Abstract
Objectives
To estimate the association between chronic hypertension and perinatal mortality and to evaluate the extent to which risks are impacted by preterm delivery.
Design
Cross-sectional analysis.
Setting
United States, 2015–18.
Population
Singleton births (20–44 weeks of gestation).
Exposure
Chronic hypertension, defined as elevated blood pressure diagnosed before pregnancy or recognised before 20 weeks of gestation.
Main outcomes and measures
We derived the risk of perinatal mortality in relation to chronic hypertension from Poisson models, adjusted for confounders. The impacts of misclassification and unmeasured confounding were assessed. Causal mediation analysis was performed to quantify the impact of preterm delivery on the association.
Results
Of the 15 090 678 singleton births, perinatal mortality rates were 22.5 and 8.2 per 1000 births in chronic hypertensive and normotensive pregnancies, respectively (adjusted risk ratio 2.05, 95% CI 2.00–2.10). Corrections for exposure misclassification and unmeasured confounding biases substantially increased the risk estimate. Although causal mediation analysis revealed that most of the association of chronic hypertension on perinatal mortality was mediated through preterm delivery, the perinatal mortality rates were highest at early term, term and late term gestations, suggesting that a planned early term delivery at 37–386/7 weeks may optimally balance risk in these pregnancies. Additionally, 87% (95% CI 84–90%) of perinatal deaths could be eliminated if preterm deliveries, as a result of chronic hypertension, were preventable.
Conclusions
Chronic hypertension is associated with increased risk for perinatal mortality. Planned early term delivery and targeting modifiable risk factors for chronic hypertension may reduce perinatal mortality rates.
Keywords: Causal mediation analysis, chronic hypertension, neonatal death, perinatal mortality, preterm delivery, stillbirth
Tweetable abstract
Maternal chronic hypertension is associated with increased risk for perinatal mortality, largely driven by preterm birth.
Introduction
Chronic hypertension complicates 1–5% of pregnancies, but there has been a 13-fold increase in the prevalence of chronic hypertension in the USA in the last four decades.1 The American obesity epidemic,2,3 and deferred childbearing leading to older age of pregnant women,4,5 contribute substantially to this trend.6 Chronic hypertension during pregnancy is associated with increased rates of maternal complications, including death,7 superimposed pre-eclampsia,8,9 placental abruption,10-12 end-stage renal disease13 and long-term cardiovascular and cerebrovascular disease.12,14,15 Chronic hypertension is also an independent risk factor for an array of perinatal complications, including stillbirth,16-18 fetal growth restriction19 and neonatal death,20,21 as well as neurological and neurodevelopmental deficits in children.22-24 Considering the increasing prevalence rates of chronic hypertension and the associated complications, there is a need to evaluate the impact of chronic hypertension on perinatal mortality in the USA, including stillbirth and neonatal death.
Chronic hypertension is associated with increased risk of preterm delivery,8 and gestational age at delivery impacts neonatal outcomes in pregnancies complicated by chronic hypertension.25 Gestational age also influences obstetric care including interventions to improve neonatal outcomes (i.e. the administration of antenatal corticosteroids to promote fetal lung maturity) as well as delivery timing.26 In the pathway between chronic hypertension and perinatal outcomes, preterm delivery features as an important component in the causal pathway, but how and to what extent preterm delivery plays a role in shaping these associations remain unknown.
We undertook a population-based cross-sectional study in a large contemporary US cohort to understand the association between chronic hypertension and perinatal mortality. The primary objective was to quantify the mediation effects of preterm birth in this causal paradigm. We hypothesised that chronic hypertension is associated with perinatal mortality and that preterm delivery plays an important role that mediates this causal association.
Methods
Study design and data sources
We performed a cross-sectional analysis of all singleton births in the USA from 2015 to 2018. We used data on fetal deaths, live births, and linked live births–infant death records ascertained by the National Center for Health Statistics of the Centers for Disease Control and Prevention. The live birth–infant death data are derived from infant death certificates linked to the live birth certificates for infants that died before reaching 1 year of age. Study variables were derived from the 2003 revision of the US standard certificates of vital events. Gestational age in these data were based on the best obstetric estimate,27 which had a 99.6% agreement within 2 weeks in comparison to data abstracted from hospital charts.28 These de-identified data are publicly available, so no ethical approval from an Institutional Review Board was sought. The study followed the STROBE reporting guidelines for cross-sectional studies.
Cohort composition
The study population included all women who delivered singleton births in the USA between 20 and 44 weeks of gestation from 2015 to 2018. We excluded multiple gestations, and pregnancies with records with missing gestational age or with gestational age <20 and ≥45 weeks, as well as pregnancies with missing chronic hypertension status. After all the exclusions, 15 090 678 singleton births were included in the study (Figure S1).
