Abstract
Objective
To investigate whether inspiratory muscle training (IMT) offered adjunctively to an exercise training program reduces symptoms of dyspnea in survivors of breast cancer.
Design
Double-blind, parallel-group, randomized controlled trial.
Setting
Outpatient rehabilitation program in a university hospital.
Participants
Ninety-eight female patients with breast cancer who completed adjuvant treatment and subsequently entered cancer rehabilitation were screened for participation. Inclusion criteria were reduced inspiratory muscle strength and/or symptoms of dyspnea. Twenty patients (N=20) were randomly assigned to an intervention group (n=10) or a control group (n=10).
Intervention
Both groups received a 3-month exercise training program in combination with either IMT (intervention) or sham-IMT (control).
Main Outcome Measures
Changes in dyspnea intensity perception (10-point Borg Scale) at comparable time points (isotime) during constant work rate cycling was the primary outcome. Secondary outcomes included changes in respiratory muscle function, exercise capacity, and changes in symptoms of dyspnea during daily life (Transitional Dyspnea Index [TDI]).
Results
The intervention group achieved a larger reduction in exertional dyspnea at isotime compared with the control group (−1.8 points; 95% CI, −3.7 to 0.13; P=.066). The intervention group also exhibited larger improvements in dyspnea during daily life (TDI score, +2.9 points; 95% CI, 0.5-5.3; P=.022) and improved both respiratory muscle endurance (+472 seconds; 95% CI, 217-728; P=.001) and cycling endurance (+428 seconds; 95% CI, 223-633; P=.001) more than the control group.
Conclusions
Because of the limited sample size all obtained findings need to be interpreted with caution. The study offers initial insights into the potential of adjunctive IMT in selected survivors of breast cancer. Larger multicenter studies should be performed to further explore the potential role and general acceptance of this intervention as a rehabilitation tool in selected patients after breast cancer treatment.
KEYWORDS: Breast neoplasms, Breathing exercises, Dyspnea, Exercise, Muscle strength, Physical therapy modalities, Randomized controlled trial, Rehabilitation
List of abbreviations: BDI, Baseline Dyspnea Index; IMT, inspiratory muscle training; PImax, maximal inspiratory pressure; MID, minimal important difference; TDI, Transitional Dyspnea Index
Breast cancer is the most prevalent type of cancer in women worldwide.1 As a result of early diagnosis and advanced treatments, the number of survivors of breast cancer increases.2 However, up to 90% of survivors of breast cancer experience long-term impairments after treatment.3 These may include decreased strength, decreased aerobic capacity, and fatigue.3, 4, 5
Additionally, dyspnea, marked by a sensation of breathing discomfort (especially on physical exertion) is a frequently reported symptom in survivors of (breast) cancer.6, 7, 8, 9 Potential causes of exertional dyspnea could be impairments in pulmonary function and respiratory muscle function.6 Kluthcovsky et al studied cancer-related fatigue in survivors of breast cancer and observed an association between fatigue and dyspnea.5 These authors noticed that patients often used the terms fatigue or exhaustion when referring to dyspnea. As a result, symptoms of dyspnea remain often undiagnosed and frequently untreated.5 Furthermore, respiratory muscle function is often not assessed, leaving the association between respiratory muscle function and dyspnea underexplored. Both limb and respiratory muscle strength is often decreased in these patients.6,7,9 Moreover, chest wall compliance is frequently reduced after cancer treatments, which increases the load on the respiratory muscles, especially during exercise.6,10 Impairments in pulmonary function are also common and will further increase respiratory muscle work during exercise.11
Exercise training programs are effective in improving physical fitness and reducing fatigue after breast cancer treatment.4,12,13 These programs typically consist of a combination of aerobic and resistance exercises.12,13 Implementing specific inspiratory muscle training (IMT) adjunctively to exercise training programs has previously resulted in larger improvements in respiratory muscle function and dyspnea in patients with chronic respiratory disease.14,15
There is currently, however, no evidence for the effects of adjunctive inspiratory muscle training added to an exercise training program in survivors of breast cancer. Therefore, this study aimed to evaluate the effectiveness of adjunctive IMT in symptomatic survivors of breast cancer with impaired respiratory muscle function. We hypothesized that adjunctive IMT would result in larger improvements in symptoms of dyspnea compared with an exercise training program offered without adjunctive IMT.
Methods
Trial design
The design of the study is a double-blind, parallel-group, randomized controlled trial. Patients who agreed to participate were randomized into an intervention group or a control group at a 1:1 ratio. Both groups participated in an exercise training program, but only the intervention group received additional respiratory muscle training. The control group received a sham treatment. This study was approved by the local ethics committee (reference no. MP003175).
Participants
Participants were recruited in the local university hospitals, Department of Physical Medicine and Rehabilitation, between May 2018 and January 2019. Stable patients with breast cancer who completed adjuvant treatment were allowed to undergo the offered rehabilitation program and were therefore eligible to participate in the study. Additionally, patients had to exhibit reduced maximal inspiratory pressure ([PImax] below predicted normal value), indicative of impaired respiratory muscle function or symptoms of dyspnea in daily life (score ≤9/12 on Baseline Dyspnea Index [BDI]) to remain eligible.16 Exclusion criteria were the presence of underlying chronic cardiac or respiratory disease that might have contributed to symptoms of dyspnea. Participants had to provide written informed consent before participation in accordance with the Declaration of Helsinki.
Group allocation was conducted using sealed opaque envelopes in random block sizes of 4 and 6 (order unknown to investigators) according to an established method.17 Participants and outcome assessors were blinded to group allocation. Therapists offering the exercise training program or the adjunctive intervention were not blinded to group allocation.
