Abstract
Background
Currently, the rapid antigen test (RAT) and reverse transcriptase–polymerase chain reaction (RT–PCR) are considered the main stakeholders in COVID-19 diagnosis. In RT–PCR, any of at least 2 evolutionary conserved genes (RdRP, E-, N-, ORF1ab gene) and S-gene of SARS-CoV-2 are endorsed, and in RAT, the nucleocapsid antigen (N-Ag) of SARS-CoV-2 is considered due to its stability and fewer chances of mutation effects. In the present work, we evaluated the performance of the AG-Q COVID-19 N-Ag self-test kit and conducted a validation study in comparison with the RT–PCR.
Methods
AG-Q COVID-19 N-Ag rapid test kit is an Indian Council of Medical Research (ICMR) approved product developed and marketed by Agappe Diagnostics Limited. The RT–PCR assay was performed with a COVIPATH COVID-19 RT–PCR kit from Thermo Fisher Scientific.
Results
We observed 19 false-negative results in antigen self-tests, including samples of threshold cycle (Ct) values 22/22 (N-gene/ORF1ab-gene) in RT–PCR, indicating inadequate sampling by the patients in self-tests, leading to false-negative results and increased chances of the disease spreading. Based on the RT–PCR Ct value vs antigen self-test comparison, it is evident that proper sampling is crucial in performing antigen self-tests. Also, there were weak positive results in antigen self-tests with a Ct value of 18/19 in RT–PCR.
Conclusions
Although the sensitivity and diagnostic accuracy offered by the AG-Q COVID-19 N-Antigen self-test in comparison with RT–PCR fulfills the ICMR tenets for RAT, this study recommends the laboratory/hospital-based RAT execution would be appropriate, rather than the self-test.
Keywords: N-antigen, self-test, RT–PCR, COVID-19, colloidal gold
IMPACT STATEMENT
This study revealed the importance of sampling in COVID-19 antigen self-tests. The self-test strategy provided a faster pace for the mass screening of SARS-CoV-2 diagnosis, but the chances for false-negative results are also high. Such false-negative results will negatively affect the population by increasing the chance of disease spreading. Hence, the study recommends laboratory/hospital-based sampling for RAT for a better outcome.
Introduction
The rapid antigen test (RAT) and reverse transcriptase–polymerase chain reaction (RT–PCR) are the major stakeholders in COVID-19 diagnosis. RAT is the primary option for mass screening and RT–PCR stands out as the confirmatory gold standard test for COVID-19 disease. Diagnosis of COVID-19 through RAT is the fastest way to screen the suspected clusters, thereby preventing the transmission of the disease to a certain extent (1, 2). To date, the Indian Council of Medical Research (ICMR) has validated and approved 52 RAT COVID-19 kits and 7 RAT self-test COVID-19 kits to use in India. The RAT can be considered as a confirmatory test in the case of positive results, but an RT–PCR test is further recommended to confirm the infection in the suspected individuals with a negative antigen test (3, 4).
The COVID-19 RAT focuses on the detection of nucleocapsid antigens (N-Ag) of SARS-CoV-2. Even though the spike protein receptor binding domain is specific to SARS-CoV-2, synonymous mutations in the spike protein receptor binding domain gene make this protein incongruous for consideration as a diagnostic marker in the RAT platform. The N-gene appears as least vulnerable for mutations, hence the nucleocapsid protein is considered to be an evolutionarily stable and reliable viral-marker for COVID-19 detection through RAT. The RT–PCR kits focus on the identification minimum of 2 genes out of the N-gene, RdRP gene, E-gene, ORF1ab, and S-gene (5–7).
Out of several preanalytical errors leading to false-negative COVID-19 diagnosis, 2 factors need more attention: proper sample collection and the storage of samples before testing. The preferred sample collection method for COVID-19 antigen test is nasopharyngeal, due to the maximum virus colonization in the nasopharyngeal region. In RAT self-tests, nasal swab-based sample collection is favored to make the test more user-friendly. Improper or inadequate sampling leads to false-negative results in RAT, which are further intensified in nasal self-sample collection. In the case of RT–PCR, a nasopharyngeal sample together with an oropharyngeal sample collection is preferred to minimize the sensitivity issue due to improper sampling.
