Abstract
Many insects use adhesive organs to climb. The ability to cling to surfaces is advantageous but is increasingly challenged as animals grow, due to the associated reduction in surface-to-volume ratio. Previous work has demonstrated that some climbing animals overcome this scaling problem by systematically altering the maximum force per area that their adhesive pads can sustain; their adhesive organs become more efficient as they grow, an observation which is also of substantial relevance for the design of bioinspired adhesives. What is the origin of this change in efficiency? In insects, adhesive contact is mediated by a thin film of a liquid, thought to increase adhesive performance via capillary and viscous forces. Here, we use interference reflection microscopy and dewetting experiments to measure the contact angle and dewetting speed of the secretion of pre-tarsal adhesive pads of Indian stick insects, varying in mass by over two orders of magnitude. Neither contact angle nor dewetting speed change significantly with body mass, suggesting that the key physical properties of the pad secretion—its surface tension and viscosity—are size-invariant. Thus, the observed change in pad efficiency is unlikely to arise from systematic changes of the physical properties of the pad secretion; the functional role of the secretion remains unclear.
Keywords: adhesion, climbing, fluid mechanics, biological attachment, pad secretion
1. Introduction
Bioinspired adhesives are an important area of biomimetic innovation [1]. However, despite significant progress [2–4], key challenges remain. Technical adhesives often need to cover large areas and carry heavy loads, resulting in a classic scaling problem: large contacts suffer reduced strength due to stress concentrations, and heavy weights lead to poorer relative performance due to changes in the support-surface-to-volume ratio [4,5]. A similar problem arises for climbing animals of various sizes [6]. How do they resolve this problem?
Previous work has revealed that some climbing animals are able to systematically alter the sustainable force per area—the efficiency of their adhesive pads—as they increase in size [6–10]. In insects [11], tree frogs [7,8] and arachnids [12,13], adhesive surface contacts are mediated via thin films of a liquid secretion, and it has been speculated that systematic changes to the physical properties of this secretion represent a possible strategy to alter pad efficiency with size [6]. The basis of this hypothesis is the long-standing assumption that the secretion’s functional significance is to promote attachment via capillary and viscous forces [14–19]. Here, we test experimentally whether Indian stick insects alter the physical properties of their pad secretion as they grow, in order to increase pad efficiency and overcome the scaling problem.
2. Material and methods
2.1. Study animals
Stick insects (Carausius morosus, Sinéty 1901, Phasmatodea, Phasmatidae) were taken from an all-female laboratory colony, fed with bramble and water ad libitum, and kept in a climate chamber at 60% RH/25°C with a 12/12 h light cycle. We randomly collected 54 individuals to cover a body mass range of 2.57–892.03 mg (EX124, Explorer Analytical Balance, OH, USA; readability = 0.1 mg), close to the maximum mass range of the colony (figure 1a). All experiments were conducted with live insects and adhesive pads (arolia) on the forelegs, which were confirmed to be free of damage by visual inspection (Leica S APO stereomicroscope, Leica Microsystems, Germany).
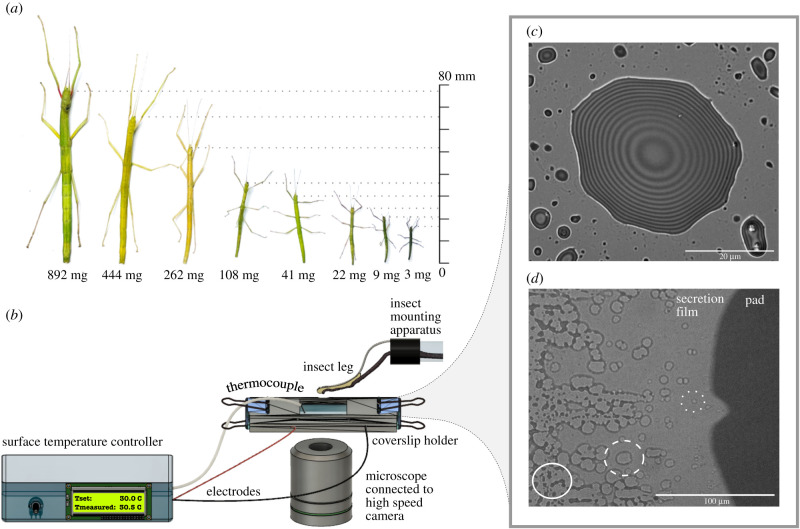
Figure 1.
