-019-11490-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chang L., Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001;410:37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- 85.Navas T.A., Mohindru M., Estes M., Ma J.Y., Sokol L., Pahanish P., et al. Inhibition of overactivated p38 MAPK can restore hematopoiesis in myelodysplastic syndrome progenitors. Blood. 2006;108:4170–4177. doi: 10.1182/blood-2006-05-023093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zou J., Zou P., Wang J., Li L., Wang Y., Zhou D., et al. Inhibition of p38 MAPK activity promotes ex vivo expansion of human cord blood hematopoietic stem cells. Ann Hematol. 2012;91:813–823. doi: 10.1007/s00277-011-1397-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bari S., Zhong Q., Fan X., Poon Z., Lim A.S.T., Lim T.H., et al. Ex vivo expansion of CD34+CD90+CD49f+ hematopoietic stem and progenitor cells from non-enriched umbilical cord blood with azole compounds. Stem Cells Transl Med. 2018;7:376–393. doi: 10.1002/sctm.17-0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Passegue E., Wagner E.F., Weissman I.L. JunB deficiency leads to a myeloproliferative disorder arising from hematopoietic stem cells. Cell. 2004;119:431–443. doi: 10.1016/j.cell.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 89.Santaguida M., Schepers K., King B., Sabnis A.J., Forsberg E.C., Attema J.L., et al. JunB protects against myeloid malignancies by limiting hematopoietic stem cell proliferation and differentiation without affecting self-renewal. Cancer Cell. 2009;15:341–352. doi: 10.1016/j.ccr.2009.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang T., Inesta-Vaquera F., Niepel M., Zhang J., Ficarro S.B., Machleidt T., et al. Discovery of potent and selective covalent inhibitors of JNK. Chem Biol. 2012;19:140–154. doi: 10.1016/j.chembiol.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xiao X., Lai W., Xie H., Liu Y., Guo W., Liu Y., et al. Targeting JNK pathway promotes human hematopoietic stem cell expansion. Cell Discov. 2019;5:2. doi: 10.1038/s41421-018-0072-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zimmermann J., Buchdunger E., Mett H., Meyer T., Lydon N.B., Traxler P. Phenylamino-pyrimidine (PAP)—derivatives: a new class of potent and highly selective PDGF-receptor autophosphorylation inhibitors. Bioorg Med Chem Lett. 1996;6:1221–1226. [Google Scholar]
- 93.Yu H., Yuan Y., Shen H., Cheng T. Hematopoietic stem cell exhaustion impacted by p18 INK4C and p21 Cip1/Waf1 in opposite manners. Blood. 2006;107:1200–1206. doi: 10.1182/blood-2005-02-0685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yuan Y., Shen H., Franklin D.S., Scadden D.T., Cheng T. In vivo self-renewing divisions of haematopoietic stem cells are increased in the absence of the early G1-phase inhibitor, p18INK4C. Nat Cell Biol. 2004;6:436–442. doi: 10.1038/ncb1126. [DOI] [PubMed] [Google Scholar]
- 95.Gao Y., Yang P., Shen H., Yu H., Song X., Zhang L., et al. Small-molecule inhibitors targeting INK4 protein p18INK4C enhance ex vivo expansion of haematopoietic stem cells. Nat Commun. 2015;6:6328. doi: 10.1038/ncomms7328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li Y., Zhang W., Zhang Y., Ding Y., Yang M., He M., et al. Enhanced self-renewal of human long-term hematopoietic stem cells by a sulfamoyl benzoate derivative targeting p18INK4C. Blood Adv. 2021;5:3362–3372. doi: 10.1182/bloodadvances.2020004054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sirin O., Lukov G.L., Mao R., Conneely O.M., Goodell M.A. The orphan nuclear receptor Nurr1 restricts the proliferation of haematopoietic stem cells. Nat Cell Biol. 2010;12:1213–1219. doi: 10.1038/ncb2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Xie X.Q., Yang P., Zhang Y., Zhang P., Wang L., Ding Y., et al. Discovery of novel INK4C small-molecule inhibitors to promote human and murine hematopoietic stem cell ex vivo expansion. Sci Rep. 2015;5:18115. doi: 10.1038/srep18115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hua P., Hester J., Adigbli G., Li R., Psaila B., Roy A., et al. The BET inhibitor CPI203 promotes ex vivo expansion of cord blood long-term repopulating HSCs and megakaryocytes. Blood. 