Abstract
The potential transmission of SARS-CoV-2 via food has been controversial since the beginning of the COVID-19 pandemic. To investigate these concerns, reliable detection methods and data on virus die-off rates in various foods are needed. Here, an FDA-standard method for the detection of enteric viruses’ RNA from soft fruits was modified for the recovery of infectious SARS-CoV-2. Then, the survival of SARS-CoV-2 on berries was investigated as well as the effectiveness of washing virus-contaminated berries with water. The modified method did not significantly reduced log infectivity titers of recovered viruses, but berries did. The detection limit of the method for infectious SARS-CoV-2 was ∼2.97 log TCID50/g of berries. On SARS-CoV-2-inoculated berries that were stored at 4 °C for 7 days, significant reductions in SARS-CoV-2 infectivity were observed over time. In contrast, on frozen berries, infectious SARS-CoV-2 was recovered for 28 days without significant reductions. Washing SARS-CoV-2-inoculated berries with water removed >90% of infectious viruses within 10 min; however, infectious viruses were detected in wash water. Therefore, on fresh berries infectious viruses are markedly inactivated over time and can be largely removed by washing with water. However, the prolonged survival of SARS-CoV-2 on frozen berries suggests that the virus can potentially spread through frozen fruits.
Keywords: SARS-CoV-2, Food, Berries, Method optimization, Viral recovery from fruits, Washing fruits, Transmission on food, Virus survival
1. Introduction
Since the onset of the COVID-19 pandemic, the potential transmission of SARS-CoV-2 via food has been controversial. Multiple surveys in several countries have reported that consumers resorted to washing fruits and vegetables using bleach or soap with the intent of preventing SARS-CoV-2 infections (Faour-Klingbeil et al., 2021; Finger et al., 2021; Gharpure et al., 2020). A CDC survey found that almost 20% of 502 respondents washed fruits and vegetables using bleach with the intent of preventing SARS-CoV-2 infections (Gharpure et al., 2020). Another survey in the USA that was performed between April to August 2020 revealed a significant increase in produce washing with water and soap during the pandemic as compared to before the pandemic (Thomas and Feng, 2021). A survey from the Middle East demonstrated that 70% of 1074 participants were concerned that COVID-19 can be transmitted through food (Faour-Klingbeil et al., 2021). Also, a survey in Brazil showed that 27.4% of 3000 participants indicated that they washed fruits with detergents due to fears of acquiring SARS-CoV-2 (Finger et al., 2021). Furthermore, the possibility of SARS-CoV-2 transmission via food was used for bioterrorism claims, suggesting that COVID-19 positive individuals can intentionally spread the virus to others by spitting on food (Desai and Amarasingam, 2020). News reports from China suggested that SARS-CoV-2 viral RNA can be detected on food products and packaging (Brown, 2020; Wilkinson, 2021), and this was later linked epidemiologically to human infections (Liu et al., 2020). Subsequently, bans were placed on imports from meat and poultry processing facilities that reported thousands of COVID-19 infected workers (Dyal et al., 2020).
Although SARS-CoV-2 is regarded mainly as a respiratory pathogen, there is ample evidence of its ability to cause enteric infections (Zhou et al., 2020) including gastrointestinal illness (GI) symptoms (Brooks and Bhatt, 2021), high expression of its receptor, ACE2, in the GI tract (Harmer et al., 2002) and the prolonged shedding of the virus in the stool of COVID-19 patients (Wu et al., 2020; Xiao et al., 2020). The presence of infectious SARS-CoV-2 from human fecal specimens was demonstrated by intranasal inoculation of ferret animals (Jeong et al., 2020). Thus, SARS-CoV-2 may be capable of limited transmission through the fecal-oral route (Jones et al., 2020), raising concerns about acquiring SARS-CoV-2 via ingestion of contaminated food. However, food can potentially get contaminated with SARS-CoV-2 through either the fecal (via unhygienic hands) and/or respiratory (droplets from coughing/sneezing) routes. Recently, a WHO report on the origins of the SARS-CoV-2 suggested that the re-introduction of SARS-CoV-2 via cold/food chain is possible (WHO, 2021). The first epidemiological evidence linking the presence of infectious SARS-CoV-2 on outer packages of frozen cod to human infections came from China (Liu et al., 2020). However, so far, no other country has reported any cases of food-associated transmission of SARS-CoV-2 (O'Brien et al., 2021).
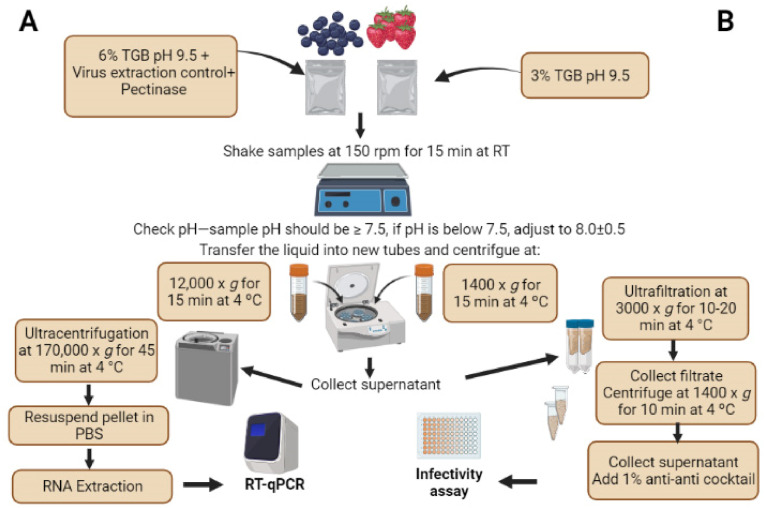
In laboratory studies, it was shown that meat (pork, beef and salmon) dipped in low titer infectious SARS-CoV-2 (104 TCID50/ml) and stored under either refrigerated or freezing conditions were positive for the pathogen for at least 9 and 20 days, respectively (Feng et al., 2021). Another study found that on salmon dipped in high-titer SARS-CoV-2 (106 TCID50/ml), the infectious virus was detectable on day 8 of cold storage (Dai et al., 2021). However, given that SARS-CoV-2 is highly sensitive to heat treatment with over a 6-log reduction in infectivity titer at 70 °C (Pastorino et al., 2020), concerns over the presence of SARS-CoV-2 on meat products are less warranted (Dhakal et al., 2021). Safe meat handling and proper cooking of meat products are expected to mitigate possible health risks associated with a number of foodborne pathogens (EFSA, 2021; Thippareddi et al., 2020). However, there is a lack of studies investigating the survival of SARS-CoV-2 transmission on frozen produce. Although, the two previous studies attempted to examine virus survival on fresh produce, these studies did not optimize methods for virus recovery from produce, used small pieces (1.5 cm) of produce to examine virus survival over a 24 h period (Dhakal et al., 2021; Haddow et al., 2020), and did not include frozen products. In addition, these studies are difficult to compare to each other's because of different viral recovery methods used. The United States' Food and Drug Administration (FDA) adopted a standard method for the detection of enteric viruses from berries. This method relies on elution of naked viruses using Tris-glycine beef extract buffer at pH 9.5 and further concentrating the viruses by ultracentrifugation (Fig. 1 A). However, the method was developed for non-enveloped viruses and relies on RT-qPCR as an end-point detection assay. It is not known whether it can be adapted to enveloped viruses, such as SARS-CoV-2, and to cell culture as an endpoint assay.