Exposure
The exposure was chronic hypertension as indicated on the US standard certificates of fetal death, live birth and infant death. The diagnosis includes elevated blood pressure before pregnancy or recognised within the first 20 weeks of gestation.29
Outcomes measures
The primary outcome was perinatal mortality, defined as a composite of stillbirth (fetal deaths at 20 weeks or more of gestation), early neonatal death (deaths within 0–6 days of life), and late neonatal death (deaths between 7 and 28 days of life). Timely obstetric interventions may prevent stillbirth, but could lead to neonatal death, so the composite was intended to capture perinatal mortalities that could be attributed to exposure to chronic hypertension. We also quantified the impact of chronic hypertension on stillbirth, as well as early and late neonatal deaths, as secondary outcomes.
Statistical analyses
We estimated perinatal mortality rates, expressed per 1000 births, for chronic hypertensive versus normotensive pregnancies. We examined the association between chronic hypertension and perinatal mortality on both the additive (risk difference) and multiplicative (risk ratio, RR) scales, derived from fitting log-linear Poisson regression models with robust variance and the identity-link for risk difference and log-link for risk ratio.30 Associations with their corresponding 95% CI were derived before and after adjusting for confounders. We also examined associations between chronic hypertension and the risks of stillbirth, early neonatal deaths and late neonatal deaths.
Confounders
We adjusted for several confounders including maternal age (<15, 15–19, 20–24, 25–29 [reference], 30–34, 35–39 and ≥40 years), education (below high school, up to high school, college and beyond college educated [reference]), race/ethnicity (non-Hispanic White [reference], non-Hispanic Black, Hispanic or other race), maternal smoking (non-smoker [reference], smoked before pregnancy only and smoked before and during pregnancy), pre-pregnancy body-mass index (BMI in kg/m2; underweight [<18.5], normal [18.5–24.9, reference], overweight [25.0–29.9], obese [30.0–34.9] and morbidly obese [≥35.0]), and pre-gestational diabetes (present, absent [reference]). We also adjusted for year of delivery to address potential temporal changes in the prevalence of chronic hypertension and maternal mortality.
Missing data
Since some of the covariates contained missing data, we imputed missing data through multiple imputation by a chained equations approach.31 We assumed that the pattern of missing data was ‘missing at random’, and created 20 imputed data sets (after 50 burn-in iterations). All analyses were performed for each of the 20 data sets, and we combined the results of the analyses of imputations based on Rubin’s principles.32 Details regarding missing covariate data are shown in Table S1.
Causal mediation analysis
Gestational age features on the causal pathway between chronic hypertension and perinatal mortality as a mediator (Figure S2); therefore, any adjustment for preterm delivery in the assessment of the perinatal mortality risk in relation to chronic hypertension would lead to collider bias.33 To resolve this issue, we undertook a causal mediation analysis based on the counterfactual framework34,35 to decompose the total effect of chronic hypertension on perinatal mortality into the natural direct effect (the effect of the exposure [chronic hypertension] on the outcome [perinatal mortality] if the mediator [preterm delivery <37 weeks] were set to what it would have been, probably contrary to the fact, in the absence of the exposure) and the natural indirect effect (the effect of the exposure on the outcome when the exposure is present after setting the mediator to what it would have been, probably contrary to the fact, with versus without the exposure).36,37 We also estimated the controlled direct effect (the effect of chronic hypertension on mortality that is not mediated through preterm delivery). The total effect, natural direct effect, natural indirect effect and controlled direct effect were estimated on a multiplicative scale with risk ratio and 95% CI as effect measure.
In addition, we estimated the proportion mediated (the proportion of the total effect that is mediated through preterm delivery), and the proportion eliminated (the proportion of the chronic hypertension–perinatal mortality association that might be eliminated by blocking the effect of chronic hypertension on preterm delivery). The proportion eliminated is the proportion of perinatal deaths that could be prevented by designing interventions to reduce preterm delivery associated with chronic hypertension. All 95% CI estimates were based on bootstrap resampling method (1000 resamples).
Sensitivity analysis: probabilistic bias analysis
Chronic hypertension is subject to misclassification in these data files, with sensitivity of 44% (and agreement of 98%) in comparison to data abstracted from hospital medical charts.28 To address potential exposure misclassification and unmeasured confounding biases, we undertook a sensitivity analysis through a probabilistic bias analysis.38,39 Exposure (chronic hypertension) misclassification was assumed to be differential with respect to the outcome (perinatal mortality). Based on a uniform distribution, we assumed the priors for sensitivity for chronic hypertension to range between 0.30 and 0.95 among those with perinatal deaths and between 0.20 and 0.90 among live births; the priors for specificity for chronic hypertension were assumed to range between 0.98 and 1.00 for both deaths and live births, respectively.