2020;136:2410–2415. doi: 10.1182/blood.2020005357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Evans R.M., Mangelsdorf D.J. Nuclear receptors, RXR, and the Big Bang. Cell. 2014;157:255–266. doi: 10.1016/j.cell.2014.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Guo B., Huang X., Lee M.R., Lee S.A., Broxmeyer H.E. Antagonism of PPARγ signaling expands human hematopoietic stem and progenitor cells by enhancing glycolysis. Nat Med. 2018;24:360–367. doi: 10.1038/nm.4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Leesnitzer L.M., Parks D.J., Bledsoe R.K., Cobb J.E., Collins J.L., Consler T.G., et al. Functional consequences of cysteine modification in the ligand binding sites of peroxisome proliferator activated receptors by GW9662. Biochemistry. 2002;41:6640–6650. doi: 10.1021/bi0159581. [DOI] [PubMed] [Google Scholar]
- 103.Liu X., Zheng H., Yu W.M., Cooper T.M., Bunting K.D., Qu C.K. Maintenance of mouse hematopoietic stem cells ex vivo by reprogramming cellular metabolism. Blood. 2015;125:1562–1565. doi: 10.1182/blood-2014-04-568949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Boyer L.A., Lee T.I., Cole M.F., Johnstone S.E., Levine S.S., Zucker J.P., et al. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell. 2005;122:947–956. doi: 10.1016/j.cell.2005.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Szabo E., Rampalli S., Risueno R.M., Schnerch A., Mitchell R., Fiebig-Comyn A., et al. Direct conversion of human fibroblasts to multilineage blood progenitors. Nature. 2010;468:521–526. doi: 10.1038/nature09591. [DOI] [PubMed] [Google Scholar]
- 106.Huang X., Lee M.R., Cooper S., Hangoc G., Hong K.S., Chung H.M., et al. Activation of OCT4 enhances ex vivo expansion of human cord blood hematopoietic stem and progenitor cells by regulating HOXB4 expression. Leukemia. 2016;30:144–153. doi: 10.1038/leu.2015.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nishino T., Miyaji K., Ishiwata N., Arai K., Yui M., Asai Y., et al. Ex vivo expansion of human hematopoietic stem cells by a small-molecule agonist of c-MPL. Exp Hematol. 2009;37:1364–1377. doi: 10.1016/j.exphem.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 108.Kao Y.R., Chen J., Narayanagari S.R., Todorova T.I., Aivalioti M.M., Ferreira M., et al. Thrombopoietin receptor-independent stimulation of hematopoietic stem cells by eltrombopag. Sci Transl Med. 2018;10 doi: 10.1126/scitranslmed.aas9563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Trowbridge J.J., Xenocostas A., Moon R.T., Bhatia M. Glycogen synthase kinase-3 is an in vivo regulator of hematopoietic stem cell repopulation. Nat Med. 2005;12:89–98. doi: 10.1038/nm1339. [DOI] [PubMed] [Google Scholar]
- 110.Ferensztajn-Rochowiak E., Rybakowski J.K. The effect of lithium on hematopoietic, mesenchymal and neural stem cells. Pharmacol Rep. 2016;68:224–230. doi: 10.1016/j.pharep.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 111.Ko K.H., Holmes T., Palladinetti P., Song E., Nordon R., O'Brien T.A., et al. GSK-3β inhibition promotes engraftment of ex vivo-expanded hematopoietic stem cells and modulates gene expression. Stem Cell. 2011;29:108–118. doi: 10.1002/stem.551. [DOI] [PubMed] [Google Scholar]
- 112.Huang J., Nguyen-McCarty M., Hexner E.O., Danet-Desnoyers G., Klein P.S. Maintenance of hematopoietic stem cells through regulation of Wnt and mTOR pathways. Nat Med. 2012;18:1778–1785. doi: 10.1038/nm.2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Li J., Zhang L., Yin L., Ma N., Wang T., Wu Y., et al. In vitro expansion of hematopoietic stem cells by inhibition of both GSK3 and p38 signaling. Stem Cells Dev. 2019;28:1486–1497. doi: 10.1089/scd.2019.0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rothbart S.B., Strahl B.D. Interpreting the language of histone and DNA modifications. Biochim Biophys Acta. 2014;1839:627–643. doi: 10.1016/j.bbagrm.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jones P.A. Functions of DNA methylation: islands, start sites, gene bodies and beyond. Nat Rev Genet. 2012;13:484–492. doi: 10.1038/nrg3230. [DOI] [PubMed] [Google Scholar]
