Abstract
Dyke-Davidoff-Masson syndrome (DMMS) is a non-inherited rare condition with a clinical constellation of hemiparesis/hemiplegia, facial asymmetry, intellectual disability, and epilepsy. The radiological features can be including unilateral cerebral atrophy, calvarial thickening, and hyper pneumatization of the paranasal sinuses. The condition can either be congenital or acquired. The presentation usually occurs during childhood or early adolescents, but there have been adult cases reported.
Here we report a 48-year-old male who was a known poorly controlled epileptic that contracted SARS-CoV-2 with subsequently developed status epilepticus and, when worked up, was shown to have features of DDMS. This case is unique as the patient had hemiatrophy and epilepsy but managed to lead a normal, physically demanding, and high functioning academic career and presented late in life. Perhaps only due to coronavirus disease 2019 (COVID-19) was this diagnosis picked up.
This report contains a case presenting atypical DDMS in status epilepticus and COVID -19 plus other complications. From our knowledge, this is the first case presenting these comorbidities reported to the medical literature.
Keywords: Covid-19, Dyke-Davidoff-Mason Syndrome, status epilepticus, hyperglycaemic hyperosmolar syndrome
Introduction
In 1580, the first respiratory pandemic was reported 1 . Up to date, millions of people died, most of them during the 20 th and 21 st centuries. The most devastating epidemic and outbreaks were the Spanish Flu (500 million infected) during the early 20th century, even bigger than Hong Kong flu, swine flu, SARS-CoV-1 (2003), and the MERS-CoV outbreak (2012) 1– 3 . However, the first pandemic causing encephalitis was reported soon after 1580 3 .
Since 1965, when human coronaviruses were discovered 4 , several types of coronavirus (CoV) have been reported, including SARS-CoV-2, SARS-CoV-1, and MERS-CoV, which are all responsible for three epidemics, plus others four types that also infect many human beings (HCoV-229E, HCoV-OC43, HCoV-NL63, and HCoV-HKU1) 5 . Based on several mechanisms, coronaviruses affect the peripheral and the central nervous system. Even before SARS-CoV-2, other types of coronavirus, such as SARS-CoV-1, HCoV-229E, and HCoV-OC43, also damage the nervous system 5 .
Wuhan is a large city and the capital of Hubei Province in the People's Republic of China. Wuhan has a population of around 11 million persons. At the beginning of December 2019, an outbreak of many persons presenting viral pneumonia of an unknown agent was reported. In the following month (January 7, 2020), some Chinese authors identified the etiological agent of that respiratory disease and called it by 2019-nCoV (for 2019 novel coronavirus) 6– 8 .
Since December 2019, documented an increasing number of cases presenting the novel coronavirus disease of 2019 (nCOVID-19) and associated neurological manifestation are published every month. CoV also caused neurological lesions like anosmia and ageusia with different prevalence in China (5%) 6 or in Italy (88%) 9 .
In a recent study by Dorche et al., the following list of neurological complications were observed: headache and dizziness (the most common on initial presentation), fatal encephalitis with HCoV-OC43 (two immunosuppressed infants), acute disseminated encephalomyelitis (one 15-year-old boy with HCoV-OC43 and four adults with SARS-CoV-2), acute flaccid paralysis (HCoV 229E) and OC43 (one 3-year-old girl), ischemic (1.3%) and hemorrhage (0.5%) strokes, encephalitis with SARS-CoV-1 RNA (one 39-year-old patient), different presentation of Guillain-Barré syndrome, cerebral venous sinus thrombosis (13 patients in nine studies), acute encephalomyelitis (four patients), acute myelitis (five patients), optic neuritis (one patient) altered level of consciousness (nonconvulsive status epilepticus, infections, parenchymal lesions, electrolyte disturbances, hypoxic, toxic and metabolic encephalopathies), leukoencephalopathy (18 patients in three studies), acute necrotizing encephalopathy (eight patients), other encephalitis (22 patients out of 13 reviews), mild encephalitis/encephalopathy with a reversible splenial lesion(MERS), posterior reversible encephalopathy syndrome (PRES), and Bickerstaff's encephalitis (BBE), 10– 14 . In patients infected by SARS-CoV-1 and SARS-CoV-2, epileptic seizures have been reported 13 also in infected patients with MERS-CoV 12 and SARS-CoV-2. In COVID-19, (48 epileptic patients out of 20 studies), visual impairments (12 patients out of 3 reviews), impaired eye movement mainly due to Abducens nerve palsy (12 patients out of 4 reviews), trigeminal neuropathy (in 9 patients out of 2 studies), Miller-Fisher syndrome (52 patients out of 36 studies), skeletal muscle injury and muscular diseases Have been reported 10 .
Nepal and colleagues 15 reported one case of Bell's palsy, as a neurological presentation of COVID-19. Another group of authors published two new patient cases but did not include enough supporting information to draw firm conclusions 16 . At the same time, other authors have published cases presenting generalized epileptic seizures 17– 22 .
One case of focal status epilepticus (SE) was reported by Vollono et al. 23 and acute epileptic encephalopathy by others 24– 26 , including the treatment for these conditions 27 .
A systematic review done by Ghannam et al. found two cases of SE, one of which had a past medical history of epilepsy from another cause 28 . Gelisse et al. established that some patients with severe SARS-CoV-2 infection are at risk of subclinical epileptic seizures or even nonconvulsive status epilepticus (NCSE) and recommend video EEG monitoring in some cases 29 .
Recently, some authors have speculated that acute epileptic seizures may be due to swelling of the brain cortex (encephalitis) and the direct damage of the brain cortex by the virus because SARS-CoV-2 can be present in the cerebrospinal fluid (CSF) of some patients 18, 30, 31 .
