Abstract
7-O-Galloyltricetifavan (7OGT), a natural flavonoid, is isolated from the leaves of Pithecellobium clypearia. The compound exhibits a variety of biological activities. This study details the evaluation of the HOO• antiradical activity of 7OGT by quantum chemistry calculations. The HOO• trapping activity of 7OGT in the gas phase (reference state) was discovered to follow the formal hydrogen transfer mechanism with a rate constant of k = 4.58 × 108 M−1 s−1. In physiological environments, 7OGT is predicted to be an excellent HOO• radical scavenger with koverall = 2.65 × 108 and 1.40 × 104 M−1 s−1 in water and pentyl ethanoate solvents, respectively. The HOO• antiradical activity of 7OGT in water at physiological pH is approximately 2000 times that of Trolox and substantially higher than that of other well-known natural antioxidants such as trans-resveratrol or ascorbic acid. Thus, 7OGT is an excellent natural antioxidant in polar environments.
Keywords: flavonoid, density functional theory, antiradical activity, antioxidant, kinetics
1. Introduction
7-O-Galloyltricetifavan (7OGT; figure 1), a natural flavonoid, was first isolated from the leaves of Pithecellobium clypearia [1–3]. 7OGT is a flavan derivative that has antiviral properties against respiratory syncytial virus, influenza H1N1 virus, herpes simplex virus type 1 and coxsackie B3 virus as well as anti-inflammatory, anti-Alzheimer, anti-allergic and antioxidant properties [1–7]. Studies showed that 7OGT has potent xanthine oxidase inhibition with an IC50 = 25.5 µmol l−1 [3] and inhibits soluble epoxide hydrolase enzymatic activity with IC50 values 10.0 ± 0.4 µM [5]. Thus, 7OGT is indicated as a good natural antioxidant with the known neuroprotective activity that is believed to underpin the prevention of Alzheimer's disease [7].
Figure 1.
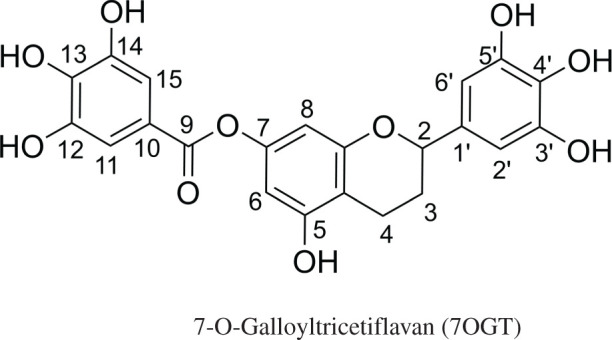
The structure of 7OGT.
Oxidative stress is now thought to play a role in several chronic diseases [8–10]. The ability of natural products to scavenge free radicals is an essential aspect of their anti-inflammatory, antibacterial and cancer-preventive properties, and it is the driving force behind the investigation of the antioxidant properties [11–13]. There is currently no information about the kinetics and mechanism of the HOO• + 7OGT in physiological conditions; however, computer calculations offer a convenient way to predict the antioxidant activity of organic compounds in physiological media [11,14,15]. Since 7OGT has exhibited a broad range of potent biological activities, this study aims to delve into the underpinning antiradical activity of 7OGT by a quantum chemical approach, using HOO• as a model radical.
2. Computational methods
The density functional theory-based quantum chemical calculations used here had been outlined in a range of former works for modelling antioxidant activities of various compounds [16–20]. In brief, the M06–2X/6-311 ++ G(d,p)//M06-2X/6-31 + G(d) method was used to calculate thermodynamic parameters in the gas phase [21]. The kinetic calculations were performed at the M06-2X/6-311 ++G(d,p) level of theory, following the quantum mechanics-based test for overall free radical scavenging activity (QM-ORSA) protocol [22–24] with the SMD solvation model [25] for water and pentyl ethanoate solvents [11,14,17,19,26–35]. This protocol delivers results in reasonably good agreement with experimental data (kcalc/kexp ratio = 1–2.9) [11,26,36], and therefore, it is commonly used to assess the radical scavenging activity of natural and synthetic compounds [14,15,20,37,38].
Using the transition state (TS) theory at 298.15 K, 1 M standard state, the rate constant (k) was computed as follows [31–34,39]:
| 2.1 |
where σ is the reaction symmetry number [29,30], κ contains the tunnelling corrections calculated using the Eckart barrier [35], kB is the Boltzmann constant, h is the Planck constant and ΔG≠ is the Gibbs free energy of activation.
The reaction barriers of single electron transfer (SET) reactions in media were determined using the Marcus theory [40,41]. The equations used to calculate the Gibbs free energy change of reaction ΔG≠ for the SET pathway are
| 2.2 |
and
| 2.3 |
where ΔESET is the non-adiabatic energy difference among reactants and vertical products for SET, and is the standard Gibbs free energy change of the reaction [42,43].
