Abstract
We investigated surface selection and adhesion of motile zoospores of a green, macrofouling alga (Enteromorpha) to self-assembled monolayers (SAMs) having a range of wettabilities. The SAMs were formed from alkyl thiols terminated with methyl (CH3) or hydroxyl (OH) groups or mixtures of CH3- and OH-terminated alkyl thiols and were characterized by measuring the advancing contact angles and by X-ray photoelectron spectroscopy. There was a positive correlation between the number of spores that attached to the SAMs and increasing contact angle (hydrophobicity). Moreover, the sizes of the spore groups (adjacent spores touching) were larger on the hydrophobic SAMs. Video microscopy of a patterned arrangement of SAMs showed that more zoospores were engaged in swimming and “searching” above the hydrophobic sectors than above the hydrophilic sectors, suggesting that the cells were able to “sense” that the hydrophobic surfaces were more favorable for settlement. The results are discussed in relation to the attachment of microorganisms to substrata having different wettabilities.
Enteromorpha spp. are commonly found throughout the world in the upper intertidal regions of shores and estuaries and are the most common macroalgae that foul man-made structures, including boats, buoys, ships, and submarines (6, 7). Colonization of substrata occurs mainly through the production and release into the water column of enormous numbers of motile spores, which may be either asexual zoospores or zygotes formed from fusion of sexual gametes (11). Zoospores are cells that are quadriflagellate, naked (i.e., they lack a cell wall), and pyriform, and the spore body is 7 to 10 μm long. The critical event involved in colonization of substrata is the transition from a motile cell to an attached, nonmotile settled cell that develops a cell wall and germinates to produce a new plant.
Prior to permanent adhesion, a swimming spore exhibits characteristic presettlement behavior that involves a change from random swimming to a “searching” pattern of exploration close to the substratum (11). During the searching phase, the spore appears to become temporarily attached to the substratum as it spins like a top on its apical dome, with the flagella acting as propellers. During spinning, a pad of elastic material is sometimes extruded, and this pad is left behind on the surface if the spore continues to search for a suitable place on which to settle. Once a suitable area for settlement is located, the spore commits itself to permanent adhesion through rapid secretion of an N-linked, polydisperse, self-aggregating glycoprotein (Mr under reducing, denaturing conditions, 110,000) that anchors the spore to the substratum (5, 29).
A number of cues are involved in surface localization by zoospores, including negative phototaxis (10), thigmotaxis (18), and chemotaxis (9). The presence of a microbial biofilm is also important in determining the number of spores that attach to a surface (14; I. Joint, M. E. Callow, J. A. Callow, and K. R. Clark, submitted for publication), possibly through the release of chemical signals and/or modification of the topography or physicochemical properties of the substratum.
In this study we examined the role of surface wettability in zoospore adhesion. Although there have been a number of reports in which the authors have related microbial attachment to surface wettability (1, 16–18, 28), there have been none on algae which employ surfaces that are fully characterized and vary systematically with respect to wettability. The substrata used in the present study were self-assembled monolayers (SAMs) of ω-substituted alkane thiolates on gold (3). SAM technology permits construction of surfaces that are chemically defined and uniform with respect to surface morphology and can present a variety of chemical functional groups. Another aspect of our study was the use of patterned SAMs which allowed the zoospores a “choice” of surfaces with different wettabilities. SAMs have been used previously to study the effects of hydrophobicity (19, 32), chemistry (19), and surface topography (33) on bacterial attachment, as well as for a number of studies on the effects on substratum physicochemistry on adsorption of proteins and mammalian cells (22, 24, 25, 27). The SAMs used in this study were formed from alkyl thiols terminated with methyl groups (CH3) or hydroxyl groups (OH) or mixtures of CH3- and OH-terminated alkyl thiols that resulted in a range of surface wettabilities.
MATERIALS AND METHODS
Preparation and transportation of SAMs.