Intervention
After baseline measurements, a 3-month intervention program was started. Both groups followed the identical exercise training program. Additionally, the intervention group performed 2 IMT sessions per day, consisting of 30 breaths against a resistance of 50% of their PImax, 4-5 minutes per session, for 7 d/wk, for 12 weeks, using an electronic tapered flow resistive loading device (POWERbreathe KHP2).a This device enables constant monitoring of training data and ensures higher performed total work during training sessions than other methods.18 Patients were instructed to fill their diaries by copying stored data from the device. Total work and training load during the training program were subsequently extracted from the diaries. Supervised training sessions, including measurements of PImax, were planned to be performed on-site every 2 weeks after the exercise training sessions of the rehabilitation program. Furthermore, training loads were increased at these visits to maintain the external load at ∼50% of PImax at respective measurements throughout the study period. Ratings of perceived inspiratory effort on a modified Borg scale (10-point Borg Scale of 4-5 of 10) were used to support decisions on increasing training load. The control group completed the same amount of IMT sessions but trained at ∼10% of their initial PImax. This training load remained unchanged to avoid training stimuli. To increase adherence, both treatments were presented as active interventions. The training was presented as strength training in the intervention group and as endurance training in the control group. Participants in the control group were able to follow the active treatment after the completion of the study. All assessments except for the maximal cardiopulmonary exercise test and the lung diffusion capacity were repeated after the intervention period.
Assessments
Supplemental table S1 presents an overview of all outcome measurements. An overview of the study design is depicted in supplemental table S2.
Pulmonary function
Full pulmonary function testing including spirometry, lung volumes, and diffusion capacity was performed at the department of pneumology according to current European Respiratory Society guidelines.19, 20, 21 Reference values from the Global Lung Function Initiative were used to interpret the outcomes.22,23
Respiratory muscle function
Respiratory muscle function was evaluated by measuring the PImax and maximal expiratory pressure using a microRPM Pressure Meterb and respiratory muscle endurance (POWERbreatheKH2)a in accordance with international guidelines.24 During assessments of maximal mouth pressures, patients had to perform maximal quasi-static inspiratory and expiratory efforts starting from either residual volume or total lung capacity for the measurements of PImax and maximal expiratory pressure, respectively. The maximum 1-second plateau pressure of the 3 best maneuvers that differed by <10% was retained and compared with reference values.19 The endurance breathing test was conducted with an established protocol.24 After standardized instructions, patients were instructed to breathe against a constant submaximal external resistance until task failure.24 Patients were encouraged to perform as many forceful and deep inhalations and complete exhalations in the device as possible. Breathing duration, number of breaths, and total external work performed during the protocol were registered.
Symptoms of dyspnea
A modified Borg Scale (0-10) was used during the endurance breathing test, constant work rate cycling test (primary outcome), and 6-minute walk test to assess the intensity of dyspnea throughout the tests. The Multidimensional Dyspnea Profile scale was used to assess dyspnea by evaluating overall breathing discomfort at the end of the constant work rate cycling test.25 To measure the change in the severity of dyspnea during daily life we used the BDI and the corresponding Transitional Dyspnea Index (TDI). The BDI/TDI consist of 3 different categories, namely functional impairment, magnitude of task, and magnitude of effort.26 All categories were rated in 5 grades, from 0 (severe) to 4 (unimpaired).26 Scores were added up to obtain a general score, ranging from 0-12 representing the severity of dyspnea at baseline. Therefore, the lower the score, the worse the severity of dyspnea.26 The TDI was subsequently used to quantify the change in dyspnea from baseline. Changes in dyspnea were rated by 7 grades, ranging from −3 (major deterioration) to +3 (major improvement) for each category.26 The change scores on all categories were added up to give a general image of the change in dyspnea during daily life, ranging between −9 and +9. The modified Medical Research Council dyspnea scale rates dyspnea intensity on a score between 0 (unimpaired) and 4 (severe) in terms of breathing possibility during daily activities.27 This dyspnea scale and the BDI/TDI explore dyspnea intensity differently; hence, they complement each other perfectly.28
Exercise capacity
Assessment of maximal exercise capacity was performed during the initial screening procedure through a cardiopulmonary exercise test, which was performed on an electronically-braked cycle ergometer (Ergoline 800s)c with detailed metabolic and cardiopulmonary measurements (Vs229d).c Endurance exercise capacity was assessed using constant work rate cycling against a workload (W) of 80% of the peak work rate achieved during the cardiopulmonary exercise test. Before the constant work rate cycling test, forced vital capacity and maximal voluntary ventilation were assessed by spirometry. Throughout the test, heart rate, oxygen saturation, minute ventilation, and other breathing and exercise parameters were recorded. Secondary parameters were extracted as 30-second averages that were subsequently used to determine values at a standardized time point (isotime) and peak exercise. In addition, minute-by-minute intensity of dyspnea and leg discomfort was evaluated using a modified Borg Scale (0-10).29 Blood pressure and inspiratory capacity were measured every 2 minutes. In addition, functional exercise capacity was evaluated using a 6-minute walk test.30 Before and after the test, patients were asked to rate leg discomfort and symptoms of dyspnea on a modified Borg Scale (0-10).29 Additionally, the walking distance was measured as well as oxygen saturation and heart rate throughout the test.
Peripheral muscle strength
Handgrip strength was measured using handheld dynamometry. Patients had to keep the elbow of the tested side in 90 degrees of flexion and a neutral position of pro- and supination while performing the test. Both sides were tested 3 times, and the maximal value was retained.31,32
Statistical analyses
A sample size of 10 patients in the intervention group and 10 patients in the control group was required to detect a between-group difference of 1.3±1 units for the change in dyspnea intensity rating on a modified Borg Dyspnea Scale (0-10) between pre-and postintervention assessments at isotime during the constant work rate cycling test with a statistical power (ß) of 80% and a risk for a type I error (α) <5%. All data were analyzed following the intention-to-treat principle. Statistical procedures were performed using SPSS version 27.0.d Postintervention between-group differences were compared adjusting for baseline differences in an analysis of covariance, and adjusted mean differences between groups are reported alongside their 95% CI.33 In addition, paired samples t tests or Wilcoxon tests were applied to examine within-group differences before and after treatment. To further investigate within-group changes from pre- to post intervention at different time points during the constant work rate cycling test, 2-way repeated-measures analyses of variance were conducted. Alongside these results, partial η2 values are reported as a measure of effect size. Furthermore, exploratory correlates of training outcomes with changes in respiratory muscle function and symptoms of dyspnea were investigated using linear bivariate correlation tests.