Corrections for unmeasured confounding bias were based on the following assumptions: (1) we assumed that the prevalence of the unmeasured confounder among those with and without chronic hypertension, under a log-normal distribution, ranged between 5% and 15%, and 3% and 10%, respectively; and (2) the risk ratio for the confounder–outcome association was varied between 1.25 and 3.00. Under these assumptions, we drew the bias parameters 100 000 times from the prior distributions to address exposure misclassification and unmeasured confounding (computational strategies are provided in the R package ‘episensr’40). From these analyses, we report the median bias-corrected risk ratio (RRbc) and 95% CIbc.
Log-linear regression models and the mediation analysis were fitted in SAS (version 9.4; SAS Institute, Cary, NC, USA) using the GENMOD and the CAUSALMED procedures, respectively. The probabilistic bias analysis was implemented in R (R Foundation for Statistical Computing, Vienna, Austria) using the ‘episensr’ package.40
Patient and public involvement
Patients and the public were not involved in the design, conduct or reporting of this study.
Results
During the study period (2015–18), there were 15 090 678 singleton births delivered between 20 and 44 weeks of gestation in the USA (Figure S1). The prevalence rate of chronic hypertension was 1.8% (n = 274 125). The distributions of socio-economic factors in relation to chronic hypertension are shown in Table S1. Women with chronic hypertension were more likely to be older, African-American, of higher parity, college-educated, obese and tobacco smokers, compared with normotensive women.
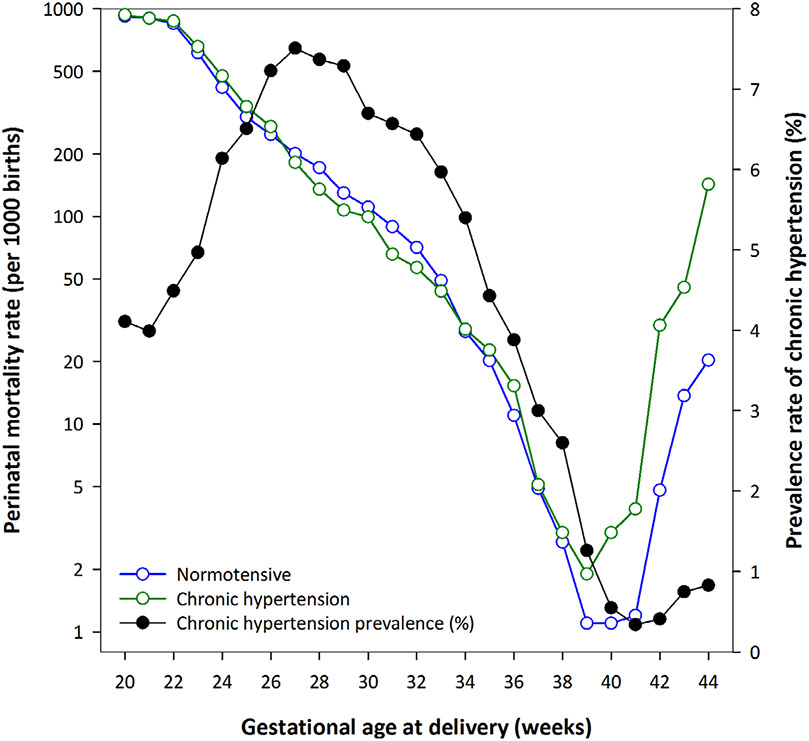
Perinatal mortality rates were higher among chronic hypertensive women (22.5 per 1000 births) compared with normotensive women (8.2 per 1000 births; Table 1). Among chronic hypertensive pregnancies, the rate of mortality was highest for stillbirth (16.1 per 1000 births), in comparison to early (4.6 per 1000 births) and late (1.8 per 1000 births) neonatal deaths. Gestational age-specific risk of perinatal mortality (per 1000 births) among normotensive and chronic hypertensive pregnancies are described in Figure 1. These rates were particularly high at early term, term and late term gestations.
Table 1.
Perinatal mortality rates by chronic hypertension among non-malformed singleton births: USA, 2015–18
| Perinatal mortality |
Perinatal mortality: Number (rate per 1000 births) |
||
|---|---|---|---|
| Total births (n = 15 090 678) |
Normotensive (n = 14 816 553) |
Chronic hypertension (n = 274 125) |
|
| Perinatal mortality | 127 355 (8.4) | 121 199 (8.2) | 6156 (22.5) |
| Stillbirth | 82 438 (5.5) | 78 037 (5.3) | 4401 (16.1) |
| Neonatal mortality | 44 917 (3.0) | 43 162 (2.9) | 1755 (6.4) |
| Early neonatal mortality | 34 620 (2.3) | 33 346 (2.3) | 1274 (4.6) |
| Late neonatal mortality | 10 297 (0.7) | 9816 (0.7) | 481 (1.8) |
Values are presented as n (%).
Figure 1.
Gestational age-specific risk of perinatal mortality (per 1000 births) among normotensive and chronic hypertensive pregnancies in the USA, between 2015 and 2018. We recognise the potential for a collider bias in this graphical presentation, but it is purely for descriptive purposes to guide the causal mediation analysis.
