Abstract
Background
Patient-reported outcomes of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection are an important measure of the full burden of coronavirus disease (COVID). Here, we examine how (1) infecting genotype and COVID-19 vaccination correlate with inFLUenza Patient-Reported Outcome (FLU-PRO) Plus score, including by symptom domains, and (2) FLU-PRO Plus scores predict return to usual activities and health.
Methods
The epidemiology, immunology, and clinical characteristics of pandemic infectious diseases (EPICC) study was implemented to describe the short- and long-term consequences of SARS-CoV-2 infection in a longitudinal, observational cohort. Multivariable linear regression models were run with FLU-PRO Plus scores as the outcome variable, and multivariable Cox proportional hazards models evaluated effects of FLU-PRO Plus scores on return to usual health or activities.
Results
Among the 764 participants included in this analysis, 63% were 18–44 years old, 40% were female, and 51% were White. Being fully vaccinated was associated with lower total scores (β = −0.39; 95% CI, −0.57 to −0.21). The Delta variant was associated with higher total scores (β = 0.25; 95% CI, 0.05 to 0.45). Participants with higher FLU-PRO Plus scores were less likely to report returning to usual health and activities (health: hazard ratio [HR], 0.46; 95% CI, 0.37 to 0.57; activities: HR, 0.56; 95% CI, 0.47 to 0.67). Fully vaccinated participants were more likely to report returning to usual activities (HR, 1.24; 95% CI, 1.04 to 1.48).
Conclusions
Full SARS-CoV-2 vaccination is associated with decreased severity of patient-reported symptoms across multiple domains, which in turn is likely to be associated with earlier return to usual activities. In addition, infection with the Delta variant was associated with higher FLU-PRO Plus scores than previous variants, even after controlling for vaccination status.
Keywords: COVID-19, SARS-CoV-2, symptoms, patient-reported outcomes, vaccine breakthrough
The ongoing severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic continues to cause significant morbidity as measured by a variety of metrics, including hospitalization [1]. However, the likelihood of hospitalization can vary for reasons not directly related to disease severity including access to care. In addition, the binary outcome of outpatient/inpatient does not capture the full burden of coronavirus disease 2019 (COVID-19) illness, particularly for those who are not hospitalized but nonetheless may experience significant impact on daily activities or work. Patient-reported outcomes (PROs) give insights into the experience of patients relative to their baseline health and can be used along with indirect health measurements to characterize the full spectrum of COVID-19 morbidity in a variety of studies. Historically, the use of symptom data in such analyses has been challenging due to a lack of a standardized and comprehensive quantitative scale.
The inFLUenza Patient-Reported Outcome (FLU-PRO) instrument was originally developed to assess patient-reported outcomes with respect to influenza-like illness [2, 3] but has been systematically evaluated for use with a range of viral respiratory infections [4, 5]. More recently, we validated the FLU-PRO Plus (FLU-PRO plus a senses domain) for the evaluation of SARS-CoV-2 [5]. FLU-PRO Plus was designed to assess respiratory symptom intensity, frequency, and duration and provides insights into the phenotype of COVID-19. In addition to symptom severity across multiple domains (eg, respiratory, systemic, nose, etc.), the survey includes questions about whether the participant has returned to usual health or activities, which can be used to determine time to recovery.
SARS-CoV-2 vaccination is associated with reduced likelihood of death, hospitalization, and visits to ambulatory care [6–9]. However, severity of symptoms can vary in the outpatient setting, and few studies have evaluated how COVID-19 vaccines reduce the occurrence and severity of specific symptoms. Currently published studies use limited symptom measurements (eg, days of symptoms, fever) that do not characterize the full patient-reported phenotype of COVID-19 [6]. Moreover, vaccine effectiveness studies have not consistently evaluated how vaccination improves the probability of faster return to baseline health and activities. Such outcomes were not measured in the pivotal phase III COVID-19 vaccine trials that led to the authorization or licensure of these products [10].
Here we fully characterized symptomology reported in SARS-CoV-2-infected US Military Health System (MHS) beneficiaries by FLU-PRO Plus domain scores. We extend our previous validation of the measurement properties of this tool and the presenting symptoms of patients with COVID-19 to examine how FLU-PRO Plus scores predict time to return to usual health and activities and identify other predictors of return to usual health and activities. We also examine how prior vaccination may impact FLU-PRO Plus symptom scores (overall and by domain) and return to prior health and activity, adjusting for demographics, comorbidities, and infecting genotype.
METHODS
Population, Setting, and Study Design
The epidemiology, immunology, and clinical characteristics of pandemic infectious diseases (EPICC) study was implemented across 10 military treatment facilities (MTFs) in the United States (Brooke Army Medical Center, Carl R. Darnall Army Medical Center, Fort Belvoir Community Hospital, Madigan Army Medical Center, Naval Medical Center Portsmouth, Naval Medical Center San Diego, Tripler Army Medical Center, William Beaumont Army Medical Center, Walter Reed National Military Medical Center, and Womack Army Medical Center) in order to explore the risk factors for and characteristics of SARS-CoV-2 infection in an observational, longitudinal cohort [11, 12]. Participants at the MTFs were enrolled based on (a) confirmed infection with SARS-CoV-2, (b) meeting the criteria for SARS-CoV-2 testing per CDC guidelines, (c) exposure to someone with confirmed SARS-CoV-2 infection, and (d) being vaccinated against SARS-CoV-2. Demographic and clinical information were collected at baseline, and swabs and blood specimens were collected at different time points (Supplementary Table 1).
Consent and Approval
Participants provided informed consent when they were enrolled into EPICC. The study was implemented according to the Declaration of Helsinki and good clinical practice guidelines. The Uniformed Services University Institutional Review Board (IDCRP-085) approved this study.
