Dear Editor, As the COVID‐19 pandemic continues to surge, the varied spectrum of clinical presentations keeps growing. The US Centers for Disease Control and Prevention (CDC) has recently described COVID‐19‐associated multisystem inflammatory syndrome in adults (MIS‐A), which presents as an amalgam of Kawasaki disease (KD) and toxic shock syndrome (TSS).1, 2 We report a 22‐year‐old Indian man with 5 days of fever, headache, myalgia and skin rash, and a one day history of lower‐extremity and upper‐abdominal pain, and vomiting.
COVID‐19 reverse‐transcriptase polymerase chain reaction (RT‐PCR) at admission was negative. He was febrile (104·6 °F), tachypnoeic (36 breaths per min) and tachycardic (122 beats per min), but was maintaining blood pressure (104/62 mmHg) and SpO2 (98% room air). Dermatological examination revealed bilateral nonexudative conjunctival injection; hyperpigmented fissured lips; generalized erythema with multiple well‐defined, discrete‐to‐coalescing, hyperpigmented macules involving the face, trunk and all extremities; and oedema of both hands and feet (Figure 1a–c). These findings were suggestive of KD as per the American Heart Association (AHA) criteria, although cervical lymphadenopathy was absent.
Figure 1.
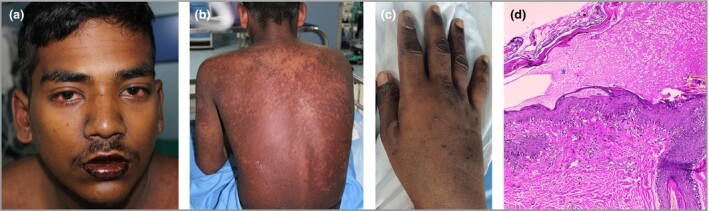
(a) Bilateral nonexudative conjunctival injection and swollen hyperpigmented chapped and fissured lips. (b) Generalized diffuse erythema and multiple well‐defined, discrete‐to‐coalescing, hyperpigmented macules involving the trunk. Nikolsky’s sign was negative. (c) Oedema of the hand is appreciable. (d) Skin biopsy from a truncal lesion shows a subcorneal split with red blood cells and fibrin within it (blue star). Stratum corneum shows parakeratosis and few haemosiderin‐laden macrophages (yellow box). Focal apoptotic keratinocytes, spongiosis with irregular acanthosis and basal cell vacuolation are seen, with the upper dermis showing perivascular oedema and a mixed inflammatory infiltrate. Haematoxylin and eosin, original magnification × 200.
Investigations (normal levels in brackets) revealed haemoglobin 9·2 g dL−1; total leucocyte count 11 820 cells μL−1 with 81% neutrophils, 10% lymphocytes and 5% eosinophils; bilirubin 1·2 mg dL−1; aspartate transaminase 162 IU L−1 (5–40); alanine aminotransferase 1171 IU L−1 (16–63); protein 6·2 g dL−1 (5·7–8·2); albumin 1·7 g dL−1 (4·0–4·7); triglycerides 253 mg dL−1 (< 150); erythrocyte sedimentation rate 92 mm h−1; C‐reactive protein 24 mg dL−1; lactate dehydrogenase 505 U L−1 (81–234); creatine phosphokinase 341 U L−1 (26–192); ferritin 7410 ng mL−1 (23–336); procalcitonin 3·48 ng mL−1 (0–0·5); troponin I 81 ng L−1 (0–50); D‐dimer 400 ng dL−1 (0–200); fibrinogen 398 mg dL−1 (200–400); interleukin‐6 54·9 pg mL−1 (< 6·4) and sinus tachycardia on electrocardiogram. Chest X‐ray and computed tomography (CT) showed bilateral pleural effusion. Transthoracic echocardiography (TTE), with 65% left ventricular ejection fraction, and CT coronary angiography did not reveal any abnormality.
Differentials of KD, TSS and haemophagocytic lymphocytosis (HLH) were considered. The skin biopsy findings are shown in Figure 1(d). Absence of hypotension and negative blood cultures ruled out TSS. Bone marrow showed no haemophagocytosis and the HLH‐2004 criteria were not met.3 Repeat COVID‐19 RT‐PCR was negative, while anti‐SARS‐CoV‐2 antibodies were raised to 18 AU mL−1, confirming prior COVID‐19 infection. With positive anti‐SARS‐CoV‐2 antibody, characteristic skin rash, and systemic features and investigations, the patient was diagnosed with COVID‐19‐associated Kawasaki‐like MIS‐A (K‐MIS‐A). He was empirically managed with low‐molecular‐weight heparin and antibiotics until initial negative blood cultures were available, and was discharged after 14 days with desquamation over the palms, soles and body, and stabilization of vital parameters and systemic symptoms. Repeat TTE and CT coronary angiography at 2 and 4 months did not show any cardiac abnormality. We plan to follow up the patient for 1 year to look for any sequelae.
Several features of our patient raised concern for K‐MIS‐A. He fulfilled the AHA criteria for KD and the CDC working MIS‐A case definition.1 MIS‐A might be a phenotype with a combination of KD, TSS and macrophage activation syndrome, which are syndromes associated with hyperinflammation and dysregulated immune response in reaction to infectious triggers or current infection.1, 2 A lot of evidence has reported differences in clinical presentation, investigations and immunological responses in KD and MIS in children (MIS‐C), concluding that the pathophysiology may be different. As such, MIS‐C and MIS‐A may be new and multifaceted entities distinct from KD.4
To date, five cases have shown features of K‐MIS‐A similar to our patient’s.1, 2 People of all ages can develop MIS either with concurrent SARS‐CoV‐2 infection or as a postinfectious phenomenon, as 30% of cases of MIS‐A had negative PCR but positive COVID‐19 antibodies.1 It took 2–5 weeks to develop MIS‐A following onset of COVID‐19 symptoms.1 Hence, it becomes pertinent to examine COVID‐19 antibodies along with RT‐PCR for recognition of MIS‐A.