Fig. 1.
A: A flowchart showing the general steps of (A) the FDA standard for “Concentration, Extraction, and Detection of Norovirus and Hepatitis A virus in Soft Fruit” with RT-qPCR as an endpoint and the (B) modifications made to optimize the recovery of infectious SARS-CoV-2, an enveloped virus, from berries with infectivity assay as an endpoint. The flowchart was created using BioRender.com.
The objectives of this study were to (a) adapt the FDA standard method used for RNA detection of enteric viruses to the recovery of infectious SARS-CoV-2 from berries, (b) examine the survival of the virus on fresh and frozen berries and (c) determine the effectiveness of washing in water to remove the virus from contaminated berries. Berries were targeted in this study because they represent a known high-risk food commodity for the spread of foodborne enteric viruses such as human norovirus and hepatitis A virus. Also, berries contaminated in one country can serve as vehicles for transport of enteric viruses to other countries [reviewed in (Bozkurt et al., 2021)]. Furthermore, these foods are mainly consumed raw without prior cooking, posing a higher risk for spreading pathogens. Blueberries and strawberries were used in this study because they represent contrasting surface morphologies and both can be consumed fresh or frozen without further processing.
2. Materials and methods
2.1. Preparation of viral stocks
The US reference strain SARS-CoV-2 USA-WA 1/2020 (BEI resources NR-52281) was propagated in African green monkey kidney cells (Vero E6 ATCC CRL-1586) originally at Dr. RJ Hogan's BSL3 laboratory (College of Veterinary Medicine, University of Georgia, Athens, GA) as described previously (Harcourt et al., 2020) and gifted to our laboratory. Handling of SARS-CoV-2 was done under strict BSL3 biosafety protocols at the Center for Food Safety BSL3 laboratory. Vero E6 cells were propagated in DMEM +10% FBS. All cell culture media were supplemented with 1% antibiotic-antimycotic cocktail. One or two-day-old 90% confluent cells were used to prepare virus stocks using a multiplicity of infection of 0.01. Harvesting the virus was done between 62 and 72 h of incubation. Infected cells were collected from the flasks and centrifuged at low speed (450×g for 5 min at 4 °C) to pellet the cell debris (Case et al., 2020), while supernatants containing the virus, were aliquoted (100–200 μl) and stored at −80 °C. SARS-CoV-2 was ultra-filtered through an Amicon® 100 K Ultra-15 (Millipore) immediately after harvest to remove virus-interfering cell culture debris and to exchange the virus cell culture media with water which is a more relevant matrix (Esseili et al., 2015). An aliquot of the virus was immediately titrated as described below. The original viral titer generated was ∼7 log TCID50/ml while the ultrafiltered virus titer was ∼8 log TCID50/ml. The ultrafiltered virus was diluted in sterile water to the viral titers used in various experiments below. Water was used because it is a common vehicle of contamination in fecal-oral route and is also a major constituent of respiratory droplets.
2.2. Spiking berries with SARS-CoV-2
Blueberries and strawberries were bought from local grocery stores. The calyx was removed from strawberries. The berries’ original plastic packaging was sterilized with 70% ethanol and allowed to dry in a biological safety cabinet. Undamaged blueberries and strawberries (∼15 g/replicate) were spiked with a total viral particles ∼6.2 log TCID50 per replicate in a total volume of 100 μl (equivalent to ∼5 log TCID50/g). The spots were pipetted randomly on the surface of the berries as 10 μl droplets. The viral droplets were allowed to dry in a biosafety cabinet for approximately 30 min. One set of berries were stored in their sterilized packaging and incubated inside an environmental chamber (GEN1000, Conviron) under 4 °C and 40–80% relative humidity and a cycle of 12 h light/12 h dark for a maximum of 7 days (approximate shelf life of berries in retail stores). A second set was placed inside sterile Whirl-Pak bags (Nasco) and stored at −20 °C for 28 days.
2.3. Recovery of SARS-CoV-2 from berries
The FDA standard method for detection of norovirus (non-enveloped virus) by RT-qPCR from soft fruits was followed with modifications to allow recovery and quantification of infectious SARS-CoV-2, an enveloped virus, in cell culture (FDA, 2021c). The virus elution buffer used was 0.05 M Tris, 0.25 M Glycine, 3% Beef Extract (TGB, pH 9.5). However, the beef extract in the elution buffer was reduced to 3% instead of 6% as reported previously (Collomb et al., 1986; Dubois et al., 2002). The latter was also found to minimize cytotoxicity to Vero cells in the TCID50 infectivity assay (Fig. 2 A and B). The cytotoxicity assay was performed using the cell proliferation MTT kit (Sigma) per manufacturer's instructions. The absorbance at 550 nm from the cell culture plates was read using a spectrophotometer (Bio Tek ELx800). Prior to performing MTT assay, images from cells in 96-well plates treated with elution buffer using 3% vs 6% beef extract or from negative berry samples eluted using 3% beef in elution buffer were captured using light microscope (Leica DMi1) at 5x magnification. The pH of all samples following the processing steps was measured at undiluted, 1:10 and 1:100 in cell culture media. The pH was measured using Orion Star A211 (Thermo Scientific, USA). The pectinase step was not followed because all SARS-CoV-2 contamination was done on the surface of produce with minimal damage to berries during inoculation or processing. In addition, centrifugation was done twice at lower speed (1400×g for 15 min) instead of once at high speed (12,000×g for 15 min) to minimize damage to the virus envelope, as reported previously for coronavirus 229 E (Yepiz-Gomez et al., 2013). Also, the ultracentrifugation step was replaced with an ultrafiltration step to restrict all handling of SARS-CoV-2 in BSL3 laboratory which did not have an ultracentrifuge. The ratio 1: 0.6 of berries to elution buffer (w/v) was similar to the FDA method but 15 g of berries were used instead of 50 g in order to reduce processing time. Using this method, every 12 samples required ∼7 h to be processed and tested by theTCID50 infectivity assay described below. The optimized method was initially tested with coronavirus 229 E-inoculated berries in a BSL2 laboratory before adopting it for SARS-CoV-2 in BSL3 lab (data not shown). Briefly, ∼15 g of berries were suspended in 9–10 ml of the elution buffer 3% TGB inside Whirl-Pak bags. The samples were shaken for 15 min at 150 rpm under room temperature (approximately 20 °C). The liquid was then collected and centrifuged at 1400×g rpm for 10 min at 4 °C. The supernatants were ultrafiltered using Amicon® 100 K Ultra-15 at 3000×g for 10–20 min at 4 °C. Approximately 500 μl of each sample were recovered and collected into new 1.5 ml tubes. The membranes of the Amicon ultrafilteration tubes were washed once with ∼500 μl sterile water which were combined with their respective samples to make the total volume of all of the samples equal to 1 ml. The samples were re-centrifuged at 1400×g for 10 min at 4 °C. The supernatants were collected, supplemented with 1% antibiotic-antimycotic cocktail and tested immediately (to avoid freeze thawing) for SARS-CoV-2 infectivity in Vero cells as described below. Sampling for all berries was done on days 0 (immediately following the 30 min drying period, 1, 3 and 7 for berries stored under refrigeration and on days 0, 14 and 28 for frozen berries. Uninoculated berries served as negative controls.