(a) Carausius morosus stick insects vary by over two orders of magnitude in mass between first instars and adults. (b) The physical properties of the secretion were determined with a custom-made set-up. (c) Interference reflection microscopy image of a droplet of the pad secretion deposited on glass, recorded at 30°C. Interference fringes indicate fixed height increments and can thus be used to estimate the contact angle and surface tension of the pad secretion. (d) When a contacting pad is slid rapidly to expose the secretion film, thermodynamic instabilities lead to dewetting; dry patches nucleate within the thin continuous film (dotted circle), then grow (dashed circle) and finally merge (full circle). The speed of dry patch growth is inversely proportional to the secretion’s viscosity.
2.2. Contact angle and dewetting speed measurements
We estimated the physical properties of the secretion—its surface tension and viscosity—by interference reflection microscopy (IRM) and dewetting experiments, performed with a five-component set-up: (i) an insect mounting apparatus as described in Labonte et al. [20]; (ii) a borosilicate glass coverslip coated with conducting indium-tin-oxide on the bottom side to enable surface temperature control via Joule heating (see electronic supplementary material for surface preparation and characterization); (iii) a coverslip holder to secure the coverslip in place and to connect it to a temperature control unit; (iv) a K-thermocouple (TP870, Extech, USA) connected to a custom-built Arduino temperature controller to maintain a constant surface temperature of 30°C during all experiments; and (v) an inverted microscope (DMi8, Leica Microsystems, Germany) connected to a high-speed camera (Blackfly S USB3, BFS-U3-16S2C-CS, FLIR, USA; figure 1b).
In order to assess whether the secretion’s surface tension changes with size, we measured contact angles of droplets deposited on glass using IRM, following earlier work [21,22]. In brief, we performed artificial ‘steps’ with the mounted arolium, using a micromanipulator (M3301R, World Precision Instruments, UK), which resulted in the deposition of small droplets (average radius approx. 5 μm, below the capillary length). The droplets were then imaged at 63× magnification, an illuminating numerical aperture of 0.8, and with a wavelength of 445 nm from a pE-300 ultra LED (filter with 10 nm bandwidth, CoolLED, UK; figure 1c). We approximated the contact angle as the slope calculated from the first two height increments derived from the interference fringes, to minimize a bias introduced by the curvature of the droplets. We measured contact angles from three droplets per individual and 23 individuals across the size range (n = 23, N = 69). The relationship between contact angle and surface tension is complex [23], but simplifies for a dominantly dispersive fluid such as the insect pad secretion (see electronic supplementary material) [24–26].
In order to estimate the viscosity of the secretion, we quantified its dewetting speed [22]. Hydrodynamic theory predicts that surface tension, γ, and dewetting speed, vd, are related to viscosity, η, via
| 2.1 |
where θ is the contact angle [27–29]. To quantify dewetting speed, we connected the micromanipulator to a hydraulic drum controller (MHW-3, Narishige, Japan), to rapidly slide pads in surface contact in parallel to the coverslip. The subsequent dewetting of the thin film left behind was recorded at 20× magnification and 226 fps (figure 1d). We recorded a minimum of three dewetting videos per stick insect. Every dewetting experiment was conducted on a clean part of the glass coverslip. A period of at least 10 min separated consecutive experiments with the same individual in order to provide time for recovery of a sufficient footprint volume [17]. The rate of growth of dry patches, the dewetting speed (vd = dR/dt), was extracted with a custom-written Fiji macro [30]. A minimum of three dry patches, tracked for at least four frames, were analysed for 54 stick insects (n = 54, N = 354).
3. Results and discussion
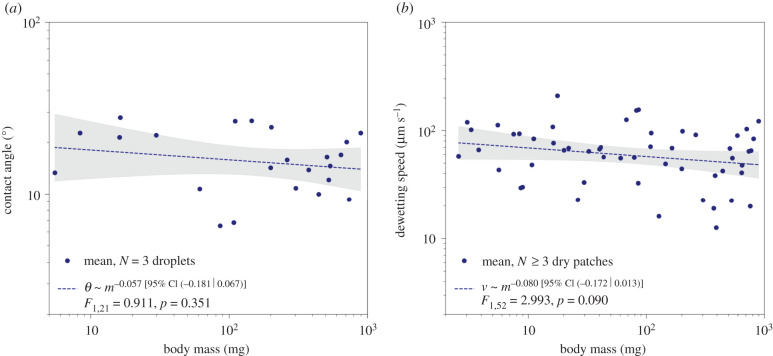
The contact angle of the pad secretion does not vary significantly with size (log–log slope obtained via ordinary least-squares (OLS) regression = −0.057 (95% CI (−0.181|0.067)), F1,21 = 0.911, p = 0.351, n = 23; figure 2a). The mean contact angle of 17 ± 6° (mean ± s.d.) is in excellent agreement with previous measurements in ants, stick insects and cockroaches (18 ± 7°, 18 ± 1° and 17 ± 1°, respectively) [17,22,31]. As the secretion is immiscible in water and almost entirely dispersive [24–26], this result strongly suggests a size-invariant surface tension (see electronic supplementary material for a detailed argument).