SAMs were prepared at the University of New Mexico on gold-coated coverslips (22 by 50 by 0.25 mm) or regular glass microscope slides (VWR Scientific). The glass supports were cleaned by immersion in a solution prepared by mixing 70% (vol/vol) concentrated H2SO4 with 30% commercial H2O2 (piranha etch) for 20 min to 1 h, thoroughly rinsed in deionized H2O, and dried under a stream of nitrogen. Note that the piranha etch solution is a powerful oxidizer, can react violently when it is placed in contact with organic compounds, and should be stored in containers which prevent pressure buildup. The samples were then placed into the chamber of a metal evaporator. The system was evacuated to a pressure of 10−6 torr, and 10 Å of chromium and then 300 Å of gold were deposited on the substrata. The system was then restored to room pressure, and the samples were removed and submerged in 1 mM ethanolic solutions of dodecane thiol (referred to below as CH3-thiol and obtained from Aldrich Chemical), mercaptoundecanol (OH-thiol; Aldrich Chemical), or a mixture of CH3- and OH-thiols. The samples were immersed in the thiol solutions overnight at 4°C. The SAMs remained in the thiol solutions at 4°C until they were shipped, at which time they were rinsed in ethanol and dried under a stream of N2. The resulting surfaces (i.e., the ω-terminated alkane thiolates) are referred to below as CH3-SAMs and OH-SAMs.
Patterned SAMs were produced by serial electrochemical desorption and reformation of the SAMs as previously described (31). A SAM was formed on gold with CH3-thiol. A laser ablation system consisting of a Nikon Diaphot 300 inverted microscope adapted with a computer-controlled, pulsed-nitrogen pumped laser (λ, 390 nm; 15 μJ/pulse; 20 pulses/s) was used to cut lines in the gold film (prepared as described above) to form electrically isolated regions in the film. The UV laser beam was focused through a ×10 objective on the microscope, and it ablated the gold and the SAM, which generated lines of exposed glass that were approximately 15 μm wide. The slide was then placed in 0.5 M ethanolic KOH, and an anode was connected to one element. A cyclic current was then applied (−1.0 to −1.5 V versus Ag/AgCl; 500 mV s−1) to the element for six cycles. Desorption of the CH3-SAM from the surface was monitored by cyclic voltammetry to ensure complete removal of the SAM. The exposed gold was then treated with a 10 mM ethanolic solution of the desired ω-substituted alkane thiol for 20 min. A series of elements could thus be addressed sequentially, which resulted in a pattern consisting of different SAMs on a single surface.
For transportation, the SAMs were removed from the thiol solutions, dried, and placed in plastic coverslip or slide boxes. The boxes were then put into a plastic desiccator that was subsequently evacuated and then flooded with N2. This cycle was repeated three times, and after the final N2 purge, the chamber was evacuated and sealed. All seams and orifices were then sealed with Parafilm, and the desiccator was placed in a package. The package was sent to the University of Birmingham, Birmingham, United Kingdom, via overnight delivery.
Surface characterization of SAMs.
Samples were tested both before and after shipment to ensure that the integrity of the samples was maintained during shipping. Advancing water contact angles (θAW) were measured both immediately before packing and upon receipt.
X-ray photoelectron spectroscopy was used to determine the surface compositions of mixed monolayers. Samples were analyzed with a model SSX-100 spectrometer (Surface Science Instruments, Mountain View, Calif.) at the National ESCA and Surface Analysis Center for Biomedical Problems at the University of Washington. Using this system, workers analyzed an elliptical area whose short axis was adjusted so that it was 1,000 μm long. An A1 Kα1,2 monochromatized X-ray source (hν, 1,486.6 eV) was used to stimulate photoemission. The energy of the emitted photoelectrons was measured with a hemispherical analyzer. Survey scans for binding energies ranging from 0 to 1,000 eV (with a pass energy of 150 eV) were performed to examine the elemental compositions of the surfaces. At this pass energy, the transmission function of the spectrometer can be assumed to be constant (Surface Science Instruments). Peak areas were normalized by the number of scans, the number of points per electron volt, the Scofield photoemission cross sections (26), and the sampling depth. X-ray photoelectron spectroscopy data were acquired at a photoelectron take-off angle of 55°; the take-off angle was defined as the angle between the surface normal and the axis of the analyzer lens. High-resolution scans were also recorded at a pass energy of 50 eV. Peak positions were assigned by referencing the hydrocarbon (CHx) peak to 285.0 eV.
Plant material.
Fertile plants of Enteromorpha linza were collected from Wembury Beach, United Kingdom (50°18′N, 4°02′W). Zoospores were released and prepared for attachment experiments as described previously (11).
Zoospore adhesion assays.
