Abstract
Background
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Omicron variant has spread rapidly throughout the world since being identified in South Africa in November 2021. Few studies have assessed primary series and booster vaccine effectiveness against Omicron among US healthcare workers
Methods
We conducted a test-negative case-control design to estimate BNT162b2 and mRNA1273 primary vaccination and booster effectiveness against SARS-CoV-2 infection and symptomatic coronavirus disease 2019 during an Omicron surge among employees of the University of Pennsylvania Health System. The study period was between 1 July 2021 and 5 April 2022. We defined the Delta period as 1 July to 12 December 2021 and the Omicron period as beginning 12 December 21.
Results
Our sample included 14 520 tests (2776 [19%] positive)—7422 (506 [7%] positive) during Delta and 7098 (2270 [32%] positive) during Omicron. Benchmarked against Delta, the vaccine effectiveness of 2 vaccine doses was lower during Omicron, with no significant protection against infection. Booster doses added significant protection, although they also showed reduced effectiveness during Omicron. Compared with findings in employees who had received 2 vaccine doses, 3 doses of BNT162b2 had a relative effectiveness of 50% (95% confidence interval, 42%–56%) during Omicron, relative to 78% (63%–87%) during Delta; 3 doses of mRNA1273 had a relative effectiveness of 56% (45%–65%) during Omicron, relative to 96% (82%–99%) during Delta. Restricting the sample to symptomatic tests yielded similar results to our primary analysis. After initial waning in BNT162b2 booster protection against infection, it remained largely stable for ≥16 weeks after vaccination.
Conclusions
Our findings provide a strong rationale for boosters among healthcare workers in the Omicron era.
Keywords: SARS-CoV-2, Omicron Variant, BNT162b2, mRNA1273, vaccine effectiveness, booster, healthcare worker
A test-negative case control study of healthcare workers with a vaccine mandate found lower vaccine effectiveness for 2 severe acute respiratory syndrome coronavirus 2 messenger RNA vaccine doses during Omicron than during a Delta period; booster doses added significant protection.
The SARS-CoV-2 Omicron variant has spread rapidly throughout the world since being identified in South Africa in November 2021 [1], and it includes common sublineages BA.1, BA.1.1, and BA.2. Omicron’s growth advantage is principally explained by increased infection rates among vaccinated people as well as increased reinfection rates [2]. Omicron displays an unprecedented amount of escape from vaccine- and infection-derived neutralizing antibodies, though with some preservation of neutralization activity after booster dosing [3]. Relatedly, observational studies of the general population have found that vaccines provide less protection against symptomatic Omicron infection than they did against the Delta variant, though there is some improvement after booster dosing [4–6].
Few studies have assessed vaccine effectiveness against Omicron among healthcare workers, a population that requires special consideration for several reasons. First, many health systems have implemented vaccine mandates [7], and as a result there is an opportunity to better understand primary and booster vaccine performance within populations with nearly universal primary vaccination coverage. Second, even mild infections among healthcare workers are consequential because of both the potential for healthcare-associated transmissions to vulnerable patients and the harmful impacts of staff shortages on health systems. To address this gap in the literature, we conducted a test-negative case-control study to estimate BNT162b2 and mRNA1273 effectiveness during an Omicron surge among approximately 28 000 employees of the University of Pennsylvania Health System (UPHS) in Pennsylvania and New Jersey.
METHODS
We conducted a test-negative case-control design to estimate BNT162b2 and mRNA1273 primary vaccination and booster effectiveness against SARS-CoV-2 infection and symptomatic coronavirus disease 2019 (COVID-19) during an Omicron (primarily sublineage BA.1) surge among UPHS employees. We benchmarked these estimates against parallel comparisons made during a period dominated by Delta. We included 5 of 6 UPHS hospitals and associated outpatient practices (Hospital of the University of Pennsylvania, Penn Presbyterian Medical Center, Pennsylvania Hospital, Chester County Hospital, and Princeton Health). Employees readily have access to free severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) polymerase chain reaction (PCR) testing within UPHS (typically same-day testing and regardless of symptoms), are required to attest to symptoms daily (and test if symptomatic) [8], can easily and promptly receive SARS-CoV-2 vaccinations from UPHS, and were mandated to be fully vaccinated by 1 September 2021 (>98% compliance). Booster doses are freely available to employees but are not mandatory.
The overall study period was between 1 July 2021 and 5 April 2022. We defined the Omicron period as beginning on 20 December 2021, the first week during which >80% of evaluated SARS-CoV-2 isolates were identified as confirmed (via sequencing) or presumptive (via S-gene target failure) Omicron (primarily sublineage BA.1) [9]. Before this, the Delta variant was dominant, accounting for nearly all sequenced isolates since July 2021. We defined the Delta period as 1 July to 12 December 2021 to allow for a 1-week transition period from Delta to Omicron. We excluded samples obtained during this transition period.
