Abstract
Background
Longer-term humoral responses to 2-dose coronavirus disease 2019 (COVID-19) vaccines remain incompletely characterized in people living with human immunodeficiency virus (HIV) (PLWH), as do initial responses to a third dose.
Methods
We measured antibodies against the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike protein receptor-binding domain, angiotensin-converting enzyme 2 (ACE2) displacement, and viral neutralization against wild-type and Omicron strains up to 6 months after 2-dose vaccination, and 1 month after the third dose, in 99 PLWH receiving suppressive antiretroviral therapy and 152 controls.
Results
Although humoral responses naturally decline after 2-dose vaccination, we found no evidence of lower antibody concentrations or faster rates of antibody decline in PLWH compared with controls after accounting for sociodemographic, health, and vaccine-related factors. We also found no evidence of poorer viral neutralization in PLWH after 2 doses, nor evidence that a low nadir CD4+ T-cell count compromised responses. Post–third-dose humoral responses substantially exceeded post–second-dose levels, though Omicron-specific responses were consistently weaker than responses against wild-type virus. Nevertheless, post–third-dose responses in PLWH were comparable to or higher than controls. An mRNA-1273 third dose was the strongest consistent correlate of higher post–third-dose responses.
Conclusion
PLWH receiving suppressive antiretroviral therapy mount strong antibody responses after 2- and 3-dose COVID-19 vaccination. Results underscore the immune benefits of third doses in light of Omicron.
Keywords: HIV, COVID-19, vaccines, immune response, humoral, antibodies, neutralization, third dose
People living with human immunodeficiency virus with well-controlled viral loads on antiretroviral therapy and preserved CD4+ T-cell counts mount strong, functional antibody responses to 2- and 3-dose coronavirus disease 2019 vaccination, including to Omicron. Monitoring responses over time remains important.
Because people living with human immunodeficiency virus (HIV) (PLWH) may be at increased risk of severe coronavirus disease 2019 (COVID-19) owing to immunosuppression, higher rates of multimorbidity, and/or social determinants of health [1–4], vaccination is expected to benefit this group. Although 2-dose COVID-19 vaccination protects against severe disease [5–7], impaired responses have been observed in certain immunocompromised groups [8–12], prompting research into COVID-19 vaccine responses in PLWH. This is because, while antiretroviral therapy can reverse HIV-induced immune dysfunction to a large extent [13–16], persistent HIV-related immunopathology can nevertheless blunt vaccine responses [17–19]. Clinical trials [20, 21] and real-world studies, however [22–28], including an initial study of the present cohort [29], have found generally strong immune responses to 2-dose COVID-19 vaccination in PLWH with controlled HIV loads on therapy and preserved CD4+ T-cell counts [20–24, 29], though weaker responses have been observed in PLWH who are not receiving therapy or who have CD4+ T-cell counts <200/μL [22, 25–27].
However, vaccine-induced antibody responses decline over time, which can increase the risk of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection [30–32], particularly with the more transmissible Omicron variant [33–37]. Although immune response durability after 2-dose COVID-19 vaccination has been examined among PLWH participants in the ChAdOx1 clinical trial [38], few real-world studies have investigated this. Furthermore, no studies to our knowledge have investigated immune responses in PLWH to third vaccine doses, despite their widespread recommendation to maintain protection [39–41]. We extend our previous report [29] to characterize humoral responses to both wild-type (WT) and Omicron SARS-CoV-2 variants up to 1 month after the third vaccine dose, in 99 PLWH and 152 controls without HIV.
METHODS
Participants
We recruited 99 adult PLWH and 152 controls without HIV, the latter predominantly healthcare workers, in British Columbia (BC), Canada [29]. Serum and plasma (collected in ethylenediaminetetraacetic acid [EDTA] for all PLWH and 16% of controls, or anticoagulant citrate dextrose for the remainder) were collected before vaccination; 1 month after the first dose; 1, 3, and 6 months after the second dose; and 1 month after the third dose. Here, we extend the previous study [29] to include all post–second-dose, and the 1-month post–third dose, study visits. A cohort flowchart is shown in Supplementary Figure 1.
Ethical Approval
All participants provided written informed consent. This study was approved by the University of British Columbia/Providence Health Care and Simon Fraser University Research Ethics Boards.
Data Sources
Sociodemographic, health and COVID-19 vaccine data were collected through self-report and medical records. We assigned a score of 1 for each of 11 chronic conditions: hypertension, diabetes, asthma, obesity (body mass index ≥30, calculated as weight in kilograms divided by height in meters squared), chronic diseases of lung, liver, kidney, heart or blood, cancer; and immunosuppression due to chronic conditions or medication. For PLWH, a recent CD4+ T-cell count <200/μL constituted immunosuppression.
Binding Antibody Assays
Binding antibodies were measured using 2 commercial assays. We measured total binding antibodies against SARS-CoV-2 nucleocapsid and spike (S) receptor-binding domain (RBD) in serum samples using the Elecsys Anti-SARS-CoV-2 and Anti-SARS-CoV-2 S assays, respectively, on a cobas e601 module analyzer (Roche Diagnostics). After infection, results of both assays should be positive, whereas after vaccination only the S assay result should be positive. The anti-S assay reports results in arbitrary units per milliliter. For the S assay, serum samples were tested undiluted, with samples above the upper limit of quantification (ULOQ) retested at 1:100 dilution, allowing a measurement range of 0.4–25 000 U/mL. Anti-RBD binding immunoglobulin G (IgG) concentrations in serum were also quantified using the V-plex SARS-CoV-2 (IgG) Panel 22 enzyme-linked immunosorbent assay kit (Meso Scale Diagnostics) on a Meso QuickPlex SQ120 instrument. This assay quantifies both WT- and Omicron-specific IgG. Serum samples were diluted 1:10 000, allowing a broader dynamic range than the Roche assay. Results are reported in arbitrary units per milliliter.
Angiotensin-Converting Enzyme 2 (ACE2) Receptor Displacement Assay
We assessed the ability of serum antibodies to block the RBD/angiotensin-converting enzyme 2 (ACE2) receptor interaction by competition enzyme-linked immunosorbent assay (Panel 22 V-plex SARS-CoV-2 [ACE2]; Meso Scale Diagnostics) on a Meso QuickPlex SQ120 instrument. This represents a higher-throughput approach to estimate potential virus neutralizing activity and is commonly used as a surrogate for this function [42]. Serum samples were diluted 1:40 and results reported as the percentage of ACE2 displacement.
Live Virus Neutralization
Neutralizing activity in plasma was examined in live SARS-CoV-2 assays using isolate USA-WA1/2020 (BEI Resources) and a local Omicron BA.1 isolate (GISAID accession no. EPI_ISL_9805779) on Vero E6–TMPRSS2 (JCRB-1819) target cells. As described elsewhere [29], viral stock was adjusted to 50 times the median tissue culture infectious dose per 200 µL in the presence of serial 2-fold plasma dilutions (from 1:20 to 1:2560) and added to target cells in 96-well plates in triplicate. The appearance of viral cytopathic effects was recorded 3 days after infection. Neutralizing activity is reported as the reciprocal of the highest plasma dilution able to prevent cytopathic effects in all triplicate wells. Samples exhibiting partial or no neutralization at 1:20 dilution were defined as below the limit of quantification (BLOQ).
