Abstract
Background
Characterising the psychiatric sequelae of coronavirus disease 2019 (COVID-19) can inform the development of long-term treatment strategies. However, few studies have examined these sequelae at different time points after COVID-19 infection.
Aims
The study aimed to investigate the incidences and risks of acute and delayed psychiatric sequelae in patients hospitalised with COVID-19 in Japan.
Methods
This retrospective cohort study was conducted using a database comprising healthcare claims data from public health insurance enrollees residing in a Japanese city. We analysed a primary cohort comprising patients hospitalised with COVID-19 between March 2020 and July 2021 and two control cohorts comprising patients hospitalised with influenza or other respiratory tract infections (RTI) during the same period. We calculated the incidences of acute (1–3 months after infection) and delayed (4–6 months after infection) psychiatric sequelae. These sequelae were identified using diagnosis codes and categorised as mood/anxiety/psychotic disorder, mood disorder, anxiety disorder, psychotic disorder or insomnia. Multivariable logistic regression models were used to estimate the odds ratios (ORs) of psychiatric sequelae occurrence after COVID-19 infection compared with influenza and other RTI.
Results
The study population with acute psychiatric sequela consisted of 662 patients with COVID-19, 644 patients with influenza, and 7369 patients with RTI who could be followed for 3 months; the study population with delayed psychiatric sequelae consisted of 371 patients with COVID-19, 546 patients with influenza, and 5397 patients with RTI who could be followed for 6 months. In the analysis of acute psychiatric sequelae, COVID-19 had significantly higher odds of mood/anxiety/psychotic disorder (OR: 1.39, p=0.026), psychotic disorder (OR: 2.13, p<0.001), and insomnia (OR: 2.59, p<0.001) than influenza, and significantly higher odds of insomnia (OR: 1.44, p=0.002) and significantly lower odds of anxiety disorder (OR: 0.56, p<0.001) than other RTI. In the analysis of delayed psychiatric sequelae, COVID-19 had significantly higher odds of psychotic disorder (OR: 2.25, p=0.007) than influenza, but significantly lower odds of anxiety disorder (OR: 0.55, p=0.011) than other RTI.
Conclusions
COVID-19 was generally associated with an increased risk of psychiatric sequelae occurring within 3 months after infection, but had a lower risk of new psychiatric sequelae developing 4–6 months after infection.
Keywords: Anxiety, Depression, Psychotic Disorders, Psychiatry, COVID-19
What is already known on this topic
COVID-19 may be associated with psychiatric sequelae, but previous studies have mostly been conducted using interviews and questionnaires that are susceptible to recall bias. Few studies have characterised the incidences and risks of clinically diagnosed psychiatric sequelae at different time points after infection.
What this study adds
Using healthcare claims data containing recorded diagnoses, we quantified the incidences and risks of acute (1–3 months after infection) and delayed (4–6 months after infection) psychiatric sequelae following COVID-19 infection relative to influenza and other respiratory tract infections.
How this study might affect research, practice or policy
Our findings shed light on the incidences and risks of various psychiatric sequelae after COVID-19 infection at different time points, which may support the development of post-acute treatment strategies.
Introduction
Coronavirus disease 2019 (COVID-19) was first reported in Wuhan, China, in December 2019 and quickly escalated from a local outbreak into an unprecedented global pandemic by March 2020.1 In addition to COVID-19’s physiological outcomes, this disease also has psychiatric consequences.2 To increase our understanding of COVID-19’s overall impact and inform the development of long-term treatment strategies,3 there is a need to characterise the psychiatric sequelae that occur at different time points after infection.
Previous studies have indicated that COVID-19 survivors have an elevated risk of developing psychiatric sequelae, such as persistent fatigue,4–10 anxiety,7 9 depression,9 and sleeping disorders.6 9 10 However, many of these studies were conducted using questionnaire-based surveys, face-to-face interviews, and telephone surveys, which are susceptible to recall bias. Furthermore, patients with severe COVID-19 symptoms who require hospitalisation would be unable to participate in such studies during or soon after the acute phase, which increases the risk of selection bias toward asymptomatic and mild cases.
To address these shortcomings, studies have used healthcare data to analyse clinically diagnosed psychiatric outcomes after COVID-19 infection. For example, a US study showed that patients with COVID-19 had a higher risk of mental health disorders when compared with a non-infected contemporary control group and a non-infected historical control group (ie, people who did not experience the pandemic).11 Similarly, a UK study reported that patients with COVID-19 had higher incidences of anxiety and mood disorders than patients with influenza or other respiratory tract infections (RTI).12
It has been proposed that post-acute COVID-19 sequelae should include conditions that persist or develop beyond 3 or 4 weeks after the onset of acute symptoms.13 A systematic review reported that many studies evaluated the incidences of psychiatric sequelae within 3 months after COVID-19 infection, whereas few studies assessed sequelae that developed after that time period.14 In Japan, a study found that patients with COVID-19 still experienced fatigue and other symptoms 4 months after infection,5 but those findings were based on self-reported subjective symptoms through telephone interviews without clinical diagnoses. Therefore, there is a need to generate evidence on the relationship between COVID-19 infection and the occurrence of clinically diagnosed psychiatric sequelae at different time points in Japan.
To contribute to this evidence, this study was conducted to investigate the incidences and risks of acute and delayed psychiatric sequelae in patients hospitalised with COVID-19 in Japan using healthcare claims data.