A correction was applied to rate constants that were close to the diffusion limit [11]. The apparent rate constants (kapp) for an irreversible bimolecular diffusion-controlled reaction were computed using the Collins–Kimball theory in solvents at 298.15 K [44]; the steady-state Smoluchowski rate constant (kD) was estimated using the literature [11,45],
| 2.4 |
and
| 2.5 |
DAB = DA + DB (DAB is the mutual diffusion coefficient of the reactants A and B) [44,46], where DA or DB is determined using the Stokes–Einstein formulation (2.6) [47,48].
| 2.6 |
η is the viscosity of the solvents (i.e. η(pentyl ethanoate) = 8.62 × 10 −4 Pa s, η(H2O) = 8.91 × 10 −4 Pa s) and a is the radius of the solute.
To avoid over-penalizing entropy losses in solution, the solvent cage effects were added using Okuno's adjustments [49], which were modified with the free volume theory according to the Benson correction [11,50–52].
For species with numerous conformers, all of them were energy minimized, with the lowest electronic energy conformer being included in the study. The existence of only one single imaginary frequency was a defining feature of all transition stages. To verify that each TS is accurately related to the pre-complex and post-complex, intrinsic coordinate calculations were completed. The calculations were carried out using Gaussian 09 software [53].
3. Results and discussions
3.1. The gas phase evaluation
Following the established protocol [19,54], the antioxidant activity of 7OGT was first evaluated according to the three main radical scavenging mechanisms: sequential electron transfer followed by proton transfer (SETPT), formal hydrogen transfer (FHT) and sequential proton loss followed by electron transfer (SPLET). In the two-step reactions such as the SETPT and SPLET pathways, the first step reaction (i.e. SET and proton loss (PL) for the SETPT and SPLET pathways, respectively) normally has the higher activation energy, with the exception of the proton dissociation of acidic moieties in water that is considered separately. Therefore, the thermochemical parameters i.e. proton affinity (PA), bond dissociation energy (BDE) and ionization energies (IE) that characterize the PL, FHT and SET reactions, respectively, were computed for all relevant bonds of 7OGT with the M06-2X/6-311++G(d,p)//M06-2X/6–31+G(d) method in the gas phase [21]. The results are presented in table 1. The results showed that the BDE values of the C−H range from 84.3 to 99.0 kcal mol−1, while the BDEs are 73.9–85.3 kcal mol−1 for O-H bonds. This suggests that the hydroxyl groups are the thermodynamically preferred sites of activity via the hydrogen transfer reaction. The lowest BDE values were presented at the O4' − H bond (73.9 kcal mol−1) and the O13−H bond (77.1 kcal mol−1). These sites are believed to play a key role in 7OGT's radical scavenging activity via the FHT mechanism. According to this data, 7OGT has lower BDE(O−H) values than e.g. vanillic acid (85.2 kcal mol−1), [16] puerarin (87.3 kcal mol−1), [54] resveratrol (83.9 kcal mol−1), [55] or viniferifuran (82.7 kcal mol−1) [56]. This suggests that 7OGT could exhibit faster antiradical activity (following the FHT mechanism) than these natural antioxidants.
Table 1.
The calculated thermodynamic parameters (BDEs, PAs and IEs, in kcal mol−1) and the ΔGo of HOO• + 7OGT reaction.
| positions | FHT |
PL |
SET |
|||
|---|---|---|---|---|---|---|
| BDE | ΔGo | PA | ΔGo | IE | ΔG° | |
| C2 − H | 84.3 | −2.3 | 178.8 | 156.4 | ||
| C3 − H | 99.0 | 12.5 | ||||
| C4 − H | 86.2 | 0.3 | ||||
| O5 − H | 85.3 | −0.3 | 345.7 | 193.1 | ||
| O12 − H | 83.4 | −2.2 | 353.6 | 200.6 | ||
| O13 − H | 77.1 | −8.4 | 340.0 | 187.4 | ||
| O3' − H | 80.5 | −5.0 | 341.7 | 189.1 | ||
| O4' − H | 73.9 | −11.3 | 341.4 | 188.7 | ||
As shown in table 1, the lowest IE and PA values are significantly higher (about 2.42 and 4.60 times, respectively) than those of the BDE. Therefore, the antiradical activity of 7OGT is expected to favour the FHT mechanism in lipid media. The calculated Gibbs free energy changes (ΔG°) of the 7OGT + HOO• reaction via the main mechanisms: FHT, PL, which is the first step of SPLET, and SET as the first step of the SETPT suggest that the FHT reaction is spontaneous (ΔG° < 0) for most of the sites, apart from the C3(4)H−bonds; however, the PL and SET reactions are not spontaneous (ΔG° > 0) in any cases. The Marcus theory was also used to estimate the reaction barriers of the SET reaction in the gas phase [40,41]; however, this reaction was negative in the studied conditions (ΔG≠(SET) = 155.6 and λ = 21.2 kcal mol−1). Thus kinetics of HOO• + 7OGT reaction were computed following the FHT mechanism at the positions that yielded negative ΔG°.