The sample transportation and storage conditions are other important factors for consideration for COVID-19 diagnosis. RAT is supposed to be performed immediately after sample collection to prevent protein degradation on transportation and storage. Transportation in a cold chain at 4 °C and storage for a few hours in the lysis buffer are bearable for RAT. For RT–PCR, the preferred method involves storing the nasopharyngeal and oropharyngeal samples in a viral transport medium (VTM). In the VTM, samples are stable for transportation in a cold chain transportation bag at 4 °C and can be stored for longer at −20 °C (8, 9). In this study, we have evaluated the performance of the AG-Q COVID-19 N-Ag self-test kit and conducted a validation study at the Noorul Islam Institute of Medical Science & Research Foundation (NIMS), Trivandrum, Kerala State, in comparison with the RT–PCR. The AG-Q COVID-19 N-Ag rapid test kit is an ICMR-approved product developed and marketed by Agappe Diagnostics Limited. The kit contents are designed to make it suitable for self-test as per the ICMR guidelines. The transformation of a laboratory-based antigen test to self-test comprises 2 major changes; the nasopharyngeal swab is substituted with a nasal swab and an artificial intelligence support with a QR code is provided to upload the patient details and result to the ICMR portal. Self-test by RAT is advised only in symptomatic individuals and immediate contacts of laboratory-confirmed positive cases.
Materials and Methods
Sample Collection
One hundred and fifty participants were recruited for this study at NIMS, Trivandrum. Institutional ethical committee approval (IEC approval number; NIMS/IEC/2021/07/02) has been taken and informed consent was obtained from each participant for using the sample for research purpose. Eighty-six COVID-19 positive samples were collected from the inpatients in the COVID care center of NIMS Medicity and 64 negative/control samples were collected from the outpatients who came for COVID-19 testing. For the COVID-19 antigen self-test, the patients themselves performed the nasal sample collection from both nostrils with the nasal swab provided under the supervision of a healthcare professional, and the nasopharyngeal and oropharyngeal sample collection for RT–PCR were done by the healthcare professional in the COVID care center. Sampling for RAT and RT–PCR were performed at the same time. Confirmed COVID-19 infected inpatients within 5 days of admission were only included for positive sample collection.
Assays and Instruments
The RT–PCR assay was performed with a COVIPATH COVID-19 RT–PCR kit from Thermo Fisher Scientific. The kit detects both the N-Gene and ORF1ab-Gene of SARS-CoV-2. The Zybio automated nucleic acid extraction kit was used for the viral RNA extraction prior to RT–PCR. The AG-Q COVID-19 N-Ag self-test kit was used to qualitatively detect the presence or absence of SARS-CoV-2. Nasopharyngeal swab samples collected in viral transport media were used for RT–PCR while nasal swab samples from the corresponding participants were used in the RAT test for the comparison study. Both samples for RAT and RT–PCR were transferred together to the central laboratory facility for performing the tests within a time period of 2 hours. On receipt, the lysis buffered sample was sent immediately for RAT analysis, and in the meantime, the VTM samples were used for RNA extraction.
Statistical Analysis
The clinical sensitivity, clinical specificity, positive predictive value, negative predictive value, and accuracy of the AG-Q COVID-19 N-Ag kit in comparison to RT–PCR were calculated using MedCalc statistical software.