Figure 2.
Neither the contact angle (a) nor the dewetting speed (b) of the pad secretion change significantly with size, indicating that both surface tension and viscosity are size-invariant (nθ = 23 individuals, nd = 54 individuals).
The dewetting speed showed a small but insignificant trend to decrease with size (OLS log–log slope = −0.080 (95% CI (−0.172|0.013)), F1,52 = 2.993, p = 0.090, n = 54; figure 2b). The mean dewetting speed was 69 ± 39 μm s−1 (mean ± s.d.), comparable to preliminary results reported for ants (vd = 60 ± 5 μm s−1) [22]. As both contact angle and dewetting speed do not vary significantly with size, neither does viscosity (OLS log–log slope = 0.004 (95% CI (−0.149|0.158)), F1,52 = 0.003, p = 0.955; see electronic supplementary material). Any change in pad efficiency is thus unlikely to arise from changes of the physical properties of the pad secretion.
Although contact angle and dewetting speed are size-invariant, they scatter notably around the mean (38% and 56% coefficient of variation, respectively). A linear mixed model with random intercept for the 23 paired individuals suggested that the dewetting speed variation arises largely from inter-individual variation (moderate intraclass correlation coefficient (ICC) of 0.56), and thus that the dewetting measurements are robust. When the variation in contact angle is included (vd/θ3 ∝ γ/η, see equation (2.1)), the ICC increases to 0.88, highlighting that the nonlinear contribution of the contact angle further accentuates the differences among individuals (see electronic supplementary material).
The insect pad secretion is a multi-phasic emulsion, consisting of aqueous droplets dispersed in an oily continuous phase [22,25]. IRM imaging of the pad contact area across the size range revealed a qualitatively similar appearance and a similar amount and size of aqueous phase droplets (electronic supplementary material, figure S3). In combination with the size-invariance of the contact angle and dewetting speed, this finding suggests that the chemical composition of the pad secretion may also vary little with size.
4. Conclusion
Our results demonstrate that stick insects do not systematically alter the physical properties of their pad secretion to avoid a size-related reduction in safety factors. Indeed, a size-specific change in efficiency is only observed in the presence of shear forces [10], and is thus likely explained by the shear-sensitivity of biological adhesive pads [9,32,33]. Potential changes in the physical properties of the pad secretion will only affect adhesive performance if attachment is dominated by capillary or viscous forces. Although this has been a long-standing assumption [15–19], strong direct evidence in support of it is absent, and recent research has put forward alternative functional interpretations [34–36]. Systematic changes in adhesive performance may also arise from a size-specific variation of elastocapillary effects [37], or the pad’s viscoelastic properties, which thus need to be investigated in future work.
Acknowledgements
The authors would like to thank Myrta N. Stoukidi and Eleni Papafilippou for their respective contributions to the design of the surface temperature controller and the Fiji dewetting analysis macro, and Fabian Plum for the three-dimensional scan of the stick insect leg appearing in figure 1b.
Data accessibility
Raw data (spreadsheet and dewetting video) are part of the submission and provided in the electronic supplementary material [38].
Authors' contributions
D.-M.K.: formal analysis, investigation, methodology, supervision, writing—original draft; C.N.S.A.: formal analysis, investigation, writing—review and editing; A.E.L.A.: formal analysis, methodology, writing—review and editing; D.L.: conceptualization, formal analysis, funding acquisition, project administration, supervision, writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
We declare we have no competing interests.
Funding
Funding for this study was provided by the Biotechnology and Biological Sciences Research Council to D.L. (BBSRC grant no. BMPF-P72408).