Kinetic studies of the HOO• + 7OGT reaction were performed following the QM-ORSA protocol with the M06-2X/6-311 ++ G(d,p) method [11,27,28], and results are presented in table 2 and figure 2.
Table 2.
Calculated ΔH, ΔG≠ (kcal mol−1), tunnelling corrections (κ) and kEck (M−1 s −1), branching ratios (Γ, %) at 298.15 K for the HOO• + 7OGT reaction.
| reactions | ΔH | ΔG≠ | κ | kEck | Γ |
|---|---|---|---|---|---|
| 7OGT − C2 − H + HOO• | 7.3 | 16.6 | 36.4 | 1.51 × 102 | 0.0 |
| 7OGT − O5 − H + HOO• | 3.7 | 13.5 | 23.1 | 1.93 × 104 | 0.0 |
| 7OGT − O12 − H + HOO• | 3.9 | 13.4 | 46.8 | 4.23 × 104 | 0.0 |
| 7OGT − O13 − H + HOO• | 4.3 | 14.6 | 53.3 | 6.63 × 103 | 0.0 |
| 7OGT − O3′ − H + HOO• | 2.6 | 12.3 | 19.3 | 1.13 × 105 | 0.0 |
| 7OGT − O4′ − H + HOO• | −2.0 | 7.4 | 19.6 | 4.58 × 108 | 100.0 |
| koverall | 4.58 × 108 |
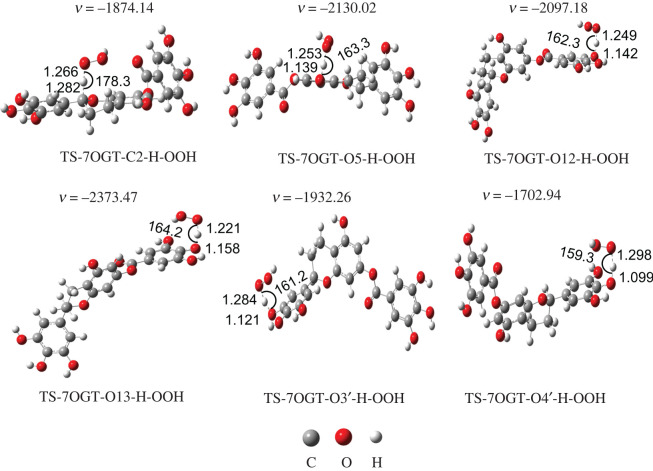
Figure 2.
The FHT TSs between the 7OGT and HOO• radical.
The energy barriers for the 7OGT + HOO• reaction following the FHT pathway are within the range of −2.0 to 7.3 kcal mol−1 (table 2). The O4' − H + HOO• reaction had the lowest barrier height with ΔH = −2.0 kcal mol−1. It can be affirmed that the HOO• scavenging activity of the O4′ − H bond is the highest among all of the studied bonds. The HOO• trapping activity of 7OGT is mainly due to the H-abstraction of the O4′−H bond (ΔG≠ = 7.4 kcal mol−1; kEck = 4.58 × 108 M−1 s−1; Γ = 100%). The activation Gibbs energies (ΔG≠) range 7.4−16.6 kcal mol−1, while the κ values vary 19.3−53.3. Thus, the κ values play an important role in the rate constants of the hydroperoxyl antiradical activity of the 7OGT. This result is consistent with previous studies on phenolic compounds [22,27]. The calculated results suggest that the HOO• trapping activity of 7OGT is defined by the FHT reaction at the O4′ position; therefore, this reaction will be further analysed in physiological media.
3.2. The radical scavenging activity of 7-O-Galloyltricetifavan in physiological environments
Previous research has shown that the antiradical activity of phenolic compounds in aqueous solutions is dominated by anion states [37,38]. The protonation states of 7OGT were investigated at physiological pH to discover potential radical scavenging mechanisms [16,38,57]. Based on the calculated data [58], pKa1 and pKa2 values were 6.87 and 8.29, respectively (figure 3). Therefore, in water at pH = 7.4, three states, including neutral (H2A: 20.7%), anion (HA−: 70.2%) and dianion (A2−: 9.1%) will be used for studying the radical scavenging activity, whereas the neutral state will be considered in the lipid medium (pentyl ethanoate solvent).
Figure 3.
