Abstract
Background
Both severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and coronavirus disease 2019 (COVID-19) vaccination contribute to population-level immunity against SARS-CoV-2. This study estimated the immunological exposure and effective protection against future SARS-CoV-2 infection in each US state and county over 2020–2021 and how this changed with the introduction of the Omicron variant.
Methods
We used a Bayesian model to synthesize estimates of daily SARS-CoV-2 infections, vaccination data and estimates of the relative rates of vaccination conditional on infection status to estimate the fraction of the population with (1) immunological exposure to SARS-CoV-2 (ever infected with SARS-CoV-2 and/or received ≥1 doses of a COVID-19 vaccine), (2) effective protection against infection, and (3) effective protection against severe disease, for each US state and county from 1 January 2020 to 1 December 2021.
Results
The estimated percentage of the US population with a history of SARS-CoV-2 infection or vaccination as of 1 December 2021 was 88.2% (95% credible interval [CrI], 83.6%–93.5%). Accounting for waning and immune escape, effective protection against the Omicron variant on 1 December 2021 was 21.8% (95% CrI, 20.7%–23.4%) nationally and ranged between 14.4% (13.2%–15.8%; West Virginia) and 26.4% (25.3%–27.8%; Colorado). Effective protection against severe disease from Omicron was 61.2% (95% CrI, 59.1%–64.0%) nationally and ranged between 53.0% (47.3%–60.0%; Vermont) and 65.8% (64.9%–66.7%; Colorado).
Conclusions
While more than four-fifths of the US population had prior immunological exposure to SARS-CoV-2 via vaccination or infection on 1 December 2021, only a fifth of the population was estimated to have effective protection against infection with the immune-evading Omicron variant.
Keywords: SARS-CoV-2, immunological exposure, effective protection
Population immunity to severe acute respiratory syndrome coronavirus 2 varies across the United States. Introduction of the immune-evading Omicron variant resulted in an effective absolute increase of approximately 30 percentage points in the fraction of the population susceptible to infection.
By 1 December 2021, >48 million coronavirus disease 2019 (COVID-19) cases and 780 000 COVID-19–associated deaths had been reported in the United States [1, 2]. Between 1 December 2021 and 1 February 2022, an additional 26 million cases (35% of cumulative US COVID-19 cases) and 100 000 deaths (11% of all US COVID-19 deaths) were reported [3]. Reducing COVID-19 morbidity and mortality rates depends largely on reaching high levels of population immunity. The emergence of the Omicron variant [4, 5] illustrates the importance of identifying areas of highest vulnerability and underscores how continued viral evolution may reduce effective protection.
The true number of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections that have occurred is unknown. Estimates of the percentage of the US population ever infected vary between 37% and 62% [6–9]. Seroprevalence estimates from a nationwide convenience sample suggested that, as of 21 December 2021, 33.5% of the US population >16 years old had infection-induced SARS-CoV-2 antibodies [10]; a nationwide blood-donor study estimated 28.8% infection-induced seroprevalence for the same period [11]. The level of protection that infection confers and the rate at which protection and seropositivity wane are incompletely understood [12–14].
By 1 December 2021, >240 million US residents (72.9%) had received ≥1 dose of a COVID-19 vaccine [1], and >80 million residents had received both the initial 1- or 2-dose schedule and a booster. Reported efficacy against symptomatic infection for the 3 vaccines available in the United States ranged from 66% (Johnson & Johnson) to 94% (Pfizer and Moderna) in clinical trials [15–17]. Vaccine efficacy against infection was estimated to be lower during the Delta surge compared with earlier waves, and further reductions in efficacy against the Omicron variant have been reported [4, 5]. Declines in vaccine efficacy may reflect both waning immunity and increased immune escape for viral variants. Despite evidence of waning efficacy [18–21], vaccination appears to provide durable protection against severe disease, and boosters partially restore vaccine efficacy [22–24].
Local estimates of population immunity are important for understanding the risks of continued SARS-CoV-2 transmission. State-level estimates of infection- and vaccine-induced SARS-CoV-2 seroprevalence based on blood donation data have been reported [25], with estimates for May 2021 ranging from 63.7% in Mississippi to 91.7% in Connecticut. While these estimates provide a direct measure of seroprevalence in the study populations, they may be affected by systematic differences between blood donors and the general population. Moreover, these data do not provide county-level estimates or account for waning of protection.
For the current study, we used state- and county-level modeled estimates of cumulative SARS-CoV-2 infections and reported coverage for initial and booster vaccination [6, 26]. We estimated the joint distribution of prior SARS-CoV-2 infection and vaccination from survey data [27]. Using these inputs in a Bayesian analytic framework, we estimated the population with SARS-CoV-2 immunological exposure (ever infected or vaccinated) for each US state and county through 1 December 2021. Incorporating evidence on the time course of natural and vaccine-induced immunity, we estimated effective population immunity against infection and against severe disease over time, as well as effective protection against the Omicron variant, accounting for immune escape.
METHODS
Data
Infections
We extracted time series estimates of SARS-CoV-2 infections from a statistical model [6] that synthesizes reported data on COVID-19 cases and deaths [3, 28], accounting for both underascertainment and time lags. We imputed missing cases and deaths data for Nebraska counties after 30 June 2021 (see Supplementary Methods). We estimated cumulative infections for each US state and 3137 counties from the first reported case date until 1 December 2021. Across all states and counties, dates for the first reported case ranged between 16 December 2019 and 12 November 2020 (interquartile range, 20 February to 7 March 2020). We excluded 6 counties owing to missing or insufficient data.
Vaccinations
We extracted estimates from a repository reporting weekly county-level vaccination coverage based on Centers for Disease Control and Prevention (CDC)–reported data, adjusted for known biases and incompleteness in several states [29, 30]. We imputed missing data and smoothed the weekly time series [31] into a daily time series of residents having received ≥1 vaccine dose (see Supplementary Methods). We summed these counts for all counties within each state to produce state-level estimates. We extracted daily state- and county-level booster coverage data from CDC reports [26]. County-level booster coverage reporting started on 16 December 2021. Booster coverages before this date were imputed proportional to the corresponding state coverage using the ratio of county to state booster coverage on 16 December 2021.
Co-occurrence of Infection and Vaccination
The Census Bureau’s Household Pulse Survey collects data on COVID-19–relevant beliefs and behaviors at 2-weekly intervals, for individuals ≥18 years old [27]. We extracted data from 2 2 February to 30 August 2021, to estimate the joint distribution of infection and vaccination among survey respondents. We extracted the following variables: had COVID (yes/no; whether a respondent has received a positive COVID-19 diagnosis), received vaccine (yes/no; whether a respondent has received ≥1 dose of a COVID-19 vaccine), state, and week. Responses other than yes or no (eg, unknown) were excluded (2.3% of respondents).