References
- 1.Favi PM, Yi S, Lenaghan SC, Xia L, Zhang M. 2014. Inspiration from the natural world: from bio-adhesives to bio-inspired adhesives. J. Adhes. Sci. Technol. 28, 290-319. ( 10.1080/01694243.2012.691809) [DOI] [Google Scholar]
- 2.Sameoto D, Menon C. 2010. Recent advances in the fabrication and adhesion testing of biomimetic dry adhesives. Smart Mater. Struct. 19, 103001. ( 10.1088/0964-1726/19/10/103001) [DOI] [Google Scholar]
- 3.Jagota A, Hui CY. 2011. Adhesion, friction, and compliance of bio-mimetic and bio-inspired structured interfaces. Mater. Sci. Eng. R: Rep. 72, 253-292. ( 10.1016/j.mser.2011.08.001) [DOI] [Google Scholar]
- 4.Arzt E, Quan H, McMeeking RM, Hensel R. 2021. Functional surface microstructures inspired by nature—from adhesion and wetting principles to sustainable new devices. Prog. Mater. Sci. 120, 100823. ( 10.1016/j.pmatsci.2021.100823) [DOI] [Google Scholar]
- 5.Bartlett MD, Croll AB, King DR, Paret BM, Irschick DJ, Crosby AJ. 2012. Looking beyond fibrillar features to scale gecko-like adhesion. Adv. Mater. 24, 1078-1083. ( 10.1002/adma.201104191) [DOI] [PubMed] [Google Scholar]
- 6.Labonte D, Federle W. 2015. Scaling and biomechanics of surface attachment in climbing animals. Phil. Trans. R. Soc. B 370, 20140027. ( 10.1098/rstb.2014.0027) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barnes WJP, Oines C, Smith JM. 2006. Whole animal measurements of shear and adhesive forces in adult tree frogs: insights into underlying mechanisms of adhesion obtained from studying the effects of size and scale. J. Comp. Physiol. A 192, 1179-1191. ( 10.1007/s00359-006-0146-1) [DOI] [PubMed] [Google Scholar]
- 8.Smith JM, Barnes WJP, Downie JR, Ruxton GD. 2006. Structural correlates of increased adhesive efficiency with adult size in the toe pads of hylid tree frogs. J. Comp. Physiol. A 192, 1193-1204. ( 10.1007/s00359-006-0151-4) [DOI] [PubMed] [Google Scholar]
- 9.Labonte D, Federle W. 2016. Biomechanics of shear-sensitive adhesion in climbing animals: peeling, pre-tension and sliding-induced changes in interface strength. J. R. Soc. Interface 13, 20160373. ( 10.1098/rsif.2016.0373) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Labonte D, Struecker MY, Birn-Jeffery AV, Federle W. 2019. Shear-sensitive adhesion enables size-independent adhesive performance in stick insects. Proc. R. Soc. B 286, 20191327. ( 10.1098/rspb.2019.1327) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walker G, Yulf AB, Ratcliffe J. 1985. The adhesive organ of the blowfly Calliphora vomitoria: a functional approach (Diptera: Calliphoridae). J. Zool. 205, 297-307. ( 10.1111/j.1469-7998.1985.tb03536.x) [DOI] [Google Scholar]
- 12.Peattie AM, Dirks JH, Henriques S, Federle W. 2011. Arachnids secrete a fluid over their adhesive pads. PLoS ONE 6, e20485. ( 10.1371/journal.pone.0020485) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wolff JO, Gorb SN. 2016. Attachment structures and adhesive secretions in arachnids. Cham, Switzerland: Springer International Publishing. [Google Scholar]
- 14.Stork NE. 1980. Experimental analysis of adhesion of Chrysolina polita (Chrysomelidae: Coleoptera) on a variety of surfaces. J. Exp. Biol. 88, 91-108. ( 10.1242/jeb.88.1.91) [DOI] [Google Scholar]
- 15.Vötsch W, Nicholson G, Müller R, Stierhof YD, Gorb S, Schwarz U. 2002. Chemical composition of the attachment pad secretion of the locust Locusta migratoria. Insect Biochem. Mol. Biol. 32, 1605-1613. ( 10.1016/S0965-1748(02)00098-X) [DOI] [PubMed] [Google Scholar]
- 16.Abou B, Gay C, Laurent B, Cardoso O, Voigt D, Peisker H, Gorb S. 2010. Extensive collection of femtolitre pad secretion droplets in the beetle Leptinotarsa decemlineata allows nanolitre microrheology. J. R. Soc. Interface 7, 1745-1752. ( 10.1098/rsif.2010.0075) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dirks JH, Federle W. 2011. Fluid-based adhesion in insects—principles and challenges. Soft Matter 7, 11 047-11 053. ( 10.1039/c1sm06269g) [DOI] [Google Scholar]
- 18.Peisker H, Heepe L, Kovalev AE, Gorb SN. 2014. Comparative study of the fluid viscosity in tarsal hairy attachment systems of flies and beetles. J. R. Soc. Interface 11, 20140752. ( 10.1098/rsif.2014.0752) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stark AY, Arstingstall K, Yanoviak SP. 2018. Adhesive performance of tropical arboreal ants varies with substrate temperature. J. Exp. Biol. 221, jeb171843. ( 10.1242/jeb.171843) [DOI] [PubMed] [Google Scholar]