FLU-PRO Plus Measurement
FLU-PRO Plus asks participants to rate the intensity and/or frequency of 34 symptoms in the past 24 hours on a 5-point scale from “not at all” to “very much” for most symptoms (“never” to “always” in the case of sneezing and coughing, and number of times for vomiting and diarrhea). In addition, the FLU-PRO Plus collects patient global assessment (PGA) information, for example, the overall severity of their symptoms and whether the participant has returned to their usual activities or health, among other questions. EPICC participants were asked to fill out the FLU-PRO Plus every day for 14 days after enrollment in the study. Total scores are derived by calculating the mean score of the symptoms for each day in each of the 7 symptom domains (throat, nose, eyes, gastrointestinal, respiratory, systemic, senses). Participants enrolled before the addition of the senses domain in May 2020 did not answer the questions about loss of taste or smell; therefore, we have calculated a total score with and without the sense domain. Maximum FLU-PRO Plus scores were used in the models considering factors associated with overall severity, whereas baseline FLU-PRO Plus scores were used in the analyses looking at whether the participant reported returning to usual health or activities.
Diagnosis of SARS-CoV-2 Cases and Determination of Infecting Genotype
Swabs were processed using quantitative polymerase chain reaction (qPCR), which utilized the SARS-CoV-2 (2019-CoV) CDC qPCR Probe Assay research use only kits (Cat. # 10006770), consistent with the Emergency Use Authorization (EUA) issued on December 1, 2020, and manufactured by Integrated DNA Technologies, Inc. (Coralville, IA, USA). Two regions of the nucleocapsid (N) gene were targeted by the assay, with an additional primer/probe set to detect the RNase P (RP) gene. Clinical samples were tested using various PCR assays available at participating MTFs. Participants were identified to be SARS-CoV-2 positive based on a PCR-positive test within 21 days post–symptom onset, using swabs collected for this study or the original clinical PCR assays.
The SARS-CoV-2-infecting genotype was determined by whole-genome sequencing using an amplicon tiling strategy on viral RNA extracted from swabs [13]. Amplified product for sequencing was prepared with NexteraXT library kits (Illumina Inc., San Diego, CA, USA). Libraries were run on the Illumina NextSeq 550 or NovaSeq 6000 platform, and genome assembly was achieved using BBMap, version 38.86, and iVar, version 1.2.2. Genotype classification was performed using the Pangolin classification tools [14].
Determination of Vaccine History and Vaccine Breakthrough Status
Vaccine breakthroughs were identified using vaccine dates collected using surveys filled in by the participants, as well as using data collected from the medical record at the site and the centralized military health system data repository (MDR). Vaccine breakthroughs were identified as SARS-CoV-2-positive people who reported COVID-19 symptoms that began 14 or more days after their final vaccine dose, not including booster doses. Participants were considered partially vaccinated if they received 1 dose of the 2-dose mRNA vaccine series or if their symptoms began <14 days after their final dose of vaccine.
Statistical Analysis
Analyses included SARS-CoV-2-infected adults who reported symptoms on at least 1 FLU-PRO Plus survey within 2 weeks of symptom onset and provided complete demographic information (age, sex, and race). Differences in FLU-PRO Plus scores between vaccine breakthrough, partially vaccinated, and unvaccinated participants were compared using Kruskal-Wallis rank-sum tests, and demographic characteristics were compared using Pearson’s chi-square tests. Mean total and domain scores were calculated and plotted by days postenrollment.
We identified factors associated with the maximum FLU-PRO Plus total and domain scores using linear regression. Models were run with and without the Delta variant variable, as information about infecting variant was only available for a subset of participants. An interaction term between the Delta variant variable and vaccine breakthrough variable was included in the model. Cox proportional hazards models that included sex, age group (18–44, 45–64, and 65+ years), race, vaccination status, and all the domains used reported return to usual health (or activities) as the outcome. Similar models evaluated total FLU-PRO Plus score instead of the FLU-PRO Plus domain scores. Partially vaccinated participants were dropped from the Cox proportional hazards model due to small numbers. Finally, total and domain scores at baseline were dichotomized, <1 and 1+, based on prior FLU-PRO findings [15] and exploratory analysis. We then evaluated whether these groups (ie, <1 and 1+) differ in returning to usual health using Kaplan-Meier survival analysis. Survival curves were generated for each of the domain scores, as well as the total, at baseline, with time to return to usual activities and health as the outcomes. Participants who did not report returning to activities or health during the follow-up period were censored at the time of their final survey. All statistical analyses were performed in R, version 4.0.4 [16].
RESULTS
EPICC enrolled 2079 participants at the MTFs between March 20, 2020, and December 15, 2021, among whom 764 SARS-CoV-2-positive participants with complete demographic information who had at least 1 FLU-PRO Plus survey collected within 2 weeks of symptom onset were included in this analysis (Supplementary Figure 1). On average, participants filled out FLU-PRO Plus surveys for 10 days. Sixty-three percent of the included participants were young adults (18–44 years old), and 60% were male (Table 1). Approximately half reported being non-Hispanic White; Hispanic/Latino was reported by 26.6%, and 12.2% were Black. The highest average maximum domain scores were noted in the senses domain (1.6), followed by the nose domain (1.2), systemic domain (1.1), and respiratory domain (1.1).
Table 1.