Estimation
For each location, we computed the percentage immunologically exposed, defined as the percentage of the population with a prior SARS-CoV-2 infection, ≥1 dose of a COVID-19 vaccine, or both. We calculated values separately for individuals aged <12 or ≥12 years old, owing to differences in vaccine eligibility for these groups over the study period.
Immunological Exposure for the Population Aged ≥12 Years
Using the Household Pulse data, we fit a logistic regression model to estimate the association between self-reported vaccination status and prior COVID-19 diagnosis. We operationalized this relationship as the odds ratio for reported vaccination, comparing individuals reporting a prior COVID-19 diagnosis to those reporting no prior diagnosis (see Supplementary Methods). Using these regression results, we created state-specific prior distributions for the odds ratio of vaccination given prior infection status, for individuals aged ≥12 years (Supplementary Table 1). This approach assumes that the odds ratio for vaccination among those with a prior undiagnosed infection is the same as for those with a prior diagnosed infection. We validated this relationship using data from the Axios-Ipsos Coronavirus Tracker [32]. We calculated the joint probability of being vaccinated or infected as the sum of the marginal probabilities for prior infection and prior vaccination, minus the probability of being both infected and vaccinated, to avoid double counting (see Supplementary Methods).
Immunological Exposure for the Population <12 Years Old
For the population <12 years old, the percentage immunologically exposed was assumed equal to the estimated percentage ever infected, as this age group was not eligible for vaccination during most of the study period. We assumed that infection prevalence in this age group was equal to that in the overall population. We combined immunity estimates for both age groups (<12 and ≥12 years) in a weighted sum to obtain the percentage immunologically exposed in the full population. We validated our results by comparing them with published population immunity estimates based on laboratory data from a blood donor sample [25].
Waning of Protection
Protection conferred by natural infection and vaccination declines over time [24, 33–35]. Findings of prior studies suggest that antibody titers decay rapidly in the 3 months after infection and more gradually thereafter [14, 36]. Neutralizing antibody activity has been observed up to 8 months after symptom onset [12], and simulation studies suggest that titers wane below 1:20 (often used to infer 50% protection) for the majority of previously infected individuals by 341 days after symptom onset [14]. Antibody titers in vaccinated individuals are believed to wane at similar rates [13], although vaccine efficacy against symptomatic SARS-CoV-2 infection has been shown to remain robust in the first 6 months after inoculation [20, 21].
Based on studies of antibody titers, clinical trials, and vaccine effectiveness studies, we formulated 3 simplified waning scenarios, designed to capture major uncertainties in waning rates (Supplementary Figure 1). For the main analysis (base-case scenario), we assumed that infection or vaccination each initially confer 80% protection against infection that declines to 25% by 12 months after exposure, and protection against severe disease starts at 95% and declines to 85% after 12 months. For individuals both infected and vaccinated, we assumed constant protection of 90% against infection and 95% against severe disease. (See Supplementary Methods for optimistic and pessimistic scenarios used in sensitivity analyses.) We assumed that booster uptake was randomly distributed in the eligible (fully vaccinated) population and that receiving a booster restored immunity to original (prewaning) levels and subsequently waned following the “both infected and vaccinated” curve [23, 24, 37].
Immune Escape Under the Omicron Variant
Early evidence indicates the Omicron variant can escape immunity acquired by immunization or infection with earlier variants [4, 38]. Available evidence suggests that the immune escape may range between a 30-fold drop [38] to halving of the protection of earlier variants, with greater protection retained by boosted individuals [22]. Protection against severe disease appears more robust [39]. We translated this evidence into high, medium (used in the main analysis), and low immune-escape scenarios, to capture a simplified yet plausible range of scenarios. In the medium escape scenario, protection against infection was reduced by 70% (40% for those who received a booster) and protection against severe disease was reduced by 20% (15% for those who received a booster) compared with immunity against pre-Omicron variants. (See Supplementary Table 2 for low and high escape scenarios.)
Model Implementation
We executed the analysis with R software [40] and the rstan package [41]. (https://github.com/covidestim/covidestim/tree/immunity-waning). For state-level results, we report uncertainty using equal-tailed 95% credible intervals (95% CrIs). We calculated national estimates and conservative uncertainty intervals by summing state-level estimates and upper and lower bounds of state-level intervals. County-level estimates were produced using an optimization routine [6] that produces point estimates without uncertainty intervals. In summarizing county-level results, we excluded counties with a population <1000 (0.9% of all counties).
RESULTS
By 1 December 2021, 59.2% (95% CrI, 46.9%–75.6%) of the US population was estimated to have been infected with SARS-CoV-2, with state-level estimates ranging from 24.0% (16.0%–40.3%; Hawaii) to 78.5% (68.7%–88.6%; New Mexico). County-level estimates ranged from 9.0% (San Juan County, Washington) to 91.3% (San Juan County, New Mexico). The percentage of the US population that received ≥1 COVID-19 vaccine dose was estimated to be 65.2%. State-level coverage varied between 32.0% (West Virginia) and 82.8% (New Hampshire), and county-level coverage between 13.3% (Morgan County, West Virginia) and 89.9% (Pitkin County, Colorado).
Based on the results of the Household Pulse Survey, individuals reporting a prior COVID-19 diagnosis were substantially less likely to report being vaccinated. The odds ratio of vaccination among individuals with a prior COVID-19 diagnosis (compared with no prior diagnosis) varied from 0.40 (95% CrI, .36–.44) in Florida to 0.58 (.53–.63) in Texas, with a national mean of 0.52 (.50–.55).
Immunological Exposure
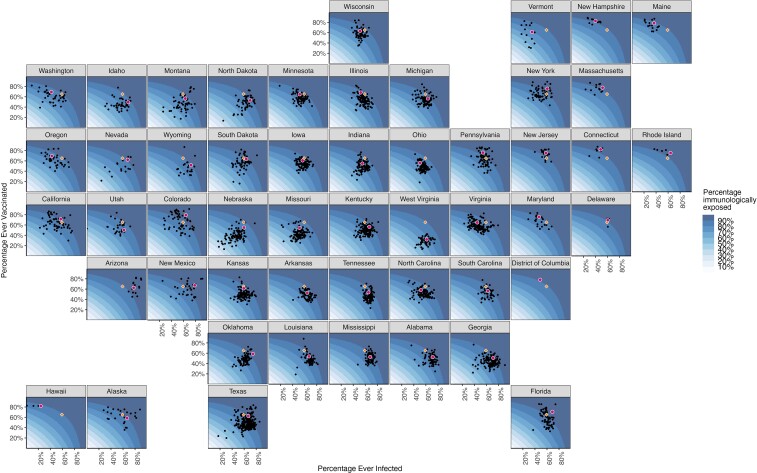
The national estimate for the population immunologically exposed was 88.2% (95% CrI, 83.6%–93.5%). State-level estimates ranged from 76.9% (95% CrI, 67.6%–87.6%; West Virginia) to 94.4% (91.2%–97.3%; New Mexico) (Table 1). Across counties, the percentage immunologically exposed ranged from 42.4% (Sioux County, Nebraska) to 98.3% (San Juan County, New Mexico; interquartile range 80.2–87.3%) (Figure 1 and Supplementary Figure 2).