Assay Standardization and Testing Protocol for AG-Q COVID-19 N-Ag Self-Test
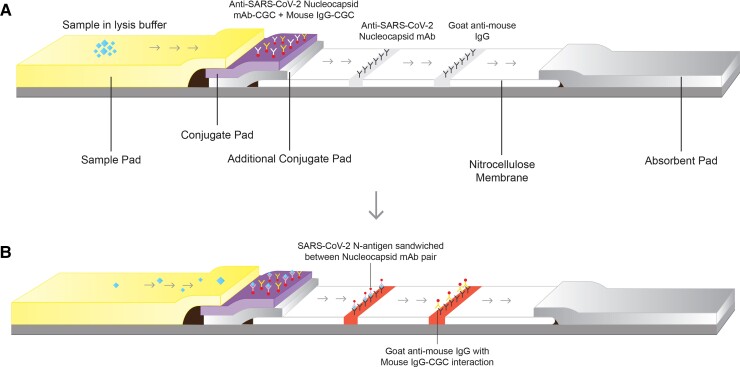
A pair of SARS-CoV-2 specific antinucleocapsid monoclonal antibodies were used as the capture and detector antibodies in the RAT assay. The test line and control line capture antibodies (antinucleocapsid antibody and goat antimouse IgG, respectively) were coated on the nitrocellulose membrane at test and control lines, respectively, and colloidal gold conjugated (CGC) detector antibodies (antinucleocapsid antibody CGC and mouse IgG CGC) were coated on the conjugate release pad. Then, 40-nm colloidal gold nanoparticles were used for detector antibodies conjugation through passive adsorption and the tri-sodium citrate reduction method was used for the preparation of a colloidal gold solution (10). The membrane system comprises overlapping alignment of a sample pad, conjugate pad, additional conjugate pad, nitrocellulose membrane, and absorbent pad. Standardization of membrane assembly was done in such a way that the strip possessed a wicking rate of 2 minutes.
The lysis buffer provided for sample treatment breaks the viral particles and releases the nucleocapsid protein from viral genetic material. For the self-test, the nasal sample has to be collected by the patient using the nasal swab provided with the kit. Three to 4 drops of sample mixed lysis buffer are dropped into the sample well of the cassette for testing. The result interpretation time for the assay is 2–20 minutes. A positive sample will give bands on both the test and control lines, whereas a negative sample produces only one band on the control line (Fig. 1, A and B).
Fig. 1.
(A), Depicts the membrane assembly and antibodies distribution in the COVID-19 antigen self-test strip; (B), Depicts the sandwich reaction between N-Antigen and anti-SARS-CoV-2 nucleocapsid antibodies on test line and goat antimouse IgG to mouse IgG interaction at the control line. mAb, monoclonal antibody; CGC, colloidal gold conjugate.
Performance Evaluation
The AG-Q COVID-19 N-Ag self-test kit was internally validated to assess the performance in terms of analytical sensitivity, linearity, accelerated stability study, and capability of detecting SARS-CoV-2 variants. Recombinant nucleocapsid antigen (N-Antigen of 17 SARS-CoV-2 variants) from Fapon Biotech, China, was used for the performance evaluation of the product. The nucleocapsid antigen was reconstituted and serially diluted in the lysis buffer with a concentration ranging from 80 000 ng/mL to 100 pg/mL. The accelerated stability study was carried out at different temperatures of 24 °C (room temperature), 37 °C, and 45 °C. The strips and lysis buffer were subjected to 60 days of accelerated stability study at 85% humidity for the aforementioned temperatures. The antigen test strip, pouched in a 5-layered aluminum pouch with activated silica particles, and the lysis buffer, prefilled in an antigen extraction tube, were used for the stability study. After 60 days of temperature and humidity exposure, the strips were tested with the recombinant nucleocapsid antigen prepared in lysis buffer kept for the stability study.
The accuracy, clinical sensitivity, clinical specificity, positive predictive value, and negative predictive value of the developed RAT self-test kit were externally assessed in comparison with the RT–PCR performed at NIMS. The samples collected in VTM for RT–PCR were subjected to RNA extraction and 10 µL of the RNA extract used for PCR amplification.
