Abstract
A man in his 40s was admitted to his local hospital 6 days after the first vague symptoms of COVID-19. His general condition deteriorated, and he was treated in the intensive care unit but did not require mechanical ventilation. During his recovery, he experienced a cough spell, after which his dyspnoea recurred and rapidly increased. CT pulmonary angiogram showed a 10×18 cm cavitary lesion with an air-fluid level and surrounding atelectasis of the right lower lobe. A one-way valve mechanism had developed, leading to the formation of a pneumatocele. The patient was treated by occlusion of all bronchial segments of the right lower lobe with endobronchial valves, and the pneumatocele was evacuated with a pigtail catheter. The valves were removed 4 weeks after insertion, and the right lower lobe re-expanded. Six months after treatment, the patient had recovered completely and almost regained his former lung function.
Keywords: Air leaks, COVID-19, Pneumothorax, Respiratory medicine, Cardiothoracic surgery
Background
Spontaneous pneumomediastinum and pneumothorax are unusual complications of pulmonary infections, acute respiratory distress syndrome, positive-pressure ventilation, chest trauma and chemical pneumonitis. Pneumatoceles represent even more unusual complications in these settings. Pneumatoceles are air-containing, intraparenchymal, cavitary lesions of various size from less than 1 cm to the entire hemithorax. We report the use of endobronchial valves to stop entry of air into the pneumatocele and the subsequent removal of the valves without recurrence of pneumatocele.
Case presentation
A Caucasian man in his 40s was admitted in the spring of 2021 to his local hospital, 6 days after the first non-specific symptoms of COVID-19. His medical history included previous hemithyroidectomy (in 2016, benign histology), surgical resection of thymoma (2017) and pneumonia caused by Mycoplasma pneumoniae (2016) and a successfully treated visceral leishmaniasis from which he suffered after a sailing holiday in the Mediterranean Sea (2017). During routine follow-up after thymectomy, a CT thorax with intravenous contrast in 2020 was unremarkable except for dependent atelectasis (figure 1A).
Figure 1.
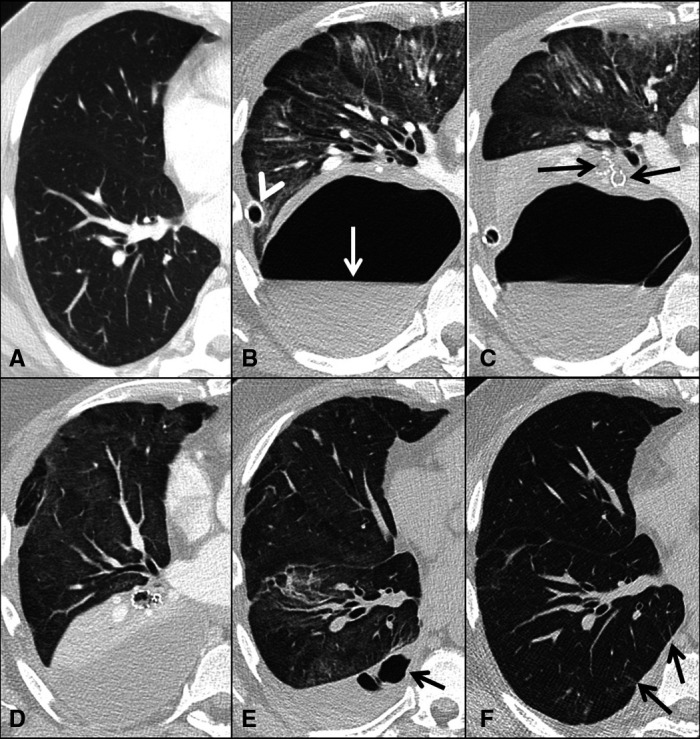
(A–F). Serial transverse CT scans of the chest sections showing right hemithorax only. One year before admission for COVID-19 pneumonia routine CT with contrast agent was unremarkable except for some dependent atelectasis (A). CT pulmonary angiogram 3 weeks after admission shows a 10×18 cm cavitary lesion with an air-fluid level (arrow) and surrounding atelectasis of the lower lobe. A chest drainage tube is positioned in the periphery of the cavity (arrowhead). There are widespread ground glass opacities in the upper and middle lobe (B). Chest CT after insertion of endobronchial valves (arrows) covering all segments of the lower lobe (C). Three days later CT scans show endobronchial valves in situ and collapse of the lower lobe. The drainage tube is removed (D). One month later the endobronchial valves have been removed. In the lower lobe, there is almost complete re-expansion but still some opacifications related to COVID-19 pneumonia in all lobes. In the pleural cavity organised fluid with small air cavities (arrow) (E). High-resolution CT scans 5 months after admission show almost complete absorption of the abnormalities; however, small parenchymal bands are observed (arrows) (F).