- 116.Cimmino L. Methylation maintains HSC division fate. Proc Natl Acad Sci U S A. 2017;114:192–194. doi: 10.1073/pnas.1619390114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhao J., Chen X., Song G., Zhang J., Liu H., Liu X. Uhrf1 controls the self-renewal versus differentiation of hematopoietic stem cells by epigenetically regulating the cell-division modes. Proc Natl Acad Sci U S A. 2017;114:142–151. doi: 10.1073/pnas.1612967114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Nishino T., Wang C., Mochizuki-Kashio M., Osawa M., Nakauchi H., Iwama A. Ex vivo expansion of human hematopoietic stem cells by garcinol, a potent inhibitor of histone acetyltransferase. PLoS One. 2011;6 doi: 10.1371/journal.pone.0024298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.TeKippe M., Harrison D.E., Chen J. Expansion of hematopoietic stem cell phenotype and activity in Trp53-null mice. Exp Hematol. 2003;31:521–527. doi: 10.1016/s0301-472x(03)00072-9. [DOI] [PubMed] [Google Scholar]
- 120.Shojaei F., Trowbridge J., Gallacher L., Yuefei L., Goodale D., Karanu F., et al. Hierarchical and ontogenic positions serve to define the molecular basis of human hematopoietic stem cell behavior. Dev Cell. 2005;8:651–663. doi: 10.1016/j.devcel.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 121.De Felice L., Tatarelli C., Mascolo M.G., Gregorj C., Agostini F., Fiorini R., et al. Histone deacetylase inhibitor valproic acid enhances the cytokine-induced expansion of human hematopoietic stem cells. Cancer Res. 2005;65:1505–1513. doi: 10.1158/0008-5472.CAN-04-3063. [DOI] [PubMed] [Google Scholar]
- 122.Bug G., Gul H., Schwarz K., Pfeifer H., Kampfmann M., Zheng X., et al. Valproic acid stimulates proliferation and self-renewal of hematopoietic stem cells. Cancer Res. 2005;65:2537–2541. doi: 10.1158/0008-5472.CAN-04-3011. [DOI] [PubMed] [Google Scholar]
- 123.Papa L., Zimran E., Djedaini M., Ge Y., Ozbek U., Sebra R., et al. Ex vivo human HSC expansion requires coordination of cellular reprogramming with mitochondrial remodeling and p53 activation. Blood Adv. 2018;2:2766–2779. doi: 10.1182/bloodadvances.2018024273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zimran E., Papa L., Djedaini M., Patel A., Iancu-Rubin C., Hoffman R. Expansion and preservation of the functional activity of adult hematopoietic stem cells cultured ex vivo with a histone deacetylase inhibitor. Stem Cells Transl Med. 2020;9:531–542. doi: 10.1002/sctm.19-0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Iancu-Rubin C., Hoffman R. Role of epigenetic reprogramming in hematopoietic stem cell function. Curr Opin Hematol. 2015;22:279–285. doi: 10.1097/MOH.0000000000000143. [DOI] [PubMed] [Google Scholar]
- 126.Chaurasia P., Gajzer D.C., Schaniel C., D'Souza S., Hoffman R. Epigenetic reprogramming induces the expansion of cord blood stem cells. J Clin Invest. 2014;124:2378–2395. doi: 10.1172/JCI70313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Seet L.F., Teng E., Lai Y.S., Laning J., Kraus M., Wnendt S., et al. Valproic acid enhances the engraftability of human umbilical cord blood hematopoietic stem cells expanded under serum-free conditions. Eur J Haematol. 2009;82:124–132. doi: 10.1111/j.1600-0609.2008.01169.x. [DOI] [PubMed] [Google Scholar]
- 128.Mahmud N., Petro B., Baluchamy S., Li X., Taioli S., Lavelle D., et al. Differential effects of epigenetic modifiers on the expansion and maintenance of human cord blood stem/progenitor cells. Biol Blood Marrow Transplant. 2014;20:480–489. doi: 10.1016/j.bbmt.2013.12.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Walasek M.A., Bystrykh L., van den Boom V., Olthof S., Ausema A., Ritsema M., et al. The combination of valproic acid and lithium delays hematopoietic stem/progenitor cell differentiation. Blood. 2012;119:3050–3059. doi: 10.1182/blood-2011-08-375386. [DOI] [PubMed] [Google Scholar]