- 20.Labonte D, Federle W. 2013. Functionally different pads on the same foot allow control of attachment: stick insects have load-sensitive ‘heel’ pads for friction and shear-sensitive ‘toe’ pads for adhesion. PLoS ONE 8, e81943. ( 10.1371/journal.pone.0081943) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wiegand G, Jaworek T, Wegner G, Sackmann E. 1997. Studies of structure and local wetting properties on heterogeneous, micropatterned solid surfaces by microinterferometry. J. Colloid Interface Sci. 196, 299-312. ( 10.1006/jcis.1997.5193) [DOI] [PubMed] [Google Scholar]
- 22.Federle W, Riehle M, Curtis ASG, Full RJ. 2002. An integrative study of insect adhesion: mechanics and wet adhesion of pretarsal pads in ants. Integr. Comp. Biol. 42, 1100-1106. ( 10.1093/icb/42.6.1100) [DOI] [PubMed] [Google Scholar]
- 23.Owens DK, Wendt RC. 1969. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 13, 1741-1747. ( 10.1002/app.1969.070130815) [DOI] [Google Scholar]
- 24.Hasenfuss I. 1999. The adhesive devices in larvae of Lepidoptera (Insecta, Pterygota). Zoomorphology 119, 143-162. ( 10.1007/s004350050088) [DOI] [Google Scholar]
- 25.Dirks JH. 2009. Mechanisms of fluid-based adhesion in insects. PhD thesis, University of Cambridge, Cambridge, UK. [Google Scholar]
- 26.Labonte D. 2010. Biomechanics of attachment in insects—the influence of surface energy. Bremen, Germany: University of Applied Sciences. [Google Scholar]
- 27.Tanner LH. 1979. The spreading of silicone oil drops on horizontal surfaces. J. Phys. D: Appl. Phys. 12, 1473-1484. ( 10.1088/0022-3727/12/9/009) [DOI] [Google Scholar]
- 28.Redon C, Brochard-Wyart F, Rondelez F. 1991. Dynamics of dewetting. Phys. Rev. Lett. 66, 715-718. ( 10.1103/PhysRevLett.66.715) [DOI] [PubMed] [Google Scholar]
- 29.Martin P, Brochard-Wyart F. 1998. Dewetting at soft interfaces. Phys. Rev. Lett. 80, 3296-3299. ( 10.1103/PhysRevLett.80.3296) [DOI] [Google Scholar]
- 30.Schindelin J, et al. 2012. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676-682. ( 10.1038/nmeth.2019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dirks JH, Clemente CJ, Federle W. 2010. Insect tricks: two-phasic foot pad secretion prevents slipping. J. R. Soc. Interface 7, 587-593. ( 10.1098/rsif.2009.0308) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Autumn K, Dittmore A, Santos D, Spenko M, Cutkosky M. 2006. Frictional adhesion: a new angle on gecko attachment. J. Exp. Biol. 209, 3569-3579. ( 10.1242/jeb.02486) [DOI] [PubMed] [Google Scholar]
- 33.Federle W, Labonte D. 2019. Dynamic biological adhesion: mechanisms for controlling attachment during locomotion. Phil. Trans. R. Soc. B 374, 20190199. ( 10.1098/rstb.2019.0199) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Betz O. 2010. Adhesive exocrine glands in insects: morphology, ultrastructure, and adhesive secretion. In Biological adhesive systems (eds von Byern J, Grunwald I), pp. 111-152. Vienna, Austria: Springer. (doi:10.1007/978-3-7091-0286-2_8) [Google Scholar]
- 35.Labonte D, Federle W. 2015. Rate-dependence of ‘wet’ biological adhesives and the function of the pad secretion in insects. Soft Matter 11, 8661-8673. ( 10.1039/C5SM01496D) [DOI] [PubMed] [Google Scholar]
- 36.Hosoda N, Nakamoto M, Suga T, Gorb SN. 2021. Evidence for intermolecular forces involved in ladybird beetle tarsal setae adhesion. Sci. Rep. 11, 1. ( 10.1038/s41598-020-79139-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gernay S, Federle W, Lambert P, Gilet T. 2016. Elasto-capillarity in insect fibrillar adhesion. J. R. Soc. Interface 13, 20160371. ( 10.1098/rsif.2016.0371) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaimaki D-M, Andrew CNS, Attipoe AEL, Labonte D. 2022. The physical properties of the stick insect pad secretion are independent of body size. FigShare. ( 10.6084/m9.figshare.c.6048365) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Raw data (spreadsheet and dewetting video) are part of the submission and provided in the electronic supplementary material [38].