Fig. 2.
(A) Light microscopy images (a to g) and cytotoxicity assay (h) on vero-E6 cells at day 4 post-treatment with recovered 3% or 6% Tris-Glycine Beef buffer (TGB, pH 9.5) or with (B) recovered 3% Tris-Glycine beef buffer (TGB, pH 9.5) from berries. All samples were subjected to all the processing steps of FDA-based method and the pH was measured for all recovered eluants before being tested for their effect on cell viability at undiluted, 1:10 or 1:100 dilutions. Control negative cells were used to calculated % viability. Comparing within each buffer or berry type: means with different letters differ significantly (p < 0.05). Comparing between buffers or berry types: significant differences are denoted with asterisks.
2.4. SARS-CoV-2 detection limit on berries
The virus inoculum level was serially (1:10) diluted in water, and triplicate samples (∼15 g/replicate) of blueberries and strawberries were spot-inoculated with 10 μl (100 μl total volume) of each serially diluted virus (∼105, 104, 103, 102, and 101 TCID50/g). The berry samples were processed immediately as described above to determine the infectious titers of recovered SARS-CoV-2. Additionally, to evaluate the effect of the FDA-based processing method on infectivity of recovered SARS-CoV-2, the viral inocula was serially diluted 10-fold in 10 ml of elution buffer and processed as described above. The recovered viruses from berry samples were tested for infectivity.
2.5. Effect of washing contaminated berries in water
Blueberries and strawberries were spot-inoculated with SARS-CoV-2 as described above. Following a drying period (∼30 min), one set of berries was washed by immersion in 50 ml of autoclaved distilled water and gently shaken at 150 rpm for 10 min at room temperature. A 10 min washing period was chosen based on previous research reporting that washing decreases ∼1 log of human norovirus from lettuce using water alone (Anfruns-Estrada et al., 2019) Washed berries were processed as described above to determine the post-wash SARS-CoV-2 infectivity titer. The wash water was collected and ultrafiltered to concentrate the virus in water to 500–1000 μl. Another set of inoculated berries was not washed but was processed immediately after a 30-min drying period to determine the pre-wash SARS-CoV-2 infectivity titer.
2.6. Virus titration by TCID50 assay
One to two-day-old 90% confluent cell monolayers in 96-well plates were infected in quadruplet with serially diluted samples (1:10) in cell culture media supplemented with 2% FBS and 1% antibiotic-antimycotic cocktail and incubated at 37 °C. The samples were kept incubated on the cells for 4–5 days when the cells were inspected for cytopathic effects. Viral titers were estimated following the Reed-Muench equation for the calculation of TCID50 (Payment and Trudel, 1993). Positive (virus with known titer) and negative controls (cell culture media) were included in each experiment.
2.7. Statistics
All experiments were performed independently three times with three technical replicates tested for each berry type, dilution, temperature, time or washing. Averages and standard errors were calculated from the technical replicates. GraphPad Prism version 5 (GraphPad Software, USA) was used for all statistical analyses. Recovery efficiencies (%) were calculated from each experiment by multiplying the titers of the recovered viruses by 100 and dividing it by the mean titer of the viral inocula. The entire data set was transformed to log10. All SARS-CoV-2 infectivity titers from berries were converted log10 per g. Linear regression analysis was used to determine deviation from linearity for the SARS-CoV-2 infectivity data obtained for the limit of detection experiments. One way or two-way analysis of variance (ANOVA) followed by Tukey or Bonferroni post-tests, respectively were used to determine significant differences in mean infectivity titers. The factors analyzed included time, treatment, dilution, berry type and virus source (recovered versus original). Differences in means were considered significant when the P value was less than 0.05 and were denoted in the figures by either letters or asterisks. Data were expressed as mean ± standard error (SE).
3. Results
3.1. The FDA-modified method for recovery of enteric foodborne viruses can be used for recovering infectious SARS-CoV-2 from berries
First, the cytotoxicity assay showed that 3% TGB buffer after recovery was not cytotoxic to Vero-E6 cells at undiluted, 1:10 or 1:100 dilutions (Fig. 2A, h). In contrast, the undiluted 6% TGB buffer after recovery was ∼20% cytotoxic to Vero-E6 cells which is significantly different than undiluted 3% TGB (Fig. 2A, h). This was also confirmed by observing the cells under the microscope (Fig. 2A). Therefore, 3% TGB was used in the adopted method to recover infectious SARS-CoV-2 from berries.
Second, when the 3% TGB buffer was used on berries, the undiluted samples eluted from strawberries showed significant reduction in cell viability (∼70%) as compared to those eluted from blueberries (∼20%) (Fig. 2B, h). Both blueberry and strawberry samples at 1:10 or higher dilutions did not show any significant changes to cell viability. Therefore, for all experiments below, SARS-CoV-2 eluted from berries was tested starting with the 1:10 dilution in the TCID50 assay. In addition, the pH values of the eluted samples from berries or TGB at 1:10 dilution were close to that of cell culture media alone (Fig. 2A and B). Therefore, the pH of each sample was not neutralized before addition to Vero-E6 cells.