The most common liver abnormality is aminotransferase elevation owing to direct, ischaemic or drug‐induced liver injury leading to cytolysis.5 Similarly, troponin elevation is attributed to mechanisms like cytokine release causing both direct and indirect myocardial injury through suppression of angiotensin‐converting enzyme 2 (ACE2) and plaque instability in coronary vessels, SARS‐CoV‐2 targeting myocardial ACE2 receptors, and oxidative‐stress‐mediated endothelial dysfunction.6 Hence, on a background of multifactorial pathogenesis, the most plausible explanation would be a virus‐mediated immunogenic response triggering multisystem inflammation. Our patient probably had systemic capillary leak syndrome secondary to COVID‐19, evident from hypoalbuminaemia and pleural effusion but without haemoconcentration or hypotension.7 The patient initially had eosinophilia, which had settled by the third day of admission. This contrasts with eosinopenia, seen in severe COVID‐19. Eosinophils have been associated with severe coronary vasculitis and aneurysms in KD.8 Hence, the presence of eosinophilia may hint towards KD or K‐MIS‐A.5, 8
Potential therapies used in MIS‐A include intravenous immunoglobulin (IVIg), aspirin, anticoagulation, corticosteroids and tocilizumab.1 With our evolving understanding of K‐MIS‐A, treatment protocols are yet to be standardized. Although we gave only anticoagulants to our patient, he recovered completely without any cardiac sequelae, as seen at follow‐up. The CDC’s detailed data on 27 cases of MIS‐A included two cases with deranged inflammatory markers, ECG and TTE changes, which recovered on only anticoagulants without IVIg or steroids.1 Hence, there might be a subset of patients with K‐MIS‐A who may recover spontaneously without conventional therapies. The focus of our case is to reiterate the possibility of COVID‐19‐associated K‐MIS‐A and timely diagnosis through early identification of dermatological manifestations and antibody testing, even when COVID‐19 RT‐PCR is negative. Further information on the investigations is available on direct request.
Acknowledgments
We are thankful to the Department of Pathology and Radiology, Command Hospital Air Force Bangalore, India for their necessary support.
Author Contribution
Rajeshwari Dabas: Conceptualization (equal); Investigation (equal); Project administration (equal); Resources (equal); Supervision (equal); Visualization (equal); Writing‐review & editing (equal). G Varadaraj: Formal analysis (equal); Investigation (equal); Methodology (equal); Resources (equal); Validation (equal); Visualization (equal); Writing‐review & editing (equal). Sunmeet Sandhu: Conceptualization (equal); Data curation (equal); Methodology (equal); Project administration (equal); Validation (equal); Writing‐original draft (equal). Anuj Bhatnagar: Formal analysis (equal); Methodology (equal); Project administration (equal); Software (equal); Supervision (equal); Visualization (equal); Writing‐review & editing (equal). Reetika Pal: Data curation (equal); Investigation (equal); Methodology (equal); Resources (equal); Writing‐original draft (equal).
Contributor Information
R. Dabas, Department of Dermatology Command Hospital Air Force Bangalore Bengaluru India
G. Varadaraj, Department of Medicine Command Hospital Air Force Bangalore Bengaluru India
S. Sandhu, Department of Dermatology Command Hospital Air Force Bangalore Bengaluru India.
A. Bhatnagar, Department of Dermatology Command Hospital Air Force Bangalore Bengaluru India
R. Pal, Department of Dermatology Command Hospital Air Force Bangalore Bengaluru India
References
- Morris SB, Schwartz NG, Patel P et al. Case series of multisystem inflammatory syndrome in adults associated with SARS‐CoV‐2 infection – UK and United States, March–August 2020. MMWR Morb Mortal Wkly Rep 2020; 69:1450–6. 10.15585/mmwr.mm6940e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaigany S, Gnirke M, Guttmann A et al. An adult with Kawasaki‐like multisystem inflammatory syndrome associated with COVID‐19. Lancet 2020; 396:e8–10. 10.1016/S0140-6736(20)31526-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henter J‐I, Horne A, Aricó M et al. HLH‐2004: diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer 2007; 48:124–31. [DOI] [PubMed] [Google Scholar]
- Consiglio CR, Cotugno N, Sardh F et al. The immunology of multisystem inflammatory syndrome in children with COVID‐19. Cell 2020; 183:968–81.e7. 10.1016/j.cell.2020.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon AM, Barritt AS 4th. Elevated liver enzymes in patients with COVID‐19: look, but not too hard. Dig Dis Sci 2021; 66:1767–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tersalvi G, Vicenzi M, Calabretta D et al. Elevated troponin in patients with coronavirus disease 2019: possible mechanisms. J Card Fail 2020; 26:470–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case R, Ramaniuk A, Martin P et al. Systemic capillary leak syndrome secondary to coronavirus disease 2019. Chest 2020; 158:e267–e268. 10.1016/j.chest.2020.06.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogan E, Foulon P, Cappeliez O et al. Multisystem inflammatory syndrome with complete Kawasaki disease features associated with SARS‐CoV‐2 infection in a young adult. A case report. Front Med 2020; 7:428. [DOI] [PMC free article] [PubMed] [Google Scholar]