Data Sources
We used the UPHS human resources database to identify all paid employees within the included UPHS hospitals during the study period. We then extracted SARS-CoV-2 PCR testing data (collection date and result) for these employees during the study period from the UPHS electronic medical record. All samples were provider collected from the anterior nares, with testing conducted at UPHS laboratories. We excluded all tests from employees who had previously tested positive (to minimize bias caused by hybrid immunity) and all negative tests from employees who had tested negative within the last 60 days (to minimize bias caused by testing frequency).
We obtained employee vaccination status from the UPHS electronic medical record (product, dose, and date of receipt) and supplemented it with data from the UPHS human resources database (where employees are required to attest to vaccination history) when data were missing from the electronic medical record (5% for BNT162b2 and 2% for mRNA1273). Our study sample included tests from employees who were (1) unvaccinated at the time of testing, (2) vaccinated with 2 doses of BNT162b2 or mRNA1273>14 days before testing (fully vaccinated), or (3) vaccinated with 3 doses of BNT162b2 or mRNA1273 >7 days before testing (boosted). We excluded tests from employees who had received a non–messenger RNA (mRNA) vaccine (<1%) or heterologous vaccination before testing (<1%) and from employees whose employment was terminated before testing.
We classified a test as symptomatic if the employee reported symptoms potentially consistent with COVID-19 within 14 days before the test, as determined by the daily UPHS symptom attestation survey, through which employees are required to attest presence or absence of symptoms daily, or the UPHS Occupational Health and Infection Control Database, which records details of investigations of employees reporting COVID-19 exposure, symptoms, or a positive test result.
We obtained employee demographics (age, sex, race/ethnicity, and county of residence) from the UPHS electronic medical record, and clinical/nonclinical job role from the UPHS human resources database. We obtained publicly available county-level rolling average COVID-19 case rates per 100 000 people from the New York Times COVID-19 database [10].
Statistical Analysis
We used logistic regression models to calculate the vaccine effectiveness of 2 doses of BNT162b2 and mRNA1273 compared with findings in unvaccinated employees and the relative effectiveness of 3 doses of BNT162b2 and mRNA1273 compared with (1) findings in unvaccinated employees and (2) 2 doses of the same vaccine product. The PCR test result was the dependent variable, cases were positive tests results, and controls were negative test results. We defined effectiveness as 1 minus the odds of vaccination in cases divided by the odds of vaccination in controls. We adjusted estimates for age, race/ethnicity, presence of symptoms, clinical versus nonclinical work role, rolling average case rates per 100 000 people on the specimen collection date for the employee’s county of residence, and specimen collection week. We conducted separate analyses for the Delta and Omicron periods.
We repeated the analyses using only symptomatic tests (as defined above) to determine effectiveness against symptomatic COVID-19. For this symptomatic analysis we were only able to estimate the effectiveness of 3 relative to 2 vaccine doses because of an insufficient sample size of tests from unvaccinated employees, which made up <5% of the study sample.
To determine booster effectiveness over time since vaccination, we calculated the effectiveness of 3 doses of BNT162b2 at <8, 8–12, 12–16, and >16 weeks from vaccination compared with (1) unvaccinated employees and (2) 2 doses of BNT162b2 <6 months before testing. There were insufficient observations to estimate mRNA1273 booster effectiveness over time since vaccination (75% had received the booster within 12 weeks) or protection against symptomatic infection over time since vaccination (19% of tests were symptomatic).
The study was approved by the University of Pennsylvania Institutional Review Board. We performed statistical analysis using SAS (version 9.4 and R (version 3.5.2) software, using the ggplot2 package.
RESULTS
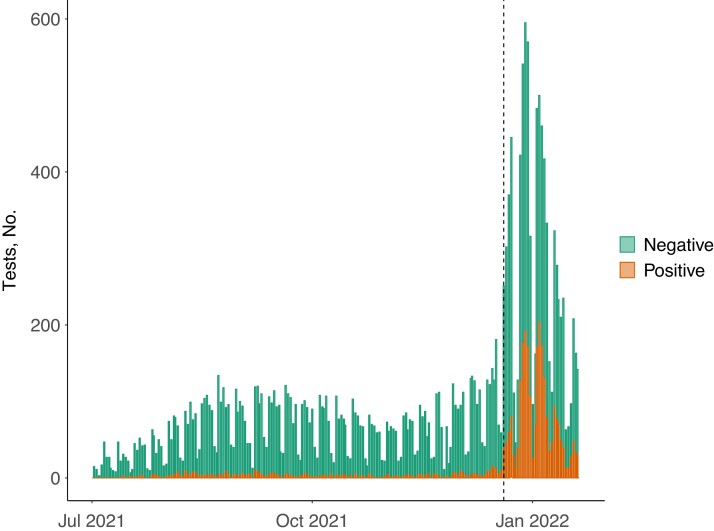
With the emergence of Omicron, UPHS experienced its largest surge of employee infections since vaccines became available (Figure 1). Our study sample included 14 520 tests (2776 [19%] positive)—7422 (506 [7%] positive) during the Delta period and 7098 (2270 [32%] positive) during the Omicron period. Study sample characteristics are provided in Tables 1 and 2, and inclusion flowcharts in Supplementary Figures 1 and 2. During the Delta period, there were 321 tests (4%) among unvaccinated employees, 4366 (59%) among employees with 2 doses of BNT162b2, 1617 (22%) among those with 2 doses of mRNA1273, 909 (12%) among those with 3 doses of BNT162b2, and 209 (3%) among those with 3 doses of mRNA1273. During the Omicron period, there were 85 tests (1%) among unvaccinated employees, 2094 (30%) among employees with 2 doses of BNT162b2, 916 (13%) among those with 2 doses of mRNA1273, 3015 (42%) among those with 3 doses of BNT162b2, and 988 (14%) those with 3 doses of mRNA1273.
