Abstract
Objective
To conduct a systematic review examining the effect of exercise and rehabilitation in people with Ehlers-Danlos syndrome (EDS).
Data Sources
The following databases were systematically searched: MEDLINE, MEDLINE In-Process/ePubs, Embase, Cochrane Central Register of Controlled Trials, PsycINFO, and Cumulative Index to Nursing and Allied Health. The final time point captured by the search is November 27, 2020.
Study Selection
Eligible study designs included case-control, case-series, prospective cohort, retrospective cohort, and intervention studies of structured exercise or rehabilitation interventions. Eligible populations included adults (18 years or older) with EDS (all subtypes) and hypermobility spectrum disorders. The search was restricted to articles published in English.
Data Extraction
Data were extracted by 2 independent reviewers. Risk of bias was assessed using the Physiotherapy Evidence Database (PEDro) scale for randomized controlled trials (RCTs) and Risk Of Bias In Nonrandomized Studies of Interventions (ROBINS-I) for non-RCTs. Reporting quality of RCTs was assessed using the Consolidated Standards for Reporting of Trials statement with the harms extension. Reporting was guided by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses checklist.
Data Synthesis
The search yielded 10 eligible studies including 330 participants. The study designs included 5 RCTs, 1 cohort, 2 single-arm interventions, 1 retrospective, and 1 feasibility study. All studies showed some improvement in a physical and/or psychological outcome after the intervention period. One adverse event (nonserious) potentially related to the intervention was reported. Of the 5 RCTs, 2 were rated as high quality with low risk of bias using PEDro, and the majority of non-RCTs were rated as critical risk of bias by ROBINS-I.
Conclusions
The results suggest that exercise and rehabilitation may be beneficial for various physical and psychological outcomes. Adequately powered and rigorous RCTs of exercise and rehabilitation interventions for people with EDS are needed.
KEYWORDS: Ehlers-Danlos Syndrome, Exercise, Joint instability, Rehabilitation
List of abbreviations: AIMS-2, Arthritis Impact Measurement Scales-2; CONSORT, Consolidated Standards for Reporting of Trials; EDS, Ehlers-Danlos syndrome; HADS, Hospital and Anxiety Depression Scale; hEDS, hypermobile EDS; HSD, hypermobility spectrum disorders; PEDro, Physiotherapy Evidence Database; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; QOL, quality of life; RCT, randomized control trial; ROBINS-I, Risk Of Bias In Nonrandomized Studies of Interventions; SF-36, Short Form-36; 6MWT, 6-minute walk test; VAS, visual analog scale
Ehlers-Danlos syndrome (EDS) is characterized by the abnormal synthesis and/or function of collagen, fibrillin, and elastin in the body and typically affects the gastrointestinal, cardiovascular, and musculoskeletal systems.1,2 The exact prevalence of EDS is unknown, but according to some estimates, EDS affects approximately 1 in 5000 to 1 in 20,000 people worldwide.3,4 EDS typically manifests as fragile skin, organ dysfunction, significant diffuse pain,5 and hypermobility that can lead to recurrent joint dislocations and other injuries.6, 7, 8
The EDS nosology has evolved over the past 30 years (1988 Berlin criteria,9 1998 Villefranche nosology,6 1998 Brighton criteria,10 2017 EDS classification11), with the most recent system comprising 13 EDS subtypes. Of these subtypes, 12 are usually confirmed by molecular analyses, which provide clarity on inheritance patterns and can guide approaches to management. The 13th subtype, known as hypermobile EDS (hEDS), does not have a known genetic marker, cannot be confirmed by molecular analysis,9 and is diagnosed by clinical assessment. The diagnostic criteria for most EDS subtypes include joint hypermobility, although people with hEDS may experience more frequent joint subluxations and/or dislocations,6 debilitating musculoskeletal symptoms,8,12 chronic limb and joint pain,3,8 and occasional dyspnea.13 Joint hypermobility directly results from deficiencies in the extracellular connective tissue matrix, which leads to increased connective tissue elasticity, pathologic range of motion and, ultimately, recurrent subluxations and/or dislocations, and tendon ruptures.9,14 Before the 2017 EDS classification, joint hypermobility syndrome was considered to be synonymous with EDS hypermobility type.15,16 However, the updated 2017 EDS classification for the various subtypes10 proposed a newer category of hypermobility spectrum disorders (HSDs). HSD includes the patients who present with generalized joint hypermobility, not otherwise specified, and do not have a complete phenotype to confirm hEDS as per the 2017 criteria. (For consistency in this article, all references to hEDS or HSD will collectively be referred to as HSD/hEDS).
Grahame17 and Celletti18 and colleagues observed that the physical sequelae experienced by people with EDS is, in part, amplified with psychological distress and behavioral responses, such as kinesiophobia, a fear of physical movement and activity19 that collectively contribute to poor quality of life (QOL) and further impairments. Specifically, Scheper et al reported that the behavioral response of kinesiophobia has been shown to lead to physical deconditioning, which can exacerbate joint laxity, and a continued cycle of weakness, joint instability, worsening pain, and further deconditioning.20 Given the numerous musculoskeletal symptoms, functional challenges, and resulting physical deconditioning that can be experienced by people with EDS, exercise and rehabilitation have emerged as important components of disease management.
Exercise and rehabilitation for people with EDS have commonly focused on optimizing physical function with improved muscular strength,21 proprioceptive acuity,18,22,23 and postural exercises, such as lumbar spinal stabilization and trunk muscle endurance exercises.24 While acknowledging exercise and rehabilitation as an important part of EDS care, the evolution of the diagnostic criteria and the diagnostic confusion regarding the type of EDS and inclusion of phenotypically similar but variable conditions9 has led to exercise and rehabilitation studies that include heterogenous populations. Previous review articles have worked to summarize the evidence related to exercise and rehabilitation interventions for adults with EDS including Palmer,25 Smith,26 Corrado27 and colleagues. While informative, these reviews excluded EDS clinical analogs and the remaining 12 EDS subtypes from their analyses. In an effort to complement these past reviews, the aim of the present systematic review is to provide an updated summary of the evidence related to exercise and rehabilitation for people with EDS, inclusive of all subtypes and clinical analogs, as well as evaluate the methodologic and reporting quality of exercise studies using both critical appraisal tools and reporting guidelines.
Methods
The search followed the Cochrane Handbook28 and the Cochrane Methodological Expectations of Cochrane Intervention Reviews29 for conducting the search, the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines30 for reporting the search (PRISMA Checklist provided in supplemental table S1, (available online only at http://www.archives-pmr.org/), and the PRESS 2015 guideline for peer-reviewing the search strategies and avoiding potential search errors.31
Search strategy
Preliminary searches were conducted, and publications known to meet the inclusion criteria were assessed for potential keywords and appropriate controlled vocabulary terms (such as Medical Subject Headings for Medline and EMTREE descriptors for Embase) (supplemental table S2, available online only at http://www.archives-pmr.org/). Using the Ovid search interface, 6 electronic databases were searched: (1) MEDLINE (1946-), (2) MEDLINE In-Process/ePubs (daily), (3) Embase (1947-), (4) Cochrane Central Register of Controlled Trials (1991-), (5) PsycINFO (1806-), and (6) Cumulative Index to Nursing and Allied Health (EbscoHost, 1982-). All searches were conducted on July 9, 2019, by an information specialist (M.E.) and updated later to include eligible studies up to and including November 27, 2020. Reference lists of all included trials and previous systematic reviews were checked manually for additional relevant publications. Eligibility criteria for the studies included in this review are detailed in table 1.
Table 1.
Inclusion and exclusion criteria for studies included in this review
| Variable | Inclusion Criteria | Exclusion Criteria |
|---|---|---|
| Publication date | Articles before November 27, 2020 | Articles after November 27, 2020 |
| Language | English | Not published in English |
| Participant diagnosis | EDS (all subtypes) as identified by Berlin 1988 nosology,9 Villefranche 1998 nosology,6 Brighton 1998,10 and/or 2017 EDS classification.11 Participants diagnosed with generalized joint hypermobility, hypermobility spectrum disorders, and other hEDS clinical analogs. |
Inclusion of participants that do not meet any diagnosis found in the Participant diagnosis inclusion criteria. |
| Participant age | Adults (18 y or older) | Age younger than 18 y |
| Intervention | Includes structured exercise or rehabilitation interventions.* | Interventions with the purpose of changing behavior (ie, exercise/physical activity engagement) without structured exercise or rehabilitation programming. |
| Study type | Case-controlProspective cohortRetrospective cohortIntervention studies including
|
Review articles Case reports Animal studies |
Exercise and rehabilitation interventions are operationally defined as interventions involving regular, structured aerobic, resistance, stability, or balance exercises with the intended purpose of improving physical fitness and/or health outcomes.
Review process
Reference management software was used to manage all citations.a Eligibility screening of each reference title and abstract was conducted by 2 reviewers (S.B-I., N.M.). Full-text articles were retrieved for all potentially eligible studies and reviewed by the same 2 reviewers (S.B-I., N.M.) for a final decision about inclusion or exclusion. Disagreements regarding eligibility of articles at the screening and full-text review stages were resolved by discussion or adjudication of a third reviewer (D.S.M.).
Data extraction
Data from the included trials were extracted by 1 author (S.B-I.) in MS Excel 2010b and cross-checked by a second author (N.M.). Extracted data included citation details, location of study, study design, inclusion criteria, sample size, participant characteristics, description of the exercise intervention, outcome measurements, and findings. The complete list of extracted parameters for each subcategory is available in supplemental table S3 (available online only at http://www.archives-pmr.org/).