Table 1.
Key Population Immunity Outcomes for Each US State on 1 December 2021
| State | % Ever Vaccinateda | % Ever Infected (95% CrI) | Ratio of % Vaccinated to % Infected | % Immunologically Exposed (95% CrI) | % Effectively Protected (95% CrI) | |||
|---|---|---|---|---|---|---|---|---|
| Pre-Omicron Variantsb | Omicron Variantc | |||||||
| Against Infection | Against Severe Disease | Against Infection | Against Severe Disease | |||||
| Alabama | 52.4 | 71.3 (60–84.2) | 0.73 | 88.6 (83–94.3) | 52.8 (50–55.9) | 74.1 (70.3–78.2) | 20.7 (19.9–21.7) | 61 (57.9–64.3) |
| Alaska | 58.3 | 66.8 (54.8–81.2) | 0.87 | 88.7 (83.3–94.2) | 57.7 (54.2–61.4) | 73.9 (70.4–77.9) | 24.2 (23.2–25.4) | 61.6 (58.8–64.8) |
| Arizona | 62.5 | 77.6 (67.6–88.1) | 0.81 | 93.4 (89.7–96.8) | 58.8 (56.1–61.5) | 77.3 (75–79.5) | 22.9 (22.1–23.8) | 63.7 (61.9–65.5) |
| Arkansas | 52.8 | 64.3 (52–79.4) | 0.82 | 85.8 (79.5–92.6) | 50.5 (47.4–54.5) | 71.5 (67.3–76.3) | 20.6 (19.8–21.9) | 59.1 (55.8–63) |
| California | 71.4 | 57.2 (44.5–74.1) | 1.25 | 89.4 (85.6–93.9) | 55.3 (51.3–60.8) | 75.3 (73.4–77.9) | 22.1 (20.9–23.8) | 62.2 (60.7–64.3) |
| Colorado | 79.1 | 63.7 (51.4–79) | 1.24 | 93.3 (90.6–96.3) | 63.2 (59.6–67.8) | 78.9 (77.9–80.1) | 26.4 (25.3–27.8) | 65.8 (64.9–66.7) |
| Connecticut | 82.3 | 48.4 (36.1–66.7) | 1.7 | 91.6 (89.2–94.8) | 58.8 (54.9–64.8) | 79.5 (78.7–80.7) | 24.1 (23–25.9) | 65.9 (65.3–66.8) |
| Delaware | 69.4 | 60.3 (47.8–76.5) | 1.15 | 89.8 (85.6–94.5) | 56.8 (53.2–61.7) | 75.8 (73.5–78.6) | 23.2 (22.2–24.7) | 62.8 (61–65.1) |
| District of Columbia | 78.3 | 48.8 (36.5–67.1) | 1.6 | 90.0 (86.9–94) | 55.7 (51.8–61.8) | 77.3 (76.2–79.1) | 21.2 (20–23) | 63.4 (62.5–64.8) |
| Florida | 70.7 | 69 (57.3–82.6) | 1.03 | 93.2 (90–96.4) | 60.5 (56.7–64.8) | 78.5 (76.5–80.7) | 23.5 (22.3–24.8) | 64.7 (63–66.5) |
| Georgia | 50.8 | 71.2 (59.9–84.1) | 0.71 | 88.4 (82.8–94.1) | 51.8 (48.7–55.1) | 73.4 (69.5–77.6) | 20.2 (19.2–21.2) | 60.3 (57.2–63.7) |
| Hawaii | 82.1 | 24 (16–40.3) | 3.43 | 86.8 (85.2–90) | 46.4 (43.5–52.6) | 77.3 (76.9–78.3) | 17.8 (16.9–19.6) | 63.2 (62.9–64) |
| Idaho | 49.4 | 68.6 (56.9–82.4) | 0.72 | 86.8 (80.4–93.4) | 54.7 (51.7–57.5) | 72.5 (68.1–77.3) | 23.4 (22.6–24.3) | 60.4 (57–64.3) |
| Illinois | 68.3 | 52.4 (39.9–70.2) | 1.3 | 87.2 (82.9–92.6) | 52.6 (48.9–58.1) | 73.3 (71–76.6) | 22.2 (21.1–23.9) | 60.9 (59.1–63.5) |
| Indiana | 54.9 | 55.2 (42.6–72.5) | 1 | 82.9 (76.6–90.4) | 47.9 (44.4–53) | 68.8 (64.9–74.2) | 19.8 (18.8–21.4) | 57 (53.8–61.3) |
| Iowa | 60.8 | 56.4 (43.8–73.5) | 1.08 | 85.3 (79.7–91.9) | 53 (50–57.3) | 71.4 (68.2–75.7) | 23.9 (23.1–25.2) | 60 (57.4–63.4) |
| Kansas | 61.4 | 59.3 (46.7–75.7) | 1.04 | 86.8 (81.4–92.8) | 52.6 (49.2–57.2) | 72.3 (69.1–76.2) | 21.7 (20.7–23.1) | 59.9 (57.4–63.1) |
| Kentucky | 56.3 | 66.8 (54.8–81.2) | 0.84 | 87.8 (82.1–93.7) | 55.1 (51.9–58.6) | 73.9 (70.1–78.1) | 23 (22.1–24.1) | 61.4 (58.4–64.8) |
| Louisiana | 53.8 | 67.2 (55.2–81.4) | 0.8 | 87.2 (81.4–93.4) | 51.1 (47.9–54.9) | 72 (68.3–76.3) | 20.5 (19.6–21.7) | 59.5 (56.5–62.8) |
| Maine | 78.3 | 36.6 (25.8–55.3) | 2.14 | 87.7 (84.7–92.1) | 55.5 (52.3–61.2) | 77.2 (75.9–79.4) | 24.2 (23.3–25.9) | 64.4 (63.4–66.2) |
| Maryland | 75.8 | 46.5 (34.3–65) | 1.63 | 88.6 (85.2–93) | 55.1 (51.6–60.9) | 75.8 (74.4–77.9) | 23.1 (22–24.8) | 62.9 (61.8–64.6) |
| Massachusetts | 77.7 | 51.7 (39.2–69.7) | 1.5 | 90.6 (87.5–94.5) | 57.4 (53.5–63.3) | 77.9 (76.5–79.9) | 23.4 (22.3–25.2) | 64.5 (63.4–66.1) |
| Michigan | 56.5 | 63.4 (51.1–78.8) | 0.89 | 87.5 (81.8–93.4) | 53.3 (50.2–57.1) | 72.6 (68.9–77.1) | 23.2 (22.4–24.5) | 60.7 (57.7–64.2) |
| Minnesota | 64.8 | 51.7 (39.2–69.6) | 1.25 | 85.4 (80.5–91.6) | 54.2 (51–58.8) | 71.9 (69.2–75.7) | 24.5 (23.7–25.9) | 60.4 (58.3–63.5) |