Figure 1.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) polymerase chain reaction tests among University of Pennsylvania Health System employees included in the study sample. Dashed vertical line indicates the start of the Omicron period—20 December 2021—after which >80% of evaluated SARS-CoV-2 isolates were identified as confirmed (via sequencing) or presumptive (via S-gene target failure) Omicron.
Table 1.
Characteristics of Study Sample During A Period Dominated by the Omicron Variant (20 December 2021 to 5 April 2022)
| Study Participants, No. (Column %)a | ||||||||
|---|---|---|---|---|---|---|---|---|
| BNT162b2 Vaccine | mRNA1273 Vaccine | |||||||
| Characteristic | Total | Unvaccinated | 2 Doses | 3 Doses | 2 Doses | 3 Doses | Symptomatic | SARS-CoV-2 PCR Positive |
| All tests, no. (row %) | 7098 (100) | 85 (1) | 2094 (30) | 3015 (42) | 916 (13) | 988 (14) | 1340 (19) | 2270 (32) |
| Female sex | 5745 (81) | 82 (96) | 1676 (80) | 2400 (80) | 779 (85) | 808 (82) | 1089 (81) | 1853 (82) |
| Location | ||||||||
| HUP | 3983 (56) | 24 (28) | 1255 (60) | 1996 (66) | 337 (37) | 371 (38) | 840 (63) | 1245 (55) |
| Presbyterian | 546 (8) | 2 (3) | 93 (4) | 125 (4) | 166 (18) | 160 (16) | 82 (6) | 206 (9) |
| PAH | 854 (12) | 6 (7) | 168 (8) | 140 (5) | 222 (24) | 318 (32) | 104 (8) | 268 (12) |
| Princeton | 943 (13) | 45 (53) | 333 (16) | 445 (15) | 70 (8) | 50 (5) | 92 (7) | 265 (12) |
| Chester | 772 (11) | 8 (9) | 245 (12) | 309 (10) | 121 (13) | 89 (9) | 222 (17) | 286 (13) |
| Age, y | ||||||||
| 18–30 | 1465 (21) | 20 (24) | 464 (22) | 649 (22) | 183 (20) | 149 (15) | 302 (23) | 526 (23) |
| 31–40 | 2454 (35) | 29 (34) | 807 (39) | 1032 (34) | 297 (32) | 289 (29) | 499 (37) | 810 (36) |
| 41–50 | 1362 (19) | 15 (18) | 436 (21) | 520 (17) | 195 (21) | 196 (20) | 268 (20) | 489 (22) |
| 51–60 | 1165 (16) | 13 (15) | 269 (13) | 500 (17) | 167 (18) | 216 (22) | 176 (13) | 318 (14) |
| >60 | 652 (9) | 8 (9) | 118 (6) | 314 (10) | 74 (8) | 138 (14) | 95 (7) | 127 (6) |
| Race/ethnicity | ||||||||
| White | 4324 (61) | 53 (62) | 1123 (54) | 2049 (68) | 481 (53) | 618 (63) | 819 (61) | 1251 (55) |
| Black | 1556 (22) | 26 (31) | 637 (30) | 381 (13) | 303 (33) | 209 (21) | 307 (23) | 627 (28) |
| Asian | 638 (9) | 0 (0) | 145 (7) | 354 (12) | 55 (6) | 84 (9) | 116 (9) | 185 (8) |
| Other | 518 (7) | 5 (6) | 165 (8) | 217 (7) | 62 (7) | 69 (7) | 86 (6) | 179 (8) |
| Latinx | 62 (1) | 1 (1) | 24 (1) | 14 (0) | 15 (2) | 8 (1) | 12 (1) | 28 (1) |
| Clinical job role | 4361 (62) | 61 (76) | 1219 (58) | 1971 (66) | 548 (60) | 562 (57) | 823 (62) | 1335 (59) |
| Time since dose 2 or 3, wk | ||||||||
| <8 | … | … | 2 (0) | 519 (17) | 1 (0) | 437 (45) | … | … |
| 8–12 | … | … | 4 (0) | 968 (32) | 0 (0) | 280 (29) | … | … |
| 12–16 | … | … | 22 (1) | 868 (29) | 10 (1) | 118 (12) | … | … |
| 16–20 | … | … | 147 (7) | 293 (10) | 34 (4) | 78 (8) | … | … |
| 20–24 | … | … | 201 (10) | 243 (8) | 28 (3) | 27 (3) | … | … |
| >24 | … | … | 1718 (82) | 124 (4) | 843 (92) | 25 (3) | … | … |
Abbreviations: Chester, Chester County Hospital; HUP, Hospital of the University of Pennsylvania; PAH, Pennsylvania Hospital; PCR, polymerase chain reaction; Presbyterian, Penn Presbyterian Medical Center; Princeton, Princeton Health; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Data represent no. (column %) of study participants unless otherwise specified.