The absolute risks and risk ratio of perinatal mortality in pregnancies complicated by chronic hypertension are described in Table 2. After adjusting for potential confounders, chronic hypertension was associated with 11.0 (95% CI 10.5–11.5) per 1000 excess perinatal deaths over the normotensive group. The increase in risk of perinatal death in chronic hypertensive pregnancies was two-fold higher (adjusted RR 2.05, 95% CI 2.00–2.10), compared with normotensive pregnancies.
Table 2.
Risk of perinatal mortality in relation to chronic hypertension among singleton births: USA, 2015–18
| Perinatal mortality | Risk difference (95% CI) for mortality |
Risk ratio (95% CI) for mortality |
|||
|---|---|---|---|---|---|
| Unadjusted | Adjusted | Unadjusted | Adjusted | Bias-corrected | |
| Perinatal mortality | 14.3 (13.7–14.8) | 11.0 (10.5–11.5) | 2.75 (2.68–2.82) | 2.05 (2.00–2.10) | 4.86 (3.42–5.98) |
| Stillbirth | 10.8 (10.3–11.3) | 7.7 (7.2–8.3) | 3.05 (2.96–3.14) | 1.86 (1.81–1.92) | 7.76 (4.52–11.12) |
| Neonatal mortality | 3.5 (3.2–3.8) | 2.8 (2.6–3.1) | 2.20 (2.10–2.30) | 1.86 (1.78–1.96) | 2.54 (1.78–3.13) |
| Early neonatal mortality | 2.4 (2.1–2.7) | 1.9 (1.6–2.1) | 2.07 (1.95–2.18) | 1.74 (1.65–1.84) | 2.20 (1.54–2.71) |
| Late neonatal mortality | 1.1 (0.9–1.3) | 1.0 (0.8–1.1) | 2.66 (2.40–2.91) | 2.29 (2.09–2.52) | 6.54 (4.57–8.18) |
Risk difference is expressed per 1000 births.
Risk difference and risk ratios are adjusted for maternal age, parity, education, race/ethnicity, pre-pregnancy smoking, smoking during pregnancy, pre-pregnancy body mass index and year of delivery, and are based on multiple imputation analysis.
Bias corrected risk ratios refer to multiple probabilistic bias-corrected risk ratio, following simultaneous corrections for exposure (chronic hypertension) misclassification and unmeasured confounding biases.
Sensitivity analysis
The bias-corrected risk ratios, with simultaneous corrections for both exposure misclassification and unmeasured confounding, revealed that the risk of perinatal mortality in chronic hypertensive pregnancies was almost five-fold higher (RRbc 4.86, 95% CI 3.42–5.98), compared with normotensive pregnancies. The risk was highest for stillbirth in relation to chronic hypertension (RRbc 7.76, 95% CI 4.52–11.12).
Causal mediation analysis
The causal mediation analysis was performed to disentangle the total effect of chronic hypertension–perinatal mortality association, with preterm delivery as the potential mediator (Table 3). Much of the total effect of perinatal mortality and its component mortalities was mediated through preterm delivery. The indirect effect was greatest for perinatal mortality and stillbirth. Given the strong mediation component of the chronic hypertension–mortality association that operates through preterm delivery, the proportion eliminated estimate is correspondingly high: 87% (95% CI 84–90%).
Table 3.
Causal mediation analysis to estimate the impact of preterm delivery (<37 weeks of gestation) on the association between chronic hypertension and perinatal mortality among singleton births: USA, 2015–18
| Perinatal mortality | Risk ratio (95% CI) for mortality |
Percent (95% CI) |
||||
|---|---|---|---|---|---|---|
| Total effect | Controlled direct effect |
Natural direct effect |
Natural indirect effect |
Mediated | Eliminated | |
| Perinatal mortality | 1.93 (1.87–1.99) | 1.12 (1.09–1.15) | 1.07 (1.04–1.12) | 1.85 (1.82–1.88) | 92 (88–96) | 87 (84–90) |
| Intrauterine fetal demise | 2.12 (2.03–2.20) | 1.15 (1.11–1.20) | 1.17 (1.12–1.22) | 1.95(1.91–1.99) | 85 (82–89) | 86 (83–90) |
| Neonatal mortality | 1.60 (1.52–1.70) | 1.08 (1.02–1.13) | 0.93 (0.88–0.99) | 1.67 (1.63–1.71) | —* | —* |
| Early neonatal mortality | 1.50 (1.41–1.62) | 1.02 (0.97–1.08) | 0.82 (0.76–0.90) | 1.68 (1.63–1.73) | —* | —* |
| Late neonatal mortality | 1.89 (1.70–1.11) | 1.23 (1.09–1.40) | 1.26 (1.11–1.43) | 1.63 (1.55–1.72) | 71 (60–85) | 74 (59–89) |
Risk ratios are adjusted for maternal age, parity, education, race/ethnicity, pre-pregnancy smoking, smoking during pregnancy, pre-pregnancy body mass index and year of delivery.
