Abstract
Background
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants of concern (VOCs), especially the Delta and Omicron variants, have been reported to show significant resistance to approved neutralizing monoclonal antibodies (mAbs) and vaccines. We previously identified a mAb named 35B5 that harbors broad neutralization to SARS-CoV-2 VOCs. Herein, we explored the protection efficacy of a 35B5-based nasal spray against SARS-CoV-2 VOCs in a small-scale clinical trial.
Methods
We enrolled 30 healthy volunteers who were nasally administered the modified 35B5 formulation. At 12, 24, 48, and 72 hours after nasal spray, the neutralization efficacy of nasal mucosal samples was assayed with pseudoviruses coated with SARS-CoV-2 spike protein of the wild-type strain or the Alpha, Beta, Delta, or Omicron variants.
Results
The nasal mucosal samples collected within 24 hours after nasal spray effectively neutralized SARS-CoV-2 VOCs (including Delta and Omicron). Meanwhile, the protection efficacy was 60% effective and 20% effective at 48 and 72 hours after nasal spray, respectively.
Conclusions
A single nasal spray of 35B5 formation conveys 24-hour effective protection against SARS-CoV-2 VOCs, including the Alpha, Beta, Delta, or Omicron variants. Thus, 35B5 nasal spray might be potential in strengthening SARS-CoV-2 prevention, especially in high-risk populations.
Clinical Trials Registration
2022-005-02-KY.
Keywords: COVID-19, SARS-CoV-2, variants of concern, antibody, nasal spray
We enrolled 30 healthy volunteers who were nasally administered a modified anti–SARS-CoV-2 antibody formulation. The nasal mucosal samples collected within 24 hours after nasal spray effectively neutralized SARS-CoV-2 variants of concern, highlighting the prophylactic effects of the antibody formulation.
The novel coronavirus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causes coronavirus disease 2019 (COVID-19) and has resulted in a global epidemic. Though a spectrum of COVID-19 vaccines has been investigated to control the epidemic [1], neutralizing monoclonal antibody (mAb) therapies targeting SARS-CoV-2, especially the Omicron (B.1.1.529) variant, are still of paramount importance due to the Omicron variant being markedly resistant to current COVID-19 vaccines [2–5] and the capacity of neutralizing mAbs to provide immediate protection for unvaccinated individuals and vaccine-unresponsive individuals.
Currently, neutralizing mAbs against SARS-CoV-2, including those approved for clinical use and being investigated in clinical trials, are mainly used through intravenous infusion [1]. However, antibody levels in the lung are 200–10 000 times lower than those in the serum after intravenous infusion [6, 7], which leads to suboptimal protection against respiratory viruses, such as SARS-CoV-2. Alternatively, intranasal delivery of neutralizing mAbs has been proved to be advantageous in preventing and treating respiratory viruses, exemplified by influenza virus [8] and respiratory syncytial virus [9, 10]. Indeed, the nasal cavity is the first and major site of infection by SARS-CoV-2 [11, 12], and nasal delivery of anti–SARS-CoV-2 mAbs should prevent the transmission of SARS-CoV-2.
Our previous studies identified an array of human mAbs that target the receptor-binding domain (RBD) protein of SARS-CoV-2 and neutralize SARS-CoV-2 [13–15]. Among them, one mAb, named as 35B5, broadly and potently neutralizes World Health Organization–stated SARS-CoV-2 VOCs, especially exhibiting the picomolar neutralizing efficacy to the Delta variant (B.1.617.2) [15] and the Omicron variant (B.1.1.529) [13]. Herein, we explored the effects of nasal spray of 35B5 mAb in protecting individuals from SARS-CoV-2 VOCs.
METHODS
Study Population
We enrolled 2 cohorts in the study; each cohort contained 15 healthy volunteers. All volunteers provided written informed consent. These volunteers were nasally administered a modified 35B5 mAb formulation (1 mg/mL 35B5 mAb, diluted in 50% Dulbecco’s phosphate-buffered saline [PBS] plus 50% glycerol) by a homemade nebulizer. For cohort 1, a nasal sample at 12 hours was collected from 1 nasal cavity of each volunteer, while a nasal sample at 24 hours was collected from the other nasal cavity. For cohort 2, each nasal sample of a timepoint was collected from 1 individual. The nasal secretions were collected by the insertion of cotton swabs into nasal cavities for 5 minutes, then hydrating for 10 minutes with 0.5 mL of PBS solution. The study received institutional review board approval at Chongqing Public Health Medical Center (2022-005-02-KY).