Zoospore suspensions were standardized as described previously (11). The concentration of spores was adjusted to 106 spores ml−1 by using natural seawater unless otherwise stated. Coverslips or microscope slides were incubated with spore suspensions in the dark at 20°C. Substrata were washed in seawater before they were fixed in 2% glutaraldehyde in seawater for 10 min and then washed as described previously (11).
Time course of zoospore adhesion.
Glass coverslips coated with SAMs generated by using either CH3- or OH-thiol solutions were placed individually into 5-cm-diameter Sterilin petri dishes to which 5-ml portions of spore suspensions were added. The θAW of the CH3- and OH-thiol surfaces were 116° and <15°, respectively. New ethanol-washed glass coverslips, as supplied by the manufacturer (VWR), were used to compare results obtained with SAMs to the results of previous experiments (11). Dishes were incubated for 20, 40, or 60 min before the coverslips were removed and processed as described above. Three replicates were used for each treatment. Attached spores were counted in 10 fields of view, located at 1-mm intervals across the midpoint of each of the three replicate coverslips. The mean ±95% confidence limit for 30 counts per mm2 of surface was calculated.
Zoospore adhesion to SAMs formed from mixtures of CH3- and OH-thiols. (i) SAM-coated coverslips.
Four coverslips containing each type of SAM were shipped as described above. We used SAMs that were formed with different solution molar fractions of OH-thiol (χOHsol), where
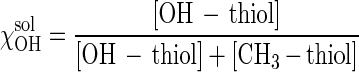 |
SAMs were formed from mixed thiol solutions having χOHsol of 0, 0.2, 0.45, 0.50, 0.55, 0.65, 0.70, 0.80, 0.90, and 1. Three replicate coverslips were used for the zoospore adhesion assay. The remaining coverslip was used to determine the contact angle. In all cases, except for the 100% OH-terminated SAM, the contact angle was found to be ±20% of the contact angle recorded prior to shipping. In the discussion of θAW below we refer to the measurements obtained at the University of New Mexico.
(ii) Patterned SAMs.
A microscope slide (76 by 26 mm) having 11 SAMs along the length of the long axis (each area, 5 by 15 mm) was placed in a compartment of a polystyrene culture dish (In Vitro Systems & Services, GmbH), and 10 ml of a spore suspension was added. After the preparations were processed as described above, spores were counted in 20 fields of view through the midpoint of the long axis at 0.5-mm intervals of each SAM by using analySIS software and a personal computer connected to an Olympus model BH2 microscope equipped with a video camera. The number of spores that were in groups (i.e., touching; 1 to 15 spores) was also recorded. Data are presented below for the mean numbers of spores that adhered ±95% confidence limits (x = 20) and also for the percentages of total spores that were present in groups. For clarity, the percentages of cells found in groups were calculated by combining group sizes as follows: 1, 2 + 3, 4 + 5, 6 + 7, 8 + 9, 10 + 11, 12 + 13, and 14 + 15.
Zoospore swimming behavior assays.
In order to investigate whether the pattern of spore swimming and searching behavior was affected by the properties of substrata, a video analysis of zoospore behavior was conducted by using a pattern consisting of SAMs in close spatial proximity, which provided spores with a choice of substrata having different wettabilities. For technical reasons, this part of the investigation was conducted at the University of Melbourne, Melbourne, Australia. The pattern was formed on a standard microscope slide and consisted of a square (16 by 16 mm) that was divided into four sectors, each of which was 8 by 8 mm. At the midpoint were corners of four sectors bearing mixed-component SAMs formed from solutions with χOHsol values of 0.2, 0.4, 0.6, and 0.8. Adjacent SAMs were separated by approximately 15 μm of bare glass. The contact angles of the sectors before dispatch to Australia were 100°, 96°, 64°, and 42°, respectively.
The slide was placed in a 9-cm-diameter petri dish to which 25 ml of a spore suspension (2 × 106 spores ml−1) was added. Zoospores were released from E. linza collected from Port Melbourne, Victoria, Australia (37°50′S, 144°55′E). The dish was positioned on the stage of a Zeiss Universal microscope under a Plan 2.5/0.08 objective; the apparatus was set up in a darkroom to prevent phototactic spore movements. Sequences were filmed by using a Panasonic model WV-F250E color video camera and were recorded with a Pioneer model V1000p rewritable video disc recorder. After 10 min the microscope was focused on a plane just above the slide surface so that both swimming and settled spores could be observed. Time-lapse images were then collected every 3 min for 60 min by using a Genesis Systems model Z84C-V1000P VDR timer controller and a Uniblitz model T132 shutter driver controller. Single images were captured on a Targa 2000 Pro video card. At the end of the filming (a total of 70 min after spores were put into the dish), the number of spores that were firmly attached to each sector was assessed by recording images of the slide after unsettled spores in seawater were washed away. Finally, as a check to ensure that the SAMs sent to Australia were performing like those sent to Birmingham, the SAM slide was fixed and processed by using the standard cell counting procedure described above.