Results
Study population
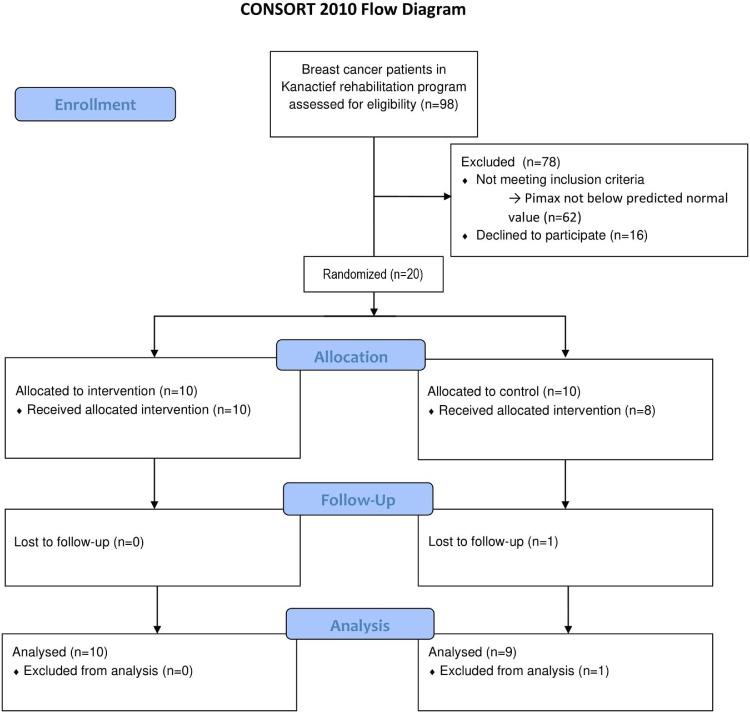
Figure 1 displays the flow of participants throughout the different phases of the study. Twenty stable patients with breast cancer were enrolled. One patient in the control group was not willing to complete the exercise training program nor the sham intervention and was subsequently dropped out of the study. Additionally, another patient from the control group did not follow the sham intervention but did perform pre and post measurements and was subsequently conserved in the analyses. Finally, the exercise and breathing pattern data of a patient in the intervention group was missing during the postintervention constant work rate cycling test because of calibration issues.
Fig 1.
Consolidated Standards of Reporting Trials flow diagram displaying the progress of participants through the phases of the study.
Baseline characteristics
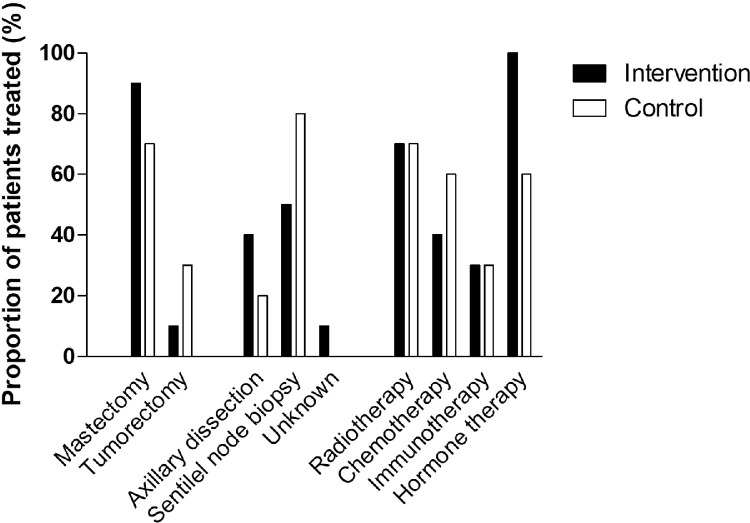
Table 1 presents an overview of the baseline characteristics. All participants were female and aged between 36 and 69 years, and except for PImax (mean difference, −17cmH2O; 95% CI, −30 to −4; P=.015), no relevant baseline differences were observed between groups. This was also true for the different adjuvant treatments that patients received. These data are presented in fig 2.
Table 1.
Baseline characteristics
| Characteristic | Intervention (n=10) | Control (n=10) |
|---|---|---|
| Age (y) | 51±5 | 55±9 |
| Height (cm) | 165±6 | 168±5 |
| Weight (kg) | 71±14 | 75±15 |
| Medical treatments | ||
| Type of breast surgery | ||
| Mastectomy (% received) | 90 | 70 |
| Tumorectomy (% received) | 10 | 30 |
| Type of axillary surgery | ||
| Axillary lymph node dissection (% received) | 40 | 20 |
| Sentinel node biopsy (% received) | 50 | 80 |
| Unknown (% received) | 10 | 0 |
| Type of adjuvant treatment | ||
| Radiotherapy (% received) | 70 | 70 |
| Chemotherapy (% received) | 40 | 60 |
| Immunotherapy (% received) | 30 | 30 |
| Hormone therapy (% received) | 100 | 60 |
| Pulmonary function | ||
| FVC, L (% predicted) | 3.7±0.5 (105±12) | 3.5±0.6 (101±14) |
| FEV1, L (% predicted) | 2.9±0.4 (103±12) | 2.8±0.7 (100±18) |
| FEV1/FVC (%) | 78.8±6.7 | 78.7±6.6 |
| RV, L (% predicted) | 1.9±0.2 (107±12) | 2.3±0.3 (121±20) |
| FRC, L (% predicted) | 3.1±0.4 (112±15) | 3.2±0.5 (114±15) |
| TLC, L (% predicted) | 5.7±0.6 (111±10) | 5.9±0.7 (112±13) |
| TLco, mmol/min/kPa (% predicted) | 6.3±0.9 (82±11) | 6.4±0.8 (86±11) |
| Respiratory muscle function | ||
| PImax, cmH2O (% predicted) | −74±11 (69±10) | −91±15 (91±15) |
| PEmax, cmH2O (% predicted) | 139±27 (77±15) | 145±26 (85±14) |
| Endurance breathing time (s) | 209±79 | 266±126 |
| External resistance (% PImax) | 62±10 | 61±7 |
| Symptoms of dyspnea | ||
| BDI, 0-12 | 8.4±2.4 | 8.6±1.9 |
| MDP, 0-10 | 6.7±1.8 | 6.4±2.9 |
| mMRC, 0-4 | 0.8±0.4 | 1.0±0.7 |
| Exercise capacity | ||
| Maximal exercise capacity | ||
| V̇o2max, L/min (% predicted) | 2.0±0.4 (91±19) | 2.0±0.4 (97±27) |
| Load (W) | 123±28 | 118±30 |
| Maximal heart rate, 1/min (% predicted) | 158±13 (93±6) | 151±17 (94±11) |
| Constant work rate cycling | ||
| Duration, min | 7.0±3.3 | 6.2±4.5 |
| Load, W (% peak work rate) | 98±20 (80±4) | 94±22 (80±2) |
| Functional capacity | ||
| 6MWD, m (% predicted) | 557±92 (84±14) | 553±105 (86±18) |
| Peripheral muscle strength | ||
| Handgrip strength, N (% predicted) | 255±53 (94±19) | 248±29 (102±21) |
NOTE. Data are presented as mean ± SD unless otherwise indicated.