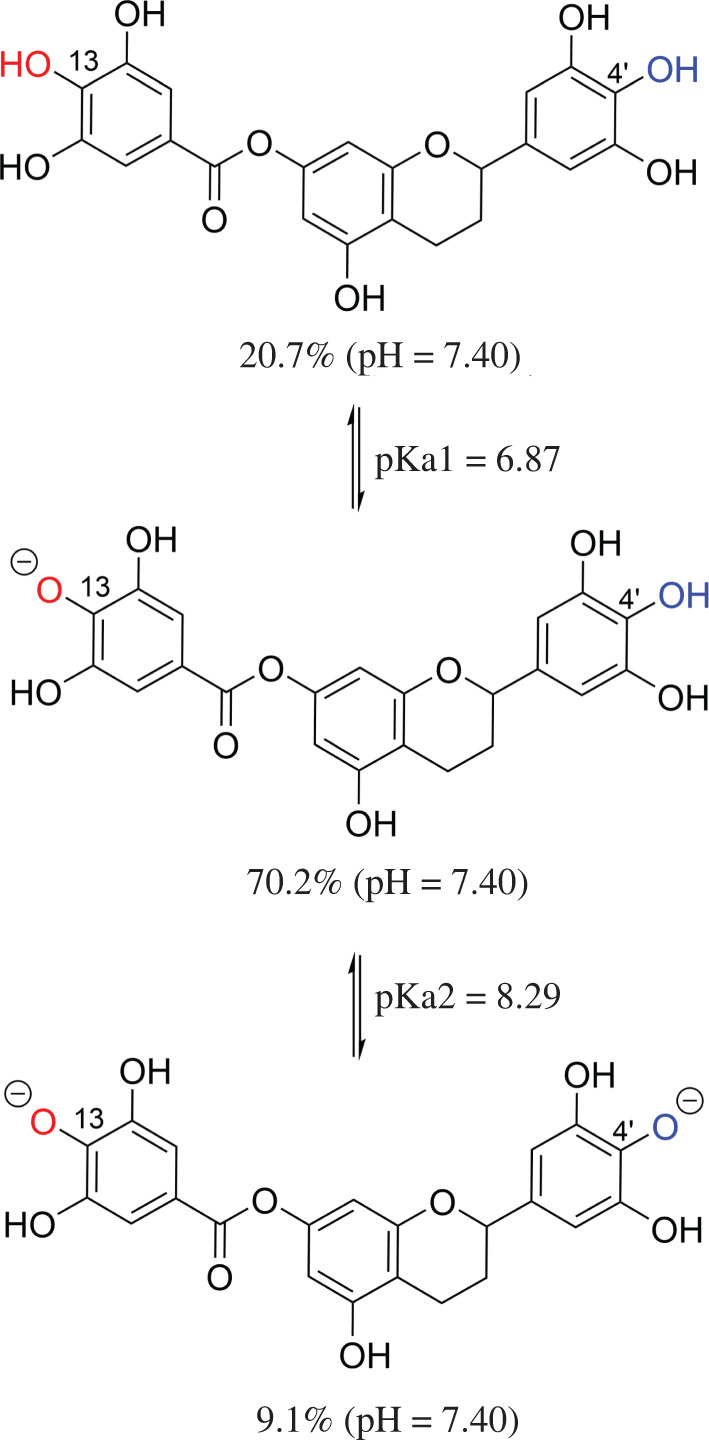
The deprotonation of 7OGT.
The calculated results in the vacuum suggest that the HOO• antiradical activity in non-polar media follows the hydrogen transfer mechanism at the O4′ − H bond. Thermodynamic evaluation in pentyl ethanoate and water (electronic supplementary material, table S2) did not differ at the most likely site of activity (BDE = 74.6 and 78.9 kcal mol−1 and ΔG° = −10.7 and −9.6 kcal mol−1 in the lipid and water media, respectively) for the neutral state (H2A), however, the PL and SET pathways of this state are not spontaneous in either media (ΔG° > 0). Previous studies showed that for a compound containing acidic moieties the SET reaction of the dissociated states should be also considered in the aqueous solution [16,18,21,37,54,57]. Thus, the kinetics of the radical scavenging activity of 7OGT against HOO• radical in physiological media were carried out following equations (3.1) and (3.2) below, and the results are presented in table 3.
Table 3.
ΔG≠ (kcal mol−1), λ, κ, kapp, kf, koverall (M−1 s−1) and Γ (%) of the 7OGT + HOO• reaction in the physiological media.
| mechanisms | pentyl ethanoate |
water |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ΔG≠ | κ | kapp | Γ | ΔG≠ | κ | kapp | f | kbf | Γ | ||
| SET | H2A | 113.2 | 19.7 | 7.10 × 10−71 | 0.0 | 31.1 | 19.0a | 9.60 × 10−11 | 0.207 | 1.99 × 10−11 | 0.0 |
| HA− | 9.0 | 17.1a | 1.40 × 106 | 0.702 | 9.83 × 105 | 0.4 | |||||
| A2− | 4.3 | 18.2a | 2.90 × 109 | 0.091 | 2.64 × 108 | 99.6 | |||||
| FHT | H2A | 14.2 | 59.9 | 1.40 × 104 | 100.0 | 15.8 | 587.0 | 9.60 × 103 | 0.207 | 1.99 × 103 | 0.0 |
| koverall | 1.40 × 104 | 2.65 × 108 | |||||||||
aλ.
bkf = f.kapp; koverall = ∑kf(kapp).