In other extensive studies involving several hundreds of COVID-19 patients, the authors concluded that none of their cases had acute symptomatic seizures or SE 20, 32– 42 .
Nevertheless, the retrospective case series published by Somani and collaborators 25 deserves special mention. These investigators published the electroencephalographic findings and clinical manifestations of two COVID-19 patients with new-onset SE without a previous history of epilepsy or acute epileptic seizures. Both patients had SARS-CoV-2 pneumonia confirmed by CT scan and PCR; however, the authors did not perform CSF and could not rule out meningoencephalitis. The second patient presented a new-onset refractory status epilepticus. The same author established the neurovirulence of SARS-CoV-1, finding the presence of viral antigen in the thalami, hippocampus, medulla oblongata, and mesencephalic regions that regulate cardiorespiratory functions in a human autopsy series 25 . Some recent good news is the excellent response of SE to levetiracetam reported by two investigators 24, 25 .
Other investigators also recommend the use of verapamil in patients presenting SE stage III and SARS-Cov-2 infection 43 . The same authors reported the first patient affected by PRES and SARS-CoV-2 without SE. In contrast, Mohammad et al. wrote about a 32-year-old male with tonic-clonic generalized SE 44 . In the meantime, other investigators delivered essential recommendations to improve the management of SE during the pandemic despite the lack of ventilators and ICU facilities 45 .
Acquired or congenital (infantile) cerebral hemiatrophy, otherwise referred to as Dyke-Davidoff-Masson syndrome (DDMS), was first described in 1933 by Dyke and colleagues 46– 48 . DDMS is a non-inherited rare condition 49 , with an unknown frequency; most of the literature stems from either case reports or series 50 . DDMS is a diagnostic constellation made up of hemiparesis/hemiplegia, facial asymmetry, intellectual disability, and treatment-resistant epilepsy, classically with distinct neuroimaging features 48 . However, according to Ayaz et al., the syndrome has varied clinical and radiological spectrum presenting at different life stages 51 . The classical imaging findings are hypoplasia of one brain hemisphere (hemiatrophy), often accompanied by volume reduction of corresponding cranial fossa and thickening of nearby bony structures and equilateral enlargement paranasal sinuses, the frontal sinus being the most involved or hyperpneumotisation of mastoid air cells. The congenital type can be due to insults suffered during fetal or early childhood development, such as ischemia, trauma, infarction, hemorrhage, and infections. However, the acquired type is usually associated with trauma, infectious diseases, or hemorrhages after one month of age 47 . We know that hemispherectomy is the best treatment for patients who have drug-resistant and disabling seizures.
At the time of writing, the coronavirus disease-19 (COVID-19) pandemic continues infecting peoples worldwide. COVID-19, caused by SARS-CoV-2, has thus far claimed 23,057,288 cases worldwide 52 and 607 045 patients in South Africa 53 . Up to date, 46 medical doctors died in the Eastern Cape province alone.
We performed an extensive search of the medical literature to answer our research question: "What is the reported frequency of status epilepticus in patients with DDMS and coronavirus infections?
Case presentation
A 48-year-old African male patient was admitted to Nelson Mandela Academic Central Hospital (NMACH) in Mthatha, South Africa. He was born out of a non-consanguineous marriage and was referred from a regional hospital with tonic-clonic-generalized status epilepticus. On initial presentation to the base hospital, he was given diazepam 10 mg IV stat (dose repeated twice) and then loaded with phenytoin 750 mg.
This patient had a past medical history of chronic epilepsy for many years but was well-controlled on valproate acid CR 500 mg PO Bd, levetiracetam 750 mg PO BD per day. There was no facial asymmetry, no hemiplegia, the rest of the cognitive functions were average, and there are no mental retardation signs.
He was also a chronic hypertensive. He worked as a police officer on further inquiry and he did not smoke, consume drugs or alcohol. We did not obtain remarkable information on birth history, developmental milestones, education history, prior admissions to hospital, and childhood illnesses.
We found no noticeable body asymmetry on examination. The patient had pink mucous membranes, was well hydrated, and afebrile with a GCS 11/15 (E3V3M5); his motor examination revealed a power 3/5 with spastic hypertonia on left upper and lower limbs, and no fits noted.
He was in respiratory distress with tachypnea of 30 breaths/minute saturating at 84% on a 40% venture face mask. The rest of the vital signs showed a BP 118/88 mmHg and Pulse 98 bpm. He had scattered crepitation on the chest bilaterally. Table 1 shows all blood test results.
Table 1. Blood test results.
| Blood test variables | Patient value | Normal range |
|---|---|---|
| White cell count | 5.30 × 10 9/L | 3.9–12.6 × 10 9/L |
| Hb | 16.1 g/dL | 12–15 g/dl |
| Platelets | 365 × 10 9/L | 186–454×10 9/L |
| Sodium | 141 mmol/L | 136 – 145 mmol/L |
| Potassium | 3.8 mmol/L | 3.5–5.1 mmol/L |
| Chloride | 113 mmol/L | 98–105 mmol/L |
| Urea | 8.8 mmol/L | 2.1–7.1 mmol/L |
| Creatinine | 122 µmol/L | 48–90 µmol/L |
| Calcium | 2.20 mmol/L | 2.15–2.5 mmol/L |
| Magnesium | 0.87 mmol/L | 0.63–1.05 mmol/L |
| Phosphate | 1.40mmol/L | 0.78–1.42 mmol/L |
| C–reactive protein | 23 mg/L | <10 mg/L |
| Erythrocyte
sedimentation rate |
12 mm/hr | 0–10 mm/hr |
| Total protein | 72 g/L | 60–78 g/L |
| Total Bilirubin | <4 µmol/L | 5–21 µmol/L |
| Alkaline phosphatase | 90 U/L | 42–98 U/L |
| Aspartate transaminase | 23 U/L | 13–35 U/L |
| Alanine transaminase | 19 U/L | 7–35 U/L |
| Total cholesterol | 4.78 mmol/L | <4.5 mmol/L |
| HbA1C | 5.1% | <7% |
| Valproate level | 427 µmol/L, | 346.70–693.40 µmol/L |
| Phenytoin level | 139 µmol/L | 20–40 µmol/L |
Serum levels of interleukine-6 were not available. PCR confirmed SARS-CoV infection, but we did not perform ferritin and procalcitonin investigations.