Spores were counted by using three consecutive video images representing each time point (i.e., 10, 13, and 16 min after the spore suspension was added [mean 13 min]; 37, 40, and 43 min after the spore suspension was added) [mean, 40 min]; and 64, 67, and 70 min after the spore suspension was added [mean, 67 min]). Spores were counted in each sector within an area (0.45 by 0.45 mm) adjacent to the midpoint of the four SAM sectors. The mean number of attached spores per square millimeter ±95% confidence limits was calculated for each group of three images. Attached spores were counted by using the single video image after the slide was washed.
Settled spores on the fixed slide were counted in 20 fields of view located at 100-μm intervals along the diagonal of each square, starting at the central corner. The mean number of attached spores per square millimeter ±95% confidence limits was calculated.
RESULTS
Surface analysis of SAMs.
Figure 1A shows θAW of SAMs as a function of the χOHsol. We chose a series of solution mole fractions so that a range of θAW from 20° to 110° was obtained with intervals of ∼10° between consecutive samples. The mole fractions of OH-terminated alkyl thiolates in the SAMs (χOHsurf) were calculated by using the O1S peak area as described elsewhere (4, 32). A trace of contaminating oxygen was observed with the 100% CH3-SAM (atomic percentage, 3.1). The relationship between χOHsurf and χOHsol is shown in Fig. 1B. The error of analysis for the instrument used was ∼10% (15).
FIG. 1.
Surface chemical properties of mixed SAMs. (A) θAW of mixed SAMs formed from OH-thiol and CH3-thiol. The data are averages for three test areas. The error bars indicate one standard deviation. (B) χOHsurf in mixed SAMs as a function of the χOHsol used to form the mixed SAMs.
Time courses of spore attachment to OH- and CH3-SAMs.
The time courses for spore attachment to OH- and CH3-SAMs on glass showed that the rate of attachment was highest on the CH3-SAM and lowest on the OH-SAM (Fig. 2). After 1 h of incubation there were approximately 2.5 times more spores attached to the CH3-SAM than to the OH-SAM. The glass control, which had an intermediate contact angle (θAW, ∼40°), exhibited intermediate levels of zoospore attachment.
FIG. 2.
Time course for zoospore adhesion to glass (θAW, ∼40°), a CH3-terminated (methyl) SAM (θAW, 116°), and a OH-terminated (hydroxyl) SAM (θAW, <15°). Each point is a mean based on 30 counts; the bars indicate 95% confidence limits.
Attachment to SAMs with different proportions of surface hydroxyl and methyl groups.
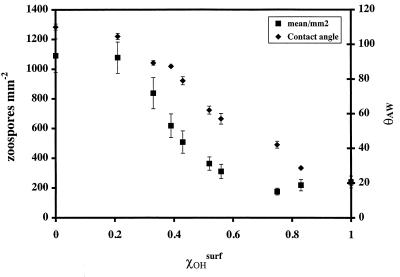
Figure 3 shows the total number of attached spores after 1 h as a function of χOHsurf. Both the θAW and the number of spores attached decreased as the χOHsurf increased and the surface became more wettable (i.e., hydrophilic). The most pronounced response of spore settlement to wettability was observed for the contact angle range from 40 to 80°, corresponding to a χOHsurf range of 0.45 to 0.80.
FIG. 3.
Zoospore adhesion to and θAW of SAMs plotted versus the χOHsurf in mixed SAMs. The mean number of zoospores attached after 1 h was derived from 30 counts.
A similar relationship between the total number of spores and wettability was observed with the patterned SAMs (Fig. 4A). Figure 4B shows that the majority of spores were attached as single spores or in small groups (maximum number of spores per group, 5 or 6) on the more hydrophilic SAMs (θAW, ≤80°). On surfaces with θAW of >80°, the proportion of spores in larger groups increased as the surfaces become less wettable (i.e., more hydrophobic) (Fig. 5). On the most hydrophobic surface (θAW, 110°), only 25% of the total spores were present as single spores, and the majority of the spores were aggregated into groups containing up to 15 spores (Fig. 4B).