Lipid medium,
| 3.1 |
Water at physiological pH,
| 3.2 |
According to the results (table 3), the HOO• + 7OGT reaction in the aqueous solution (koverall = 2.65 × 108 M−1 s−1) is approximately 104 times faster than that (koverall = 1.40 × 104 M−1 s−1) in the lipid medium. The SET of dianion A2− plays a principal role (kf = 2.64 × 108 M−1 s−1, Γ = 99.6%) in the HOO• antiradical activity of 7OGT in the aqueous solution. Compared with typical antioxidants indicated that the HOO• scavenging activity of 7OGT is faster than those of Trolox (approx. 2000 times, k = 1.30 × 105 M−1 s−1), [24] trans-resveratrol (approx. five times, k = 5.62 × 107 M−1 s−1) [55], ascorbic acid (approx. two times, k = 1.00 × 108 M−1 s−1) [11], ramalin (approx. 1692 times, k = 1.56 × 105 M−1 s−1) [57], deoxynimbidiol (approx. 1.5 times, k = 1.69 × 108 M−1 s−1) [24] and 8-hydroxyconiothyrinone B (approx. 4.5 times, k = 5.80 × 107 M−1 s−1) [18]. Hence, 7OGT is one of the most excellent natural antioxidants in polar environments.
4. Conclusion
The hydroperoxyl antiradical activity of 7OGT was successfully evaluated by computational chemistry. The results showed that the antiradical activity of the 7OGT in non-polar media such as gas phase and pentyl ethanoate follows the FHT reaction (k = 4.58 × 108 and 1.40 × 104 M−1 s−1, respectively). 7OGT also presented excellent antiradical activity (koverall = 2.65 × 108 M−1 s−1) in the aqueous solution. The HOO• radical scavenging of 7OGT is faster than that of typical antioxidants such as trans-resveratrol, Trolox, deoxynimbidiol, ramalin, 8-hydroxyconiothyrinone B and ascorbic acid. Thus, 7OGT is one of the most potent natural antioxidants identified thus far in polar environments.
Data accessibility
Data are available at the Dryad Digital Repository: https://doi.org/10.5061/dryad.jh9w0vtcq [59].
Authors' contributions
L.T.H.: conceptualization, data curation, formal analysis, investigation and writing—original draft; T.T.V.T.: conceptualization, data curation, formal analysis and investigation; N.T.H.: conceptualization, data curation, formal analysis, investigation and validation; A.M.: software, supervision and writing—review and editing; Q.V.V.: formal analysis, methodology, project administration, software, supervision, validation, visualization, writing—original draft and writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
We declare we have no competing interests.
Funding
The authors acknowledge the support of Hue University under the Core Research Group Program, grant no. NCM.DHH.2020.05. This research was funded by the Vietnamese Ministry of Education and Training under project number B2020-DHH-15 (T.T.V.T).
References
- 1.Li Y, Leung KT, Yao F, Ooi LS, Ooi VE. 2006. Antiviral flavans from the leaves of Pithecellobium clypearia. J. Nat. Prod. 69, 833-835. ( 10.1021/np050498o) [DOI] [PubMed] [Google Scholar]
- 2.Bao L, Yao X, Xu J, Guo X, Liu H, Kurihara H. 2009. Effects of Pithecellobium clypearia Benth extract and its main components on inflammation and allergy. Fitoterapia 80, 349-353. ( 10.1016/j.fitote.2009.04.009) [DOI] [PubMed] [Google Scholar]
- 3.Duong NT, Vinh PD, Thuong PT, Hoai NT, Bach TT, Nam NH, Anh NH. 2017. Xanthine oxidase inhibitors from Archidendron clypearia (Jack.) I.C. Nielsen: results from systematic screening of Vietnamese medicinal plants. Asian Pac. J. Trop. Med. 10, 549-556. ( 10.1016/j.apjtm.2017.06.002) [DOI] [PubMed] [Google Scholar]
- 4.Mercader AG, Pomilio AB. 2010. QSAR study of flavonoids and biflavonoids as influenza H1N1 virus neuraminidase inhibitors. Eur. J. Med. Chem. 45, 1724-1730. ( 10.1016/j.ejmech.2010.01.005) [DOI] [PubMed] [Google Scholar]
- 5.Thao NP, Luyen BTT, Kim JH, Jo AR, Dat NT, Van Kiem P, Van Minh C, Kim YH. 2016. Identification, characterization, kinetics, and molecular docking of flavonoid constituents from Archidendron clypearia (Jack.) Nielsen leaves and twigs. Bioorg. Med. Chem. 24, 3125-3132. ( 10.1016/j.bmc.2016.05.034) [DOI] [PubMed] [Google Scholar]