| Mississippi | 52.8 | 68.3 (56.5–82.2) | 0.77 | 87.7 (81.8–93.7) | 50.8 (47.7–54.5) | 72.4 (68.6–76.7) | 20 (19.1–21.2) | 59.6 (56.6–63.1) |
| Missouri | 54.2 | 51.4 (38.9–69.3) | 1.06 | 80.8 (74.3–89.1) | 46.8 (43.4–52) | 67.8 (63.6–73.6) | 19.7 (18.8–21.3) | 56.2 (52.9–60.9) |
| Montana | 56.6 | 62.9 (50.5–78.4) | 0.9 | 86.6 (80.6–93) | 55.1 (52–58.7) | 72.9 (69.1–77.6) | 24.1 (23.2–25.2) | 61 (57.9–64.7) |
| Nebraska | 55 | 59.8 (42.8–79.1) | 0.92 | 84.6 (76.2–92.8) | 51.8 (46.9–57.8) | 70.3 (64.9–76.2) | 22.4 (21.1–24.3) | 58.7 (54.4–63.4) |
| Nevada | 62.3 | 67.7 (49.2–86.5) | 0.92 | 90.1 (82.9–96.3) | 55.4 (49.2–62.1) | 75 (70.4–79.6) | 21.5 (19.7–23.6) | 61.7 (58–65.5) |
| New Hampshire | 82.8 | 39.9 (28.7–58.7) | 2.08 | 90.5 (88.3–93.8) | 54.9 (50.9–61.8) | 80.2 (79.5–81.4) | 18.1 (16.9–20.1) | 64.7 (64.1–65.7) |
| New Jersey | 75.3 | 58.3 (45.7–74.9) | 1.29 | 91.1 (87.8–95) | 57.6 (53.6–63) | 76.8 (75.3–78.7) | 22.8 (21.6–24.5) | 63.4 (62.2–64.9) |
| New Mexico | 67.2 | 78.5 (68.7–88.6) | 0.86 | 94.4 (91.2–97.3) | 62.4 (60–64.7) | 78.4 (76.6–80.1) | 25.9 (25.2–26.7) | 65.2 (63.8–66.6) |
| New York | 75.8 | 60.6 (48–76.7) | 1.25 | 91.8 (88.7–95.4) | 57.8 (53.5–63.5) | 77.4 (76–79.2) | 21.4 (20.1–23.2) | 63.4 (62.2–64.8) |
| North Carolina | 58.1 | 51.7 (37.6–71.7) | 1.12 | 83.2 (76.5–91.2) | 46.2 (41.1–53.9) | 69.4 (65.1–75.4) | 17.3 (15.8–19.6) | 56.7 (53.3–61.5) |
| North Dakota | 53.1 | 70 (58.4–83.3) | 0.76 | 88.4 (82.6–94.2) | 53.2 (50.6–56) | 72.6 (68.9–76.6) | 22.8 (22.1–23.8) | 60.5 (57.6–63.7) |
| Ohio | 56 | 50.3 (38–70.7) | 1.11 | 81.3 (75.2–90) | 48.3 (44.9–54.7) | 68.2 (64.3–74.5) | 21.3 (20.3–23.2) | 56.9 (53.8–62) |
| Oklahoma | 58.7 | 74.6 (63.8–86.3) | 0.79 | 91.4 (86.9–95.8) | 57.8 (55–60.5) | 76 (73.1–79) | 22.6 (21.8–23.5) | 62.7 (60.3–65.1) |
| Oregon | 69.6 | 42.4 (29.7–62.9) | 1.64 | 85.0 (80.4–91.3) | 52.8 (48.7–59.9) | 73.1 (70.6–77.1) | 22.4 (21.3–24.5) | 60.8 (58.8–64) |
| Pennsylvania | 76.1 | 54.2 (41.6–71.7) | 1.4 | 90.6 (87.4–94.5) | 56.3 (51.9–62.5) | 77.3 (75.7–79.4) | 20.7 (19.4–22.6) | 63.2 (62–64.9) |
| Rhode Island | 75.4 | 64.1 (51.8–79.2) | 1.18 | 92.6 (89.3–96.1) | 61.2 (57.8–65.7) | 78.7 (77.1–80.6) | 25.2 (24.2–26.6) | 65.3 (64.1–66.9) |
| South Carolina | 56.4 | 62.3 (49.9–78) | 0.91 | 86.3 (80.3–92.8) | 51.9 (48.5–56.2) | 72.2 (68.4–76.8) | 21 (20–22.3) | 59.7 (56.7–63.4) |
| South Dakota | 63.5 | 63.6 (51.3–78.9) | 1 | 88.8 (83.9–94.1) | 55.4 (52.6–59.1) | 73.3 (70.7–76.5) | 22.9 (22.1–24.1) | 60.9 (58.8–63.4) |
| Tennessee | 53.8 | 65.2 (51.3–85.3) | 0.82 | 86.6 (79.9–94.9) | 51.7 (47.6–58.2) | 72.3 (67.6–78.9) | 21.6 (20.4–23.7) | 60 (56.3–65.4) |
| Texas | 62.6 | 66.9 (54.9–81.2) | 0.94 | 89.4 (84.9–94.3) | 54.4 (50.9–58.6) | 73.6 (71–76.7) | 21.1 (20.1–22.4) | 60.6 (58.5–63) |
| Utah | 50.5 | 61.5 (49.1–77.4) | 0.82 | 83.7 (77.1–91.2) | 49 (45.3–53.3) | 68.8 (64.5–74) | 19.7 (18.6–21) | 56.8 (53.4–61) |
| Vermont | 61.4 | 35.3 (25.8–52.7) | 1.74 | 78 (73.2–85.6) | 50.4 (47.6–55.8) | 67.8 (64.9–72.9) | 24.2 (23.5–25.7) | 57.4 (55.2–61.4) |
| Virginia | 64.9 | 44.7 (32.7–63.4) | 1.45 | 83 (78.1–89.7) | 50.1 (46.6–55.7) | 70.7 (67.9–74.9) | 21.2 (20.3–22.9) | 58.7 (56.6–62.1) |
| Washington | 69.2 | 41.7 (30.1–60.5) | 1.66 | 84.5 (80.2–90.5) | 50.9 (47.3–57) | 72.3 (70.1–75.7) | 21.6 (20.6–23.3) | 60 (58.3–62.8) |
| West Virginia | 32 | 61.3 (48.8–77.2) | 0.52 | 76.9 (67.6–87.6) | 41.6 (37.6–46.2) | 65.4 (58.3–74.2) | 14.4 (13.2–15.8) | 53 (47.3–60) |
| Wisconsin | 63.4 | 51.4 (39.6–71) | 1.23 | 84.7 (79.7–91.7) | 52.9 (49.7–58.6) | 71.7 (68.8–76.4) | 23.5 (22.7–25.3) | 60.1 (57.8–63.8) |
| Wyoming | 51.7 | 72.3 (55.3–91.4) | 0.71 | 89.1 (80.5–97.1) | 56.2 (50.7–62.6) | 74.7 (68.5–81.3) | 23.7 (22.1–25.8) | 62.1 (57.2–67.5) |
Abbreviation: CrI, credible interval.
