Abstract
Objectives
To appraise the existing literature reporting an association between retinal markers and cognitive impairment in adults aged 65 years and over and to provide directions for future use of retinal scanning as a potential tool for dementia diagnosis.
Design
Systematic review of peer-reviewed empirical articles investigating the association of retinal markers in assessing cognitive impairment.
Data sources
Three electronic databases, Medline, PsycINFO and EMBASE were searched from inception until March 2022.
Eligibility criteria
All empirical articles in English investigating the association between retinal markers and cognition in humans aged ≥65 years using various retinal scanning methodologies were included. Studies with no explicit evaluation of retinal scanning and cognitive outcomes were excluded. Risk of bias was assessed using the Quality Assessment of Diagnostic Accuracy Studies tool.
Data extraction and synthesis
Data extraction was conducted by two authors (VJ, RS) and reviewed by another author (JS). Results were synthesised and described narratively.
Results
Sixty-seven eligible studies examining 6815 older adults were included. Majority of studies were cross-sectional (n=60; 89.6%). Optical coherence tomography (OCT) was the most commonly used retinal scanning methodology to measure the thickness of retinal nerve fibre layer, the ganglion cell complex, choroid and macula. 51.1% of cross-sectional studies using OCT reported an association between the thinning of at least one retinal parameter and poor cognition. Longitudinal studies (n=6) using OCT also mostly identified significant reductions in retinal nerve fibre layer thickness with cognitive decline. Study quality was overall moderate.
Conclusion
Retinal nerve fibre layer thickness is linked with cognitive performance and therefore may have the potential to detect cognitive impairment in older adults. Further longitudinal studies are required to validate our synthesis and understand underlying mechanisms before recommending implementation of OCT as a dementia screening tool in clinical practice.
PROSPERO registration number
CRD42020176757.
Keywords: ophthalmology, medical ophthalmology, neuro-ophthalmology, dementia, haematology
Strengths and limitations of this study.
This systematic review provides an in-depth evaluation of the relationship between retinal markers identified using various scanning methods and early detection of cognitive impairment in older adults to inform future research and clinical practice.
This review includes a substantially larger number of empirical articles than previous systematic reviews, as well as the inclusion of six longitudinal studies to establish cause-and-effect relationships between retinal scanning and cognitive performance.
The included studies were methodologically rated using appropriate tools.
Majority of the included studies were cross-sectional and have used different retinal imaging devices, therefore it is not possible to compare measurements across devices.
Introduction
The last decade has seen a substantial increase in research focused on the identification, development and validation of diagnostic and prognostic retinal biomarkers for dementia, particularly Alzheimer’s disease (AD).1 AD is the most common form of dementia and affects 60%–70% dementia cases. With 1 in 10 Australians aged over 65 with dementia and 50 million people affected worldwide,2 cognitive impairment is a prevalent issue in our ageing population. The worldwide cost of dementia is estimated to be US$818 billion in 2015,2 and therefore, early detection of AD that could reflect the deposition of amyloid-beta (Aβ, a pathological hallmark feature found in AD brain) in the brain and the resulting cognitive impairment will be of high economic benefit. It is now evident that deposition of Aβ in the brain occurs 15–20 years earlier than the onset of cognitive decline.3 Early diagnosis could help develop preventive or delaying strategies, lower mortality rates, allow timely access to medication, improve quality of life, stabilise cognitive decline and minimise preventable hospital visits.4 However, to date, there is no cost-effective, clinically established early AD diagnostic marker.
Retinal biomarkers may be advantageous because they are cost and time efficient, can be assessed non‐invasively, and present a minimal degree of patient risk and a high degree of accessibility.5As the retina forms as an outgrowth of the brain during embryological development, retinal cells reflects that of the brain and spinal cord.6 Therefore, retinal changes may exhibit brain changes and allow detection of dementia before symptoms manifest, unlike traditional neuropsychological screening tests which primarily detect cognitive impairment following presentation of warning signs, such as memory loss.7 Apart from the effects of normal ageing, marked interindividual differences in the rate of cognitive decline indicate that other age-associated pathologies may be involved, such as macrovascular or microvascular disease.