Statistical Analysis
Continuous variables were compared using the Mann-Whitney U test (unpaired data) or Wilcoxon test (paired data). Relationships between continuous variables were assessed using Spearman's correlation. Multiple linear regression was used to investigate the relationship between HIV infection and COVID-19 vaccine–related immune outcomes using a confounder model that adjusted for variables that could influence vaccine responses and/or that differed in prevalence between PLWH and controls. For neutralization at 6 months after the second dose, we used multiple logistic regression owing to the high proportion of results that were BLOQ. Variables included HIV infection (controls as reference group), age (per year), sex at birth (female as reference), ethnicity (nonwhite as reference), number of chronic conditions (per additional), interval between first and second doses (per day), sampling date after vaccination (per day), dual ChAdOx1 as the initial regimen (messenger RNA [mRNA] or mixed [ChAdOx1/mRNA] regimen as the combined reference group), and prior COVID-19 (COVID-19 naive as reference). Plasma neutralization models also corrected for anticoagulant (anticoagulant citrate dextrose as reference), and post–third-dose analyses also corrected for third dose mRNA vaccine brand (BNT162b2 as reference) and the interval between second and third doses (per day). All tests were 2 tailed, with differences considered statistically significant at P < .05. Analyses were conducted using Prism v9.2.0 software (GraphPad).
RESULTS
Cohort Characteristics
As described elsewhere [29], all PLWH had suppressed plasma HIV loads while on therapy (Table 1). Recent and nadir CD4+ T-cell counts were a median of 715/μL (interquartile range [IQR], 545–943/μL; range, 130–1800/μL) and 280/μL (123–490/μL; range, <10/μL to 1010/μL), respectively. PLWH and controls were broadly similar in age and number of chronic conditions, but the PLWH group included more participants who were male and more who were white. Because the majority of controls were healthcare workers who were vaccinated before the general population (including PLWH), some vaccine-related variables differed between groups. The interval between first and second doses was longer for controls (median, 89 vs 58 days for PLWH), because those vaccinated earlier in Canada’s campaign waited up to 112 days between doses owing to initially limited vaccine supply [43]. Fewer controls received dual ChAdOx1 vaccines as their initial regimen (<1% vs 8% of PLWH), because initially only mRNA vaccines were available in Canada. Vaccine timing also indirectly influenced postvaccination SARS-CoV-2 infection incidence. While a comparable number of PLWH (8%) and controls (10%) were anti-nucleocapsid seropositive at study entry, a larger proportion of PLWH (18%) experienced postvaccination infections (18% vs 9% of controls). This is unlikely to be due to increased infection susceptibility but rather because the first Omicron “wave” occurred just before the post–third-dose study visit for many PLWH, whereas most controls had already completed this visit.
Table 1.
Participant Characteristics
| Characteristic | Participants, No. (%)a | |
|---|---|---|
| PLWH (n = 99) | Controls (n = 152) | |
| HIV-related variables | ||
| ȃReceiving antiretroviral therapy | 99 (100) | … |
| ȃMost recent plasma viral load, median (IQR), HIV RNA copies/mL | <50 (<50 to <50) | … |
| ȃMost recent CD4+ T-cell count, median (IQR), cells/μL | 715 (545–943) | … |
| ȃNadir CD4+ T-cell count, median (IQR), cells/μL | 280 (123–490) | … |
| Sociodemographic and health variables | ||
| ȃAge, median (IQR), y | 54 (40–61) | 47 (35–70) |
| ȃMale sex at birth | 87 (88) | 50 (33) |
| ȃEthnicity | ||
| ȃȃWhite | 69 (69) | 78 (51) |
| ȃȃBlack | 5 (5) | 1 (0.7) |
| ȃȃAsian | 10 (10) | 59 (38) |
| ȃȃLatin American | 8 (8) | 4 (2.6) |
| ȃȃMiddle Eastern/Arab | 3 (3) | 0 (0) |
| ȃȃMixed ethnicity | 3 (3) | 8 (5.3) |
| ȃȃNot disclosed | 1 (1) | 2 (1.3) |
| ȃNo. of chronic conditions, median (IQR) | 0 (0–1) | 0 (0–1) |
| ȃChronic condition | ||
| ȃȃHypertension | 15 (15) | 22 (14.5) |
| ȃȃDiabetes | 6 (6) | 6 (3.9) |
| ȃȃAsthma | 7 (7) | 15 (9.9) |
| ȃȃObesity | 15 (15) | 14 (9.2) |
| ȃȃChronic lung disease | 4 (4) | 3 (2) |
| ȃȃChronic liver disease | 4 (4) | 1 (0.7) |
| ȃȃChronic kidney disease | 1 (1) | 1 (0.7) |
| ȃȃChronic heart disease | 1 (1) | 4 (2.6) |
| ȃȃChronic blood disease | 1 (1) | 2 (1.3) |
| ȃȃCancer | 4 (4) | 4 (2.6) |
| ȃȃImmunosuppression | 4 (4) | 0 (0) |
| ȃȃ≥1 Condition | 45 (45) | 50 (33) |
| COVID-19 status | ||
| ȃCOVID-19 convalescent (anti–nucleocapsid antibody positive) at study entry | 8 (8) | 15 (10) |
| ȃCOVID-19 after vaccination | 18 (18) | 13 (9) |
| Vaccine details | ||
| ȃInitial 2-dose regimen | ||
| ȃȃmRNA-mRNA | 82 (82) | 148 (97) |
| ȃȃChAdOx1-mRNA (heterologous) | 8 (8) | 3 (2) |
| ȃȃChAdOx1-ChAdOx1 | 8 (8) | 1 (0.7) |
| ȃȃChAdOx1–not disclosed | 1 (1) | … |
| ȃTime between 1st and 2nd doses, median (IQR), d | 58 (53–67) | 89 (65–98) |
| ȃ3rd dose | ||
| ȃȃBNT162b2 | 23/80 (29) | 56/137 (41) |
| ȃȃmRNA-1273 | 56/80 (70) | 81/137 (59) |
| ȃȃUnknown | 1/80 (1) | … |
| ȃTime between 2nd and 3rd doses, median (IQR), d | 183 (143–191) | 198 (173–216) |
| Specimen collection | ||
| ȃSpecimen 1 mo after 2nd dose | 97 (97) | 151 (99) |
| ȃDay of collection 1 mo after 2nd dose, median (IQR) | 30 (29–30) | 30 (29–32) |
| ȃSpecimen 3 mo after 2nd dose | 96 (96) | 148 (97) |
| ȃDay of collection 3 mo after 2nd dose, median (IQR) | 90 (90–91) | 90 (89–91) |
| ȃSpecimen 6 mo after 2nd dose | 62 (62) | 136 (89) |
| ȃDay of collection 6 mo after 2nd dose, median (IQR) | 180 (177–182) | 180 (178–182) |
| ȃSpecimen 1 mo after 3rd dose | 80 (80) | 137 (90) |
| ȃDay of collection 1 mo after 3rd dose, median (IQR) | 30 (30–32) | 30 (29–32) |
Abbreviations: COVID-19, coronavirus disease 2019; HIV, human immunodeficiency virus; IQR, interquartile range; mRNA, messenger RNA; PLWH, people living with human immunodeficiency virus.
Data represent no. (%) of participants unless identified as median (IQR).