Abbreviations: FEV1, forced expiratory volume in 1 second; FRC, functional residual capacity; FVC, forced vital capacity; MDP, multidimensional dyspnea profile; mMRC Modified Medical Research Council Scale; PEmax, maximal expiratory pressure; % PImax, percentage of the mean inspiratory load relative to the PImax; % predicted, percentage of the predicted normal value; RV, residual volume; 6MWD, 6-minute walking distance; TLC, total lung capacity; TLco, diffusing capacity of the lungs for carbon monoxide; VC, vital capacity; V̇o2max, maximal oxygen uptake.
Fig 2.
Adjuvant treatments received by study participants.
Respiratory muscle training
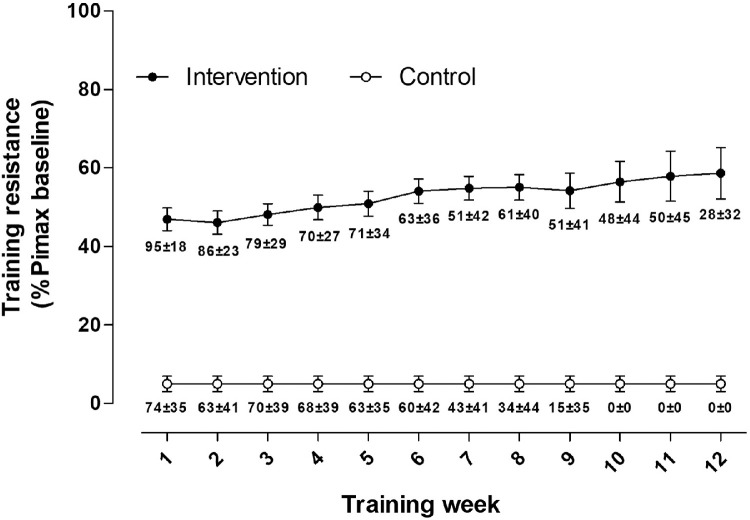
Supplemental table S3 presents an overview of the mean training data for each group. Adherence with prescribed training sessions was 63%±18% and 41%±28% in the intervention and control group, respectively (total sessions performed, 105±49 vs 68±37). Total work performed throughout the training intervention was higher in the intervention group than the control group (21670J±12266J vs 2813J±1781J; 95% CI, −27615 to −10099; P=.002). In the intervention group, training resistance started at 47%±9% of their baseline PImax in the first week of training and ended at 59%±16% in the last week of training. Weekly mean inspiratory resistance (%PImax baseline) is shown in fig 3.
Fig 3.
Mean inspiratory resistance during weekly inspiratory muscle training sessions throughout the intervention period. Training resistance is expressed as percentage baseline maximal inspiratory pressure measured from residual volume. Percentage adherence to prescribed training sessions is displayed under weekly averages of training resistance. Values are mean ± SE.
Main outcomes
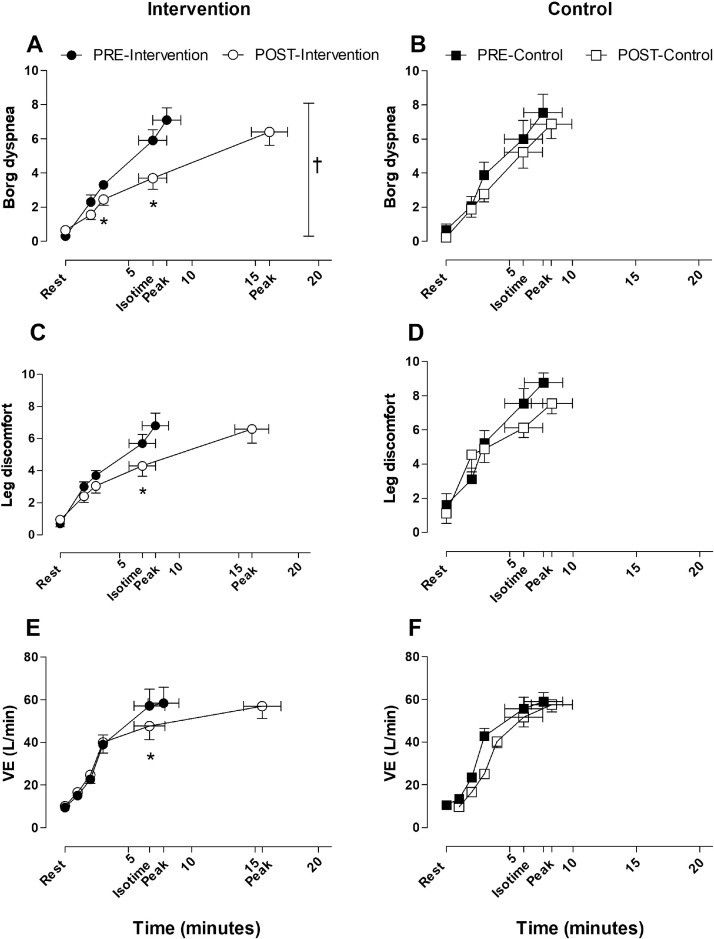
After the intervention period, exertional dyspnea scores at isotime were significantly lower only in the intervention group, while between-group differences were not statistically significant (P=.066) in analogy with between-group differences in multidimensional dyspnea profile scores of dyspnea unpleasantness recorded at peak exercise (P=.091) (table 2). The intervention group exhibited a significantly larger increase in constant work rate endurance cycling time than the control group (see table 2 and fig 4). Reductions in sensations of leg fatigue and minute ventilation during exercise were comparable between groups (see table 2 and fig 3). Changes in breathing pattern were also comparably small in both groups (see table 2 and supplemental fig S1). The scores on the TDI questionnaire increased significantly in the intervention group compared with the control group (P=.022) (see table 2). As displayed in table 2, PImax increased from −74±11 cmH2O to −93±19 cmH2O in the intervention group and from −91±16 cmH2O to −98±13 cmH2O in the control group (unadjusted mean difference, 12cmH2O; 95% CI, −5 to 30; P=.164; d=0.668). Furthermore, there was a significant and very large (d=1.962) increase in respiratory muscle endurance time in favor of the intervention group (see table 2). Improvements in functional exercise capacity as assessed by the 6-minute walk distance and changes in handgrip strength were comparable between groups (see table 2).
