Abstract
Cyclooxygenases play a vital role in inflammation and are responsible for the production of prostaglandins. Two cyclooxygenases are described, the constitutive cyclooxygenase-1 and the inducible cyclooxygenase-2, for which the target inhibitors are the non-steroidal anti-inflammatory drugs (NSAIDs). Prostaglandins are a class of lipid compounds that mediate acute and chronic inflammation. NSAIDs are the most frequent choices for treatment of inflammation. Nevertheless, currently used anti-inflammatory drugs have become associated with a variety of adverse effects which lead to diminished output even market withdrawal. Recently, more studies have been carried out on searching novel selective COX-2 inhibitors with safety profiles. In this review, we highlight the various structural classes of organic and natural scaffolds with efficient COX-2 inhibitory activity reported during 2011–2021. It will be valuable for pharmaceutical scientists to read up on the current chemicals to pave the way for subsequent research.
KEY WORDS: Inflammation, Cyclooxygenase, Prostaglandins, Adverse effects, COX-2 inhibitors
Graphical abstract
As a crucial enzyme for catalyzing the synthesis of prostaglandins, cyclooxygenase-2 (COX-2) was identified as a promising target for inflammation treatment. This review highlights the current progress of developing COX-2 inhibitors with various synthetic and natural structures as NSAIDs candidates.
1. Introduction
Inflammation is an important part of the immune system's response against hostile world and has been linked to a variety of immunological diseases1. Chemical agents, physical injuries, immunological reactions and Infection by pathogenic organisms usually cause acute or chronic inflammations2. Non-steroidal anti-inflammatory drugs (NSAIDs) have long been known to alleviate inflammation. They treat a variety of diseases caused by inflammation, such as rheumatoid arthritis, acute fever as well as relieving common daily pains3. In the history of medicine development, the first therapeutic NSAID was aspirin, which has been used for more than 100 years since 1898. For more than a century, more typical NSAIDs, such as celecoxib, indomethacin, ibuprofen, and diclofenac (Fig. 1), have been latterly developed, and approved by the U.S. Food and Drug Administration (FDA) for clinical treatment. NSAIDs competitively inhibits the activity of cyclooxygenases (COXs) and thereby interferes with the bioconversion of the downstream inflammatory mediators. In 1971, Vane et al.4,5 first confirmed that the therapeutic target of NSAIDs is cyclooxygenase, further investigation found that the inhibition of cyclooxygenase directly resulted in termination of the biosynthesis of prostaglandins (PGs), which are crucial mediators of inflammation.
Figure 1.
Structure formulas of clinically used COX-1 and COX-2 inhibitors.
Four major bioactive PGs, prostaglandin E2 (PGE2), prostacyclin I2 (PGI2), prostaglandin D2 (PGD2), prostaglandin F2α (PGF2α), along with a thromboxane A2 (TXA2) are generated during inflammation6. The formation of PGs was shown in Fig. 2, initially, arachidonic acid (AA) is released from the phospholipid by the catalysis of the phospholipase A2 (PLA2), and then a prostaglandin H2 (PGH2) is subsequently formed by the actions of both COX-1 and COX-2. These four PGs and TXA2 are eventually produced through the bioconversion of PGs and TXA2 synthases in downstream mechanism. The level of prostaglandin production mainly depends on the expression of cyclooxygenases (COXs) in inflammatory tissues, especially cyclooxygenases-27. PGs are hormone-like lipid compounds and are involved in many physiological reactions and play a key role in the generation of inflammatory responses8. In general, PGs exert their effects by mediating the body's responses to tissue injury or inflammation. Among them, PGE2 is the dominated prostaglandin that induces typical symptoms of inflammation, such as pain, fever, tumor, and anaphylactic reaction9.
Figure 2.
A diagram of the biochemistry of prostanoids.
Two functional COXs are identified and defined as COX-1 and COX-2 according to their different structures and functions. COX-1 belongs to constitutive isoenzyme and extensively existed in most cells. Prostaglandins catalyzed by COX-1 have protective effects on gastrointestinal tract. COX-2 is an inducible enzyme which acts as the most important source of prostaglandins, so it is always regarded as a pathologic enzyme chiefly responsible for inflammation10, 11, 12. NSAIDs exhibits anti-inflammatory effects by the non-selective/selective inhibition of cyclooxygenase (COX) activity and subsequently blocks the biosynthesis of prostaglandins in lesion sites13, 14, 15. However, due to simultaneous inhibition of COX-1 and COX-2, non-selective NSAIDs not only achieve anti-inflammatory and analgesic purposes, but also cause serious adverse effects, such as digestive tract damage and platelet function disorder. While selective NSAIDs only inhibits COX-2 but does not affect the protective effect of prostaglandins catalyzed by COX-1 on gastrointestinal tract and platelet, thus greatly decreasing the risk of gastrointestinal side effects16,17. The traditional anti-inflammatory mechanism of COX-2 inhibitor has been confirmed by recent studies. As depicted in Fig. 3, COX-2 inhibitors exert pharmacological activity through inhibition of the NF-κB pathway. Since COX-2 is responsible for producing reactive oxygen species (ROS), COX-2 inhibition causes a sharp drop in the amount of reactive oxygen species (ROS) in the upstream mechanism and keep NF-κB in an inactive state of bondage to P-IκB in the downstream, and thereby prohibit the production of pro-inflammatory cytokines, including NO, PGE2, IL-6, and TNF-α18. Although selective COX-2 inhibitors are the most common choice of treatment for inflammatory diseases, they are often found to be associated with potential adverse effects of cardiovascular disorder, and a possible increased risk of heart attack, blood clots and stroke. Therefore, discovering new selective COX-2 inhibitors that can reduce such side effects appear more popular19,20.
Figure 3.
A diagram of the putative anti-inflammatory mechanism of the COX-2 inhibitor in RAW264.7 cells.
To prevent undesirable outcomes, finding new NSAIDs with improved safety profiles remains the most effective approach to inflammation treatment. This paper reviews the classification and pharmacological action of new COX-2 inhibitors which have been reported in organic synthesis in the last ten years. In addition, new compounds from natural origins with potent COX-2 inhibitory and anti-inflammatory activity are also included, which are the promising COX-2 inhibitor for drug design and clinical use.
2. Chemistry and pharmacology of new synthetic COX-2 inhibitors
2.1. Compounds having a pyrazole ring
Pyrazole is a π-excess aromatic heterocycle, which has been recognized as a pharmacologically important active scaffold for organic synthesis, especially for new COX-2 inhibitor development. Clinical agents containing pyrazole fragments are celecoxib, antipyrine, aminopyrine, and metamizole21. During the past ten years, a number of pyrazole derivatives were reported and screened for their COX-2 inhibition and anti-inflammatory activity (Fig. 4). In 2014, a new compound 1 containing pyrazole fragment was synthesized by Bansal et al.22. Compound 1 showed excellent selective inhibition of COX-2 [IC50 = 0.31 μmol/L, selectivity index (SI) > 222] and potential anti-inflammatory activity with ED50 of 74.3 mg/kg in a carrageenan-induced rat paw edema model. The COXs inhibition activity of compound 1 was obtained by using a COX fluorescent inhibitor screening assay kit consisting of ovine COX-1 and human recombinant COX-2 enzymes [an enzyme immunoassay (EIA)]. Further investigation indicated that compound 1 presented suppression of acetic acid-induced writhes, and it showed better gastro-spasm profile compare to that of aspirin. In a molecular docking study, compound 1 showed higher selective binding affinity towards COX-2 than to COX-1. An important hydrogen bond between the oxygen of the nitro group and the hydrogen of Arg120 was observed, which is important for the interaction with COX-2. El-Sayed et al.23 reported the synthesis of a series of potentially useful 1,5-diphenyl pyrazoles. Compounds 2 and 3 displayed a considerable COX-2 inhibitory activity and a good selectivity (EIA) (IC50 = 0.45 μmol/L, SI = 111.1). Compounds 2 and 3 also presented high anti-inflammatory activity (ED50 = 118 and 120 mg/kg) comparable with diclofenac (ED50 = 114 mg/kg) in carrageenan-induced rat raw paw oedema assay. Notably, replacement of cycloalkanone moiety can significantly influence their activity. Meanwhile, molecular docking analysis indicated that compounds 2 and 3 bind into the active site of COX-2 in a similar manner to SC-558, a selective COX-2 inhibitor. Xu's group24 reported a novel class of molecules, adopting a new pyrazole N-aryl sulfonate synthetic approach. This inspiration came from the selective COX-2 inhibitor-celecoxib, which has a typical sulfonamide fragment. In vitro EIA experiments indicated that compounds 4, 5, 6, and 8 have strong COX-2 inhibitory activity (Table 1). According to the selectivity index on COXs, compounds 4–8 displayed comparable selective COX-2 inhibition with that of celecoxib. Importantly, compounds 4, 5, 7, and 8 showed excellent in vivo anti-inflammatory activity (5, 7, and 8: % inhibition of auricular edemas = 27.0, 27.0 and 25.7, respectively; 4 and 7: % inhibition ratios of writhing = 50.7 and 48.5, separately, at the oral dose of 30 mg/kg, 8 mice/test group). El-Sayed et al.25 continued to report some pyrazole derivatives based on the skeleton of SC-558 and celecoxib in 2012. Some of the newly synthesized compounds showed increased COX-2 inhibitory and anti-inflammatory activity. Example as compound 9, exhibiting excellent COX-2 inhibitory activity (EIA) with IC50 of 0.26 μmol/L and selectivity index (SI = 192.3). Furthermore, the carrageenan-induced rat paw edema assay showed that compound 9 exerted equivalent anti-inflammatory activity with ED50 of 0.170 mmol/kg in comparison to the reference drug (diclofenac: ED50 = 0.198 and celecoxib: 0.185 mmol/kg, respectively). Per the docking result, the trifluoromethyl moiety of compound 9 inserts deep inside the COX-2 pocket and forming hydrogen bond with Gln192 and Arg513, this result was consistent with COX-2 inhibition. A novel series of pyrazole derivatives that have unusual flexible fragments were reported by Gopi's group26, of these, compounds 10 and 11 exhibited moderate selective COX-2 inhibitory potency by using a chromogenic assay (IC50 = 16.8 and 14.3 μmol/L, SI = 0.5100 and 0.4400, respectively). In docking calculations, an interaction between the ligands and Arg513 was observed, which is required for the dependent inhibition of COX-2. In 2020, on the basis of the structure of celecoxib, several halogenated triarylpyrazoles were prepared by Abdellatif et al.27. In vitro COX-2 inhibition assay indicated that three fluorinated compounds 12–14 exerted excellent efficacies (IC50 = 0.049, 0.057, and 0.054 μmol/L, respectively) close to that of celecoxib (IC50 = 0.055 μmol/L) and showed better selective index (SI = 253.1, 201.8, and 214.8, respectively) than celecoxib (SI = 179.4). Moreover, compounds 12–14 exhibited close gastric profile (ulcer index (UI) = 1.25–2.5) to celecoxib (UI = 1.75). In this study, halogenated aryl ring was found to be crucial to affect activity and selectivity, and halogen atom fluoro derivatives showed better COX-2 selectivity than celecoxib. Abdelall et al.28 prepared a new series of 1,5-diaryl pyrazoles as both COX-2 and 15-lipoxygenase inhibitors. Compound 15 was more effective (ED50 = 0.98 μmol/L) on COX-2 inhibition than that of references celecoxib (ED50 = 1.54 μmol/L) and meclofenamate sodium (ED50 = 5.64 μmol/L) in an EIA assay. Meanwhile, compound 15 showed good anti-inflammatory activity and selectivity index (SI = 4.89) in in vivo assay, which was almost identical to that of celecoxib (SI = 4.93). Moreover, the in vivo ulcerliability activity assay was explored in this study, compound 15 presented good ulcerous profile (UI = 2.78) and it was as safe as the reference celecoxib (UI = 2.9). In addition, the results suggested that presence of a (CF3) moiety in pyrazoles had no effect on COX-2 selectivity. Aiming to directly inhibit the production of PGE2 in serum samples of rats, some novel pyrazole derivatives were recently designed by Mohammed et al.29. Of these, compound 16, which contains an acylamino linker, presented COX-2 inhibition with IC50 = 1.76 μmol/L and a good selectivity index value of 11.1. Moreover, compound 16 showed potential anti-inflammatory activity (% edema inhibition = 81) and was less ulcerogenic than indomethacin in the in vivo ulcer liability assay. Unfortunately, the potency and selectivity of compound 16 cannot be compared with that of celecoxib, further structural modification is required to improve the activity.
Figure 4.
Chemical structures of compounds 1–16.
Table 1.