- 6.Zhang W, Xue W, Jia Y, Wen G, Lian X, Shen J, Liu A, Wu S. 2018. A concise synthesis of (±)-7-O-galloyltricetiflavan. RSC Adv. 8, 14 389-14 392. ( 10.1039/C8RA01606B) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shi S, et al. 2020. Semi-synthesis and biological evaluation of flavone hybrids as multifunctional agents for the potential treatment of Alzheimer's disease. Bioorg. Chem. 100, 103917. ( 10.1016/j.bioorg.2020.103917) [DOI] [PubMed] [Google Scholar]
- 8.Loscalzo J. 2003. Oxidant stress: a key determinant of atherothrombosis. Biochem. Soc. Trans. 31, 1059-1061. ( 10.1042/bst0311059) [DOI] [PubMed] [Google Scholar]
- 9.Ferroni P, et al. 2017. Oxidant stress as a major determinant of platelet activation in invasive breast cancer. Int. J. Cancer 140, 696-704. ( 10.1002/ijc.30488) [DOI] [PubMed] [Google Scholar]
- 10.Harned AM, Volp KA. 2011. The sorbicillinoid family of natural products: Isolation, biosynthesis, and synthetic studies. Nat. Prod. Rep. 28, 1790-1810. ( 10.1039/c1np00039j) [DOI] [PubMed] [Google Scholar]
- 11.Galano A, Alvarez-Idaboy JR. 2013. A computational methodology for accurate predictions of rate constants in solution: application to the assessment of primary antioxidant activity. J. Comput. Chem. 34, 2430-2445. ( 10.1002/jcc.23409) [DOI] [PubMed] [Google Scholar]
- 12.Rebollar-Zepeda AM, Campos-Hernández T, Ramírez-Silva MT, Rojas-Hernández A, Galano A. 2011. Searching for computational strategies to accurately predict pKas of large phenolic derivatives. J. Chem. Theory Comput. 7, 2528-2538. ( 10.1021/ct2001864) [DOI] [PubMed] [Google Scholar]
- 13.Moore JD, Harned AM, Henle J, Flynn DL, Hanson PR. 2002. Scavenge–ROMP–filter: a facile strategy for soluble scavenging via norbornenyl tagging of electrophilic reagents. Org. Lett. 4, 1847-1849. ( 10.1021/ol0257880) [DOI] [PubMed] [Google Scholar]
- 14.Carreon-Gonzalez M, Vivier-Bunge A, Alvarez-Idaboy JR. 2019. Thiophenols, promising scavengers of peroxyl radicals: mechanisms and kinetics. J. Comput. Chem. 40, 2103-2110. ( 10.1002/jcc.25862) [DOI] [PubMed] [Google Scholar]
- 15.Galano A, Mazzone G, Alvarez-Diduk R, Marino T, Alvarez-Idaboy JR, Russo N. 2016. Food antioxidants: chemical insights at the molecular level. Annu. Rev. Food Sci. Technol. 7, 335-352. ( 10.1146/annurev-food-041715-033206) [DOI] [PubMed] [Google Scholar]
- 16.Hoa NT, Hang DTN, Hieu DP, Van Truong H, Hoang LP, Mechler A, Vo QV. 2021. The hydroperoxyl radical scavenging activity of sulfuretin: insights from theory. R. Soc. Open Sci. 8, 210626. ( 10.1098/rsos.210626) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boulebd H, Mechler A, Hoa NT, Vo QV. 2020. Thermodynamic and kinetic studies of the antiradical activity of 5-hydroxymethylfurfural: computational insights. New J. Chem. 44, 9863-9869. ( 10.1039/D0NJ01567A) [DOI] [Google Scholar]
- 18.Vo QV, Thong NM, Le Huyen T, Nam PC, Tam NM, Hoa NT, Mechler A. 2020. A thermodynamic and kinetic study of the antioxidant activity of natural hydroanthraquinones. RSC Adv. 10, 20 089-20 097. ( 10.1039/D0RA04013D) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vo QV, Van Bay M, Nam PC, Mechler A. 2019. Is indolinonic hydroxylamine a promising artificial antioxidant? J. Phys. Chem. B 123, 7777-7784. [DOI] [PubMed] [Google Scholar]
- 20.Vo QV, Hoa NT, Mechler A. 2021. Modelling the mechanism and kinetics of the radical scavenging activity of iminostilbene. Polym. Degrad. Stab. 185, 109483. ( 10.1016/j.polymdegradstab.2021.109483) [DOI] [Google Scholar]
- 21.Rakita A, Nikolić N, Mildner M, Matiasek J, Elbe-Bürger A. 2020. Re-epithelialization and immune cell behaviour in an ex vivo human skin model. Sci. Rep. 10, 1-11. ( 10.1038/s41598-019-56847-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galano A, Alvarez-Idaboy JR. 2014. Kinetics of radical-molecule reactions in aqueous solution: a benchmark study of the performance of density functional methods. J. Comput. Chem. 35, 2019-2026. ( 10.1002/jcc.23715) [DOI] [PubMed] [Google Scholar]