Table 2.
Changes in primary and secondary outcome measurements
| Outcome | Intervention |
Control |
|||
|---|---|---|---|---|---|
| Pretraining | Post Training | Pretraining | Post Training | Adjusted Difference (95% CI) at Post Training | |
| Respiratory muscle strength | |||||
| PImax (cmH2O) | −74±11 | −93±19⁎ | −91±16 | −98±13 | −1 (−19 to 18) |
| PEmax (cmH2O) | 139±25 | 144±28 | 143±26 | 141±27 | 6 (-14 to 25) |
| Respiratory muscle endurance test | |||||
| Endurance breathing time (s) | 209±79 | 741±282⁎ | 269±133 | 321±236 | 472 (217 to 728)† |
| Total work (J) | 103±61 | 560±403⁎ | 206±131 | 326±157⁎ | 336 (24 to 648)† |
| Average power (W) | 2.0±1.2 | 5.9±2.5⁎ | 4.5±2.2 | 6.9±1.9⁎ | 1.4 (−1.2 to 4.0) |
| Average volume (L) | 1.8±0.7 | 2.6±0.7⁎ | 2.1±0.6 | 2.5±0.4⁎ | 0.3 (−0.3 to 0.8) |
| CWR cycle ergometer exercise test | |||||
| Work rate (W) | 99±23 | 98±23 | 94±24 | 94±24 | |
| Reason stopping (% dyspnea) | 57±17 | 53±19 | 41±26 | 48±15 | −10 (−32 to 12) |
| Isotime | |||||
| Exercise capacity (s) | 400±218 | 367±272 | |||
| Dyspnea isotime (Borg units) | 5.8±2.1 | 3.3±1.9⁎ | 6.0±3.3 | 5.2±2.8 | −1.8 (−3.7 to 0.13) |
| Leg discomfort (Borg units) | 5.4±1.7 | 4.0±1.9⁎ | 7.6±2.6 | 7.6±1.8 | −1.3 (−3.2 to 0.6) |
| Heart rate (beats/min) | 150±21 | 139±22⁎ | 127±28 | 134±26 | −15 (−27 to −3)† |
| VE (L/min) | 57.2±23.2 | 47.7±19.0⁎ | 55.7±16.0 | 51.7±13.6 | −5.1 (−12.7 to 2.5) |
| VT (L) | 1.86±0.52 | 1.73±0.52 | 1.69±0.22 | 1.65±0.24 | −0.05 (−0.30 to 0.19) |
| RR (breaths/min) | 31±6 | 28±6 | 34±10 | 32±10 | −2 (−7 to 2) |
| V̇o2 (L/min) | 1.71±0.46 | 1.48±0.44⁎ | 1.56±0.38 | 1.49±0.30 | −0.1 (−0.3 to 0.0) |
| VCo2 (L/min) | 1.95±0.62 | 1.55±0.51⁎ | 1.68±0.41 | 1.64±0.34 | −0.27 (−0.55 to 0.00)† |
| RQ | 1.13±0.16 | 1.03±0.15 | 1.08±0.08 | 1.11±0.10 | −0.11 (−0.21 to −0.00)† |
| IC (L) | 2.43±0.35 | 2.57±0.47 | 2.47±0.29 | 2.49±0.36 | 0.12 (−0.18 to 0.42) |
| Peak exercise | |||||
| Exercise time (s) | 467±218 | 933±267⁎ | 460±272 | 500±294 | 428 (223 to 633)† |
| Dyspnea (Borg units) | 6.9±2.3 | 6.0±2.2 | 7.6±3.2 | 6.9±2.6 | −0.5 (−2.6 to 1.5) |
| Leg discomfort (Borg units) | 6.4±2.4 | 6.2±2.7 | 8.8±1.6 | 7.6±1.8 | −1.1 (−3.8 to 1.7) |
| Heart rate (beats/min) | 155±16 | 145±29 | 134±26 | 139±24 | −16 (−31 to 0) |
| VE (L/min) | 58.4±22.2 | 57.0±17.3 | 59.0±12.6 | 57.6±10.2 | −0.2 (−10.6 to 10.1) |
| V̇o2 (L/min) | 1.76±0.44 | 1.64±0.32 | 1.64±0.33 | 1.59±0.26 | −0.00 (−0.25 to 0.24) |
| Symptoms of dyspnea | |||||
| TDI total score (−9 to +9) | 7.0±1.2 | 4.1±3.0 | 2.9 (0.5 to 5.3)† | ||
| MDP (A1, 0 to 10) | 6.7±1.9 | 4.8±3.5⁎ | 6.4±3.1 | 6.6±2.6 | −2.0 (−4.3 to 0.4) |
| mMRC (0 to 4) | 0.8±0.4 | 0.2±0.4⁎ | 1.0±0.7 | 0.7±0.7 | −0.4 (−0.8 to 0.1) |
| Functional exercise capacity | |||||
| 6MWD (m), dyspnea post 6MWD, leg discomfort post 6MWD (N), handgrip strength |
557±87 2.9±1.4 2.7±1.7 255±53 |
584±71⁎ 2.4±1.6 |
545±101 3.3±2.4 |
580±85 2.2±0.9 |
−5 (−45 to 35) 0.2 (−1.2 to 1.6) 1.1 (−1.1 to 3.5) 4 (−15 to 23) |
| 2.6±1.6 255±50 |
4.8±2.9 248±29 |
3.6±2.2 256±34 |
|||
NOTE. Data are presented as mean ± SD.