- 130.Huang X., Guo B., Liu S., Wan J., Broxmeyer H.E. Neutralizing negative epigenetic regulation by HDAC5 enhances human haematopoietic stem cell homing and engraftment. Nat Commun. 2018;9:2741. doi: 10.1038/s41467-018-05178-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Milhem M., Mahmud N., Lavelle D., Araki H., DeSimone J., Saunthararajah Y., et al. Modification of hematopoietic stem cell fate by 5aza 2′deoxycytidine and trichostatin A. Blood. 2004;103:4102–4110. doi: 10.1182/blood-2003-07-2431. [DOI] [PubMed] [Google Scholar]
- 132.Araki H., Mahmud N., Milhem M., Nunez R., Xu M., Beam C.A., et al. Expansion of human umbilical cord blood SCID-repopulating cells using chromatin-modifying agents. Exp Hematol. 2006;34:140–149. doi: 10.1016/j.exphem.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 133.Araki H., Yoshinaga K., Boccuni P., Zhao Y., Hoffman R., Mahmud N. Chromatin-modifying agents permit human hematopoietic stem cells to undergo multiple cell divisions while retaining their repopulating potential. Blood. 2007;109:3570–3578. doi: 10.1182/blood-2006-07-035287. [DOI] [PubMed] [Google Scholar]
- 134.Saraf S., Araki H., Petro B., Park Y., Taioli S., Yoshinaga K.G., et al. Ex vivo expansion of human mobilized peripheral blood stem cells using epigenetic modifiers. Transfusion. 2015;55:864–874. doi: 10.1111/trf.12904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.V M S., Kale V.P., Limaye L.S. Expansion of cord blood CD34 cells in presence of zVADfmk and zLLYfmk improved their in vitro functionality and in vivo engraftment in NOD/SCID mouse. PLoS One. 2010;5 doi: 10.1371/journal.pone.0012221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Chute J.P., Muramoto G.G., Whitesides J., Colvin M., Safi R., Chao N.J., et al. Inhibition of aldehyde dehydrogenase and retinoid signaling induces the expansion of human hematopoietic stem cells. Proc Natl Acad Sci U S A. 2006;103:11707–11712. doi: 10.1073/pnas.0603806103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Park G.B., Kim M.J., Vasileva E.A., Mishchenko N.P., Fedoreyev S.A., Stonik V.A., et al. Echinochrome a promotes ex vivo expansion of peripheral blood-derived CD34+ cells, potentially through downregulation of ROS production and activation of the Src-Lyn-p110δ Pathway. Mar Drugs. 2019;17:526. doi: 10.3390/md17090526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhang Q.S., Marquez-Loza L., Eaton L., Duncan A.W., Goldman D.C., Anur P., et al. Fancd2‒/‒ mice have hematopoietic defects that can be partially corrected by resveratrol. Blood. 2010;116:5140–5148. doi: 10.1182/blood-2010-04-278226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Rimmele P., Lofek-Czubek S., Ghaffari S. Resveratrol increases the bone marrow hematopoietic stem and progenitor cell capacity. Am J Hematol. 2014;89:235–238. doi: 10.1002/ajh.23837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Heinz N., Ehrnstrom B., Schambach A., Schwarzer A., Modlich U., Schiedlmeier B. Comparison of different cytokine conditions reveals resveratrol as a new molecule for ex vivo cultivation of cord blood-derived hematopoietic stem cells. Stem Cells Transl Med. 2015;4:1064–1072. doi: 10.5966/sctm.2014-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Liu Y., Zhang B., Zhang J., Wang S., Yao H., He L., et al. CAPE promotes the expansion of human umbilical cord blood-derived hematopoietic stem and progenitor cells in vitro. Sci China Life Sci. 2014;57:188–194. doi: 10.1007/s11427-014-4611-8. [DOI] [PubMed] [Google Scholar]
- 142.Chen X., Han Y., Zhang B., Liu Y., Wang S., Liao T., et al. Caffeic acid phenethyl ester promotes haematopoietic stem/progenitor cell homing and engraftment. Stem Cell Res Ther. 2017;8:255. doi: 10.1186/s13287-017-0708-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tanhuad N., Thongsa-Ad U., Sutjarit N., Yoosabai P., Panvongsa W., Wongniam S., et al. Ex vivo expansion and functional activity preservation of adult hematopoietic stem cells by a diarylheptanoid from Curcuma comosa. Biomed Pharmacother. 2021;143:112102. doi: 10.1016/j.biopha.2021.112102. [DOI] [PubMed] [Google Scholar]