Table 2.
Characteristics of Study Sample During a Period Dominated by the Delta Variant (1 July to 12 December 2021)a
| Study Participants, No. (Column %) | ||||||||
|---|---|---|---|---|---|---|---|---|
| BNT162b2 Vaccine | mRNA1273 Vaccine | |||||||
| Characteristic | Total | Unvaccinated | 2 Doses | 3 Doses | 2 Doses | 3 Doses | Symptomatic | SARS-CoV-2 PCR Positive |
| All tests, no. (row %) | 7422 (100) | 321 (4) | 4366 (59) | 909 (12) | 1617 (22) | 209 (3) | 1361 (18) | 506 (7) |
| Female sex | 6020 (81) | 299 (93) | 3493 (80) | 717 (79) | 1343 (83) | 168 (80) | 1070 (81) | 388 (79) |
| Location | ||||||||
| HUP | 4563 (61) | 129 (40) | 3055 (70) | 636 (70) | 659 (41) | 84 (40) | 825 (63) | 300 (61) |
| Presbyterian | 540 (7) | 19 (6) | 183 (4) | 49 (5) | 262 (16) | 27 (13) | 77 (6) | 31 (6) |
| PAH | 850 (11) | 37 (12) | 237 (5) | 40 (4) | 464 (29) | 72 (34) | 102 (8) | 54 (11) |
| Princeton | 780 (11) | 96 (30) | 484 (11) | 100 (11) | 90 (6) | 10 (5) | 74 (6) | 41 (8) |
| Chester | 689 (9) | 40 (12) | 407 (9) | 84 (9) | 142 (9) | 16 (8) | 240 (18) | 63 (13) |
| Age, y | ||||||||
| 18–30 | 1714 (23) | 93 (29) | 1084 (25) | 187 (21) | 321 (20) | 29 (14) | 358 (26) | 137 (27) |
| 31–40 | 2685 (36) | 124 (39) | 1633 (37) | 355 (39) | 516 (32) | 57 (27) | 490 (36) | 170 (34) |
| 41–50 | 1208 (16) | 55 (17) | 692 (16) | 129 (14) | 297 (18) | 35 (17) | 242 (18) | 91 (18) |
| 51–60 | 1143 (15) | 30 (9) | 612 (14) | 144 (16) | 317 (20) | 40 (19) | 187 (14) | 72 (14) |
| >60 | 672 (9) | 19 (6) | 345 (8) | 94 (10) | 166 (10) | 48 (23) | 84 (6) | 36 (7) |
| Race/ethnicity | ||||||||
| White | 4736 (64) | 173 (54) | 2810 (64) | 639 (70) | 971 (60) | 143 (68) | 906 (67) | 341 (67) |
| Black | 1493 (20) | 120 (37) | 836 (19) | 95 (10) | 406 (25) | 36 (17) | 230 (17) | 104 (21) |
| Asian | 611 (8) | 4 (1) | 372 (9) | 104 (11) | 112 (7) | 19 (9) | 111 (8) | 25 (5) |
| Other | 522 (7) | 22 (7) | 316 (7) | 67 (7) | 106 (7) | 11 (5) | 97 (7) | 32 (6) |
| Latinx | 60 (1) | 2 (1) | 32 (1) | 4 (0) | 22 (1) | 0 (0) | 17 (1) | 4 (1) |
| Clinical job role | 4728 (64) | 201 (65) | 2855 (66) | 609 (67) | 942 (59) | 121 (59) | 860 (64) | 318 (63) |
| Time since dose 2 or 3, wk | ||||||||
| <8 | … | … | 152 (3) | 711 (78) | 45 (3) | 162 (87) | … | … |
| 8–12 | … | … | 131 (3) | 161 (18) | 21 (1) | 10 (5) | … | … |
| 12–16 | … | … | 132 (3) | 132 (3) | 38 (2) | 2 (1) | … | … |
| 16–20 | … | … | 130 (3) | 130 (3) | 51 (3) | 0 (0) | … | … |
| 20–24 | … | … | 171 (4) | 171 (4) | 114 (7) | 1 (1) | … | … |
| >24 | … | … | 3650 (84) | 30 (3) | 1348 (83) | 11 (6) | … | … |
Abbreviations: Chester, Chester County Hospital; HUP, Hospital of the University of Pennsylvania; PAH, Pennsylvania Hospital; PCR, polymerase chain reaction; Presbyterian, Penn Presbyterian Medical Center; Princeton, Princeton Health; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Data represent no. (column %) of study participants unless otherwise specified.