FIG. 4.
Zoospore adhesion after 1 h of settling versus θAW for patterned SAMs. (A) Mean number of zoospores attached in each sector of the pattern deposited on a microscope slide. Each point is a mean based on 20 counts for each sector; the bars indicate 95% confidence limits. (B) Percentages of total zoospores in groups of various sizes.
FIG. 5.
Images of zoospores (after 1 h of settling) attached to SAMs with different θAW.
Spore behavior on SAMs as revealed by video microscopy.
Counts of motile spores could not be obtained until the majority of the spores were in the same focal plane; thus, the sample was left for 10 min before the microscope was focused on the plane of the surface. The subsequent spore counts obtained from the video images thus represented both swimming and settled spores. Figure 6 shows that the distribution of spores associated with the four sectors was not random; there was a negative correlation between spore number and increasing content of OH-terminated groups (i.e., increasing hydrophilicity). The highest number of spores associated with all sectors was recorded at 10 min. At all time points the hydrophobic sectors had more spores associated with them than the hydrophilic sectors. The order of response was as follows: θAW of 101° >θAW of 96° > θAW of 64° > θAW of 42°. The numbers of adherent spores counted in the different sectors of the slide washed after 70 min revealed the same correlation between attachment and wettability seen in other experiments. A comparison of the values for the washed slide and the 67-min incubation showed that by this time between 50 and 70% of the total spores counted were attached spores.
FIG. 6.
Distribution of spores on patterned SAMs with θAW of 101°, 96°, 64°, and 42°. The counts represent both cells that settled on the surface and swimming cells in the same focal plane. The washed counts represent settled cells only, which were recorded on video after the 70-min sample was washed.
Finally, the mean numbers of zoospores that adhered to each sector after fixation (±95% confidence limits), as assessed by the standard cell counting procedure were, 733 ± 48, 446 ± 50, 209 ± 59, and 173 ± 51 spores mm−2 for θAW of 101°, 96°, 64°, and 42°, respectively. Thus, the SAM slide used in this experiment performed in the same way that the slides used in the other attachment assays performed.
DISCUSSION
Effect of wettability on spore attachment.
The surfaces used in this study provided ranges of wettabilities and known chemical compositions. The composition and surface properties of the SAMs are comparable to those described previously (2, 4, 32). The time course for spore adhesion to uncoated glass was similar to the time course reported previously (8, 11). Settlement on the hydrophobic CH3-SAM was most rapid, and the highest number of attached spores was associated with this surface. A positive correlation between the number of spores attached and a high θAW (i.e., hydrophobicity) was seen in all experiments.
The pronounced effect of wettability on spore attachment observed at contact angles between 40° and 80° may be explained by thermodynamic models, such as the model proposed for bacterial adhesion (1). In such models the free energy of microbial adhesion (ΔFadh) is determined by the relationships of the interfacial tensions between the organism and the substratum (γBS), between the organism and the bulk liquid (γBL), and between the surface and the liquid (γSL):
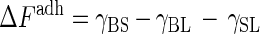 |
In studies such as this study, the only variable is γSL, which is directly related to the contact angle (θ) by using Young's equation:
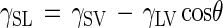 |
where γSV and γLV are the substratum-vapor and liquid-vapor interfacial tensions, respectively. Since in our experimental procedures, γSV and γLV were presumably consistent, the differences in surface energy depended only on cosθ. When the data from Fig. 3, for example, are plotted as spore attachment versus cosθAW, the regression of the resulting plot is nearly linear, and the regression coefficient (R2) is 0.912. Thus, the results obtained are consistent with thermodynamic models. However, such models were originally developed for equilibrium situations with inert, colloidal particles, and their limitations when they are applied to living cells that exhibit complex attachment biology have been extensively discussed (17). Nevertheless, the model is consistent with the results and suggests that spore attachment may be dominated by the same forces which control adhesion of colloidal particles and that hydrophobic interactions are important. The favorable effects of hydrophobic surfaces on promoting spore attachment are also demonstrated by an analysis of group size. Gregarious settlement of spores onto glass was observed previously when high concentrations of spores were employed (11) or when settlement occurred in the presence of detritus associated with a microbial biofilm (10). However, in the experiments described here, the spore concentration employed was relatively low (106 spores ml−1). On glass substrata, this concentration would not have produced the extensive level of gregarious settlement that was associated with the more hydrophobic SAMs (contact angles greater than approximately 80°).