Enzyme-Linked Immunosorbent Assay
Enzyme-linked immunosorbent assay (ELISA) plates (Thermo Fisher, 446469) were coated with 50 ng SARS-CoV-2 RBD protein (Sino Biological, 40592-V08H) in 100 µL PBS per well overnight. Then, the ELISA plates were incubated with blocking buffer (5% fetal bovine serum + 0.1% Tween 20 in PBS) for 1 hour. Nasal mucosal samples (50 µL) were next added to each well and incubated for 1 hour. The ELISA plates were then washed with PBST (PBS + 0.1% Tween 20), incubated with horseradish peroxidase–conjugated goat antihuman immunoglobulin G (IgG) antibody (Bioss Biotech), and washed with PBST, and then TMB (Beyotime) was added. The ELISA plates were allowed to react with TMB for approximately 5 minutes and then stopped by 1 M sulfuric acid stop buffer. The optical density value was determined at 450 nm. In each ELISA assay, a range of serially diluted original/unmodified 35B5 mAb (0.00064, 0.0032, 0.016, 0.08, 0.4, 2, 10, and 50 µg/mL; 5-fold dilution) was used as positive control.
SARS-CoV-2 Pseudovirus Neutralization Assay
SARS-CoV-2 pseudotype particles (0.01 multiplicity of infection), including the wild-type (WT) strain (SinoBiological, PSV001), the Alpha (B.1.1.7) strain (SinoBiological, PSV006), the Beta (B.1.351) strain (SinoBiological, PSV008), the Delta (B.1.617.2) strain (SinoBiological, PSV011), and the Omicron (B.1.1.529) strain (SinoBiological, PSV016), were preincubated with nasal mucosal samples for 1 hour at 37°C. Afterward, hACE2-expressing HEK-293 (hACE2/293) T cells were incubated with the mixtures overnight and then cultured with fresh media. At 48 hours after incubation, the luciferase activity of SARS-CoV-2 typed pseudovirus-infected hACE2/293 T cells were determined by a luciferase reporter assay kit (Promega, E1910). Such a SARS-CoV-2 pseudovirus neutralization assay has been proven to be highly consistent with the authentic SARS-CoV-2 neutralization assay [3, 13, 16, 17].
Statistical Analysis
The 35B5 concentrations of nasal mucosal samples of each individual were compared by the paired t test. The cutoff value in each pseudovirus neutralization assay was determined by the receiver operating characteristic curve analysis and was of the highest likelihood ratio. P values < .05 were defied as statistically significant. GraphPad Prism version 6.0 software was used for statistical analysis.
RESULTS
Mucosal Swabs Sampled Post–Nasal Spray of 35B5 mAb Preserve Potent Neutralization Against SARS-CoV-2 VOCs Within 24 Hours
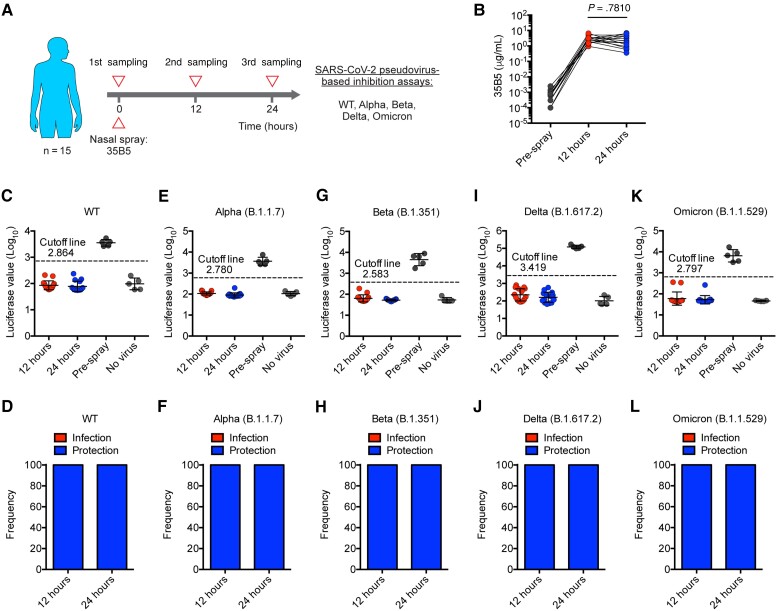
We recruited 15 healthy volunteers (cohort 1; median age, 29 [range, 23–46] years) who had no history of SARS-CoV-2 infection and received COVID-19 vaccines (Supplementary Table 1). Prior to antibody spray, nasal samples of all volunteers were collected as blank controls (Figure 1A). Then, volunteers were intranasally administered a modified 35B5 mAb formulation (see details in the Materials and Methods) and were subsequently performed with nasal sampling at 12 hours and 24 hours after antibody spray (Figure 1A).