Results
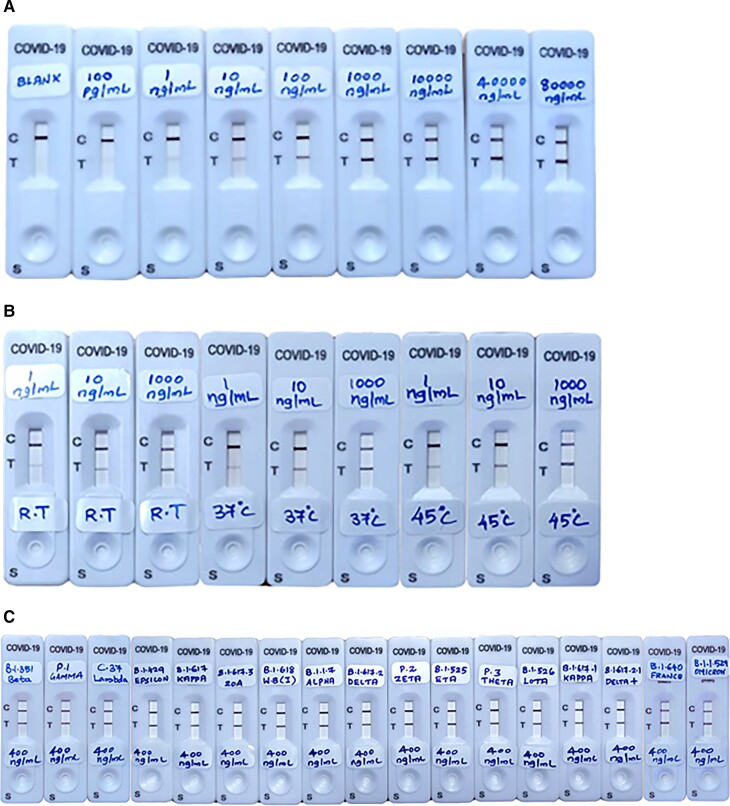
The AG-Q COVID-19 N-Antigen self-test offers an analytical sensitivity of 100 pg/mL and a linearity of ≥80 000 ng/mL with the recombinant nucleocapsid protein (Fig. 2, A). The product is not affected by the prozone effect at a higher concentration of N-Antigen. The product successfully withstands the high temperature and humidity during an accelerated stability study for 60 days. Even after 60 days at 37°C and 45°C, the strips showed the same efficacy as the strips at room temperature (Fig. 2, B). The N-Ag of SARS-CoV-2 variants (variants of concern and variants of interest) were detectable using the AG-Q COVID-19 N-Antigen self-test kit (Fig. 2, C and Table 1). A concentration of 400 ng/mL of N-Antigen was prepared for each variant in the lysis buffer and used as the sample (3 drops = 75 µL) to check the efficiency of the kit.
Fig. 2.
(A), COVID-19 positive result with recombinant nucleocapsid protein. Analytical sensitivity, 100 pg/mL and linearity ≥80 000 ng/mL; (B), Accelerated stability study result at room temperature, 37 °C, and 45 °C. The performance test was done using recombinant nucleocapsid protein (1000 ng/mL, 10 ng/mL, 1 ng/mL) in lysis buffer; (C), SARS-CoV-2 variants check in an AG-Q COVID-19 N-Antigen self-test kit.
Table 1.
SARS-CoV-2 variants detectable by AG-Q COVID-19 N-Antigen RAT.