All 95% CI estimates are based on bootstrap resampling (1000 replications).
Percent mediated and percent eliminated are not shown because risk ratio estimates for natural direct effect and natural indirect effects are on the opposite sides of the null.
Discussion
Principal findings
In this population-based study that included over 15 million singleton births in the USA from 2015 to 2018, we found that chronic hypertension was associated with substantially increased rates of perinatal mortality, including stillbirth and neonatal deaths. The causal mediation analysis suggested that the major driver of mortality risk in chronic hypertensive pregnancies was mediated through preterm delivery. There was also higher risk of perinatal mortality after 39 weeks of gestation. These results suggest that a planned, early term delivery at 37–386/7 weeks may optimally balance risk in these pregnancies.
Interpretation
An understanding of the perinatal risks associated with chronic hypertension is largely informed by older studies. For example, a previous population-based study from the USA, which utilised Nationwide Inpatient Sample data from 1995 to 2008, reported a higher risk for stillbirth (odds ratio [OR] 2.31, 95% CI 2.11–2.53) for pregnancies complicated by chronic hypertension compared with normotensive pregnancies.6 In a population-based prospective cohort study from Sweden (1992–2004), an increase in stillbirth risk (OR 2.71, 95% CI 1.96–3.73) and neonatal death (OR 2.89, 95% CI 1.95–4.30) was observed.21 The results of our study, which showed increased perinatal mortality associated with chronic hypertension, are consistent with these studies, yet the magnitude of risk was larger. After adjusting for bias due to misclassification and unmeasured confounders, we found the risk of perinatal mortality was 4.86 (95% CI 3.42–5.98), which was largely due to stillbirth at term gestations. The reason for this difference is uncertain, but may be a result of advances in neonatal care that minimise the risk of neonatal deaths.
The mediation analysis suggested that a substantial driver of perinatal mortality risk is preterm delivery. Although most deliveries in our cohort occurred at term, there was an association between chronic hypertension and preterm delivery. A previous population-based study from the USA found that the odds of preterm delivery for chronic hypertensive versus normotensive pregnancies was 3.01 (95% CI 2.88–3.14).6 A meta-analysis on chronic hypertension and pregnancy outcomes showed that pregnancies complicated by chronic hypertension are at a higher risk of preterm delivery before <37 weeks of gestation (RR 2.7, 95% CI 1.9–36).8 Most often, preterm births are clinician-initiated secondary to superimposed pre-eclampsia or fetal growth restriction,41,42 and cannot be avoided. However, whenever possible, and only if maternal and fetal status allow, avoiding preterm delivery in pregnancies complicated by chronic hypertension may have a large impact on perinatal mortality. This is especially prudent in the late preterm period when providers may have different thresholds to pursue delivery for pregnancies complicated by chronic hypertension.
The results of our study are consistent with previous work43 and professional society recommendations44 that suggest the optimal timing of delivery for pregnancies complicated by chronic hypertension is 37–38 weeks of gestation. It is critical to avoid early preterm delivery in these pregnancies (because of preterm delivery-associated complications and long-term chronic health conditions), but term and late term pregnancies also carry a high risk of stillbirth. Despite national efforts to avoid early term deliveries,45 the findings from this study suggest that early term deliveries may minimise the risks of stillbirths associated with chronic hypertension.
Women with chronic hypertension often have risk factors, such as advanced maternal age, obesity and tobacco smoking,1,46,47 which in turn increase the risks of adverse perinatal outcomes.48-52 Public health strategies that target modifiable risk factors to reduce the burden of chronic hypertension may be the best strategy to reduce perinatal mortality associated with this disease. The mediation analysis quantified the preventable proportion of perinatal deaths associated with chronic hypertension by avoiding preterm deliveries associated with chronic hypertension.
Although this study improves our understanding of the magnitude of chronic hypertension’s impact on perinatal mortality, there is a need for further study: (1) to develop preventive strategies such as weight loss and preconception blood pressure management for women at risk; (2) to assess the effectiveness of these strategies on perinatal outcomes; and (3) to evaluate whether treating mild chronic hypertension in pregnancy has the potential to reduce the risk of adverse pregnancy outcomes.53
Strengths and weaknesses
The main strengths of the study were that it was large and contemporary. This not only increases the generalisability of the findings, but also reflects current practices in the USA. In addition, although we quantified the association between chronic hypertension and perinatal mortality, we also performed a causal mediation analysis to evaluate the impact of preterm delivery on this association. An application of causal inference methodology in this context provides insight into what drives the risk, which can be used to generate novel approaches to reduce the risks associated with chronic hypertension.