Figure 1.
The mucosal swabs sampled post nasal spray of 35B5 monoclonal antibody (mAb) preserves potent neutralization against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants of concern within 24 hours. A, Schematic diagram. B, 35B5 mAb concentrations in nasal mucosal samples at different timepoints as determined by enzyme-linked immunosorbent assay. C–L, Neutralization of nasal mucosal samples against SARS-CoV-2 wild-type (WT) (C and D), Alpha (E and F), Beta (G and H), Delta (I and J), and Omicron (K and L) as determined by pseudovirus neutralization assays. Nasal secretions from individuals with a luciferase value under the cutoff line were considered protective. The cutoff value in each pseudovirus-neutralizing function assay was determined by receiver operating characteristic curve analysis and was of the highest likelihood ratio to distinguish samples of positive controls (pre–antibody spray) and samples of negative controls (no virus). Error bars (C, E, G, I, and K) indicate standard deviation.
First, we used IgG ELISA to detect 35B5 mAb in nasal mucosal samples of 3 different timepoints (pre–antibody spray, 12 hours and 24 hours). We found that mAbs specific for SARS-CoV-2 RBD were undetected in nasal mucosal samples of pre–antibody spray timepoint, suggesting no preexisting anti–SARS-CoV-2 RBD mAbs in the nasal mucus of these volunteers who received COVID-19 vaccines (Figure 1B). Remarkably, abundant amounts of mAbs specific for SARS-CoV-2 RBD were observed in the nasal mucosal samples at 12 hours and 24 hours after antibody spray (Figure 1B). Given no detectable anti–SARS-CoV-2 RBD mAbs in pre–antibody spray samples, nasal spray of 35B5 mAb acted as the sole contributor for the anti–SARS-CoV-2 RBD mAb-rich nasal mucus at 12 hours and 24 hours. We further observed that the 35B5 mAb concentration in nasal mucus varied in the range from 1 to 10 µg/mL (Figure 1B), which might be due to nonunified nasal administration. However, the 35B5 mAb concentrations were comparable between the 12-hour and 24-hour samples (Figure 1B), which suggests that modified 35B5 mAbs were stably adhered to the nasal mucosal surface for at least 24 hours.
We next assessed the neutralizing activity of 35B5 mAb-rich nasal mucus against SARS-CoV-2 variants by pseudovirus neutralization assays as previously described [18, 19]. Notably, we found that 100% of nasal mucosal samples from both 12 hours and 24 hours were able to protect hACE2/293 T cells from being infected by SARS-CoV-2 pseudoviruses, including WT, B.1.1.7 (Alpha), B.1.351 (Beta), B.1.617.2 (Delta), and B.1.1.529 (Omicron) (Figure 1C–L). These findings echo the ultrapotent neutralizing activities of 35B5 mAb in treating authentic SARS-CoV-2–infected mice [15] and highlight the highly potent prophylactic effects of modified 35B5 mAb spray in protecting individuals from SARS-CoV-2 (especially the Omicron variant) infection.