In vitro COX-1/COX-2 inhibition (IC50, μmol/L) and selectivity index for compounds 4–8, and standard agent.
| Compd. | COX-1a | COX-2a | COX-2 selectivityb |
|---|---|---|---|
| 4 | 0.5 | 0.0011 | 455 |
| 5 | 96.57 | 0.0092 | 10,479 |
| 6 | >100.00 | 0.092 | >1087 |
| 7 | >100.00 | 0.53 | >189 |
| 8 | >100.00 | <0.01 | ‒ |
| Celecoxib | >100.00 | 0.056 | 295 |
The result (IC50, μmol/L) is the mean of three determinations acquired using a COX fluorescent inhibitor screening assay kit (Cayman Chemical, MI, USA).
COX-2 selectivity index (COX-1 IC50/COX-2 IC50).
2.2. Compounds having imidazole and imidazoline rings
The imidazole and imidazoline groups, as structurally similar pharmacophores, have been widely explored in the development of NSAIDs30. From 2014 to 2021, several literatures have reported new COX-2 inhibitors containing imidazole and imidazoline moieties (Fig. 5). In 2014, Sarnpitak et al.31 designed and synthesized an active imidazoline analog 17. Compound 17 displayed prominent COX-2 inhibitory activity (IC50 = 0.3 μmol/L) comparable to clinically used celecoxib (IC50 = 0.091 μmol/L) upon in vitro evaluation. This study also proved that replacement of methylsulfonyl group by sulfonamide showed no pronounced suppressive effect on COX-2 inhibition. Four years later, Abdellatif et al.32 reported a number of 4-substituted-imidazoline analogs. Compounds 18–20 were more active towards COX-2 compare to celecoxib. Compounds 18, 20, and 21 were less ulcerogenic than clinical drugs including ibuprofen and celecoxib (Table 2). Structure‒activity relationship study (SAR) revealed that multiple ‒OCH3 (18) substituent on benzene ring has more favorable effect on the COX-2 inhibition and selectivity than other analogs. Some new substituted imidazoline-5-one derivatives 22, 23, and 24 were prepared by Metwally et al.33 Compounds 22–24 showed similar anti-inflammatory activity (% inhibition of edema = 43.1, 41.8, and 49.0) compared to celecoxib (% inhibition of edema = 43.1%), which suggested that keeping the same sulfonamide (SO2NH2) moiety in new structures is crucial to maintain or increase the anti-inflammatory activity. Further study indicated that compounds 22–24 exhibited a high efficacy towards COX-2 inhibition (EIA) with IC50 of 0.090, 0.087, and 0.092 μmol/L, respectively. In addition, several substituted 1,5-diarylimidazole derivatives having the thioalkyl group at position 2 were reported by Navidpour et al.34 in 2014. Of these, compound 25 showed the moderate inhibition (EIA) (IC50 = 14.2 μmol/L) of COX-2 and displayed less selectivity (SI = 3.1) than celecoxib (IC50 = 0.544 μmol/L; SI = 19.4). The results suggested that compounds bearing thiomethyl group at position 2 have better activity as compared with thioethyl derivatives in this study.
Figure 5.
Chemical structures of compounds 17–25.
Table 2.
In vitro COX-1/COX-2 inhibition (IC50, μmol/L), selectivity index, ulcerogenic evaluation for compounds 18–21, and standard agents.
| Compd. | COX-1a COX-2a | COX-2 selectivityb | Ulcer index | |
|---|---|---|---|---|
| 18 | 4.52 | 0.42 | 10.76 | 1.22 |
| 19 | 6.74 | 0.62 | 10.87 | 3.02 |
| 20 | 4.52 | 0.52 | 8.69 | 2.60 |
| 21 | 7.86 | 0.86 | 9.14 | 2.61 |
| Celecoxib | 7.23 | 0.84 | 8.61 | 2.93 |
| Ibuprofen | ‒ | ‒ | ‒ | 20.25 |
The result (IC50, μmol/L) is the mean of three determinations acquired using a COX fluorescent inhibitor screening assay kit (Cayman Chemical, MI, USA).
COX-2 selectivity index (COX-1 IC50/COX-2 IC50).
2.3. Compounds having an indole ring
The indole moiety belongs to an important pharmacophore core for the synthesis of novel selective COX-2 inhibitors35. To discover novel selective COX-2 inhibitors, Hayashi et al.36 designed a new acid-type compound 26 in 2012 (Fig. 6). Compound 26 maintained the basis structure of indomethacin and exerted potent selective COX-2 inhibition with IC50 of 0.009 and 0.155 μmol/L in human cells and HWB cells, respectively. Moreover, compound 26 had good oral anti-inflammation efficacy and potent in vivo anti-oedematous effect. Meanwhile, a new N-1 and C-3 substituted indole derivative 27 was synthesized by Kaur’s group37 that showed selective COX-2 inhibitory activity (EIA) with IC50 of 0.32 μmol/L and SI of >312. According to the docking result, the phenyl CF3 substituent attached to the C N is located near the COX-2 active site and formed an important hydrogen bond to His90, which is crucial for the COX-2 inhibition. Bhat's group38 reported a new COX-2 inhibitor 28, compound 28 not only inhibited COX-2 expression but also possessed desirable gastral safety profile. This work provided valuable information for exploring gastro-protective COX-2 inhibitors. Recently, Singh et al.39 reported several new compounds containing tosyl and dipeptide groups at N-1 and C-3 position, respectively, which were developed for COX-2 inhibitors. Of all the compounds, compounds 29–31 showed similar in vivo anti-inflammatory activities to diclofenac. Moreover, an in vitro COX-2 selectivity assay showed that compounds 29 and 30 displayed competitive inhibition and selectivity of COX-2 (IC50 = 0.006 and 0.099 μmol/L; SI = 351 and 440, respectively). However, compound 31 showed excellent COX-2 inhibitory activity IC50 of 0.54 μmol/L, but the selectivity (SI = 24) is poor. Additionally, Estevão et al.40 synthesized a new indole derivative 32. In comparison with indomethacin, the methoxy at C-5 was replaced by a sulfonamide, the C-2 and C-3 positions were substituted by two 4-fluro benzyls, respectively. Compound 32 showed selective COX-2 inhibitory activity with 67 ± 6% (50 μmol/L) which is close to indomethacin (78 ± 3%). More recently, Jung group41 designed and synthesized a novel N-1, C-3 substituted indole analog 33 that merge the structural motifs of anti-inflammatory ascidian metabolites, herdmanines. To form two vital hydrogen bonds with Tyr355 and Arg120, the acid in indomethacin was replaced by a hydrazone moiety based on bioisosteric replacement drug design strategy. Compound 33 showed a considerable COX-2 inhibitory activity (7.59 μmol/L) and selectivity (SI = 5.16) compared with diclofenac (IC50 = 1.21 μmol/L, SI = 15.18). The indole-containing analogues were mostly designed based on the structure of indomethacin. Consequently, replacement of the C-3 acetic acid moiety in indomethacin by various substitutes is an effective strategy to improves their activity and selectivity. In addition, the modification at N-1 and C-2 is also a reasonable option.
Figure 6.
Chemical structures of compounds 26–33.
2.4. Compounds having a thiazole ring
Thiazole is a privileged pharmacophore in medicinal chemistry and bear high potential for the anti-inflammatory therapeutic option. In the past 10 years, three groups have reported several thiazole derivatives which were developed as selective COX-2 inhibitors42 (Fig. 7). Sağlık et al.43 recently designed and synthesized novel derivatives bearing thiazolyl-hydrazine-methyl sulfonyl moiety as selective COX-2 inhibitors. Compound 34 demonstrated significant and selective COX-2 inhibition potency with an IC50 value of 0.140 ± 0.006 μmol/L and selectivity index of >714.28 comparable to nimesulide (IC50 = 1.684 ± 0.079 μmol/L) and celecoxib (IC50 = 0.132 ± 0.005 μmol/L) in in vitro COX-2 inhibition assay (EIA). Per the molecular docking results, compound 34 bounded in a similar manner as celecoxib with COX-2 enzyme. Later, Abdel-Aziz et al.44 synthesized a few novel anti-inflammatory EGFR inhibitors with cardiac and gastric safety profiles. Chemically, these compounds were formed with pyrimidine-5-carbonitrile hybrids with 2-amino-4-aryl-1,3-thiazole through an acetamide group linker. Compounds 35–37 displayed good and selective COX-2 inhibitions (EIA) (IC50 = 1.17, 1.13, and 1.03 μmol/L; SI = 5.78, 7.84, and 8.21, respectively) relative to celecoxib (IC50 = 0.88 μmol/L, SI = 8.31). Further study indicated that compounds 35–37 exhibited anti-inflammatory activity (the percentage of edema inhibition) up to 90%, 94%, and 86% of meloxicam after 4 h interval and higher gastric safety profiles than meloxicam. Compounds 36 and 37 had a superior safety profile with an ulcer index of 2.70 and 2.40, respectively, compared to meloxicam (UI = 18). In addition, Hofmann's group45 designed a new thiazole analogue, with 4-chloro- and 2-hydroxy-substituted compound 38, which displayed good and selective COX-2 inhibition with activity of 9.1 ± 1.1% (COX-2 product formation) measured at a concentration of 10 μmol/L.
Figure 7.
Chemical structures of compounds 34–38.
2.5. Compounds having a tetrazole ring
Since 2011, two groups have reported the synthesis and pharmacological studies of anti-inflammatory tetrazole derivatives (Fig. 8). Labib et al.46 designed several tetrazole derivatives based on bio-isosteric replacement of SO2NH2 in celecoxib. Structurally, two classes of compounds were designed: isoxazoles (39, 40) and pyrazoles (41–44). Compounds 39–44 are active and displayed potential in vitro COX-2 inhibitory activity in an EIA assay (IC50 = 0.039–0.065 μmol/L). Notably, compounds 40, 42, and 44 attained significant COX-2 selectivity index values which were as selective as celecoxib. Moreover, compounds 40 and 44 showed similar anti-inflammatory activity to celecoxib at different time intervals and were less ulcerogenic than celecoxib (Table 3). Downstream inflammatory factors were also detected, compounds 40 and 44 significantly decrease the production of PGE2 (% inhibition = 81.042 and 82.724 in sequent) which is comparable to celecoxib (% inhibition = 79.666). The collected data indicated that the derivatives with methoxy are more active than those with hydrogen on the benzene ring. Al-Hourani et al.47 reported a tetrazole-containing compound 45, which exhibited potent COX-2 inhibition with IC50 value of 2.0 μmol/L, but the SI value of compound 45 (SI = 210) was less than celecoxib (SI = 313). Five years later, this group48 prepared more 1,5-diaryl-substituted tetrazoles by further modifications to the methylsulfonyl unit. The collected biological data showed that compounds 46 and 47 exhibited moderate COX-2 inhibitory activity (IC50 = 24 and 38 μmol/L, respectively) and selectivity (SI = 0.87 and 5.2, respectively); compound 48 displayed enhanced COX-2 inhibitory activity and selectivity towards COX-2 (EIA) (IC50 = 3 μmol/L, SI = > 67). The acquired results suggested that the presence of the methylsulfonyl unit, methylene spacer at C-1, and longer linker make the new derivatives more active towards COX-2 enzyme.
Figure 8.
Chemical structures of compounds 39–48.
Table 3.
In vitro COX-1/COX-2 inhibition (IC50, μmol/L), selectivity index, ulcerogenic evaluation and in vivo anti-inflammation activity (dose = 50 mg/kg) for compounds 39‒44, and standard agents.
| Compd. | COX-1a | COX-2a | COX-2 selectivityb | Ulcer index | Rat paw edema (mm) (% edema inhibition, 6 h) |
|---|---|---|---|---|---|
| 39 | 11.3 | 0.045 | 251.11 | 0.21 ± 0.02 | 7.27 ± 0.14 |
| 40 | 12.4 | 0.041 | 302.44 | 0.123 ± 0.01 | 5.10 ± 0.36 |
| 41 | 10.5 | 0.064 | 164.06 | 0.26 ± 0.01 | 6.54 ± 0.0.14 |
| 42 | 12.8 | 0.043 | 297.67 | 0.21 ± 0.01 | 5.47 ± 0.23 |
| 43 | 10.9 | 0.065 | 167.69 | 0.55 ± 0.03 | 7.05 ± 0.35 |
| 44 | 12.4 | 0.039 | 317.95 | 0.11 ± 0.01 | 4.99 ± 0.19 |
| Celecoxib | 12.7 | 0.045 | 282.22 | 0.167 ± 0.01 | 5.21 ± 0.19 |
| Indomethacin | 0.10 | 0.080 | 1.25 | 0.88 ± 0.04 | ‒ |
‒Not applicable.
The result (IC50, μmol/L) is the mean of three determinations acquired using a COX fluorescent inhibitor screening assay kit (Cayman Chemical, MI, USA).
COX-2 selectivity index (COX-1 IC50/COX-2 IC50).