Risk of bias assessment
Risk of bias is considered the degree of systematic error in the design, conduct, and analysis contributing to an estimate of effect. It is considered by Cochrane as one of several factors that must be considered when judging study quality (the extent to which one can be confident that an estimate of effect is near the true value for an outcome).28 Risk of bias of randomized control trials (RCTs) and the degree to which the level of bias influenced study quality were assessed using the Physiotherapy Evidence Database (PEDro) scale, a widely used scale developed to assess exercise and rehabilitation studies.32 The risk of bias of nonrandomized study designs was assessed using Risk Of Bias In Nonrandomized Studies of Interventions (ROBINS-I),33 a tool developed for undertaking systematic reviews that include nonrandomized studies. ROBINS-I evaluates risk of bias across 7 domains: confounding, selection of participants into the study, classification of interventions, deviations of intended interventions, missing data, measurement of outcomes, and the selection of reported results.33 For RCTs and non-RCTs, studies were independently assessed by 2 reviewers (S.B-I., N.M.). For RCTs, reviewers rated each domain as “yes” or “no” to yield a summary score. PEDro has classified the assessment scores as indicators of “poor quality” (PEDro score 3), “fair quality” (PEDro score 4-5), or “high quality” (PEDro score 6-10).30 For non-RCTs, risk of bias elements were rated as “yes,” “possibly yes,” “no,” “possibly no,” “no information,” or “not applicable.” Non-RCTs were then classified using the ROBINS-I classification as “low,” “moderate,” “serious,” or “critical risk of bias” based on whether the level of bias in domains may have led to material bias in the outcomes of interest. Any disagreements between the reviewers were resolved by discussion and reassessment of the full texts, with arbitration by a third reviewer (D.S.M.) if necessary. Interrater reliability was evaluated using the κ coefficient and percentage agreement across guidelines.
Reporting quality assessment
The reporting quality of RCTs was assessed by the Consolidated Standards for Reporting of Trials (CONSORT) Statement.34,35 The CONSORT Statement has been specifically developed for the critical assessment of the quality of evidence provided and the reporting of harms-related issues. The CONSORT Statement checklist comprises 22 items addressing domains such as objectives, trial design, participant flow, data analysis, harms and unintended effects, and interpretation of results.31 The evaluation was independently completed by 2 reviewers (S.B-I., N.M.). Unlike the PEDro scale, the CONSORT Statement does not result in summary scores and thus serves to reveal thematic results. The quality and harms-related issues of the remaining 5 studies that were not included in the CONSORT evaluation were reviewed and are narratively discussed.
Data synthesis and analysis
Given the heterogeneity of study designs and outcome measures used in the literature, a meta-analysis was not conducted. A descriptive synthesis approach was used with findings presented in both narrative form and in tables relative to the data extracted.
Results
Study selection
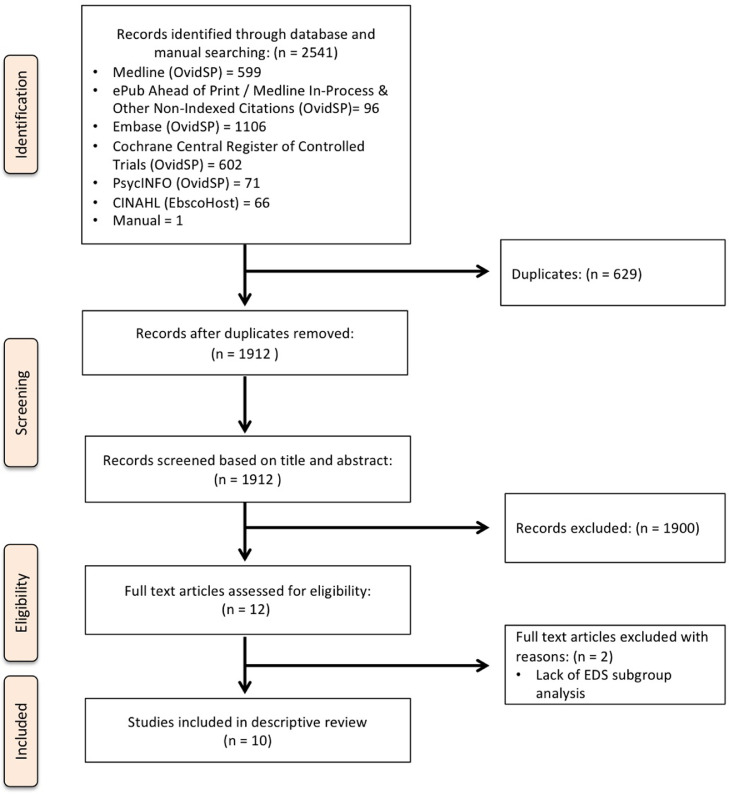
The PRISMA diagram is presented in fig 1. The search strategy yielded 2541 records. After removal of duplicates, 1912 citations were screened for eligibility and 11 full-text articles were retrieved. Of these, 2 articles were excluded36,37 and 10 met the inclusion criteria for this review.
Fig 1.
PRISMA diagram summarizing evidence search and selection (inclusive of secondary search and thus, 710 studies up to November 27, 2020).
Study characteristics
Table 2 summarizes the characteristics of the included studies. The studies were conducted between 2004 and 2020 and occurred in the UK (N=3),21,38,39 Turkey (N=2),23,24 Iran,22 France,40 Denmark,40 Belgium,41 and Norway.42 Across the 10 studies, 330 participants were included, all of whom were diagnosed with HSD/hEDS as defined by the 2017 EDS classification,11 the Brighton 1998,10 and the Villefranche 1998 diagnostic criteria.6 The following study designs were used in the included studies: RCTs,22, 23, 24,41 pilot RCT,38 cohort study,39 single-armed intervention trial,21,42 single-armed retrospective study,43 and single-armed feasibility study.40 The median sample size was 22 (range, 11-102).
Table 2.
Study characteristics
| Author/Setting (Country/Hospital) | Type of Study | Sample Size (Per Group) and Participant Mean Age ± SD | Diagnostic Criteria or nosology(± additional inclusion criteria) |
|---|---|---|---|
| Bathen et al42 Oslo, Norway |
Single-arm, pilot study | N=12 Age: median=35, range=20-51 y |
Brighton 199810 or Villefranche 19986 |
| Toprak et al24 Izmir, Turkey |
RCT | IG: n=23 Age: 20.3±2.2 y CG: n=23 Age: 21.0±2.2 y |
Brighton 199810 |
| Daman et al22 Shiraz, Iran |
RCT | IG: n=12 Age: 22.25±1.28 y CG: n=12 Age: 21.66±2.0 y |
Brighton 199810 |
| Ferrell et al21 Glasgow, Scotland |
Single-arm, intervention trial | N=20 Age: 27.3±10.4 y |
Brighton 199810 and knee joint pain |
| Hakimi et al43 Lille, France |
Single-arm, retrospective study | N=21 Age: 45±13 y |
Conducted by EDS specialist, with specific nosology/criteria not provided |
| Liaghat et al40 Odense, Denmark |
Single-arm, feasibility study | N=11 Age: 39.3±13.9 y |
2017 EDS classification11 |
| Palmer et al38 London, England |
Pilot RCT | IG: n=15 Age: 37.2±14.1 y CG: n=14 Age: 33.3±9.7 y |
Brighton 199810 |
| Reychler et al41 Brussels, Belgium |
RCT | IG: n=10 Age: 45.8±5.5 y CG: n=10 Age: 53.1±3.4 y |
Brighton 1998,10 2017 EDS classification,11 and reduced inspiratory muscle strength |
| Sahin et al23 Istanbul, Turkey |
RCT | IG: n=15 Age: 25.60±6.0 y CG: n=25 Age: 27.68±7.8 y |
Brighton 199810 |
| To and Alexander39 London, England |
Cohort study | IG: n=47 Age: 34.6±10.6 y CG.a: n=26 Age: 31.8±10.5 y CG.b: n=29 Age: 38.2±9.3 y |
Brighton 199810 and interior knee pain |
Abbreviations: CG, control group; CG.a, control group with generalized joint hypermobility (ie, joint flexibility without multiple site symptoms experience in those with HSD); CG.b, control group without generalized joint hypermobility; IG, intervention group.
Risk of bias in included studies
The PEDro scale was used to evaluate RCTs for their risk of bias and its contributions to study quality. Across these RCTs, 1 study was rated as poor quality,23 2 as fair quality,22,24 and 2 as high quality (table 3).38,41
Table 3.
Risk of bias of RCTs using PEDro scale
| Variable | Toprak et al24 | Daman et al22 | Palmer et al38 | Reychler et al41 | Sahin et al23 |
|---|---|---|---|---|---|
| Eligibility criteria were specified | + | + | + | + | + |
| Participants were randomly allocated to groups | + | + | + | + | + |
| Allocation was concealed | − | − | + | + | − |
| The groups were similar at baseline regarding the most important prognostic indicators | + | + | + | + | + |
| There was blinding of all assessors who measured at least 1 key outcome | + | + | − | + | − |
| There was blinding of all participants | − | − | − | − | − |
| There was blinding of all therapists who administered the therapy | − | − | − | − | − |
| Measures of at least 1 key outcome were obtained from more than 85% of the participants initially allocated to groups | − | − | − | + | − |
| All participants for whom outcome measures were available received the treatment or control condition as allocated | − | − | + | + | − |
| The results of between-group statistical comparisons are reported for at least 1 key outcome | + | + | + | + | − |
| The study provides both point measures and measures of variability for at least 1 key outcome | + | + | + | + | + |
| Total score | 5 | 5 | 6 | 8 | 3 |
NOTE. PEDro score: high quality=PEDro score 6-10, fair quality=PEDro score 4-5, poor quality=PEDro score=<5.