The patient presented with a one-day SE type II (established), characterized by recurrent tonic-clonic generalized seizures with impaired awareness. As he did not recover, progressive doses of anti-seizure medication (ASM) were administered, reaching a total of 20 mg of diazepam (10 mg IV twice), 1500 mg of phenytoin (IV bolus), and 1500 mg of valproate (25 mg/kg), without recovering. An urgent cranial CT scan of the brain revealed atrophy on the right cerebral hemisphere with associated thickening of the calvarium on the same side without hyperpneumotisation of paranasal sinuses or mastoid air cells ( Figure 1, Figure 2 and Figure 3), suggestive of Dyke-Davidoff-Masson Syndrome; otherwise, there was no bleed or area of infarct, and there was no space-occupying lesion.
Figure 1. CT scan of the brain (coronal view).

Shows a notable atrophy of the right cerebral hemisphere with enlargement of the ipsilateral lateral ventricle.
Figure 2. CT scan of the brain (axial view).

Shows asymmetry of the lateral ventricles (right to left) with a notable atrophy of the right cerebral hemisphere.
Figure 3. CT scan of the head (axial view).
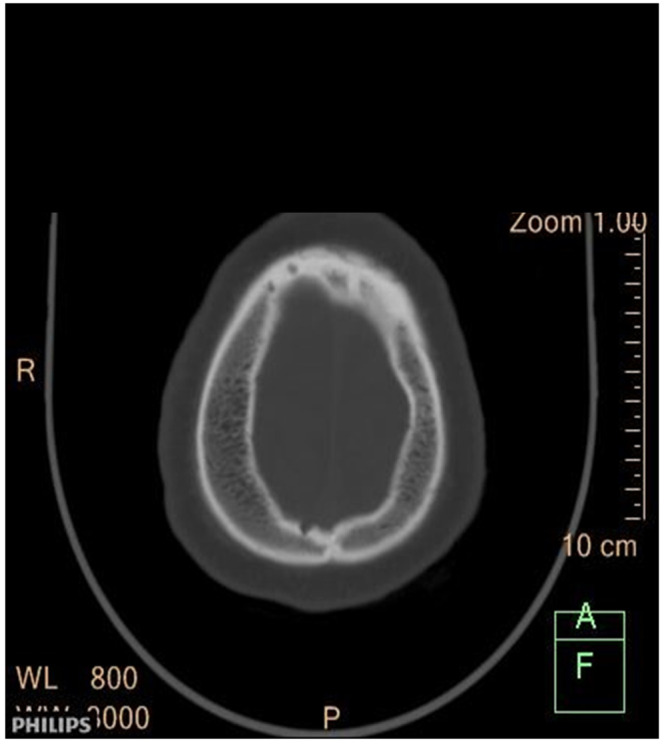
Shows a marked thickness on the right side of the skull.
On the second day of admission, the patient was admitted to the COVID ward, put on high-flow nasal oxygen (60% at 15 L/min), dexamethasone 8 mg IV daily, Clexane 60 mg SC 12-hourly, ceftriaxone 1 g IV every day, azithromycin 500 mg Po daily, vitamin D 50000 U PO weekly, vitamin C 250 mg PO 8-hourly, diazepam 10 mg IV if fitting, valproate 500 mg IV 12-hourly, phenytoin 100 mg IV 8-hourly, amlodipine 10 mg orally daily, Ridaq 25 mg orally daily and intravenous fluids (1 L Ringers lactate IV 8-hourly).
After two days of admission, the patient improved neurologically and presented no more seizures, but his respiratory distress continued progressively getting worse, and his septic markers were rising. Two days later, blood levels showed values of Na 159 mmol/L, K 5.1 mmol/L, urea 42 mmol/L, creatinine 275 µmol/L, CRP 117 mg/dL, T protein 88 g/L, Alb 38 g/L, ALT 48 U/L, AST 168 U/L, GGT 190 U/L, ALP 58U/L, white cell count 14.30 × 10 9/L, Hb 17.1g/dl, platelet count 316 × 10 9/L cholesterol level no done, Blood gas showed Ph. 7.46, PaCO 2 36 mmHG, PaO 2 66mmHg, HCO 3 27mmol/L, Na 160 mmol/L, K 3.5 mmol/L, Ca 1.05 mmol/L, Hgt 25.6 mmol/L, blood oxygen saturation 84%. The patient's urine did not contain ketone bodies.
On the fifth day after admission, the patient was assessed as having ARDS secondary to COVID-19 and hyperglycemic hyperosmolar state (HHS), and high-flow nasal O 2 was increased to 100% concentration at 20 L/min. One and a half hours after the onset of the symptoms, the patient had not recovered yet. The patient began to fit again and under the suspicion of refractory status epilepticus secondary to HHS and neuro-COVID 19; when another round of 20 mg of diazepam (10 mg IV twice), 1500 mg of phenytoin (IV bolus), and 1500 mg of valproate (25 mg/kg) was started, the patient developed cardiac arrest and demised.