Received ≥1 coronavirus disease 2019 vaccine dose.
Assuming the base-case waning functions.
Assuming the base-case waning functions and the medium immune evasion scenario.
Figure 1.
Estimated percentage immunologically exposed on 1 December 2021, for each US county and state. Background coloring indicates the state-specific distribution of immunity as a function of infections and vaccinations. Black dots represent counties in a state; red dots, the state average. Orange diamonds represent the state population–weighted national averages of the percentage ever infected and vaccinated (this does not represent the national average of immunity because the calculation for immunity is state specific).
Effective Protection
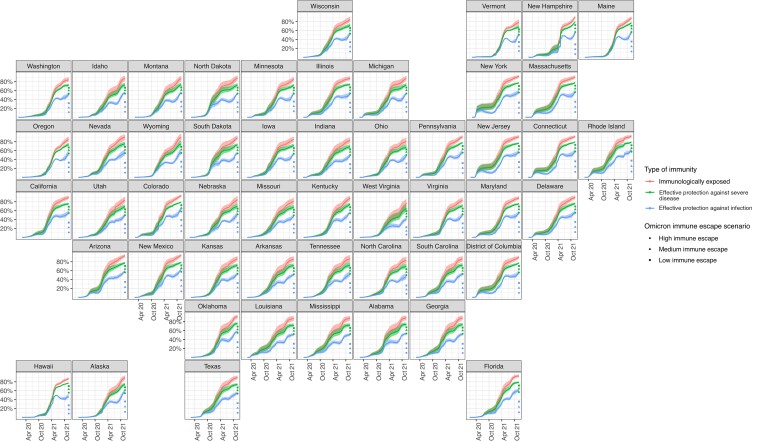
Accounting for waning of immunity, the percentage of the US population with effective protection against infection with pre-Omicron variants increased from 14.7% (95% CrI, 11.1%–19.8%) on 1 January 2021 to 54.1% (50.5%–59.2%) by 1 December 2021. On 1 December 2021, effective protection against infection with the Omicron variant was estimated to be 21.8% (95% CrI, 20.7%–23.4%). The percentage of the population with effective protection against severe disease was estimated to be 74.1% (95% CrI, 71.4%–77.6%) for pre-Omicron variants and 61.2% (59.1%–64.0%) for the Omicron variant.
Effective protection against infection with Omicron varied across states between 14.4% (95% CrI, 13.2%–15.8%; West Virginia) and 26.4% (25.3%–27.8%; Colorado). Effective protection against severe disease ranged between 53.0% (95% CrI, 47.3%–60.0%; West Virginia) and 65.8% (64.9%–66.7%; Colorado) (Table 1).
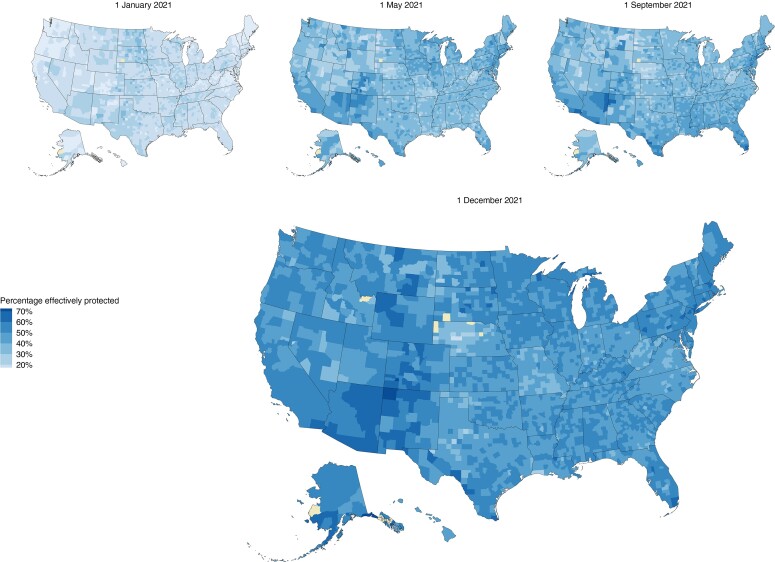
Figure 2 shows how state-level percentages of those immunologically exposed, effectively protected against infection, and effectively protected against severe disease have evolved over the course of the epidemic and with the introduction of Omicron. For counties, the percentage of the population with effective protection against infection and severe disease caused by pre-Omicron variants, respectively, varied between 26.6% and 48.4% (Cameron Parish, Louisiana) and 72.5% and 77.4% (Fairfax City, Virginia; Figure 3). Estimates of effective protection against infection and severe disease, respectively, from the Omicron variant varied between 8.4% and 26.4% (McPherson County, Nebraska), and 33.1% and 71.3% (Mineral County, Colorado) (Supplementary Figure 3).
Figure 2.
State-level estimates and uncertainty intervals of the percentages immunologically exposed, effectively protected against infection, and effectively protected against severe disease over time, with estimates of effective protection against Omicron infection under 3 immune-escape scenarios.
Figure 3.
County-level estimates of the percentage of the population effectively protected against infection at 4 time points between 1 January and 1 December 2021. Counties excluded owing to missing or insufficient data are colored yellow.