Third, when tested in elution buffer, the virus infectivity was not significantly changed after the processing steps of the FDA-based method. Specifically, the infectivity titers of the recovered viruses were not significantly different from the original viral inocula at any of the ten-fold serial dilutions tested (Table 1 ). The % recovery efficiency varied between experiments, but on average did not differ significantly between different viral inocula and ranged between 7 and 38% (Table 1). Furthermore, there was a linear relationship between the infectivity titers of the viral inocula and recovered viruses (R-square 0.84) (Fig. 3 A), suggesting that titers of recovered viruses can be used to predict original viral titers.
Table 1.
Mean viral titers (log TCID50/ml or g) for SARS-CoV-2 that was inoculated and recovered at time zero in elution buffer alone or on berries. Recovery efficiencies (%) were calculated from each experiment by multiplying the titers of the recovered viruses by 100 and dividing it by the mean titer of the viral inocula. Comparing within TGB or within berries: means with different letters differ significantly (p < 0.05). Comparing between berries or between inoculated and recovered viruses: significant differences are denoted with asterisks when p value < 0.05. Data were expressed as mean ± standard error (SE).
| Virus eluted from | Mean Inoculated Viral Titers (TCID50/ml or g) | Mean Recovered Viral titer (TCID50/ml or g) | No. of detected/No. of Replicates | Mean log reduction | Mean Recovery Efficiency (%) |
|---|---|---|---|---|---|
| 3%TGB | 5.55 ± 0.14 | 5.06 ± 0.13 | 9/9 | 0.62 ± 0.15 abc | 38.5 ± 10.8 a |
| 4.52 ± 0.20 | 3.99 ± 0.15 | 9/9 | 0.31 ± 0.08 c | 32.4 ± 10.2 a | |
| 3.35 ± 0.19 | 2.96 ± 0.21 | 9/9 | 0.26 ± 0.10 bc | 36.2 ± 11.2 a | |
| 2.32 ± 0.24 | 1.37 ± 0.42 | 6/9 | 1.03 ± 0.30 a | 27.1 ± 12.2 a | |
| 1.24 ± 0.35 |
0.48 ± 0.18 |
4/9 |
0.83 ± 0.15 abc |
7.4 ± 5.7 a |
|
| Blueberries | 5.01 ± 0.16 | 3.79 ± 0.25 | 9/9 | 1.20 ± 0.25 a | 15.6 ± 5.6 a |
| 4.01 ± 0.23 | 2.18 ± 0.15 | 9/9 | 1.81 ± 0.15 a | 2.25 ± 0.6 b | |
| 2.97 ± 0.27 |
0.56 ± 0.08 |
6/9 |
2.59 ± 0.11 b |
0.29 ± 0.08 bc |
|
| Strawberries | 5.01 ± 0.16 | 3.36 ± 0.22* | 9/9 | 1.64 ± 0.22 a | 4.9 ± 2.3 a* |
| 4.01 ± 0.23 | 1.85 ± 0.24* | 9/9 | 2.14 ± 0.24 ab | 1.8 ± 0.6 a | |
| 2.97 ± 0.27 | 0.58 ± 0.02* | 7/9 | 2.51 ± 0.08 b | 0.3 ± 0.1 a |
Fig. 3.
Scatter plots showing the detection limit for SARS-CoV-2 infectivity in (A) elution buffer alone (3% TGB, pH 9.5) and (B) on berries. The virus was serially diluted tenfold before inoculating elution buffer or berries. All samples were processed similarly as described in the methods section. The infectivity titers of the recovered viruses were plotted against the infectivity of the original serially diluted viral inocula. The data represent the mean of three independent experiments, with three technical replicates per berry type. Error bars represent standard error.
Fourth, similarly, evaluation of the FDA-based method on recovery of SARS-CoV-2 inoculated on berries revealed a linear relationship between the infectivity titer of the inoculated virus on berries and those recovered from blueberries (R-square 0.84) and strawberries (R-square 0.79) (Fig. 3B). However, there were significant losses in the infectivity titers of recovered viruses from both berries in comparison to titers of inoculated viruses (Table 1). Specifically, the log reduction in virus infectivity on berries ranged on average from 1.2 (blueberry) to 1.64 (strawberry) log TCID50/g for the 5 log TCID50/g inoculum and about 2.5 log TCID50/g for the ∼3 log TCID50/g inoculum (Table 1). The % recovery efficiency varied significantly between blueberries and strawberries at the higher inoculum only (15.6 vs 4.9, respectively) (Table 1). However, at the lower viral inocula, the recovery efficiency was similar between berries (Table 1). Therefore, this FDA-based modified method is suitable for detecting infectious SARS-CoV-2 when the virus is present on berries at ∼ ≥ 3 log TCID50/g of which 0.5 TCID50/g can be recovered (Table 1).
3.2. SARS-CoV-2 remains infectious for a prolonged time on frozen berries
Infectious SARS-CoV-2 particles were recovered through day 7 of cold storage from both berries (Fig. 4 A). For both blueberries and strawberries stored at 4 °C, the infectivity titers of SARS-CoV-2 started to decrease significantly on day 3 (Fig. 4A). Within 3 days at 4 °C, on average, ∼1 and 2 log TCID50/g of the virus were inactivated on blueberries and strawberries, respectively (Table 2 ). Comparing berries to each other revealed significantly higher infectious particles on blueberries on day 3 and 7 as compared to strawberries. In contrast, on frozen berries, SARS-CoV-2 was highly stable and did not show significant changes in infectivity titers (Fig. 4B) or log reduction (Table 2) throughout the 28-day storage period for either blueberries or strawberries.
Fig. 4.
Survival of infectious SARS-CoV-2 on berries stored at (A) 4 °C for 7 days or (B) −20 °C for 28 days. The x-axis starts at the detection limit of 0.5 log TCID50/g. Comparing within a berry type: means with different letters differ significantly (p < 0.05). Comparing corresponding time points between berries: significant differences are denoted with asterisks when p value < 0.05. The data represent the mean of three independent experiments, with three technical replicates per storage temperature, time point or berry type. Error bars represent standard error.