- 23.Zhao Y, Truhlar DG. 2008. How well can new-generation density functionals describe the energetics of bond-dissociation reactions producing radicals? J. Phys. Chem. A 112, 1095-1099. ( 10.1021/jp7109127) [DOI] [PubMed] [Google Scholar]
- 24.Vo QV, Tam NM, Van Bay M, Thong NM, Le Huyen T, Hoa NT, Mechler A. 2020. The antioxidant activity of natural diterpenes: theoretical insights. RSC Adv. 10, 14 937-14 943. ( 10.1039/D0RA02681F) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marenich AV, Cramer CJ, Truhlar DG. 2009. Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 113, 6378-6396. ( 10.1021/jp810292n) [DOI] [PubMed] [Google Scholar]
- 26.Alvarez-Idaboy JRl, Galano A. 2012. On the chemical repair of DNA radicals by glutathione: hydrogen vs electron transfer. J. Phys. Chem. B 116, 9316-9325. ( 10.1021/jp303116n) [DOI] [PubMed] [Google Scholar]
- 27.Dzib E, Cabellos JL, Ortíz-Chi F, Pan S, Galano A, Merino G. 2019. Eyringpy: a program for computing rate constants in the gas phase and in solution. Int. J. Quantum Chem. 119, e25686. ( 10.1002/qua.25686) [DOI] [Google Scholar]
- 28.Dzib E, Cabellos J, Ortiz-Chi F, Pan S, Galano A, Merino E. 2018. Eyringpy 1.0.2. Mérida, Yucatán: Cinvestav.
- 29.Pollak E, Pechukas P. 1978. Symmetry numbers, not statistical factors, should be used in absolute rate theory and in Broensted relations. J. Am. Chem. Soc. 100, 2984-2991. ( 10.1021/ja00478a009) [DOI] [Google Scholar]
- 30.Fernández-Ramos A, Ellingson BA, Meana-Pañeda R, Marques JM, Truhlar DG. 2007. Symmetry numbers and chemical reaction rates. Theor. Chem. Acc. 118, 813-826. ( 10.1007/s00214-007-0328-0) [DOI] [Google Scholar]
- 31.Eyring H. 1935. The activated complex in chemical reactions. J. Chem. Phys. 3, 107-115. ( 10.1063/1.1749604) [DOI] [Google Scholar]
- 32.Truhlar DG, Hase WL, Hynes JT. 1983. Current status of transition-state theory. J. Phys. Chem. 87, 2664-2682. ( 10.1021/j100238a003) [DOI] [Google Scholar]
- 33.Furuncuoglu T, Ugur I, Degirmenci I, Aviyente V. 2010. Role of chain transfer agents in free radical polymerization kinetics. Macromolecules 43, 1823-1835. ( 10.1021/ma902803p) [DOI] [Google Scholar]
- 34.Vélez E, Quijano J, Notario R, Pabón E, Murillo J, Leal J, Zapata E, Alarcón G. 2009. A computational study of stereospecifity in the thermal elimination reaction of menthyl benzoate in the gas phase. J. Phys. Org. Chem. 22, 971-977. ( 10.1002/poc.1547) [DOI] [Google Scholar]
- 35.Eckart C. 1930. The penetration of a potential barrier by electrons. Phys. Rev. 35, 1303-1309. ( 10.1103/PhysRev.35.1303) [DOI] [Google Scholar]
- 36.Alberto ME, Russo N, Grand A, Galano A. 2013. A physicochemical examination of the free radical scavenging activity of Trolox: mechanism, kinetics and influence of the environment. Phys. Chem. Chem. Phys. 15, 4642-4650. ( 10.1039/c3cp43319f) [DOI] [PubMed] [Google Scholar]
- 37.Galano A, Raúl Alvarez-Idaboy J. 2019. Computational strategies for predicting free radical scavengers' protection against oxidative stress: where are we and what might follow? Int. J. Quantum Chem. 119, e25665. ( 10.1002/qua.25665) [DOI] [Google Scholar]
- 38.Boulebd H. 2021. Is cannabidiolic acid an overlooked natural antioxidant? Insights from quantum chemistry calculations. New J. Chem. 46, 162-168. ( 10.1039/D1NJ04771J) [DOI] [Google Scholar]
- 39.Evans MG, Polanyi M. 1935. Some applications of the transition state method to the calculation of reaction velocities, especially in solution. Trans. Faraday Soc. 31, 875-894. ( 10.1039/tf9353100875) [DOI] [Google Scholar]
- 40.Marcus RA. 1964. Chemical and electrochemical electron-transfer theory. Annu. Rev. Phys. Chem. 15, 155-196. ( 10.1146/annurev.pc.15.100164.001103) [DOI] [Google Scholar]