Methods
Study design and settings
This retrospective cohort study was conducted using data from September 2019 to October 2021. The data were provided by the Longevity Improvement & Fair Evidence (LIFE) Study, which is a research database project managed by Kyushu University (Fukuoka, Japan).15 The LIFE Study collects claims data from enrollees of Japan’s National Health Insurance System and Latter-Stage Older Persons Health Care System residing in participating municipalities. The National Health Insurance System is a public insurance programme for persons aged 0–74 years, and the Latter-Stage Older Persons Health Care System is a public insurance programme for all persons aged ≥75 years and persons aged 65–74 years with specific diseases. The claims data include recorded diagnoses based on the International Classification of Diseases, 10th Revision (ICD-10) codes, drug prescriptions and medical treatments during clinical encounters.
Study participants
The study participants comprised National Health Insurance System enrollees and Latter-Stage Older Persons Health Care System enrollees residing in Kobe City (Hyogo, Japan), which is a LIFE Study participant municipality. To comparatively evaluate the psychiatric sequelae of COVID-19 infection, we constructed a primary cohort and two control cohorts.
The primary cohort consisted of patients who had been hospitalised with a diagnosis of COVID-19 (ICD-10 codes: U071, U072, B34.2) between March 2020 and July 2021. Next, we constructed an influenza control cohort comprising patients who had been hospitalised with a diagnosis of influenza (ICD-10 codes: J09-11) between March 2020 and July 2021, as well as an RTI control cohort comprising patients who had been hospitalised with a diagnosis of any other RTI (J00-06, J12-18, J20-22) between March 2020 and July 2021. The use of patients with influenza and patients with other RTI as the control cohorts is similar to the method employed in a previous study.12 We chose this approach because patients with influenza or other RTI may experience acute symptoms similar to patients with COVID-19 and could also be susceptible to the same post-acute sequelae.
We limited the analysis to patients who did not die during the index hospitalisation. Patients with a diagnosis of influenza or other RTI were excluded from the primary cohort, patients with a diagnosis of COVID-19 or other RTI were excluded from the influenza control cohort and patients with a diagnosis of COVID-19 or influenza were excluded from the RTI control cohort. The patients were followed for 3 months and 6 months after the index infection. Using the claims data, we identified patients hospitalised between 1 March 2020 and 31 July 2021 for the 3-month follow-up (until 31 October 2021), and patients hospitalised between 1 March 2020 and 30 April 2021 for the 6-month follow-up (until 31 October 2021).
Outcome measures
The study outcome was the occurrence of psychiatric sequelae after COVID-19 infection, influenza infection or other RTI.
At present, there is no universally accepted definition for post-acute COVID-19 symptoms. WHO has proposed the following clinical case definition: ‘Post COVID-19 condition occurs in individuals with a history of probable or confirmed SARS-CoV-2 infection, usually 3 months from the onset of COVID-19 with symptoms and that last for at least 2 months and cannot be explained by an alternative diagnosis.’16 Other studies have also reported that a large proportion of patients still experience psychiatric symptoms 6 months after COVID-19 onset.9 14 Based on these reports, we examined the occurrence of psychiatric sequelae 1–3 months after the index infection (designated ‘acute psychiatric sequelae’) and 4–6 months after the index infection (designated ‘delayed psychiatric sequelae’).
The psychiatric sequelae were identified using ICD-10 codes and grouped into five categories: (1) mood/anxiety/psychotic disorder (ICD-10 codes: F20–29, F30–F39, F40–F48), (2) mood disorder (F30–F39), (3) anxiety disorder (F40–F48), (4) psychotic disorder (F20–F29), and (5) insomnia (F51.0 or G47.0). Patients who had received diagnoses of any of these psychiatric disorders 6 months before the index infection were excluded from the analysis. For the analysis of delayed psychiatric sequelae (4–6 months after the index infection), we also excluded patients who received diagnoses of any of the psychiatric disorders in the first 3 months after the index infection.
Statistical analysis
The patient characteristics were described using means with SD for continuous variables and numbers with percentages for categorical variables. To account for variations in patient characteristics, we acquired information on patient sex, age and comorbidities. Age was analysed using three categories (≤64 years, 65–74 years, and ≥75 years). These categories were selected because Japan’s health insurance system categorises persons aged 65–74 years and ≥75 years as ‘early elderly’ and ‘late elderly’, respectively.17 The following comorbidities were identified and included in the analysis: hypertension (ICD-10 codes: I10–I16), diabetes (E10–E14), chronic lower respiratory disease (J40–J47), heart disease (I20–I25, I30–I52), chronic kidney disease (N18, I12), chronic liver disease (K70, K72, K73, K74, K76.0, K76.1, K76.6, K76.8), stroke (I63), dementia (F01, F02, F03, G31.0, G31.83), cancer (C00–D49, C81–C96), and rheumatoid arthritis (M05, M06).
This analysis sought to compare the incidences of psychiatric sequelae after COVID-19 infection with the incidences after influenza infection or other RTI. To produce comparable estimates, we used propensity score matching to control for variations between the primary cohort and control cohorts.18 We first calculated the propensity scores for becoming infected with COVID-19 and influenza using multivariable logistic regression models that adjusted for patient sex, age and comorbidities. Patients with COVID-19 were then matched with patients with influenza using these propensity scores, and the incidences of psychiatric sequelae were compared. The same process was used to match the patients with COVID-19 with the patients with RTI. Matching was performed through 1:1 nearest neighbour matching without replacement using a calliper width of 0.2 SDs of the logit of the propensity score. Absolute standardised differences below 0.10 were regarded as showing good balance between the cohorts.