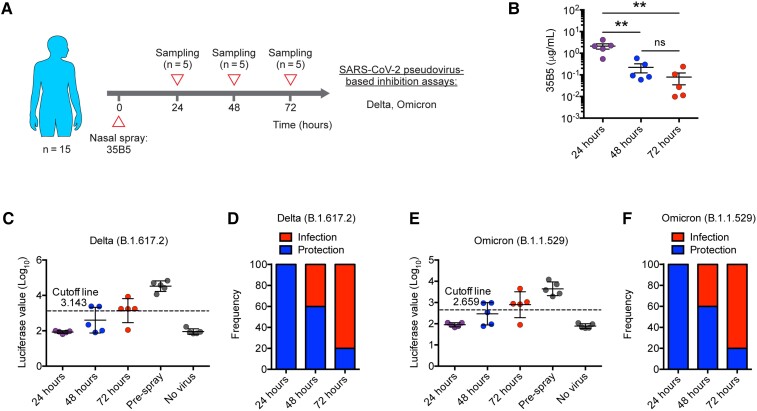
Protection Efficacy of a Single Dose of 35B5 Nasal Spray Wanes After 24 Hours
To further explore the protection duration of a single dose of 35B5 mAb formation, we recruited another cohort of 15 healthy volunteers (cohort 2; median age, 28 [range, 26–31] years) (Supplementary Table 1). These volunteers were nasally administered the 35B5 mAb formation and the nasal mucosal samples were collected at 24, 48, or 72 hours (Figure 2A). As evaluated by the IgG ELISA assay, 35B5 mAb concentrations of 48-hour samples and 72-hour samples were reduced 10-fold and 100-fold, respectively, compared to that of 24-hour samples (Figure 2B). Additionally, we also examined the neutralizing activities of these mucosal samples against the SARS-CoV-2 Delta and Omicron variants. Consistently, we found that 24-hour samples show highly potent neutralizing capacity to the Delta and Omicron variants (Figure 2C–F). However, the frequency of protection dropped to 60% at 48 hours post–nasal spray and eventually descended to 20% at 72 hours post–nasal spray (Figure 2C–F). Thus, these findings suggest that a single dose of 35B5 nasal spray conveys protection against SARS-CoV-2 within the first 24 hours and a second dose of 35B5 nasal spray is needed for longer duration of protection.
Figure 2.
Protection efficacy of a single dose of 35B5 nasal spray wanes after 24 hours. A, Schematic diagram. B, 35B5 monoclonal antibody concentrations in nasal mucosal samples at different timepoints as determined by enzyme-linked immunosorbent assay. C–F, Neutralization of nasal mucosal samples against the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Delta (C and D) and Omicron (E and F) variants as determined by pseudovirus neutralization assays. Nasal secretions from individuals with luciferase value under the cutoff line were considered protective. The cutoff value in each pseudovirus-neutralizing function assay was determined by receiver operating characteristic curve analysis and was of the highest likelihood ratio to distinguish samples of positive controls (pre–antibody spray) and samples of negative controls (no virus). Error bars in B indicate standard error of the mean, and error bars in C and E indicate standard deviation. **P < .01. Abbreviation: ns, not significant.
DISCUSSION
Neutralizing mAbs convey immediate protection to individuals exposed to SARS-CoV-2. Our previous works identified a human mAb 35B5 that exhibits picomolar pan-neutralization against SARS-CoV-2 VOCs in vitro and in vivo [13, 15]. In this small-scale clinical trial, we show that nasal secretions of individuals nasally administered 35B5 mAb formulation within the first 24 hours convey efficient in vitro neutralization against SARS-CoV-2 VOCs, including the Alpha (B.1.1.7), Beta (B.1.351), Delta (B.1.617.2), and Omicron (B.1.1.529) variants. To our knowledge, this is the first clinical study to report the prophylactic efficacy of neutralizing mAb against SARS-CoV-2 infection through nasal spray.