Relative Contributions of Prior Infection and Vaccination
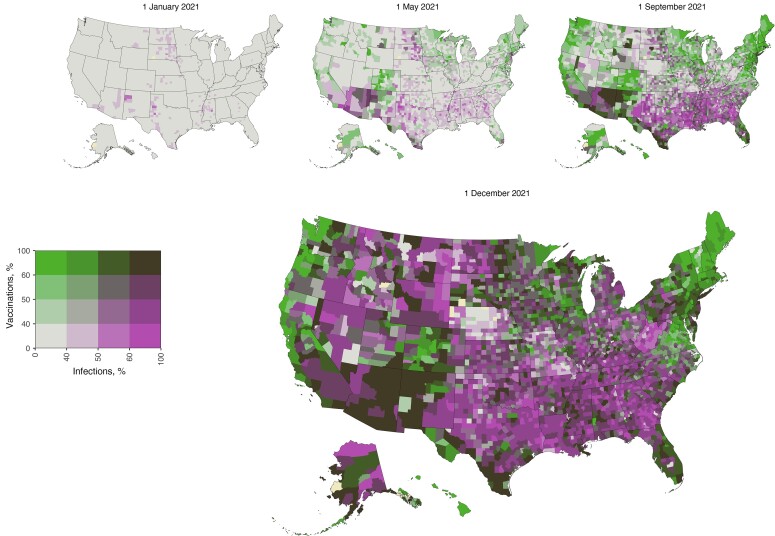
On 1 December 2021, 23.0% (95% CrI, 18.4%–28.3%) of the US population was estimated to have been infected but not vaccinated, 29.0% (18.0%–36.8%) was estimated to have been vaccinated but not infected, and 36.2% (28.4%–47.2%) was estimated to have been both vaccinated and infected. Relative contributions of vaccination and prior infection varied widely across states and counties and over time (Supplementary Figures 4 and 5). Figure 4 highlights regional patterns in the different pathways to overall immunity on 1 December 2021. The state-population weighted regional averages of the population immunologically exposed were 89.1% in the West (20.4% only infected and 30.8% only vaccinated), 84.7% in the Midwest (24.8% and 30.0%, respectively), 88.3% in the South (27.5% and 24.4%), and 91.0% in the Northeast (14.5% and 35.5%).
Figure 4.
Relative contributions of prior infection and vaccination to population immunity for each county at 4 time points between 1 January and 1 December 2021. The percentage ever infected and the percentage vaccinated are categorized with cutoff scores of 40%, 50%, and 60%, values that roughly correspond to the quantile breakpoints of the estimates of those ever infected and vaccinated on 1 December 2021.
Validation
We reestimated the odds ratio of vaccination given prior infection using independent survey data, allowing biweekly national estimates between January and June 2021 [42] (Supplementary Methods). For the 13 survey waves included in this period, the odds ratio of vaccination given prior infection varied between 0.35 and 0.98, and the mean odds ratio was 0.51 (95% CrI, .44–.59), similar to the estimated national value in the main analysis. We compared our estimates of the percentage of the population immunologically exposed with blood donor seroprevalence estimates (Supplementary Figure 6). Our estimates of the percentage immunologically exposed were generally lower than seroprevalence estimates from Jones et al [25].
Sensitivity Analyses
We conducted additional analyses evaluating the sensitivity of our effectively protected estimates to waning assumptions, with pessimistic and optimistic scenarios (Supplementary Methods and Supplementary Figure 1), in combination with the Omicron immune-escape scenarios (Supplementary Table 2). In these analyses, national estimates of effective protection against infection from pre-Omicron variants ranged between 47.1% and 64.3%, and protection against infection with Omicron between 12.3% and 28.4% (Supplementary Table 3). Effective protection against severe disease from pre-Omicron variants ranged between 67.5% and 79.0%, and protection against severe disease from the Omicron variant between 57.4% and 61.7% (Supplementary Table 4).
DISCUSSION
We analyzed the joint distribution of COVID-19 vaccination and prior SARS-CoV-2 infection in each US state and county since the beginning of the COVID-19 epidemic and estimated how population immunity changed over this period. By 1 December 2021, more than three-quarters of the US population had prior immunological exposure to SARS-CoV-2 via vaccination or infection; half of the population retained effective protection against infection with previously circulating variants, while only a fifth of the population had effective protection against infection with the Omicron variant.
The current study has several limitations. First, we chose to model separately the populations <12 and ≥12 years old separately. On 1 November 2021, children 5–11 years old became eligible for vaccination; however, we did not account explicitly for the initial vaccination scale-up through 1 December 2021 in this group. For other ages, we used vaccination coverage data from Tiu et al and Merritt et al [29, 30], which endeavor to address known biases in CDC vaccination data. We further adjusted these data to assure that no greater than 100% of the ≥12-year-old population could have been vaccinated by the end of our study period. Furthermore, we assumed the cumulative infections to be proportional between the <12- and ≥12-year-old populations. While some indicators suggest lower cumulative infections among children, other evidence shows the opposite pattern, which contradicts this and suggests a higher seroprevalence for children compared to adults [43–46].
Second, to estimate effective protection, we made assumptions about how natural and vaccine-induced immunity wanes over time. Despite accumulating evidence, these assumptions are still uncertain. In sensitivity analyses, we examined additional waning scenarios, providing a range of plausible values for the level of effective protection. We did not account for differences in waning for the pre-Omicron SARS-CoV-2 variants. Our assumptions regarding the immune escape of the Omicron variant are preliminary. The presented range of plausible scenarios demonstrates that SARS-CoV-2 variants that evade immune protection may spread widely despite high prevalence of prior infection and vaccine coverage.
Third, the model for infections assumes individuals can only be infected once, so possible reinfections and breakthrough infections among vaccinated individuals are not accounted for in our estimates of immunity. For this reason, and because reinfections and breakthrough infections with Omicron are common, we used estimates of infections only up until 1 December 2021.
Fourth, we estimated the relationship between prior infection and vaccination status using survey data that have been criticized for nonrepresentativeness [32]. While this relationship was confirmed in independent survey data validated against external benchmarks [32], it is still possible that reporting biases could have distorted this relationship. If there is greater overlap between vaccinated and previously infected populations, then overall population immunity will be lower than estimated in our analyses. Finally, we assumed that booster uptake was randomly distributed among the eligible (vaccinated) population.
Existing and new SARS-CoV-2 variants will likely continue circulating, since neither natural infection nor offers permanent immunity against infection. Recent CDC recommendations for local COVID-19 monitoring focus on hospitalizations per capita [47]. However, monitoring community outbreaks through signals such as testing volume and surveillance of wastewater data remains important [48, 49]. Estimates of effective protection against infection and severe disease in the population presented in this study provide valuable insight into how to assess local (counties and states) risks in the United States.
In conclusion, as of 1 December 12021, the fraction of the US population that had ever been infected with SARS-CoV-2 and/or received ≥1 dose of a COVID-19 vaccine varied between counties and states. Accounting for waning of population immunity, effective protection against infection by pre-Omicron variants in US states was 27.6%–40.4% lower than the percentage immunologically exposed. Introduction and takeover of the Omicron variant reduced effective protection against infection by another 26.2%–37.0% across US states.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author Contributions. T. C., J. A. S., and N. A. M. and conceived and supervised the project. T. C., J. A. S., and N. A. M. acquired funding. F. K. wrote the model code, drafted the original manuscript, and visualized the results. F. K. and M. R. curated the data and executed the analysis. All authors contributed to the development of the methods and reviewed and edited the original manuscript.