Description of SARS-CoV-2 (+) EPICC Participants Included in FLU-PRO Plus Analysis, by Vaccination Status
| Total (n = 764) |
Unvaccinated (n = 587) |
Partially Vaccinated (n = 25) |
Fully Vaccinated (n = 152) |
P Value | |
|---|---|---|---|---|---|
| Age, No. (%) | .74a | ||||
| 18–44 y | 480 (62.8) | 365 (62.2) | 18 (72.0) | 97 (63.8) | |
| 45–64 y | 219 (28.7) | 174 (29.6) | 5 (20.0) | 40 (26.3) | |
| 65+ y | 65 (8.5) | 48 (8.2) | 2 (8.0) | 15 (9.9) | |
| Sex, No. (%) | .29a | ||||
| Male | 460 (60.2) | 345 (58.8) | 15 (60.0) | 100 (65.8) | |
| Female | 304 (39.8) | 242 (41.2) | 10 (40.0) | 52 (34.2) | |
| Race, No. (%) | .01a | ||||
| White | 386 (50.5) | 276 (47.0) | 12 (48.0) | 98 (64.5) | |
| Hispanic or Latino | 203 (26.6) | 170 (29.0) | 8 (32.0) | 25 (16.4) | |
| Black | 93 (12.2) | 75 (12.8) | 1 (4.0) | 17 (11.2) | |
| Asian | 34 (4.5) | 29 (4.9) | 1 (4.0) | 4 (2.6) | |
| Other | 48 (6.3) | 37 (6.3) | 3 (12.0) | 8 (5.3) | |
| Military status, No. (%) | .13a | ||||
| Active duty | 400 (52.4) | 296 (50.4) | 17 (68.0) | 87 (57.2) | |
| Dependent | 200 (26.2) | 163 (27.8) | 6 (24.0) | 31 (20.4) | |
| Retired military | 164 (21.5) | 128 (21.8) | 2 (8.0) | 34 (22.4) | |
| Delta variant (among those with variant information), No. (%) | 115 (23.1) | 18 (5.0) | 4 (26.7) | 93 (76.2) | <.01a |
| Missing variant information, No. | 267 | 227 | 10 | 30 | |
| Days since symptom onset first FLU-PRO was completed | .31b | ||||
| Mean (SD) | 8.6 (2.9) | 8.7 (2.8) | 7.8 (3.3) | 8.4 (3.1) | |
| Days FLU-PRO was completed | .01b | ||||
| Mean (SD) | 10.2 (3.6) | 10.0 (3.7) | 10.2 (3.8) | 10.9 (3.3) | |
| Poorest physical health reported on FLU-PRO, No. (%) | <.01a | ||||
| Poor | 136 (17.8) | 121 (20.6) | 1 (4.0) | 14 (9.2) | |
| Fair | 318 (41.6) | 245 (41.7) | 13 (52.0) | 60 (39.5) | |
| Good | 211 (27.6) | 159 (27.1) | 6 (24.0) | 46 (30.3) | |
| Very good | 76 (9.9) | 49 (8.3) | 4 (16.0) | 23 (15.1) | |
| Excellent | 23 (3.0) | 13 (2.2) | 1 (4.0) | 9 (5.9) | |
| Returned to activities by last FLU-PRO Plus survey, No. (%) | 564 (73.8) | 412 (70.2) | 22 (88.0) | 130 (85.5) | <.01a |
| Returned to health by last FLU-PRO Plus survey, No. (%) | 489 (64.0) | 352 (60.0) | 21 (84.0) | 116 (76.3) | <.01a |
| Maximum scores, mean (SD) | |||||
| Total score (no senses) | 0.9 (0.6) | 0.9 (0.6) | 0.7 (0.6) | 0.7 (0.6) | <.01b |
| Total score (including senses) | 0.9 (0.6) | 0.9 (0.6) | 0.8 (0.7) | 0.7 (0.6) | <.01b |
| Throat score | 0.7 (0.9) | 0.8 (0.9) | 0.8 (1.0) | 0.6 (0.8) | .04b |
| Eyes score | 0.6 (0.8) | 0.6 (0.8) | 0.7 (0.9) | 0.5 (0.8) | .01b |
| Nose score | 1.2 (0.9) | 1.2 (0.8) | 1.1 (0.8) | 1.3 (0.9) | .45b |
| Systemic score | 1.1 (0.9) | 1.2 (0.9) | 0.8 (1.0) | 0.8 (0.8) | <.01b |
| Gastrointestinal score | 0.7 (0.7) | 0.7 (0.7) | 0.5 (0.6) | 0.4 (0.5) | <0.01b |
| Respiratory score | 1.1 (0.8) | 1.2 (0.8) | 1.0 (0.8) | 0.9 (0.8) | <0.01b |
| Senses score | 1.6 (1.6) | 1.7 (1.6) | 1.7 (1.6) | 1.4 (1.6) | .05b |
Abbreviations: EPICC, epidemiology, immunology, and clinical characteristics of pandemic infectious diseases study; FLU-PRO Plus, inFLUenza Patient-Reported Outcome Plus; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Pearson’s chi-square test.
Kruskal-Wallis rank-sum test.
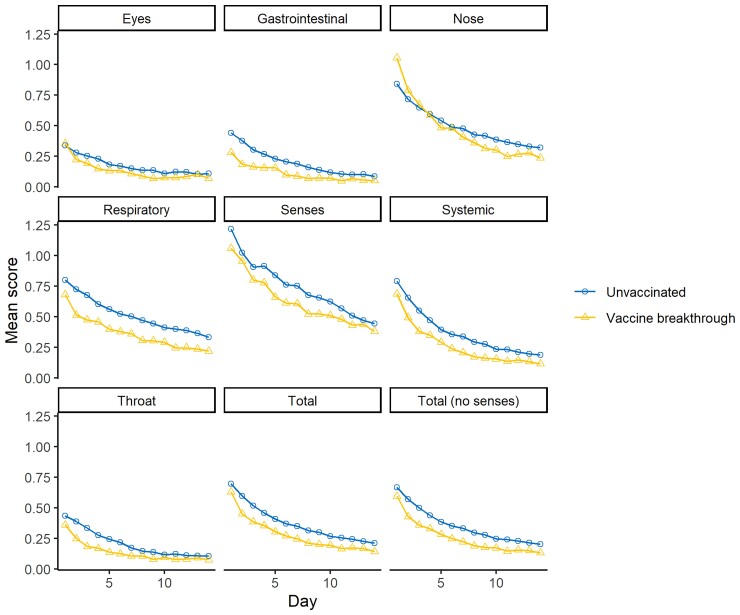
When evaluating the effect of vaccination status on symptoms, 70% of the unvaccinated participants reported returning to usual activities, and 60% reported returning to usual health by the end of their FLU-PRO Plus follow-up; a greater percentage of the participants who were fully or partially vaccinated reported returning to usual activities (85.5% and 88.0%, respectively) and health (76.3% and 84.0%, respectively). Unvaccinated participants reported higher maximum total scores, as well as higher maximum systemic, gastrointestinal, and respiratory scores than participants who had been fully vaccinated. Figure 1 depicts the trends in the daily scores reported by participants (see Supplementary Figure 2 for symptoms by days post–symptom onset).
Figure 1.
Mean FLU-PRO Plus domain and total scores by time since enrollment and vaccine breakthrough status (partially vaccinated participants not shown). Abbreviation: FLU-PRO Plus, inFLUenza Patient-Reported Outcome Plus.