To evaluate the risk of psychiatric sequelae occurrence after COVID-19 infection, we constructed multivariable logistic regression models for each of the five psychiatric sequelae categories during 1–3 months (acute psychiatric sequelae) and 4–6 months (delayed psychiatric sequelae) after infection. These models included COVID-19 infection as the independent variable of interest, with influenza infection or other RTI as the reference category. The covariates included patient sex, age and comorbidities. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated.
We then conducted a sensitivity analysis to assess the robustness of our results. In Japanese claims data, patients may receive suspected diagnoses of psychiatric disorders. Therefore, such patients may not be undergoing any pharmacological treatment despite having recorded diagnoses. To provide a more stringent identification of the outcomes, we defined the psychiatric sequelae not only through ICD-10 codes but also through corresponding prescriptions of hypnotics/sedatives, anxiolytics, antipsychotics and other central nervous system agents. Furthermore, the inclusion of these prescriptions would enable us to focus on more severe psychiatric disorders that require pharmacological treatment. These drug prescriptions were identified using Japanese drug codes recorded in the claims data. Based on these newly defined outcomes, we recalculated the ORs and 95% CIs of psychiatric sequelae occurrence after COVID-19 infection compared with influenza or other RTI.
All statistical analyses were performed using Stata V.17 (Stata Corp, College Station, Texas, USA). Statistical significance (two sided) was set at 5%.
Results
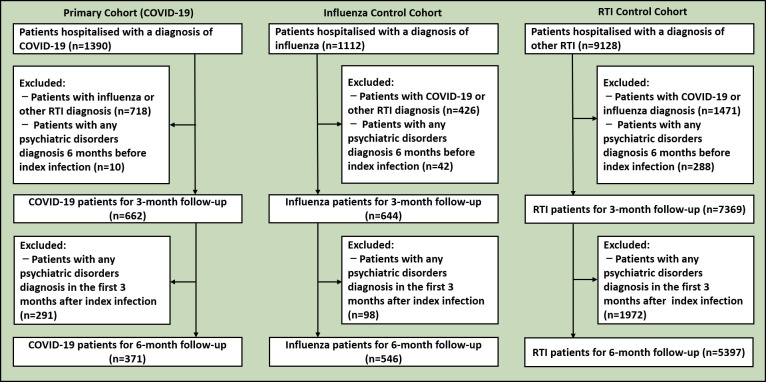
Figure 1 shows the flowchart of patient selection in the primary cohort and control cohorts for the 3-month and 6-month follow-up periods. For the 3-month follow-up, we identified 662 patients who had been hospitalised with COVID-19, 644 patients who had been hospitalised with influenza, and 7369 patients who had been hospitalised with other RTI. For the 6-month follow-up, we identified 371 patients who had been hospitalised with COVID-19, 546 patients who had been hospitalised with influenza, and 5397 patients who had been hospitalised with other RTI. Table 1 summarises the characteristics of the primary and control cohorts according to follow-up period. For the 3-month follow-up, patients with COVID-19 had a mean age (SD) of 72.7 (16.5) years, and women accounted for 54.8% of the cohort. For the 6-month follow-up, patients with COVID-19 had a mean age (SD) of 72.5 (17.1) years, and women accounted for 55.3% of the cohort. The most prevalent comorbidity among the patients with COVID-19 was hypertension for both the 3-month follow-up (55.9%) and 6-month follow-up (58.0%).
Figure 1.
Flowchart of patient selection. RTI, respiratory tract infection.
Table 1.
Patient characteristics
| 3-Month follow-up | 6-Month follow-up | |||||
| Hospitalised with COVID-19 |
Hospitalised with influenza | Hospitalised with other RTI | Hospitalised with COVID-19 |
Hospitalised with influenza | Hospitalised with other RTI | |
| No. of patients | 662 | 644 | 7369 | 371 | 546 | 5397 |
| Sex | ||||||
| Female | 363 (54.8) | 345 (53.6) | 3901 (52.9) | 205 (55.3) | 296 (54.2) | 2874 (53.3) |
| Age | ||||||
| Mean (SD), years | 72.7 (16.5) | 78.5 (14.4) | 79.0 (14.2) | 72.5 (17.1) | 78.8 (13.8) | 78.6 (14.1) |
| ≤64 years | 144 (21.8) | 52 (8.1) | 702 (9.5) | 85 (22.9) | 38 (7.0) | 555 (10.3) |
| 65–74 years | 149 (22.5) | 123 (19.1) | 1189 (16.1) | 75 (20.2) | 108 (19.8) | 906 (16.8) |
| ≥75 years | 369 (55.7) | 469 (72.8) | 5478 (74.3) | 211 (56.9) | 400 (73.3) | 3936 (72.9) |
| Comorbidities | ||||||
| Hypertension | 370 (55.9) | 415 (64.4) | 4805 (65.2) | 215 (58.0) | 357 (65.4) | 3535 (65.5) |
| Diabetes | 306 (46.2) | 299 (46.4) | 3439 (46.7) | 169 (45.6) | 261 (47.8) | 2509 (46.5) |
| Chronic lower respiratory disease | 111 (16.8) | 151 (23.4) | 2256 (30.6) | 65 (17.5) | 133 (24.4) | 1702 (31.5) |
| Heart disease | 227 (34.3) | 325 (50.5) | 3896 (52.9) | 140 (37.7) | 276 (50.5) | 2857 (52.9) |
| Chronic kidney disease | 40 (6.0) | 64 (9.9) | 746 (10.1) | 27 (7.3) | 53 (9.7) | 542 (10.0) |
| Chronic liver disease | 76 (11.5) | 80 (12.4) | 831 (11.3) | 47 (12.7) | 69 (12.6) | 623 (11.5) |
| Stroke | 53 (8.0) | 94 (14.6) | 950 (12.9) | 37 (10.0) | 78 (14.3) | 704 (13.0) |
| Dementia | 89 (13.4) | 106 (16.5) | 1461 (19.8) | 55 (14.8) | 90 (16.5) | 1063 (19.7) |
| Cancer | 113 (17.1) | 194 (30.1) | 1993 (27.0) | 58 (15.6) | 160 (29.3) | 1454 (26.9) |
| Rheumatoid arthritis | 18 (2.7) | 35 (5.4) | 368 (5.0) | 10 (2.7) | 29 (5.3) | 281 (5.2) |
Values are presented as number (%) unless otherwise indicated.