| SARS-CoV-2 | WHO Label | Pango Lineage | Detected by AG-Q COVID-19 N-Ag Kit |
|---|---|---|---|
| Variants of concern | Alpha | B.1.1.7 | Yes |
| Beta | B.1.351 | Yes | |
| Gamma | P.1 | Yes | |
| Delta | B.1.617.2 | Yes | |
| Delta Plus | B.1.617.2.1 | Yes | |
| Omicron | B.1.1.529 | Yes | |
| Variants under monitoring | France Strain | B.1.640 | Yes |
| Variants of interest | Lambda | C.37 | Yes |
| Formerly monitored variants | Epsilon | B.1.429 | Yes |
| Kappa | B.1.617 | Yes | |
| 20A | B.1.617.3 | Yes | |
| West Bengal Strain | B.1.618 | Yes | |
| Zeta | P.2 | Yes | |
| Eta | B.1.525 | Yes | |
| Theta | P.3 | Yes | |
| Lota | B.1.526 | Yes | |
| Kappa | B.1.617.1 | Yes |
The third-party evaluation study at NIMS in comparison with the RT–PCR reveals the clinical sensitivity and specificity of the antigen self-test. Based on the report, the kit offers a clinical sensitivity of 77.91% and clinical specificity of 100% in comparison with the gold standard RT–PCR assay. The Thermo Fisher RT–PCR kit considered a threshold cycle (Ct) value up to 37 as positive for the N-Gene and ORF1ab-Gene. As per the ICMR guidelines, sensitivity >50% and specificity >95% are mandatory for the approval of the antigen self-test when comparing with RT–PCR. In total, the AG-Q COVID-19 N-Ag self-test offers an accuracy of 87.33% with a positive predictive value of 100% and a negative predictive value of 77.11% (Table 2).
Table 2.
Depicts the diagnostic efficacy of AG-Q COVID-19 N-Antigen self-test in comparison with RT–PCR (done by MedCalc statistical software).
| Gold standard real time PCR for COVID-19 (Ct value range: < 37) for N-Gene and ORF 1ab | AG-Q COVID-19 N-antigen self-test card | ||
|---|---|---|---|
| Test positive | Test negative | ||
| True positive | 86 | 67 (false negative: 19) | 83 (false positive: 0) |
| True negative | 64 | ||
| Value | 95% CI | ||
| Kit sensitivity | 77.91% | 67.67% to 86.14% | |
| Kit specificity | 100.00% | 94.4% to 100.00% | |
| Positive predictive value: the probability that the disease is present when the test is positive | 100.00% | ||
| Negative predictive value: the probability that the disease is not present when the test is negative | 77.11% | 69.37% to 83.36% | |
| Accuracy | 87.33% | 80.93% to 92.20% | |
Even though the kit satisfied the ICMR guidelines of RAT approval strategies, the RT–PCR Ct value compared with the antigen self-test results put forward uncertainty for sampling efficiency in an antigen self-test. The 19 false-negative results in the antigen self-test included samples of Ct values 22/22 (N-gene/ORF1ab-gene) in RT–PCR. Also, there were weak positive results in antigen self-test with a Ct value of 18/19 in RT–PCR. In general, the positive test line band intensity is high in low Ct values (high viral load) and vice versa. The positive result obtained in AG-Q COVID-19 N-Ag self-test even at a RT–PCR Ct value of 36/35 proved the diagnostic efficacy of the product for COVID-19 (Table 3).
Table 3.
COVID-19 RT–PCR positive samples Ct value comparison with the AG-Q COVID-19 N-Antigen self-test result.