- 41.Marcus RA. 1993. Electron transfer reactions in chemistry: theory and experiment. Rev. Mod. Phys. 65, 599-610. ( 10.1103/RevModPhys.65.599) [DOI] [Google Scholar]
- 42.Nelsen SF, Blackstock SC, Kim Y. 1987. Estimation of inner shell Marcus terms for amino nitrogen compounds by molecular orbital calculations. J. Am. Chem. Soc. 109, 677-682. ( 10.1021/ja00237a007) [DOI] [Google Scholar]
- 43.Nelsen SF, Weaver MN, Luo Y, Pladziewicz JR, Ausman LK, Jentzsch TL, O'Konek JJ. 2006. Estimation of electronic coupling for intermolecular electron transfer from cross-reaction data. J. Phys. Chem. A 110, 11 665-11 676. ( 10.1021/jp064406v) [DOI] [PubMed] [Google Scholar]
- 44.Collins FC, Kimball GE. 1949. Diffusion-controlled reaction rates. J. Colloid Sci. 4, 425-437. ( 10.1016/0095-8522(49)90023-9) [DOI] [Google Scholar]
- 45.Von Smoluchowski M. 1917. Versucheiner Mathematischen Theorie der Koagulations Kinetic Kolloider Lousungen. Z. Phys. Chem 92, 129-168. [Google Scholar]
- 46.Truhlar DG. 1985. Nearly encounter-controlled reactions: the equivalence of the steady-state and diffusional viewpoints. J. Chem. Educ. 62, 104. ( 10.1021/ed062p104) [DOI] [Google Scholar]
- 47.Einstein A. 1905. Über die von der molekularkinetischen Theorie der Wärme geforderte Bewegung von in ruhenden Flüssigkeiten suspendierten Teilchen. Ann. Phys. 322, 549-560. ( 10.1002/andp.19053220806) [DOI] [Google Scholar]
- 48.Stokes GG. 1905. Mathematical and physical papers. Cambridge, UK: University Press. [Google Scholar]
- 49.Okuno Y. 1997. Theoretical investigation of the mechanism of the Baeyer-Villiger reaction in nonpolar solvents. Chem. Eur. J. 3, 212-218. ( 10.1002/chem.19970030208) [DOI] [PubMed] [Google Scholar]
- 50.Benson S. 1960. The foundations of chemical kinetics. New York, NY: McGraw-Hill. [Google Scholar]
- 51.Iuga C, Alvarez-Idaboy JR, Vivier-Bunge A. 2011. ROS initiated oxidation of dopamine under oxidative stress conditions in aqueous and lipidic environments. J. Phys. Chem. B 115, 12 234-12 246. ( 10.1021/jp206347u) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alvarez-Idaboy JR, Reyes L, Mora-Diez N. 2007. The mechanism of the Baeyer–Villiger rearrangement: quantum chemistry and TST study supported by experimental kinetic data. Org. Biomol. Chem. 5, 3682-3689. ( 10.1039/b712608e) [DOI] [PubMed] [Google Scholar]
- 53.Frisch M, et al. 2009. Gaussian 09. Wallingford, CT: Gaussian Inc.
- 54.Zhou H, Li X, Shang Y, Chen K. 2019. Radical scavenging activity of puerarin: a theoretical study. Antioxidants 8, 590. ( 10.3390/antiox8120590) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cordova-Gomez M, Galano A, Alvarez-Idaboy JR. 2013. Piceatannol, a better peroxyl radical scavenger than resveratrol. RSC Adv. 3, 20 209-20 218. ( 10.1039/c3ra42923g) [DOI] [Google Scholar]
- 56.Shang Y, Zhou H, Li X, Zhou J, Chen K. 2019. Theoretical studies on the antioxidant activity of viniferifuran. New J. Chem. 43, 15 736-15 742. ( 10.1039/C9NJ02735A) [DOI] [Google Scholar]
- 57.Vo QV, Tam NM, Van Bay M, Mechler A. 2020. The radical scavenging activity of natural ramalin: a mechanistic and kinetic study. Chem. Phys. Lett. 739, 137004. ( 10.1016/j.cplett.2019.137004) [DOI] [Google Scholar]
- 58.Galano A, et al. 2016. Empirically fitted parameters for calculating pKa values with small deviations from experiments using a simple computational strategy. J. Chem. Inf. Model. 56, 1714-1724. ( 10.1021/acs.jcim.6b00310) [DOI] [PubMed] [Google Scholar]
- 59.Hieu LV, Van Thi TT, Hoa NT, Mechler A, Vo QV. 2022. Data from: 7-O-Galloyltricetifavan: a promising natural radical scavenger. Dryad Digital Repository. ( 10.5061/dryad.jh9w0vtcq) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available at the Dryad Digital Repository: https://doi.org/10.5061/dryad.jh9w0vtcq [59].