Third dose eligibility and timing also differed between groups. In October 2021, third doses began to be offered in BC to priority populations, including PLWH who had ≥1 of the following: age ≥65 years, prior AIDS-defining illness, prior CD4+ T-cell count <200/μL or prior CD4 fraction ≤ 15%, any plasma HIV load >50 copies/mL in 2021, or perinatally acquired HIV. The majority of PLWH in BC met ≥1 criterion, though not all eligible individuals were vaccinated immediately. By January 2022, all remaining adults in BC aged ≥18 years were eligible for a third dose 6 months after their second dose. At the time of writing, 80% of PLWH participants and 88% of controls had received a third dose, on average 6.3 months after their second dose. All third doses were mRNA vaccines, and more PLWH than controls received mRNA-1273 (70% vs 59%, respectively). Third mRNA-1273 dose recommendations also differed by risk group: 100 μg was recommended for adults aged ≥70 years and PLWH meeting any priority criterion, whereas the standard 50-μg booster was recommended for all other adults.
Binding Antibody Responses
Initial responses to 2-dose vaccination in this cohort have been described elsewhere [29]. Briefly, 1 month after 2-dose vaccination, anti-RBD antibody concentrations were a median (IQR) of 3.9 (3.7–4.1) log10 U/mL in PLWH, compared with 4.0 (3.8–4.2) log10 in controls (P = .04; Figure 1A ). Only 2 participants were nonresponders: 1 in the PLWH group with non–HIV-related immunodeficiency and 1 >80-year-old control participant with 3 chronic conditions. By 3 months after the second dose, antibody concentrations had declined to a median (IQR) of 3.4 (3.2–3.6) log10 U/mL in PLWH, compared with 3.6 (3.4–3.8) log10 U/mL in controls (P < .001). HIV infection, however, did not remain significantly associated with antibody concentrations at these 2 time points after controlling for sociodemographic, health-related, and vaccine-related variables (HIV-related estimates and P values shown in Table 2; full models shown in Supplementary Table 1). Rather, older age, more chronic conditions, and dual ChAdOx1 vaccination were associated with lower antibody concentrations at both time points, while a longer dose interval was associated with higher antibody concentrations, regardless of HIV status.
Figure 1.
Concentrations of total binding antibodies in serum to spike receptor-binding domain (RBD) after 2 and 3 coronavirus disease 2019 (COVID-19) vaccine doses. A, Binding antibody responses to the severe acute respiratory syndrome coronavirus 2 spike RBD in serum samples 1, 3, and 6 months after the second vaccine dose and 1 month after the third dose, in people living with human immunodeficiency virus (PLWH) (orange) and controls (blue) who were COVID-19 naive at the studied time point, as well as in individuals who had recovered from COVID-19 at the studied time point (COVID group [black]). Participants who experienced a postvaccination infection were relocated from their original group into the COVID group at their first postinfection study visit, where they are denoted by red symbols. Numbers of participants are shown at the bottom of the plot. The thick horizontal red bar represents the median; thinner horizontal red bars, the interquartile range (IQR). P values were computed using the Mann-Whitney U test (for comparisons between groups) or the Wilcoxon matched-pairs test (for comparisons across time points within a group) and are uncorrected for multiple comparisons. Abbreviations: LLOD, lower limit of detection; ULOQ, upper limit of quantification. B, Temporal declines in serum binding antibody responses to spike RBD after 2 vaccine doses in individual PLWH (light orange) and controls (light blue) who remained COVID-19 naive during this period. Thick lines in corresponding colors denote averages for each group. Only participants with a complete longitudinal data series with no values above the ULOQ are shown. C, Binding antibody half-lives after 2 COVID-19 vaccine doses, in PLWH (orange) and controls (blue) who remained COVID-19 naive during this period. Values were calculated by fitting an exponential curve to the data shown in B. The 2 outliers are individuals whose antibody levels did not decay or decayed exceedingly slowly, producing half-lives >500 days. For visualization purposes, their half-lives are shown as 500 days. Numbers are indicated at the bottom of the plot. Red bars and whiskers represent medians and interquartile ranges, and P values were computed using the Mann-Whitney U test.
Table 2.
Impact of Human Immunodeficiency Virus Infection on Humoral Responses to Coronavirus Disease 2019 Vaccination: Summary of Estimates from Multivariable Analysesa
| Humoral Measure | SARS-CoV-2 Strain | Study Time Point | HIV Infection Estimates From Multivariable Model | |||
|---|---|---|---|---|---|---|
| Estimate | 95% CI | P Value | Full Model | |||
| Log10 anti-RBD antibodiesb | WT | 1 mo after 2nd dose | −0.017 | −.18 to .14 | .83 | Supplementary Table 1 |
| WT | 3 mo after 2nd dose | −0.13 | −.27 to .019 | .09 | Supplementary Table 1 | |
| WT | 6 mo after 2nd dose | −0.036 | −.19 to .11 | .64 | Supplementary Table 2 | |
| Antibody half-life after 2nd dose (in days)c | WT | Calculated from all post–2nd-dose time points | 6.33 | −19.92 to 32.59 | .63 | Supplementary Table 2 |
| Log2 viral neutralizationd | WT | 1 mo after 2nd dose | 0.20 | −.47 to .86 | .56 | Supplementary Table 1 |
| WT | 3 mo after 2nd dose | −0.063 | −.74 to .62 | .86 | Supplementary Table 1 | |
| WT | 6 mo after 2nd dosee | OR = 0.51e | .12 to 1.77 | .32 | Supplementary Table 3 | |
| WT | 1 mo after 3rd dose | 0.58 | −.22 to 1.37 | .15 | Supplementary Table 4 | |
| Omicron-specific log10 binding IgGf | Omicron | 1 mo after 3rd dose | 0.36 | .14 to .58 | .002g | Supplementary Table 5 |
| Omicron-specific ACE2 displacement (as %)f | Omicron | 1 mo after 3rd dose | 3.49 | −8.48 to 15.47 | .57 | Supplementary Table 5 |
Abbreviations: ACE2, angiotensin-converting enzyme 2; CI, confidence interval; HIV, human immunodeficiency virus; Ig, immunoglobulin; RBD, receptor-binding domain; OR, odds ratio; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; WT, wild type.
This table summarizes the estimates (coefficients or OR, as appropriate), 95% CI, and P values of the relationship between HIV infection and specific humoral responses to coronavirus disease 2019 (COVID-19) vaccination at the time points shown. All estimates were calculated using multivariable linear regression, except for viral neutralization at 6 months after the 2nd dose (see footnote d). Full models, adjusted for clinical, demographic and SARS-CoV-2 vaccination variables, are shown in supplementary tables as indicated.
Quantified in serum samples using the Roche Elecsys anti–spike protein assay.
Antibody half-lives were calculated from anti-RBD antibody concentrations from all participants with a complete longitudinal data series after the second vaccine dose, with no values above the upper limit of quantification and no evidence of prior COVID-19.
For viral neutralization, reciprocal plasma dilutions were log2 transformed before multivariable analysis.
Results for the time point 6 months after the second dose are presented as the adjusted OR and associated 95% CI of detectable viral neutralization activity at this time point, calculated using multivariable logistic regression.
Quantified in serum using the Meso Scale Diagnostics V-Plex assay (panel 22).
Significant at P < .05.
By 6 months after the second dose, anti-RBD antibody concentrations had declined to a median (IQR) of 3.1 (2.9–3.3) log10 U/mL in PLWH versus 3.2 (3.0–3.4) log10 U/mL in controls (P = .002; Figure 1A ), though no effect of HIV infection on antibody concentrations remained after multivariable correction (P = .64; Table 2; full model in Supplementary Table 2). Rather, dual ChAdOx1 vaccination was the strongest correlate of poorer responses at this time point, while a longer time between vaccination and sampling was associated with marginally higher antibody concentrations. The latter observation is likely due to confounding by age; 13 controls aged ≥ 70 years did not contribute samples at this time point because they had already received third doses per BC’s age-based rollout, and another 25 participants aged ≥ 65 years contributed this sample early owing to imminently scheduled third doses. Prior COVID-19 was also associated with superior antibody concentrations at this time point, though 11 recent infections (red symbols in Figure 1A ) influenced this result.