- 144.Lim W., Ryu S., Bazer F.W., Kim S.M., Song G. Chrysin attenuates progression of ovarian cancer cells by regulating signaling cascades and mitochondrial dysfunction. J Cell Physiol. 2018;233:3129–3140. doi: 10.1002/jcp.26150. [DOI] [PubMed] [Google Scholar]
- 145.Li Y., He M., Zhang W., Yang M., Ding Y., Xu S., et al. Antioxidant small molecule compound chrysin promotes the self-renewal of hematopoietic stem cells. Front Pharmacol. 2020;11:399. doi: 10.3389/fphar.2020.00399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Yang M., Li K., Ng P.C., Chuen C.K., Lau T.K., Cheng Y.S., et al. Promoting effects of serotonin on hematopoiesis: ex vivo expansion of cord blood CD34+ stem/progenitor cells, proliferation of bone marrow stromal cells, and antiapoptosis. Stem Cell. 2007;25:1800–1806. doi: 10.1634/stemcells.2007-0048. [DOI] [PubMed] [Google Scholar]
- 147.Nogueira-Pedro A., Barbosa C.M., Segreto H.R., Lungato L., D'Almeida V., Moraes A.A., et al. α-Tocopherol induces hematopoietic stem/progenitor cell expansion and ERK1/2-mediated differentiation. J Leukoc Biol. 2011;90:1111–1117. doi: 10.1189/jlb.0611282. [DOI] [PubMed] [Google Scholar]
- 148.Purton L.E., Bernstein I.D., Collins S.J. All-trans retinoic acid delays the differentiation of primitive hematopoietic precursors (lin–c-kit+Sca-1+) while enhancing the terminal maturation of committed granulocyte/monocyte progenitors. Blood. 1999;94:483–495. [PubMed] [Google Scholar]
- 149.Purton L.E., Bernstein I.D., Collins S.J. All-trans retinoic acid enhances the long-term repopulating activity of cultured hematopoietic stem cells. Blood. 2000;95:470–477. [PubMed] [Google Scholar]
- 150.Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 151.He N., Chan C.K., Sobhian B., Chou S., Xue Y., Liu M., et al. Human polymerase-associated factor complex (PAFc) connects the super elongation complex (SEC) to RNA polymerase II on chromatin. Proc Natl Acad Sci U S A. 2011;108:636–645. doi: 10.1073/pnas.1107107108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Li Y., Wen H., Xi Y., Tanaka K., Wang H., Peng D., et al. AF9 YEATS domain links histone acetylation to DOT1L-mediated H3K79 methylation. Cell. 2014;159:558–571. doi: 10.1016/j.cell.2014.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hentze M.W., Castello A., Schwarzl T., Preiss T. A brave new world of RNA-binding proteins. Nat Rev Mol Cell Biol. 2018;19:327–341. doi: 10.1038/nrm.2017.130. [DOI] [PubMed] [Google Scholar]
- 154.Byers R.J., Currie T., Tholouli E., Rodig S.J., Kutok J.L. MSI2 protein expression predicts unfavorable outcome in acute myeloid leukemia. Blood. 2011;118:2857–2867. doi: 10.1182/blood-2011-04-346767. [DOI] [PubMed] [Google Scholar]
- 155.de Andres-Aguayo L., Varas F., Kallin E.M., Infante J.F., Wurst W., Floss T., et al. Musashi 2 is a regulator of the HSC compartment identified by a retroviral insertion screen and knockout mice. Blood. 2011;118:554–564. doi: 10.1182/blood-2010-12-322081. [DOI] [PubMed] [Google Scholar]
- 156.Park S.M., Deering R.P., Lu Y., Tivnan P., Lianoglou S., Al-Shahrour F., et al. Musashi-2 controls cell fate, lineage bias, and TGF-β signaling in HSCs. J Exp Med. 2014;211:71–87. doi: 10.1084/jem.20130736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Rentas S., Holzapfel N.T., Belew M.S., Pratt G.A., Voisin V., Wilhelm B.T., et al. Musashi-2 attenuates AHR signalling to expand human haematopoietic stem cells. Nature. 2016;532:508–511. doi: 10.1038/nature17665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Minuesa G., Antczak C., Shum D., Radu C., Bhinder B., Li Y., et al. A 1536-well fluorescence polarization assay to screen for modulators of the MUSASHI family of RNA-binding proteins. Comb Chem High Throughput Screen. 2014;17:596–609. doi: 10.2174/1386207317666140609122714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Minuesa G., Albanese S.K., Xie W., Kazansky Y., Worroll D., Chow A., et al. Small-molecule targeting of MUSASHI RNA-binding activity in acute myeloid leukemia. Nat Commun. 2019;10:2691. doi: 10.1038/s41467-019-10523-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zhao B.S., Roundtree I.A., He C. Post-transcriptional gene regulation by mRNA modifications. Nat Rev Mol Cell Biol. 2017;18:31–42. doi: 10.1038/nrm.2016.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zhang C., Chen Y., Sun B., Wang L., Yang Y., Ma D., et al. m6A modulates haematopoietic stem and progenitor cell specification. Nature. 2017;549:273–276. doi: 10.1038/nature23883. [DOI] [PubMed] [Google Scholar]