Disclaimer. The findings, conclusions, and views expressed are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC), Council of State and Territorial Epidemiologists, or the National Institutes of Health. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Financial support. This work was supported by the Centers for Disease Control and Prevention (contract 200-2016-91779; grant 75D30121F0003 to N. A. M.), including through the Council of State and Territorial Epidemiologists (support to F. K. and N. A. S.; grants NU38OT000297-03 to T. C. and N. A. M. and NU38OT000297-02 to J. A. S.); the National Institute of Allergy and Infectious Diseases (support to F. K. and grants R01 AI112438 to T. C., R01 AI137093 to V. E. P., and R01 AI146555-01A1 to N. A. M.); and the National Institute on Drug Abuse (grant 3R37DA01561217S1 to J. A. S.).
Supplementary Material
Contributor Information
Fayette Klaassen, Department of Global Health and Population, Harvard T.H. Chan School of Public Health, Boston, Massachusetts, USA.
Melanie H Chitwood, Department of Epidemiology of Microbial Diseases and Public Health Modeling Unit, Yale School of Public Health, New Haven, Connecticut, USA.
Ted Cohen, Department of Epidemiology of Microbial Diseases and Public Health Modeling Unit, Yale School of Public Health, New Haven, Connecticut, USA.
Virginia E Pitzer, Department of Epidemiology of Microbial Diseases and Public Health Modeling Unit, Yale School of Public Health, New Haven, Connecticut, USA.
Marcus Russi, Department of Epidemiology of Microbial Diseases and Public Health Modeling Unit, Yale School of Public Health, New Haven, Connecticut, USA.
Nicole A Swartwood, Department of Global Health and Population, Harvard T.H. Chan School of Public Health, Boston, Massachusetts, USA.
Joshua A Salomon, Department of Health Policy, Stanford University School of Medicine, Stanford, California, USA.
Nicolas A Menzies, Department of Global Health and Population, Harvard T.H. Chan School of Public Health, Boston, Massachusetts, USA.
References
- 1. The New York Times . Coronavirus in the U.S.: latest map and case count. 2021. Available at: https://www.nytimes.com/interactive/2021/us/covid-cases.html. Accessed 1 December 2021.
- 2. Washington Post . U.S. coronavirus cases and state maps: tracking cases, deaths. 2021. Available at: https://www.washingtonpost.com/graphics/2020/national/coronavirus-us-cases-deaths/?itid=hp_pandemic%20test. Accessed 1 December 2021.
- 3. The COVID Tracking Project . 2021. Available at: https://covidtracking.com/. Accessed 29 October 2021.
- 4. Pulliam JRC, van Schalkwyk C, Govender N, et al. Increased risk of SARS-CoV-2 reinfection associated with emergence of the Omicron variant in South Africa. medRxiv [Preprint]. March 06, 2022 [accessed 11 March 2022]. Available from: 10.1101/2021.11.11.21266068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Torjesen I. Covid-19: Omicron may be more transmissible than other variants and partly resistant to existing vaccines, scientists fear. BMJ 2021; 375:n2943. doi: 10.1136/bmj.n2943. [DOI] [PubMed] [Google Scholar]
- 6. Chitwood MH, Russi M, Gunasekera K, et al. Reconstructing the course of the COVID-19 epidemic over 2020 for US states and counties: results of a Bayesian evidence synthesis model. medRxiv [Preprint]. 22 July 2021 [accessed 23 February 2022]. Available from: 10.1101/2020.06.17.20133983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sen P, Yamana TK, Kandula S, et al. Burden and characteristics of COVID-19 in the United States during 2020. Nature 2021; 598:338–41. doi: 10.1038/s41586-021-03914-4. [DOI] [PubMed] [Google Scholar]
- 8. Irons NJ, Raftery AE. Estimating SARS-CoV-2 infections from deaths, confirmed cases, tests, and random surveys. Proc Natl Acad Sci USA 2021; 118:e2103272118. doi: 10.1073/pnas.2103272118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. covidestim: COVID-19 nowcasting. 2021. Available at: https://covidestim.org/. Accessed 29 October 2021.
- 10. Centers for Disease Control and Prevention . COVID data tracker. Antibody seroprevalence: nationwide commercial lab seroprevalence. 2020. Available at: https://covid.cdc.gov/covid-data-tracker/#national-lab. Accessed 23 February 2022.
- 11. Centers for Disease Control and Prevention . COVID data tracker. Antibody seroprevalence: nationwide blood donor seroprevalence. 2020. Available at: https://covid.cdc.gov/covid-data-tracker/#nationwide-blood-donor-seroprevalence. Accessed 23 February 2022.
- 12. Anand SP, Prévost J, Nayrac M, et al. Longitudinal analysis of humoral immunity against SARS-CoV-2 spike in convalescent individuals up to 8 months post-symptom onset. Cell Rep Med 2021; 2:100290. doi: 10.1016/j.xcrm.2021.100290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med 2021; 27:1205–211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 14. Wheatley AK, Juno JA, Wang JJ, et al. Evolution of immune responses to SARS-CoV-2 in mild-moderate COVID-19. Nat Commun 2021; 12:162. doi: 10.1038/s41467-021-21444-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 2020; 384:403–16. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020; 383:2603–15. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sadoff J, Gray G, Vandebosch A, et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. N Engl J Med 2021; 384:2187–201. doi: 10.1056/NEJMoa2101544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Harder T, Koch J, Vygen-Bonnet S, et al. Efficacy and effectiveness of COVID-19 vaccines against SARS-CoV-2 infection: interim results of a living systematic review, 1 January to 14 May 2021. Euro Surveill 2021; 26:2100563. doi: 10.2807/1560-7917.es.2021.26.28.2100563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shapiro J, Dean NE, Madewell ZJ, et al. Efficacy estimates for various COVID-19 vaccines: what we know from the literature and reports. medRxiv [Preprint]. 18 June 2021. [accessed 22 October 2021] Available from: 10.1101/2021.05.20.21257461. [DOI] [Google Scholar]
- 20. Thomas SJ, Moreira ED, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine through 6 months. N Engl J Med 2021; 385:1761–73. doi: 10.1056/nejmoa2110345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Widge AT, Rouphael NG, Jackson LA, et al. Durability of responses after SARS-CoV-2 mRNA-1273 vaccination. N Engl J Med 2021; 384:80–2. doi: 10.1056/nejmc2032195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Andrews N, Stowe J, Kirsebom F, et al. Effectiveness of COVID;19, vaccines against the Omicron (B.1.1.529) variant of concern. medRxiv [Preprint]. 14 December 2021 [accessed 23 February 2021]. Available from: 10.1101/2021.12.14.21267615. [DOI] [Google Scholar]
- 23. Gruell H, Vanshylla K, Tober-Lau P, et al. mRNA booster immunization elicits potent neutralizing serum activity against the SARS-CoV-2 Omicron variant. Nat Med 2022; 28:477–80. doi: 10.1038/s41591-021-01676-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hall V, Foulkes S, Insalata F, et al. Effectiveness and durability of protection against future SARS-CoV-2 infection conferred by COVID-19 vaccination and previous infection; findings from the UK SIREN prospective cohort study of healthcare workers March 2020 to September 2021. medRxiv [Preprint]. 1 December 2021 [accessed 23 February 2022]. Available from: 10.1101/2021.11.29.21267006. [DOI]
- 25. Jones JM, Stone M, Sulaeman H, et al. Estimated US infection- and vaccine-induced SARS-CoV-2 seroprevalence based on blood donations, July 2020-May 2021. JAMA 2021; 326:1400–9. doi: 10.1001/jama.2021.15161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Centers for Disease Control and Prevention . COVID data tracker. 2020. Available at: https://covid.cdc.gov/covid-data-tracker. Accessed 22 October 2021.