Discussion and literature review
Our literature review utilized the Preferred Reporting Items for Systemic review and Meta-Analysis statement. However, we did not conduct a classical systematic review.
We reviewed the databases published before August 20, 2020, such as Medline EMBASE, Scopus online databases, Google Scholar, to identify articles evaluating COVID-19 and SE in DDMS. All items about "neurologic complications* OR epilepsy* OR brain* OR status epilepticus* OR fits* OR neuronal lesion* OR Neuro-Covid* OR cortical lesions* OR DDMS OR * OR seizure* OR COVID-19* OR unconsciousness* OR acute epileptic seizure*, OR Duke Davidoff Mason Syndrome*" where * is the PubMed wildcard for every possible word beginning or ending. Other neurological combinations were considered beyond the scope of the current work and no included. Finally, we did not find a publication related to COVID-19, SE and DDMS.
Our patient complained of chronic arterial hypertension, and this condition and diabetes mellitus is associated with a significant risk of lung disease leading to COVID-19 severity. Despite the patient's condition, antihypertensive therapy should continue in COVID-19 patients 54 . Concerning our patient, it's important to highlight that diabetes mellitus by itself is one of the most relevant comorbidities associated with the severity of all coronavirus infections, including the current SARS-CoV-2, and affected cases have an increased risk to develop severe complications such as acute respiratory distress syndrome and systemic organ failure 55 . COVID-19 patients with hyperglycemia are at risk of developing other infections, including influenza and pneumonia with increasing mortality rate; this is also applicable to other SARS coronavirus, pandemic influenza A 2009 (H1N1), and middle east respiratory syndrome coronavirus 56– 59 .
Here we discuss elevated urea and creatinine in our patient. Therefore, it is essential to mention that apart from diabetes and hypertension, acute kidney injury has also been documented in some patients with COVID-19. ACE2 gene expression in renal cells and bladders cells has been investigated, and the results confirmed damage of the renal proximal tubule cells and the bladder epithelial cells by COVID-19 infection 60 . SARS-CoV-2 affects the kidneys 61 , which has been confirmed by examining viral nucleocapsid protein accumulated in the renal tubules by post-mortem examination proved that 62 .
DDMS is due to atrophy of one cerebral hemisphere and usually occurs due to an insult to the brain in utero or an early period of childhood 63 . In the first description, Dyke, Davidoff, and Masson described nine patients who had a constellation of seizures, facial asymmetry, mental retardation, and hemiparesis with bare skull X-ray changes (ipsilateral osseous hypertrophy and calvarial thickening) 64 . We can understand intracranial pathology with MRI and CT scans, which results in such clinical presentation. Our patient is atypical because he did not complain of weakness on the left half body, was strong enough to work as a police officer, and there was no evidence of mental retardation was observed.
Some patients with DDMS can complain of psychiatric manifestations in rare instances 65 . The radiological features can include unilateral cerebral atrophy, calvarial thickening, and hyperpneumotisation of the paranasal sinuses 66 . As with our patient, mental retardation does not always need to be present, and the seizures may develop years after the initial insult 67 .
Some authors classify DDMS as either congenital/primary or acquired/secondary. The congenital form occurs due to an insult that happens in utero. It could be infections or vascular disorders occurring during the gestational period (unilateral cerebral artery pathologies or mid aortic arch coarctation) 68 . The congenital forms usually present during the perinatal period. The acquired form occurs due to early childhood infections, trauma, tumors, asphyxia, intracranial ischemia, or hemorrhage.
To better understand the anatomical changes occurring, it is crucial to understand the brain's growth and surrounding structure. The mail sulci form around 3 months’ gestation up to approximately eight months of pregnancy. If there are no prominent sulci visible on imaging, the congenital form of DDMS is present. Most brain and skull development occurs during the first three years of life (reaching 75% of adult size). The outward pressure of the brain parenchyma on the skull contributes to this growth. If there is unilateral atrophy, then the surrounding structures will grow inwards (calvarial thickening, enlarged sinuses, increased width of diploid spaces 69, 70 . Hangmen et al. proposed that the congenital form of DDMS be named unilateral cerebral hypoplasia because there is hypoplasia instead of atrophy 71 .
A literature review done by Unal et al. showed that in the pediatric presentations, there is a male predisposition towards DDMS, and the left hemisphere is more commonly affected; the mean age at diagnosis was 11 in this review 72 . However, a literature review done by Diestro et al. in 2018 comprising 21 patients with a mean age at presentation being 31 years old showed a slight female despondence, and these adult presentations more commonly involving the right cerebral hemisphere. In 28% of cases (6/21), there was no mental retardation, and in 14% (3/21), it was unknown whether there was mental retardation 73 .
The association of SARS-CoV and seizures was known even before the current pandemic. In 2003 authors published the first observed case 74 . The following year, other patients were reported 75 , and later another case was associated with a different coronavirus 76 . However, the total number of reported cases is small. SE and encephalopathy have been reported in children as a presentation of COVID-19. The mechanism of production of seizures is also known 77 . These authors 77, 78 proposed that epileptic seizures can be due to several mechanisms, such as direct infection of the virus, a post-infectious mechanism, an autoimmune response, hematogenous pathway and thrombosis 79 , by dysregulated cytokine storm 79 , and by the retrograde neural way, hypoxia, and via the ACE-2 enzyme 80 .
Association between DDMS and SE is hugely uncommon, and before the current COVID-19 pandemic, only three adolescent patients have been reported: one in 2015 81 and the other two in 2018 82 . Other recent systematic reviews, case series, and case reports did not mention any association of DDMS and SE 51, 63, 73, 83– 90 . At the time of writing (August 25, 2020), no patients presenting DDMS and SE infected by COVID-19 have been reported to the medical literature.