Despite these strengths, the study has some limitations. There may be a difference in risk for pregnancies complicated by mild chronic hypertension versus pregnancies complicated by severe disease that require antihypertensive medications, but we lacked data on the severity of chronic hypertension. There is also possible misclassification bias due to inaccuracy in recording diagnosis of chronic hypertension, inability to distinguish women who may have developed superimposed pre-eclampsia and type of preterm birth (clinician-initiated versus spontaneous), in these data. We have attempted to account for these misclassification and unmeasured confounder biases in the sensitivity analysis.
Conclusion
Compared with normotensive pregnancies, pregnancies complicated by chronic hypertension are associated with increased risk for perinatal mortality, including stillbirth and neonatal death. A large driver of this risk was preterm delivery, but there was also a substantial increased risk of perinatal mortality at term and late term gestations. The results of this study suggest that the best strategies to reduce the risk of perinatal mortality include planned, early term deliveries and targeting modifiable risk factors for chronic hypertension such as advanced maternal age and obesity that may impact the prevalence of chronic hypertension.
Supplementary Material
Figure S1. Study flow diagram.
Figure S2. Simplified directed acyclic graph showing the relationship between chronic hypertension and perinatal mortality with preterm delivery as the mediator and unmeasured confounders.
Table S1. Distribution of maternal characteristics in relation to chronic hypertension among singleton births: USA 2015–18.
Funding
Dr. Ananth is supported in part by the National Heart, Lung and Blood Institute (R01-HL150065) and the National Institute of Environmental Health Sciences (R01-ES033190), National Institutes of Health.
Footnotes
Disclosures of interests
None declared. Completed disclosure of interests form available to view online as supporting information.
Supporting Information
Additional supporting information may be found online in the Supporting Information section at the end of the article.
Details of ethics approval
Ethics approval was not sought because the data used in this study are publicly available in de-identified form; therefore, these data do not qualify as human subjects research.
Data availability statement
The data used in this study are publicly available.
References
- 1.Ananth CV, Duzyj CM, Yadava S, Schwebel M, Tita ATN, Joseph KS. Changes in the prevalence of chronic hypertension in pregnancy, United States, 1970 to 2010. Hypertension 2019;74:1089–95. [DOI] [PubMed] [Google Scholar]
- 2.Kim SY, Dietz PM, England L, Morrow B, Callaghan WM. Trends in pre-pregnancy obesity in nine states, 1993–2003. Obesity (Silver Spring) 2007;15:986–93. [DOI] [PubMed] [Google Scholar]
- 3.Hales CM, Fryar CD, Carroll MD, Freedman DS, Ogden CL. Trends in obesity and severe obesity prevalence in US Youth and Adults by Sex and Age, 2007–2008 to 2015–2016. JAMA 2018;319:1723–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martin JA, Hamilton BE, Ventura SJ, Osterman MJ, Kirmeyer S, Mathews TJ, et al. Births: final data for 2009. Natl Vital Stat Rep 2011;60:1–70. [PubMed] [Google Scholar]
- 5.Misra DP, Ananth CV. Infant mortality among singletons and twins in the United States during 2 decades: effects of maternal age. Pediatrics 2002;110:1163–8. [DOI] [PubMed] [Google Scholar]
- 6.Bateman BT, Bansil P, Hernandez-Diaz S, Mhyre JM, Callaghan WM, Kuklina EV. Prevalence, trends, and outcomes of chronic hypertension: a nationwide sample of delivery admissions. Am J Obstet Gynecol 2012;206:134.e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Creanga AA, Berg CJ, Syverson C, Seed K, Bruce FC, Callaghan WM. Pregnancy-related mortality in the United States, 2006–2010. Obstet Gynecol 2015;125:5–12. [DOI] [PubMed] [Google Scholar]
- 8.Bramham K, Parnell B, Nelson-Piercy C, Seed PT, Poston L, Chappell LC. Chronic hypertension and pregnancy outcomes: systematic review and meta-analysis. BMJ 2014;348:g2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sutton EF, Hauspurg A, Caritis SN, Powers RW, Catov JM. Maternal outcomes associated with lower range stage 1 hypertension. Obstet Gynecol 2018;132:843–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williams MA, Mittendorf R, Monson RR. Chronic hypertension, cigarette smoking, and abruptio placentae. Epidemiology 1991;2:450–3. [DOI] [PubMed] [Google Scholar]
- 11.Ananth CV, Vintzileos AM. Ischemic placental disease: epidemiology and risk factors. Eur J Obstet Gynecol Reprod Biol 2011;159:77–82. [DOI] [PubMed] [Google Scholar]