Abbreviations: −, low risk of bias; +, high risk of bias.
Thematically, higher risk of bias was commonly attributed to (1) a lack of concealed allocation of participants; (2) a lack of measures of at least 1 key outcome for more than 85% of participants; (3) failure to deliver intervention as allocated; and (4) a lack of blinding of assessors, participants, and therapists. However, the nature of exercise intervention models used by the included studies led to an unavoidable nonblinding of personnel delivering interventions and participants in all 7 studies. The κ coefficient for interrater assessment reliability when using the PEDro scale was interpreted as “good agreement” (κ=0.78).44
Among the 5 non-RCTs, 1 was considered to have moderate risk of bias39 and 4 were at critical risk of bias21,40,42,43 (table 4). Higher risk of bias was commonly attributed to (1) bias because of confounding, (2) bias because of deviations from intended interventions, (3) bias because of missing data, and (4) bias in measurement of outcomes. The κ coefficient for interrater assessment reliability was interpreted as “good agreement” (κ=0.79).44
Table 4.
Risk of bias of nonrandomized studies using ROBINS-I tool
| Bias Domain | Bathen et al42 | Ferrell et al21 | Hakimi et al43 | Liaghat et al40 | To & Alexander39 | |
|---|---|---|---|---|---|---|
| Bias because of confounding | 1.1 | Y | Y | Y | Y | Y |
| 1.2 | NA | NA | Y | NA | N | |
| 1.3 | NA | NA | Y | NA | N | |
| 1.4 | N | NA | NA | NA | Y | |
| 1.5 | NA | NA | NA | NA | N | |
| 1.6 | NA | NA | NA | NA | NA | |
| 1.7 | NA | NA | N | NA | NA | |
| 1.8 | NA | NA | N | NA | NA | |
| RoB judgment |
Critical |
Critical |
Critical |
Critical |
Moderate |
|
| Bias in selection of participants into the study | 2.1 | N | N | N | N | N |
| 2.2 | NA | NA | NA | NA | NA | |
| 2.3 | NA | NA | NA | NA | NA | |
| 2.4 | Y | PN | Y | Y | Y | |
| 2.5 | NA | N | NA | NA | NA | |
| RoB judgment |
Low |
Serious |
Low |
Low |
Low |
|
| Bias in classification of interventions | 3.1 | Y | PY | N | Y | Y |
| 3.2 | Y | Y | NI | y | Y | |
| 3.3 | PN | PY | NI | PN | N | |
| RoB judgment |
Low |
Moderate |
Serious |
Low |
Low |
|
| Bias because of deviations from intended interventions | 4.1 | N | NI | NI | N | N |
| 4.2 | NA | NA | NI | NA | NA | |
| 4.3 | Y | NI | NI | NI | NA | |
| 4.4 | Y | NI | NI | Y | Y | |
| 4.5 | PY | NI | NI | PY | N | |
| 4.6 | NA | NI | NI | NA | Y | |
| RoB judgment |
Low |
Serious |
NI |
Moderate |
Moderate |
|
| Bias because of missing data | 5.1 | Y | N | N | N | N |
| 5.2 | PN | Y | Y | N | N | |
| 5.3 | PN | N | NI | N | N | |
| 5.4 | NA | NA | N | NA | N | |
| 5.5 | NA | N | N | Y | Y | |
| RoB judgment |
Low |
Serious |
Critical |
Moderate |
Moderate |
|
| Bias in measurement of outcomes | 6.1 | PY | PY | N | PY | N |
| 6.2 | PY | Y | N | Y | Y | |
| 6.3 | Y | PY | Y | Y | Y | |
| 6.4 | PN | PN | N | PN | N | |
| RoB judgment |
Serious |
Serious |
Low |
Moderate |
Moderate |
|
| Bias in selection of reported results | 7.1 | PN | PN | N | N | N |
| 7.2 | PN | PN | N | N | N | |
| 7.3 | N | N | N | N | N | |
| RoB judgment |
Low |
Low |
Low |
Low |
Low |
|
| Overall bias: low/moderate/serious/critical/NI | Critical | Critical | Critical | Critical | Moderate | |
Abbreviations: N, no; NA, not applicable; NI, no information; PN, probably no; PY, probably yes; RoB; risk of bias; Y, yes.
Reporting quality of included studies
Only 1 of the 5 studies evaluated using the CONSORT Statement met over 50% of the items (table 5).24 Adherence to key methodological items of the CONSORT Statement was as follows: 3 of 5 studies described sample size determination methodology;24,22,38 all 5 provided data for patient baseline characteristics;22, 23, 24,38,41 4 of 5 adhered to random sequence generation;22,24,38,41 1 of 5 for allocation concealment;41 3 of 5 for blinding of patients, care providers, or assessors;22,24,41 3 of 5 for reporting of adverse effects;22, 23, 24 4 of 5 for providing a balanced discussion of benefits and harms;23,25,38,41 and 2 of 5 for describing the generalizability of their results.24,41 Overall, the 5 studies demonstrated a compromise in reporting quality through the absence of a method to implement random allocation sequence and the absence of blinding of patients, care providers, and assessors. In contrast, all 5 studies provided the method of allocation in the abstract, sufficient scientific background in the introduction, eligibility criteria for participants, details of interventions, specific objectives, baseline demographics, and clinical characteristics of each group.
Table 5.
Reporting quality assessment of included RCTs using the CONSORT statement and harms-assessment extension
| Variable | Toprak et al24 | Daman et al22 | Palmer et al38 | Reychler et al41 | Sahin et al23 |
|---|---|---|---|---|---|
| Title and abstract* | + | + | − | + | + |
| Background* | + | + | + | + | + |
| Participants | + | + | + | + | + |
| Interventions | + | + | + | + | + |
| Objectives | + | + | + | + | + |
| Outcomes* | + | − | − | − | − |
| Sample size | + | + | + | − | − |
| Randomization: sequence generation | + | + | + | + | − |
| Randomization: allocation concealment | − | − | − | + | − |
| Randomization: implementation | + | − | − | − | − |
| Blinding (masking) | + | + | − | + | − |
| Statistical methods* | − | − | + | − | − |
| Participant flow* | + | − | + | + | − |
| Recruitment | + | − | + | + | − |
| Baseline data | + | + | + | + | + |
| Nos. analyzed* | + | − | + | + | − |
| Outcomes and estimation* | + | − | + | − | − |
| Ancillary analysis* | − | − | − | − | − |
| Adverse events* | + | + | − | − | + |
| Interpretation* | + | + | + | + | − |
| Generalizability* | + | − | − | + | − |
| Overall evidence* | + | − | + | − | − |
Abbreviations: −, does not meet requirements; +, meets requirements.
Assessment based on CONSORT statement and harm-extension.34
Study findings
Physical and functional health
All studies included at least 1 parameter of physical and functional health as a main study outcome (table 6). The 4 studies21,40, 41, 42 that investigated muscle strength demonstrated benefits associated with exercise. Ferrell et al21 measured peak and average leg strength using an isokinetic leg dynamometer before and after 8 weeks of progressive closed kinetic chain exercises performed 3 × /wk. They found an increase in the median peak and average torque for the quadriceps and hamstrings (quadriceps: peak=+3Nm, P=.038; average=+14Nm, P<.001; hamstring: peak=+13Nm, P=.002; average=+5Nm, P=.007).21 However, given that this study is considered to have critical risk of bias, in part because of confounding and missing data (see table 4), these findings are particularly vulnerable to overestimation. In an RCT of 20 participants, Reychler et al measured maximal sniff nasal inspiratory pressure and observed an increase in inspiratory muscle strength after 6 weeks of inspiratory muscle exercise (using a threshold inspiratory muscle trainer 5 × /wk) compared with the controls (+16cm H2O±3, P<.001).41 Liaghat et al measured shoulder strength using a dynamometer before and after 16 weeks of a heavy shoulder strengthening exercise programme targeting scapular and rotator cuff muscles. They found an increase in the median shoulder strength after 16 weeks compared with baseline for scaption in 45°, internal rotation, and external rotation (scaption: +0.51Nm/kg; 95% CI, 0.23-0.78; internal rotation: +1.32Nm/kg; 95% CI, 0.70-1.95; external rotation: +0.89Nm/kg; 95% CI, 0.37-1.40).40 While the findings described by Liaghat40 are statistically significant, this study is vulnerable to a critical risk of bias because of possible confounding (see table 4), which may have influenced the relationship seen between their exercise program and muscle strength. Bathen et al measured lower extremity muscle strength using the stair-walking-up-and-down test and up-on-toes test. After 2.5 weeks of inpatient and 3 months of at-home multidisciplinary rehabilitation programming combining physical and cognitive behavioral therapy, they found an increase in lower extremity muscle strength (stair-walking-up: −0.13 seconds, P=.004; up-on-toes: +4.50 seconds, P=.004) compared with baseline.42 Similar to the studies of Ferrell21 and Liaghat41 and colleagues, the study of Bathen et al44 is considered to have a critical risk of bias because of possible confounding (table 4) and is vulnerable to influence of extraneous variables on the dependent variables and a weakened relationship between the exercise intervention and muscle strength.
Table 6.