Table 2.
log reductions of SARS-CoV-2 (TCID50/g) on berries stored at different temperatures for various time points. Comparing within berries: means with different letters differ significantly (p < 0.05). Comparing between berries: significant differences are denoted with asterisks when p value < 0.05. Data were expressed as mean ± standard error (SE).
| Storage condition | Time point | Blueberries |
Strawberries |
||
|---|---|---|---|---|---|
| Virus log reduction (TCID50/g) | No. of detected/No. of Replicates | Virus log reduction on (TCID50/g) | No. of detected/No. of Replicates | ||
| 4 °C | Day 1 | 0.28 ± 0.16 a | 9/9 | 0.52 ± 0.20 a | 9/9 |
| Day 3 | 1.03 ± 0.32 ab | 9/9 | 1.97 ± 0.42 b | 8/9 | |
| Day 7 |
2.51 ± 0.33 c |
6/9 |
3.18 ± 0.10 c |
5/9 |
|
| −20 °C | Day 14 | 0.60 ± 0.19 a | 9/9 | 0.61 ± 0.19 a | 9/9 |
| Day 28 | 0.51 ± 0.22 a | 9/9 | 0.54 ± 0.22 a | 9/9 | |
3.3. Washing fresh berries in water removed >90% of SARS-CoV-2 from berries
Washing fresh berries for 10 min in sterile water with gentle shaking (150 rpm) reduced significantly the infectivity titer of SARS-CoV-2 on blueberries and strawberries on average by ∼1.2 and 1 log TCID50/g, respectively (Fig. 5 A). There were no significant differences in virus infectivity titers when comparing blueberry to strawberry for either pre- or post-wash virus titers. However, the wash water was found to harbor SARS-CoV-2 at a significantly higher virus titer for blueberry in comparison to strawberry wash water (∼2.5 and 0.5 log TCID50/ml; respectively) (Fig. 5B).
Fig. 5.
Infectious SARS-CoV-2 (A) on berries pre- and post-washing with water at room temperature (under gentle shaking at 150 rpm for 10 min) and (B) in wash water. The x-axis in (A) starts at the detection limit of 0.54 log TCID50/g. Significant differences in infectivity titers as compared with pre-wash titers within each berry type are denoted with asterisks when p value < 0.05. The data represent the mean of three independent experiments, with three technical replicates per berry type. Error bars represent standard error.
4. Discussion
The European Food Safety Authority acknowledges that there is a possibility of food directly contaminated with SARS-CoV-2 to cause infection if the virus comes in contact with the mucous membranes of the mouth (BfR, 2021; EFSA, 2021). In contrast, the United FDA maintains that touching surfaces and objects, not food, can potentially transmit the virus (FDA, 2021a). Regardless, consumers have been worried about infected people touching their food and whether this can potentially transmit the virus to them (Thomas and Feng, 2021). Early in the pandemic, almost half of the surveyed individuals in the United States had concerns about food prepared outside their homes, fearing that the virus survives on raw foods (Thomas and Feng, 2021). In addition, many surveys around the world showed that consumers resorted to improper washing of fruits and vegetables to prevent acquiring the virus from food. Our results showed that for fresh berries, washing with clean water can reduce the viral load by 1–1.2 log (90–93%) per gram. The latter is consistent with previous studies showing that washing leafy greens with water removed 1 to 1.3 log per gram of human norovirus within 10 min at room temperature (Anfruns-Estrada et al., 2019), while washing strawberries for 2 min in potable water with gentle stirring removed approximately 1 log of hepatitis A virus and murine norovirus (a surrogate for human norovirus) (Zhou et al., 2017). Our results support the WHO, EFSA and FDA recommendation for consumers to wash produce with water before consumption (FDA, 2021b).
To our knowledge, our study is the first to show that SARS-CoV-2 remains infectious for at least a week on fresh berries and for at least a month on frozen berries, a food category that can be consumed without further processing/cooking by consumers. However, reductions in infectivity are more important than actual duration of the virus survival, because the survival duration will vary based on the initial viral load of contamination. Our results suggest that in case of suspicion of berry contamination with SARS-CoV-2, leaving berries at 4 °C for 3 days would be expected to reduce the viral load by at least one to two log (90–99%) per gram of berries. Previous laboratory studies have shown that SARS-CoV-2 survives on apple and spinach for at least 24 h without significant reductions in infectivity (Dhakal et al., 2021). In addition, a common cold human coronavirus, namely 229 E, has been shown to survive between 1 and 4 days on lettuce, cucumber, apples and tomatoes (Blondin-Brosseau et al., 2021; Yepiz-Gomez et al., 2013). It is difficult to compare the results of these studies to our results because different viral recovery methods were used as well as different viral inocula. For example, Dhakal et al. used SARS-CoV-2 at 1 × 105 PFU per 1.5 × 1.5 cm pieces of produce, while 229 E was used by Yepiz-Gomez at 1.2 × 106 PFU per various masses of produce (8–10 g lettuce, 30–40 g strawberries and 16–20 g raspberries), and by Blondin-Brosseau at 5 × 105 TCID50/ml per 5 × 5 cm pieces of produce. Interestingly, our results showed that by day 3 more inactivation occurred on the surface of strawberries as compared to blueberries. In addition, recovery of infectious virus particles (per ml of wash water) from the strawberry wash water was less than recovery from blueberry wash water. The latter is potentially due to the lower viral recovery from strawberries as compared to blueberries. Blueberries has a smoother surface and waxy skin which makes it easier to elute viruses and at the same time limit the exudation of antiviral substances (Bozkurt et al., 2021). Taken together, our results suggest that some factors associated with the surface of strawberries are more damaging to the virus than are those of blueberries. This requires further investigation to identify the factors that affect SARS-CoV-2 persistence/inactivation on the surface of different fruits.
Contamination of frozen berries with SARS-CoV-2 is more concerning, because there were no significant reductions in SARS-CoV-2 infectivity for at least 28 days. Before freezing, berries are usually washed with water which, as shown in our study, is expected to reduce the viral load significantly. In addition, wash water is usually supplemented with sanitizers which are expected to reduce microbial loads both on produce and in wash water (Ortiz-Sola et al., 2020; Zhou et al., 2017). However, as also shown in our study, it is well known that wash water can become a source of cross-contamination. In our study, SARS-CoV-2 at approximately 3 log TCID50/g was consistently detected on berries throughout the 28 days period, suggesting that low level of contamination post freezing is of concern. Therefore, further investigations are also needed to determine the effect of commonly used sanitizers in wash water on the inactivation SARS-CoV-2-contaminated berries. It is widely accepted that enteric viruses, such as human noroviruses, survive for a long time (>90 days) with little reduction (<1 log) in infectivity on frozen berries [reviewed in (Bozkurt et al., 2021)]. This is partially why frozen berries have been implicated historically in many large geographically dispersed outbreaks of norovirus and hepatitis A virus globally (A). Our results expand on previous studies done on frozen meat (Dai et al., 2021; Feng et al., 2021) and suggest that SARS-CoV-2 can be potentially transmitted on frozen fruits such as berries due to its high stability on these frozen commodities.