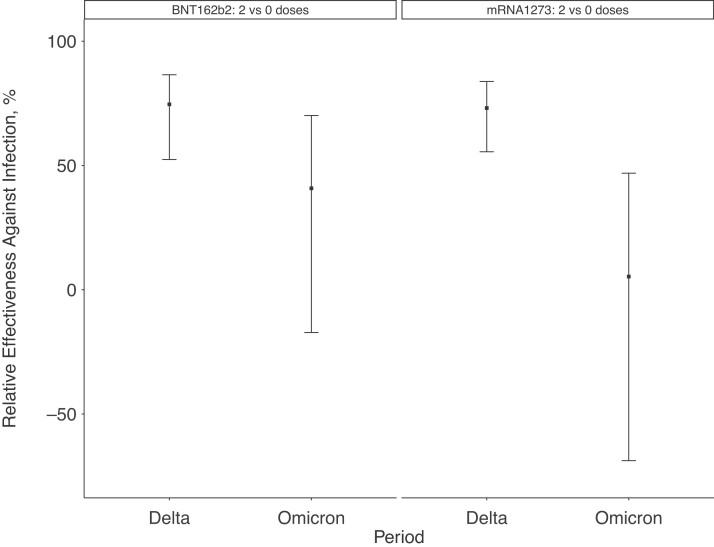
Benchmarked against the Delta period, vaccine effectiveness of 2 vaccine doses was lower during the Omicron period (Figure 2). Compared with findings in unvaccinated employees, 2 doses of BNT162b2 had a vaccine effectiveness of 41% (95% confidence interval [CI], −17% to 87%) during the Omicron period, versus 75% (52%–87%) during the Delta period; 2 doses of mRNA1273 had a vaccine effectiveness of 5% (−69% to 47%) during the Omicron period, versus 73% (56%–84%) during the Delta period.
Figure 2.
Effectiveness of 2 doses of BNT162b2 or mRNA1273 vaccine relative to findings in unvaccinated employees, comparing a period dominated by Omicron (20 December 2021 to 5 April 2022) to one dominated by Delta (1 July to 12 December 2021). Estimates were adjusted for age, sex, race/ethnicity, presence of symptoms, work role (clinical or nonclinical), rolling average case rates per 100 000 people on the specimen collection date for the employee’s county of residence, and the specimen collection week.
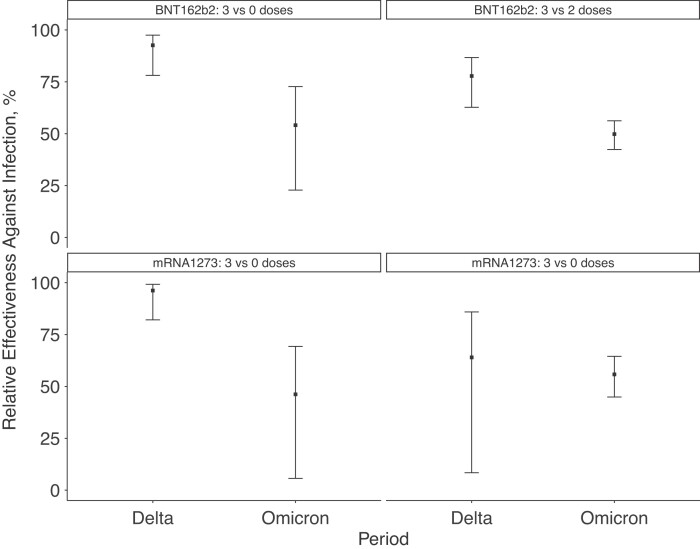
Booster doses, on the other hand, continued to display a significant amount of protection, although they also showed reduced effectiveness during the Omicron period (Figure 3). Compared with findings in unvaccinated employees, 3 doses of BNT162b2 had a relative effectiveness of 54% (95% CI, 23%–73%) during the Omicron period, relative to 93% (78%–98%) during Delta; 3 doses of mRNA1273 had a relative effectiveness of 46% (6%–69%) during the Omicron period, relative to 96% (82%–99%) during Delta. Compared with findings in employees who had received 2 vaccine doses, 3 doses of BNT162b2 had a relative effectiveness of 50% (95% CI, 42%–56%) during the Omicron period, relative to 78% (63%–87%) during Delta; 3 doses of mRNA1273 had a relative effectiveness of 56% (45%–65%) during the Omicron period, versus 96% (82%–99%) during Delta.
Figure 3.
Effectiveness of 3 doses of BNT162b2 or mRNA1273 vaccine relative to findings in (1) unvaccinated employees and (2) employees who had received 2 doses of the same vaccine, comparing a period dominated by Omicron (20 December 2021 to 5 April 2022) to one dominated by Delta (1 July to 12 December 2021). Estimates were adjusted for age, sex, race/ethnicity, presence of symptoms, work role (clinical or nonclinical), rolling average case rates per 100 000 people on the specimen collection date for the employee’s county of residence, and the specimen collection week.