COVID-19, coronavirus disease 2019; RTI, respiratory tract infection.
Table 2 shows the characteristics of the cohorts after propensity score matching. After matching the primary cohort and influenza control cohort for the 3-month follow-up, the patients with COVID-19 and patients with influenza had a mean age (SD) of 78.9 (11.5) years and 77.5 (14.1) years, respectively. Women accounted for 57.5% of patients with COVID-19 and 53.8% of patients with influenza. For the 6-month follow-up, patients with COVID-19 and influenza had a mean age (SD) of 78.5 (11.7) years and 78.1 (12.6) years, respectively. Women accounted for 56.8% of patients with COVID-19 and 55.4% of patients with influenza. After matching the primary cohort and RTI control cohort for the 3-month follow-up, the patients with COVID-19 and other RTI had a mean age (SD) of 74.7 (14.7) years and 74.9 (16.1) years, respectively. Women accounted for 54.4% of patients with COVID-19 and 55.3% of patients with other RTI. For the 6-month follow-up, the patients with COVID-19 and other RTI had a mean age (SD) of 74.4 (15.9) years and 73.7 (17.2) years, respectively. Women accounted for 55.0% of patients with COVID-19 and 54.7% of patients with other RTI. All absolute standardised differences were below 0.1, with the exception of age in the matched primary cohort and influenza control cohort for the 3-month follow-up.
Table 2.
Patient characteristics after propensity score matching
| After infection in the matched primary cohort and influenza control cohort | After infection in the matched primary cohort and RTI control cohort | |||||||||||
| 3-Month follow-up | 6-Month follow-up | 3-Month follow-up | 6-Month follow-up | |||||||||
| Hospitalised with COVID-19 | Hospitalised with influenza | Standardised differences | Hospitalised with COVID-19 | Hospitalised with influenza | Standardised differences | Hospitalised with COVID-19 | Hospitalised with other RTI | Standardised differences | Hospitalised with COVID-19 | Hospitalised with other RTI | Standardised differences | |
| No. of patients | 457 | 457 | 285 | 285 | 597 | 597 | 333 | 333 | ||||
| Sex | ||||||||||||
| Female | 263 (57.5) | 246 (53.8) | 0.075 | 162 (56.8) | 158 (55.4) | 0.028 | 325 (54.4) | 330 (55.3) | −0.017 | 183 (55.0) | 182 (54.7) | 0.006 |
| Age | ||||||||||||
| Mean (SD), years | 78.9 (11.5) | 77.5 (14.1) | 0.107 | 78.5 (11.7) | 78.1 (12.6) | 0.028 | 74.7 (14.7) | 74.9 (16.1) | −0.016 | 74.4 (15.9) | 73.7 (17.2) | 0.040 |
| Comorbidities | ||||||||||||
| Hypertension | 300 (65.6) | 294 (64.3) | 0.027 | 192 (67.4) | 183 (64.2) | 0.066 | 370 (62.0) | 392 (65.7) | −0.077 | 215 (64.6) | 206 (61.9) | 0.056 |
| Diabetes | 216 (47.3) | 228 (49.9) | −0.052 | 147 (51.6) | 148 (51.9) | −0.007 | 305 (51.1) | 297 (49.7) | 0.027 | 169 (50.8) | 163 (48.9) | 0.036 |
| Chronic lower respiratory disease | 99 (21.7) | 97 (21.2) | 0.011 | 59 (20.7) | 53 (18.6) | 0.053 | 111 (18.6) | 109 (18.3) | 0.009 | 65 (19.5) | 66 (19.8) | −0.008 |
| Heart disease | 222 (48.6) | 209 (45.7) | 0.057 | 138 (48.4) | 133 (46.7) | 0.035 | 227 (38.0) | 229 (38.4) | −0.007 | 140 (42.0) | 129 (38.7) | 0.067 |
| Chronic kidney disease | 39 (8.5) | 39 (8.5) | −0.000 | 26 (9.1) | 28 (9.8) | −0.024 | 40 (6.7) | 38 (6.4) | 0.014 | 27 (8.1) | 27 (8.1) | −0.000 |
| Chronic liver disease | 59 (12.9) | 51 (11.2) | 0.054 | 36 (12.6) | 33 (11.6) | 0.032 | 76 (12.7) | 56 (9.4) | 0.107 | 47 (14.1) | 39 (11.7) | 0.072 |
| Stroke | 52 (11.4) | 59 (12.9) | −0.047 | 37 (13.0) | 39 (13.7) | −0.021 | 53 (8.9) | 47 (7.9) | 0.036 | 37 (11.1) | 38 (11.4) | −0.009 |
| Dementia | 82 (17.9) | 75 (16.4) | 0.041 | 52 (18.2) | 54 (18.9) | −0.018 | 89 (14.9) | 99 (16.6) | −0.046 | 55 (16.5) | 59 (17.7) | −0.032 |
| Cancer | 111 (24.3) | 111 (24.3) | −0.000 | 57 (20.0) | 54 (18.9) | 0.027 | 113 (18.9) | 109 (18.3) | 0.017 | 58 (17.4) | 62 (18.6) | −0.031 |
| Rheumatoid arthritis | 17 (3.7) | 19 (4.2) | −0.022 | 9 (3.2) | 10 (3.5) | −0.020 | 18 (3.0) | 10 (1.7) | 0.089 | 10 (3.0) | 9 (2.7) | 0.018 |
Values are presented as number (%) unless otherwise indicated.