Differential diagnoses, such as Silver-Russell syndrome, basal ganglia germinoma, neurofibromatosis, Parry-Romberg Syndrome, Sturge-Weber syndrome, Rasmussen encephalitis, Fishman syndrome, linear nevus syndrome, and Rasmussen encephalitis, should be considered during the management of these patients.
Finally, we want to highlight some precautions to be considered when treating patients in SE and COVID-19. As aforementioned, DDMS causes epilepsy, epileptic seizures, and even SE. On the other hand, COVID-19 can also cause epileptic seizures and SE. However, the use of some ASM and anti-COVID medicines may cause complications. Therefore, some medications should be used with caution. For example, lacosamide is recommended for the adjunctive treatment of partial-onset seizures, diabetic neuropathic pain, and to control attacks in refractory SE. However, it can prolong the PR interval on an electrocardiogram. Hydroxychloroquine extends the QT interval 91 . Elongation of the QT interval can also be caused by azithromycin, phenytoin, carbamazepine, and rufinamide, leading to cardiac conduction disturbances 13 . Combining ASM with hydroxychloroquine and azithromycin can be harmful. Therefore, we recommend EKG monitoring.
Conclusion
In our opinion, the SE in our patient had a multifactorial origin, including HHS and atypical DDMS. This could have created a susceptible environment in which the new coronavirus's disease acted as a SE trigger. These hypotheses make this case a unique report.
To our knowledge, this patient is the first case of SARS-CoV-2 infection leading to TCG-SE type 2 on a DDMS patient published in the medical literature.
Data availability
All data underlying the results are available as part of the article and no additional source data are required.
Consent
Written informed consent for publication of their clinical details and clinical images was obtained from the relatives of the patient.
Acknowledgments
Special thanks to Dr. A Anwary from the Department of Radiology, NMACH. Mthatha, South Africa, for his report on images.
Funding Statement
The author(s) declared that no grants were involved in supporting this work.
[version 1; peer review: 2 approved]
References
- 1. Martin PMV, Martin-Granel E: 2,500-year evolution of the term epidemic. Emerg Infect Dis. 2006;12(6):976–980. 10.3201/eid1206.051263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Troyer EA, Kohn JN, Hong S: Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19. Neuropsychiatric symptoms and potential immunologic mechanisms. Brain Behav Immun. 2020;87:34–39. 10.1016/j.bbi.2020.04.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cunha BA: Influenza: historical aspects of epidemics and pandemics. Infect Dis Clin North Am. 2004;18(1):141–155. 10.1016/S0891-5520(03)00095-3 [DOI] [PubMed] [Google Scholar]
- 4. Kahn JS, McIntosh K: History and recent advances in coronavirus discovery. Pediatr Infect Dis J. 2005;24(11 Suppl):S223– S227. 10.1097/01.inf.0000188166.17324.60 [DOI] [PubMed] [Google Scholar]
- 5. Zhu N, Zhang D, Wang W, et al. : A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. 10.1056/NEJMoa2001017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schwartz DA, Graham AL: Potential Maternal and Infant Outcomes from Coronavirus 2019-nCoV (SARS-CoV-2) Infecting Pregnant Women: Lessons from SARS, MERS, and Other Human Coronavirus Infections. Viruses. 2020;12(2):194. 10.3390/v12020194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ma K, chen T, Han MF: [Management and clinical thinking of Coronavirus Disease 2019]. Zhonghua Gan Zang Bing Za Zhi. 2020;28(0):E002. 10.3760/cma.j.issn.1007-3418.2020.0002 [DOI] [PubMed] [Google Scholar]
- 8. Mao L, Jin H, Wang M, et al. : Neurologic Manifestations of Hospitalized Patients With Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683–690. 10.1001/jamaneurol.2020.1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lechien JR, Chiesa-Estomba CM, Cabaraux P, et al. : Features of Mild-to-Moderate COVID-19 Patients with Dysphonia. J Voice. 2020; S0892-1997(20)30183-1. 10.1016/j.jvoice.2020.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dorche MS, Huot Ph, Osherov M, et al. : Neurological complications of coronavirus infection; a comparative review and lessons learned during the COVID-19 pandemic. J Neurol Sci. 2020;417:117085. 10.1016/j.jns.2020.117085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pinna P, Grewal P, Hall JP, et al. : Neurological manifestations, and COVID-19: Experiences from a tertiary care center at the Frontline. J Neurol Sci. 2020;415:116969. 10.1016/j.jns.2020.116969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jain R, Young M, Dogra S, et al. : COVID-19 related neuroimaging findings: A signal of thromboembolic complications a strong prognostic marker of poor patient outcome. J Neurol Sci. 2020;414:116923. 10.1016/j.jns.2020.116923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Asadi-Pooya AA: Seizures associated with coronavirus infections. Seizure. 2020;79:49–52. 10.1016/j.seizure.2020.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen T, Wu D, Chen H: Clinical characteristics of 113 deceased patients with coronavirus disease 2019: a retrospective study. BMJ. 2020;368: m1091. 10.1136/bmj.m1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nepal G, Rehrig JH, Shrestha GS, et al. : Neurological manifestations of COVID-19: a systematic review. Crit Care. 2020;24(1):421. 