We next assessed temporal reductions in antibody concentrations after 2 vaccine doses (Figure 1B ). Assuming exponential decay, and restricting the analysis to COVID-19–naive participants with a complete post–second-dose longitudinal series with no values above the assay ULOQ, we estimated antibody half-lives to be a median of (IQR) of 53 (47–70) days in PLWH versus 59 (51–75) days in controls (P = .02; Figure 1C ). This difference, however, did not remain significant after multivariable correction (P = .63; Table 2; full model in Supplementary Table 2).
A third vaccine dose boosted antibody concentrations to an average of 0.4–0.5 log10 U/mL higher than peak post–second dose levels (within-group P < .001 for both PLWH and controls), to a median (IQR) of 4.3 (4.2 to >ULOQ) log10 U/mL in PLWH and 4.4 (4.2 to >ULOQ) log10 U/mL in controls (between-group P = .83), values comparable to those in participants with prior COVID-19 (Figure 1A ). Multivariable analyses were not performed, because nearly 50% of values were above the ULOQ.
Consistent with previous observations at 1 and 3 months after the second vaccine dose [29], we observed no significant relationship between the most recent or nadir CD4+ T-cell count and antibody concentrations either 6 months after the second dose or 1 month after the third dose in PLWH (Supplementary Figure 2). We also observed no significant relationship between these CD4 parameters and antibody half-life after the second dose (Spearman ρ ≤ .16; P ≥ .3; not shown).
Viral Neutralization
One month after the second vaccine dose, SARS-CoV-2 neutralization was achieved at a median (IQR) reciprocal plasma dilution of 160 (40–320) in PLWH, compared with 80 (40–160) in controls (Mann-Whitney P = .06; Figure 2A ). By 3 months after the second dose this activity declined to a median (IQR) of 40 (20–80) in both PLWH and controls (P = .23). Multivariable analyses confirmed no association between HIV infection and neutralization at these time points (HIV-related estimates in Table 2; full model in Supplementary Table 1). Rather, older age, more chronic conditions, and dual ChAdOx1 vaccination were associated with weaker neutralization at one or both of these time points, while prior COVID-19 was associated with stronger neutralization. By 6 months after the second dose, neutralization had declined to BLOQ in 52% of COVID-19–naive participants, to a median (IQR) reciprocal dilution of 20 (BLOQ to 40) in PLWH, compared with BLOQ (BLOQ to 20) in controls (P = .07; Figure 2A ). Owing to the large proportion of BLOQ values, we applied multivariable logistic regression with neutralization as a binary variable, and we confirmed no association between HIV infection and neutralization at this time point (HIV-related estimates in Table 2; full model in Supplementary Table 3). We identified only prior COVID-19 as a biological correlate of neutralization at this time point, though this is influenced by 11 recent infections.
Figure 2.
Live virus neutralization activities after 2 and 3 coronavirus disease 2019 (COVID-19) vaccine doses. Viral neutralization activity in plasma at 1, 3, and 6 months after the second dose, and 1 month after the third vaccine dose, in people living with human immunodeficiency virus (PLWH) (orange) and controls (blue) who were COVID-19 naive at the studied time point, as well as individuals who had recovered from COVID-19 at the studied time point (COVID group [black]). Plasma neutralization was defined as the reciprocal of the highest plasma dilution at which vial cytopathic effect was prevented in all triplicate assay wells. Plasma samples showing neutralization in <3 wells at the lowest plasma dilution of 1:20 were coded as having a reciprocal dilution of 10, corresponding to the lower limit of quantification (LLOQ) in this assay. The highest dilution tested was 1:2560, which corresponds to the upper limit of quantification (ULOQ). Participants who experienced a postvaccination infection were relocated from their original group into the COVID-19 group at their first postinfection study visit, where they are denoted by a red symbol. Numbers of participants are shown at the bottom of the plot. Thick horizontal red bars represent medians; thinner horizontal red bars, interquartile ranges. P values were computed using the Mann-Whitney U test (for comparisons between groups) or the Wilcoxon matched-pairs test (for comparisons across time points within a group) and are uncorrected for multiple comparisons.
A third COVID-19 vaccine dose boosted neutralization to an average of 4-fold higher than peak post–second-dose levels (within-group P < .001 for PLWH and controls; Figure 2A ). In fact, neutralization activities in PLWH (median reciprocal dilution [IQR], 640 [160–1280]) exceeded those in controls (median 320 [160–320]; Mann-Whitney P < .001) at this time point, though this difference did not remain significant after multivariable adjustment (P = .15; Table 2; full model in Supplementary Table 4). Rather, having received mRNA-1273 as a third dose and having prior COVID-19 were correlated with better neutralization at this time point, though this is again influenced by numerous recent infections.
We observed no significant relationship between the most recent CD4+ T-cell count and neutralization, neither 6 months after the second dose nor 1 month after the third dose in COVID-19–naive PLWH, nor any relationship between nadir CD4+ T-cell count and neutralization 6 months after the second dose (Supplementary Figure 2). An inverse relationship between nadir CD4+ T-cell count and neutralization 1 month after the third dose was found, however (Spearman ρ = −.28; P = .04).
Omicron-Specific Responses
To estimate the extent to which a third vaccine dose boosts protection against the now-dominant Omicron variant, we compared peak WT- and Omicron-specific responses 1 month after the second and third doses, using a platform that simultaneously quantifies responses to both antigens (Meso Scale Diagnostics V-plex assay; see Methods). To avoid confounding by infection-induced immunity, we restricted this analysis to COVID-19–naive individuals. For both PLWH and controls, Omicron-specific anti-RBD serum IgG concentrations were on average approximately 0.6 log10 U/mL lower than WT-specific ones at both time points (all within-group comparisons, P < .001; Figure 3A ). Nevertheless, the third dose significantly boosted anti-Omicron IgG concentrations to an average of 0.3–0.5 log10 U/mL higher than post–second-dose levels in both groups (within-group comparisons, P < .001).