Abbreviations: CWR, constant work rate; IC, inspiratory capacity; isotime, the time of the post measurement equal to the end of time of the premeasurement; MDP, multidimensional dyspnea profile; mMRC, modified medical research council scale; peak exercise, averaged last 30 s of loaded cycling; PEmax, maximal expiratory pressure; RQ, respiratory quotient; RR, respiratory rate; 6MWD, 6-minute walking distance; 10-point Borg, modified Borg Dyspnea Scale (0-10); VCo2, carbon dioxide production; VE, ventilation; V̇o2, oxygen consumption; VT, tidal volume.
P<.05, within-group differences pre- vs post intervention by paired t test or Wilcoxon test.
P<.05, between-group differences intervention vs control by analysis of covariance.
Fig 4.
Dyspnea intensity, sensation of leg discomfort, and VE assessed during constant work rate cycling tests. Pre- and-postactive intervention measures of (A) dyspnea intensity, (C) leg discomfort, and (E) VE. Pre- and postcontrol intervention measures of (B) dyspnea intensity, (C) leg discomfort, and (E) VE. Values are mean ± SE. Abbreviation: VE, ventilation. *Paired-samples t test: P<.05, post vs preintervention. †Two-way repeated measures analysis of variance: P=.01 for pre- to postassessment effect.
External work performed during the respiratory muscle training intervention correlated significantly with changes in exercise time during the constant work rate cycling test (r=0.785, P<.001), changes in respiratory muscle endurance time (r=0.544, P=.020), and TDI scores (r=0.697, P=.001). Furthermore, changes in training load significantly correlated with changes in PImax (r=−0.558, P=.020).
Discussion
This study investigated the effects of adjunctive IMT on respiratory muscle function, symptoms of dyspnea, and exercise capacity in selected survivors of breast cancer. We observed relevant additional improvements in respiratory muscle function, endurance cycling time, and symptoms of dyspnea during daily activities after adjunctive IMT. Moreover, this study implemented a sham treatment, effectively blinding the control group and accounting for placebo treatment effects in the process.
Respiratory muscle endurance improved considerably more (adjusted mean difference, +472 seconds; 95% CI, 217-728) after adjunctive IMT in contrast to the sham control intervention. This constitutes a very large effect size (d=1.96). Average improvements in PImax in the intervention group of 19 cmH2O exceeded previously established minimal important differences (MID) of changes in inspiratory muscle strength in heart failure (MID, 11.4 cmH2O)34 and chronic obstructive pulmonary disease (MID, 17.2 cmH2O).35 This did, however, not result in a significant difference between groups, despite an unadjusted difference of 12 cmH2O (95% CI, −5 to 30; P=.164) and a moderate to large effect size (d=0.668). Improvements in PImax in our control group were larger than studies in chronic obstructive pulmonary disease lacking a sham control intervention (7.4±4.9 cmH2O vs 1.3±0.9 cmH2O).36 This together with the relatively small sample size might have contributed to this observation.
We hypothesized that adjunctive IMT would reduce symptoms of exertional dyspnea and increase exercise capacity. There was evidence for a reduction of self-reported dyspnea symptoms during daily life as shown by the significant improvement on the TDI questionnaire in the intervention group compared with the control group. Clinical relevance of this finding is illustrated by comparing the adjusted difference (2.9 points) with the previously established MID of 1 point.37 Although there was no significant between-group difference in change scores for the perceived intensity of dyspnea at comparable time points during the constant work rate cycling test, a statistically significant reduction within the intervention group was observed (see fig 3).
The adjusted difference in dyspnea reduction of −1.8 points on the modified Borg Scale (0-10) scores at isotime seems clinically relevant compared with the MID of 1 point established in previous work.38
Improvements in endurance exercise capacity during a constant work rate cycling test showed a substantial between-group difference (adjusted difference, 428 seconds; 95% CI, 223-633; P=.001). This additional improvement largely exceeds the MID of 46-105 seconds previously established in patients with chronic lung disease.15
While both groups showed relevant improvements, no between-group difference was observed in the 6-minute walk distance (adjusted mean difference, −5 m; 95% CI, −45 to 35). The lack of between-group differences on this outcome provides further evidence that constant work rate tests might be more suitable when investigating the effects of adjunctive interventions.15,39 Regarding handgrip strength, no changes were observed, indicating the specificity of IMT to affect respiratory but not peripheral muscles.
Study limitations
In this study, training adherence was lower (62.7% and 40.7% for intervention and control groups, respectively) than previous studies using comparable IMT protocols.15,40 Because of limited staffing and larger physical distance between the rehabilitation center and the hospital, we offered less regular supervised sessions than initially planned (every 2 weeks). Nevertheless, the average total number of training sessions performed (105±49 in the intervention group vs 68±37 in the control group) was considerable and comparable with previous studies.15,40 However, for future research we recommend implementing regular supervised sessions to optimize treatment adherence and take full advantage of IMT programs.