At this stage it is not possible to identify in cell biological terms the precise point(s) in the whole spore attachment process at which hydrophobic interactions could be important. As determined by other researchers working on aquatic adhesion systems (reviewed in reference 16), hydrophobic interactions assist in the displacement of water molecules from interfaces and therefore facilitate the substratum-adhesive bonding process. On this basis, spores which committed themselves to attachment to a hydrophilic surface presumably were not able to form an adhesive bond and, thus, under the experimental conditions used, could be readily detached by the slide rinsing procedure and would not be detected as settled cells.
However, hydrophobic interactions could also be important in the preadhesion, surface selection phase of attachment, during which swimming spores actively probe the surface before engaging in a spinning behavior, in which a spore rotates on its apical papilla through a small elastic pad consisting of temporary adhesive. The spore may then commit itself to permanent adhesion, which involves discharge of a permanent adhesive, or it may detach and move to another site. Displacement of water between the apical papilla and the substratum may be assisted by hydrophobic interactions, which thus allows closer proximity between the plasma membrane of the spore and the surface and which may facilitate the transfer of any signals required by the spore to trigger the release of the permanent adhesive (11).
Effect of substratum wettability on swimming spore behavior.
Support for the hypothesis that surface wettability had an effect on the surface selection phase of settlement was obtained from the video time-lapse studies on the accumulation of swimming spores over the different SAM sectors. The results suggest that exploration was not random. Between 10 and 16 min after a spore suspension was introduced above a 2-by-2 pattern of SAMs, there were approximately three times more spores (swimming and settled) associated with the most hydrophobic sector than with the most hydrophilic sector. The number of spores that settled in the first 16 min was probably no more than approximately 10% of the total (Fig. 2), so the total spore count at this time mainly represented swimming spores. An unusual feature of these results is that the total numbers of spores associated with the four sectors all decreased over time. The observed decreases may have been due to a number of factors, including a change in the surface properties of the SAMs (i.e., the hydrophobic surfaces became more hydrophilic with time and vice versa) and the possibility that the flashes of light necessary for time-lapse video recording caused some spores to swim away from the area being observed as this area was subjected to the most intense illumination.
Although the results described above are consistent with the hypothesis that surface wettability may influence attachment at the preadhesion, surface selection stage, they could also be explained by other mechanisms. It is known that Enteromorpha spores respond positively to signals from previously settled spores during gregarious settlement behavior (11), possibly because of diffusible chemical signals. Other observations have also shown that zoospores exhibit chemoattractive behavior (9, 10). It is possible, therefore, that on the more hydrophobic SAMs the attached spores provide a ready source of diffusible signals that attract more spores to the interface compared with hydrophilic surfaces. This explanation is also supported by the data which showed that gregarious settlement was greater on hydrophobic surfaces.
It is becoming increasingly apparent that in aquatic bacterial systems attachment to surfaces having different hydrophobicities proceeds by seemingly different cellular mechanisms. Enzymatic and detergent treatment of Vibrio proteolytica, for example, leads to differential attachment to hydrophobic surfaces but not hydrophilic surfaces (23). Observations of attachment of the marine bacteria Pseudomonas sp. strain NCIMB 2021 (32, 33) and Halomonas marina ATCC 25374 (L. K. Ista, unpublished data) to SAMs have revealed that these bacteria attach to CH3-SAMs by their cell bodies and to OH-SAMs in the polar region. Furthermore, studies performed with H. marina and surfaces whose hydrophobicity can be switched in response to an environmental cue have shown that there are probably different mechanisms for attachment to hydrophobic and hydrophilic surfaces (20). The hydrophobicity of the substratum can even alter the way in which cells of an individual bacterial species arrange themselves on a surface. Dalton and colleagues have shown that the marine organism strain SW5 aggregates as tightly packed layers on hydrophobic surfaces and forms chains on hydrophilic surfaces (13).
The present study, for the first time, allowed us to investigate the effect of surface wettability on Enteromorpha zoospore settlement and primary adhesion independent of other surface characteristics and in situations in which the spores have a choice of surfaces. The results of several other studies have indicated that in general, the strength of adhesion of both micro- and macroorganisms is lower on hydrophobic surfaces with low surface free energy (12, 21, 30). This property is being exploited commercially as coatings with low surface energies, such as silicone elastomers, are now being employed to control biofouling.