COVID-19, coronavirus disease 2019; RTI, respiratory tract infection.
The incidences of acute and delayed psychiatric sequelae after propensity score matching are presented in table 3. Among the psychiatric sequelae that occurred 1–3 months after infection in the matched primary cohort and influenza control cohort, insomnia had the highest incidence in both patients with COVID-19 (61.7%) and patients with influenza (38.5%). Similarly, insomnia also had the highest incidence in both patients with COVID-19 (46.3%) and patients with influenza (37.9%) 4–6 months after infection. Among the psychiatric sequelae that occurred 1–3 months after infection in the matched primary cohort and RTI control cohort, insomnia had the highest incidence in both patients with COVID-19 (61.1%) and patients with other RTI (52.8%). Similarly, insomnia also had the highest incidence in both patients with COVID-19 (44.4%) and patients with other RTI (48.9%) 4–6 months after infection.
Table 3.
Incidences of psychiatric sequelae after propensity score matching
| After infection in the matched primary cohort and influenza control cohort | After infection in the matched primary cohort and RTI control cohort | |||||||
| Acute: 1–3 months after infection | Delayed: 4–6 months after infection | Acute: 1–3 months after infection | Delayed: 4–6 months after infection | |||||
| Hospitalised with COVID-19 (n=457) |
Hospitalised with influenza (n=457) | Hospitalised with COVID-19 (n=285) |
Hospitalised with influenza (n=285) | Hospitalised with COVID-19 (n=597) |
Hospitalised with other RTI (n=597) | Hospitalised with COVID-19 (n=333) |
Hospitalised with other RTI (n=333) | |
| Mood/anxiety/psychotic disorder | 167 (36.5) | 130 (28.4) | 89 (31.2) | 74 (26.0) | 207 (34.7) | 247 (41.4) | 104 (31.2) | 123 (36.9) |
| Mood disorder | 92 (20.1) | 68 (14.9) | 47 (16.5) | 39 (13.7) | 114 (19.1) | 136 (22.8) | 53 (15.9) | 66 (19.8) |
| Anxiety disorder | 58 (12.7) | 55 (12.0) | 29 (10.2) | 32 (11.2) | 75 (12.6) | 123 (20.6) | 34 (10.2) | 55 (16.5) |
| Psychotic disorder | 87 (19.0) | 44 (9.6) | 42 (14.7) | 21 (7.4) | 103 (17.3) | 121 (20.3) | 47 (14.1) | 54 (16.2) |
| Insomnia | 282 (61.7) | 176 (38.5) | 132 (46.3) | 108 (37.9) | 365 (61.1) | 315 (52.8) | 148 (44.4) | 163 (48.9) |
Values are presented as number (%).
COVID-19, coronavirus disease 2019; RTI, respiratory tract infection.
Table 4 presents the results of the multivariable logistic regression analyses of acute and delayed psychiatric sequelae in the matched cohorts (ie, patients with COVID-19 matched with patients with influenza and patients with COVID-19 matched with patients with other RTI). For acute psychiatric sequelae occurring 1–3 months after infection, COVID-19 had significantly higher odds of mood/anxiety/psychotic disorder (OR: 1.39, 95% CI: 1.04 to 1.85, p=0.026), psychotic disorder (OR: 2.13, 95% CI: 1.42 to 3.18, p<0.001) and insomnia (OR: 2.59, 95% CI: 1.97 to 3.41, p<0.001) when compared with influenza. In contrast, COVID-19 had significantly higher odds of insomnia (OR: 1.44, 95% CI: 1.14 to 1.82, p=0.002) and significantly lower odds of anxiety disorder (OR: 0.56, 95% CI: 0.40 to 0.76, p<0.001) when compared with other RTI. For delayed psychiatric sequelae occurring 4–6 months after infection, COVID-19 had significantly higher odds of psychotic disorder (OR: 2.25, 95% CI: 1.25 to 4.04, p=0.007) when compared with influenza. In contrast, COVID-19 had significantly lower odds of anxiety disorder (OR: 0.55, 95% CI:0.34 to 0.87, p=0.011) when compared with other RTI.
Table 4.
Results of the multivariable logistic regression analyses of psychiatric sequelae after COVID-19 infection
| Acute: 1–3 months after COVID-19 infection | Delayed: 4–6 months after COVID-19 infection | |||||||
| COVID-19 vs influenza | COVID-19 vs other RTI | COVID-19 vs influenza | COVID-19 vs other RTI | |||||
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | |
| Mood/anxiety/psychotic disorder | 1.39 (1.04 to 1.85) | 0.026 | 0.76 (0.59 to 0.96) | 0.023 | 1.30 (0.88 to 1.91) | 0.183 | 0.75 (0.54 to 1.05) | 0.097 |
| Mood disorder | 1.37 (0.96 to 1.94) | 0.083 | 0.80 (0.60 to 1.06) | 0.116 | 1.24 (0.77 to 1.98) | 0.380 | 0.76 (0.51 to 1.14) | 0.190 |
| Anxiety disorder | 1.02 (0.68 to 1.53) | 0.919 | 0.56 (0.40 to 0.76) | <0.001 | 0.86 (0.50 to 1.48) | 0.583 | 0.55 (0.34 to 0.87) | 0.011 |
| Psychotic disorder | 2.13 (1.42 to 3.18) | <0.001 | 0.83 (0.62 to 1.12) | 0.231 | 2.25 (1.25 to 4.04) | 0.007 | 0.84 (0.54 to 1.31) | 0.437 |
| Insomnia | 2.59 (1.97 to 3.41) | <0.001 | 1.44 (1.14 to 1.82) | 0.002 | 1.40 (1.00 to 1.96) | 0.052 | 0.81 (0.59 to 1.11) | 0.187 |
COVID-19, coronavirus disease 2019; RTI, respiratory tract infection.