The linear regression model results demonstrate that men reported lower FLU-PRO Plus scores than women, and scores tended to decrease with time (Table 2; Supplementary Table 2). Full vaccination status was statistically significantly associated with lower total scores, as well as lower throat, eyes, systemic, gastrointestinal, and respiratory domain scores (Table 2; Supplementary Table 3). Infection with the Delta variant was associated with higher total, throat, eyes, systemic, and respiratory domain scores. Among those infected with Delta, those who had been vaccinated had lower total scores than those who were unvaccinated (difference in total score, −0.47; 95% CI, −0.76 to −0.18; P = .006), although the interaction term between the Delta variant and vaccination status was not significant (Supplementary Tables 4 and 5).
Table 2.
Linear Regression Model Output Considering Maximum Reported FLU-PRO Plus Total and Domain Scores as the Outcome Variables (n = 497 With Genotype Information)
| Statistical Models | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | Total (No Senses) | Nose | Throat | Eyes | Systemic | Gastrointestinal | Respiratory | Senses | |
| Age group 18–44 y | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| 45–64 y | 0.05 (0.06) | 0.11 (0.06) | −0.11 (0.08) | 0.03 (0.09) | 0.11 (0.08) | 0.15 (0.08) | 0.13 (0.06)* | 0.20 (0.08)* | −0.59 (0.15)*** |
| 65+ y | −0.18 (0.10) | −0.06 (0.10) | −0.17 (0.13) | −0.11 (0.14) | −0.08 (0.13) | −0.12 (0.13) | 0.11 (0.10) | 0.11 (0.12) | −1.14 (0.24)*** |
| Male | −0.18 (0.06)** | −0.18 (0.06)** | −0.27 (0.08)*** | −0.12 (0.08) | −0.15 (0.08) | −0.26 (0.08)*** | −0.30 (0.06)*** | −0.14 (0.07) | −0.48 (0.14)*** |
| Days since onset of symptoms | −0.03 (0.01)** | −0.04 (0.01)*** | −0.07 (0.01)*** | −0.05 (0.01)*** | −0.03 (0.01)* | −0.07 (0.01)*** | −0.01 (0.01) | −0.01 (0.01) | −0.06 (0.02)* |
| Vaccination status Unvaccinated | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Fully vaccinated | −0.39 (0.09)*** | −0.42 (0.09)*** | −0.12 (0.13) | −0.50 (0.13)*** | −0.41 (0.13)** | −0.56 (0.13)*** | −0.39 (0.10)*** | −0.52 (0.12)*** | −0.32 (0.23) |
| Partially vaccinated | −0.11 (0.16) | −0.12 (0.16) | −0.30 (0.22) | 0.28 (0.23) | 0.27 (0.22) | −0.19 (0.23) | −0.27 (0.17) | −0.12 (0.21) | −0.16 (0.39) |
| Delta variant | 0.25 (0.10)** | 0.27 (0.10)** | 0.16 (0.13) | 0.41 (0.14)** | 0.27 (0.13)* | 0.34 (0.13)* | 0.13 (0.10) | 0.32 (0.13)* | 0.05 (0.23) |
Abbreviation: FLU-PRO Plus, inFLUenza Patient-Reported Outcome Plus.
***P < .001; **P < .01; *P < .05.
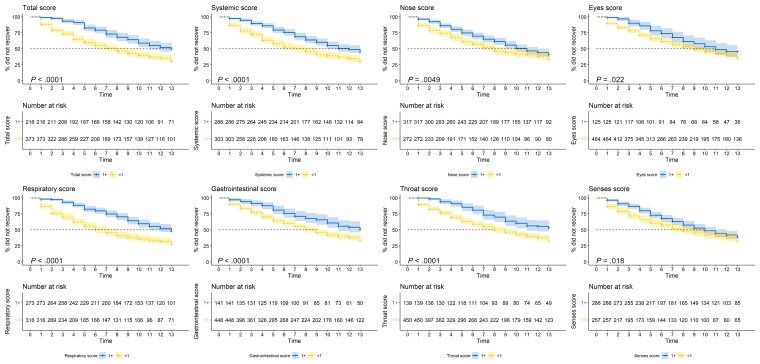
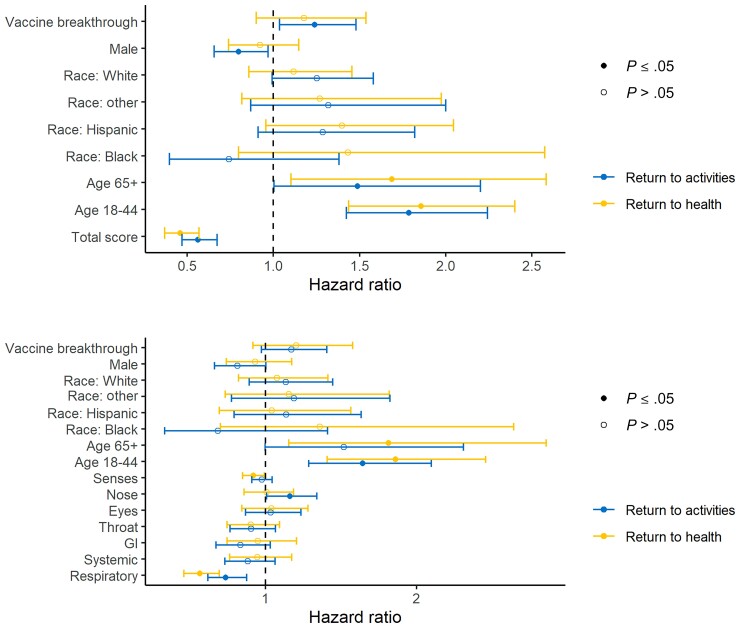
Kaplan-Meier curves indicate longer time to return to usual health and activities among those with higher total FLU-PRO Plus scores, as well as domain-specific FLU-PRO Plus scores (Figure 2; Supplementary Figure 3). To estimate the probability and predictors of returning to usual activities or health after COVID-19, we fit Cox proportional hazards models that included age group, race, sex, vaccination status, and FLU-PRO Plus scores (Figure 3) as predictors. For every unit increase in total FLU-PRO Plus score, participants were 54% less likely to return to usual health and 44% less likely to return to activities. Participants who had been fully vaccinated were more likely to return to usual activities and health during the 14-day survey period than participants who had not been fully vaccinated, although this was only statistically significant for activities (from the model with total FLU-PRO Plus scores: return to activities: hazard ratio [HR], 1.24; 95% CI, 1.04 to 1.48; return to health: HR 1.17; 95% CI, 0.9 to 1.54). Participants who were 18–44 years of age were more likely to report returning to usual health and activities than those who were 45–64 years of age (return to activities: HR, 1.79; 95% CI, 1.42 to 2.24; return to health: HR, 1.86; 95% CI, 1.44 to 2.40). Men were less likely to report returning to their daily activities than women (HR, 0.80; 95% CI, 0.66 to 0.97). Finally, we examined whether symptom intensity by specific FLU-PRO Plus domains was associated with return to usual activities or health, controlling for the other domains. Participants with a 1-unit increase in the respiratory domain score were 44% and 27% less likely to report returning to usual health and activities, respectively, during the survey period. In addition, those who had higher nose symptom scores were more likely to return to usual activities (HR, 1.16; 95% CI, 1.01 to 1.34).