Conclusions
Because of the limited sample size all obtained findings need to be interpreted with caution. The study offers initial insights into the potential of adjunctive IMT in selected survivors of breast cancer. Larger multicenter studies should be performed to further explore the potential role and general acceptance of this intervention as a rehabilitation tool in selected patients after breast cancer treatment.
Suppliers
-
a.
POWERbreathe KHP2, HaB International Ltd, Southam, Warwickshire, England, UK.
-
b.
microRPM Pressure Meter; BD-CareFusion, San Diego, CA.
-
c.
Ergoline 800s; Vs229d; SensorMedics Corporation, Yorba Linda, CA.
-
d.
SPSS Version 27.0; IBM, Armonk, NY.
Acknowledgments
We thank Laura Rommens, MSc, and Jente Geerts, MSc, for their contribution in the collection and analysis of data as well as for their contribution in drafting the manuscript. Additionally, we thank Lies Serrien, PT, for her assistance during patient screening, supervision of training sessions, and collection of additional data. Lastly, we acknowledge HaB International Ltd (Southam, UK) for providing training devices on loan for the study duration.
Footnotes
Clinical Trial Registration No.: MP003175.
Disclosures: none.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.arrct.2022.100196.
Appendix. Supplementary materials
References
- 1.Salehiniya H, Ghoncheh M, Pournamdar Z. Incidence and mortality and epidemiology of breast cancer in the world. Asian Pacific J Cancer Prev. 2016;17:43–46. doi: 10.7314/apjcp.2016.17.s3.43. [DOI] [PubMed] [Google Scholar]
- 2.Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66:271–289. doi: 10.3322/caac.21349. [DOI] [PubMed] [Google Scholar]
- 3.Lovelace DL, McDaniel LR, Golden D. Long-term effects of breast cancer surgery, treatment, and survivor care. J Midwifery Womens Health. 2019;64:713–724. doi: 10.1111/jmwh.13012. [DOI] [PubMed] [Google Scholar]
- 4.Loh SY, Musa AN. Methods to improve rehabilitation of patients following breast cancer surgery: a review of systematic reviews. Breast Cancer Targets Ther. 2015;7:81–98. doi: 10.2147/BCTT.S47012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garabeli Cavalli Kluthcovsky AC, Urbanetz AA, de Carvalho DS, Pereira Maluf EM, Schlickmann Sylvestre GC, Bonatto Hatschbach SB. Fatigue after treatment in breast cancer survivors: Prevalence, determinants and impact on health-related quality of life. Support Care Cancer. 2012;20:1901–1909. doi: 10.1007/s00520-011-1293-7. [DOI] [PubMed] [Google Scholar]
- 6.O'Donnell DE, Webb KA, Langer D, Elbehairy AF, Neder JA, Dudgeon DJ. Respiratory factors contributing to exercise intolerance in breast cancer survivors: a case-control study. J. Pain Symptom Manage. 2016;52:54–63. doi: 10.1016/j.jpainsymman.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 7.Travers J, Dudgeon DJ, Amjadi K, et al. Mechanisms of exertional dyspnea in patients with cancer. J Appl Physiol. 2008;104:57–66. doi: 10.1152/japplphysiol.00653.2007. [DOI] [PubMed] [Google Scholar]
- 8.Bruera E, Schmitz B, Pither J, Neumann CM, Hanson J. The frequency and correlates of dyspnea in patients with advanced cancer. J Pain Symptom Manage. 2000;19:357–362. doi: 10.1016/s0885-3924(00)00126-3. [DOI] [PubMed] [Google Scholar]
- 9.Dudgeon DJ, Lertzman M, Askew GR. Physiological changes and clinical correlations of dyspnea in cancer outpatients. J Pain Symptom Manage. 2001;21:373–379. doi: 10.1016/s0885-3924(01)00278-0. [DOI] [PubMed] [Google Scholar]
- 10.Langer D, Webb K, Elbehairy A, Neder A, Dudgeon D, O'Donnell D. Dyspnea and exercise intolerance in breast cancer survivors: the role of inspiratory muscle weakness. Eur Respir J. 2015;46:PA4816. [Google Scholar]
- 11.Suesada MM, Carvalho HA, Albuquerque ALP, Salge JM, Stuart SR, Takagaki TY. Impact of thoracic radiotherapy on respiratory function and exercise capacity in patients with breast cancer. J Bras Pneumol. 2018;44:469–476. doi: 10.1590/S1806-37562017000000120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meneses-Echávez JF, González-Jiménez E, Ramírez-Vélez R. Effects of supervised exercise on cancer-related fatigue in breast cancer survivors: a systematic review and meta-analysis. BMC Cancer. 2015;15:1–13. doi: 10.1186/s12885-015-1069-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Volaklis KA, Halle M, Tokmakidis SP. Exercise in the prevention and rehabilitation of breast cancer. Wien Klin Wochenschr. 2013;125:297–301. doi: 10.1007/s00508-013-0365-8. [DOI] [PubMed] [Google Scholar]
- 14.Gosselink R, De Vos J, Van Den Heuvel SP, Segers J, Decramer M, Kwakkel G. Impact of inspiratory muscle training in patients with COPD: what is the evidence? Eur Respir J. 2011;37:416–425. doi: 10.1183/09031936.00031810. [DOI] [PubMed] [Google Scholar]
- 15.Charususin N, Gosselink R, Decramer M, et al. Randomised controlled trial of adjunctive inspiratory muscle training for patients with COPD. Thorax. 2018;73:942–950. doi: 10.1136/thoraxjnl-2017-211417. [DOI] [PubMed] [Google Scholar]
- 16.Neder JA, Andreoni S, Lerario MC, Nery LE. Reference values for lung function tests. II. Maximal respiratory pressures and voluntary ventilation. Brazilian J Med Biol Res. 1999;32:719–727. doi: 10.1590/s0100-879x1999000600007. [DOI] [PubMed] [Google Scholar]