ACKNOWLEDGMENTS
This work was supported by Office of Naval Research grant N00014-96-1-0373 to J.A.C. and M.E.C. by ONR grant N00014-95-1-0901 to G.P.L., and by NIGMS grant 5S06GM5276-04 supporting A.C.N. The National ESCA and Surface Analysis Center for Biomedical Problems at the University of Washington is funded through NIH grant RR01296.
We thank Ruth Perry (University of Birmingham) for technical assistance, J. A. Finlay (University of Birmingham) for contact angle measurements, Tim Spurck (University of Melbourne, Melbourne, Australia) for assistance with video microscopy, Deborah Leach-Scampavia (University of Washington) for providing the X-ray photoelectron spectroscopy data, and Víctor Pérez-Luna (University of New Mexico) for discussions concerning data interpretation.
REFERENCES
- 1.Absolom D R, Lamberti F V, Policova Z, Zingg W, van Oss C J, Neumann A W. Surface thermodynamics of bacterial adhesion. Appl Environ Microbiol. 1983;46:90–97. doi: 10.1128/aem.46.1.90-97.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bain C D, Evall J, Whitesides G M. Formation of monolayers by the coabsorption of thiols on gold: variations in the head group, tail group, and solvent. J Am Chem Soc. 1989;111:7155–7164. [Google Scholar]
- 3.Bain C D, Troughton E B, Tao Y-T, Evall J, Whitesides G M. Formation of monolayer films by the spontaneous assembly of organic thiols from solution onto gold. J Am Chem Soc. 1989;111:321–335. [Google Scholar]
- 4.Bain C D, Whitesides G M. Formation of two-component surfaces by the spontaneous assembly of monolayers on gold from solutions containing mixtures of organic thiols. J Am Chem Soc. 1988;110:6560–6561. [Google Scholar]
- 5.Callow, J. A., M. S. Stanley, R. Wetherbee, and M. E. Callow. Cellular and molecular approaches to understanding primary adhesion in Enteromorpha. Biofouling, in press.
- 6.Callow M E. Fouling algae from “in-service” ships. Bot Mar. 1986;29:351–357. [Google Scholar]
- 7.Callow M E. Ship-fouling: the problem and method of control. Biodeterior Abstr. 1996;10:411–421. [Google Scholar]
- 8.Callow M E, Callow J A. Attachment of zoospores of the fouling alga Enteromorpha in the presence of zosteric acid. Biofouling. 1998;13:87–95. [Google Scholar]
- 9.Callow M E, Callow J A. Enhanced adhesion and chemoattraction of zoospores of the fouling alga Enteromorpha to some foul-release silicone elastomers. Biofouling. 1998;13:157–172. [Google Scholar]
- 10.Callow, M. E., and J. A. Callow. Substratum location and zoopspore behaviour in the fouling alga, Enteromorpha. Biofouling, in press. [DOI] [PubMed]
- 11.Callow M E, Callow J A, Pickett-Heaps J D, Wetherbee R. Primary adhesion of Enteromorpha (Chlorophyta, Ulvales) propagules: quantitative settlement studies and video microscopy. J Phycol. 1997;33:938–947. [Google Scholar]
- 12.Callow M E, Fletcher R L. The influence of low surface energy materials on bioadhesion: a review. Int Biodeterior Biodegrad. 1994;34:333–343. [Google Scholar]
- 13.Dalton H M, Poulsen L K, Halasz P, Angles M L, Goodman A E, Marshall K C. Substratum-induced morphological changes in a marine bacterium and their relevance to biofilm structure. J Bacteriol. 1994;176:6900–6902. doi: 10.1128/jb.176.22.6900-6906.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dillon P S, Maki J S, Mitchell R. Adhesion of Enteromorpha swarmers to microbial films. Microb Ecol. 1989;17:39–47. doi: 10.1007/BF02025592. [DOI] [PubMed] [Google Scholar]
- 15.Favio P, Perez-Luna V H, Boland T, Castner D G, Ratner B D. Surface chemical composition and fibrinogen adsorption-retention of fluoropolymer films deposited from and RF glow discharge. Plasmas Polymers. 1996;1:299–326. [Google Scholar]
- 16.Fletcher M. Bacterial attachment in aquatic environments: a diversity of surfaces and adhesion strategies. In: Fletcher M, editor. Bacterial adhesion: molecular and ecological diversity. New York, N.Y: Wiley-Liss; 1996. pp. 1–24. [Google Scholar]