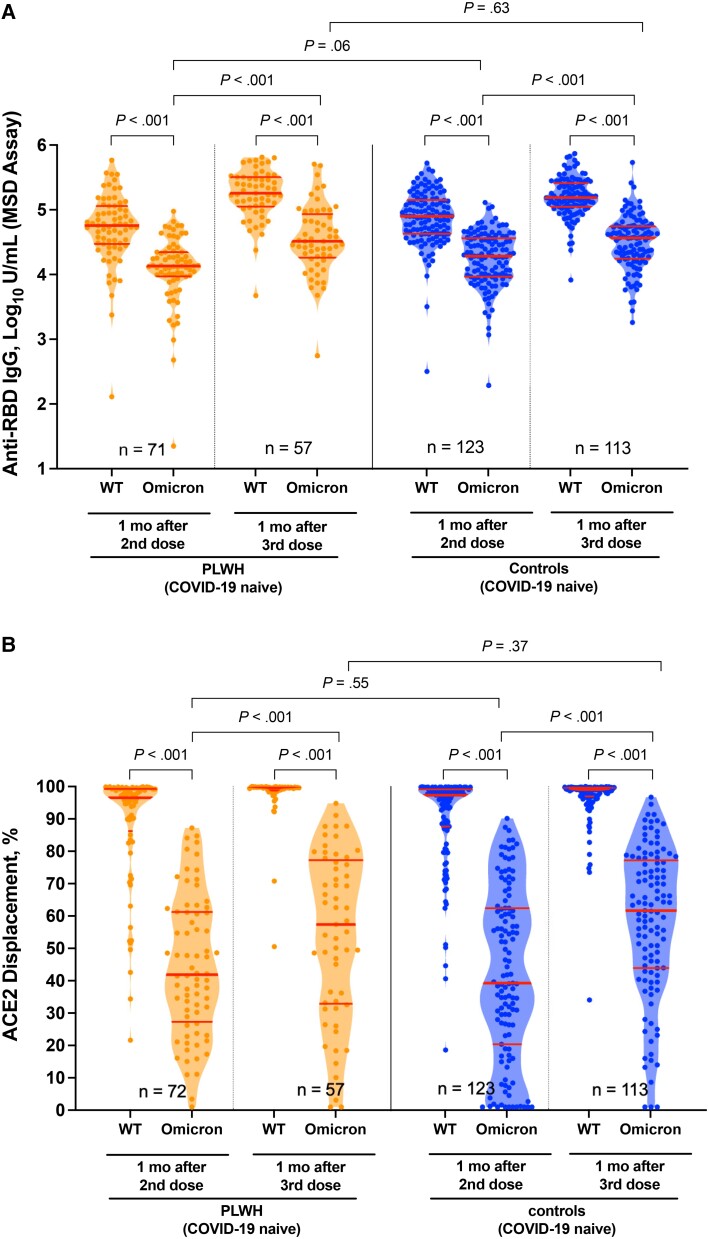
Figure 3.
Omicron-specific immunoglobulin G (IgG) binding and angiotensin-converting enzyme 2 (ACE2) displacement activities 1 month after the second and third coronavirus disease 2019 (COVID-19) vaccine doses. A, Binding IgG responses in plasma to the wild-type (WT) and Omicron spike receptor binding domain (RBD), measured using the Meso Scale Diagnostics (MSD) V-Plex assay, in people living with human immunodeficiency virus (PLWH) (orange) and controls (blue) who remained COVID-19 naive throughout the study. Numbers of participants are shown at the bottom of the plot. Thick horizontal red bars represent medians; thinner horizontal red bars, interquartile ranges. P values were computed using the Wilcoxon matched-pairs test (for all within-group comparisons) or the Mann-Whitney U test (for between-group comparisons) and are uncorrected for multiple comparisons. B, Same as A but for ACE2 displacement activity, measured using the V-plex severe acute respiratory syndrome coronavirus 2 ACE2 assay, with results reported as the percentage of ACE2 displacement.
One month after the second dose, anti-Omicron IgG concentrations were a median (IQR) of 4.13 (3.95–4.35) log10 U/mL in PLWH and 4.28 (3.97–4.56) log10 U/mL in controls (P = .06). After 3 doses however, these responses reached equivalence, with a median (IQR) of 4.51 (4.26–4.93) log10 U/mL in PLWH versus 4.56 (4.24–4.74) log10 U/mL in controls (P = .63). In fact, a multivariable analysis of Omicron-specific IgG concentrations after the third dose identified HIV infection as being associated with an adjusted 0.36 log10 U/mL higher Omicron-specific IgG concentration (P = .002; Table 2; full model in Supplementary Table 5). Having received mRNA-1273 for the third dose and a longer interval between second and third doses were also significantly associated with higher Omicron-specific anti-RBD IgG responses, while male sex was associated with lower responses. We also confirmed that total WT-specific anti-RBD antibody concentrations (measured by the Roche Elecsys assay in Figure 1) and total WT-specific anti-RBD IgG concentrations (measured by Meso Scale Diagnostics assay in Figure 3) were strongly correlated, as expected (Supplementary Figure 3).
We also assessed the ability of plasma to block the RBD-ACE2 interaction, which estimates potential viral neutralization [42]. This activity was significantly weaker against Omicron compared with WT for both groups at both time points (all within-group comparisons, P < .001; Figure 3B ), and the discrepancy was most pronounced after 2 doses (eg, median WT- and Omicron-specific activities in PLWH were 97% and 42%, respectively, at this time). The third dose nevertheless universally boosted Omicron-specific responses to above second-dose levels (all within-group comparisons, P < .001), with median Omicron-specific activity in PLWH rising from 42% after 2 doses to 57% after 3 (P < .001). Omicron-specific ACE2% displacement activities were comparable between groups at both time points: 1 month after the second dose these were a median (IQR) of 42% (27%–61%) in PLWH versus 39% (20%–62%) in controls (P = .55), rising to a median of 57% (33%–77%) in PLWH versus 62% (44%–77%) in controls 1 month after the third dose (P = .37). Multivariable analyses confirmed no association between HIV infection and Omicron-specific ACE2 displacement activity after 3 doses (P = .57, Table 2; full model in Supplementary Table 5). After 3 doses, we observed a weak inverse relationship between nadir CD4+ T-cell count and Omicron-specific ACE2% displacement (Spearman ρ = −.3; P = .02) but no relationship between other CD4+ T-cell count measures and Omicron-specific responses (Supplementary Figure 2).
Finally, we assessed plasma neutralization against live WT and Omicron viruses at 1 month after the second and third doses in a subset of COVID-19–naive participants (Figure 4). While Omicron-specific neutralization was significantly weaker than wild type at both time points in both PLWH and controls (all within-group comparisons, P < .001), the third dose significantly boosted Omicron-specific neutralization above second-dose levels (both within-group comparisons, P < .001). One month after the second dose, both PLWH and controls neutralized Omicron at a median reciprocal dilution of 20 (IQR, BLOQ to 40) (P = .71). One month after the third dose, anti-Omicron neutralization increased to a median (IQR) reciprocal dilution of 80 (40–160) in PLWH, compared with 40 (40–80) in controls (P = .03). This was consistent with the superior neutralization of WT virus observed in PLWH at this time point (Figure 2). Neutralization of WT and Omicron viruses were significantly correlated with ACE2 displacement activities, as expected (all P < .001; Supplementary Figure 4).
Figure 4.
Omicron-specific neutralization activities 1 month after the second and third coronavirus disease 2019 (COVID-19) vaccine doses. Neutralization activities, reported as the reciprocal of the highest plasma dilution at which neutralization was observed in all triplicate assay wells, against the wild-type (WT) and Omicron virus isolates in a subset of people living with human immunodeficiency virus (PLWH) (orange) and controls (blue) who remained COVID-19 naive throughout the study. Numbers of participants are shown at the bottom of the plot. Thick horizontal red bars represent medians; thinner horizontal red bars, interquartile ranges. P values were computed using the Wilcoxon matched-pairs test (for within-group comparisons) or the Mann-Whitney U test (for between-group comparisons) and are uncorrected for multiple comparisons. Abbreviations: LLOQ, lower limit of quantification; ULOQ, upper limit of quantification.
DISCUSSION
Our study confirms that anti-SARS-CoV-2 antibody concentrations and neutralizing activities naturally decline after 2-dose COVID-19 vaccination [32, 44]. Nevertheless, we found no evidence that PLWH receiving suppressive antiretroviral therapy exhibited lower antibody concentrations at any time point up to 6 months after 2-dose vaccination, nor did they exhibit faster rates of antibody decline during this period compared with controls, after accounting for sociodemographic, health-related, and vaccine-related factors. Similarly, we found no evidence that PLWH exhibited poorer neutralization at any time point after 2 doses compared to controls. The lack of significant difference in immune response decline between PLWH and controls after 2-dose vaccination is consistent with data from PLWH participants in the original ChAdOx1 trial [38].