Nasal spray of neutralizing mAbs was recently reported to be of high efficacy to protect animals against SARS-CoV-2 [6, 20–22]. Indeed, nasal delivery of neutralizing mAbs mainly targets the airway [6], including nasal cavity and lung, and was approved to be more effective than systemic application (eg, intraperitoneal) in curtailing SARS-CoV-2 load and ameliorating lung pathology [22]. In addition, nasal delivery of neutralizing mAbs was also suggested to be superior to systemic application in protecting animals against influenza [8] and respiratory syncytial virus [9, 10]. Herein, we found that nasal spray of 35B5 antibody generates enriched antibodies in the nasal mucosa, thus conveying potent prophylactic protection against SARS-CoV-2 by nasal spray of neutralizing mAbs in a clinical setting. Further studies are needed to explore whether the nasal spray of 35B5 antibody could efficiently delivery antibodies to lung of volunteers, as reported in the animal study [6], and thus further support the usage of 35B5 nasal spray in clinical practice.
The portal of entry for SARS-CoV-2 is the respiratory tract, and the lung is the primary organ for pathogenesis. Immunoglobulin M (IgM) and immunoglobulin A (IgA) are mucosal antibodies that defend against respiratory viruses locally. Remarkably, nasal delivery of an IgM mAb conferred higher respiratory protection than the IgG1 counterpart for protection against SARS-CoV-2 in mice [6]. Given the high prophylactic efficacy of nasal spray of 35B5 IgG1 mAb in the study, the effects of nasal spray of engineered IgM- or IgA-typed 35B5 mAb warrant further investigation.
The Omicron (B.1.1.529) variant is a novel VOC identified in December 2021 and has rapidly increased in frequency worldwide [23], attributing to its remarkable escape from current COVID-19 vaccines and therapeutic neutralizing mAbs approved for clinical use [2–5]. In this study, we found that the nasal secretions of individuals administered the modified 35B5 mAb formulation efficiently neutralize the Omicron variant in vitro, thus highlighting the modified 35B5 mAb formulation as an emergency medical countermeasure in coping with the Omicron variant.
Given the fact that some studies indicate higher SARS-CoV-2 titers in the throat/saliva than nasopharyngeal swabs and that the throat/saliva was suggested to be more sensitive than the corresponding nasopharyngeal swabs for SARS-CoV-2 detection [24–27], we assume that a delivery of 35B5 mAb to the respiratory tract via both the nose and mouth could convey a reinforced protection against SARS-CoV-2 infection.
There are some limitations to our study. One limitation is the relatively small population size, even though we obtained consistent results in 2 cohorts. Another limitation is the lack of functional validation of 35B5 nasal spray with the authentic SARS-CoV-2 inhibition assays or mouse experiments, which need further investigation. In addition, we found that the effective protection duration of 1 single dose of 35B5 nasal spray only covers the first 24 hours, which requires daily inhalation in clinical practice. More strategies, such as modifying the initial antibody concentration or optimizing the formulation for extended duration of antibody at nasal mucosa, are needed to improve the protection duration of 35B5 nasal spray in the future.
In conclusion, we showed the first clinical evidence that nasal spray of modified neutralizing mAb may efficiently protect individuals from SARS-CoV-2, including WT and the B.1.1.7 (Alpha), B.1.351 (Beta), B.1.617.2 (Delta), and B.1.1.529 (Omicron) variants. We advise to take nasal spray of neutralizing mAb as a strategy to strengthen COVID-19 prevention, especially for high-risk populations.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author Contributions. Y. L., S. Y., Y. Y., Z. P., and X. Y. performed the experiments. S. Y., Z. L., L. H., J. T., Q. W., S. L., and Q. T. assisted in recruiting the healthy participants. L. G., J. Z., Y. W., Y. H., L. X., Q. H., and B. Z. helped to discuss the results. X. C. designed the study, analyzed the data, and drafted the manuscript with L. Y. and Y. C.; and L. Y. and Y. C. supervised the study.
Acknowledgments. The authors thank all of the members in L. Y.’s laboratory for technical assistance and discussion.
Financial support. This work was supported by the National Science and Technology Major Project of China (grant number 2021YFC2300502 to L. Y.) and the National Natural Science Foundation of China (grant number 31825011 to L. Y.), with payments made to institution.
Supplementary Material
Contributor Information
Yao Lin, Institute of Immunology, Third Military Medical University, Chongqing, China.
Shuai Yue, Institute of Immunology, Third Military Medical University, Chongqing, China.