Interventions, efficacy, and risk of bias score (as determine by way of PEDro assessment) or risk of bias judgment (as determined by way of ROBINS-I) for clinical exercise and rehabilitation interventions of included studies
| Author | Form of Exercise Intervention | Outcome Measures | P Value or Δ Mean (95% CI) | PEDro Score | ROBINS-I Judgment |
|---|---|---|---|---|---|
| Bathen et al42 | 2.5 wk inpatient and 8 wk 5 × /wk multidisciplinary rehabilitation program combining physical therapy (ie, strength training, aquatic exercise, endurance training, pain management) and cognitive behavioral therapy (ie, focusing on functioning with a diagnosis, increased awareness of the importance of prioritizing activity, and living with pain). | COPM performance COPM satisfaction Tandem walking backwards Stair walking up Stair walking down Up on toes TSK-13 NPRS |
.008 .005 .006 .004 .065 .045 .022 .213 |
NA | Critical |
| Toprak et al24 | IG: 8 wk, 3 × /wk, lumbar spinal stabilization exercise program CG: no lumbar spinal stabilization exercise program |
VAS (pain intensity) SMEO stability index SMEC stability index DMEO stability index DMEC stability index FLEX EXT LATr LATl |
.022 .884 .447 .036 .070 <.001 .003 .001 <.001 |
7 | NA |
| Daman et al22 | IG: 4 wk, 3d/wk, received combined exercise therapy of closed kinetic chain exercises and proprioception exercises CG: no combined exercise therapy |
Goniometer, angle error (weight-bearing) Goniometer, angle error (nonweight-bearing) VAS (pain intensity) SF-36 (physical functioning) SF-36 (mental health) |
.030 .009 <.001 <.010 .420 |
8 | NA |
| Ferrell et al21 | 8 wk, 3 × /wk, progressive closed kinetic chain exercises |
Threshold angle Balance board Isokinetic dynamometer (average strength, quadricep) Isokinetic dynamometer (peak strength, quadricep) Isokinetic dynamometer (average strength, hamstring) Isokinetic dynamometer (peak strength, hamstring) VAS (musculoskeletal pain) SF-36 (physical functioning) SF-36 (mental health) |
<.001 <.001 <.001 .030 .007 .002 .003 .029 .008 |
NA | Critical |
| Hakimi et al43 | 4 wk, 2 × /wk, multidisciplinary rehabilitation program (ie, balneotherapy, ergometer exercises, occupational therapy, physical activity, physiotherapy, walking, proprioception exercises, sophrology, yoga exercises) and therapeutic patient education workshops led by several professionals (ie, dieticians, physiotherapists, doctors, psychologists) Rest 1 wk. Followed by 4 wk 3 × /wk continuation of program and workshops |
6MWT TSK MFI-20 (general) MFI-20 (physical) MFI-20 (mental) MFI-20 (reduced activity) MFI-20 (reduced motivation) BPI (pain at its least) BPI (pain at its worst) BPI (pain on the average) BPI (pain right now) BPI (pain interference) SF-36 (physical functioning) SF-36 (mental health) |
.001 .033 NS (not available) NS (not available) NS (not available) .010 NS (not available) NS (not available) .230 NS (not available) NS (not available) NS (not available) NS (not available) .022 |
NA | Critical |
| Liaghat et al40 | 16 wk 3 × /wk heavy shoulder strengthening exercise program targeting scapular and rotator cuff muscles | Shoulder stability Average pain intensity WOSI total score NPRS (lowest in past 7 d) NPRS (highest in past 7 d) NPRS (average in past 7 d) CIS, fatigue subscale COOP/WONCA TSK Global perceived effect EQ-5D-3L (index score) EQ-5D-3L (EQ-VAS) Internal rotation passive Internal rotation active External rotation passive External rotation active Isometric scaption Isometric internal rotation Isometric external rotation Isometric external/internal rotation ratio Angle error (low range) Angle error (midrange) Angle error (high range) |
−528 (−738 to −318) −2.4 (−3.7 to −1.2) −538 (−738 to −318) −0.9 (−1.7 to −0.2) −2.5 (−3.8 to −1.2) −2.4 (−3.7 to −1.2) −9 (−16 to −2) −1.2 (−4.5 to 2.1) −3.3 (−5.7 to −0.8) Not available 0.01 (−0.08 to 0.09) 7 (−7 to 21) −8.9 (−18.8 to 0.9) −3.5 (−12.4 to 5.3) −0.1 (−13.3 to 13.2) 1.9 (−9.3 to 13.1) 0.51 (0.23 to 0.78) 1.32 (0.70 to 1.95) 0.89 (0.37 to 1.40) −0.02 (−0.10 to 0.05) −1.2 (−2.4 to 0.0) −0.9 (−2.2 to 0.3) 0.6 (−2.0 to 3.2) |
NA | Critical |
| Palmer et al38 | IG: 1 × advice intervention session with physiotherapist supplemented by advice booklets and 4-mo composed of 6 physiotherapy sessions CG: 1 × advice intervention session with physiotherapist supplemented by advice booklets |
MDHAQ (function) MDHAQ (pain) MDHAQ (global) MDHAQ (RADAI) MDHAQ (fatigue) BRAF (average fatigue) BRAF (effect of fatigue) BRAF (coping with fatigue) VAS (most affected joints at rest) VAS (most affected joints on movement) VAS (all joints at rest) VAS (all joints on movement) 18-item Exercise Self-efficacy |
0.52 (−0.69 to 1.74) −0.81 (−4.47 to 2.85) −0.78 (−4.54 to 2.97) 3.01 (−3.83 to 9.84) −0.04 (−4.15 to 4.07) −0.65 (−4.31 to 3.01) −1.68 (−6.61 to 3.26) −2.49 (−5.84 to 0.86) 5.90 (−30.88 to 42.68) 6.64 (−29.98 to 43.26) −18.37 (−51.57 to 14.84) −14.34 (−49.21 to 20.52) 7.03 (−18.59 to 32.65) |
5 |
NA |
| Reychler et al41 | IG: 6 wk 5 × /wk unsupervised sessions of inspiratory muscle training CG: no inspiratory muscle training |
SNIP Forced expiratory vital capacity Forced expiratory volume in 1 s 6MWT HADS-Anxiety HADS-Depression |
<.001 .237 .009 .003 .830 .408 |
7 | NA |
| Sahin et al23 | IG: 8 wk 3 × /wk proprioceptive exercise program CG: no proprioceptive exercise program |
Isokinetic dynamometer, angle error (right extremity) Isokinetic dynamometer, angle error (left extremity) VAS (during movement) VAS (resting) AIMS-2 (physical) AIMS-2 (emotional) AIMS-2 (symptoms) AIMS-2 (social) AIMS-2 (occupational) |
.001 .001 .010 .027 .358 .596 .206 .917 .006 |
6 | NA |
| To and Alexander39 | IG: 16 wk 3 × /wk individualized leg exercises CG.a & CG.b: 16 wk 3 × /wk individualized leg exercises |
VAS HAP Lysholm scale Concentric torque Eccentric torque |
<.010 <.010 <.010 .310 .150 |
NA | Moderate |
Abbreviations: BPI, Brief Pain Inventory; BRAF, Bristol Rheumatoid Arthritis Fatigue; CG, control group; CG.a, control group with generalized joint hypermobility (ie, joint flexibility without multiple site symptoms experience in those with HSD); CG.b, control group without generalized joint hypermobility; CIS, Checklist of Individual Strength; COOP, Dartmouth Primary Care Cooperative Research Network; COPM, Canadian Occupational Performance Measure; DMEC, dynamic mode eyes closed; DMEO, dynamic mode eyes open; EQ-5D-3L, European Quality of Life 5 Dimensions 3-Level Scale; EQ-VAS, European Quality of Life visual analog scale; EXT, back extensor musculature; FLEX, trunk flexor musculature; HAP, Human Activity Profile; IG, intervention group; LATl, left lateral trunk musculature; LATr, right lateral trunk musculature; MDHAQ, Multidimensional Health Assessment Questionnaire; MFI, Multidimensional Fatigue Inventory; NA, not applicable; NPRS, Numerical Pain Rating Scale; NS, not significant; RADAI, Rheumatoid Arthritis Disease Activity Index; SMEC, static mode eyes closed; SMEO, static mode eyes open; SNIP, Sniff nasal inspiratory pressure; TSK-13, 13-item Tampa Scale of Kinesiophobia; WONCA, World Organization of National Colleges Academies and Academic Associations of General Practitioners/Family Physicians; WOSI, Western Ontario Shoulder Instability Index.
Three of the 4 studies that investigated the effect of exercise on proprioception acuity found significant improvements.21, 22, 23,40 The study by Ferrell et al, described above, found improved posttest performance in proprioceptive acuity measured using a threshold detection angle error,45 which required the participant to detect the direction of displacement of the knee joint (−0.28°±0.03, P<.001).21 While the study by Ferrell shows significant changes in proprioceptive acuity, again these findings should be interpreted with caution because of the aforementioned risk of bias in the study. In an RCT of 8 weeks of kinesthesia (awareness of the position and movement of limbs) and balance exercises 3 × /wk vs no intervention, Sahin et al tested knee proprioception using an isokinetic dynamometer and measured the absolute angle error values for reproducing predetermined knee angles. They found that only the intervention group improved proprioceptive acuity (right knee: −0.09°±0.08, P<.001; left knee: −0.79±0.05, P=.001).23 Sahin23 shows significant improvement in proprioceptive acuity; however, this study demonstrated a compromise in study quality and high risk of bias because of limitations in allocation, outcome measurements, and between-group comparisons (see tables 3 and 5), making it vulnerable to confounding and weakened statistical power. In agreement, Daman et al observed greater knee joint proprioception using a goniometer to measure angle error under weight-bearing and nonweight-bearing conditions after 4 weeks of closed kinetic chain exercises 3 × /wk compared with the controls (weight-bearing: −3.01°±2.42, P=.030; nonweight-bearing: −1.89°±1.51, P=.009).22 The nonrandomized study by Liaghat et al, described above, did not find significant postprogram improvements in proprioception acuity at low range (considered 55°±10°), midrange (90°±10°), or high range (125°±10°), measured using a digital inclinometer.40 However, the findings of Liaghat41 should again be cautiously interpreted because of a critical risk of bias.