At the time of admission, he experienced fever, cough and increasing dyspnoea. His general condition deteriorated further, and he was treated in the intensive care unit with non-invasive ventilation and high-flow nasal cannula oxygen therapy. However, he was at no point in need of invasive mechanical ventilation. Three weeks after admission, at which time he was clearly improving, he experienced a heavy sneeze or cough spell. Following this, his dyspnoea recurred and increased. To rule out pulmonary embolism, a CT scan was performed. The CT scan revealed a thoracic air collection initially diagnosed as a right-sided pneumothorax and widespread ground-glass opacities but no pulmonary embolism. Multiple chest drainage tubes were inserted into the pleural space; however, despite appropriate placement, no evacuation of air ensued (figure 1B).
Investigations
Four days after the finding of suspected pneumothorax, the patient was referred to our tertiary university hospital due to suspected pneumatocele. At admission, the patient was dependent on oxygen at 8–10 L/min and desaturated to SpO2 65% when moving from his bed to a chair. To definitively rule out the suspicion of pneumothorax, an ultrasound-guided thoracic pigtail drain was inserted into the basal pleural space. Because this additional drain only resulted in the drainage of pleural fluid and not air, the suspicion of pneumatocele was strengthened, but the drain was left in place. Furthermore, despite the pleural drainage, a new CT scan revealed further expansion of the intrapulmonary air collection.
Hence, it was concluded that the patient suffered from a pneumatocele without communication to the pleural space. A one-way valve mechanism seemed to have developed, presumably due to airway obstruction by inflammation and oedema leading to distal air trapping and pneumatocele. The pneumatocele was located in the right lower lobe, and it was assumed that the trapped air originated from the bronchi of the lower lobe (figure 1B).
Treatment
In an attempt to attenuate the air leak into the pneumatocele, three endobronchial valves (Zephyr, Pulmonx Inc., Redwood City, California, USA) were inserted. One small valve (4.0-Low Profile (LP)) in the superior segment of the lower lobe, one small valve (4.0-LP) in the medial basal segment bronchus and one large valve (5.0) covering the other basal segments (anterior, lateral and posterior segment bronchi) (figure 1C). Three days later, a CT scan confirmed complete atelectasis of the lower lobe, but the pneumatocele remained unchanged in shape and size (figure 1D). Another CT-guided pigtail catheter was inserted into the pneumatocele. During the ensuing 5 hours, the pneumatocele was evacuated for 2.0–2.5 L of air and 0.4 L of fluid. This catheter was removed after 7 hours. The following day, a CT scan confirmed the complete disappearance of the cavity in the right lung. The remaining apical pleural drain was retained for an additional 4 days due to a small pneumothorax. Repeated X-rays of the thorax confirmed that the cavity did not recur, and the patient’s dyspnoea and need of extra oxygen supply decreased. However, pleural fluid re-accumulated, and serosanguinous pleural fluid was drained twice from the right thorax. Twelve days after insertion of the bronchial valves, the patient was transferred back to his local hospital without the need for supplemental oxygen. Prior to valve insertion, the patient was treated with intravenous cefotaxime, and this was changed to per oral amoxicillin 14 days following valve insertion. Antibiotics were continued until valve removal.