Reliable methods to detect infectious SARS-CoV-2 in food are lacking. Usually the recovery of viruses from food is accompanied by significant losses occurring due to various inactivating ingredients in foods (Blondin-Brosseau et al., 2021; Esseili et al., 2012). Previous research that exposed apples, tomatoes and pepper to SARS-CoV-2 inoculum at 3.1 log PFU/L was not successful in detecting infectious virus 1 h post-inoculation (Haddow et al., 2020). The latter could be due to low viral inocula (3 log PFU/l), mode of viral application (nebulizing), method employed to recover the viruses (swabbing), and the elution buffer used (cell culture media), none of which were initially evaluated for their effect on the recovery of infectious SARS-CoV-2. In contrast, Dhakal et al. spot-inoculated ∼5 log of SARS-CoV-2 on cut skins of apples and was able to show viral recovery within 1 h by pipetting 1 ml of elution buffer (cell culture media) five times on inoculated produce (Dhakal et al., 2021). Yet again, the latter method cannot be applied to field sampling, because the authors used very small amounts of test food (1.5 × 1.5 cm), hence there was no need for viral concentration and further processing. Here, we showed that the FDA standard method for the recovery and concentration of foodborne enteric human norovirus and hepatitis A viruses from berries could be modified for the detection of infectious SARS-CoV-2 when the virus is present at ≥ 950 infectious particles (∼2.97 log TCID50/g) per gram of berries. However, given the detection limit and viral loss occurring at low contamination levels, a 5-log TCID50/g as a starting inoculum was used in our study for the purpose of studying SARS-CoV-2 survival/reduction on berries over time.
A single cough event is expected to generate ∼105 SARS-CoV-2 particles (Wang et al., 2020). Therefore, even if all of the 105 particles from a single or multiple coughs landed on fresh berries, at least two-log of reduction in infectious SARS-CoV-2 on berries would be expected to occur if simple measures such as storage at 4 °C and washing in water are applied. However, whether the remaining viral particles result in infection cannot be ruled out based on the current available data from SARS-CoV-2 animal models. For example, in the SARS-CoV-2 ferret model, intranasal inoculation of the animals with low dose of SARS-CoV-2 (∼2 log10 RNA copies/ml in a fecal specimen) caused mild symptoms (mainly slight increase in body temperature and rhinorrhea) (Jeong et al., 2020). A more relevant study showed that in a SARS-CoV-2 hamster model, a 105 infectious SARS-CoV-2 dose needs to be ingested to cause a mild infection of the respiratory and GI tract (Lee et al., 2020). A low viral RNA load was detectable at 12 h in the oral mucosa only on day 4, while infectious viruses were detectable in the oral mucosa, esophagus and stomach but not in intestinal tissues which showed histopathological changes. This suggests that infection through the oral route can be a source of virus transmission and mild infections. Additionally, there could be limited virus transmission through feces because orally-infected hamsters shed the viral RNA in their feces over 12 days and their oral swabs continued to be positive for the virus for 6 days. In contrast, at a higher infectious dose (107 PFU) and in a nonhuman primate model, the virus given intragastrically causes impairment of the GI barrier and severe infections in both the lung and the intestine (Jiao et al., 2021). Taken together, accidental or intentional (bioterrorism) contamination of frozen berries with SARS-CoV-2 might pose higher risk because the virus survived longer on frozen vs fresh berries. Since it is unethical to try challenging human volunteers orally with the virus on foods, more animal and in vitro digestion studies are needed to understand whether different levels of the virus contamination on food can initiate infections of oral mucosa and/or the small intestine. These studies are essential not only from a food safety perspective but also for claims of virus re-introduction through the cold-chain food trade and for the control of possible bioterrorism using food as a vehicle.
5. Conclusions
Storing fresh berries for 3 days in the refrigerator and washing in water is expected to reduce the infectivity of SARS-CoV-2 by > 2 log (99%). Our results emphasize that implementation of proper respiratory hygiene and food safety measures is essential in preventing initial contamination of frozen berries that are subsequently not cooked before consumption. The latter is of paramount importance when the food is served to an already-at risk population such as the elderly, immunocompromised, hospitalized individuals and people with underlying medical conditions.
Funding
Esseili MA Faculty startup money from the University of Georgia.
Author contribution
Esseili MA: designed and conducted all the experiments, analyzed the data and wrote the manuscript. Amy Mann propagated Vero E6 cells and prepared tissue culture plates. Revati Narwankar made various buffers, captured light microscopy images, performed MTT assay and helped with method optimization using common cold coronavirus 229 E. Dr. Issmat Kassem and Francisco Diez provided critical discussion and editing of the manuscript. Robert Hogan provided SARS-CoV-2 and infectivity assay protocol.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors would like thank Dr. Larry Beuchat for reviewing the manuscript.