Additional markers of physical health including balance, functional exercise capacity, joint function, stability, and endurance were investigated across 3 RCTs.23,24,41 Reychler et al reported greater functional exercise capacity measured using the 6-minute walk test (6MWT)46 and greater pulmonary function using a spirometer47 after 6 weeks of inspiratory muscle exercise 5 × /wk compared with the controls (6MWT=+56m±52, P=.003; forced expiratory volume in 1 second=11.3±0.8, P=.009).41 Sahin et al observed an improvement in knee joint function using the Arthritis Impact Measurement Scales-2 (AIMS-2) occupational activity in only the exercise group after 8 weeks of kinesthesia and balance exercises 3 × /wk compared with baseline (−1.12±1.29, P<.01), but there were no significant differences found in other AIMS-2 subscales (P>.05)23 Again, given the poor methodological quality and high risk of bias of this study, cautious interpretation of these findings is recommended.23 Toprak Celenay and Ozer Kaya observed improvements in the median dynamic postural stability, measured using the Biodex Balance System SD,c and the median trunk muscle endurance, measured using McGill's trunk muscle endurance tests,48 after 8 weeks of spinal stabilization 3 × /wk compared with the controls (postural stability: dynamic mode eyes open, P=.036; muscle endurance: trunk flexor musculature, P<.001; back extensor musculature, P=.003; right lateral trunk musculature, P=.001; left lateral trunk musculature, P<.001).24
In addition, 4 nonrandomized studies investigated markers of physical health.21,40,42,43 Similar to the findings of Reychler et al, Hakimi et al reported greater functional exercise capacity using the 6MWT45 after the completion of a rehabilitation program of 4 weeks at 2 × /wk and a subsequent 4 weeks at 3 × /wk. Improvements were seen at the 6-week follow-up time point compared with baseline (+52.4m, P<.001).43 Hakimi also recorded the effects of their rehabilitation program on fatigue measured using Multidimensional Fatigue Inventory,49 which assesses fatigue in 5 dimensions: general fatigue, physical fatigue, mental fatigue, reduced activity, and reduced motivation. Here, there was significant improvement in only the Multidimensional Fatigue Inventory reduced activity subscale at the end of the 8-week program compared with baseline (−2.8, P=.01), which was not maintained at the 6-week follow-up.43 While these findings are significant, the study conducted by Hakimil43 is considered to have a critical risk of bias because of possible confounding and missing data (see table 4), which may reduce the statistical power of these results. Liaghat et al also found improvement in fatigue measured using the Checklist of Individual Strength50 subscale fatigue after a 16-week heavy shoulder strengthening exercise program 3 × /wk compared with baseline (−9; 95% CI, −16 to −2).40 Similar to the findings of improved postural stability found in the RCT by Toprak Celenay and Ozer Kaya,24 Liaghat reported a 51% improvement in the mean change of shoulder stability after the abovementioned program measured using the Western Ontario Stability Index51 compared with baseline (total score=−528; 95% CI, −738 to −318; physical symptoms=−245; 95% CI, − 337 to −152; sports/recreation/work=−117; 95% CI, − 172 to −61; lifestyle=−67; 95% CI, −113 to −22; emotions=−100; 95% CI, −159 to −40).40 Liaghat also measured functional health, range of motion, and joint mobility after the 16-week heavy shoulder strengthening program but found no significant change in functional health or range of motion compared with baseline. Statistics indicating change in joint mobility were not available.40 Ferrell21 and Bathen44 and colleagues assessed balance as an outcome. Ferrell assessed balance using an instrumented balance board to assess the individual's percentage of time spent out of balance (outside 8° of the horizontal plane). They found improved posttest performance after their 8 weeks of progressive closed kinetic chain exercise intervention (−4.5%±0.9, P=.008).21 After the previously described multidisciplinary rehabilitation program, Bathen reported an improvement in dynamic balance using the tandem-walking-backward test compared with baseline (−9.05 seconds, P=.006).42 While the findings described by Liaghat,40 Ferrell,21 and Bathen42 and colleagues are statistically significant, these studies are considered to have a high risk of bias because of confounding and are therefore vulnerable to influence of extraneous variables on the dependent variables.
Bathen42 was the only study to investigate activities of daily living and reported an improvement in performance of and satisfaction in performance of activities of daily living compared with baseline (performance: +2.13, P=.008; satisfaction in performance: +2.15, P=.005). This was measured using the Canadian Occupational Performance Measure52 after their 14.5-week multidisciplinary rehabilitation program, but again the critical risk of bias in this study should be considered when interpreting these results.42
Pain and kinesiophobia
Pain was measured in 7 studies,22, 23, 24,38,40,42,43 and of these, 4 studies found significant improvements in pain outcomes (see table 6).22, 23, 24,40 Daman et al reported a decrease in pain intensity using the visual analog scale (VAS)53 after 4 weeks of closed kinetic chain and proprioception exercises 3 × /wk compared with the controls (−2.78±0.05, P<.001).23 Sahin et al measured pain intensity, again using VAS, during rest and movement and found improvements in only the exercise group after 8 weeks of proprioceptive exercise 3 × /wk compared with baseline (VAS during rest: −1.87±0.33, P=.027; VAS during movement: −4.04±1.63, P=.01).23 While the study by Sahin et al23 shows significant changes in pain intensity, the compromise in study quality and high risk of bias in this study should again be considered when interpreting these findings. Toprak Celenay and Ozer Kaya reported an improvement in pain intensity using the VAS50 after an 8-week lumbar spinal stabilization exercise program compared with the control group (+1.6, P=.022).24 In agreement, Liaghat et al measured participants’ lowest, highest, and average shoulder pain during the last 7 days at baseline and after the 16-week shoulder strengthening exercise program using the numeric pain rating scale. It was reported that the lowest, highest, and average pain decreased after the 16-week program compared with baseline (lowest=−0.9; 95% CI, − 1.7 to − 0.2; highest=−2.5; 95% CI, −3.8 to −1.2; average=−2.4; 95% CI, − 3.7 to −1.2).40 Hakimi et al reported an increase in pain at its worst, as defined by the Brief Pain Inventory54 6 weeks after the previously described 8-week rehabilitation program (+0.9, P=.023).43 Palmer et al measured pain intensity using the Multidimensional Health Assessment Questionnaire55 after 16 weeks of physiotherapy (involving postural, resistance, and cardiovascular exercises) and 4 (individually spaced) advice therapy sessions (involving information on physical activity and joint protection). No significant changes were found in the physiotherapy and advice group compared with the advice only group.38
The effect of exercise and rehabilitation interventions on kinesiophobia were reported in nonrandomized studies (see table 6). Hakimi evaluated kinesiophobia using the Tampa Scale for Kinesiophobia56 and reported significant improvement in kinesiophobia at the end of the 8-week program compared with baseline (−3.1, P=.033), which was not maintained at the 6-week follow-up.43 Similarly, Liaghat40 and Bathen42 and colleagues found improvements in kinesiophobia measured using the Tampa Scale for Kinesiophobia. After their 16-week shoulder exercise program, Liaghat et al found improvement compared with baseline (−3.3; 95% CI, −5.7 to −0.8).40 In agreement, Bathen et al reported significant improvement in kinesiophobia at the end of their 14.5-week multidisciplinary rehabilitation program compared with baseline (−4, P=.022).42
While the improvements in pain and kinesiophobia reported by Liaghat,40 Hakimi,43 and Bathen42 and colleagues are statistically significant, these findings should again be cautiously interpreted because of a high risk of bias.
Quality of life and psychological health
QOL and psychological well-being were measured in 5 studies and are summarized in table 6.21,22,40,41,43 Ferrell et al measured QOL for physical function and mental health using the Short Form-36 (SF-36) questionnaire,57 finding improvements in the both summary scores after an 8-week program of progressive closed kinetic chain exercises compared with baseline (physical functioning: +9.10, P=.029; mental health: +27.80, P=.008).21 Daman et al similarly found improvements in the physical function subscale summary score of the SF-36 (+11.94±2.96, P=.010) but no significant change in the mental health subscale summary score.22 Reychler et al measured anxiety and depression using the Hospital and Anxiety Depression Scale (HADS)58 and observed no difference between intervention and control participants for either HADS-anxiety or HADS-depression.41 Liaghat measured QOL using the European Quality of Life 5 Dimensions 3-Level Scale and the European Quality of Life VAS59 and observed no significant change for either scales between baseline and after a 16-week heavy shoulder strengthening exercise program 3 × /wk.40 Hakimi et al measured QOL using the SF-36. Significant improvement in QOL was observed at the end of the 8-week rehabilitation program in only the vitality subscale compared with baseline (+14.2, P=.001), which then returned to baseline 6 weeks after the end of intervention.43 However, the QOL results from Ferrell,21 Liaghat et al,40 and Hakimi39 and colleagues should again be cautiously interpreted because of a high risk of bias.