- 17.Doig GS, Simpson F. Randomization and allocation concealment: a practical guide for researchers. J Crit Care. 2005;20:187–191. doi: 10.1016/j.jcrc.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 18.Langer D, Charususin N, Jácome C, et al. Efficacy of a novel method for inspiratory muscle training in people with chronic obstructive pulmonary disease. Phys Ther. 2015;95:1264–1273. doi: 10.2522/ptj.20140245. [DOI] [PubMed] [Google Scholar]
- 19.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 20.Wanger J, Clausen JL, Coates A, et al. Standardisation of the measurement of lung volumes. Eur Respir J. 2005;26:511–522. doi: 10.1183/09031936.05.00035005. [DOI] [PubMed] [Google Scholar]
- 21.Graham BL, Brusasco V, Burgos F, et al. 2017 ERS/ATS standards for single-breath carbon monoxide uptake in the lung. Eur Respir J. 2017;49:1–31. doi: 10.1183/13993003.00016-2016. [DOI] [PubMed] [Google Scholar]
- 22.Quanjer PH, Stanojevic S, Cole TJ, et al. Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40:1324–1343. doi: 10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stanojevic S, Graham BL, Cooper BG, et al. Official ERS technical standards: Global Lung Function Initiative reference values for the carbon monoxide transfer factor for Caucasians. Eur Respir J. 2017;50 doi: 10.1183/13993003.00010-2017. [DOI] [PubMed] [Google Scholar]
- 24.Laveneziana P, Albuquerque A, Aliverti A, et al. ERS statement on respiratory muscle testing at rest and during exercise. Eur Respir J. 2019;53 doi: 10.1183/13993003.01214-2018. [DOI] [PubMed] [Google Scholar]
- 25.Banzett RB, O'Donnell CR, Guilfoyle TE, et al. Multidimensional dyspnea profile: an instrument for clinical and laboratory research. Eur Respir J. 2015;45:1681–1691. doi: 10.1183/09031936.00038914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mahler DA, Weinberg DH, Wells CK, Feinstein AR. The measurement of dyspnea. Contents, interobserver agreement, and physiologic correlates of two new clinical indexes. Chest. 1984;85:751–758. doi: 10.1378/chest.85.6.751. [DOI] [PubMed] [Google Scholar]
- 27.Milačić N, Milačić B, Dunjić O, Milojković M. Validity of cat and MMRC - dyspnea score in evaluation of COPD severity. Acta Medica Median. 2015;54:66–70. [Google Scholar]
- 28.Perez T, Burgel PR, Paillasseur JL, et al. Modified medical research council scale vs baseline dyspnea index to evaluate dyspnea in chronic obstructive pulmonary disease. Int J COPD. 2015;10:1663–1672. doi: 10.2147/COPD.S82408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Borg GA. Pyschophysical bases of percieved exertion. Med Sci Sport Exerc. 1982;14:377–381. [PubMed] [Google Scholar]
- 30.Schmidt K, Vogt L, Thiel C, Jäger E, Banzer W. Validity of the six-minute walk test in cancer patients. Int J Sports Med. 2013;34:631–636. doi: 10.1055/s-0032-1323746. [DOI] [PubMed] [Google Scholar]
- 31.Trampisch US, Franke J, Jedamzik N, Hinrichs T, Platen P. Optimal Jamar dynamometer handle position to assess maximal isometric hand grip strength in epidemiological studies. J Hand Surg Am. 2012;37:2368–2373. doi: 10.1016/j.jhsa.2012.08.014. [DOI] [PubMed] [Google Scholar]
- 32.Leong DP, Teo KK, Rangarajan S, et al. Reference ranges of handgrip strength from 125,462 healthy adults in 21 countries: a Prospective Urban Rural Epidemiologic (PURE) study. J Cachexia Sarcopenia Muscle. 2016;7:535–546. doi: 10.1002/jcsm.12112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vickers AJ, Altman DG. Analysing controlled trials with baseline and follow up measurements. Br Med J. 2001;323:1123–1124. doi: 10.1136/bmj.323.7321.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Täger T, Schell M, Cebola R, et al. Biological variation, reference change value (RCV) and minimal important difference (MID) of inspiratory muscle strength (PImax) in patients with stable chronic heart failure. Clin Res Cardiol. 2015;104:822–830. doi: 10.1007/s00392-015-0850-3. [DOI] [PubMed] [Google Scholar]
- 35.Iwakura M, Okura K, Kubota M, et al. Estimation of minimal clinically important difference for quadriceps and inspiratory muscle strength in older outpatients with chronic obstructive pulmonary disease: a prospective cohort study. Phys Ther Res. 2021;24:35–42. doi: 10.1298/ptr.E10049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang K, Zeng GQ, Li R, et al. Cycle ergometer and inspiratory muscle training offer modest benefit compared with cycle ergometer alone: a comprehensive assessment in stable COPD patients. Int J COPD. 2017;12:2655–2668. doi: 10.2147/COPD.S140093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Witek TJ, Jr, Mahler DA. Minimal important difference of the transition dyspnoea index in a multinational clinical trial. Eur Respir J. 2003;21:267–272. doi: 10.1183/09031936.03.00068503a. [DOI] [PubMed] [Google Scholar]
- 38.Khair RM, Nwaneri C, Damico RL, Kolb T, Hassoun PM, Mathai SC. The minimal important difference in borg dyspnea score in pulmonary arterial hypertension. Ann Am Thorac Soc. 2016;13:842–849. doi: 10.1513/AnnalsATS.201512-824OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Camillo CA, Osadnik CR, Van Remoortel H, Burtin C, Janssens W, Troosters T. Effect of “add-on” interventions on exercise training in individuals with COPD: a systematic review. ERJ Open Res. 2016;2:00078–02015. doi: 10.1183/23120541.00078-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Langer D, Ciavaglia C, Faisal A, et al. Inspiratory muscle training reduces diaphragm activation and dyspnea during exercise in COPD. J Appl Physiol. 2018;125:381–392. doi: 10.1152/japplphysiol.01078.2017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.