Yang Yang, Guangdong Provincial Key Laboratory of Immune Regulation and Immunotherapy, School of Laboratory Medicine and Biotechnology, Southern Medical University, Guangzhou, China.
Sen Yang, Division of Infectious Diseases, Chongqing Public Health Medical Center, Chongqing, China.
Zhiwei Pan, Institute of Immunology, Third Military Medical University, Chongqing, China.
Xiaofan Yang, Dermatology Hospital, Southern Medical University, Guangzhou, China.
Leiqiong Gao, Institute of Immunology, Third Military Medical University, Chongqing, China.
Jing Zhou, Institute of Immunology, Third Military Medical University, Chongqing, China.
Zhirong Li, Institute of Immunology, Third Military Medical University, Chongqing, China.
Li Hu, Institute of Immunology, Third Military Medical University, Chongqing, China.
Jianfang Tang, Institute of Immunology, Third Military Medical University, Chongqing, China.
Qing Wu, Institute of Immunology, Third Military Medical University, Chongqing, China.
Shun Lei, Institute of Immunology, Third Military Medical University, Chongqing, China.
Qin Tian, Dermatology Hospital, Southern Medical University, Guangzhou, China.
Yifei Wang, Guangdong Provincial Key Laboratory of Immune Regulation and Immunotherapy, School of Laboratory Medicine and Biotechnology, Southern Medical University, Guangzhou, China.
Yaxing Hao, Institute of Immunology, Third Military Medical University, Chongqing, China.
Lifan Xu, Institute of Immunology, Third Military Medical University, Chongqing, China.
Qizhao Huang, Guangdong Provincial Key Laboratory of Immune Regulation and Immunotherapy, School of Laboratory Medicine and Biotechnology, Southern Medical University, Guangzhou, China.
Bo Zhu, Institute of Cancer, Xinqiao Hospital, Third Military Medical University, Chongqing, China.
Yaokai Chen, Division of Infectious Diseases, Chongqing Public Health Medical Center, Chongqing, China.
Xiangyu Chen, Guangdong Provincial Key Laboratory of Immune Regulation and Immunotherapy, School of Laboratory Medicine and Biotechnology, Southern Medical University, Guangzhou, China; Institute of Cancer, Xinqiao Hospital, Third Military Medical University, Chongqing, China.
Lilin Ye, Institute of Immunology, Third Military Medical University, Chongqing, China.
References
- 1. Corti D, Purcell LA, Snell G, Veesler D. Tackling COVID-19 with neutralizing monoclonal antibodies. Cell 2021; 184:3086–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Liu L, Iketani S, Guo Y, et al. Striking antibody evasion manifested by the omicron variant of SARS-CoV-2. Nature 2021; 602:676–81. [DOI] [PubMed] [Google Scholar]
- 3. Cameroni E, Bowen JE, Rosen LE, et al. Broadly neutralizing antibodies overcome SARS-CoV-2 Omicron antigenic shift. Nature 2021; 602:664–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hoffmann M, Krüger N, Schulz S, et al. The Omicron variant is highly resistant against antibody-mediated neutralization: implications for control of the COVID-19 pandemic. Cell 2021; 185:447–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Garcia-Beltran WF, St Denis KJ, Hoelzemer A, et al. mRNA-based COVID-19 vaccine boosters induce neutralizing immunity against SARS-CoV-2 Omicron variant. Cell 2022; 185:457–66.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ku Z, Xie X, Hinton PR, et al. Nasal delivery of an IgM offers broad protection from SARS-CoV-2 variants. Nature 2021; 595:718–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Respaud R, Vecellio L, Diot P, Heuzé-Vourc'h N. Nebulization as a delivery method for mAbs in respiratory diseases. Expert Opin Drug Deliv 2015; 12:1027–39. [DOI] [PubMed] [Google Scholar]
- 8. Leyva-Grado VH, Tan GS, Leon PE, Yondola M, Palese P. Direct administration in the respiratory tract improves efficacy of broadly neutralizing anti-influenza virus monoclonal antibodies. Antimicrob Agents Chemother 2015; 59:4162–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Weltzin R, Traina-Dorge V, Soike K, et al. Intranasal monoclonal IgA antibody to respiratory syncytial virus protects rhesus monkeys against upper and lower respiratory tract infection. J Infect Dis 1996; 174:256–61. [DOI] [PubMed] [Google Scholar]