Figure 2.
Time to return to usual health using Kaplan-Meier survival curves among EPICC participants with SARS-CoV-2 who did not report returning to usual health at day 1 on the FLU-PRO Plus survey. Participants were split into 2 groups according to whether their baseline FLU-PRO score was 1+ or <1. P values presented were calculated using a log-rank test. Abbreviations: EPICC, epidemiology, immunology, and clinical characteristics of pandemic infectious diseases study; FLU-PRO Plus, inFLUenza Patient-Reported Outcome Plus; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Figure 3.
Cox proportional hazard model results of return to usual activities or health as a function of total FLU-PRO Plus scores (top figure) or domain-specific FLU-PRO Plus scores (bottom figure). Partially vaccinated participants (n = 25) were dropped from the data set for this analysis. Abbreviations: FLU-PRO Plus, inFLUenza Patient-Reported Outcome Plus; GI, gastrointestinal.
DISCUSSION
Postvaccination SARS-CoV-2 infections have been associated with decreased risk of hospitalization or death, but there is limited knowledge on the impact of vaccinations on patient-reported outcomes evaluated using standardized and comprehensive symptom measurements. Here we show that SARS-CoV-2 vaccination is associated with decreased severity of patient-reported symptoms using a quantitative multidomain score previously validated and recommended for use in COVID-19 [5, 17]. These findings are consistent with other studies that have shown a reduced duration of symptoms and a reduced frequency of febrile symptoms in COVID-19 vaccine breakthrough cases [6]. However, our analysis offers a more granular characterization of the association between vaccination and symptom phenotype by prospectively evaluating symptoms in the cohort using a standardized, comprehensive, and validated measure. In addition, we showed that vaccination was associated with a quicker return to baseline function. We demonstrate that acute quantitatively scored symptoms via the FLU-PRO Plus score predicted return to prior activities, even after adjusting for variables such as age. Taken together, these findings extend our knowledge that COVID-19 vaccination mitigates illness and support the use of patient-reported outcomes as enrollment criteria and outcome measures in clinical trials [17].
We evaluated the impact on returning to usual health (or activities) during the 14-day survey period as an outcome, considering the participant’s symptom score and vaccination status as independent variables. Participants with higher symptom scores were less likely to report returning to usual health or activities during the 2-week FLU-PRO Plus follow-up, which underscores the validity of this measurement tool and potential use as an enrollment criterion in trials and as a predictor of disease course in natural history observational studies. In addition, even after controlling for symptom intensity, participants who had been fully vaccinated were more likely to report returning to usual activities during the 2-week FLU-PRO Plus follow-up. Further research is needed to explore this finding.
This study had several limitations. EPICC is a longitudinal cohort study with comprehensive data on participants’ experience of SARS-CoV-2 infection. However, many participants were enrolled beyond 14 days post–symptom onset, when symptoms may have already decreased or disappeared. Therefore, we limited the analysis to those with their first FLU-PRO Plus survey submission within 14 days of symptom onset, excluding a significant number of subjects. We controlled for time since onset of symptoms, and we performed a sensitivity analysis in those with FLU-PRO Plus collected within 60 days of symptom onset and obtained similar results (data not shown). Given that a meta-analysis has determined that 80% of individuals infected with SARS-CoV-2 have symptoms that persist beyond 14 days [18] and participants were asked to fill out 2 weeks of FLU-PRO Plus surveys, we have captured the earliest, highly symptomatic period; however, future COVID-19 studies may benefit from longer-term follow-up. Finally, we did not have variant data for all of the participants’ infections; therefore, our ability to detect differences by variant was limited. The evolution of the pandemic may also affect symptom severity; teasing apart the differences in host response, variant evolution, and interactions among such factors over time is an ongoing challenge.
When comparing adults who were included in the analysis with those who were excluded, there were some differences (Supplementary Table 6). Those who were included in this analysis were more likely to have been infected by the Delta variant and were less likely to be fully vaccinated when compared with those who were not included in this analysis. Although this does not impact the internal validity of the results, it may affect generalizability to other groups of patients. Some subgroups of participants may have complied better with study procedures or may have been enrolled earlier in their illness than others; because we control for other factors in the multivariable analyses, this should not impact the generalizability of these results. Further work is needed to enroll a wider range of participants closer to the time of onset.
CONCLUSIONS
In conclusion, the Delta variant was associated with higher symptom severity when compared with prior variants among EPICC participants after controlling for vaccination and other factors. In addition, vaccination decreased the severity of patient-reported symptoms. Such reductions in patient-reported symptoms were, in turn, likely to be associated with earlier return to usual health or activities. This research underscores the importance of SARS-CoV-2 vaccination, not only for preventing hospitalization and death, but also to decrease symptom burdens and lost work time. These findings also serve as further validation of the FLU-PRO Plus structured patient-reported outcome tool in evaluating medical countermeasures to COVID-19.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Acknowledgments
We appreciate the EPICC participants for their central role in this study. Many thanks to the IDCRP team at the clinical research sites—physician/clinical investigators, site managers, regulatory staff, clinical research coordinators, and laboratory personnel—for their support of this study and contributions to its success under very challenging circumstances.