2.6. Compounds having an oxadiazole ring
Oxadiazole moiety has precedent for use as a bioisosteric substitute in drug design and synthesis. In the past ten years, three groups had reported the synthesis of COX-2 inhibitors containing oxadiazole group. As summarized in Fig. 9, El-Sayed et al.49 designed a novel heterocyclic oxadiazoles 49, which exhibited prominent COX-2 inhibitory activity (IC50 = 0.041 μmol/L) and selectivity (SI = 89.72) comparably to celecoxib (IC50 = 0.049 μmol/L, SI = 308.16) by using an enzyme immunoassay. In 2020, Alfayomy et al.50 reported two new selective COX-2 inhibitors 50 and 51, which belong to pyrimidine-5-carbonitrile hybrids with 1,3,4-oxadiazole scaffold. Compounds 50 and 51 showed significant and selectivity on COX-2 inhibition. Further investigation indicated that compounds 50 and 51 displayed good in vivo anti-inflammatory activity up to 89.5% inhibition at 4 h in carrageenan-induced rat paw edema assay. Moreover, compound 50 displayed superior safety profile than celecoxib. The results revealed that the pyrimidinyl substituent markedly affected the activity against COX-2. Grover et al.51 synthesized a new series of oxadiazole-comprising derivatives 52–55. Compounds 52–55 exhibited good and selective inhibition of COX-2 (EIA), but the efficacy and selectivity were less than reference drug celecoxib. Besides, compounds 53 and 55 had better in vivo anti-inflammatory activity than celecoxib (Table 4). The results confirmed that tert-butyl is an indispensable moiety to enhance COX-2 inhibitory activity and selectivity. This approach provides an alternative inspiration for new COX-2 inhibitor development.
Figure 9.
Chemical structures of compounds 49–55.
Table 4.
In vitro COX-1/COX-2 inhibition (IC50, μmol/L), selectivity index and in vivo anti-inflammation activity (dose = 150 μmol/kg) for compounds 52–55, and standard agents.
| Compd. | COX-1a | COX-2a | COX-2 selectivityb | Rat paw edema (% edema inhibition, 5 h) |
|---|---|---|---|---|
| 52 | 54.99 | 0.74 | 74.31 | 41.06 ± 2.64 |
| 53 | 63.76 | 0.48 | 132.83 | 58.04 ± 1.30 |
| 54 | 55.05 | 0.81 | 67.96 | 46.38 ± 2.47 |
| 55 | 60.61 | 0.89 | 68.10 | 59.33 ± 2.19 |
| Celecoxib | 37.98 | 0.10 | 379.80 | 49.81 ± 1.92 |
| Indomethacin | 98.23 | 50.99 | ‒ | ‒ |
The result (IC50, μmol/L) is the mean of three determinations acquired using a COX fluorescent inhibitor screening assay kit (Cayman Chemical, MI, USA).
COX-2 selectivity index (COX-1 IC50/COX-2 IC50).
2.7. Derivatives having fused heterocyclic fragments
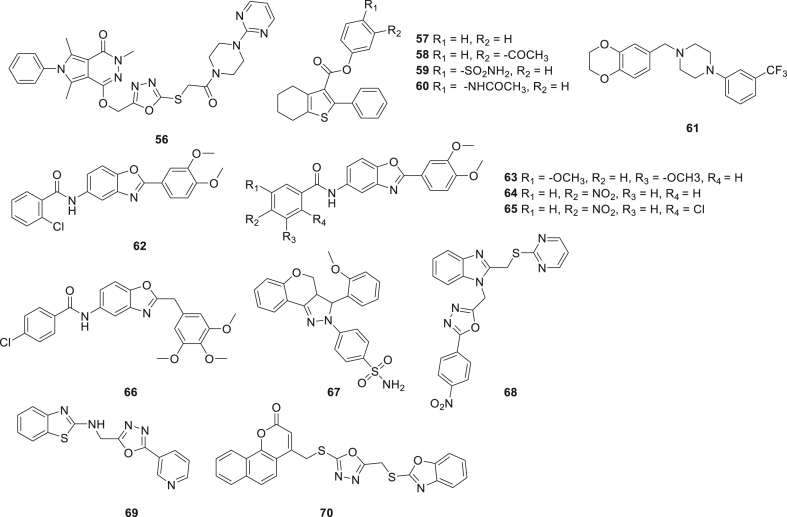
Recently, fused heterocyclic rings have been flexibly used as crucial core for COX-2 inhibitors (Figure 10, Figure 11). Szczukowski et al.52 produced a number of novel hybrid pyrrolo[3,4-d]pyridazinone derivatives bearing 4-aryl-1-(1-oxoethyl)piperazine pharmacophores. Compound 56 exerted no cytotoxicity and had significant selective COX-2 inhibition at lower concentrations. Structurally, the arylpiperazine pharmacophore is connected with 1,3,4-oxadiazole ring via sulfur. The results indicated that elongating the linker part is important to enhance the anti-inflammatory activity. Khatri et al.53 prepared several benzothiophene derivatives, and compounds 57, 58, 59, and 60 showed potent and selective COX-2 inhibition (EIA) (IC50 = 0.33, 0.31, 0.67, and 1.40 μmol/L respectively, selectivity index: 48.8–183.8). Analysis of SAR indicated that various substitutions of benzyl were the major determinants for COX-2 inhibition, such as compounds 59 (4-SO2NH2) and 60 (‒NHCOCH3), which showed enhanced activity compared with 57 and 58. Moreover, compounds 57–60 showed considerable anti-inflammatory activity in vivo. Sun et al.54 reported a series of novel selective inhibitors of enzyme COX-2. Compound 61 had potential anti-inflammatory activity with no cytotoxicity. Moreover, compound 61 showed selective inhibition towards COX-2 (IC50 = 0.2 μmol/L) and COX-1 (IC50 = 8.35 μmol/L) in an enzyme immuno assay (Bio-Swamp). Four bioactive benzoxazole analogs were prepared by Kaur et al.55. Compounds 62–65 showed significant COX-2 inhibitory activity and selectivity towards COX-2 over COX-1. Of all the compounds, compound 62 was the most active compound with excellent inhibition of COX-2 (EIA) (IC50 = 0.04 μmol/L) and good selectivity (SI = 25.5). The in vivo assays results indicated that compounds 62–65 had significant anti-inflammatory activity (% inhibition = 84.09%, 68.18%, 79.54% and 72.72%, respectively), greater than reference drug ibuprofen (% inhibition = 65.90, dose = 60 mg/kg). More importantly, they demonstrated a more significant gastric tolerance than ibuprofen, the pharmacokinetic profile of compounds 62–65 showed their available druggability. Later, a group of benzoxazole-benzamide analogs 66 was reported by the same group56. Compound 66 exhibited potent and selective COX-2 inhibition (EIA) with IC50 of 0.14 μmol/L as compared to celecoxib (IC50 = 0.15 μmol/L). Compound 66 also exhibited in vivo anti-inflammatory activity (79.54%) superior to ibuprofen (65.90%) (dose = 20 mg/kg); The ulcerogenic activity results indicated that compound 66 had significant more gastric tolerance than ibuprofen. The collected data revealed that electron withdrawing substitutions at ortho and para positions to phenyl ring aide in improving activity. Molecular docking results suggested that the benzoxazole ring is a crucial moiety to interact with Tyr355 and Arg120 of the COX-2 enzyme. All the experimental date demonstrated that compound 66 is a potential COX-2 inhibitor and valuable for further clinical investigation. Chen's group57 reported a novel dihydropyrazole sulfonamide derivative 67, which exhibited remarkable and selective COX-2 inhibition (IC50 = 0.33 μmol/L), the potency almost identical to that of celecoxib. Several new benzimidazole derivatives endowed with oxadiazole were described by Rathore et al.58 in 2014. Compound 68 displayed reasonable COX-2 inhibition (EIA) (IC50 = 8.2 μmol/L) and selectivity (SI > 12.1). Moreover, compound 68 was much safer in terms of gastric toxicity with a severity index of 0.48, lower than that of indomethacin. The SAR studies revealed that the electron-withdrawing compounds showed better COX-2 inhibitory activity than those of the electron-releasing ones. An oxadiazole analog 69 was reported by Iyer et al.59 in 2016 and exerted good COX-2 inhibition but associated with moderate COX-1 inhibition in vitro. The undesirable COX-2 selectivity indicated that compound 69 need further modification. In the same year, Nesaragi's group60 prepared some novel coumarinyl-1,3,4-oxadiazolyl-2-mercaptobenzoxazoles. Of these, compounds 70–72 displayed moderate and selective COX-2 inhibitions (IC50 = 23.71, 33.47 and 23.95 μmol/L, respectively, SI = 33.95, 20.25 and 24.98, respectively). The results suggested that the activity is influenced by the bulkiness and lipophilicity of substituent on the benzene ring. Abdu-Allah et al.61 synthesized two novel 4-aminosalicylate based thiazolinone derivatives 73 and 74, both of which showed excellent COX-2 inhibitory efficacy (IC50 = 44 and 39 nmol/L) and selectivity indexes (SI = 66.82 and 68.46). Unfortunately, the selectivity indices of tested compounds (73 and 74) were lower than celecoxib but still higher than diclofenac sodium and indomethacin. Additionally, compounds 73 and 74 showed improved safety profiles than indomethacin. Analysis of the biological data revealed that the bulkiness of the substituent at heterocyclic ring enhanced the COX-2 inhibition activity. Murahari et al.62 introduced five active azomethine derivatives 75–79. Compounds 75–79 showed potent and selective COX-2 inhibition revealing that substitution with electron donors such as methoxyl and hydroxyl has unfavorable effect on anti-inflammatory activity. Meanwhile, compounds 75–79 were subjected to an ulcerogenic activity assay, and showed safer profiles with low ulcer indices when compared to the clinically used drug celecoxib (Table 5).
Figure 10.
Chemical structures of compounds 56–70.
Figure 11.
Chemical structures of compounds 71–79.
Table 5.
In vitro COX-1/COX-2 inhibition (IC50, μmol/L), selectivity index, ulcerogenic evaluation for compounds 75‒79, and standard agent.
| Compd. | COX-1a | COX-2a | COX-2 selectivityb | Ulcer index |
|---|---|---|---|---|
| 75 | 276.44 | 4.320 | 63.99 | 0.603 ± 0.15 |
| 76 | 76.67 | 25.87 | 2.963 | ‒ |
| 77 | 300.72 | 11.48 | 26.19 | 0.642 ± 0.25 |
| 78 | 33.58 | 7.750 | 4.332 | ‒ |
| 79 | 225.68 | 21.87 | 10.31 | 1.991 ± 0.34 |
| Celecoxib | >50 | 0.34 | 147.05 | 1.204 ± 0.06 |
The result (IC50, μmol/L) is the mean of three determinations acquired using a COX fluorescent inhibitor screening assay kit (Cayman Chemical, MI, USA).
COX-2 selectivity index (COX-1 IC50/COX-2 IC50).