| False Negative Results (n=19) | Weak Positive Results (n=10) | ||||||
|---|---|---|---|---|---|---|---|
| Ct value N/ORF1ab | RT–PCR | N-Antigen self | Interpretation | Ct value N/ORF1ab | RT–PCR | N-Antigen self | Interpretation |
| 25/25 | P | N | FN | 36/34 | P | WP | TP |
| 33/34 | P | N | FN | 26/26 | P | WP | TP |
| 22/22 | P | N | FN | 28/30 | P | WP | TP |
| 27/27 | P | N | FN | 18/19 | P | WP | TP |
| 27/37 | P | N | FN | 31/32 | P | WP | TP |
| 24/23 | P | N | FN | 29/30 | P | WP | TP |
| 35/35 | P | N | FN | 27/28 | P | WP | TP |
| 29/30 | P | N | FN | 34/34 | P | WP | TP |
| 30/31 | P | N | FN | 36/35 | P | WP | TP |
| 30/32 | P | N | FN | 27/28 | P | WP | TP |
| 36/34 | P | N | FN | True positive results | |||
| 32/33 | P | N | FN | Ct value N/ORF1ab | RT–PCR | N-Antigen self | Interpretation |
| 31/31 | P | N | FN | 17/18 | P | P | TP |
| 27/28 | P | N | FN | 25/29 | P | P | TP |
| 25/26 | P | N | FN | 22/23 | P | P | TP |
| 26/27 | P | N | FN | 28/30 | P | P | TP |
| 26/27 | P | N | FN | 24/25 | P | P | TP |
| 36/35 | P | N | FN | 18/20 | P | P | TP |
| 31/32 | P | N | FN | 30/32 | P | P | TP |
| True Positive results (n = 57) | 30/31 | P | P | TP | |||
| Ct value N/ORF1ab | RT–PCR | N-Antigen self | Interpretation | 25/26 | P | P | TP |
| 29/31 | P | P | TP | 28/29 | P | P | TP |
| 27/28 | P | P | TP | 28/28 | P | P | TP |
| 29/30 | P | P | TP | 29/32 | P | P | TP |
| 31/31 | P | P | TP | 36/35 | P | P | TP |
| 25/26 | P | P | TP | 17/18 | P | P | TP |
| 32/32 | P | P | TP | 29/30 | P | P | TP |
| 29/29 | P | P | TP | 31/33 | P | P | TP |
| 22/21 | P | P | TP | 24/25 | P | P | TP |
| 19/18 | P | P | TP | 23/24 | P | P | TP |
| 29/28 | P | P | TP | 24/25 | P | P | TP |
| 22/21 | P | P | TP | 26/26 | P | P | TP |
| 22/23 | P | P | TP | 23/23 | P | P | TP |
| 26/25 | P | P | TP | 29/31 | P | P | TP |
| 30/29 | P | P | TP | 34/34 | P | P | TP |
| 31/31 | P | P | TP | 28/30 | P | P | TP |
| 24/23 | P | P | TP | 28/28 | P | P | TP |
| 27/32 | P | P | TP | 21/21 | P | P | TP |
| 21/29 | P | P | TP | 21/21 | P | P | TP |
| 21/29 | P | P | TP | 29/30 | P | P | TP |
| 23/20 | P | P | TP | 20/21 | P | P | TP |
| 28/30 | P | P | TP | 25/25 | P | P | TP |
| 23/22 | P | P | TP | 29/30 | P | P | TP |
| 26/27 | P | P | TP | ||||
| 14/15 | P | P | TP | ||||
| 22/26 | P | P | TP | ||||
| 21/21 | P | P | TP | ||||
P = Positive, N = Negative, FP = False Positive, FN = False Negative, WP = Weak Positive, TP = True Positive (WP is considered as TP in result reporting).
Based on the RT–PCR Ct value vs antigen self-test comparison, it is evident that proper sampling and the region of sampling are crucial in performing antigen self-tests. Improper sampling by the patients, or less virus colonization in the nasal region, may lead to false-negative results in the self-test and may increase the chance of viral transmission. It should also be noted that this study compared nasopharyngeal swab sampling by healthcare professionals (for RT–PCR) vs the nasal swab collection (for antigen self-test) by the participants. Our previous product validation data on AG-Q COVID-19 N-Antigen laboratory-based RAT vs RT–PCR gave a sensitivity of 91.2% and specificity of 100%. In comparison, the self-test reduces the sensitivity of RAT to 77.91%, which is due to the insufficient viral load obtained by the nasal swab during self-sample collection.