10.1186/s13054-020-03121-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pinzon RT, Wijaya VO, Buana RB, et al. : Neurologic Characteristics in Coronavirus Disease 2019 (COVID-19): A Systematic Review and Meta-Analysis. Front Neurol. 2020;11:565. 10.3389/fneur.2020.00565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hao X, Zhou D, Li Z, et al. : Severe psychological distress among epilepsy patients during the COVID-19 outbreak in southwest China. Epilepsia. 2020;61(6):1166–1173. 10.1111/epi.16544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Moriguchi T, Harii N, Goto J, et al. : A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int J Infect Dis. 2020;94:55–58. 10.1016/j.ijid.2020.03.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Duong L, Xu P, Liu A: Meningoencephalitis without respiratory failure in a young female patient with COVID-19 infection in downtown Los Angeles, early April 2020. Brain Behav Immun. 2020;87:33. 10.1016/j.bbi.2020.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lu L, Xiong W, Liu D, et al. : New-onset acute symptomatic seizure and risk factors in coronavirus disease 2019: a retrospective multicenter study. Epilepsia. 2020;61(6):e49–e53. 10.1111/epi.16524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fasano A, Cavallieri F, Canali E, et al. : First motor seizure as presenting symptom of SARS-CoV-2 infection. Neurol Sci. 2020;41(7):1651–1653. 10.1007/s10072-020-04460-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Leonardi M, Padovani A, McArthur JC: Neurological manifestations associated with COVID-19: a review and a call for action. J Neurol. 2020;267(6):1573–1576. 10.1007/s00415-020-09896-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vollono C, Rollo E, Romozzi M, et al. : Focal status epilepticus as a unique clinical feature of COVID-19: a case report. Seizure. 2020;78:109–112. 10.1016/j.seizure.2020.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Balloy G, Leclair-Visonneau L, Péréon Y, et al. : Non-lesional status epilepticus in a patient with coronavirus disease 2019. Clin Neurophysiol. 2020 ;131(8):2059–2061. 10.1016/j.clinph.2020.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Somani S, Pati S, Gaston T, et al. : De Novo status epilepticus in patients with COVID-19. Ann Clin Transl Neurol. 2020;7(7):1240–1244. 10.1002/acn3.51071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Katal S, Balakrishnan S, Gholamrezanezhad A: Neuroimaging and neurologic findings in COVID-19 and other coronavirus infections: A systematic review in 116 patients. J Neuroradiol. 2020; S0150-9861(20)30204-2. 10.1016/j.neurad.2020.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Orsucci D, Ienco EC, Nocita G, et al. : Neurological features of COVID-19 and their treatment: a review. Drugs Context. 2020;9: 2020-5-1. 10.7573/dic.2020-5-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ghannam M, Alshaer Q, Al-Chalabi M, et al. : Neurological involvement of coronavirus disease 2019: a systematic review. J Neurol. 2020;267(11):3135–3153. 10.1007/s00415-020-09990-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gelisse P, Rossetti AO, Genton P, et al. : How to carry out and interpret EEG recordings in COVID-19 patients in ICU. Clin Neurophysiol. 2020;131( 8):2023–2031. 10.1016/j.clinph.2020.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Niazkar HR, Zibaee B, Nasimi A, et al. : The neurological manifestations of COVID-19: a review article. Neurol Sci. 2020;41(7):1667–1671. 10.1007/s10072-020-04486-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chen X, Laurent S, Onur OA, et al. : A systematic review of neurological symptoms and complications of COVID-19. J Neurol. 2020;1–11. 10.1007/s00415-020-10067-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang L, Shen Y, Li M, et al. : Clinical manifestations and evidence of neurological involvement in 2019 novel coronavirus SARS-CoV-2: a systematic review and meta-analysis. J Neurol. 2020;267(10):2777–2789. 10.1007/s00415-020-09974-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Butala N: Neurological Aspects of Coronavirus Infectious Disease 2019 (COVID-19). Innov Clin Neurosci. 2020;17(4–6):13–15. [PMC free article] [PubMed] [Google Scholar]
- 34. García-Azorín D, Martínez-Pías E, Trigo J, et al. : Neurological Comorbidity Is a Predictor of Death in Covid-19 Disease: A Cohort Study on 576 Patients. Front Neurol. 2020;11:781. 10.3389/fneur.2020.00781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bridwell R, Long B, Gottlieb M: Neurologic complications of COVID-19. Am J Emerg Med. 2020;38(7):1549.e3–1549.e7. 10.1016/j.ajem.2020.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Alomari SO, Abou-Mrad Z, Bydon A: COVID-19 and the Central Nervous System. Clin Neurol Neurosurg. 2020;198:106116. 10.1016/j.clineuro.2020.106116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yavarpour-Bali H, Ghasemi-Kasman M: Update on neurological manifestations of COVID-19. Life Sci. 2020;257:118063. 10.1016/j.lfs.2020.118063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Needham EJ, Chou SHY, Coles AJ, et al. : Neurological Implications of COVID-19 Infections. Neurocrit Care. 2020;32(3):667–671. 10.1007/s12028-020-00978-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sheraton M, Deo N, Kashyap R, et al. : A Review of Neurological Complications of COVID-19. Cureus. 2020;12(5):e8192. 10.7759/cureus.8192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mao L, Jin H, Wang M, et al. : Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683–690. 10.1001/jamaneurol.2020.1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ellul MA, Benjamin L, Singh B, et al. : Neurological associations of COVID-19. Lancet Neurol. 2020;19(9):767–783. 10.1016/S1474-4422(20)30221-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Garg RK: Spectrum of Neurological Manifestations in Covid-19: A Review. Neurol India. 2020;68(3):560–572. 10.4103/0028-3886.289000 [DOI] [PubMed] [Google Scholar]