- 162.Vu L.P., Pickering B.F., Cheng Y., Zaccara S., Nguyen D., Minuesa G., et al. The N6-methyladenosine (m6A)-forming enzyme METTL3 controls myeloid differentiation of normal hematopoietic and leukemia cells. Nat Med. 2017;23:1369–1376. doi: 10.1038/nm.4416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Weng H., Huang H., Wu H., Qin X., Zhao B.S., Dong L., et al. METTL14 inhibits hematopoietic stem/progenitor differentiation and promotes leukemogenesis via mRNA m6A Modification. Cell Stem Cell. 2018;22:191–205. doi: 10.1016/j.stem.2017.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Li Z., He X.C., Li L. Hematopoietic stem cells: self-renewal and expansion. Curr Opin Hematol. 2019;26:258–265. doi: 10.1097/MOH.0000000000000506. [DOI] [PubMed] [Google Scholar]
- 165.Zhu T., Roundtree I.A., Wang P., Wang X., Wang L., Sun C., et al. Crystal structure of the YTH domain of YTHDF2 reveals mechanism for recognition of N6-methyladenosine. Cell Res. 2014;24:1493–1496. doi: 10.1038/cr.2014.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Li F., Zhao D., Wu J., Shi Y. Structure of the YTH domain of human YTHDF2 in complex with an m6A mononucleotide reveals an aromatic cage for m6A recognition. Cell Res. 2014;24:1490–1492. doi: 10.1038/cr.2014.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Du H., Zhao Y., He J., Zhang Y., Xi H., Liu M., et al. YTHDF2 destabilizes m6A-containing RNA through direct recruitment of the CCR4-NOT deadenylase complex. Nat Commun. 2016;7:12626. doi: 10.1038/ncomms12626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Wang H., Zuo H., Liu J., Wen F., Gao Y., Zhu X., et al. Loss of YTHDF2-mediated m6A-dependent mRNA clearance facilitates hematopoietic stem cell regeneration. Cell Res. 2018;28:1035–1038. doi: 10.1038/s41422-018-0082-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Paris J., Morgan M., Campos J., Spencer G.J., Shmakova A., Ivanova I., et al. Targeting the RNA m6A reader YTHDF2 selectively compromises cancer stem cells in acute myeloid leukemia. Cell Stem Cell. 2019;25:137–148. doi: 10.1016/j.stem.2019.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Fonseca-Pereira D., Arroz-Madeira S., Rodrigues-Campos M., Barbosa I.A., Domingues R.G., Bento T., et al. The neurotrophic factor receptor RET drives haematopoietic stem cell survival and function. Nature. 2014;514:98–101. doi: 10.1038/nature13498. [DOI] [PubMed] [Google Scholar]
- 171.Grey W., Chauhan R., Piganeau M., Huerga Encabo H., Garcia-Albornoz M., McDonald N.Q., et al. Activation of the receptor tyrosine kinase RET improves long-term hematopoietic stem cell outgrowth and potency. Blood. 2020;136:2535–2547. doi: 10.1182/blood.2020006302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Sauvageau G., Iscove N.N., Humphries R.K. In vitro and in vivo expansion of hematopoietic stem cells. Oncogene. 2004;23:7223–7232. doi: 10.1038/sj.onc.1207942. [DOI] [PubMed] [Google Scholar]
- 173.Wang L., Guan X., Wang H., Shen B., Zhang Y., Ren Z., et al. A small-molecule/cytokine combination enhances hematopoietic stem cell proliferation via inhibition of cell differentiation. Stem Cell Res Ther. 2017;8:169. doi: 10.1186/s13287-017-0625-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Jiang M., Chen H., Lai S., Wang R., Qiu Y., Ye F., et al. Maintenance of human haematopoietic stem and progenitor cells in vitro using a chemical cocktail. Cell Discov. 2018;4:59. doi: 10.1038/s41421-018-0059-5. [DOI] [PMC free article] [PubMed] [Google Scholar]