3. Chemistry and pharmacology of structurally modified COX-2 inhibitors
3.1. Derivatives of existing market drugs
Structural modification of existing drugs is an effective approach for drug development. The structures of bumetanide, celecoxib, indomethacin, and nimesulide have also been altered to develop new derivatives (Fig. 12). In 2020, Ibrahim et al.63 have reported several novel benzenesulfonamide analogs which aim to developed as COX-2 inhibitors. Structurally, bumetanide was used as a precursor to synthesized new analogues. Of interest, the replacement of an acetic group by the bulky triazole moieties led to the potent COX-2 inhibitors, compounds 80 and 81. Compounds 80 and 81 exhibited excellent inhibition (Cayman's COX (ovine) Colorimetric Inhibitor Screening Assay) of COX-2 with IC50 values of 0.28 and 0.17 μmol/L, and a considerable selectivity index (SI = 71.93 and 115.82) in comparison to celecoxib (SI = 4.93). Further investigation indicated that compounds 80 and 81 showed good anti-inflammatory activity and lower ulcerogenicity when administered orally. On the basis of the structure of indomethacin. Ikeda's group64 reported a fluorinated analog 82 of indomethacin, which bearing a lipophilic 3,3,3-trifluoroprop-1-enyl group at C-2 position. Compound 82 displayed greater COX-2 inhibitory activity and selectivity than indomethacin. Molecular docking results indicated that fluorine substituent of compound 82 contributed to a significant gain of the binding affinity for COX-2 by increasing van der Waals contacts. Aiming to discover novel selective COX-2 inhibitor. Chandna et al.65 designed two series of celecoxib derivatives containing 1,5-diaryl fragments by bioisosteric replacement. The first series of celecoxib analogue were synthesized bearing a cyano group in place of sulfonamide moiety and then carbothioamide moiety was introduced and prepared the second series of analogues. Among these compounds, 83–86 exhibited potential selective COX-2 inhibitions (IC50 = 7.07–19.22 μmol/L), but the activity of compounds 83–86 is weaker than that of celecoxib (Table 6). Nevertheless, compounds 83–86 showed potent in vivo anti-inflammatory activity which is comparable to indomethacin. Based on SAR study, the carbothioamide substituent compounds displayed better activity and selectivity than those of cyano substituent ones. In another study, Hassan et al.66 reported a series of anti-inflammatory celecoxib analogs 87–91 by introducing a benzofuran moiety. It's worth noting that phenyl sulfonamide is an indispensable pharmacophore to maintain COX-2 selectivity. Accordingly, compounds 87–91 presented potent and selective COX-2 inhibitions with IC50 values of 0.34–0.52 μmol/L. Meanwhile, changing the hydrogen in 87 into methyl in 89 led to minor decrease in COX-2 inhibition. The celecoxib analogue 91 with trifluoromethyl also had better COX-2 inhibition than fluoro analogue 90. Compounds 87–91 also possess better gastric safety profile and less gastric ulceration effect compared to clinical drug celecoxib (Table 7). Renard et al.67 prepared a series of nimesulide analogs 92–94 in accordance with its favorable gastric and cardiovascular safety profile. Chemically, these derivatives were designed in which the nitrobenzene ring was replaced by pyridine nucleus based on isosteric replacement. The oxygen atom also has been replaced with nitrogen to construct a new linker between two aromatic rings. As a consequence, compounds 92–94 exhibited remarkable inhibitory activity associated to a COX-2/COX-1 selectivity ratio (7.46, 15.35, and 7.67, respectively; IC50 = 0.26, 0.09, and 0.30 μmol/L) similar or higher than that of celecoxib (ratio: 7.46, IC50 = 0.35 μmol/L) in a human whole blood model. The SAR study indicated that the various substitutions on the benzene ring are the main factor affecting their activity towards COXs.
Figure 12.
Chemical structures of compounds 80–94.
Table 6.
In vitro COX-1/COX-2 inhibition (IC50, μmol/L), selectivity index and in vivo anti-inflammation activity for compounds 83–86, and standard agents.
| Compd. | COX-1a | COX-2a | COX-2 selectivityb | Rat paw edema (% edema inhibition, 4 h) |
|---|---|---|---|---|
| 83 | >30 | 19.22 | >1.56 | 0.24 ± 0.05 |
| 84 | >30 | 7.07 | >4.24 | 0.84 ± 0.01 |
| 85 | >30 | 9.07 | >3.31 | 0.7 ± 0.07 |
| 86 | >30 | 17.43 | >1.72 | 0.3 ± 0.01 |
| Celecoxib | >30 | 0.15 | >200 | ‒ |
| Indomethacin | 0.18 | >30 | ‒ | 0.17 ± 0.03 |
The result (IC50, μmol/L) is the mean of three determinations acquired using a COX fluorescent inhibitor screening assay kit (Cayman Chemical, MI, USA).
COX-2 selectivity index (COX-1 IC50/COX-2 IC50).
Table 7.
In vitro COX-1/COX-2 inhibition (IC50, μmol/L), selectivity index, ulcerogenic evaluation (rat 50 mg/kg) and for compounds 87‒91, and standard agent.
| Compd. | COX-1a COX-2a | COX-2 selectivityb | Ulcer index | |
|---|---|---|---|---|
| 87 | >50 | 0.40 | >6.67 | 13.82 ± 0.62 |
| 88 | >50 | 0.52 | >96.15 | 11.56 ± 0.54 |
| 89 | >50 | 0.36 | >138.90 | 10.50 ± 0.63 |
| 90 | >50 | 0.46 | >108.70 | 11.75 ± 0.63 |
| 91 | >50 | 0.34 | >147.06 | 10.50 ± 0.59 |
| Celecoxib | >50 | 0.28 | >178.57 | 16.12 ± 0.86 |
The result (IC50, μmol/L) is the mean of three determinations acquired using a COX fluorescent inhibitor screening assay kit (Cayman Chemical, MI, USA).
COX-2 selectivity index (COX-1 IC50/COX-2 IC50).
3.2. Derivatives having fragments of natural products
Pharmaceutical chemists continuously design new chemical scaffolds inspired by reported natural products from 2011 to 2021, including examples that were displayed in Fig. 13. Ribeiro et al.68 designed a series of cinnamic acid derivatives, and found three active compounds 95–97 as new COX-2 inhibitors. Compounds 95–97 exhibited moderate inhibition of COX-2 (human whole blood assay) (IC50 = 3.0, 2.4, and 1.09 μmol/L; SI ≥ 33, 10.0, and 3.9, respectively). The results confirmed that phenolic hydroxyl fragment is a potential pharmacological core for COXs inhibition. The data acquired also indicated that by introducing a couple of bulky hydrophobic groups may be a fruitful approach to increase the COX-2 selective inhibition. Takahash et al.69 reported a new synthetic serotonin derivative: compound 98. Compound 98 showed weak inhibition on COX-2 (IC50 = 42.5 μmol/L) and considerable selectivity in serotonin derivatives testing assay. In this study, it was confirmed that extending amide linkage of 98 is crucial to increase COX-2 inhibitory activity. Rayar et al.70 prepared a cyclocoumarol analog 99, which exhibited good inhibitory activity against PGE2 production, and no inhibitory activity against the COX-1 was observed. Further study indicated that compound 99 showed considerable anti-inflammatory activity in a concentration-dependent manner.
Figure 13.
Chemical structures of compounds 95–99.
4. Chemistry and pharmacology of potential COX-2 inhibitors from nature origin
Naturally occurring compounds have been reported to inhibit COX-2 enzyme, thereby possessing beneficial effects against inflammation. In the past ten years, a large number of natural compounds were identified as COX-2 inhibitors or exerting COX-2 inhibitory activity, examples as natural phenols, flavonoids, terpenoids, alkaloids, and other hybrids. The characteristics of their structural core scaffolds, COX-2 inhibitory activity, anti-inflammatory effects, and structure‒activity relationships are introduced as follows:
4.1. Phenols
Čulenová et al.71 investigated a phenolic compound 100 from Morus alba root bark. Compound 100 showed significant more in vitro COX-2 inhibition with the IC50 value of 15.85 μmol/L than indomethacin (IC50 = 27.04 μmol/L, SI = 0.16), but the selectivity of COX-2 is low (SI = 0.47). Natu's group72 isolated an anti-inflammatory compound 101 from Alpinia officinarum Hance. Biological investigation indicated that compound 101 exhibited potent anti-inflammatory ability by the inhibition of the release and/or action of histamine, serotonin and kinin, and by COX-2 inhibition. Liu et al.73 recently discovered two novel anti-inflammatory compounds 102 and 103 from Carissa spinarum. Compounds 102 and 103 were identified and showed good COX-2 inhibition by the COX-2 inhibition screening method (EIA) and the activity of compound 102 (IC50 value = 0.3 μmol/L) was comparable to indomethacin (IC50 = 1.1 μmol/L). Nile et al.74 investigated the anti-inflammatory potency of three natural acids: ferulic acid (104), caffeicacid (105), and gallic acid (106). Compounds 104–106 showed potent COX-2 inhibitory activity (EIA) (IC50 = 68.5, 62.5, and 65.2 μg/mL, respectively). Further investigation suggested that compounds 104–106 exerted anti-inflammatory effect through suppressing the activity of xanthine oxidase and COX-2 enzyme. Paulino et al.75 extracted and analyzed the phenols of propolis and grape pomace from Uruguayan species. Z-Fertaric acid 107 was identified and demonstrated good anti-inflammatory activity, and COX-2 inhibitory activity. The potential pharmacological activity of curcumin 108 was investigated76. Briefly, curcumin exhibited anti-inflammatory activity by significantly reducing the production of pro-inflammatory mediators including COX-2 and PGE2 through NF-κB pathway. A new homoegonol 109 was isolated from the extracts of Mamuyo (Styrax ramirezii Greenm)77. 109 displayed anti-inflammatory activity by nitric oxide reduction. In addition, 109 was able to decrease the LPS-induced transcription of inducible pro-inflammatory enzyme coding genes of COX-2. Cheng's group78 reported the isolation and anti-inflammatory evaluation of two phenols, Periplanetol A (110) and Periplanetol B (111) (Fig. 14), from Periplaneta americana. 110 and 111 exhibited good COX-2 inhibition activity with IC50 values of 0.768 and 0.617 μmol/L, but it is lower than that of celecoxib (IC50 = 0.041 μmol/L).
Figure 14.
Chemical structures of compounds 100–111.
4.2. Flavonoids
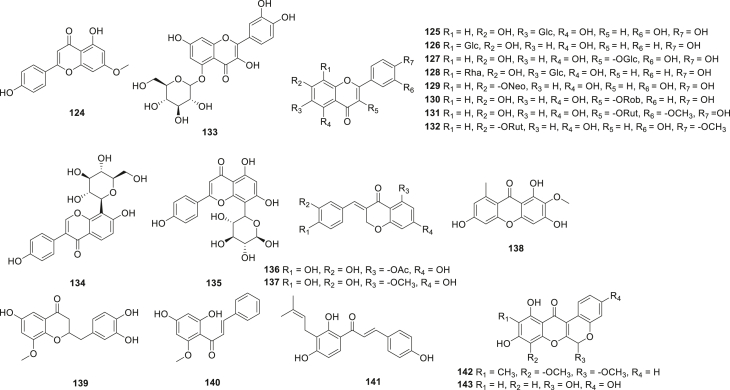
Honmore et al.72 discovered a flavonoid derivative 112, which exhibited selective COX-2 inhibition. The in vivo mice assay showed that 112 had potential anti-inflammatory activity in paw edema in comparison with diclofenac. Paulino et al.75 extracted several flavonoids from propolis and grape pomace, flavonoid glycosylate (113), pinobanksin (114), and anthocyanin (115). 113–115 showed potent anti-inflammatory and selective COX-2 inhibitory activity (EIA) (SI = 1.82, 1.52, and 1.64, respectively). Hu et al.79 identified a flavonoid, kaempferol-3-O-rutinoside 116, which showed VEGF-C-mediated anti-inflammation by interfering with VEGF-C-related signal transduction and interfered with the NF-κB signaling pathway. Compound 116 exhibited high potency to trigger the receptor activation and inhibited the production of IL-6, TNF-α, and the expression of iNOS and COX-2. The anti-inflammatory effects of compound 116 was investigated in LPS-induced macrophages, and indicated that 116, as a natural compound, could be developed as an anti-inflammatory agent with good drug likeness. Nobiletin 117 (NOB) is a potential anti-inflammatory candidate. Xiao's group80 recently reported their work on the anti-inflammatory potency of 117. 117 showed significant anti-inflammatory activity by inhibiting the expression of pro-inflammatory markers. Further investigation demonstrated that compound 117 sharply reduced the levels of iNOS and COX-2 protein in a concentration-dependent manner. Three new flavonoids 18–20 (Fig. 15) were found from the leaves of Myrica rubra sieb81, which can inhibit the expression levels of iNOS and COX-2 protein in a dose-dependent manner, and they also showed significant anti-inflammatory activity by inhibiting LPS-stimulated pro-inflammatory cytokines. Hanákova et al.82 isolated a new geranylated flavanone 121 from Paulownia tomentosa fruits. Compound 121 had moderate COX-2 inhibition activity (EIA) (IC50 = 9.5 μmol/L, SI = 2.8) and was more selective than ibuprofen (IC50 = 4.2 μmol/L, SI = 1.5). Hosek et al.83 reported a new geranylated flavonoid, diplacone 122, which can significantly down-regulated the expression of COX-2 in Western blot assay. Meanwhile, a new flavonoid 123 was purified from licorice residues84, which displayed potent NO inhibitory effect (IC50 = 9.89 μmol/L) compared with minocycline (IC50 = 33.20 μmol/L). Compound 123 also notably exhibited IL-1β, IL-6, iNOS, and COX-2 inhibition. A new compound 124 was identified from Daphne genkwa Sieb and showed anti-inflammatory activity through NF-κB signaling pathway85, decreased expression levels of iNOS and COX-2 mRNA were observed. A large family of flavonoids, comprising compounds 125‒132 (Fig. 16), was isolated from lotus plumule86. Compounds 125‒132 displayed significant anti-inflammatory activity by inhibiting the production of pro-inflammatory cytokines. Further study demonstrated that compounds 125‒132 were considered as potential COX-2 ligands by computer modeling calculations. An et al.87 analyzed the anti-inflammatory effect of Saxifragin (133) which is founded abundantly in plants, especially in Saxifrage stolonifera. Compound 133 showed outstanding anti-inflammatory activity and decreased the production of PGE2 through suppressing the level of protein expression of COX-2. Puerarin (134) is a flavonoid derivative and possesses antipyretic and sedation activity. Pharmacological experiments indicated that puerarin exerted anti-inflammatory activity via the ERK/Nrf2/ARE pathway by inhibiting the production of pro-inflammatory markers including iNOS and COX-288, it has the potential to be a COX-2 inhibitor. Toyama's group89 reported their work on the anti-inflammatory activity of 8-C-rhamnosyl apigenin 135, which demonstrated selective COX-2 inhibitory activity with an IC50 value of 28.6 μmol/L and potent in vivo anti-inflammatory activity. Waller et al.90 evaluated the COX-2 inhibitory activity of four flavonoids 136–139 from the bulbs of the Southern African Ledebouria socialis. Compounds 136–139 showed good activity towards COX-2. Notably, compounds 136 and 137 had reasonable and selective COX-2 inhibitory activity (EIA) (IC50 = 1.12 and 2.87 μmol/L, respectively). Kim et al.91 evaluated the anti-inflammatory activity of a new chalcone 140 from Alpinia species. Compound 140 demonstrated inhibition of COX-2 expression and NF-κB activation in a luciferase transcriptional assay. Recently, Zhou et al.92 detected the anti-inflammatory therapeutic effects of a natural chalcone, isobavachalcone 141, which showed strong iNOS, COX-2, and NF-κB p65 inhibitory activity and attenuated the production levels of pro-inflammatory cytokine PGE2. This compound is also a potential lead for further modifications and pharmacological evaluation. In addition, the methanol extract of Boerhaavia diffusa roots was investigated and led to the discovery of two active rotenoids 142 and 14393, both of which showed moderate COX-2 inhibition with IC50 value of 31.4 and 25.5 μmol/L, respectively. However, both compounds 142 and 143 exerted less COX-2 selectivity indices of 1.09 and 0.79.