We also conducted a sensitivity analysis in which the psychiatric sequelae were defined using both ICD-10 codes and drug prescriptions. The results of this sensitivity analysis are shown in table 5. For acute psychiatric sequelae occurring 1–3 months after infection, COVID-19 had significantly higher odds of mood/anxiety/psychotic disorder (OR: 1.38, 95% CI: 1.02 to 1.87, p=0.038), psychotic disorder (OR: 1.79, 95% CI: 1.19 to 2.70, p=0.005) and insomnia (OR: 2.88, 95% CI: 2.18 to 3.81, p<0.001) when compared with influenza. Furthermore, COVID-19 had significantly higher odds of insomnia (OR: 1.80, 95% CI: 1.42 to 2.27, p<0.001) when compared with other RTI. For delayed psychiatric sequelae occurring 4–6 months after infection, COVID-19 had higher odds for all psychotic sequelae (except mood disorder) when compared with influenza, but these relationships were not statistically significant (mood/anxiety/psychotic disorder: OR: 1.09, 95% CI: 0.70 to 1.70, p=0.688; anxiety disorder: OR: 1.15, 95% CI: 0.54 to 2.43, p=0.715; psychotic disorder: OR: 1.27, 95% CI: 0.69 to 2.35, p=0.436; insomnia: OR: 1.22, 95% CI: 0.82 to 1.80, p=0.322). Finally, COVID-19 had significantly lower odds of anxiety disorder (OR: 0.56, 95% CI: 0.30 to 1.03, p=0.011), psychotic disorder (OR: 0.56, 95% CI: 0.34 to 0.94, p=0.027), and insomnia (OR: 0.69, 95% CI: 0.49 to 0.98, p=0.036) occurring 4–6 months after infection when compared with other RTI.
Table 5.
Sensitivity analysis: results of the multivariable logistic regression analyses of psychiatric sequelae after COVID-19 infection
| Acute: 1–3 months after COVID-19 infection | Delayed: 4–6 months after COVID-19 infection | |||||||
| COVID-19 vs influenza | COVID-19 vs other RTI | COVID-19 vs influenza | COVID-19 vs other RTI | |||||
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | |
| Mood/anxiety/psychotic disorder | 1.38 (1.02 to 1.87) | 0.038 | 0.77 (0.60 to 0.99) | 0.045 | 1.09 (0.70 to 1.70) | 0.688 | 0.62 (0.42 to 0.91) | 0.097 |
| Mood disorder | 1.55 (1.05 to 2.29) | 0.026 | 0.97 (0.71 to 1.32) | 0.823 | 0.76 (0.42 to 1.38) | 0.372 | 0.50 (0.29 to 0.85) | 0.190 |
| Anxiety disorder | 1.04 (0.62 to 1.74) | 0.885 | 0.51 (0.35 to 0.76) | 0.001 | 1.15 (0.54 to 2.43) | 0.715 | 0.56 (0.30 to 1.03) | 0.011 |
| Psychotic disorder | 1.79 (1.19 to 2.70) | 0.005 | 0.77 (0.56 to 1.05) | 0.097 | 1.27 (0.69 to 2.35) | 0.436 | 0.56 (0.34 to 0.94) | 0.027 |
| Insomnia | 2.88 (2.18 to 3.81) | <0.001 | 1.80 (1.42 to 2.27) | <0.001 | 1.22 (0.82 to 1.80) | 0.322 | 0.69 (0.49 to 0.98) | 0.036 |
In this sensitivity analysis, psychiatric sequelae were defined using both recorded diagnoses and drug prescriptions.
COVID-19, coronavirus disease 2019; RTI, respiratory tract infection.
Discussion
Main findings
In this study, we evaluated the incidences and risks of acute and delayed psychiatric sequelae among patients hospitalised with COVID-19 in a major Japanese city. By using healthcare claims data, we were able to identify clinically diagnosed infections and psychiatric disorders in the study patients. Furthermore, the data allowed us to follow the patients for a relatively long period of time with fewer dropouts than a previous Japanese study that used telephone survey data.5 Our analysis indicated that patients with COVID-19 were more likely to develop mood/anxiety/psychotic disorder, psychotic disorder and insomnia within 3 months than patients with influenza. Next, patients with COVID-19 were more likely to develop insomnia and less likely to develop anxiety disorder within 3 months than patients with other RTI. In contrast, patients with COVID-19 were more likely to develop psychotic disorder 4–6 months after infection than patients with influenza but less likely to develop anxiety disorder 4–6 months after infection than patients with other RTI. To our knowledge, this is the first study to characterise the differences in new and delayed psychiatric sequelae among COVID-19 survivors.