Several pathobiological markers have been suggested as potential predictors of cognitive dysfunction and of these, retinal microvascular signs may offer the most promise. A study by Ong et al found an association between retinal neuronal damage and grey matter atrophy, which indicates that retinal changes may reflect cerebral neurodegenerative changes and thus, predict cognitive decline.8 Nester et al demonstrated that cerebral ventricular enlargement due to cerebral atrophy seen characteristically in AD as indicated by MRI studies,9 is mirrored in retinal microvasculature changes as measured through retinal scanning tools, such as optical coherence tomography (OCT). OCT is a non-invasive technique that acquires high-resolution, cross-sectional images of the retina and is the most common tool used clinically to assess neurodegenerative changes in the retina.5 The OCT devices often vary, with some users adopting swept-source OCT (SS-OCT) devices while others used spectral-domain OCT, which can impact light source, acquisition speed and resolution.10 Therefore, as a common tool in clinical practice, retinal OCT scanning could be used routinely as an accessible alternative to brain imaging that is both faster to administer and less stressful to the patient with the potential to measure and quantify cognitive decline.
A recent cross-sectional observation study has demonstrated the value of OCT in detecting dementia, identifying OCT measurements of the macula as an ‘useful diagnostic biomarker of cognitive function’11 (pg. 117). However, there has been conflicting evidence on the effectiveness of ophthalmic scanning in mild cognitive impairment (MCI), the precursor of dementia. A significant correlation between OCT measurements in the inner retinal layers with cognitive screening assessments12 has been reported, although Ito et al saw no changes on OCT in MCI individuals, recommending further research.11 13
Recent systematic reviews have attempted to analyse the association between cognitive functioning and retinal nerve fibre layer thickness (RNFL).12 14 Thomson et al conducted a systematic review and meta-analysis of 17 articles and found a statistically significant reduction in RNFL in both AD and MCI patients when compared with healthy controls.12 This study identified OCT as a potential diagnostic tool in assessing cognitive impairment, particularly for AD and MCI syndromes. However, the study did not consider the direct comparisons of RNFL thickness to that of cognitive domains assessed using neuropsychological assessments and which the respective studies included in the review would have used to make a diagnosis of AD and MCI. Similarly, in another meta-analysis study, Wang et al evaluated the relationship of peripheral RNFL thickness in AD and MCI from 19 studies and found a progressive reduction in total RNFL thickness, particularly in the inferior and superior quadrants, suggesting RNFL thickness as a candidate biomarker for early detection of AD.14 However, both reviews conducted in 2015 appraised only a small number of cross-sectional studies with no consideration of cognitive impairment in forms other than AD and MCI. The role of the retinal layers other than the nerve fibre layer such as the ganglion cell complex (GCC) thickness and macular thickness as biomarkers in the assessment of cognitive impairment were also not evaluated.
More recent systematic reviews and meta-analysis studies have reported similar findings as per the aforementioned 2015 reviews. The study by Chan et al15 identified 30 cross-sectional studies to report that the thickness of ganglion cell and inner plexiform layer (GC-IPL), GCC, macular volume was significantly different between AD and the control group. AD group also showed reduced peripapillary RNFL (pRNFL) thickness and choroidal thickness.15 In another systematic review and meta-analysis study by Mejia-Vergara et al,16 15 studies that included MCI individuals only were included to report that pRNFL and macular GCL-IPL thinning with reduced macular volume was prominent in MCI when compared with the controls. A large effect size was observed for reduced macular thickness in MCI individuals with significant heterogeneity for macular thickness. The study concluded that more standardised and longitudinal studies were needed to support the role of OCT in identifying reduced retinal layer and/or macular thickness as a biomarker for MCI due to AD.16
The study by Ge et al17 was broader in scope as the authors included retinal markers per se and not just the RNFL thickness assessed using OCT. The study aimed to identify signature retinal markers in AD, MCI and preclinical AD population. Of the 126 studies included in this systematic review and meta-analysis, the authors reported reduced pRNFL, subfoveal choroid and total macular thickness in the AD and MCI groups when compared with the control group. Overall, the study concluded that structural retinal changes such as RNFL, choroidal thinning; optic nerve degeneration and possibly Aβ deposition; vascular retinal changes such as blood flow, vessel density and morphology and electrophysiological changes showing dysfunction of the retinal layers could be helpful markers in the diagnosis, prognosis and/or risk assessment for AD, MCI and/or preclinical AD population.17 While the study findings are broad and inconclusive, it gives an indication of studies that have explored retinal markers other than the RNFL and reported an association in AD, MCI and/or preclinical AD population.