Figure 15.
Chemical structures of compounds 112–123.
Figure 16.
Chemical structures of compounds 124–143.
4.3. Terpenoids
Bauer's group94 reported two new terpenoid derivatives: 144 and 145 from Hypericum cistifolium. Compounds 144 and 145 exhibited anti-inflammatory activity by inhibiting COXs activities (EIA). However, both of compounds 144 and 145 displayed relative low COX-1 and COX-2 inhibition. Zhang et al.95 isolated and evaluated the ethyl acetate fraction of the ethanol extract from Mallotus conspurcatus croizat, and found two new terpenoids 146 and 147, both of which demonstrated marked suppression of the secretion of PGE2 and the expression of TNF-α, iNOS, NF-κB, and COX-2 proteins. Three new sesquiterpenoids 148, 149, and 150 were isolated from the rhizomes and roots of Nardostachys jatamansi (Fig. 15)96, which inhibit the expression of pro-inflammatory mediators COX-2 protein and cytokine PGE2. An's group97 reported a novel triterpenoid 151 from Rosa rugosa root, which potently inhibited the expression of COX-2 protein and also suppressed the production of PGE2. Five new anti-inflammatory sesquiterpenes 152–156 (Fig. 17) were found from the leaves of Artemisia lavandulaefolia98, the biological evaluation results showed that compounds 152–156 had weak COX-2 inhibitory activity with IC50 values of 43.29–236.33 μmol/L. In 2020, Choo group99 investigated the potential activity of a sesquiterpene lactone, costunolide 157. Compound 157 decreased the expression level of COX-2 protein and have good anti-inflammatory activity through NF-κB signaling pathway. Gao's group100 recently isolated several new cyathane diterpenoids from the bird's nest fungus Cyathus africanus. Of the new compounds, compound 158 exhibited the most active COX-2 and iNOS inhibitory effects. Kim's group101 reported two anti-inflammatory compounds, elatoside (159) and kalopanax-saponin F (160), which were first isolated from Aralia elata. Compounds 159 and 160 suppressed the NF-κB activation induced by TNF-α with IC50 values of 4.1 and 9.5 μmol/L, respectively. Compounds 159 and 160 also showed inhibitory activity towards COX-2 in a dose-dependent manner.
Figure 17.
Chemical structures of compounds 144-160.
4.4. Alkaloids
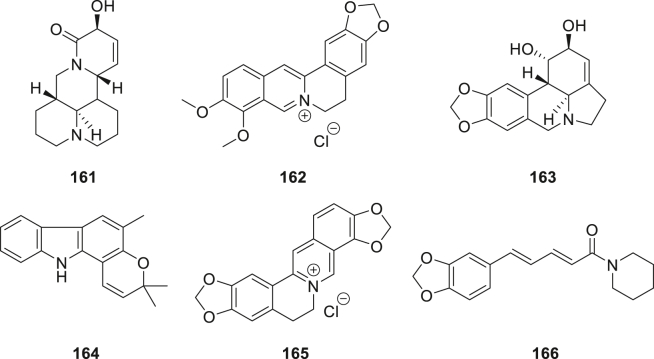
More recently, a large family of quinolizidine alkaloids were purified from the seeds of Sophora alopecuroides (Fig. 18)102. Among them, a new anti-inflammatory alkaloid 161 exhibited higher NO inhibition with IC50 values of 29.19 μmol/L than matrine (IC50 = 38.90 μmol/L). Compound 161 also showed anti-inflammatory activity through decreasing the protein levels of COX-2. Feng et al.103 had investigated the COX-2 inhibitory activity of berberine hydrochloride 162, which showed inhibitory activity of the overexpressed COX-2 through PPAR-γ pathway. Kang et al.104 reported a new alkaloid 163 extracted from Amaryllidaceae, which exhibited anti-inflammatory activity via the P38 and STATs signal pathways. The expression levels of iNOS and COX-2 protein inhibited by 163 were observed, but didn't suppress the transcription of the COX-2 gene, suggesting that 163 may serve as a COX-2 inhibitor. Mohan et al.105 investigated and assessed a major carbazole alkaloid girinimbine 164 presents in curry leaves, which exhibited potential anti-inflammatory activity. Compound 164 demonstrated suppressing effect on COX-2 enzyme, but no effect on COX-1 in an EIA assay. The result indicated that girinimbine 164 significantly inhibited COX-2 enzyme (% inhibition = 52.5; Dose = 25 μg/mL). Rui's group106 reported an isoquinoline alkaloid (coptisine, 165) from Coptidis rhizome. Similarly, compound 165 showed anti-inflammatory activity via the inhibition of NF-κB pathway. In particular, compound 165 effectively blocked the production of PGE2 through COX-2 inhibition. Lee's group107 evaluated and assessed the anti-platelet activity of a major alkaloid of black pepper and long pepper: piperine 166, which showed anti-inflammatory activity by regulating the AA-metabolizing enzymes. In the downstream mechanism, compound 166 decreased the production of PGE2 and PGD2 via COX-2 inhibition.
Figure 18.
Chemical structures of compounds 161–166.
4.5. Others
In addition to the typical classes of natural products as introduced above, hybrid natural compounds (Fig. 19) were also discovered and showed COX-2 inhibitory and anti-inflammatory activity. Lin et al.108 isolated and identified a novel quinone 167 from soft coral Sinularia flexibilis. The expression of COX-2 protein was significantly inhibited by compound 167 at 20 μmol/L with no cytotoxicity. Choi et al.109 examined the anti-inflammatory activity of several naturally occurring anthraquinone derivatives, which were isolated from the Rhubarb Rhizome. The results indicated that compound 168 was the most potent of the compounds in inhibiting the protein expression of COX-2. Liu's group110 reported a new phenyl compound 169, which was identified from a mangrove plant derived fungus Botryosphaeria sp. Compound 169 exhibited remarkable COX-2 inhibitory activity (IC50 = 1.12 μmol/L). A few phenylpropanoid derivatives 170–172 were purified from Chinese Olive by He's group111. Western blot analyses were performed in this study and found that they significantly and dose-dependently reduced the expression level of COX-2 protein. Shen's group112 isolated a new phenylpropanoid (+)-episesaminone 173 from Cinnamomum camphora, which prominently suppressed the expression levels of COX-2 protein. Additionally, three phenylpropanoids 174–176 were isolated from Lilium Asiatic hybrids flowers by Baek's group113. At a concentration of 50 μg/mL, compounds 174–176 can effectively decreased COX-2 expressions.
Figure 19.
Chemical structures of compounds 167–176.
5. Conclusions and future perspectives
COX-2 is a bio-functional enzyme that catalyzes the biosynthesis of PGs during inflammation, and has become a significant therapeutic target when searching for anti-inflammatory drugs. Since 2011, more efforts have been focused on mining new chemical scaffolds as COX-2 inhibitors. The main emphasis of this review was on the potent COX-2 inhibitory and anti-inflammatory activity of various structural families of compounds, which have been reported within the last decade. With respect to the SAR, pyrazole analogs showed the most potent and selective inhibition of COX-2. Derivatives having fragments of natural products only showed moderate COX-2 inhibition and thereby demand more structural modification to improve their activity. Moreover, derivatives having indole, oxadiazole, thiazole, and tetrazole pharmacological cores also displayed acceptable COX-2 inhibitory activity. His90, Arg120 and Arg513 were found to be most important amino acids for the inhibition and selectivity of COX-2. Meanwhile, extensive in vitro and in vivo pharmacological tests were performed and aim to discover new selective COX-2 inhibitors with safety profiles. In addition, a lot of natural compounds with good COX-2 inhibitory and anti-inflammatory activity were included herein. Natural products described in this review may provide inspiration for pharmaceutical chemists and also could serve as a foundation for novel COX-2 inhibitor design to avoid undesirable adverse effects.
Selective inhibition of COX-2 is a major feature of the new generation of NSAIDs, there are several prospects need to be considered for the development of next-generation of NSAIDs.
-
1)
The new COX-2 inhibitors must be able to reduce stomach irritation and the risk of peptic ulcers.
-
2)
In some respects, COX-1/COX-2 balanced inhibitors maybe a new direction for the development of NSAIDs, when the serious adverse effects of either non-selective or selective inhibitors are considered.
-
3)
Majority of derivatives require the presence of aryl group as the basic scaffold for COX-2 inhibitors and thereby the solubility of the new compounds need to be further considered in clinical use. Introducing hydrophilic groups into the structures may be helpful to address this problem.
The above discussion will undoubtedly attract more interest in the coming years. Regarding the future research on COX-2 inhibitor, we strongly feel that utilizing the traditional medicinal chemistry approach seems to be insufficient for current clinical needs, combing genetic engineering, enzyme engineering, and computer science may be a fruitful way to confront future challenges.
Acknowledgments
The author would like to thank Dr. K. Kaliyaperumal, Dr. M. Fredimoses, and Dr. B. Sachin for their carful revisions of this work.
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences
Author contributions
Zhiran Ju and Fen-Er Chen generated the manuscript draft. Menglan Li, Junde Xu, Daniel C. Howell, and Zhiyun Li edited and revised the manuscript. All authors reviewed and approved the final version of the manuscript.
Conflicts of interest
The authors have no conflicts of interest to declare.