- 43. Gómez-Enjuto S, Hernando-Requejo V, Lapeña-Motilva J, et al. : Verapamil as treatment for refractory status epilepticus secondary to PRES syndrome on a SARS-Cov-2 infected patient. Seizure. 2020;80:157–158. 10.1016/j.seizure.2020.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Abdulsalam MA, Abdulsalam AJ, Shehab D: Generalized status epilepticus as a possible manifestation of COVID-19. Acta Neurol Scand. 2020;142(4):297–298. 10.1111/ane.13321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kinney MO, Brigo F, Kaplan PW: Optimizing status epilepticus care during the COVID-19 pandemic. Epilepsy Behav. 2020;109:107124. 10.1016/j.yebeh.2020.107124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dyke C, Davidoff L, Masson C: Cerebral hemiatrophy & homolateral atrophy of the skull and sinuses. Surg Gynecol Obs. 1932;57:588–600. [Google Scholar]
- 47. Dilber B, Sahin S, Eyüboğlu I: Two Different Manifestations of Neonatal Vascular Injury: Dyke-Davidoff-Masson Syndrome and Crossed Cerebellar Atrophy. J Stroke Cerebrovasc Dis. 2020;29(3):104600. 10.1016/j.jstrokecerebrovasdis.2019.104600 [DOI] [PubMed] [Google Scholar]
- 48. Adebayo PB, Bakare A, Bello MM, et al. : Dyke-Davidoff-Masson syndrome in a Nigerian. Epilepsy Behav Case Rep. 2016;7:10–12. 10.1016/j.ebcr.2016.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kalaskar R, Kalaskar AR: Classical oral manifestations of Dyke-Davidoff-Masson syndrome: A case report with review of the literature. J Korean Assoc Oral Maxillofac Surg. 2018;44(4):198–203. 10.5125/jkaoms.2018.44.4.198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rashid AMA, Md Noh MSF: Dyke-Davidoff-Masson syndrome: a case report. BMC Neurol. 2018[cited 2020 Aug 15];18(1):76. 10.1186/s12883-018-1079-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bjelan M, Kozic D, Njagulj V, et al. : DYKE-DAVIDOFF-MASSON SYNDROME - TYPICAL IMAGING FEATURES. Med Pregl. 2016;69(11–12):373–5. 10.2298/mpns1612373b [DOI] [PubMed] [Google Scholar]
- 52. Coronavirus disease (COVID-19). [cited 2020 Aug 25]. Reference Source [Google Scholar]
- 53. COVID-19 South African coronavirus news and information. [cited 2020, August 20]. Reference Source [Google Scholar]
- 54. Sanchis-Gomar F, Lavie CJ, Perez-Quilis C, et al. : Angiotensin-Converting Enzyme 2 and Antihypertensives (Angiotensin Receptor Blockers and Angiotensin-Converting Enzyme Inhibitors) in Coronavirus Disease 2019. Mayo Clin Proc. 2020;95(6):1222–1230. 10.1016/j.mayocp.2020.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bornstein SR, Rubino F, Khunti K, et al. : Practical recommendations for the management of diabetes in patients with COVID-19. Lancet Diabetes Endocrinol. 2020;8(6):546–550. 10.1016/S2213-8587(20)30152-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gupta R, Ghosh A, Singh AK, et al. : Clinical considerations for patients with diabetes in times of COVID-19 epidemic. Diabetes Metab Syndr. 2020;14(3):211–212. 10.1016/j.dsx.2020.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Yang JK, Feng Y, Yuan MY, et al. : Plasma glucose levels and diabetes are independent predictors for mortality and morbidity in patients with SARS. Diabet Med. 2006;23(6):623–628. 10.1111/j.1464-5491.2006.01861.x [DOI] [PubMed] [Google Scholar]
- 58. Schoen K, Horvat N, Guerreiro NFC, et al. : Spectrum of clinical and radiographic findings in patients diagnosed with H1N1 and correlation with clinical severity. BMC Infect Dis. 2019;19(1):964. 10.1186/s12879-019-4592-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Song Z, Xu Y, Bao L, et al. : From SARS to MERS, thrusting coronaviruses into the spotlight. Viruses. 2019;11(1):59. 10.3390/v11010059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lin W, Hu L, Zhang Y, et al. : Single-cell analysis of ACE2 expression in human kidneys and bladders reveals a potential route of 2019-nCoV infection. bioRxiv. 2020; (Reviewed on August 23, 2020). 10.1101/2020.02.08.939892 [DOI] [Google Scholar]
- 61. Diao B, Feng Z, Wang C, et al. : Human kidney is a target for novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. medRxiv. 2020. 10.1101/2020.03.04.20031120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Perico L, Benigni A, Remuzzi G: Should COVID-19 concern nephrologists? Why and to what extent? The emerging impasse of angiotensin blockade. Nephron. 2020;144(5):213–221. 10.1159/000507305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Jilowa CS, Meena PS, Rohilla J, et al. : Dyke-Davidoff-Masson syndrome. Neurol India. 2017;65(2):413–4. 10.4103/neuroindia.NI_1004_15 [DOI] [PubMed] [Google Scholar]
- 64. Dyke CG, Davidoff LM, Masson CB: Cerebral hemiatrophy and homolateral hypertrophy of the skull and sinuses. Surg Gynecol Obstet. 1933;57:588–600. [Google Scholar]