Our results also showed that a third vaccine dose boosted binding antibody concentrations and function to significantly higher than post–second-dose levels [45], including against Omicron [46]. Consistent with accumulating evidence [34, 36, 37, 47], Omicron-specific antibody responses remained universally weaker than WT-specific ones at all times tested. Nevertheless, after 3 doses, antibody concentrations in PLWH were equivalent to controls, while neutralization activities (including against Omicron) were slightly higher. The latter is likely attributable to PLWH more frequently receiving mRNA-1273 (vs BNT162b2) third doses, which was the strongest correlate of higher neutralization after 3-dose vaccination (Supplementary Table 3). In fact, most PLWH were eligible for 100-μg mRNA-1273 third doses, which likely boosted responses still further, though we could not confirm this directly.
Our study has several limitations. Our results may not be generalizable to PLWH who are not receiving antiretroviral therapy, who have multiple chronic conditions or who have CD4+ T-cell counts <200/μL, though we found no evidence that a low nadir CD4+ T-cell count negatively influenced COVID-19 vaccine response (in fact, initial post–third-dose viral neutralization and Omicron-specific ACE2 displacement functions were slightly higher in PLWH with lower nadir CD4+ T-cell counts, possibly owing to their eligibility for 100-μg mRNA-1273 third doses). We did not investigate T-cell responses, which may play critical roles, particularly against variants [48, 49]. Canada’s decision to increase the interval between first and second vaccine doses to 112 days [43] and to mix mRNA and viral-vector vaccines may affect generalizability. Of the participants who received mRNA-1273 third doses, 36% received 100 μg, 23% received 50 μg, and data for the remainder were unavailable, so we could not assess dose effects on vaccine responses. Because immune correlates of vaccine-mediated protection are still being elucidated [50], the implications of our results on individual-level protection remain uncertain, particularly in light of Omicron.
In conclusion, adult PLWH with well-controlled viral loads and preserved CD4+ T-cell counts mount strong and functional antibody responses to 2- and 3-dose COVID-19 vaccination, including to Omicron, though it will be important to monitor these responses over time. Studies of PLWH who are not receiving antiretroviral treatment or who have low CD4+ T-cell counts are also needed.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Supplementary Material
Contributor Information
Hope R Lapointe, British Columbia Centre for Excellence in HIV/AIDS, Vancouver, British Columbia, Canada.
Francis Mwimanzi, Faculty of Health Sciences, Simon Fraser University, Burnaby, British Columbia, Canada.
Peter K Cheung, British Columbia Centre for Excellence in HIV/AIDS, Vancouver, British Columbia, Canada; Faculty of Health Sciences, Simon Fraser University, Burnaby, British Columbia, Canada.
Yurou Sang, Faculty of Health Sciences, Simon Fraser University, Burnaby, British Columbia, Canada.
Fatima Yaseen, Department of Molecular Biology and Biochemistry, Simon Fraser University, Burnaby, British Columbia, Canada.
Gisele Umviligihozo, Faculty of Health Sciences, Simon Fraser University, Burnaby, British Columbia, Canada.
Rebecca Kalikawe, Faculty of Health Sciences, Simon Fraser University, Burnaby, British Columbia, Canada.
Sarah Speckmaier, British Columbia Centre for Excellence in HIV/AIDS, Vancouver, British Columbia, Canada.
Nadia Moran-Garcia, British Columbia Centre for Excellence in HIV/AIDS, Vancouver, British Columbia, Canada.
Sneha Datwani, Faculty of Health Sciences, Simon Fraser University, Burnaby, British Columbia, Canada.
Maggie C Duncan, British Columbia Centre for Excellence in HIV/AIDS, Vancouver, British Columbia, Canada; Faculty of Health Sciences, Simon Fraser University, Burnaby, British Columbia, Canada.
Olga Agafitei, Faculty of Health Sciences, Simon Fraser University, Burnaby, British Columbia, Canada.
Siobhan Ennis, Faculty of Health Sciences, Simon Fraser University, Burnaby, British Columbia, Canada.
Landon Young, Division of Medical Microbiology and Virology, St Paul’s Hospital, Vancouver, British Columbia, Canada.
Hesham Ali, John Ruedy Clinic, St Paul’s Hospital, Vancouver, British Columbia, Canada.
Bruce Ganase, AIDS Research Program, St Paul’s Hospital, Vancouver, British Columbia, Canada.
F Harrison Omondi, British Columbia Centre for Excellence in HIV/AIDS, Vancouver, British Columbia, Canada; Faculty of Health Sciences, Simon Fraser University, Burnaby, British Columbia, Canada.
Winnie Dong, British Columbia Centre for Excellence in HIV/AIDS, Vancouver, British Columbia, Canada.
Junine Toy, British Columbia Centre for Excellence in HIV/AIDS, Vancouver, British Columbia, Canada.
Paul Sereda, British Columbia Centre for Excellence in HIV/AIDS, Vancouver, British Columbia, Canada.
Laura Burns, Department of Pathology and Laboratory Medicine, Providence Health Care, Vancouver, British Columbia, Canada.
Cecilia T Costiniuk, Division of Infectious Diseases and Chronic Viral Illness Service, McGill University Health Centre and Research Institute of the McGill University Health Centre, Montreal, Quebec, Canada.
Curtis Cooper, Department of Medicine, University of Ottawa, Ottawa, Ontario, Canada; Ottawa Hospital Research Institute, Ottawa, Ontario, Canada.
Aslam H Anis, School of Population and Public Health, University of British Columbia, Vancouver, British Columbia, Canada; CIHR Canadian HIV Trials Network, University of British Columbia, Vancouver, British Columbia, Canada; Centre for Health Evaluation and Outcome Sciences, Vancouver, British Columbia, Canada.
Victor Leung, Division of Medical Microbiology and Virology, St Paul’s Hospital, Vancouver, British Columbia, Canada; Department of Pathology and Laboratory Medicine, Providence Health Care, Vancouver, British Columbia, Canada; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, British Columbia, Canada.
Daniel T Holmes, Department of Pathology and Laboratory Medicine, Providence Health Care, Vancouver, British Columbia, Canada; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, British Columbia, Canada.
Mari L DeMarco, Department of Pathology and Laboratory Medicine, Providence Health Care, Vancouver, British Columbia, Canada; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, British Columbia, Canada.
Janet Simons, Department of Pathology and Laboratory Medicine, Providence Health Care, Vancouver, British Columbia, Canada; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, British Columbia, Canada.
Malcolm Hedgcock, Spectrum Health, Vancouver, British Columbia, Canada.
Natalie Prystajecky, Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, British Columbia, Canada; British Columbia Centre for Disease Control Public Health Laboratory, Vancouver, British Columbia, Canada.
Christopher F Lowe, Division of Medical Microbiology and Virology, St Paul’s Hospital, Vancouver, British Columbia, Canada; Department of Pathology and Laboratory Medicine, Providence Health Care, Vancouver, British Columbia, Canada; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, British Columbia, Canada.
Ralph Pantophlet, Faculty of Health Sciences, Simon Fraser University, Burnaby, British Columbia, Canada; Department of Molecular Biology and Biochemistry, Simon Fraser University, Burnaby, British Columbia, Canada.