Safety
In only 1 of the 10 studies was an adverse event reported to be potentially associated with study participation (<1% of all intervention participants). In the study by Reychler et al, a single participant reported thoracic pain and withdrew from the study after 3 weeks of participation.41 Palmer et al also reported adverse events (such as back pain and Achilles pain) yet found difficulty in attempting attribution of adverse events in the intervention group to the physiotherapy treatment given. This was because of the participants’ ongoing experiences with adverse events outside of the trial and because there was no baseline assessment of adverse events against which to compare adverse events during the trial. Overall, Palmer found the adverse event rate to be lower in the physiotherapy and advice group than in the advice only group, although attribution could not be firmly established.38
Discussion
This systematic review highlights the potential value of exercise and rehabilitation for people with EDS; however, it has also shown the dearth of RCTs in this field and a high risk of bias across the retrieved randomized and nonrandomized trials. While our findings show statistical significance in the potential benefits to physical and psychological well-being, there is a need for reduced risk of bias in future studies to confirm or refute these findings. Accordingly, interpretation of our findings should be made with caution. Thorough clinical reasoning, consultation, and routine examination of the literature is recommended when providing such care for those with EDS. Despite these cautions, these studies suggest that exercise and rehabilitation appear to be feasible and safe and that the rate of evidence has grown in recent years, contributing to continued clarity and specificity of the findings.
Our results suggest that exercise and rehabilitation can help to achieve benefits earlier than 6 weeks as previously described.25,27 Although exercise interventions were observed to be effective in the relative short-term, To and Alexander observed that the relationship between the length of the intervention and strength gains for individuals with HSD/hEDS were linear, suggesting that interventions continuing over a longer period of time will effect greater change in strength.39 Therefore, to optimize therapeutic outcomes, future programs should consider the incorporation of education and strategies to further enhance long-term maintenance of exercise and rehabilitation engagement. In agreement, Hakimi et al reported that apart from functional exercise capacity, many physical improvements gained during the intervention period seemed to return to baseline when assessed at a 6-week follow up, highlighting again the importance of emphasizing health behavior change and program maintenance plans.43 Fortunately, Liaghat et al demonstrated that the recommended long-term participation in an exercise program is feasible for people with EDS. Here, participants completed a 3-month shoulder strengthening exercise program and participant retention and adherence were reported to be 100% and 83%, respectively. The high retention and adherence rates were attributed to the high number of sessions supervised by a physiotherapist and the participants reporting their exercises as relevant to reducing their EDS-related symptoms.40
The results of the harms assessment among RCTs included in this review indicated a low occurrence of adverse events, where only 1 event was potentially attributable to the intervention and 2 others had undetermined or unlikely links to the intervention. In those studies that did not meet the study design criteria for assessment using the CONSORT harms assessment, there were no reports of adverse events during the intervention time frame.43,40 Accordingly, the adverse event rate associated with exercise and rehabilitation among people with EDS is conservatively estimated at <1% of the participants; however, more studies are needed to confirm and add precision to this estimate.
An important limitation of the current evidence is the limited number of studies that capture joint subluxation and/or dislocation and functional capacity outcomes. Joint stability and functional capacity have each only been examined by a single study.43,40 Although the results are limited in their breadth, these studies have shown exercise interventions that target stabilizing muscles surrounding hypermobile joints can enhance joint support throughout movement,40,60 and generalized exercise interventions that addresses cardiorespiratory aspects of movement can reduce general deconditioning.43,61 Given the known high rates of subluxations and/or dislocations in people with EDS (72% prevalence),7 the prevalence of comorbid cardiovascular autonomic dysfunction, and the known limitations in functional capacity,62 these outcomes should be prioritized in future research. Further, these conditions indicate a need for interventions composed of comprehensive exercise tailored to those living with EDS. Persons living with EDS may also have comorbid orthostatic intolerance, fatigue, chronic pain, anxiety and depression, proprioceptive disturbances, dyspnea, gastrointestinal symptoms, urogenital complications, cognitive dysfunction, autoimmune dysfunction, and cardiovascular symptoms.13,19,63, 64, 65, 66, 67 The severity of these comorbidities can range from mild to severely disabling, with grave effect on exercise and rehabilitation engagement levels.68,69 Given the complex nature of EDS-related symptoms, Bathen et al demonstrated the influence of a multidisciplinary approach to exercise therapy with a range of intervention components including cognitive behavioral therapy, energy conservation techniques, joint protection education, nutrition, and pain coping strategies.42 Here, all participants adhered to the full intervention, no harms were reported, and significant changes were found in both physical health (increased muscle strength and endurance) and metal health domains (improved perceived performance of daily activities, increased participation in daily life, and reduction in kinesiophobia).42 These results suggest benefits of a multidisciplinary approach in the treatment of EDS, specifically, the consideration of cognitive and psychological influences on exercise and rehabilitation effects for patients with EDS. The support for a multidisciplinary approach can been seen throughout additional studies70, 71, 72 as well as in practice at the GoodHope EDS Clinic at the Toronto General Hospital, providing the first 1-stop interdisciplinary model of health care delivery for EDS.73 Together, emerging research and multidisciplinary program delivery may help to inform future screening, risk assessment, and intervention design and delivery in EDS.
Although the study methodology remains modest, improvements in study rigor are noteworthy. Since 2016, more RCTs (vs nonrandomized controlled trials and other quasi-experimental and observational studies) have been conducted and assessor blinding has become more prevalent. The use of randomization and blinding helps to reduce bias and provides increasingly rigorous tools to examine potential cause-effect relationships between exercise and rehabilitation interventions and measured outcomes.74 All 10 studies included in this review were published after 2004 and originate from 7 different countries, indicating both recent growth and international efforts toward growing the body of evidence in this domain.
Study limitations
There are several limitations that justify cautious interpretation of this study. First, although we sought to capture the effect of exercise and rehabilitation interventions on all EDS subtypes, only those with joint hypermobility syndrome, EDS hypermobility type, and hEDS (and the clinical analog HSD) met the inclusion criteria. The findings of this review are therefore limited to what is now referred to as hEDS and cannot be generalized to the remaining 12 EDS subtypes. These subtypes can be complicated by comorbid presentations that can lead to injury or death (such as mast cell activation disorders, fibromyalgia, and osteopenia)11 and thus can affect participation in exercise interventions.75 Recognizing that the exercise and rehabilitation interventions used in these studies might not be feasible depending on patients’ EDS subtype, comorbid conditions, or functional health, we recommend that future studies examine interventions involving various intensities, durations, frequencies, and modifications. Second, we were unable to separately categorize and measure the effects of interventions in patients with HSD vs hEDS because of recent change of nomenclature and diagnostic criteria. Although synonymous for the purpose of this review, individuals with hEDS experience symptoms and conditions outside of those associated with HSD (such as postural orthostatic tachycardia syndrome and dyspnea).13,76 Symptom- and condition-specific consideration should therefore be undertaken before starting any exercise intervention. Third, the methodological heterogeneity in the included studies and in particular the variability in intervention design (eg, physiotherapy, inspiratory muscle training, closed kinetic chain exercises, lumbar spinal stabilization exercise) and outcome measures (eg, AIMS-2, HADS, Multidimensional Health Assessment Questionnaire) was not conducive to a meta-analysis. Lastly, the outcome measures used across the included studies have been validated for clinical trials but not specifically for people with EDS. Despite the importance of such validated outcome measures in the population with EDS, there are no condition-specific outcome measurement tools available. Recent systematic reviews have been largely inconclusive and meta-analyses have yet to be completed, partially because of the heterogeneity in outcome measurements. To address this gap, beginning in January 2019 the International Consortium on EDS and HSD has been building Common Data Elements (standardized key terms or concepts) to facilitate data sharing so that data can be compared and combined across research studies and institutions.77
Conclusions
The body of evidence on exercise and rehabilitation interventions for people with EDS supports its benefit for various physical and psychological outcomes; however, the scarcity of eligible studies and inconsistency in outcome measures used precludes definitive statements regarding intervention efficacy, feasibility, and safety. At present, exercise and rehabilitation research pertains specifically to those with HSD/hEDS, highlighting an important gap in this literature given the diverse range of physical limitations and symptoms experienced across other EDS subtypes. This review provides a platform on which to further develop research initiatives and evidence in this area. Further high-quality trials with consistent methodological approaches and larger sample sizes that are powered for primary outcomes (in particular, those that address physical function), consider a multidisciplinary intervention design, and include various EDS subgroups are needed to evaluate the effect of exercise and rehabilitation for people with EDS.
Suppliers
a. Covidence; Veritas Health Innovation, Melbourne, Australia. b. MS Excel 2010; Microsoft Corporation, Redmond, WA. c. Biodex Balance System SD; Biodex Medical Systems Inc, Shirley, NY.
Footnotes
Supported by the GoodHope Ehlers Danlos Clinic, which is supported by the Ontario Ministry of Health and Long-term Care and by the Toronto General and Western Hospital Foundation.
Systematic Review Registration No.: CRD42020140218.
Disclsoures: Dr Rozenberg reports research support from Sandra Faire and Ivan Fecan Professorship in Rehabilitation Medicine. The other authors have nothing to disclose.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.arrct.2022.100189.