We observed that patients with COVID-19 had a higher risk of developing psychiatric sequelae within 3 months than patients with other infections, which is consistent with the results of a previous study.12 Among our patients, insomnia had the highest incidence within 3 months after COVID-19 infection. The incidence of insomnia was also higher in patients with COVID-19 than in patients with influenza, although this difference was not statistically significant. A meta-analysis reported that sleep disturbances (including insomnia) occurred in approximately 34% of patients with COVID-19.19 In addition, other studies have indicated that patients with COVID-19 experience higher rates of insomnia than those without COVID-19.11 20 The higher risk of insomnia after COVID-19 infection could be influenced by the onset and persistence of physical symptoms. The common sequelae of COVID-19 include fatigue,4–10 dyspnoea4–6 8 10 and cough.4–8 Moreover, patients with COVID-19 can experience decreased respiratory function even after hospital discharge.9 21 The persistence of such symptoms during sleep may contribute to the development of insomnia and other sleep disorders.
This study also found that patients with COVID-19 had a generally lower risk of anxiety disorder 1–3 months and 4–6 months after infection when compared with patients with other RTI. These findings differ from those of previous studies.11 12 It has been reported that the majority of patients who exhibited anxiety and depressive symptoms 3–6 months after COVID-19 onset had already experienced these symptoms during the first 3 months.22 It is possible that people were generally experiencing more anxiety during the COVID-19 pandemic22 23 and may not have complained of additional anxiety after COVID-19 infection. Patients with COVID-19 also had lower incidences of the other psychiatric sequelae (except for anxiety disorder) than patients with other RTI, although these differences were not significant. Other RTI may be associated with a higher incidence of psychiatric disorders over time. Our findings suggest that COVID-19 has a long-term disease progression that is stronger than influenza and weaker than other RTI.
The sensitivity analysis, which identified psychiatric sequelae using drug prescriptions in addition to diagnosis codes, produced similar results to the base case analysis. Patients with COVID-19 who survived the first 30 days after diagnosis have shown an increased use of sleep medications,11 which indicates a higher incidence of insomnia. Also, the use of antidepressants may suggest a higher incidence of mood/anxiety/psychotic disorder.24 Patients with COVID-19 may require pharmacological treatment for psychiatric sequelae that develop in the first 1–3 months after infection.
Limitations
Our findings should be interpreted with consideration of several limitations. First, the study was conducted using data from a single Japanese city, and our results may lack generalisability. Kobe city has a population of 1.5 million, which makes it the seventh most populous city in Japan. Nevertheless, further studies that cover more regions are needed to verify or refute our findings. Second, our analysis used healthcare claims data, which do not include clinical data such as disease course or laboratory test results. For example, we did not have access to the results of pulmonary function tests and therefore could not ascertain disease severity. Studies have reported that long COVID-19 symptoms are more likely to occur in patients who experienced more severe COVID-19.9 12 25 In Japan, patients with COVID-19 with mild disease are usually treated at home or in residential care facilities without medical care, and only those with severe symptoms are hospitalised. As our study focused on hospitalised patients, our study population may contain more severe COVID-19 cases with a higher susceptibility to long COVID-19 symptoms. In addition, we did not include a healthy control group sampled from the general population. Therefore, our findings should be interpreted with caution. Third, our database did not include information on family background and socioeconomic factors, which could influence the development of psychiatric sequelae. For example, insomnia is reportedly associated with income level and employment status.26 Furthermore, we could not examine any changes in these factors during the COVID-19 pandemic. Finally, we were unable to determine the severity of the psychiatric disorders. Therefore, we could not consider any differences in this aspect between the acute and delayed psychiatric sequelae.
Implications
This is the first study to show that patients with COVID-19 in Japan have a higher risk of developing various psychiatric sequelae (especially insomnia) soon after infection, but are less likely to develop such disorders in the post-acute phase. Our findings suggest that it is important to monitor the development of psychiatric disorders soon after COVID-19 infection.
Conclusion
In this study of hospitalised patients in Japan, COVID-19 infection was found to be generally associated with an increased risk of mood/anxiety/psychotic disorder, psychotic disorder and insomnia within 3 months when compared with influenza, as well as an increased risk of insomnia and reduced risk of anxiety disorder within 3 months when compared with other RTI. However, there was a generally low risk of developing new psychiatric sequelae 4–6 months after COVID-19 infection. Healthcare professionals should be especially vigilant for the onset of psychiatric sequelae during the first 3 months after COVID-19 onset. Further research is needed to explore the development of psychiatric sequelae at different time points after hospitalisation for COVID-19.
Acknowledgments
We gratefully acknowledge and thank AMED, JST FOREST Program, and JSPS KAKENHI Grant-in-Aid for Scientific Research for financial support for this study. We also thank Kobe city for providing the data in this study.
Biography
Fumiko Murata obtained a master's degree of Public Health, and doctorate in Medical Science from Kyushu University, Japan. Since April 2021, she has been working as an assistant professor at the Department of Health Care Administration and Management, Kyushu University Graduate School of Medical Sciences in Japan. Prior to this, she served as a public health nurse at Kasuga City Hall. Her main research interests include mental health and adverse events of vaccination.

Footnotes
Contributors: FM, MM, CI and HF contributed to the study’s conception and design. FM carried out the analysis of the data and drafted the manuscript. All authors were involved in the interpretation of the results, as well as in the editing and revision of the manuscript. HF was responsible for the overall content as the guarantor.
Funding: This work was supported by AMED under grant number JP21nf0101635 and grants from the JST FOREST Program (grant no. JPMJFR205J) and JSPS KAKENHI (grant no. JP20H00563 and no. JP19K21590).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data may be obtained from a third party and are not publicly available. Data cannot be shared for privacy or ethical reasons.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
The study was approved by the Kyushu University Institutional Review Board for Clinical Research (Approval No. 2021-424).