References
- Anfruns-Estrada E., Bottaro M., Pinto R.M., Guix S., Bosch A. Effectiveness of consumers washing with sanitizers to reduce human norovirus on mixed salad. Foods. 2019;8 doi: 10.3390/foods8120637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BfR . 2021. Can the New Type of Coronavirus Be Transmitted via Food and Objects?https://www.bfr.bund.de/cm/349/can-the-new-type-of-coronavirus-be-transmitted-via-food-and-objects.pdf Retrieved July 7, 2021, from. [Google Scholar]
- Blondin-Brosseau M., Harlow J., Doctor T., Nasheri N. Examining the persistence of human Coronavirus 229E on fresh produce. Food Microbiol. 2021;98 doi: 10.1016/j.fm.2021.103780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozkurt H., Phan-Thien K.Y., van Ogtrop F., Bell T., McConchie R. Outbreaks, occurrence, and control of norovirus and hepatitis a virus contamination in berries: a review. Crit. Rev. Food Sci. Nutr. 2021;61:116–138. doi: 10.1080/10408398.2020.1719383. [DOI] [PubMed] [Google Scholar]
- Brooks E.F., Bhatt A.S. The gut microbiome: a missing link in understanding the gastrointestinal manifestations of COVID-19? Cold Spring Harb. Mol. Case Stud. 2021;7 doi: 10.1101/mcs.a006031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown C.H. 2020. China Suspends Chicken Imports from Covid-Impacted Tyson Plant in Arkansas.https://thecounter.org/china-suspends-meat-imports-tyson-arkansas-covid-19/ Retrieved 06.23.2020, from. [Google Scholar]
- Case J.B., Bailey A.L., Kim A.S., Chen R.E., Diamond M.S. Growth, detection, quantification, and inactivation of SARS-CoV-2. Virology. 2020;548:39–48. doi: 10.1016/j.virol.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collomb J., Laporte J., Vautherot J.F., Schwartzbrod L. [Research on coronaviruses in water. I. Adsorption and elution of the coronavirus on glass powder] Virologie. 1986;37:95–105. [PubMed] [Google Scholar]
- Dai M., Li H., Yan N., Huang J., Zhao L., Xu S., Wu J., Jiang S., Pan C., Liao M. Long-term survival of SARS-CoV-2 on salmon as a source for international transmission. J. Infect. Dis. 2021;223:537–539. doi: 10.1093/infdis/jiaa712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai S., Amarasingam A. 2020. #CoronaJihad: COVID-19, Misinformation, and Anti-muslim Violence in India, researchgate.Net.https://www.researchgate.net/publication/341651003_CoronaJihad_COVID-19_Misinformation_and_Anti-Muslim_Violence_in_India [Google Scholar]
- Dhakal J., Jia M., Joyce J.D., Moore G.A., Ovissipour R., Bertke A.S. Survival of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and herpes simplex virus 1 (HSV-1) on foods stored at refrigerated temperature. Foods. 2021;10 doi: 10.3390/foods10051005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois E., Agier C., Traore O., Hennechart C., Merle G., Cruciere C., Laveran H. Modified concentration method for the detection of enteric viruses on fruits and vegetables by reverse transcriptase-polymerase chain reaction or cell culture. J. Food Protect. 2002;65:1962–1969. doi: 10.4315/0362-028x-65.12.1962. [DOI] [PubMed] [Google Scholar]
- Dyal J.W., Grant M.P., Broadwater K., Bjork A., Waltenburg M.A., Gibbins J.D., Hale C., Silver M., Fischer M., Steinberg J., Basler C.A., Jacobs J.R., Kennedy E.D., Tomasi S., Trout D., Hornsby-Myers J., Oussayef N.L., Delaney L.J., Patel K., Shetty V., Kline K.E., Schroeder B., Herlihy R.K., House J., Jervis R., Clayton J.L., Ortbahn D., Austin C., Berl E., Moore Z., Buss B.F., Stover D., Westergaard R., Pray I., DeBolt M., Person A., Gabel J., Kittle T.S., Hendren P., Rhea C., Holsinger C., Dunn J., Turabelidze G., Ahmed F.S., deFijter S., Pedati C.S., Rattay K., Smith E.E., Luna-Pinto C., Cooley L.A., Saydah S., Preacely N.D., Maddox R.A., Lundeen E., Goodwin B., Karpathy S.E., Griffing S., Jenkins M.M., Lowry G., Schwarz R.D., Yoder J., Peacock G., Walke H.T., Rose D.A., Honein M.A. COVID-19 among workers in meat and poultry processing facilities - 19 States, April 2020. MMWR Morb. Mortal. Wkly. Rep. 2020;69 doi: 10.15585/mmwr.mm6918e3. [DOI] [PubMed] [Google Scholar]
- EFSA . 2021. EFSA and COVID-19.https://www.efsa.europa.eu/en/topics/efsa-and-covid-19 Retrieved July 7, 2021, from. [Google Scholar]
- Esseili M.A., Saif L.J., Farkas T., Wang Q. Feline calicivirus, murine norovirus, porcine sapovirus, and tulane virus survival on postharvest lettuce. Appl. Environ. Microbiol. 2015;81:5085–5092. doi: 10.1128/AEM.00558-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esseili M.A., Wang Q., Zhang Z., Saif L.J. Internalization of sapovirus, a surrogate for norovirus, in romaine lettuce and the effect of lettuce latex on virus infectivity. Appl. Environ. Microbiol. 2012;78:6271–6279. doi: 10.1128/AEM.01295-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faour-Klingbeil D., Osaili T.M., Al-Nabulsi A.A., Jemni M., Todd E.C.D. The public perception of food and non-food related risks of infection and trust in the risk communication during COVID-19 crisis: a study on selected countries from the Arab region. Food Control. 2021;121 doi: 10.1016/j.foodcont.2020.107617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDA . 2021. Food Safety and Availability during the Coronavirus Pandemic.https://www.fda.gov/consumers/consumer-updates/food-safety-and-availability-during-coronavirus-pandemic Retrieved July 7, 2021, from. [Google Scholar]
- FDA . 2021. 7 Tips for Cleaning Fruits, Vegetables.https://www.fda.gov/consumers/consumer-updates/7-tips-cleaning-fruits-vegetables Retrieved July 20, 2021, from. [Google Scholar]
- FDA . 2021. Concentration, Extraction, and Detection of Norovirus and Hepatitis A Virus in Soft Fruit.https://www.fda.gov/media/114183/download Retrieved, 2021, from. [Google Scholar]