References
- 1.Nathan C., Ding A. Nonresolving inflammation. Cell. 2010;140:871–882. doi: 10.1016/j.cell.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 2.Lichtenberger L.M., Wang Z.M., Romero J.J., Ulloa C., Perez J.C., Giraud M.N., et al. Non-steroidal anti-inflammatory drugs (NSAIDs) associate with zwitterionic phospholipids: insight into the mechanism and reversal of NSAID-induced gastrointestinal injury. Nat Med. 1995;1:154–158. doi: 10.1038/nm0295-154. [DOI] [PubMed] [Google Scholar]
- 3.Bindu S., Mazumder S., Bandyopadhyay U. Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: a current perspective. Biochem Pharmacol. 2020:114147. doi: 10.1016/j.bcp.2020.114147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vane J.R., Botting R.M. Mechanism of action of nonsteroidal anti-inflammatory drugs. Am J Med. 1998;10:2S–8S. doi: 10.1016/s0002-9343(97)00203-9. [DOI] [PubMed] [Google Scholar]
- 5.Vane J.R., Botting R.M. Mechanism of action of anti-inflammatory drugs. Scand J Rheumatol. 1996;25(sup102):9–21. doi: 10.3109/03009749609097226. [DOI] [PubMed] [Google Scholar]
- 6.Ricciotti E., FitzGerald G.A. Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol. 2011;31:986–1000. doi: 10.1161/ATVBAHA.110.207449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morteau O. Prostaglandins and inflammation: the cyclooxygenase controversy. Arch Immunol Ther Exp (Warsz) 2000;48:473–480. [PubMed] [Google Scholar]
- 8.Rossi A., Kapahi P., Natoli G., Takahashi T., Chen Y., Karin M., et al. Anti-inflammatory cyclopentenone prostaglandins are direct inhibitors of IκB kinase. Nature. 2000;403:103–108. doi: 10.1038/47520. [DOI] [PubMed] [Google Scholar]
- 9.Funk C.D. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294:1871–1875. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- 10.Smith W.L., Dewitt D.L. Prostaglandin endoperoxide H synthases-1 and -2. Adv Immunol. 1996;62:167–215. doi: 10.1016/s0065-2776(08)60430-7. [DOI] [PubMed] [Google Scholar]
- 11.Xie W., Robertson D.L., Simmons D.L. Mitogen-inducible prostaglandin G/H synthase: a new target for nonsteroidal antiinflammatory drugs. Drug Dev Res. 1992;25:249–265. [Google Scholar]
- 12.Nantel F., Denis D., Gordon R., Northey A., Cirino C., Chan C.C. Distribution and regulation of cyclooxygenase-2 in carrageenan-induced inflammation. Br J Pharmacol. 1999;128:853–859. doi: 10.1038/sj.bjp.0702866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gupta A.K., Gupta R.A., Soni L.K., Kaskhedikar S.G. Exploration of physicochemical properties and molecular modelling studies of 2-sulfonyl-phenyl-3-phenyl-indole analogs as cyclooxygenase-2 inhibitors. Eur J Med Chem. 2008;43:1297–1303. doi: 10.1016/j.ejmech.2007.06.022. [DOI] [PubMed] [Google Scholar]
- 14.Tanaka A., Araki H., Komoike Y., Hase S., Takeuchi K. Inhibition of both COX-1 and COX-2 is required for development of gastric damage in response to nonsteroidal antiinflammatory drugs. J Physiol Paris. 2001;95:21–27. doi: 10.1016/s0928-4257(01)00005-5. [DOI] [PubMed] [Google Scholar]
- 15.Ahlström M.M., Ridderström M., Zamora I., Luthman K. CYP2C9 structure metabolism relationships: optimizing the metabolic stability of COX-2 inhibitors. J Med Chem. 2007;50:4444–4452. doi: 10.1021/jm0705096. [DOI] [PubMed] [Google Scholar]
- 16.Wallace J.L., McKnight W., Reuter B.K., Vergnolle N. NSAID-induced gastric damage in rats: requirement for inhibition of both cyclooxygenase 1 and 2. Gastroenterology. 2000;119:706–714. doi: 10.1053/gast.2000.16510. [DOI] [PubMed] [Google Scholar]
- 17.Lamie P.F., Ali W.A.M., Bazgier V., Rarova L. Novel N-substituted indole Schiff bases as dual inhibitors of cyclooxygenase-2 and 5-lipoxygenase enzymes: synthesis, biological activities in vitro and docking study. Eur J Med Chem. 2016;123:803–813. doi: 10.1016/j.ejmech.2016.08.013. [DOI] [PubMed] [Google Scholar]
- 18.Ramalho T.C., Rocha M.V.J., da Cunha E.F.F., Freitas M.P. The search for new COX-2 inhibitors: a review of 2002–2008 patents. Expert Opin Ther Pat. 2009;50:1193–1228. doi: 10.1517/13543770903059125. [DOI] [PubMed] [Google Scholar]
- 19.Meade E.A., Smith W.L., Dewitt D.L. Differential inhibition of prostaglandin endoperoxide synthase (cyclooxygenase) isozymes by aspirin and other non-steroidal anti-inflammatory drugs. J Biol Chem. 1993;268:6610–6614. [PubMed] [Google Scholar]
- 20.Charlier C., Michaux C. Dual inhibition of cyclooxygenase-2 (COX-2) and 5-lipoxygenase (5-LOX) as a new strategy to provide safer non-steroidal anti-inflammatory drugs. Eur J Med Chem. 2003;38:645–659. doi: 10.1016/s0223-5234(03)00115-6. [DOI] [PubMed] [Google Scholar]
- 21.Somakala K., Amir M. Synthesis, characterization and pharmacological evaluation of pyrazolyl urea derivatives as potential anti-inflammatory agents. Acta Pharm Sin B. 2017;7:230–240. doi: 10.1016/j.apsb.2016.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bansal S., Bala M., Suthar S.K., Choudhary S., Bhattacharya S., Bhardwaj V., et al. Design and synthesis of novel 2-phenyl-5-(1,3-diphenyl-1H-pyrazol-4-yl)-1,3,4-oxadiazoles as selective COX-2 inhibitors with potent anti-inflammatory activity. Eur J Med Chem. 2014;80:167–174. doi: 10.1016/j.ejmech.2014.04.045. [DOI] [PubMed] [Google Scholar]
- 23.El-Sayed M.A., Abdel-Aziz N.I., Abdel-Aziz A.A., El-Azab A.S., Asiri Y.A., Eltahir K.E. Design, synthesis, and biological evaluation of substituted hydrazone and pyrazole derivatives as selective COX-2 inhibitors: molecular docking study. Bioorg Med Chem. 2011;19:3416–3424. doi: 10.1016/j.bmc.2011.04.027. [DOI] [PubMed] [Google Scholar]
- 24.Yao H.Y., Guo Q., Wang M.R., Wang R., Xu Z.Q. Discovery of pyrazole N-aryl sulfonate: a novel and highly potent cyclooxygenase-2 (COX-2) selective inhibitors. Bioorg Med Chem. 2021;46:116344. doi: 10.1016/j.bmc.2021.116344. [DOI] [PubMed] [Google Scholar]
- 25.El-Sayed M.A.A., Abdel-Aziz N.I., Abdel-Aziz A.A.M., El-Azab A.S., ElTahir K.E.H. Synthesis, biological evaluation and molecular modeling study of pyrazole and pyrazoline derivatives as selective COX-2 inhibitors and anti-inflammatory agents, Part 2. Bioorg Med Chem. 2012;20:3306–3316. doi: 10.1016/j.bmc.2012.03.044. [DOI] [PubMed] [Google Scholar]
- 26.Tewari A.K., Singh V.P., Yadav P., Gupta G., Singh A., Goel R.K., et al. Synthesis, biological evaluation and molecular modeling study of pyrazole derivatives as selective COX-2 inhibitors and anti-inflammatory agents. Bioorg Chem. 2014;56:8–15. doi: 10.1016/j.bioorg.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 27.Abdellatif K.R.A., Abdelall E.K.A., Labib M.B., Fadaly W.A.A., Zidan T.H. Synthesis of novel halogenated triarylpyrazoles as selective COX-2 inhibitors: anti-inflammatory activity, histopatholgical profile and in-silico studies. Bioorg Chem. 2020;105:104418. doi: 10.1016/j.bioorg.2020.104418. [DOI] [PubMed] [Google Scholar]
- 28.Abdelall E.K., Kamel G.M. Synthesis of new thiazolo-Celecoxib analogues as dual cyclooxygenase-2/15-lipoxygenase inhibitors: determination of regio-specific different pyrazole cyclization by 2D NMR. Eur J Med Chem. 2016;118:250–258. doi: 10.1016/j.ejmech.2016.04.049. [DOI] [PubMed] [Google Scholar]
- 29.Mohammed K.O., Nissan Y.M. Synthesis, molecular docking, and biological evaluation of some novel hydrazones and pyrazole derivatives as anti-inflammatory agents. Chem Biol Drug Des. 2014;84:473–488. doi: 10.1111/cbdd.12336. [DOI] [PubMed] [Google Scholar]
- 30.Tyagi R. Imidazoline and its derivatives: an overview. J Oleo Sci. 2007;56:211–222. doi: 10.5650/jos.56.211. [DOI] [PubMed] [Google Scholar]
- 31.Sarnpitak P., Mujumdar P., Morisseau C., Hwang S.H., Hammock B., Iurchenko V., et al. Potent, orally available, selective COX-2 inhibitors based on 2-imidazoline core. Eur J Med Chem. 2014;84:160–172. doi: 10.1016/j.ejmech.2014.07.023. [DOI] [PubMed] [Google Scholar]
- 32.Abdellatif K.R.A., Fadaly W.A.A. New 1,2-diaryl-4-substituted-benzylidene-5-4H-imidazolone derivatives: design, synthesis and biological evaluation as potential anti-inflammatory and analgesic agents. Bioorg Chem. 2017;72:123–129. doi: 10.1016/j.bioorg.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 33.Metwally N.H., Mohamed M.S. New imidazolone derivatives comprising a benzoate or sulfonamide moiety as anti-inflammatory and antibacterial inhibitors: design, synthesis, selective COX-2, DHFR and molecular-modeling study. Bioorg Chem. 2020;99:103438. doi: 10.1016/j.bioorg.2019.103438. [DOI] [PubMed] [Google Scholar]
- 34.Navidpour L., Amini M., Miri R., Firuzi O., Tavakkoli M., Shafiee A. Synthetic approaches towards the sulfonamide substituted-1,5-diarylimidazole-2-thiones as selective cyclooxygense-2 inhibitors. J Heterocycl Chem. 2014;51:71–79. [Google Scholar]
- 35.Liu H., Du D.M. Recent advances in the synthesis of 2-imidazolines and their applications in homogeneous catalysis. Adv Synth Catal. 2009;351:489–519. [Google Scholar]
- 36.Hayashi S., Ueno N., Murase A., Nakagawa Y., Takada J. Novel acid-type cyclooxygenase-2 inhibitors: design, synthesis, and structure‒activity relationship for anti-inflammatory drug. Eur J Med Chem. 2012;50:179–195. doi: 10.1016/j.ejmech.2012.01.053. [DOI] [PubMed] [Google Scholar]
- 37.Kaur J., Bhardwaj A., Huang Z., Knaus E.E. N-1 and C-3 substituted indole Schiff bases as selective COX-2 inhibitors: synthesis and biological evaluation. Bioorg Med Chem Lett. 2012;22:2154–2159. doi: 10.1016/j.bmcl.2012.01.130. [DOI] [PubMed] [Google Scholar]
- 38.Bhat M.A., Al-Omar M.A., Raish M., Ansari M.A., Abuelizz H.A., Bakheit A.H., et al. Indole derivatives as cyclooxygenase inhibitors: synthesis, biological evaluation and docking studies. Molecules. 2018;23:1250. doi: 10.3390/molecules23061250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Singh P., Prasher P., Dhillon P., Bhatti R. Indole based peptidomimetics as anti-inflammatory and anti-hyperalgesic agents: dual inhibition of 5-LOX and COX-2 enzymes. Eur J Med Chem. 2015;97:104–123. doi: 10.1016/j.ejmech.2015.04.044. [DOI] [PubMed] [Google Scholar]
- 40.Estevão M.S., Carvalho L.C., Freitas M., Gomes A., Viegas A., Manso J., et al. Indole based cyclooxygenase inhibitors: synthesis, biological evaluation, docking and NMR screening. Eur J Med Chem. 2012;54:823–833. doi: 10.1016/j.ejmech.2012.06.040. [DOI] [PubMed] [Google Scholar]
- 41.Ju Z.R., Su M., Hong J., La Kim E., Moon H.R., Chung H.Y., et al. Design of balanced COX inhibitors based on anti-inflammatory and/or COX-2 inhibitory ascidian metabolites. Eur J Med Chem. 2019;180:86–98. doi: 10.1016/j.ejmech.2019.07.016. [DOI] [PubMed] [Google Scholar]