- 10. Prince GA, Hemming VG, Horswood RL, Baron PA, Chanock RM. Effectiveness of topically administered neutralizing antibodies in experimental immunotherapy of respiratory syncytial virus infection in cotton rats. J Virol 1987; 61:1851–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gengler I, Wang JC, Speth MM, Sedaghat AR. Sinonasal pathophysiology of SARS-CoV-2 and COVID-19: a systematic review of the current evidence. Laryngoscope Invest Otolaryngol 2020; 5:354–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Killingley B, Mann AJ, Kalinova M, et al. Safety, tolerability and viral kinetics during SARS-CoV-2 human challenge in young adults. Nat Med 2022; 28:1031–41. [DOI] [PubMed] [Google Scholar]
- 13. Wang X, Chen X, Tan J, et al. 35B5 antibody potently neutralizes SARS-CoV-2 Omicron by disrupting the N-glycan switch via a conserved spike epitope [manuscript published online ahead of print 29 March 2022]. Cell Host Microbe 2022. doi:10.1016/j.chom.2022.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen X, Li R, Pan Z, et al. Human monoclonal antibodies block the binding of SARS-CoV-2 spike protein to angiotensin converting enzyme 2 receptor. Cell Mol Immunol 2020; 17:647–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang X, Hu A, Chen X, et al. A potent human monoclonal antibody with pan-neutralizing activities directly dislocates S trimer of SARS-CoV-2 through binding both up and down forms of RBD. Signal Transduct Targeted Ther 2022; 7:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ju B, Zhang Q, Ge J, et al. Human neutralizing antibodies elicited by SARS-CoV-2 infection. Nature 2020; 584:115–9. [DOI] [PubMed] [Google Scholar]
- 17. Shi R, Shan C, Duan X, et al. A human neutralizing antibody targets the receptor-binding site of SARS-CoV-2. Nature 2020; 584:120–4. [DOI] [PubMed] [Google Scholar]
- 18. Yue S, Li Z, Lin Y, et al. Sensitivity of SARS-CoV-2 variants to neutralization by convalescent sera and a VH3-30 monoclonal antibody. Front Immunol 2021; 12:751584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chen X, Pan Z, Yue S, et al. Disease severity dictates SARS-CoV-2-specific neutralizing antibody responses in COVID-19. Signal Transduct Targeted Ther 2020; 5:180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang H, Yang Z, Xiang J, Cui Z, Liu J, Liu C. Intranasal administration of SARS-CoV-2 neutralizing human antibody prevents infection in mice. bioRxiv [Preprint]. Posted online 9 December 2020. doi:10.1101/2020.12.08.416677. [Google Scholar]
- 21. Piepenbrink MS, Park JG, Oladunni FS, et al. Therapeutic activity of an inhaled potent SARS-CoV-2 neutralizing human monoclonal antibody in hamsters. Cell Rep Med 2021; 2:100218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Halwe S, Kupke A, Vanshylla K, et al. Intranasal administration of a monoclonal neutralizing antibody protects mice against SARS-CoV-2 infection. Viruses 2021; 13:1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Scott L, Hsiao NY, Moyo S, et al. Track Omicron's spread with molecular data. Science 2021; 374:1454–5. [DOI] [PubMed] [Google Scholar]
- 24. Wong SCY, Tse H, Siu HK, et al. Posterior oropharyngeal saliva for the detection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clin Infect Dis 2020; 71:2939–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yang Y, Yang M, Yuan J, et al. Laboratory diagnosis and monitoring the viral shedding of SARS-CoV-2 infection. Innovation 2020; 1:100061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wyllie AL, Fournier J, Casanovas-Massana A, et al. Saliva or nasopharyngeal swab specimens for detection of SARS-CoV-2. N Engl J Med 2020; 383:1283–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Teo AKJ, Choudhury Y, Tan IB, et al. Saliva is more sensitive than nasopharyngeal or nasal swabs for diagnosis of asymptomatic and mild COVID-19 infection. Sci Rep 2021; 11:3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.