- Feng X.L., Li B., Lin H.F., Zheng H.Y., Tian R.R., Luo R.H., Liu M.Q., Jiang R.D., Zheng Y.T., Shi Z.L., Bi Y.H., Yang X.L. Stability of SARS-CoV-2 on the surfaces of three meats in the setting that simulates the cold chain transportation. Virol. Sin. 2021 doi: 10.1007/s12250-021-00367-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger J., Lima E.M.F., Coelho K.S., Behrens J.H., Landgraf M., Franco B., Pinto U.M. Adherence to food hygiene and personal protection recommendations for prevention of COVID-19. Trends Food Sci. Technol. 2021;112:847–852. doi: 10.1016/j.tifs.2021.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gharpure R.H.C., Schnall A.H., et al. Knowledge and practices regarding safe household cleaning and disinfection for COVID-19 prevention- United States. MMWR Morb. Mortal. Wkly. Rep. 2020:705–709. doi: 10.15585/mmwr.mm6923e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddow A.D., Watt T.R., Bloomfield H.A., Shamblin J.D., Dyer D.N., Harbourt D.E. Stability of SARS-CoV-2 on produce following a low-dose aerosol exposure. Am. J. Trop. Med. Hyg. 2020;103:2024–2025. doi: 10.4269/ajtmh.20-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harcourt J., Tamin A., Lu X., Kamili S., Sakthivel S.K., Murray J., Queen K., Tao Y., Paden C.R., Zhang J., Li Y., Uehara A., Wang H., Goldsmith C., Bullock H.A., Wang L., Whitaker B., Lynch B., Gautam R., Schindewolf C., Lokugamage K.G., Scharton D., Plante J.A., Mirchandani D., Widen S.G., Narayanan K., Makino S., Ksiazek T.G., Plante K.S., Weaver S.C., Lindstrom S., Tong S., Menachery V.D., Thornburg N.J. Severe acute respiratory syndrome coronavirus 2 from patient with coronavirus disease, United States. Emerg. Infect. Dis. 2020;26:1266–1273. doi: 10.3201/eid2606.200516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmer D., Gilbert M., Borman R., Clark K.L. Quantitative mRNA expression profiling of ACE 2, a novel homologue of angiotensin converting enzyme. FEBS Lett. 2002;532:107–110. doi: 10.1016/s0014-5793(02)03640-2. [DOI] [PubMed] [Google Scholar]
- Jeong H.W., Kim S.M., Kim H.S., Kim Y.I., Kim J.H., Cho J.Y., Kim S.H., Kang H., Kim S.G., Park S.J., Kim E.H., Choi Y.K. Viable SARS-CoV-2 in various specimens from COVID-19 patients. Clin. Microbiol. Infect. 2020;26:1520–1524. doi: 10.1016/j.cmi.2020.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao L., Li H., Xu J., Yang M., Ma C., Li J., Zhao S., Wang H., Yang Y., Yu W., Wang J., Yang J., Long H., Gao J., Ding K., Wu D., Kuang D., Zhao Y., Liu J., Lu S., Liu H., Peng X. The gastrointestinal tract is an alternative route for SARS-CoV-2 infection in a nonhuman primate model. Gastroenterology. 2021;160:1647–1661. doi: 10.1053/j.gastro.2020.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D.L., Baluja M.Q., Graham D.W., Corbishley A., McDonald J.E., Malham S.K., Hillary L.S., Connor T.R., Gaze W.H., Moura I.B., Wilcox M.H., Farkas K. Shedding of SARS-CoV-2 in feces and urine and its potential role in person-to-person transmission and the environment-based spread of COVID-19. Sci. Total Environ. 2020;749 doi: 10.1016/j.scitotenv.2020.141364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A.C., Zhang A.J., Chan J.F., Li C., Fan Z., Liu F., Chen Y., Liang R., Sridhar S., Cai J.P., Poon V.K., Chan C.C., To K.K., Yuan S., Zhou J., Chu H., Yuen K.Y. Oral SARS-CoV-2 inoculation establishes subclinical respiratory infection with virus shedding in golden Syrian hamsters. Cell Rep. Med. 2020;1 doi: 10.1016/j.xcrm.2020.100121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P., Yang M., Zhao X., Guo Y., Wang L., Zhang J., Lei W., Han W., Jiang F., Liu W.J., Gao G.F., Wu G. Cold-chain transportation in the frozen food industry may have caused a recurrence of COVID-19 cases in destination: successful isolation of SARS-CoV-2 virus from the imported frozen cod package surface. Biosaf. Health. 2020;2:199–201. doi: 10.1016/j.bsheal.2020.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien B., Goodridge L., Ronholm J., Nasheri N. Exploring the potential of foodborne transmission of respiratory viruses. Food Microbiol. 2021;95 doi: 10.1016/j.fm.2020.103709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz-Sola J., Abadias M., Colas-Meda P., Sanchez G., Bobo G., Vinas I. Evaluation of a sanitizing washing step with different chemical disinfectants for the strawberry processing industry. Int. J. Food Microbiol. 2020;334 doi: 10.1016/j.ijfoodmicro.2020.108810. [DOI] [PubMed] [Google Scholar]
- Pastorino B., Touret F., Gilles M., de Lamballerie X., Charrel R.N. Heat inactivation of different types of SARS-CoV-2 samples: what protocols for biosafety, molecular detection and serological diagnostics? Viruses. 2020;12 doi: 10.3390/v12070735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payment P., Trudel M. In: Methods and Techniques in Virology. Payment P., Trudel M., editors. Mercel Deckker Inc.; 1993. Isolation and identification of viruses; p. 14. [Google Scholar]
- Thippareddi H., Balamurugan S., Patel J., Singh M., Brassard J. Coronaviruses - potential human threat from foodborne transmission? Lebensm. Wiss. Technol. 2020 doi: 10.1016/j.lwt.2020.110147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M.S., Feng Y. Food handling practices in the era of COVID-19: a mixed-method longitudinal needs assessment of consumers in the United States. J. Food Protect. 2021;84:1176–1187. doi: 10.4315/JFP-21-006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Xu G., Huang Y.W. Modeling the load of SARS-CoV-2 virus in human expelled particles during coughing and speaking. PLoS One. 2020;15 doi: 10.1371/journal.pone.0241539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . 2021. WHO-Convened Global Study of Origins of SARS-CoV-2: China Part; pp. 116–118.https://www.who.int/publications/i/item/who-convened-global-study-of-origins-of-sars-cov-2-china-part Retrived from. [Google Scholar]
- Wilkinson J. 2021. Ice Cream in China Tests Positive for COVID.https://www.nydailynews.com/news/world/ny-covid-ice-cream-china-20210116-ko73ui4yobbblbvmhyz7jysmq4-story.html Retrieved, from. [Google Scholar]
- Wu Y., Guo C., Tang L., Hong Z., Zhou J., Dong X., Yin H., Xiao Q., Tang Y., Qu X., Kuang L., Fang X., Mishra N., Lu J., Shan H., Jiang G., Huang X. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroenterol. Hepatol. 2020 doi: 10.1016/S2468-1253(20)30083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao F., Sun J., Xu Y., Li F., Huang X., Li H., Zhao J., Huang J., Zhao J. Infectious SARS-CoV-2 in feces of patient with severe COVID-19. Emerg. Infect. Dis. 2020;26 doi: 10.3201/eid2608.200681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yepiz-Gomez M.S., Gerba C.P., Bright K.R. Survival of respiratory viruses on fresh produce. Food Environ. Virol. 2013 doi: 10.1007/s12560-013-9114-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Li C., Liu X., Chiu M.C., Zhao X., Wang D., Wei Y., Lee A., Zhang A.J., Chu H., Cai J.P., Yip C.C., Chan I.H., Wong K.K., Tsang O.T., Chan K.H., Chan J.F., To K.K., Chen H., Yuen K.Y. Infection of bat and human intestinal organoids by SARS-CoV-2. Nat. Med. 2020;26:1077–1083. doi: 10.1038/s41591-020-0912-6. [DOI] [PubMed] [Google Scholar]
- Zhou Z., Zuber S., Cantergiani F., Butot S., Li D., Stroheker T., Devlieghere F., Lima A., Piantini U., Uyttendaele M. Inactivation of viruses and bacteria on strawberries using a levulinic acid plus sodium dodecyl sulfate based sanitizer, taking sensorial and chemical food safety aspects into account. Int. J. Food Microbiol. 2017;257:176–182. doi: 10.1016/j.ijfoodmicro.2017.06.023. [DOI] [PubMed] [Google Scholar]