- 65. Amann B, Garcia de la Iglesia C, Mckenna P, et al. : Treatment-refractory schizoaffective disorder in a patient with dyke-davidoff-masson syndrome. CNS Spectr. 2009;14(1):36–9. 10.1017/s1092852900020034 [DOI] [PubMed] [Google Scholar]
- 66. Pendse NA, Bapna P, Menghani V, et al. : Dyke-Davidoff-Masson syndrome (DDMS). Indian J Pediatr. 2004;71(10):943. 10.1007/BF02830843 [DOI] [PubMed] [Google Scholar]
- 67. Sener RN, Jinkins JR: MR of craniocerebral hemiatrophy. Clin Imaging. 1992;16(2):93–7. 10.1016/0899-7071(92)90119-t [DOI] [PubMed] [Google Scholar]
- 68. Graham A, Molnar Z: Development of the nervous system. In Standring S (ed): Gray's Anatomy, ed 40. London, Churchill Livingstone, Elsevier.2008;385. [Google Scholar]
- 69. Solomon GE, Hilal SK, Gold AP, et al. : Natural history of acute hemiplegia of childhood. Brain. 1970;93(1):107–120. 10.1093/brain/93.1.107 [DOI] [PubMed] [Google Scholar]
- 70. Sharma S, Goyal D, Negi A, et al. : Dyke–Davidoff–Masson syndrome. Indian J Radiol Imaging. 2006;16(2):165–6. 10.4103/0971-3026.29077 [DOI] [Google Scholar]
- 71. Hageman G, Gooskens RH, Willemse J: A cerebral cause of arthrogryposis: Unilateral cerebral hypoplasia. Clin Neurol Neurosurg. 1985;87(2):119–22. 10.1016/0303-8467(85)90108-8 [DOI] [PubMed] [Google Scholar]
- 72. Unal O, Tombul T, Cirak B, et al. : Left hemisphere and male sex dominance of cerebral hemiatrophy (Dyke-Davidoff-Masson Syndrome). Clin Imaging. 2004;28(3):163–5. 10.1016/S0899-7071(03)00158-X [DOI] [PubMed] [Google Scholar]
- 73. Diestro JDB, Dorotan MKC, Camacho AC, et al. : Clinical spectrum of Dyke-Davidoff-Masson syndrome in the adult: an atypical presentation and review of literature. BMJ Case Rep. 2018;2018: bcr2018224170. 10.1136/bcr-2018-224170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hung ECW, Chim SSC, Chan PKS, et al. : Detection of SARS coronavirus RNA in the cerebrospinal fluid of a patient with severe acute respiratory syndrome. Clin Chem. 2003;49(12):2108–2109. 10.1373/clinchem.2003.025437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Lau KK, Chu CM, Lau ST, et al. : Possible central nervous system infection by SARS coronavirus. Emerg Infect Dis. 2004;10(2):342–344. 10.3201/eid1002.030638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Dominguez SR, Robinson CC, Holmes KV: Detection of four human coronaviruses in respiratory infections in children: a one-year study in Colorado. J Med Virol. 2009;81(9):1597–1604. 10.1002/jmv.21541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Swarz JA, Daily S, Niemi E, et al. : COVID-19 Infection Presenting as Acute-Onset Focal Status Epilepticus. Pediatr Neurol. 2020;112:7. 10.1016/j.pediatrneurol.2020.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. McAbee GN, Brosgol Y, Pavlakis S, et al. : Encephalitis Associated with COVID-19 infection in an 11-Year-Old Child. Pediatr Neurol. 2020;109:94. 10.1016/j.pediatrneurol.2020.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Pedersen SF, Ho YC: SARS-CoV-2: a storm is raging. J Clin Invest. 2020;130(5):2202–2205. 10.1172/JCI137647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Wu Y, Xu X, Chen Z, et al. : Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav Immun. 2020;87:18–22. 10.1016/j.bbi.2020.03.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Zawar I, Khan AA, Sultan T, et al. : Dyke-Davidoff-Masson Syndrome. An unusual cause of status epilepticus. Neurosciences (Riyadh). 2015;20(4):385–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Alam M, Haq MAU, Ali F, et al. : Dyke-Davidoff-Masson Syndrome: An Unusual Cause of Status Epilepticus and Refractory Seizures. J Coll Physicians Surg Pak. 2018;28(6):S99–101. [DOI] [PubMed] [Google Scholar]
- 83. Roy U, Panwar A, Mukherjee A, et al. : Adult Presentation of Dyke-Davidoff-Masson Syndrome: A Case Report. Case Rep Neurol. 2016;8(1):20–6. 10.1159/000443521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Shahid R: An unusual presentation of Dyke-Davidoff Masson syndrome. Neurosciences (Riyadh). 2018;23(3):254–257. 10.17712/nsj.2018.3.20170232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Malik P, Garg R, Gulia AK, et al. : Dyke-Davidoff-Masson Syndrome- a rare cause of refractory epilepsy. Iran J Psychiatry. 2014;9(1):42–4. [PMC free article] [PubMed] [Google Scholar]
- 86. Park KI, Chung JM, Kim JY: Dyke-Davidoff-Masson Syndrome: cases of two brothers and literature review. J Epilepsy Res. 2014;4(1):24–7. 10.14581/jer.14006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Kumar NV, Gugapriya TS, Guru AT, et al. : Dyke-Davidoff-Masson syndrome. Int J App Basic Med Res. 2016;6(1):57–59. 10.4103/2229-516X.174016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Ayas ZÖ, Asil K, Öcal R: The clinico-radiological spectrum of Dyke-Davidoff-Masson syndrome in adults. Neurol Sci. 2017;38(10):1823–8. 10.1007/s10072-017-3074-7 [DOI] [PubMed] [Google Scholar]
- 89. Sarangi P, Mangaraj PD, Mohanty J: Dyke-Davidoff-Masson Syndrome (DDMS): A rare preventable cause of refractory epilepsy. American Journal of Diagnostic Imaging. 2017;1(1):28. 10.5455/ajdi.20170612101959 [DOI] [Google Scholar]
- 90. Li Y, Zhang T, Li B, et al. : A potential cause of adolescent onset Dyke-Davidoff-Masson syndrome: A case report. Medicine (Baltimore). 2019;98(51): e18075. 10.1097/MD.0000000000018075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. https://www.drugbank.ca/drugs/DB06218/. accessed on August 25 2020. [Google Scholar]