Appendix. Supplementary materials
References
- 1.Superti-Furga A, Steinmann B, Ramirez F BP. Molecular defects of type III procollagen in Ehlers-Danlos syndrome type IV. Hum Genet. 1989;82:104–108. doi: 10.1007/BF00284038. [DOI] [PubMed] [Google Scholar]
- 2.Michael Pope F, Martin GR, Lichtenstein JR, et al. Patients with Ehlers-Danlos syndrome type IV lack type III collagen. Proc Natl Acad Sci U S A. 1975;72:1314–1316. doi: 10.1073/pnas.72.4.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pyeritz R, Saunders W. In: Cecil textbook of medicine. 21st ed. Goldman L, Bennett JC, editors. W.B. Saunders; Philadelphia, PA: 2000. Ehlers-Danlos syndromes. [Google Scholar]
- 4.Byers PH. Disorders of collagen biosynthesis and structure. Metab Mol Bases Inherit Dis. 2001:1065–1068. [Google Scholar]
- 5.Byers PH. Ehlers-Danlos syndrome: recent advances and current understanding of the clinical and genetic heterogeneity. J Invest Dermatol. 1994;103(5 Suppl):S47–S52. doi: 10.1111/1523-1747.ep12399038. [DOI] [PubMed] [Google Scholar]
- 6.Beighton P, De Paepe A, Steinmann B, Tsipouras P, Wenstrup RJ. Ehlers-Danlos syndromes: revised nosology, Villefranche, 1997. Am J Med Genet. 1998;77:31–37. doi: 10.1002/(sici)1096-8628(19980428)77:1<31::aid-ajmg8>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 7.Stanitski DF, Nadjarian R, Stanitski CL, Bawle E, Tsipouras P. Orthopaedic manifestations of Ehlers-Danlos syndrome. Clin Othop Relat Res. 2000;376:213–221. doi: 10.1097/00003086-200007000-00029. [DOI] [PubMed] [Google Scholar]
- 8.Rombaut L, Malfait F, Cools A, De Paepe A, Calders P. Musculoskeletal complaints, physical activity and health-related quality of life among patients with the Ehlers-Danlos syndrome hypermobility type. Disabil Rehabil. 2010;32:1339–1345. doi: 10.3109/09638280903514739. [DOI] [PubMed] [Google Scholar]
- 9.Beighton P, De Paepe A, Danks, et al. International nosology of heritable disorders of connective tissue, Berlin, 1986. Am J Med Genet. 1988;29:581–594. doi: 10.1002/ajmg.1320290316. [DOI] [PubMed] [Google Scholar]
- 10.Grahame R, Bird HA, Child A, et al. The revised (Brighton 1998) criteria for the diagnosis of benign joint hypermobility syndrome (BJHS) J Rheumatol. 2000;27:1777–1779. [PubMed] [Google Scholar]
- 11.Malfait F, Francomano C, Byers P, et al. The 2017 international classification of the Ehlers-Danlos syndromes. Am J Med Genet Part C Semin Med Genet. 2017;175:8–26. doi: 10.1002/ajmg.c.31552. [DOI] [PubMed] [Google Scholar]
- 12.Ainsworth SR, Aulicino PL. A survey of patients with Ehlers-Danlos syndrome. Clin Orthop Relat Res. 1993;286:250–256. [PubMed] [Google Scholar]
- 13.Castori M, Camerota F, Celletti C, et al. Natural history and manifestations of the hypermobility type Ehlers-Danlos syndrome: a pilot study on 21 patients. Am J Med Genet Part A. 2010;152:556–564. doi: 10.1002/ajmg.a.33231. [DOI] [PubMed] [Google Scholar]
- 14.Johnson SM, Robinson CM. Shoulder instability in patients with joint hyperlaxity. J Bone Jt Surg Am. 2010;92:1545–1557. doi: 10.2106/JBJS.H.00078. [DOI] [PubMed] [Google Scholar]
- 15.Grahame R. Joint hypermobility: emerging disease or illness behaviour. Clin Med (London) 2013;13(Suppl 6):s50–s52. doi: 10.7861/clinmedicine.13-6-s50. [DOI] [PubMed] [Google Scholar]
- 16.Tinkle B, Bird H, Grahame R. The lack of clinical distinction between the hypemobility type of Ehlers-Danlos syndrome and the joint hypermobility syndrome (a.k.a hypermobility syndrome) Am J Med Genet Part A. 2009;149:2368–2370. doi: 10.1002/ajmg.a.33070. [DOI] [PubMed] [Google Scholar]
- 17.Grahame R. Joint hypermobility syndrome pain. Curr Pain Headache Rep. 2009;13(13):427–433. doi: 10.1007/s11916-009-0070-5. [DOI] [PubMed] [Google Scholar]
- 18.Celletti C, Castori M, La Torre G, Camerota F. Evaluation of kinesiophobia and its correlations with pain and fatigue in joint hypermobility syndrome/Ehlers-Danlos syndrome hypermobility type. Biomed Res Int. 2013;2013:58460. doi: 10.1155/2013/580460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gregg CD, McIntosh G, Hall H, Watson H, Williams D, Hoffman CW. The relationship between the Tampa Scale of Kinesiophobia and low back pain rehabilitation outcomes. Spine J. 2015;15:2466–2471. doi: 10.1016/j.spinee.2015.08.018. [DOI] [PubMed] [Google Scholar]
- 20.Scheper MC, De Vries JE, Verbunt J, Engelbert RHH. Chronic pain in hypermobility syndrome and Ehlers-Danlos syndrome (hypermobility type): it is a challenge. J Pain Res. 2015;8:591–601. doi: 10.2147/JPR.S64251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferrell WR, Tennant N, Sturrock RD, et al. Amelioration of symptoms by enhancement of proprioception in patients with joint hypermobility syndrome. Arthritis Rheum. 2004;50:3323–3328. doi: 10.1002/art.20582. [DOI] [PubMed] [Google Scholar]
- 22.Daman M, Shiravani F, Hemmati L, Taghizadeh S. The effect of combined exercise therapy on knee proprioception, pain intensity and quality of life in patients with hypermobility syndrome: a randomized clinical trial. J Bodyw Mov Ther. 2019;23:202–205. doi: 10.1016/j.jbmt.2017.12.012. [DOI] [PubMed] [Google Scholar]
- 23.Sahin N, Baskent A, Cakmak A, Salli A, Ugurlu H, Berker E. Evaluation of knee proprioception and effects of proprioception exercise in patients with benign joint hypermobility syndrome. Rheumatol Int. 2008;28:995–1000. doi: 10.1007/s00296-008-0566-z. [DOI] [PubMed] [Google Scholar]
- 24.Toprak Celenay S, Ozer Kaya D. Effects of spinal stabilization exercises in women with benign joint hypermobility syndrome: a randomized controlled trial. Rheumatol Int. 2017;37:1461–1468. doi: 10.1007/s00296-017-3713-6. [DOI] [PubMed] [Google Scholar]
- 25.Palmer S, Bailey S, Barker L, Barney L, Elliott A. The effectiveness of therapeutic exercise for joint hypermobility syndrome: a systematic review. Physiotherapy. 2014;100:220–227. doi: 10.1016/j.physio.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 26.Smith TO, Bacon H, Jerman E, et al. Physiotherapy and occupational therapy interventions for people with benign joint hypermobility syndrome: a systematic review of clinical trials. Disabil Rehabil. 2014;36:797–803. doi: 10.3109/09638288.2013.819388. [DOI] [PubMed] [Google Scholar]
- 27.Corrado B, Ciardi G. Hypermobile Ehlers-Danlos syndrome and rehabilitation: taking stock of evidence based medicine: a systematic review of the literature. J Phys Ther Sci. 2018;30:843–847. doi: 10.1589/jpts.30.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration, 2011. Available at: www.handbook.cochrane.org. Accessed April 4, 2022.
- 29.Higgins J, Lasserson T, Chandler J, Tovey D, Churchill R. Standards for the conduct and reporting of new Cochrane Intervention Reviews, reporting of protocols and the planning, conduct and reporting of updates. Methodological Expectations of Cochrane Intervention Reviews (MECIR). Available at: https://community.cochrane.org/mecir-manual. Accessed April 22, 2022.