- 17.Fletcher M, Pringle J H. The effect of surface free energy and medium surface tension on bacterial attachment to solid surfaces. J Colloid Interf Sci. 1985;104:5–14. [Google Scholar]
- 18.Fletcher R L, Callow M E. The settlement, attachment and establishment of marine algal spores. Br Phycol J. 1992;27:303–329. [Google Scholar]
- 19.Ista L K, Fan H, Baca O, López G P. Attachment of bacteria to model solid surfaces: oligo(ethylene glycol) surfaces inhibit bacterial attachment. FEMS Microb Lett. 1996;142:59–63. doi: 10.1111/j.1574-6968.1996.tb08408.x. [DOI] [PubMed] [Google Scholar]
- 20.Ista L K, Pérez-Luna V H, López G P. Surface-grafted, environmentally sensitive polymers for biofilm release. Appl Environ Microbiol. 1999;65:1603–1609. doi: 10.1128/aem.65.4.1603-1609.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lindner E. A low surface energy approach in the control of marine biofouling. Biofouling. 1992;6:193–205. [Google Scholar]
- 22.López G P, Albers M W, Schreiber S L, Carroll R, Peralta E, Whitesides G M. Convenient methods for patterning the adhesion of mammalian cells to surfaces using self-assembled monolayers of alkanethiolates on gold. J Am Chem Soc. 1993;115:5877–5878. [Google Scholar]
- 23.Paul J H, Jeffery W H. Evidence for separate adhesion mechanisms for hydrophilic and hydrophobic surfaces in Vibrio proteolytica. Appl Environ Microbiol. 1985;50:431–437. doi: 10.1128/aem.50.2.431-437.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prime K L, Whitesides G M. Self-assembled organic monolayers: model systems for studying adsorption of proteins at surfaces. Science. 1991;252:1164–1167. doi: 10.1126/science.252.5009.1164. [DOI] [PubMed] [Google Scholar]
- 25.Roberts C, Chen C S, Mrksich M, Martichonok V, Ingber D E, Whitesides G M. Using mixed self-assembled monolayers presenting RGD and (EG3)OH groups to characterize long-term attachment of bovine capillary endothelium cells to surfaces. J Am Chem Soc. 1998;120:6548–6555. [Google Scholar]
- 26.Scofield J H. Hartree-Slater subshell photoionization cross sections at 1254 and 1487 eV. J Electron Spectroscop Relat Phenom. 1976;8:129–137. [Google Scholar]
- 27.Sigal G B, Mrksich M, Whitesides G M. Effect of surface wettability on the adsorption of proteins and detergents. J Am Chem Soc. 1998;120:3464–3473. [Google Scholar]
- 28.Sorongon M L, Bloodgood R G, Burchard R F. Hydrophobicity, adhesion, and surface exposed proteins of gliding bacteria. Appl Environ Microbiol. 1991;57:3193–3199. doi: 10.1128/aem.57.11.3193-3199.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stanley M S, Callow M E, Callow J A. Monoclonal antibodies to adhesive cell coat glycoproteins secreted by zoospores of the green alga Enteromorpha. Planta. 1999;210:61–71. doi: 10.1007/s004250050654. [DOI] [PubMed] [Google Scholar]
- 30.Swain G W, Nelson W G, Preedeekanit S. The influence of biofouling adhesion and biotic disturbance on the development of fouling communities on non-toxic surfaces. Biofouling. 1998;12:257–269. [Google Scholar]
- 31.Tender L M, Worley R L, Fan H Y, López G P. Electrochemical patterning of self-assembled monolayers onto microscopic arrays of gold electrodes fabricated by laser-ablation. Langmuir. 1996;12:5515–5518. [Google Scholar]
- 32.Weincek K M, Fletcher M. Bacterial adhesion to hydroxyl- and methyl-terminated alkanethiol self-assembled monolayers. J Bacteriol. 1995;177:1959–1966. doi: 10.1128/jb.177.8.1959-1966.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weincek K M, Fletcher M. Effects of substratum wettability and molecular topography on the initial adhesion of bacteria to chemically defined substrata. Biofouling. 1997;11:293–311. [Google Scholar]