References
- 1.Cucinotta D, Vanelli M, Maurizio V. WHO declares COVID-19 a pandemic. Acta Biomed 2020;91:157–60. 10.23750/abm.v91i1.9397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rogers JP, Chesney E, Oliver D, et al. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry 2020;7:611–27. 10.1016/S2215-0366(20)30203-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vindegaard N, Benros ME. COVID-19 pandemic and mental health consequences: systematic review of the current evidence. Brain Behav Immun 2020;89:531–42. 10.1016/j.bbi.2020.05.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carfì A, Bernabei R, Landi F, et al. Persistent symptoms in patients after acute COVID-19. JAMA 2020;324:603. 10.1001/jama.2020.12603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miyazato Y, Morioka S, Tsuzuki S, et al. Prolonged and late-onset symptoms of coronavirus disease 2019. Open Forum Infect Dis 2020;7:ofaa507. 10.1093/ofid/ofaa507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arnold DT, Hamilton FW, Milne A, et al. Patient outcomes after hospitalisation with COVID-19 and implications for follow-up: results from a prospective UK cohort. Thorax 2021;76:399–401. 10.1136/thoraxjnl-2020-216086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xiong Q, Xu M, Li J, et al. Clinical sequelae of COVID-19 survivors in Wuhan, China: a single-centre longitudinal study. Clin Microbiol Infect 2021;27:89–95. 10.1016/j.cmi.2020.09.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Logue JK, Franko NM, McCulloch DJ, et al. Sequelae in adults at 6 months after COVID-19 infection. JAMA Netw Open 2021;4:e210830. 10.1001/jamanetworkopen.2021.0830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang C, Huang L, Wang Y, et al. 6-Month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet 2021;397:220–32. 10.1016/S0140-6736(20)32656-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sigfrid L, Drake TM, Pauley E, et al. Long Covid in adults discharged from UK hospitals after Covid-19: a prospective, multicentre cohort study using the ISARIC WHO Clinical Characterisation Protocol. Lancet Reg Health Eur 2021;8:100186. 10.1016/j.lanepe.2021.100186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xie Y, Xu E, Al-Aly Z. Risks of mental health outcomes in people with covid-19: cohort study. BMJ 2022;376:e068993. 10.1136/bmj-2021-068993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taquet M, Geddes JR, Husain M, et al. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: a retrospective cohort study using electronic health records. Lancet Psychiatry 2021;8:416–27. 10.1016/S2215-0366(21)00084-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nalbandian A, Sehgal K, Gupta A, et al. Post-acute COVID-19 syndrome. Nat Med 2021;27:601–15. 10.1038/s41591-021-01283-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sanchez-Ramirez DC, Normand K, Zhaoyun Y, et al. Long-term impact of COVID-19: a systematic review of the literature and meta-analysis. Biomedicines 2021;9:900. 10.3390/biomedicines9080900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fukuda H, Ishiguro C, Ono R. The Longevity Improvement & Fair Evidence (LIFE) study: overview of the study design and baseline participant profile. J Epidemiol 2022. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soriano JB, Murthy S, Marshall JC, et al. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect Dis 2022;22:e102–7. 10.1016/S1473-3099(21)00703-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Orimo H, Ito H, Suzuki T, et al. Reviewing the definition of "elderly". Geriatr Gerontol Int 2006;6:149–58. 10.1111/j.1447-0594.2006.00341.x [DOI] [Google Scholar]
- 18.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res 2011;46:399–424. 10.1080/00273171.2011.568786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deng J, Zhou F, Hou W, et al. The prevalence of depression, anxiety, and sleep disturbances in COVID‐19 patients: a meta‐analysis. Ann N Y Acad Sci 2021;1486:90–111. 10.1111/nyas.14506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Orrù G, Bertelloni D, Diolaiuti F, et al. Long-COVID syndrome? A study on the persistence of neurological, psychological and physiological symptoms. Healthcare 2021;9:575. 10.3390/healthcare9050575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bellan M, Soddu D, Balbo PE, et al. Respiratory and psychophysical sequelae among patients with COVID-19 four months after hospital discharge. JAMA Netw Open 2021;4:e2036142. 10.1001/jamanetworkopen.2020.36142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taquet M, Dercon Q, Luciano S, et al. Incidence, co-occurrence, and evolution of long-COVID features: a 6-month retrospective cohort study of 273,618 survivors of COVID-19. PLoS Med 2021;18:e1003773. 10.1371/journal.pmed.1003773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.GBD 2017 Disease and Injury Incidence and Prevalence Collaborators . Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet 2018;392:1789–858. 10.1016/S0140-6736(18)32279-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Al-Aly Z, Xie Y, Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature 2021;594:259–64. 10.1038/s41586-021-03553-9 [DOI] [PubMed] [Google Scholar]
- 25.Craparo G, La Rosa VL, Marino G, et al. Risk of post-traumatic stress symptoms in hospitalized and non-hospitalized COVID-19 recovered patients. A cross-sectional study. Psychiatry Res 2022;308:114353. 10.1016/j.psychres.2021.114353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lallukka T, Sares-Jäske L, Kronholm E, et al. Sociodemographic and socioeconomic differences in sleep duration and insomnia-related symptoms in Finnish adults. BMC Public Health 2012;12:565. 10.1186/1471-2458-12-565 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data may be obtained from a third party and are not publicly available. Data cannot be shared for privacy or ethical reasons.