- 42.Vazzana I., Terranova E., Mattioli F., Sparatore F. Aromatic Schiff bases and 2,3-disubstituted-1,3-thiazolidin-4-one derivatives as anti-inflammatory agents. Arkivoc. 2004;5:364–374. [Google Scholar]
- 43.Sağlık B.N., Osmaniye D., Levent S., Çevik U.A., Çavusoğlu B.K., Ozkay Y., et al. Design, synthesis and biological assessment of new selective COX-2 inhibitors including methyl sulfonyl moiety. Eur J Med Chem. 2021;209:112918. doi: 10.1016/j.ejmech.2020.112918. [DOI] [PubMed] [Google Scholar]
- 44.Abdel-Aziz S.A., Taher E.S., Lan P., Asaad G.F., Gomaa H.A.M., El-Koussi N.A., et al. Design, synthesis, and biological evaluation of new pyrimidine-5-carbonitrile derivatives bearing 1,3-thiazole moiety as novel anti-inflammatory EGFR inhibitors with cardiac safety profile. Bioorg Chem. 2021;111:104890. doi: 10.1016/j.bioorg.2021.104890. [DOI] [PubMed] [Google Scholar]
- 45.Rodl C.B., Vogt D., Kretschmer S.B., Ihlefeld K., Barzen S., Bruggerhoff A., et al. Multi-dimensional target profiling of N,4-diaryl-1,3-thiazole-2-amines as potent inhibitors of eicosanoid metabolism. Eur J Med Chem. 2014;84:302–311. doi: 10.1016/j.ejmech.2014.07.025. [DOI] [PubMed] [Google Scholar]
- 46.Labib M.B., Fayez A.M., El-Nahass E.S., Awadallah M., Halim P.A. Novel tetrazole-based selective COX-2 inhibitors: design, synthesis, anti-inflammatory activity, evaluation of PGE2, TNF-α, IL-6 and histopathological study. Bioorg Chem. 2020;104:104308. doi: 10.1016/j.bioorg.2020.104308. [DOI] [PubMed] [Google Scholar]
- 47.Al-Hourani B.J., Sharma S.K., Mane J.Y., Tuszynski J., Baracos V., Kniess T., et al. Synthesis and evaluation of 1,5-diaryl-substituted tetrazoles as novel selective cyclooxygenase-2 (COX-2) inhibitors. Bioorg Med Chem Lett. 2011;21:1823–1826. doi: 10.1016/j.bmcl.2011.01.057. [DOI] [PubMed] [Google Scholar]
- 48.Al-Hourani B.J., Al-Awaida W., Matalka K.Z., El-Barghouthi M.I., Alsoubani F., Wuest F. Structure‒activity relationship of novel series of 1,5-disubstituted tetrazoles as cyclooxygenase-2 inhibitors: design, synthesis, bioassay screening and molecular docking studies. Bioorg Med Chem Lett. 2016;26:4757–4762. doi: 10.1016/j.bmcl.2016.08.034. [DOI] [PubMed] [Google Scholar]
- 49.El-Sayed N.A., Nour M.S., Salem M.A., Arafa R.K. New oxadiazoles with selective-COX-2 and EGFR dual inhibitory activity: design, synthesis, cytotoxicity evaluation and in silico studies. Eur J Med Chem. 2019;183:111693. doi: 10.1016/j.ejmech.2019.111693. [DOI] [PubMed] [Google Scholar]
- 50.Alfayomy A.M., Abdel-Aziz S.A., Marzouk A.A., Shaykoon M.S.A., Narumi A., Konno H., et al. Design and synthesis of pyrimidine-5-carbonitrile hybrids as COX-2 inhibitors: anti-inflammatory activity, ulcerogenic liability, histopathological and docking studies. Bioorg Chem. 2021;108:104555. doi: 10.1016/j.bioorg.2020.104555. [DOI] [PubMed] [Google Scholar]
- 51.Grover J., Bhatt N., Kumar V., Patel N.K., Gondaliya B.J., Elizabeth Sobhia M., et al. 2,5-Diaryl-1,3,4-oxadiazoles as selective COX-2 inhibitors and anti-inflammatory agents. RSC Adv. 2015;5:45535–45544. [Google Scholar]
- 52.Ł Szczukowski, Krzyżak E., Zborowska A., Zajac P., Potyrak K., Peregrym K., et al. Design, synthesis and comprehensive investigations of pyrrolo [3,4-d] pyridazinone-based 1,3,4-oxadiazole as new class of selective COX-2 inhibitors. Int J Mol Sci. 2020;21:9623. doi: 10.3390/ijms21249623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Khatri C.K., Indalkar K.S., Patil C.R., Goyal S.N., Chaturbhuj G.U. Novel 2-phenyl-4,5,6,7-tetrahydro[b]benzothiophene analogues as selective COX-2 inhibitors: design, synthesis, anti-inflammatory evaluation, and molecular docking studies. Bioorg Med Chem Lett. 2017;27:1721–1726. doi: 10.1016/j.bmcl.2017.02.076. [DOI] [PubMed] [Google Scholar]
- 54.Sun J.A., Wang S., Sheng G.H., Lian Z.M., Liu H.Y., Zhu H.L. Synthesis of phenylpiperazine derivatives of 1,4-benzodioxan as selective COX-2 inhibitors and anti-inflammatory agents. Bioorg Med Chem. 2016;24:5626–5632. doi: 10.1016/j.bmc.2016.09.023. [DOI] [PubMed] [Google Scholar]
- 55.Kaur A., Pathak D.P., Sharma V., Wakode S. Synthesis, biological evaluation and docking study of a new series of di-substituted benzoxazole derivatives as selective COX-2 inhibitors and anti-inflammatory agents. Bioorg Med Chem. 2018;26:891–902. doi: 10.1016/j.bmc.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 56.Kaur A., Pathak D.P., Sharma V., Narasimhan B., Sharma P., Mathur R., et al. Synthesis, biological evaluation and docking study of N-(2-(3,4,5-trimethoxybenzyl)benzoxazole-5-yl) benzamide derivatives as selective COX-2 inhibitor and anti-inflammatory agents. Bioorg Chem. 2018;81:191–202. doi: 10.1016/j.bioorg.2018.07.007. [DOI] [PubMed] [Google Scholar]
- 57.Chen Z., Wang Z.C., Yan X.Q., Wang P.F., Lu X.Y., Chen L.W., et al. Design, synthesis, biological evaluation and molecular modeling of dihydropyrazole sulfonamide derivatives as potential COX-1/COX-2 inhibitors. Bioorg Med Chem Lett. 2015;25:1947–1951. doi: 10.1016/j.bmcl.2015.03.022. [DOI] [PubMed] [Google Scholar]
- 58.Rathore A., Rahman M.U., Siddiqui A.A., Ali A., Shaharyar M. Design and synthesis of benzimidazole analogs endowed with oxadiazole as selective COX-2 inhibitor. Arch Pharm. 2014;347:923–935. doi: 10.1002/ardp.201400219. [DOI] [PubMed] [Google Scholar]
- 59.Iyer V.B., Gurupadayya B., Koganti V.S., Inturi B., Chandan R.S. Design, synthesis and biological evaluation of 1,3,4-oxadiazoles as promising anti-inflammatory agents. Med Chem Res. 2016;26:190–204. [Google Scholar]
- 60.Nesaragi A.R., Kamble R.R., Dixit S., Kodasi B., Hoolageri S.R., Bayannavar P.K., et al. Green synthesis of therapeutically active 1,3,4-oxadiazoles as antioxidants, selective COX-2 inhibitors and their in silico studies. Bioorg Med Chem Lett. 2021;43:128112. doi: 10.1016/j.bmcl.2021.128112. [DOI] [PubMed] [Google Scholar]
- 61.Abdu-Allah H.H.M., Abdelmoez A.A.B., Tarazi H., El-Shorbagi A.A., El-Awady R. Conjugation of 4-aminosalicylate with thiazolinones afforded non-cytotoxic potent in vitro and in vivo anti-inflammatory hybrids. Bioorg Chem. 2020;94:103378. doi: 10.1016/j.bioorg.2019.103378. [DOI] [PubMed] [Google Scholar]
- 62.Murahari M., Mahajan V., Neeladri S., Kumar M.S., Mayur Y.C. Ligand based design and synthesis of pyrazole based derivatives as selective COX-2 inhibitors. Bioorg Chem. 2019;86:583–597. doi: 10.1016/j.bioorg.2019.02.031. [DOI] [PubMed] [Google Scholar]
- 63.Ibrahim T.S., Salem I.M., Mostafa S.M., El-Sabbagh O.I., ElKhamisi M.K.M., Hegazy L., et al. Design, synthesis, and pharmacological evaluation of novel and selective COX-2 inhibitors based on bumetanide scaffold. Bioorg Chem. 2020;100:103878. doi: 10.1016/j.bioorg.2020.103878. [DOI] [PubMed] [Google Scholar]
- 64.Ikeda A., Funakoshi E., Araki M., Ma B., Karuo Y., Tarui A., et al. Structural modification of indomethacin toward selective inhibition of COX-2 with a significant increase in van der Waals contributions. Bioorg Med Chem. 2019;27:1789–1794. doi: 10.1016/j.bmc.2019.03.021. [DOI] [PubMed] [Google Scholar]
- 65.Chandna N., Kumar S., Kaushik P., Kaushik D., Roy S.K., Gupta G.K., et al. Synthesis of novel celecoxib analogues by bioisosteric replacement of sulfonamide as potent anti-inflammatory agents and cyclooxygenase inhibitors. Bioorg Med Chem. 2013;21:4581–4590. doi: 10.1016/j.bmc.2013.05.029. [DOI] [PubMed] [Google Scholar]
- 66.Hassan G.S., Abou-Seri S.M., Kamel G., Ali M.M. Celecoxib analogs bearing benzofuran moiety as cyclooxygenase-2 inhibitors: design, synthesis and evaluation as potential anti-inflammatory agents. Eur J Med Chem. 2014;76:482–493. doi: 10.1016/j.ejmech.2014.02.033. [DOI] [PubMed] [Google Scholar]
- 67.Renard J.F., Lecomte F., Hubert P., de Leval X., Pirotte B. N-(3-Arylaminopyridin-4-yl)alkanesulfonamides as pyridine analogs of nimesulide: cyclooxygenases inhibition, anti-inflammatory studies and insight on metabolism. Eur J Med Chem. 2014;74:12–22. doi: 10.1016/j.ejmech.2013.12.033. [DOI] [PubMed] [Google Scholar]
- 68.Ribeiro D., Proenca C., Varela C., Janela J., Tavares da Silva E.J., Fernandes E., et al. New phenolic cinnamic acid derivatives as selective COX-2 inhibitors. Design, synthesis, biological activity and structure-activity relationships. Bioorg Chem. 2019;91:103179. doi: 10.1016/j.bioorg.2019.103179. [DOI] [PubMed] [Google Scholar]
- 69.Takahashi T., Miyazawa M. N-Caffeoyl serotonin as selective COX-2 inhibitor. Bioorg Med Chem Lett. 2012;22:2494–2496. doi: 10.1016/j.bmcl.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 70.Rayar A.M., Lagarde N., Martin F., Blanchard F., Liagre B., Ferroud C., et al. New selective cyclooxygenase-2 inhibitors from cyclocoumarol: synthesis, characterization, biological evaluation and molecular modeling. Eur J Med Chem. 2018;146:577–587. doi: 10.1016/j.ejmech.2018.01.054. [DOI] [PubMed] [Google Scholar]
- 71.Čulenová M., Sychrová A., Hassan S.T.S., Berchová-Bímová K., Svobodová P., Helclová A., et al. Multiple in vitro biological effects of phenolic compounds from Morus alba root bark. J Ethnopharmacol. 2020;248:112296. doi: 10.1016/j.jep.2019.112296. [DOI] [PubMed] [Google Scholar]
- 72.Honmore V.S., Kandhare A.D., Kadam P.P., Khedkar V.M., Sarkar D., Bodhankar S.L., et al. Isolates of Alpinia officinarum Hance as COX-2 inhibitors: evidence from anti-inflammatory, antioxidant and molecular docking studies. Int Immunopharm. 2016;33:8–17. doi: 10.1016/j.intimp.2016.01.024. [DOI] [PubMed] [Google Scholar]
- 73.Liu Y., Zhang Y.L., Muema F.W., Kimutai F., Chen G.L., Guo M.Q. Phenolic compounds from Carissa spinarum are characterized by their antioxidant, anti-inflammatory and hepatoprotective activities. Antioxidants. 2021;10:652. doi: 10.3390/antiox10050652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nile S.H., Ko E.Y., Kim D.H., Keum Y.S. Screening of ferulic acid related compounds as inhibitors of xanthine oxidase and cyclooxygenase-2 with anti-inflammatory activity. Rev Bras Farmacogn. 2016;26:50–55. [Google Scholar]
- 75.Paulino M., Alvareda E., Iribarne F., Miranda P., Espinosa V., Aguilera S., et al. Toward the understanding of the molecular basis for the inhibition of COX-1 and COX-2 by phenolic compounds present in Uruguayan propolis and grape pomace. J Biomol Struct Dyn. 2016;34:2643–2657. doi: 10.1080/07391102.2015.1124808. [DOI] [PubMed] [Google Scholar]
- 76.Dai C.S., Ciccotosto G.D., Cappai R., Tang S., Li D., Xie S., et al. Curcumin attenuates colistin-induced neurotoxicity in N2a cells via anti-inflammatory activity, suppression of oxidative stress, and apoptosis. Mol Neurobiol. 2018;55:421–434. doi: 10.1007/s12035-016-0276-6. [DOI] [PubMed] [Google Scholar]