Restricting the sample to the 2701 symptomatic tests yielded similar results to our primary analysis. Compared with findings in employees who had received 2 vaccine doses, 3 doses of BNT162b2 had a relative effectiveness against symptomatic COVID-19 of 39% (95% CI, 23%–52%) during the Omicron period, relative to 80% (62%–89%) during Delta; 3 doses of mRNA1273 had a relative effectiveness against symptomatic COVID-19 of 62% (43%–75%) during the Omicron period, versus 90% (49%–98%) during Delta.
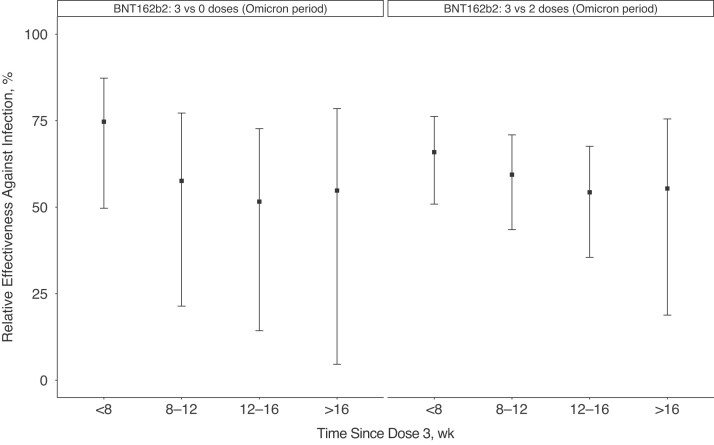
BNT162b2 booster protection during the Omicron period initially decreased and then became stable over time since vaccination (Figure 4). Compared with findings in unvaccinated employees, 3 doses of BNT162b2 had a relative effectiveness of 75% (95% CI, 50%–87%) within 8 weeks after vaccination, decreasing to 55% (5%–69%) at >16 weeks. Compared with findings in employees who had received 2 doses of BNT162b2 within the last 6 months, 3 doses of BNT162b2 had a relative effectiveness of 66% (95% CI, 51%–76%) within 8 weeks after vaccination, decreasing to 55% (19%–76%) at >16 weeks.
Figure 4.
Effectiveness of 3 doses of BNT162b2 vaccine over time since vaccination during a period dominated by Omicron (20 December 2021 to 5 April 2022) relative to findings in (1) unvaccinated employees and (2) employees who had received 2 doses of BNT162b2 <6 months before testing. Estimates were adjusted for age, sex, race/ethnicity, presence of symptoms, work role (clinical or nonclinical), rolling average case rates per 100 000 people on the specimen collection date for the employee’s county of residence, and the specimen collection week.
DISCUSSION
In this test-negative case-control study, we report one of the first assessments of SARS-CoV-2 mRNA vaccine effectiveness against Omicron (primarily sublineage BA.1) among healthcare workers in a large health system who were mandated to receive a primary vaccination series. In this highly vaccinated and closely monitored population, we found a substantial loss in protection against Omicron compared with Delta after a primary vaccine series with BNT162b2 or mRNA1273. Importantly, a third vaccine dose restored a meaningful though reduced degree of protection against both overall and symptomatic infection. Our findings are consistent with findings of immunologic and household transmission studies [2, 3], as well as other vaccine effectiveness studies conducted in the general population [4–6].
Our study is also one of the first to show that, after an initial waning in booster protection against infection, booster protection is largely stable for ≥16 weeks after vaccination. However, relatively imprecise estimates at later time points after vaccination do not rule out an ongoing gradual waning in protection over time, as seen after the primary vaccination series [11–13], a possibility that requires ongoing follow-up in the months ahead. The pattern we identified is consistent with other vaccine effectiveness studies with shorter-term follow up [6, 14]. Notably, the US Food and Drug Administration recently approved a second booster dose for older or immunocompromised individuals, starting 4 months (ie, approximately 16 weeks) after their third dose [15].
Like many health systems across the United States, UPHS experienced a wave of staff infections during the 2021 Omicron surge that was unprecedented in the vaccine era. Staffing shortages due to SARS-CoV-2 infections forced UPHS to enter contingency staffing mode for the first time during the pandemic. The consequences of this burden of illness among healthcare workers over such a short period of time are important even if individual disease severity is generally mild in the context of nearly universal receipt of a primary vaccination series. There is emerging evidence that the Omicron surge was associated with large increases in nosocomial transmission [16], which has the potential to lead to significant morbidity among vulnerable patients who are hospitalized or require frequent healthcare interactions [17]. Even beyond specific impacts related to COVID-19, many simultaneous staff absences due to illness lead to operational strain that can indirectly harm patients relying on either immediate or long-term services from a health system. In this context, our findings provide a strong rationale for the role of boosters among even lower-risk healthcare workers who have received a primary vaccination series.