- 27. Household Pulse Survey. US Census Bureau . 2021. Available at: https://www.census.gov/data/experimental-data-products/household-pulse-survey.html. Accessed 29 October 2021.
- 28. Center for the Ecology of Infectious Diseases, University of Georgia . COVID-19 portal. Available at: https://www.covid19.uga.edu/nowcast.html. Accessed 29 October 2021.
- 29. Tiu A, Susswein Z, Merritt A, et al. Characterizing the spatiotemporal heterogeneity of the COVID-19 vaccination landscape. medRxiv [Preprint]. 17 December 2021 [accessed 12 February 2022]. Available from: 10.1101/2021.10.04.21263345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Merritt A, Tiu A, Bansal S. Integrated US COVID-19 vaccination data. Harvard Dataverse. Version 1. 2021. Available at: 10.7910/DVN/BFRIKI. Accessed 11 March 2022 . [DOI]
- 31. Sax C, Steiner P. Temporal disaggregation of time series. The R Journal 2013; 5:80–7. doi: 10.32614/rj-2013-028. [DOI] [Google Scholar]
- 32. Bradley VC, Kuriwaki S, Isakov M, et al. Unrepresentative big surveys significantly overestimated US vaccine uptake. Nature 2021; 600:695–700. doi: 10.1038/s41586-021-04198-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gazit S, Shlezinger R, Perez G, et al. Comparing SARS-CoV-2 natural immunity to vaccine-induced immunity: reinfections versus breakthrough infections. medRxiv [Preprint]. 25 August 2021 [accessed 23 February 2022] Available from: 10.1101/2021.08.24.21262415. [DOI] [Google Scholar]
- 34. Goldberg Y, Mandel M, Bar-On YM, et al. Protection and waning of natural and hybrid COVID-19 immunity. medRxiv [Preprint]. 5 December 2021 [accessed 23 February 2022]. Available from: 10.1101/2021.12.04.21267114. [DOI] [Google Scholar]
- 35. Goldberg Y, Mandel M, Bar-On YM, et al. Waning immunity after the BNT162b2 vaccine in Israel. N Engl J Med 2021; 385:e85. doi: 10.1056/NEJMoa2114228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Crawford KHD, Dingens AS, Eguia R, et al. Dynamics of neutralizing antibody titers in the months after severe acute respiratory syndrome coronavirus 2 infection. J Infect Dis 2020; 223:197–205. doi: 10.1093/infdis/jiaa618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bar-On YM, Goldberg Y, Mandel M, et al. Protection against Covid-19 by BNT162b2 booster across age groups. N Engl J Med 2021; 385:2421–30. doi: 10.1056/NEJMoa2115926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dejnirattisai W, Shaw RH, Supasa P, et al. Reduced neutralisation of SARS-COV;2, Omicron-B.1.1.529 variant by post-immunisation serum. medRxiv [Preprint]. 11 December 2021 [accessed 23 February 2022]. Available from: 10.1101/2021.12.10.21267534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cele S, Jackson L, Khoury DS, et al. SARS-CoV-2 Omicron has extensive but incomplete escape of Pfizer BNT162b2 elicited neutralization and requires ACE2 for infection. medRxiv [Preprint]. 17 December 2021 [accessed 23 February 2022]. Available from: 10.1101/2021.12.08.21267417. [DOI] [Google Scholar]
- 40. R Core Team . R: A language and environment for statistical computing [computer program] Vienna, Austria: R Foundation for Statistical Computing; 2021. https://www.R-project.org/. Version 4.1.0. [Google Scholar]
- 41.Stan Development Team. RStan: the R interface to Stan [computer program] 2022. Version R package version 2.21.22020. http:/mc-stan.org/.
- 42. Ipsos . Axios-Ipsos Coronavirus Index, waves 34-48. Ann Arbor, MI: Inter-university Consortium for Political and Social Research [distributor], June 12 2021 [accessed December 1, 2021] . 10.3886/E144903V1 [DOI]
- 43. Bajema KL, Wiegand RE, Cuffe K, et al. Estimated SARS-CoV-2 seroprevalence in the US as of September 2020. JAMA Intern Med 2021; 181:450–60. doi: 10.1001/jamainternmed.2020.7976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Smith BK, Janowski AB, Danis JE, et al. Seroprevalence of SARS-CoV-2 antibodies in children and adults in St. Louis, Missouri, USA. mSphere 2021; 6:e01207–20. doi: 10.1128/mSphere.01207-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Smith BK, Janowski AB, Fremont AC, et al. Progression of SARS-CoV-2 seroprevalence in St. Louis. Missouri, through January 2021. mSphere 2021; 6:e0045021. doi: 10.1128/mSphere.00450-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Brotons P, Launes C, Buetas E, et al. Susceptibility to severe acute respiratory syndrome Coronavirus 2 infection among children and adults: a seroprevalence study of family households in the Barcelona metropolitan region, Spain. Clin Infect Dis 2021; 72:e970–7. doi: 10.1093/cid/ciaa1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Center for Disease Control and Prevention . Science brief: indicators for monitoring COVID-19 community levels and making public health recommendations. 2022. Available at: https://www.cdc.gov/coronavirus/2019-ncov/science/science-briefs/indicators-monitoring-community-levels.html. Accessed 29 April 2022. [PubMed]
- 48. Emanuel EJ, Osterholm M, Gounder CR. A national strategy for the “new normal” of life with COVID. JAMA 2022; 327:211–2. doi: 10.1001/jama.2021.24282. [DOI] [PubMed] [Google Scholar]
- 49. Michaels D, Emanuel EJ, Bright RA. A national strategy for COVID-19: testing, surveillance, and mitigation strategies. JAMA 2022; 327:213–4. doi: 10.1001/jama.2021.24168. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.