We sincerely thank the members of the EPICC COVID-19 Cohort Study Group for their many contributions in conducting the study and ensuring effective protocol operations. The authors wish to also acknowledge all who have contributed to the EPICC COVID-19 study: Brooke Army Medical Center, Fort Sam Houston, TX: Col J. Cowden; LTC M. Darling; S. DeLeon; Maj D. Lindholm; LTC A. Markelz; K. Mende; S. Merritt; T. Merritt; LTC N. Turner; CPT T. Wellington. Carl R. Darnall Army Medical Center, Fort Hood, TX: LTC S. Bazan; P.K. Love. Fort Belvoir Community Hospital, Fort Belvoir, VA: N. Dimascio-Johnson; MAJ E. Ewers; LCDR K. Gallagher; LCDR D. Larson; A. Rutt. Henry M. Jackson Foundation, Inc., Bethesda, MD: P. Blair; J. Chenoweth; D. Clark. Madigan Army Medical Center, Joint Base Lewis McChord, WA: S. Chambers; LTC C. Colombo; R. Colombo; CAPT C. Conlon; CAPT K. Everson; COL P. Faestel; COL T. Ferguson; MAJ L. Gordon; LTC S. Grogan; CAPT S. Lis; COL C. Mount; LTC D. Musfeldt; CPT D. Odineal; LTC M. Perreault; W. Robb-McGrath; MAJ R. Sainato; C. Schofield; COL C. Skinner; M. Stein; MAJ M. Switzer; MAJ M. Timlin; MAJ S. Wood. Naval Medical Center Portsmouth, Portsmouth, VA: S. Banks; R. Carpenter; L. Kim; CAPT K. Kronmann; T. Lalani; LCDR T. Lee; LCDR A. Smith; R. Smith; R. Tant; T. Warkentien. Naval Medical Center San Diego, San Diego, CA: CDR C. Berjohn; S. Cammarata; N. Kirkland; D. Libraty; CAPT (Ret.) R. Maves; CAPT (Ret.) G. Utz. Tripler Army Medical Center, Honolulu, HI: S. Chi; LTC R. Flanagan; MAJ M. Jones; C. Lucas; LTC (Ret.) C. Madar; K. Miyasato; C. Uyehara. Uniformed Services University of the Health Sciences, Bethesda, MD: B. Agan; L. Andronescu; A. Austin; C. Broder; CAPT T. Burgess; C. Byrne; COL (Ret.) K. Chung; J. Davies; C. English; N. Epsi; C. Fox; M. Fritschlanski; A. Hadley; COL P. Hickey; E. Laing; LTC C. Lanteri; LTC J. Livezey; A. Malloy; R. Mohammed; C. Morales; P. Nwachukwu; C. Olsen; E. Parmelee; S. Pollett; S. Richard; J. Rozman; J. Rusiecki; E. Samuels; M. Sanchez; A. Scher; CDR M. Simons; A. Snow; K. Telu; D. Tribble; M. Tso; L. Ulomi; M. Wayman.
United States Air Force School of Aerospace Medicine, Dayton, OH: TSgt T. Chao; R. Chapleau; M. Christian; A. Fries; C. Harrington; V. Hogan; S. Huntsberger; K. Lanter; E. Macias; J. Meyer; S. Purves; K. Reynolds; J. Rodriguez; C. Starr. United States Coast Guard, Washington, DC: CAPT J. Iskander; CDR I. Kamara. Womack Army Medical Center, Fort Bragg, NC: B. Barton; LTC D. Hostler; LTC J. Hostler; MAJ K. Lago; C. Maldonado; J. Mehrer. William Beaumont Army Medical Center, El Paso, TX: MAJ T. Hunter; J. Mejia; J. Montes; R. Mody; R. Resendez; P. Sandoval. Walter Reed National Military Medical Center, Bethesda, MD: I. Barahona; A. Baya; A. Ganesan; MAJ N. Huprikar; B. Johnson. Walter Reed Army Institute of Research, Silver Spring, MD: S. Peel.
Disclaimer. The views expressed are those of the authors and do not reflect the official policy of the Department of the Army, Department of the Navy, Department of the Air Force, Department of Defense, US Government, or the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc. (HJF). The investigators have adhered to the policies for protection of human subjects as prescribed in 45 CFR 46.
Group authors. We thank the members of the EPICC COVID-19 Cohort Study Group for their many contributions in conducting the study and ensuring effective protocol operations. The following members were all closely involved with the design, implementation, and/or oversight of the study and have met group authorship criteria for this manuscript: Brooke Army Medical Center, Fort Sam Houston, TX: T. Merritt; CPT T. Wellington. ACESO, Henry M. Jackson Foundation, Inc., Bethesda, MD: D. Clark. Madigan Army Medical Center, Joint Base Lewis McChord, WA: S. Chambers; COL P. Faestel; COL C. Mount; LTC D. Musfeldt; C. Schofield. Naval Medical Center San Diego, San Diego, CA: N. Kirkland. Tripler Army Medical Center, Honolulu, HI: LTC (Ret.) C. Madar; C. Uyehara. Uniformed Services University of the Health Sciences, Bethesda, MD: C. Broder; C. Byrne; COL (Ret.) K. Chung; C. English; COL P. Hickey; E. Laing; LTC C. Lanteri; LTC J. Livezey; P. Nwachukwu; E. Parmelee; E. Samuels; M. Sanchez; A. Scher; M. Tso; M. Wayman. United States Air Force School of Aerospace Medicine, Dayton, OH: TSgt T. Chao; K. Lanter; J. Meyer; K. Reynolds; C. Starr. United States Coast Guard, Washington, DC: CAPT J. Iskander; CDR I. Kamara. Womack Army Medical Center, Fort Bragg, NC: LTC D. Hostler; MAJ K. Lago.
Financial support. This work was supported by awards from the Defense Health Program (HU00012020067) and the National Institute of Allergy and Infectious Disease (HU00011920111). The protocol was executed by the Infectious Disease Clinical Research Program (IDCRP), a Department of Defense (DoD) program executed by the Uniformed Services University of the Health Sciences (USUHS) through a cooperative agreement by the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc. (HJF). This project has been funded in part by the National Institute of Allergy and Infectious Diseases at the National Institutes of Health under an interagency agreement (Y1-AI-5072).