- 12.Wu P, Chew-Graham CA, Maas AH, Chappell LC, Potts JE, Gulati M, et al. Temporal changes in hypertensive disorders of pregnancy and impact on cardiovascular and obstetric outcomes. Am J Cardiol 2020;125:1508–16. [DOI] [PubMed] [Google Scholar]
- 13.Wu CC, Chen SH, Ho CH, Liang FW, Chu CC, Wang HY, et al. End-stage renal disease after hypertensive disorders in pregnancy. Am J Obstet Gynecol 2014;210:147.e1–8. [DOI] [PubMed] [Google Scholar]
- 14.Cirillo PM, Cohn BA. Pregnancy complications and cardiovascular disease death: 50-year follow-up of the Child Health and Development Studies pregnancy cohort. Circulation 2015;132:1234–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grandi SM, Filion KB, Yoon S, Ayele HT, Doyle CM, Hutcheon JA, et al. Cardiovascular disease-related morbidity and mortality in women with a history of pregnancy complications. Circulation 2019;139:1069–79. [DOI] [PubMed] [Google Scholar]
- 16.Ankumah NA, Cantu J, Jauk V, Biggio J, Hauth J, Andrews W, et al. Risk of adverse pregnancy outcomes in women with mild chronic hypertension before 20 weeks of gestation. Obstet Gynecol 2014;123:966–72. [DOI] [PubMed] [Google Scholar]
- 17.Ananth CV, Savitz DA, Bowes WA Jr. Hypertensive disorders of pregnancy and stillbirth in North Carolina, 1988 to 1991. Acta Obstet Gynecol Scand 1995;74:788–93. [DOI] [PubMed] [Google Scholar]
- 18.Panaitescu AM, Syngelaki A, Prodan N, Akolekar R, Nicolaides KH. Chronic hypertension and adverse pregnancy outcome: a cohort study. Ultrasound Obstet Gynecol 2017;50:228–35. [DOI] [PubMed] [Google Scholar]
- 19.Nzelu D, Dumitrascu-Biris D, Kay P, Nicolaides KH, Kametas NA. Severe hypertension, pre-eclampsia and small for gestational age in women with chronic hypertension diagnosed before and during pregnancy. Pregnancy Hypertens 2018;14:200–4. [DOI] [PubMed] [Google Scholar]
- 20.Ananth CV, Basso O. Impact of pregnancy-induced hypertension on stillbirth and neonatal mortality. Epidemiology 2010;21:118–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zetterstrom K, Lindeberg SN, Haglund B, Hanson U. The association of maternal chronic hypertension with perinatal death in male and female offspring: a record linkage study of 866,188 women. BJOG 2008;115:1436–42. [DOI] [PubMed] [Google Scholar]
- 22.Tuovinen S, Eriksson JG, Kajantie E, Lahti J, Pesonen AK, Heinonen K, et al. Maternal hypertensive disorders in pregnancy and self-reported cognitive impairment of the offspring 70 years later: the Helsinki Birth Cohort Study. Am J Obstet Gynecol 2013;208:200.e1–9. [DOI] [PubMed] [Google Scholar]
- 23.Tuovinen S, Aalto-Viljakainen T, Eriksson JG, Kajantie E, Lahti J, Pesonen AK, et al. Maternal hypertensive disorders during pregnancy: adaptive functioning and psychiatric and psychological problems of the older offspring. BJOG 2014;121:1482–91. [DOI] [PubMed] [Google Scholar]
- 24.Grace T, Bulsara M, Pennell C, Hands B. Maternal hypertensive diseases negatively affect offspring motor development. Pregnancy Hypertens 2014;4:209–14. [DOI] [PubMed] [Google Scholar]
- 25.Harper LM, Biggio JR, Anderson S, Tita AT. Gestational age of delivery in pregnancies complicated by chronic hypertension. Obstet Gynecol 2016;127:1101–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.American College of Obstetricians Gynecologists. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol 2013;122:1122–31. [DOI] [PubMed] [Google Scholar]
- 27.Martin JA, Osterman MJ, Kirmeyer SE, Gregory EC. Measuring gestational age in vital statistics data: transitioning to the obstetric estimate. Natl Vital Stat Rep 2015;64:1–20. [PubMed] [Google Scholar]
- 28.Gregory ECW, Martin JA, Argov EL, Osterman MJK. Assessing the quality of medical and health data from the 2003 birth certificate revision: results from New York City. Natl Vital Stat Rep 2019;68:1–20. [PubMed] [Google Scholar]
- 29.American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Obstetrics. ACOG Practice Bulletin No. 203: chronic hypertension in pregnancy. Obstet Gynecol. 2019;133:e26–50. [DOI] [PubMed] [Google Scholar]
- 30.Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol 2005;162:199–200. [DOI] [PubMed] [Google Scholar]
- 31.Harel O, Mitchell EM, Perkins NJ, Cole SR, Tchetgen Tchetgen EJ, Sun B, et al. Multiple imputation for incomplete data in epidemiologic studies. Am J Epidemiol 2018;187:576–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rubin DB. Inference and missing data. Biometrika 1976;63:581–92. [Google Scholar]