Discussion
The positive samples were obtained from COVID-19 confirmed admitted patients with symptoms and other comorbidities. The admission of patients to the COVID care center is based on the RT–PCR positive results. Special attention has been taken to minimize the preanalytical errors of improper sample collection, transportation, and storage of samples. Testing within 2 hours after sample collection in the lysis buffer minimized results variation due to protein degradation in RAT. It is already validated that the AG-Q COVID-19 N-Antigen RAT can perform well with samples stored at 4 °C after 24 hours of storage in the lysis buffer provided with the kit. The lysis buffer is standardized with stabilizers to slow down protein degradation during storage. The nasopharyngeal and oropharyngeal sample collection in VTM for RT–PCR, provided adequate stability for the viral genetic material during transportation and storage before RNA extraction.
Live virus presence in patients is required to get a positive result with the RAT. Even after recovery from the disease, the residual viral nucleic acid particles may give positive results with RT–PCR. In this study, inpatients within 5 days of admission were only included for positive sample collection, ensuring that live viral load in the body minimized the effect of residual nucleic acid amplification.
Limitations of the Study
This study required sample collection from the same patient at the same time for RAT and RT–PCR, which limits the chance of an additional nasopharyngeal sample collection for laboratory-based RAT. The laboratory-based RAT analysis with the same patient samples could have provided a direct comparison between the self-test and laboratory test. Moreover, this study did not focus on correlating symptoms of the patients to the diagnostic results.
Conclusion
The sensitivity and diagnostic accuracy offered by the AG-Q COVID-19 N-Antigen self-test in comparison with RT–PCR fulfilled the ICMR tenets for RAT. Still, the disparity in correlation between the Ct values and antigen self-tests shows the critical concern regarding nasal sampling by the user. Overall, this study recommends laboratory-/hospital-based RAT execution rather than self-test.
Acknowledgment
Figure 1 is courtesy of Mr. Shine Raghavan, Agappe Diagnostics Limited, Kochi.
Contributor Information
Ajaikumar Sukumaran, Research & Development (Reagent) Department, Agappe Diagnostics Limited, Agappe Hills, Pattimattom P O, Ernakulam, Kerala, India.
Vemparthan Suvekbala, Genetics & Molecular Diagnostics Laboratory, Noorul Islam Institute of Medical Science & Research Foundation (NIMS), Trivandrum, Kerala, India.
Arun Krishnan R, Research & Development (Reagent) Department, Agappe Diagnostics Limited, Agappe Hills, Pattimattom P O, Ernakulam, Kerala, India.
Rhema Elizabeth Thomas, Research & Development (Reagent) Department, Agappe Diagnostics Limited, Agappe Hills, Pattimattom P O, Ernakulam, Kerala, India.
Aneesh Raj, Genetics & Molecular Diagnostics Laboratory, Noorul Islam Institute of Medical Science & Research Foundation (NIMS), Trivandrum, Kerala, India.
Thushara Thomas, Research & Development (Reagent) Department, Agappe Diagnostics Limited, Agappe Hills, Pattimattom P O, Ernakulam, Kerala, India.
B.L. Abhijith, Research & Development (Reagent) Department, Agappe Diagnostics Limited, Agappe Hills, Pattimattom P O, Ernakulam, Kerala, India
Jisha Jose, Research & Development (Reagent) Department, Agappe Diagnostics Limited, Agappe Hills, Pattimattom P O, Ernakulam, Kerala, India.
Jofy K. Paul, Research & Development (Reagent) Department, Agappe Diagnostics Limited, Agappe Hills, Pattimattom P O, Ernakulam, Kerala, India
D.M. Vasudevan, Research & Development (Reagent) Department, Agappe Diagnostics Limited, Agappe Hills, Pattimattom P O, Ernakulam, Kerala, India
Nonstandard Abbreviations: RAT, rapid antigen test; RT–PCR, reverse transcriptase–PCR; N-Ag, nucleocapsid antigen; ICMR, Indian Council of Medical Research; VTM, viral transport medium; NIMS, Noorul Islam Institute of Medical Science & Research Foundation; CGC, colloidal gold conjugated; Ct, threshold cycle.