- 77.Timmers M.A., Guerrero-Medina J.L., Esposito D., Grace M.H., Paredes-Lopez O., Garcia-Saucedo P.A., et al. Characterization of phenolic compounds and antioxidant and anti-inflammatory activities from Mamuyo (Styrax ramirezii Greenm.) Fruit. J Agric Food Chem. 2015;63:10459–10465. doi: 10.1021/acs.jafc.5b04781. [DOI] [PubMed] [Google Scholar]
- 78.Bai H.F., Li Y.P., Qin F.Y., Yan Y.M., Wang S.M., Zhang H.X., et al. Periplanetols A-F, phenolic compounds from Periplaneta americana with potent COX-2 inhibitory activity. Fitoterapia. 2020;143:104589. doi: 10.1016/j.fitote.2020.104589. [DOI] [PubMed] [Google Scholar]
- 79.Hu W.H., Dai D.K., Zheng B.Z., Duan R., Chan G.K., Dong T.T., et al. The binding of kaempferol-3-O-rutinoside to vascular endothelial growth factor potentiates anti-inflammatory efficiencies in lipopolysaccharide-treated mouse macrophage RAW264.7 cells. Phytomedicine. 2021;80:153400. doi: 10.1016/j.phymed.2020.153400. [DOI] [PubMed] [Google Scholar]
- 80.Wu X., Song M., Rakariyatham K., Zheng J., Wang M., Xu F., et al. Inhibitory effects of 4'-demethylnobiletin, a metabolite of nobiletin, on 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced inflammation in mouse ears. J Agric Food Chem. 2015;63:10921–10927. doi: 10.1021/acs.jafc.5b05156. [DOI] [PubMed] [Google Scholar]
- 81.Kim H.H., Kim D.H., Kim M.H., Oh M.H., Kim S.R., Park K.J., et al. Flavonoid constituents in the leaves of Myrica rubra sieb. et zucc. with anti-inflammatory activity. Arch Pharmacal Res. 2013;36:1533–1540. doi: 10.1007/s12272-013-0147-x. [DOI] [PubMed] [Google Scholar]
- 82.Hanákova Z., Hosek J., Kutil Z., Temml V., Landa P., Vanek T., et al. Anti-inflammatory activity of natural geranylated flavonoids: cyclooxygenase and lipoxygenase inhibitory properties and proteomic analysis. J Nat Prod. 2017;80:999–1006. doi: 10.1021/acs.jnatprod.6b01011. [DOI] [PubMed] [Google Scholar]
- 83.Hosek J., Toniolo A., Neuwirth O., Bolego C. Prenylated and geranylated flavonoids increase production of reactive oxygen species in mouse macrophages but inhibit the inflammatory response. J Nat Prod. 2013;76:1586–1591. doi: 10.1021/np400242e. [DOI] [PubMed] [Google Scholar]
- 84.Bai M., Yao G.D., Ren Q., Li Q., Liu Q.B., Zhang Y., et al. Triterpenoid saponins and flavonoids from licorice residues with anti-inflammatory activity. Ind Crop Prod. 2018;125:50–58. [Google Scholar]
- 85.Sun Y.W., Bao Y.G., Yu H., Chen Q.J., Lu F., Zhai S., et al. Anti-rheumatoid arthritis effects of flavonoids from Daphne genkwa. Int Immunopharm. 2020;83:106384. doi: 10.1016/j.intimp.2020.106384. [DOI] [PubMed] [Google Scholar]
- 86.Chen G.L., Fan M.X., Wu J.L., Li N., Guo M.Q. Antioxidant and anti-inflammatory properties of flavonoids from lotus plumule. Food Chem. 2019;277:706–712. doi: 10.1016/j.foodchem.2018.11.040. [DOI] [PubMed] [Google Scholar]
- 87.Cheon S.Y., Chung K.S., Jeon E., Nugroho A., Park H.J., An H.J. Anti-inflammatory activity of Saxifragin via inhibition of NF-κB involves caspase-1 activation. J Nat Prod. 2015;78:1579–1585. doi: 10.1021/acs.jnatprod.5b00145. [DOI] [PubMed] [Google Scholar]
- 88.Ma J.Q., Ding J., Xiao Z.H., Liu C.M. Puerarin ameliorates carbon tetrachloride-induced oxidative DNA damage and inflammation in mouse kidney through ERK/Nrf2/ARE pathway. Food Chem Toxicol. 2014;71:264–271. doi: 10.1016/j.fct.2014.06.017. [DOI] [PubMed] [Google Scholar]
- 89.Tamayose C.I., Romoff P., Toyama D.O., Gaeta H.H., Costa C.R.C., Belchor M.N., et al. Non-clinical studies for evaluation of 8-C-rhamnosyl apigenin purified from Peperomia obtusifolia against acute edema. Int J Molecul Sci. 2017;18:1972. doi: 10.3390/ijms18091972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Waller C.P., Thumser A.E., Langat M.K., Crouch N.R., Mulholland D.A. COX-2 inhibitory activity of homoisoflavanones and xanthones from the bulbs of the Southern African Ledebouria socialis and Ledebouria ovatifolia (Hyacinthaceae: Hyacinthoideae) Phytochemistry. 2013;95:284–290. doi: 10.1016/j.phytochem.2013.06.024. [DOI] [PubMed] [Google Scholar]
- 91.Kim A.Y., Shim H.J., Kim S.Y., Heo S., Youn H.S. Differential regulation of MyD88- and TRIF-dependent signaling pathways of Toll-like receptors by cardamonin. Int Immunopharm. 2018;64:1–9. doi: 10.1016/j.intimp.2018.08.018. [DOI] [PubMed] [Google Scholar]
- 92.Zhou Y.S., Zhong B.L., Min X.J., Hou Y., Lin L.G., Wu Q., et al. Therapeutic potential of isobavachalcone, a natural flavonoid, in murine experimental colitis by inhibiting NF-κB p65. Phytother Res. 2021;35:5861–5870. doi: 10.1002/ptr.7246. [DOI] [PubMed] [Google Scholar]
- 93.Bairwa K., Singh I.N., Roy S.K., Grover J., Srivastava A., Jachak S.M. Rotenoids from Boerhaavia diffusa as potential anti-inflammatory agents. J Nat Prod. 2013;76:1393–1398. doi: 10.1021/np300899w. [DOI] [PubMed] [Google Scholar]
- 94.Crockett S.L., Kunert O., Pferschy-Wenzig E.M., Jacob M., Schuehly W., Bauer R. Phloroglucinol and terpenoid derivatives from Hypericum cistifolium and H. galioides (Hypericaceae) Front Plant Sci. 2016;7:961. doi: 10.3389/fpls.2016.00961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang Y.J., Huang X.S., Chen H.C., Zhou D.X., Yang Z.M., Wang K., et al. Discovery of anti-inflammatory terpenoids from Mallotus conspurcatus croizat. J Ethnopharmacol. 2019;231:170–178. doi: 10.1016/j.jep.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 96.Yoon C.S., Kim D.C., Park J.S., Kim K.W., Kim Y.C., Oh H. Isolation of novel sesquiterpeniods and anti-neuroinflammatory metabolites from Nardostachys jatamansi. Molecules. 2018;23:2367. doi: 10.3390/molecules23092367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.An H.J., Kim I.T., Park H.J., Kim H.M., Choi J.H., Lee K.T. Tormentic acid, a triterpenoid saponin, isolated from Rosa rugosa, inhibited LPS-induced iNOS, COX-2, and TNF-alpha expression through inactivation of the nuclear factor-κB pathway in RAW 264.7 macrophages. Int Immunopharm. 2011;11:504–510. doi: 10.1016/j.intimp.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 98.Lv J.L., Li Z., Guo L.M. Sesquiterpene lactones with COX-2 inhibition activity from Artemisia lavandulaefolia. Chem Biodivers. 2018;15:E1700548. doi: 10.1002/cbdv.201700548. [DOI] [PubMed] [Google Scholar]
- 99.Nan L., Nam H.H., Choo B.K. Costunolide inhibits inflammation in LPS-induced RAW264.7 cells and ameliorates gastric acid reflux-induced esophageal injury in rat model. Appl Biol Chem. 2020;63:1–9. [Google Scholar]
- 100.Yin X., Wei J., Wang W.W., Gao Y.Q., Stadler M., Kou R.W., et al. New cyathane diterpenoids with neurotrophic and anti-neuroinflammatory activity from the bird's nest fungus Cyathus africanus. Fitoterapia. 2019;134:201–209. doi: 10.1016/j.fitote.2019.02.013. [DOI] [PubMed] [Google Scholar]
- 101.Nhiem N.X., Lim H.Y., Kiem P.V., Minh C.V., Thu V.K., Tai B.H., et al. Oleanane-type triterpene saponins from the bark of Aralia elata and their NF-κB inhibition and PPAR activation signal pathway. Bioorg Med Chem Lett. 2011;21:6143–6147. doi: 10.1016/j.bmcl.2011.08.024. [DOI] [PubMed] [Google Scholar]
- 102.Li J.C., Dai W.F., Liu D., Zhang Z.J., Jiang M.Y., Rao K.R., et al. Quinolizidine alkaloids from Sophora alopecuroides with anti-inflammatory and anti-tumor properties. Bioorg Chem. 2021;110:104781. doi: 10.1016/j.bioorg.2021.104781. [DOI] [PubMed] [Google Scholar]
- 103.Feng A.W., Gao W., Zhou G.R., Yu R., Li N., Huang X.L., et al. Berberine ameliorates COX-2 expression in rat small intestinal mucosa partially through PPARgamma pathway during acute endotoxemia. Int Immunopharm. 2012;12:182–188. doi: 10.1016/j.intimp.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 104.Kang J.J., Zhang Y.S., Cao X., Fan J., Li G.L., Wang Q., et al. Lycorine inhibits lipopolysaccharide-induced iNOS and COX-2 up-regulation in RAW264.7 cells through suppressing P38 and STATs activation and increases the survival rate of mice after LPS challenge. Int Immunopharm. 2012;12:249–256. doi: 10.1016/j.intimp.2011.11.018. [DOI] [PubMed] [Google Scholar]
- 105.Mohan S., Hobani Y.H., Shaheen E., Abou-Elhamd A.S., Abdelhaleem A., Alhazmi H.A., et al. Girinimbine from curry leaves promotes gastro protection against ethanol induced peptic ulcers and improves healing via regulation of anti-inflammatory and antioxidant mechanisms. Food Funct. 2020;11:3493–3505. doi: 10.1039/d0fo00053a. [DOI] [PubMed] [Google Scholar]
- 106.Zhou K., Hu L., Liao W.J., Yin D.F., Rui F. Coptisine prevented IL-beta-induced expression of inflammatory mediators in chondrocytes. Inflammation. 2016;39:1558–1565. doi: 10.1007/s10753-016-0391-6. [DOI] [PubMed] [Google Scholar]
- 107.Son D.J., Akiba S., Hong J.T., Yun Y.P., Hwang S.Y., Park Y.H., et al. Piperine inhibits the activities of platelet cytosolic phospholipase A2 and thromboxane A2 synthase without affecting cyclooxygenase-1 activity: different mechanisms of action are involved in the inhibition of platelet aggregation and macrophage inflammatory response. Nutrients. 2014;6:3336–3352. doi: 10.3390/nu6083336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lin Y.F., Kuo C.Y., Wen Z.H., Lin Y.Y., Wang W.H., Su J.H., et al. Flexibilisquinone, a new anti-inflammatory quinone from the cultured soft coral Sinularia flexibilis. Molecules. 2013;18:8160–8167. doi: 10.3390/molecules18078160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Choi R.J., Ngoc T.M., Bae K., Cho H.J., Kim D.D., Chun J., et al. Anti-inflammatory properties of anthraquinones and their relationship with the regulation of P-glycoprotein function and expression. Eur J Pharm Sci. 2013;48:272–281. doi: 10.1016/j.ejps.2012.10.027. [DOI] [PubMed] [Google Scholar]
- 110.Ju Z.R., Qin X.C., Lin X.P., Wang J.F., Kaliyaperumal K., Tian Y.Q., et al. New phenyl derivatives from endophytic fungus Botryosphaeria sp. SCSIO KcF6 derived of mangrove plant Kandelia candel. Nat Prod Res. 2016;30:192–198. doi: 10.1080/14786419.2015.1050670. [DOI] [PubMed] [Google Scholar]
- 111.Zhang S.J., Huang Y.Y., Li Y., Wang Y.H., He X.J. Anti-neuroinflammatory and antioxidant phenylpropanoids from Chinese olive. Food Chem. 2019;286:421–427. doi: 10.1016/j.foodchem.2019.02.031. [DOI] [PubMed] [Google Scholar]
- 112.Li Y.R., Fu C.S., Yang W.J., Wang X.L., Feng D., Wang X.N., et al. Investigation of constituents from Cinnamomum camphora (L.) J. Presl and evaluation of their anti-inflammatory properties in lipopolysaccharide-stimulated RAW 264.7 macrophages. J Ethnopharmacol. 2018;221:37–47. doi: 10.1016/j.jep.2018.04.017. [DOI] [PubMed] [Google Scholar]
- 113.Thi N.N., Song H.S., Oh E.J., Lee Y.G., Ko J.H., Kwon J.E., et al. Phenylpropanoids from Lilium Asiatic hybrid flowers and their anti-inflammatory activities. Appl biol chem. 2017;60:527–533. [Google Scholar]