- 33.Ananth CV, Schisterman EF. Confounding, causality, and confusion: the role of intermediate variables in interpreting observational studies in obstetrics. Am J Obstet Gynecol 2017;217:167–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robins JM, Greenland S. Identifiability and exchangeability for direct and indirect effects. Epidemiology 1992;3:143–55. [DOI] [PubMed] [Google Scholar]
- 35.Pearl J. Direct and indirect effects. In: Proceedings of the Seventeenth Conference on Uncertainty in Artificial Intelligence; 2001. pp. 411–20. [Google Scholar]
- 36.Valeri L, Vanderweele TJ. Mediation analysis allowing for exposure-mediator interactions and causal interpretation: theoretical assumptions and implementation with SAS and SPSS macros. Psychol Methods 2013;18:137–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ananth CV, VanderWeele TJ. Placental abruption and perinatal mortality with preterm delivery as a mediator: disentangling direct and indirect effects. Am J Epidemiol 2011;174:99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lash TL, Fox MP, Fink AK. Applying Quantitative Bias Analysis to Epidemiologic Data. New York, NY: Springer-Verlag; 2009. [Google Scholar]
- 39.Lash TL, Fox MP, MacLehose RF, Maldonado G, McCandless LC, Greenland S. Good practices for quantitative bias analysis. Int J Epidemiol 2014;43:1969–85. [DOI] [PubMed] [Google Scholar]
- 40.Episensr HD. Basic sensitivity analysis of epidemiological results. R package 0.9.999. 2020. https://cran.r-project.org/web/packages/episensr/index.html. Accessed 10 August 2020. [Google Scholar]
- 41.Premkumar A, Baer RJ, Jelliffe-Pawlowski LL, Norton ME. Hypertensive disorders of pregnancy and preterm birth rates among black women. Am J Perinatol 2019;36:148–54. [DOI] [PubMed] [Google Scholar]
- 42.Kase BA, Carreno CA, Blackwell SC, Sibai BM. The impact of medically indicated and spontaneous preterm birth among hypertensive women. Am J Perinatol 2013;30:843–8. [DOI] [PubMed] [Google Scholar]
- 43.Hutcheon JA, Lisonkova S, Magee LA, Von Dadelszen P, Woo HL, Liu S, et al. Optimal timing of delivery in pregnancies with pre-existing hypertension. BJOG 2011;118:49–54. [DOI] [PubMed] [Google Scholar]
- 44.American College of Obstetricians Gynecologists, Committee on Practice Bulletins, Obstetrics. ACOG Practice Bulletin No. 203: chronic hypertension in pregnancy. Obstet Gynecol 2019;133:e26–50. [DOI] [PubMed] [Google Scholar]
- 45.Oshiro BT, Kowalewski L, Sappenfield W, Alter CC, Bettegowda VR, Russell R, et al. A multistate quality improvement program to decrease elective deliveries before 39 weeks of gestation. Obstet Gynecol 2013;121:1025–31. [DOI] [PubMed] [Google Scholar]
- 46.Niskanen L, Laaksonen DE, Nyyssonen K, Punnonen K, Valkonen VP, Fuentes R, et al. Inflammation, abdominal obesity, and smoking as predictors of hypertension. Hypertension 2004;44:859–65. [DOI] [PubMed] [Google Scholar]
- 47.Gurven M, Blackwell AD, Rodriguez DE, Stieglitz J, Kaplan H. Does blood pressure inevitably rise with age?: longitudinal evidence among forager-horticulturalists. Hypertension 2012;60:25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen A, Feresu SA, Fernandez C, Rogan WJ. Maternal obesity and the risk of infant death in the United States. Epidemiology 2009;20:74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jacobsson B, Ladfors L, Milsom I. Advanced maternal age and adverse perinatal outcome. Obstet Gynecol 2004;104:727–33. [DOI] [PubMed] [Google Scholar]
- 50.Delbaere I, Verstraelen H, Goetgeluk S, Martens G, De Backer G, Temmerman M. Pregnancy outcome in primiparae of advanced maternal age. Eur J Obstet Gynecol Reprod Biol 2007;135:41–6. [DOI] [PubMed] [Google Scholar]
- 51.McDonald SD, Han Z, Mulla S, Beyene J, Knowledge SG. Overweight and obesity in mothers and risk of preterm birth and low birth weight infants: systematic review and meta-analyses. BMJ 2010;341:c3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pineles BL, Hsu S, Park E, Samet JM. Systematic review and meta-analyses of perinatal death and maternal exposure to tobacco smoke during pregnancy. Am J Epidemiol 2016;184:87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.NIH. Chronic Hypertension and Pregnancy (CHAP) Project. NIH; 2020. https://clinicaltrials.gov/ct2/show/NCT02299414. Accessed 4 September 2020. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Study flow diagram.
Figure S2. Simplified directed acyclic graph showing the relationship between chronic hypertension and perinatal mortality with preterm delivery as the mediator and unmeasured confounders.
Table S1. Distribution of maternal characteristics in relation to chronic hypertension among singleton births: USA 2015–18.
Data Availability Statement
The data used in this study are publicly available.