Outcome and follow-up
The three valves were removed during bronchoscopy 4 weeks after insertion. By the time of valve removal, the patient was no longer in need of oxygen supply. Spirometry showed a forced vital capacity of 2.3 L (45% of expected) and forced expiratory volume in the first 1 s (FEV1) of 1.8 L (43% of expected).
Following valve removal, the patient was examined after 1 and 5 months. The initial ground-glass opacities regressed and were no longer visible 6 months after treatment (figure 1E, F). The pneumatocele did not reappear. His lung function increased steadily to near-normal values (table 1). The patient returned to his full-time job 6 months after treatment.
Table 1.
Lung function prior to and 1, 2 and 6 months following endobronchial valve treatment for pneumatocele
| Lung function—prior to and after treatment | ||||
| Prior (2017) | 1 month | 2 months | 6 months | |
| FVC, litres (% expected) | 4.8 (92) | 2.3 (45) | 3.2 (61) | 4.1 (80) |
| FEV1, litres (% expected) | 3.5 (83) | 1.8 (43) | 2.4 (59) | 3.2 (80) |
| DLCO (% expected) | 8.3 (77) | 4.0 (40) | 5.7 (57) | 7.1 (71) |
DLCO, diffusing capacity for carbon monoxide; FEV1, forced expiratory volume in the first 1 s; FVC, forced vital capacity.
Discussion
In the course of severe COVID-19 pneumonia, spontaneous pneumomediastinum and pneumothorax unrelated to positive-pressure ventilation have been reported as unusual complications.1 However, these complications are also seen in several other conditions including bacterial pneumonia, positive-pressure ventilation, chest trauma and chemical pneumonitis.2 In patients with COVID-19, lung injury is thought to result from exaggerated inflammation induced by viral replication and then sustained by a cytokine storm associated with disease severity.3 4 The inflammation may lead to increased vulnerability of the lung parenchyma.
Pneumatoceles are thin-walled, focal or multifocal air-filled structures in the lung parenchyma with or without air-fluid levels. In this case, the gas-filled lesion appeared thin walled but became large enough to compress adjacent lung tissue. Differential diagnoses for thin-walled cysts include blebs, bullae, bronchogenic cysts, congenital pulmonary airway malformation and primary cystic lung diseases, which were all possible to rule out in this patient because of the normal chest CT 1 year before admission. The thin and regular wall with smooth inner margins in our case, in addition to the clinical context, favoured a pneumatocele over an abscess. Pneumatoceles may be difficult to distinguish from loculated pneumothorax in which a pocket of pleural air is trapped within a localised area. In our case, however, the lesion was surrounded by a thin rim of lung parenchyma.
Most pneumatoceles do not require specific treatment and disappear spontaneously within weeks.5 Decompression with percutaneous drainage or surgical intervention may be considered when the pneumatocele is large enough to cause cardiorespiratory instability. However, there is a risk of introducing bacteria or other infective agents into the pneumatocele, which may contribute to bronchopleural fistulation.
Lung volume reduction with endobronchial valves is an established method in the treatment of patients with severe emphysema.6 7 However, the development of pneumatoceles is reported also to be induced by the insertion of endobronchial valves.8 Persistent air leak after lung surgery or radiological interventions has also been successfully treated with endobronchial valves.9–11 Persistent air leak related to SARS-CoV-2 infection has successfully been treated with endobronchial valve placement.12
Our case illustrates the novel use of endobronchial valves in the treatment of pneumatocele. After first confirming that our patient with severe COVID-19 suffered from a pneumatocele and not a pneumothorax, we applied endobronchial valves to attenuate the increasing volume of the pneumatocele causing cardiopulmonary instability. Second, the pneumatocele was evacuated using a pigtail drain, without complications. Thereafter, the patient recovered steadily, and the valves were removed 1 month after insertion. The clinical condition of the patient improved further, and 6 month after treatment, he was fully recovered from both COVID-19 and pneumatocele.