Marc G Romney, Division of Medical Microbiology and Virology, St Paul’s Hospital, Vancouver, British Columbia, Canada; Department of Pathology and Laboratory Medicine, Providence Health Care, Vancouver, British Columbia, Canada; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, British Columbia, Canada.
Rolando Barrios, British Columbia Centre for Excellence in HIV/AIDS, Vancouver, British Columbia, Canada; School of Population and Public Health, University of British Columbia, Vancouver, British Columbia, Canada.
Silvia Guillemi, British Columbia Centre for Excellence in HIV/AIDS, Vancouver, British Columbia, Canada; Department of Family Practice, Faculty of Medicine, University of British Columbia, Vancouver, British Columbia, Canada.
Chanson J Brumme, British Columbia Centre for Excellence in HIV/AIDS, Vancouver, British Columbia, Canada; Department of Medicine, University of British Columbia, Vancouver, British Columbia, Canada.
Julio S G Montaner, British Columbia Centre for Excellence in HIV/AIDS, Vancouver, British Columbia, Canada; Department of Medicine, University of British Columbia, Vancouver, British Columbia, Canada.
Mark Hull, British Columbia Centre for Excellence in HIV/AIDS, Vancouver, British Columbia, Canada; Department of Medicine, University of British Columbia, Vancouver, British Columbia, Canada.
Marianne Harris, British Columbia Centre for Excellence in HIV/AIDS, Vancouver, British Columbia, Canada; Department of Family Practice, Faculty of Medicine, University of British Columbia, Vancouver, British Columbia, Canada.
Masahiro Niikura, Faculty of Health Sciences, Simon Fraser University, Burnaby, British Columbia, Canada.
Mark A Brockman, British Columbia Centre for Excellence in HIV/AIDS, Vancouver, British Columbia, Canada; Faculty of Health Sciences, Simon Fraser University, Burnaby, British Columbia, Canada; Department of Molecular Biology and Biochemistry, Simon Fraser University, Burnaby, British Columbia, Canada.
Zabrina L Brumme, British Columbia Centre for Excellence in HIV/AIDS, Vancouver, British Columbia, Canada; Faculty of Health Sciences, Simon Fraser University, Burnaby, British Columbia, Canada.
Notes
Acknowledgments. We thank the leadership and staff of Providence Health Care for their support of this study. We thank the phlebotomists and laboratory staff at St Paul’s Hospital, the British Columbia Centre for Excellence in HIV/AIDS, and Simon Fraser University for assistance. Above all, we thank the participants, without whom this study would not have been possible.
Disclaimer. The views expressed in this publication are those of the authors and not necessarily those of the Public Health Agency of Canada, the COVID-19 Immunity Task Force, the African Academy of Sciences, the New Partnership for Africa’s Development Planning and Coordinating Agency, Wellcome Trust, the Canadian or UK governments, or other funders.
Financial support. This work was supported by Genome BC, the Michael Smith Foundation for Health Research, and the BCCDC Foundation for Public Health (rapid severe acute respiratory syndrome coronavirus 2 vaccine research initiative in British Columbia award VAC-009 to M. A. B. and Z. L. B.); the Public Health Agency of Canada through two COVID-19 Immunity Task Force coronavirus disease 2019 (COVID-19) awards to M. G. R., M. A. B., and Z. L. B. and to C. T. C., C. C., and A. H. A.); the Canadian Institutes of Health Research, through the Coronavirus Variants Rapid Response Network (grant FRN-175622 to M. A. B.); the Canada Foundation for Innovation, through Exceptional Opportunities Fund–COVID-19 awards (to M. L. D., C. F. L., R. P., C. J. B., M. N., M. A. B., and Z. L. B.); the British Columbia Ministry of Health–Providence Health Care Research Institute (COVID-19 Research Priorities Grant to C. F. L. and C. J. B.); the CIHR Canadian HIV Trials Network (support to A. H. A.); the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (grant R01AI134229 to R. P.); the Michael Smith Foundation for Health Research (scholar awards to M. L. D. and Z. L. B.); Simon Fraser University (undergraduate research award to F. Y.); and the Sub-Saharan African Network for TB/HIV Research Excellence, through the DELTAS Africa Initiative (grant DEL-15-006) (PhD fellowships to G. U. and F. H. O.). The DELTAS Africa Initiative is an independent funding scheme of the African Academy of Sciences’ Alliance for Accelerating Excellence in Science in Africa and supported by the New Partnership for Africa’s Development Planning and Coordinating Agency, with funding from the Wellcome Trust [grant 107752/Z/15/Z] and the UK government. Funding to pay the Open Access publication charges for this article was provided by Genome BC, the Michael Smith Foundation for Health Research, and the BCCDC Foundation for Public Health.
References
- 1. Geretti AM, Stockdale AJ, Kelly SH, et al. Outcomes of COVID-19 related hospitalization among people with HIV in the ISARIC WHO Clinical Characterization Protocol (UK): a prospective observational study. Clin Infect Dis 2021; 73:e2095–e2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Boulle A, Davies MA, Hussey H, et al. Risk factors for coronavirus disease 2019 (COVID-19) death in a population cohort study from the Western Cape Province, South Africa. Clin Infect Dis 2021; 73:e2005–e2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tesoriero JM, Swain CE, Pierce JL, et al. COVID-19 outcomes among persons living with or without diagnosed HIV infection in New York State. JAMA Network Open 2021; 4:e2037069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bhaskaran K, Rentsch CT, MacKenna B, et al. HIV infection and COVID-19 death: a population-based cohort analysis of UK primary care data and linked national death registrations within the OpenSAFELY platform. Lancet HIV 2021; 8:e24–e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Poland GA, Ovsyannikova IG, Kennedy RB. SARS-CoV-2 immunity: review and applications to phase 3 vaccine candidates. Lancet 2020; 396:1595–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baden LR, El Sahly HM, Essink B, et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 2021; 384:403–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020; 383:2603–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Massarweh A, Eliakim-Raz N, Stemmer A, et al. Evaluation of seropositivity following BNT162b2 messenger RNA vaccination for SARS-CoV-2 in patients undergoing treatment for cancer. JAMA Oncol 2021; 7:1133–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Apostolidis SA, Kakara M, Painter MM, et al. Cellular and humoral immune responses following SARS-CoV-2 mRNA vaccination in patients with multiple sclerosis on anti-CD20 therapy. Nat Med 2021; 27:1990–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moor MB, Suter-Riniker F, Horn MP, et al. Humoral and cellular responses to mRNA vaccines against SARS-CoV-2 in patients with a history of CD20 B-cell-depleting therapy (RituxiVac): an investigator-initiated, single-centre, open-label study. Lancet Rheumatol 2021; 3:e789–e797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Deepak P, Kim W, Paley MA, et al. Effect of Immunosuppression on the immunogenicity of mRNA Vaccines to SARS-CoV-2: a prospective cohort study. Ann Intern Med 2021; 174:1572–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Grupper A, Rabinowich L, Schwartz D, et al. Reduced humoral response to mRNA SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients without prior exposure to the virus. Am J Transplant 2021; 21:2719–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Plana M, García F, Gallart T, et al. Immunological benefits of antiretroviral therapy in very early stages of asymptomatic chronic HIV-1 infection. AIDS 2000; 14:1921–33. [DOI] [PubMed] [Google Scholar]