Potential conflicts of interest. S.D.P., T.H.B, and M.P.S. report that the Uniformed Services University (USU) Infectious Diseases Clinical Research Program (IDCRP), a US Department of Defense institution, and the Henry M. Jackson Foundation (HJF) were funded under a Cooperative Research and Development Agreement to conduct an unrelated phase III COVID-19 monoclonal antibody immunoprophylaxis trial sponsored by AstraZeneca. The HJF, in support of the USU IDCRP, was funded by the Department of Defense Joint Program Executive Office for Chemical, Biological, Radiological, and Nuclear Defense to augment the conduct of an unrelated phase III vaccine trial sponsored by AstraZeneca. Both of these trials were part of the US Government COVID-19 response. Neither is related to the work presented here. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Stephanie A Richard, Infectious Disease Clinical Research Program, Department of Preventive Medicine and Biostatistics, Uniformed Services University of the Health Sciences, Bethesda, Maryland, USA; The Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., Bethesda, Maryland, USA.
Nusrat J Epsi, Infectious Disease Clinical Research Program, Department of Preventive Medicine and Biostatistics, Uniformed Services University of the Health Sciences, Bethesda, Maryland, USA; The Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., Bethesda, Maryland, USA.
David A Lindholm, Brooke Army Medical Center, Fort Sam Houston, Texas, USA; Uniformed Services University of the Health Sciences, Bethesda, Maryland, USA.
Allison M W Malloy, Uniformed Services University of the Health Sciences, Bethesda, Maryland, USA.
Ryan C Maves, Infectious Disease Clinical Research Program, Department of Preventive Medicine and Biostatistics, Uniformed Services University of the Health Sciences, Bethesda, Maryland, USA; Naval Medical Center San Diego, San Diego, California, USA.
Catherine M Berjohn, Uniformed Services University of the Health Sciences, Bethesda, Maryland, USA; Naval Medical Center San Diego, San Diego, California, USA.
Tahaniyat Lalani, Infectious Disease Clinical Research Program, Department of Preventive Medicine and Biostatistics, Uniformed Services University of the Health Sciences, Bethesda, Maryland, USA; The Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., Bethesda, Maryland, USA; Naval Medical Center Portsmouth, Portsmouth, Virginia, USA.
Alfred G Smith, Naval Medical Center Portsmouth, Portsmouth, Virginia, USA.
Rupal M Mody, William Beaumont Army Medical Center, El Paso, Texas, USA.
Anuradha Ganesan, Infectious Disease Clinical Research Program, Department of Preventive Medicine and Biostatistics, Uniformed Services University of the Health Sciences, Bethesda, Maryland, USA; The Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., Bethesda, Maryland, USA; Walter Reed National Military Medical Center, Bethesda, Maryland, USA.
Nikhil Huprikar, Uniformed Services University of the Health Sciences, Bethesda, Maryland, USA; Walter Reed National Military Medical Center, Bethesda, Maryland, USA.
Rhonda E Colombo, Infectious Disease Clinical Research Program, Department of Preventive Medicine and Biostatistics, Uniformed Services University of the Health Sciences, Bethesda, Maryland, USA; The Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., Bethesda, Maryland, USA; Madigan Army Medical Center, Joint Base Lewis McChord, Washington, USA.
Christopher J Colombo, Uniformed Services University of the Health Sciences, Bethesda, Maryland, USA; Madigan Army Medical Center, Joint Base Lewis McChord, Washington, USA.
Cristian Madar, Tripler Army Medical Center, Honolulu, Hawaii, USA.
Milissa U Jones, Uniformed Services University of the Health Sciences, Bethesda, Maryland, USA; Tripler Army Medical Center, Honolulu, Hawaii, USA.
Derek T Larson, Naval Medical Center San Diego, San Diego, California, USA; Fort Belvoir Community Hospital, Fort Belvoir, Virginia, USA.
Evan C Ewers, Fort Belvoir Community Hospital, Fort Belvoir, Virginia, USA.
Samantha Bazan, Carl R. Darnall Army Medical Center, Fort Hood, Texas, USA.
Anthony C Fries, US Air Force School of Aerospace Medicine, Dayton, Ohio, USA.
Carlos J Maldonado, Womack Army Medical Center, Fort Bragg, North Carolina, USA.
Mark P Simons, Infectious Disease Clinical Research Program, Department of Preventive Medicine and Biostatistics, Uniformed Services University of the Health Sciences, Bethesda, Maryland, USA.
Julia S Rozman, Infectious Disease Clinical Research Program, Department of Preventive Medicine and Biostatistics, Uniformed Services University of the Health Sciences, Bethesda, Maryland, USA; The Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., Bethesda, Maryland, USA.
Liana Andronescu, Infectious Disease Clinical Research Program, Department of Preventive Medicine and Biostatistics, Uniformed Services University of the Health Sciences, Bethesda, Maryland, USA; The Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., Bethesda, Maryland, USA.
Katrin Mende, Infectious Disease Clinical Research Program, Department of Preventive Medicine and Biostatistics, Uniformed Services University of the Health Sciences, Bethesda, Maryland, USA; The Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., Bethesda, Maryland, USA; Brooke Army Medical Center, Fort Sam Houston, Texas, USA.
David R Tribble, Infectious Disease Clinical Research Program, Department of Preventive Medicine and Biostatistics, Uniformed Services University of the Health Sciences, Bethesda, Maryland, USA.
Brian K Agan, Infectious Disease Clinical Research Program, Department of Preventive Medicine and Biostatistics, Uniformed Services University of the Health Sciences, Bethesda, Maryland, USA; The Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., Bethesda, Maryland, USA.
Timothy H Burgess, Infectious Disease Clinical Research Program, Department of Preventive Medicine and Biostatistics, Uniformed Services University of the Health Sciences, Bethesda, Maryland, USA.
Simon D Pollett, Infectious Disease Clinical Research Program, Department of Preventive Medicine and Biostatistics, Uniformed Services University of the Health Sciences, Bethesda, Maryland, USA; The Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., Bethesda, Maryland, USA.