Based on the present case report, we conclude that the use of endobronchial valves is feasible in the treatment of pneumatocele causing cardiopulmonary instability.
Learning points.
Endobronchial valves may be considered in the management of pneumatocele causing cardiopulmonary instability.
The less invasive interventional bronchoscopy compared with a surgical approach is advantageous.
The treatment is reversible because the valves may be removed.
Footnotes
Contributors: Patient primarily admitted under LHJ, ASB and AS for medical management. The interpretation of the radiological examinations was performed by TMA. Endobronchial intervention and management by AS, LHJ and ASB. ASB drafted the initial manuscript and reviewed the literature. All authors finalised the manuscript and approved the final manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Case reports provide a valuable learning resource for the scientific community and can indicate areas of interest for future research. They should not be used in isolation to guide treatment choices or public health policy.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s).
References
- 1.Elhakim TS, Abdul HS, Pelaez Romero C, et al. Spontaneous pneumomediastinum, pneumothorax and subcutaneous emphysema in COVID-19 pneumonia: a rare case and literature review. BMJ Case Rep 2020;13. 10.1136/bcr-2020-239489. [Epub ahead of print: 12 Dec 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chennu G, Przydzial P, Tchao Y, et al. Pneumatocele after acute respiratory distress syndrome in an adult patient: a case report. Case Reports in Acute Medicine 2020;3:73–8. 10.1159/000509870 [DOI] [Google Scholar]
- 3.Ye Q, Wang B, Mao J. The pathogenesis and treatment of the Cytokine Storm in COVID-19. J Infect 2020;80:607–13. 10.1016/j.jinf.2020.03.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054–62. 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jamil A. Pneumatocele. In: StatPearls. Treasure Island (FL): StatPearls Publishing, 2020. [Google Scholar]
- 6.Herth FJF, Noppen M, Valipour A, et al. Efficacy predictors of lung volume reduction with Zephyr valves in a European cohort. Eur Respir J 2012;39:1334–42. 10.1183/09031936.00161611 [DOI] [PubMed] [Google Scholar]
- 7.Labarca G, Uribe JP, Pacheco C, et al. Bronchoscopic lung volume reduction with endobronchial Zephyr valves for severe emphysema: a systematic review and meta-analysis. Respiration 2019;98:268–78. 10.1159/000499508 [DOI] [PubMed] [Google Scholar]
- 8.Skowasch D, Pizarro C, Valipour A, et al. Endobronchial valve-induced pneumatocele: a case report. Pneumologie 2013;67:639–40. 10.1055/s-0033-1344640 [DOI] [PubMed] [Google Scholar]
- 9.Hoeijmakers F, Hartemink KJ, Verhagen AF, et al. Variation in incidence, prevention and treatment of persistent air leak after lung cancer surgery. Eur J Cardiothorac Surg 2021;61:110–7. 10.1093/ejcts/ezab376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang X, Ding L, Xu H. Bronchoscopic valve placement for the treatment of persistent air leaks. Medicine 2018;97:e0183. 10.1097/MD.0000000000010183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gillespie CT, Sterman DH, Cerfolio RJ, et al. Endobronchial valve treatment for prolonged air leaks of the lung: a case series. Ann Thorac Surg 2011;91:270–3. 10.1016/j.athoracsur.2010.07.093 [DOI] [PubMed] [Google Scholar]
- 12.Donatelli P, Trentacosti F, Pellegrino MR, et al. Endobronchial valve positioning for alveolar-pleural fistula following ICU management complicating COVID-19 pneumonia. BMC Pulm Med 2021;21:307. 10.1186/s12890-021-01653-w [DOI] [PMC free article] [PubMed] [Google Scholar]