- 30.Moher D, Liberati A, Tetzlaff J. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. Int J Surg. 2010;8:336–341. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 31.McGowan J, Sampson M, Salzwedel D. PRESS peer review of electronic search strategies: 2015 guideline statement. J Clin Epidemiol. 2016;75:40–46. doi: 10.1016/j.jclinepi.2016.01.021. [DOI] [PubMed] [Google Scholar]
- 32.Maher CG, Sherrington C, Herbert RD, Moseley AM, Elkins M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys Ther. 2003;83:713–721. [PubMed] [Google Scholar]
- 33.Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:14919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schulz K, Altman D, Moher D, CONSORT Group CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:332. doi: 10.3736/jcim20100702. [DOI] [PubMed] [Google Scholar]
- 35.Hopewell S, Clarke M, Moher D. CONSORT for reporting randomized controlled trials in journal and conference abstracts: explanation and elaboration. PLoS Med. 2008;5:e20. doi: 10.1371/journal.pmed.0050020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bagherian S, Rahnama N, Wikstrom E. Corrective exercises improve movement efficiency and sensorimotor function but not fatigue sensitivity in chronic ankle instability patients: a randomized controlled trial. Clin J Sport Med. 2019;29:193–202. doi: 10.1097/JSM.0000000000000511. [DOI] [PubMed] [Google Scholar]
- 37.Bateman M, Osborne S, Smith B. Physiotherapy treatment for atraumatic recurrent shoulder instability: updated results of the Derby Shoulder Instability Rehabilitation Programme. J Arthrosc Jt Surg. 2019;6:35–41. [Google Scholar]
- 38.Palmer S, Cramp F, Clark E, et al. The feasibility of a randomised controlled trial of physiotherapy for adults with joint hypermobility syndrome. Health Technol Assess. 2016;20:1–264. doi: 10.3310/hta20470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.To M, Alexander CM. Are people with joint hypermobility syndrome slow to strengthen? Arch Phys Med Rehabil. 2019;100:1243–1250. doi: 10.1016/j.apmr.2018.11.021. [DOI] [PubMed] [Google Scholar]
- 40.Liaghat B, Skou ST, Jørgensen U, Sondergaard J, Søgaard K, Juul-Kristensen B. Heavy shoulder strengthening exercise in people with hypermobility spectrum disorder (HSD) and long-lasting shoulder symptoms: a feasibility study. Pilot Feasibility Stud. 2020;6:1–13. doi: 10.1186/s40814-020-00632-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reychler G, Liistro G, Piérard GE, Hermanns-Lê T, Manicourt D. Inspiratory muscle strength training improves lung function in patients with the hypermobile Ehlers-Danlos syndrome: a randomized controlled trial. Am J Med Genet Part A. 2019;179:356–364. doi: 10.1002/ajmg.a.61016. [DOI] [PubMed] [Google Scholar]
- 42.Bathen T, Hångmann AB, Hoff M, Andersen LØ, Rand-Hendriksen S. Multidisciplinary treatment of disability in Ehlers-Danlos syndrome hypermobility type/hypermobility syndrome: a pilot study using a combination of physical and cognitive-behavioral therapy on 12 women. Am J Med Genet Part A. 2013;161:3005–3011. doi: 10.1002/ajmg.a.36060. [DOI] [PubMed] [Google Scholar]
- 43.Hakimi A, Bergoin C, Mucci P. Immediate and 6-week after effects of a rehabilitation program for Ehlers-Danlos syndrome hypermobile type patients: a retrospective study. Am J Med Genet Part A. 2020;182:2263–2271. doi: 10.1002/ajmg.a.61772. [DOI] [PubMed] [Google Scholar]
- 44.McHugh M. Interrater reliability: the kappa statistic. Biochem Med (Zagreb) 2012;22:276–282. [PMC free article] [PubMed] [Google Scholar]
- 45.Hall M, Ferrell W, Sturrock R. The effect of the hypermobility syndrome on knee joint proprioception. Br J Rheumatol. 1995;34:121–125. doi: 10.1093/rheumatology/34.2.121. [DOI] [PubMed] [Google Scholar]
- 46.Enright PL. The six-minute walk test. Respir Care. 2003;48:783–785. [PubMed] [Google Scholar]
- 47.Miller M, Hankinson J, Brusasco V, et al. ATS/ERS Task Force. Standardization of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 48.McGill S, Childs A, Liebenson C. Endurance time for low back stabilization exercises: clinical targets for testing and training from a normal database. Arch Phys Med Rehabil. 1999;80:941–944. doi: 10.1016/s0003-9993(99)90087-4. [DOI] [PubMed] [Google Scholar]
- 49.Gentile S, Delaroziere J, Favre F, et al. Validation of the French ‘multidimensional fatigue inventory’(MFI 20) Eur J Cancer Care (Engl) 2003;12:58–64. doi: 10.1046/j.1365-2354.2003.00295.x. [DOI] [PubMed] [Google Scholar]
- 50.Voermans N, Knoop H. Both pain and fatigue are important possible determinants of disability in patients with the Ehlers-Danlos syndrome hypermobility type. Disabil Rehabil. 2011;33:706–707. doi: 10.3109/09638288.2010.531373. [DOI] [PubMed] [Google Scholar]
- 51.Eshoj H, Bak K, Blond L. Juul-Kristensen B. Translation, adaptation and measurement properties of an electronic version of the Danish Western Ontario Shoulder Instability Index (WOSI) BMJ Open. 2017;7 doi: 10.1136/bmjopen-2016-014053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Law M, Baptiste S, McColl M, Opzoomer A, Polatajko H, Pollock N. The Canadian occupational performance measure: an outcome measure for occupational therapy. Can J Occup Ther. 1990;57:82–87. doi: 10.1177/000841749005700207. [DOI] [PubMed] [Google Scholar]
- 53.Crichton N. Visual analogue scale (VAS) J Clin Nurs. 2001;10:706. 6. [Google Scholar]
- 54.Poundja J, Fikretoglu D, Guay S, Brunet A. Validation of the French version of the Brief Pain Inventory in Canadian veterans suffering from traumatic stress. J Pain Symptom Manage. 2007;33:720–726. doi: 10.1016/j.jpainsymman.2006.09.031. [DOI] [PubMed] [Google Scholar]
- 55.Pincus TA. A Multidimensional Health Assessment Questionnaire (MDHAQ) for all patients with rheumatic diseases to complete at all visits in standard clinical care. Bull NYU Hosp Jt Dis. 2007;65:150. [PubMed] [Google Scholar]
- 56.French DJ, Roach PJ, Mayes S. Fear of movement in victims of occupational accidents: French-Canadian version of the Tampa Scale of Kinesiophobia. Can J Behav Sci. 2002;34:8–33. [Google Scholar]
- 57.Saris-Baglama RN, Dewey CJ, Chisholm GB, et al. Quality Metric Health OutcomesTM Scoring Software 4.0 Installation Guide. 2004. Available at: https://www.amihealthy.com/download/installationguide_scoringsoftwarev4.pdf. Accessed Jan 20, 2020.
- 58.Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 59.Janssen MF, Pickard AS, Golicki D, et al. Measurement properties of the EQ-5D-5L compared to the EQ-5D-3L across eight patient groups: a multi-country study. Qual Life Res. 2013;22:1717–1727. doi: 10.1007/s11136-012-0322-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Graves JE, Pollock ML, Jones AE, et al. Specificity of limited range of motion variable resistance training. Med Sci Sports Exerc. 1989;21:84–89. doi: 10.1249/00005768-198902000-00015. [DOI] [PubMed] [Google Scholar]
- 61.Ross J, Grahame R. Joint hypermobility syndrome. Br Med J. 2011;342:275–281. doi: 10.1136/bmj.c7167. [DOI] [PubMed] [Google Scholar]
- 62.Engelbert RH, van Bergen M, Henneken T, et al. Exercise tolerance in children and adolescents with musculoskeletal pain in joint hypermobility and joint hypomobility syndrome. Pediatrics. 2006;118:e690–e696. doi: 10.1542/peds.2005-2219. [DOI] [PubMed] [Google Scholar]
- 63.Rowe PC, Barron DF, Calkins H, et al. Orthostatic intolerance and chronic fatigue syndrome associated with Ehlers-Danlos syndrome. J Pediatr. 1999;135:494–499. doi: 10.1016/s0022-3476(99)70173-3. [DOI] [PubMed] [Google Scholar]
- 64.Voermans NC, Knoop H, Bleijenberg G, van Engelen BG. Pain in Ehlers-Danlos syndrome is common, severe, and associated with functional impairment. J Pain Symptom Manage. 2010;40:370–378. doi: 10.1016/j.jpainsymman.2009.12.026. [DOI] [PubMed] [Google Scholar]
- 65.Beckers AB, Keszthelyi D, Fikree A, et al. Gastrointestinal disorders in joint hypermobility syndrome/Ehlers-Danlos syndrome hypermobility type: a review for the gastroenterologist. Neurogastroenterol Motil. 2017;29:e13013. doi: 10.1111/nmo.13013. [DOI] [PubMed] [Google Scholar]
- 66.Gilliam E, Hoffman JD, Yeh G. Urogenital and pelvic complications in the Ehlers-Danlos syndromes and associated hypermobility spectrum disorders: a scoping review. Clin Genet. 2020;97:168–178. doi: 10.1111/cge.13624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kindgren E, Perez AQ, Knez R. Prevalence of ADHD and autism spectrum disorder in children with hypermobility spectrum disorders or hypermobile Ehlers-Danlos syndrome: a retrospective study. Neuropsychiatr Dis Treat. 2021;17:379. doi: 10.2147/NDT.S290494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Clark JE, Getchell N, Smiley-Oyen AL, Smiley-Oyen AL. Developmental coordination disorder: issues, identification, and intervention. J Phys Educ Recreat Dance. 2005;76:49–53. [Google Scholar]
- 69.Bulbena A, Baeza‐Velasco C, Bulbena‐Cabré A, et al. Psychiatric and psychological aspects in the Ehlers–Danlos syndromes. Am J Med Genet C Semin Med Genet. 2017;175:237–245. doi: 10.1002/ajmg.c.31544. [DOI] [PubMed] [Google Scholar]
- 70.Kalisch L, Hamonet C, Bourdon C, Montalescot L, Ecile De Cazotte C, Baeza-Velasco C. Disability and rehabilitation predictors of pain and mobility disability in the hypermobile Ehlers-Danlos syndrome. Disabil Rehabil. 2020;42:3679–3686. doi: 10.1080/09638288.2019.1608595. [DOI] [PubMed] [Google Scholar]
- 71.Zhou Z, Rewari A, Shanthanna H. Management of chronic pain in Ehlers-Danlos syndrome: two case reports and a review of literature. Medicine (Baltimore) 2018;97:e13115. doi: 10.1097/MD.0000000000013115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Castori M. Pain in Ehlers-Danlos syndromes: manifestations, therapeutic strategies and future perspectives. Expert Opin Orphan Drugs. 2016;4:1145–1158. [Google Scholar]
- 73.Mittal N, Mina DS, McGillis L, et al. The GoodHope Ehlers-Danlos Syndrome Clinic: development and implementation of the first interdisciplinary program for multi-system issues in connective tissue disorders at the Toronto General Hospital. Orphanet J Rare Dis. 2021;16:1–9. doi: 10.1186/s13023-021-01962-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hariton E, Locascio JJ. Randomised controlled trials—the gold standard for effectiveness research. BJOG. 2018;125:1716. doi: 10.1111/1471-0528.15199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Grigoriou E, Boris JR, Dormans JP. Postural orthostatic tachycardia syndrome (POTS): association with Ehlers-Danlos syndrome and orthopaedic considerations. Clin Orthop Relat Res. 2015;473:722–728. doi: 10.1007/s11999-014-3898-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fu Q, Levine BD. Exercise in the postural orthostatic tachycardia syndrome. Auton Neurosci Basic Clin. 2015;188:86–89. doi: 10.1016/j.autneu.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.The Ehlers-Danlos Society. The International Consortium on EDS and HSD. Available at: https://www.ehlers-danlos.com/international-consortium/. Accessed December 20, 2022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.