- 14. Kaufmann GR, Zaunders JJ, Cunningham P, et al. Rapid restoration of CD4 T cell subsets in subjects receiving antiretroviral therapy during primary HIV-1 infection. AIDS 2000; 14:2643–51. [DOI] [PubMed] [Google Scholar]
- 15. Bart PA, Rizzardi GP, Tambussi G, et al. Immunological and virological responses in HIV-1-infected adults at early stage of established infection treated with highly active antiretroviral therapy. AIDS 2000; 14:1887–97. [DOI] [PubMed] [Google Scholar]
- 16. Lundgren JD, Babiker AG, Gordin F, et al. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med 2015; 373:795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chaer F E, Sahly HM E. Vaccination in the adult patient infected with HIV: a review of vaccine efficacy and immunogenicity. Am J Med 2019; 132:437–46. [DOI] [PubMed] [Google Scholar]
- 18. Kernéis S, Launay O, Turbelin C, Batteux F, Hanslik T, Boëlle PY. Long-term immune responses to vaccination in HIV-infected patients: a systematic review and meta-analysis. Clin Infect Dis 2014; 58:1130–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Geretti AM, Doyle T. Immunization for HIV-positive individuals. Curr Opin Infect Dis 2010; 23:32–8. [DOI] [PubMed] [Google Scholar]
- 20. Frater J, Ewer KJ, Ogbe A, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 in HIV infection: a single-arm substudy of a phase 2/3 clinical trial. Lancet HIV 2021; 8:e474–e485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Madhi SA, Koen AL, Izu A, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 in people living with and without HIV in South Africa: an interim analysis of a randomised, double-blind, placebo-controlled, phase 1B/2A trial. Lancet HIV 2021; 8:e568–e580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Levy I, Wieder-Finesod A, Litchevsky V, et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in people living with HIV-1. Clin Microbiol Infect 2021; 27:1851–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Woldemeskel BA, Karaba AH, Garliss CC, et al. The BNT162b2 mRNA vaccine elicits robust humoral and cellular immune responses in people living with HIV. Clin Infect Dis 2022; 74:1268–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ruddy JA, Boyarsky BJ, Bailey JR, et al. Safety and antibody response to two-dose SARS-CoV-2 messenger RNA vaccination in persons with HIV. AIDS 2021; 35:2399–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Noe S, Ochana N, Wiese C, et al. Humoral response to SARS-CoV-2 vaccines in people living with HIV. Infection 2022; 50:617–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Balcells ME, Le Corre N, Durán J, et al. Reduced immune response to inactivated Severe Acute Respiratory Syndrome Coronavirus 2 vaccine in a cohort of immunocompromised patients in Chile. Clin Infect Dis 2022; 75:e594–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Haidar G, Agha M, Bilderback A, et al. Prospective evaluation of Coronavirus Disease 2019 (COVID-19) vaccine responses across a broad spectrum of immunocompromising conditions: the COVID-19 Vaccination in the Immunocompromised Study (COVICS). Clin Infect Dis 2022; 75:e630–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Oyaert M, De Scheerder MA, Van Herrewege S, et al. Evaluation of humoral and cellular responses in SARS-CoV-2 mRNA vaccinated immunocompromised patients. Front Immunol 2022; 13:858399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Brumme ZL, Mwimanzi F, Lapointe HR, et al. Humoral immune responses to COVID-19 vaccination in people living with HIV receiving suppressive antiretroviral therapy. NPJ Vaccines 2022; 7:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mizrahi B, Lotan R, Kalkstein N, et al. Correlation of SARS-CoV-2-breakthrough infections to time-from-vaccine. Nat Commun 2021; 12:6379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Goldberg Y, Mandel M, Bar-On YM, et al. Waning immunity after the BNT162b2 vaccine in Israel. N Engl J Med 2021; 385:e85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Levin EG, Lustig Y, Cohen C, et al. Waning immune humoral response to BNT162b2 Covid-19 vaccine over 6 months. N Engl J Med 2021; 385:e84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pajon R, Doria-Rose NA, Shen X, et al. SARS-CoV-2 Omicron variant neutralization after mRNA-1273 booster vaccination. N Engl J Med 2022; 386:1088–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Garcia-Beltran WF, St Denis KJ, Hoelzemer A, et al. mRNA-based COVID-19 vaccine boosters induce neutralizing immunity against SARS-CoV-2 Omicron variant. Cell 2022; 185:457–66.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schmidt F, Muecksch F, Weisblum Y, et al. Plasma neutralization of the SARS-CoV-2 Omicron variant. N Engl J Med 2022; 386:599–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Collie S, Champion J, Moultrie H, Bekker LG, Gray G. Effectiveness of BNT162b2 vaccine against Omicron variant in South Africa. N Engl J Med 2022; 386:494–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cele S, Jackson L, Khoury DS, et al. Omicron extensively but incompletely escapes Pfizer BNT162b2 neutralization. Nature 2022; 602:654–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ogbe A, Pace M, Bittaye M, et al. Durability of ChAdOx1 nCoV-19 vaccination in people living with HIV. JCI Insight 2022; 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bar-On YM, Goldberg Y, Mandel M, et al. Protection of BNT162b2 vaccine booster against Covid-19 in Israel. N Engl J Med 2021; 385:1393–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Choi A, Koch M, Wu K, et al. Safety and immunogenicity of SARS-CoV-2 variant mRNA vaccine boosters in healthy adults: an interim analysis. Nat Med 2021; 27:2025–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Falsey AR, FrenckRW, Jr., Walsh EE, et al. SARS-CoV-2 neutralization with BNT162b2 vaccine dose 3. N Engl J Med 2021; 385:1627–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tan CW, Chia WN, Qin X, et al. A SARS-CoV-2 surrogate virus neutralization test based on antibody-mediated blockage of ACE2-spike protein-protein interaction. Nat Biotechnol 2020; 38:1073–8. [DOI] [PubMed] [Google Scholar]
- 43. National Advisory Committee on Immunization (NACI). An Advisory Committee Statement (ACS) National Advisory Committee on Immunization (NACI); Extended dose intervals for COVID-19 vaccines to optimize early vaccine rollout and population protection in Canada in the context of limited vaccine supply. Ottawa, Ontario, Canada: Public Health Agency of Canada, 2021.
- 44. Notarte KI, Guerrero-Arguero I, Velasco JV, et al. Characterization of the significant decline in humoral immune response six months post-SARS-CoV-2 mRNA vaccination: a systematic review. J Med Virol 2022; 94:2939–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lustig Y, Gonen T, Meltzer L, et al. Superior immunogenicity and effectiveness of the third compared to the second BNT162b2 vaccine dose. Nat Immunol 2022. [DOI] [PubMed] [Google Scholar]
- 46. Belik M, Jalkanen P, Lundberg R, et al. Comparative analysis of COVID-19 vaccine responses and third booster dose-induced neutralizing antibodies against Delta and Omicron variants. Nat Commun 2022; 13:2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Planas D, Saunders N, Maes P, et al. Considerable escape of SARS-CoV-2 Omicron to antibody neutralization. Nature 2022; 602:671–5. [DOI] [PubMed] [Google Scholar]
- 48. Keeton R, Tincho MB, Ngomti A, et al. T cell responses to SARS-CoV-2 spike cross-recognize Omicron. Nature 2022; 603:488–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Liu J, Chandrashekar A, Sellers D, et al. Vaccines elicit highly conserved cellular immunity to SARS-CoV-2 Omicron. Nature 2022; 603:493–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Feng S, Phillips DJ, White T, et al. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat Med 2021; 27:2032–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.