John H Powers, III, Clinical Research Directorate, Frederick National Laboratory for Cancer Research, Frederick, Maryland, USA.
for the EPICC COVID-19 Cohort Study Group:
J Cowden, M Darling, S DeLeon, D Lindholm, A Markelz, K Mende, S Merritt, T Merritt, N Turner, T Wellington, R Carl, S Bazan, P K Love, N Dimascio-Johnson, E Ewers, K Gallagher, D Larson, A Rutt, P Blair, J Chenoweth, D Clark, S Chambers, C Colombo, R Colombo, C Conlon, K Everson, P Faestel, T Ferguson, L Gordon, S Grogan, S Lis, C Mount, D Musfeldt, D Odineal, M Perreault, W Robb-McGrath, R Sainato, C Schofield, C Skinner, M Stein, M Switzer, M Timlin, S Wood, S Banks, R Carpenter, L Kim, K Kronmann, T Lalani, T Lee, A Smith, R Smith, R Tant, T Warkentien, C Berjohn, S Cammarata, N Kirkland, D Libraty, R Maves, (Ret.), G Utz, (Ret.), S Chi, R Flanagan, M Jones, C Lucas, C Madar, (Ret.), K Miyasato, C Uyehara, B Agan, L Andronescu, A Austin, C Broder, T Burgess, C Byrne, K Chung, (Ret.), J Davies, C English, N Epsi, C Fox, M Fritschlanski, A Hadley, P Hickey, E Laing, C Lanteri, J Livezey, A Malloy, R Mohammed, C Morales, P Nwachukwu, C Olsen, E Parmelee, S Pollett, S Richard, J Rozman, J Rusiecki, E Samuels, M Sanchez, A Scher, M Simons, A Snow, K Telu, D Tribble, M Tso, L Ulomi, M Wayman, T Merritt, T Wellington, D Clark, S Chambers, P Faestel, C Mount, D Musfeldt, C Schofield, N Kirkland, C Madar, (Ret.), C Uyehara, C Broder, C Byrne, K Chung, (Ret.), C English, P Hickey, E Laing, C Lanteri, J Livezey, P Nwachukwu, E Parmelee, E Samuels, M Sanchez, A Scher, M Tso, M Wayman, T Chao, K Lanter, J Meyer, K Reynolds, C Starr, J Iskander, I Kamara, D Hostler, and K Lago
References
- 1. Fillmore N, La J, Zheng C, et al. The COVID-19 hospitalization metric in the pre- and post-vaccination eras as a measure of pandemic severity: a retrospective, nationwide cohort study. Infect Control Hosp Epidemiol 2022. doi: 10.1017/ice.2022.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Powers JH, Guerrero ML, Leidy NK, et al. Development of the Flu-PRO: a patient-reported outcome (PRO) instrument to evaluate symptoms of influenza. BMC Infect Dis 2016; 16:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Powers JH 3rd, Bacci ED, Guerrero ML, et al. Reliability, validity, and responsiveness of InFLUenza patient-reported outcome (FLU-PRO(c)) scores in influenza-positive patients. Value Health 2018; 21:210–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Powers JH 3rd, Bacci ED, Leidy NK, et al. Performance of the inFLUenza Patient-Reported Outcome (FLU-PRO) diary in patients with influenza-like illness (ILI). PLoS One 2018; 13:e0194180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Richard SA, Epsi NJ, Pollett S, et al. Performance of the inFLUenza Patient-Reported Outcome Plus (FLU-PRO Plus) instrument in patients with coronavirus disease 2019. Open Forum Infect Dis 2021; 8:XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Thompson MG, Burgess JL, Naleway AL, et al. Prevention and attenuation of Covid-19 with the BNT162b2 and mRNA-1273 vaccines. New Engl J Med 2021; 385:320–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pilishvili T, Gierke R, Fleming-Dutra KE, et al. Effectiveness of mRNA Covid-19 vaccine among U.S. health care personnel. New Engl J Med 2021; 385:e90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rinott E, Youngster I, Lewis YE. Reduction in COVID-19 patients requiring mechanical ventilation following implementation of a national COVID-19 vaccination program – Israel, December 2020-February 2021. MMWR Morb Mortal Wkly Rep 2021; 70:326–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tenforde MW, Self WH, Adams K, et al. Association between mRNA vaccination and COVID-19 hospitalization and disease severity. JAMA 2021; 326:2043–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Thomas SJ, Moreira ED Jr, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine through 6 months. New Engl J Med 2021; 385:1761–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Epsi NJ, Richard SA, Laing ED, et al. Clinical, immunological and virological SARS-CoV-2 phenotypes in obese and non-obese military health system beneficiaries. J Infect Dis 2021; 224:1462–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Richard SA, Pollett SD, Lanteri CA, et al. COVID-19 outcomes among US Military Health System beneficiaries include complications across multiple organ systems and substantial functional impairment. Open Forum Infect Dis 2021; 8:XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Freed NE, Vlkova M, Faisal MB, Silander OK. Rapid and inexpensive whole-genome sequencing of SARS-CoV-2 using 1200 bp tiled amplicons and Oxford Nanopore Rapid Barcoding. Biol Methods Protoc 2020; 5:bpaa014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. O’Toole A, Scher E, Underwood A, et al. Assignment of epidemiological lineages in an emerging pandemic using the pangolin tool. Virus Evol 2021; 7:veab064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yu J, Powers JH 3rd, Vallo D, Falloon J. Evaluation of efficacy endpoints for a phase IIb study of a respiratory syncytial virus vaccine in older adults using patient-reported outcomes with laboratory confirmation. Value Health 2020; 23:227–35. [DOI] [PubMed] [Google Scholar]
- 16. R: A Language and Environment for Statistical Computing. R Core Team; 2017.
- 17. Seligman WH, Fialho L, Sillett N, et al. Which outcomes are most important to measure in patients with COVID-19 and how and when should these be measured? Development of an international standard set of outcomes measures for clinical use in patients with COVID-19: a report of the International Consortium for Health Outcomes Measurement (ICHOM) COVID-19 Working Group. BMJ Open 2021; 11:e051065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lopez-Leon S, Wegman-Ostrosky T, Perelman C, et al. More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Sci Rep 2021; 11:16144. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.