This study has several notable strengths. Our study population is closely monitored, with daily symptom attestation and easy access to testing, and there have been few vaccine effectiveness studies in populations with a vaccine requirement. The data sources we relied on are comprehensive, and use of multiple inputs for vaccination and symptom status will minimize the likelihood of misclassification, a major source of potential bias in vaccine effectiveness studies that use routinely collected healthcare data. The test-negative design reduces confounding caused by differences in health-seeking behavior. Because healthcare workers were among the first people in the United States eligible for both the primary vaccination series and booster doses, we have some of the longest possible follow-up since vaccination, particularly relevant for determining booster effectiveness by time since vaccination.
Limitations of this study include lack of comprehensive viral sequencing; it is possible that some positive tests were misclassified. However, the extremely low degree of variant heterogeneity seen in surveillance sequencing during the considered study periods would suggest that this was unlikely to affect our findings. Although UPHS hospitals involve multiple states, the health system is in a region that has broadly experienced different waves of the pandemic similarly. Health system and publicly reported surveillance data suggest no major differences that have affected how each location experienced the Delta and Omicron waves [9].
In addition, our study was conducted primarily in the setting of the BA.1 sublineage of Omicron. The BA.2 sublineage became dominant in our region during the last week of the study period and has increased in prevalence in many areas of the world, indicating a likely growth advantage over BA.1. However, early evidence suggests that this growth advantage is related principally to an increase in transmissibility, rather than additional immune escape [18–20]. As a result, we hypothesize that our findings would be similar in the context of BA.2.
Information on medical comorbid conditions is not comprehensively collected from employees by any of our source databases because of existing employee privacy protection policies. We were therefore unable to include comorbid conditions (including immunosuppressed status) as potential confounders in our models. Our analysis was able to consider only PCR testing conducted at UPHS locations, but the availability and use of at-home antigen testing increased during the later part of our study period. Because there were few hospitalizations and deaths among UPHS employees, and because the outcomes would not be well captured by our data sources, we did not consider these outcomes in this analysis.
In conclusion, in this test-negative case-control study estimating BNT162b2 and mRNA1273 effectiveness during an Omicron surge among approximately 28 000 employees of the UPHS in Pennsylvania and New Jersey, we found a substantial loss in protection against Omicron compared with Delta after a primary vaccine series, with a meaningful though reduced restoration of protection after a booster dose. Our findings provide a strong rationale for the role of boosters in the Omicron era among healthcare workers who have received a primary vaccination series.
Supplementary Material
Contributor Information
Aaron Richterman, The University of Pennsylvania Perelman School of Medicine, Philadelphia, Pennsylvania, USA; The University of Pennsylvania Health System, Philadelphia, Pennsylvania, USA.
Amy Behrman, The University of Pennsylvania Perelman School of Medicine, Philadelphia, Pennsylvania, USA; The University of Pennsylvania Health System, Philadelphia, Pennsylvania, USA.
Patrick J Brennan, The University of Pennsylvania Perelman School of Medicine, Philadelphia, Pennsylvania, USA; The University of Pennsylvania Health System, Philadelphia, Pennsylvania, USA.
Judith A O’Donnell, The University of Pennsylvania Perelman School of Medicine, Philadelphia, Pennsylvania, USA; The University of Pennsylvania Health System, Philadelphia, Pennsylvania, USA.
Christopher K Snider, The University of Pennsylvania Perelman School of Medicine, Philadelphia, Pennsylvania, USA; The University of Pennsylvania Health System, Philadelphia, Pennsylvania, USA.
Krisda H Chaiyachati, The University of Pennsylvania Perelman School of Medicine, Philadelphia, Pennsylvania, USA; The University of Pennsylvania Health System, Philadelphia, Pennsylvania, USA.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Financial support. This work was supported by the National Institutes of Health (grant K08AG065444 to K. H. C.)