Author Contributions: All authors confirmed they have contributed to the intellectual content of this paper and have met the following 4 requirements: (a) significant contributions to the conception and design, acquisition of data, or analysis and interpretation of data; (b) drafting or revising the article for intellectual content; (c) final approval of the published article; and (d) agreement to be accountable for all aspects of the article thus ensuring that questions related to the accuracy or integrity of any part of the article are appropriately investigated and resolved.
Authors’ Disclosures or Potential Conflicts of Interest: Upon manuscript submission, all authors completed the author disclosure form. Disclosures and/or potential conflicts of interest: Employment or Leadership: None declared. Consultant or Advisory Role: None declared. Stock Ownership: None declared. Honoraria: None declared. Research Funding: The AG-Q COVID-19 N-Antigen self-test development and performance evaluation were conducted at the research and development department of Agappe Diagnostics Limited under the supervision of the first author, A. Sukumaran. The RT–PCR-based accuracy study was performed at NIMS Medicity under the supervision of V. Suvekbala. The financial support for this study was given by Agappe Diagnostics Limited. Expert Testimony: None declared. Patents: None declared. Other Remuneration: A. Sukumaran, support for attending meetings and/or travel from Agappe Diagnostics Limited; V. Suvekbala, support for attending meetings and/or travel from NIMS Medicity.
Role of Sponsor: The funding organizations played no role in the design of study, choice of enrolled patients, review and interpretation of data, preparation of manuscript, or final approval of the manuscript.
REFERENCES
- 1. Mak GC, Cheng PK, Lau SS, Wong KK, Lau CS, Lam ET, et al. Evaluation of rapid antigen test for detection of SARS-CoV-2 virus. J Clin Virol 2020;129:104500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Diao B, Wen K, Zhang J, Chen J, Han C, Chen Y, et al. Accuracy of a nucleocapsid protein antigen rapid test in the diagnosis of SARS-CoV-2 infection. Clin Microbiol Infect 2021;27:289.e1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yamayoshi S, Sakai-Tagawa Y, Koga M, Akasaka O, Nakachi I, Koh H, et al. Comparison of rapid antigen tests for COVID-19. Viruses 2020;12:1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chaimayo C, Kaewnaphan B, Tanlieng N, Athipanyasilp N, Sirijatuphat R, Chayakulkeeree M, et al. Rapid SARS-CoV-2 antigen detection assay in comparison with real-time RT-PCR assay for laboratory diagnosis of COVID-19 in Thailand. Virol J 2020;17:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hirotsu Y, Maejima M, Shibusawa M, Nagakubo Y, Hosaka K, Amemiya K, et al. Comparison of automated SARS-CoV-2 antigen test for COVID-19 infection with quantitative RT-PCR using 313 nasopharyngeal swabs, including from seven serially followed patients. Int J Infect Dis 2020;99:397–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Krüttgen A, Cornelissen CG, Dreher M, Hornef MW, Imöhl M, Kleines M. Comparison of the SARS-CoV-2 rapid antigen test to the real star Sars-CoV-2 RT PCR kit. J Virol methods 2021;288:114024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dinnes J, Deeks JJ, Berhane S, Taylor M, Adriano A, Davenport C, et al. Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection. Cochrane Database Syst Rev 2021;3:CD013705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lippi G, Simundic AM, Plebani M. Potential preanalytical and analytical vulnerabilities in the laboratory diagnosis of coronavirus disease 2019 (COVID-19). Clin Chem Lab Med 2020;58:1070–6. [DOI] [PubMed] [Google Scholar]
- 9. Wang X, Tan L, Wang X, Liu W, Lu Y, Cheng L, Sun Z. Comparison of nasopharyngeal and oropharyngeal swabs for SARS-CoV-2 detection in 353 patients received tests with both specimens simultaneously. Int J Infect Dis 2020;94:107–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sukumaran A, Thomas T, Thomas R, Thomas RE, Paul JK, Vasudevan DM. Development and troubleshooting in lateral flow immunochromatography assays. Indian J Clin Biochem 2021;36:208–12. [DOI] [PMC free article] [PubMed] [Google Scholar]