Potential conflicts of interest. A. B. reports a single-day consulting fee regarding adult influenza vaccines (August 2021), from Sanofi and an honorarium for online discussion of COVID-19 vaccines with JPMorgan Chase employee military veterans (May 2021), from US Veteran’s Health, a special interest group of JPMorgan Chase employees. K. H. C. reports receiving grant support from the National Cancer Institute and the National Institute on Aging (grant K08AG065444) of the National Institutes of Health, the Robert Wood Johnson Foundation, and Roundtrip, Independence Blue Cross, and the Patient-Centered Outcomes Research Institute (grant COVID-2020C2-10830), all paid to the institution; consultancy fees from Verily and Vizient, paid to the individual; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from RAND Corporation; a leadership or fiduciary role with Intend Health Strategies (formerly Primary Care Progress); and nonfinancial support from RAND Corporation and Independence Blue Cross, outside the submitted work. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Tegally H, Wilkinson E, Giovanetti M, et al. Detection of a SARS-CoV-2 variant of concern in South Africa. Nature 2021; 592:438–43. doi: 10.1038/s41586-021-03402-9. [DOI] [PubMed] [Google Scholar]
- 2. Lyngse FP, Mortensen LH, Denwood MJ, et al. SARS-CoV-2 omicron VOC transmission in Danish households. medRxiv 2021. doi: 10.1101/2021.12.27.21268278. [DOI]
- 3. Schmidt F, Muecksch F, Weisblum Y, et al. Plasma neutralization of the SARS-CoV-2 Omicron variant. N Engl J Med 2022; 386:599–601. doi: 10.1056/NEJMc2119641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Thompson M, Natarajan K, Irving S, et al. Effectiveness of a third dose of mRNA vaccines against COVID-19–associated emergency department and urgent care encounters and hospitalizations among adults during periods of Delta and Omicron variant predominance—VISION network, 10 states, August 2021–January 2022. MMWR Morb Mortal Wkly Rep 2022; 71:139–45. doi: 10.15585/mmwr.mm7104e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Accorsi EK, Britton A, Fleming-Dutra KE, et al. Association between 3 doses of mRNA COVID-19 vaccine and symptomatic infection caused by the SARS-CoV-2 Omicron and Delta variants. JAMA 2022; 327:639–51. doi: 10.1001/jama.2022.0470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Andrews N, Stowe J, Kirsebom F, et al. Covid-19 vaccine effectiveness against the Omicron (B.1.1.529) variant. N Engl J Med 2022; 386:1532–46. doi: 10.1056/NEJMoa2119451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gooch K, Mitchell H.. Hospitals, health systems mandating vaccines for workers: Becker’s hospital review. 2022. Available at: https://www.beckershospitalreview.com/workforce/hospitals-health-systems-mandating-vaccines-for-workersjune17.html. Accessed 5 April 2022.
- 8. Mahraj K, Chaiyachati Krisda H, Asch David A, et al. Developing a large-scale Covid-19 surveillance system to reopen campuses. NEJM Catalyst 2021; 2(6). doi: 10.1056/CAT.21.0049. [DOI] [Google Scholar]
- 9. Everett J, Rodino K, Marques A, et al. SARS-CoV-2 variants circulating in the Delaware River Valley tracked by surveillance sequencing. 2022. Available at:https://microb120.med.upenn.edu/data/SARS-CoV-2/. Accessed 7 April 2022.
- 10. The New York Times . Coronavirus (Covid-19) data in the United States. 2022. Available at: https://github.com/nytimes/covid-19-data. Accessed 22 January 2022.
- 11. Baden LR, El Sahly HM, Essink B, et al. Phase 3 trial of mRNA-1273 during the Delta-variant surge. N Engl J Med 2021; 385:2485–7. doi: 10.1056/NEJMc2115597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Thomas SJ, MoreiraED, Jr, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine through 6 months. N Engl J Med 2021; 385:1761–73. doi: 10.1056/NEJMoa2110345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. El Sahly HM, Baden LR, Essink B, et al. Efficacy of the mRNA-1273 SARS-CoV-2 vaccine at completion of blinded phase. N Engl J Med 2021; 385:1774–85. doi: 10.1056/NEJMoa2113017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chemaitelly H, Ayoub HH, AlMukdad S, et al. Duration of protection of BNT162b2 and mRNA-1273 COVID-19 vaccines against symptomatic SARS-CoV-2 Omicron infection in Qatar. medRxiv [Preprint: not peer reviewed]. 8 February 2022. doi: 10.1101/2022.02.07.22270568. [DOI]
- 15. FDA news release . Coronavirus (COVID-19) update: FDA authorizes second booster dose of two COVID-19 vaccines for older and immunocompromised individuals. 2022. Available at: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-second-booster-dose-two-covid-19-vaccines-older-and. Accessed 9 April 2022.
- 16. Klompas M, Karan A. Preventing SARS-CoV-2 transmission in health care settings in the context of the Omicron variant. JAMA 2022; 327:619–20. doi: 10.1001/jama.2022.0262. [DOI] [PubMed] [Google Scholar]
- 17. Richterman A, Meyerowitz EA, Cevik M. Hospital-acquired SARS-CoV-2 infection: lessons for public health. JAMA 2020; 324:2155–6. doi: 10.1001/jama.2020.21399. [DOI] [PubMed] [Google Scholar]
- 18. Yu J, Collier ARY, Rowe M, et al. Neutralization of the SARS-CoV-2 Omicron BA.1 and BA.2 variants. N Engl J Med 2022; 386:1579–80. doi: 10.1056/NEJMc2201849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lyngse FP, Kirkeby CT, Denwood M, et al. Transmission of SARS-CoV-2 Omicron VOC subvariants BA.1 and BA.2: evidence from Danish households. medRxiv [Preprint: not peer reviewed]. 30 January 2022. doi: 10.1101/2022.01.28.22270044. [DOI]
- 20. Kirsebom FCM, Andrews N, Stowe J, et al. COVID-19 vaccine effectiveness against the Omicron BA.2 variant in England. Lancet Infect Dis. Published 24 May 2022. doi: 10.1016/S1473-3099(22)00309-7. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.