Despite the aforementioned review studies, the evidence is limited due to the small sample sizes and comparison of retinal markers directly to AD and/or MCI diagnosis, making the findings inconclusive as it under-represents the target population and does not reflect the associated cognitive domains. Another limitation is the extensive exclusion criteria and high comorbidity rate in the older adult population with the prevalence of concomitant eye and systemic disease such as glaucoma and diabetes respectively making them unsuitable candidates. Nevertheless, retinal scanning may be valuable in monitoring disease progression and response to treatment.
To date, no systematic review and/or meta-analysis study has analysed the specific relationship between retinal markers and cognitive screening tests that assess the functions of respective cognitive domains. This systematic review aims to summarise the available evidence on the use of retinal markers using various retinal scanning methodologies in older adults as an alternative to comprehensive cognitive assessments used in dementia diagnosis and provide directions for future research and clinical practice.
Methods
We drafted a protocol for this review ‘a priori’ and inclusion criteria were developed prior to commencing the search. We report according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA 2020) guidelines, and a checklist of PRISMA items is presented in online supplemental data S1.
bmjopen-2021-054657supp001.pdf (395.2KB, pdf)
Search strategy
A search strategy was developed using medical subject headings and key search terms related to cognitive impairment and retinal scanning. Studies were identified through Medline (1806–2022), PsycINFO (1905–2022) and EMBASE (1974–2022) databases. An updated literature search was undertaken on 17 March 2022 prior to the final analysis to ensure up-to-date and relevant articles were included. The search strategy (available in online supplemental data S2) was deliberately broad in an effort to gather all eligible studies and was developed in collaboration with the clinical librarian and reviewed by the project team. Reference lists of all included studies were handsearched for additional records. This search strategy was then adapted to the other databases.
bmjopen-2021-054657supp002.pdf (218.3KB, pdf)
Eligibility criteria
All peer-reviewed empirical articles in English and using human subjects, including but not limited to cross-sectional, population-based, case–control and longitudinal studies. Studies with no explicit evaluation of cognition and retinal scanning outcomes were excluded.
Participants
Inclusion criteria comprised adults aged 65 years and over with diagnosed cognitive impairment of any form and severity, including AD and MCI, and a control group of cognitively healthy participants. The study was limited to subjects aged over 65 as diagnosis of dementia is more prevalent in this age group. Exclusion criteria includes those with pre-existing ophthalmological, metabolic, cardiovascular, cerebrovascular, psychiatric or other disease that could affect the visual field or neurological system. Other exclusion criteria include previous intraocular surgery or trauma, the inability of the participant to collaborate sufficiently to perform an OCT scan and/or use of medications that could affect visual function.
Types of index and reference standard tests
All participants in the chosen studies were screened using standard, traditional cognitive screening tests such as Mini-Mental State Examination (MMSE) and retinal scanning using OCT, OCT-Angiography (OCTA) or another technique (available in online supplemental data S2).
Controls or comparators
Cross-sectional and cohort studies will not have a comparator, but a case–control study should have an age- and sex-matched control group of cognitively healthy participants.
Data extraction
The search results from Medline, PsycINFO and EMBASE were exported to Microsoft Excel sheet and duplicates were removed. Two authors (VJ and JS) reviewed titles, abstracts and full-text papers for eligibility. Authors resolved disagreement by discussion or, where necessary, a third author (JC) offered their view. Extraction was completed (VJ, RS) using a standardised data sheet that was piloted with three papers and revised. All data extraction was verified by JS, and disagreement resolved via discussion. Extracted data included, study design, participant demographics (including mean age, country of study), sample size, method of and parameters measured on retinal scanning, measure of cognitive function, type and degree of cognitive impairment and relevant statistical data.
Risk of bias assessment
The Quality Assessment of Diagnostic Accuracy Studies tool18 was used as it assesses the quality of studies looking at diagnostic accuracy. This covers spectrum, disease progression, partial verification, differential verification, incorporation and review bias, and incomplete data outcomes for example, withdrawals. Three reviewers (VJ, RS and JS) partook in the studies’ quality assessment and any discrepancy between reviewers was resolved through discussion and if an agreement could not be reached, a third individual was consulted (JC).
Statistical analysis
Owing to a high degree of heterogeneity that exists between studies, including study designs, population type, measures of retinal scanning and cognition, a meta-analysis of study results was not possible. A descriptive synthesis approach was utilised.
Patient and public involvement
No patient involved.
Results
Study design and population
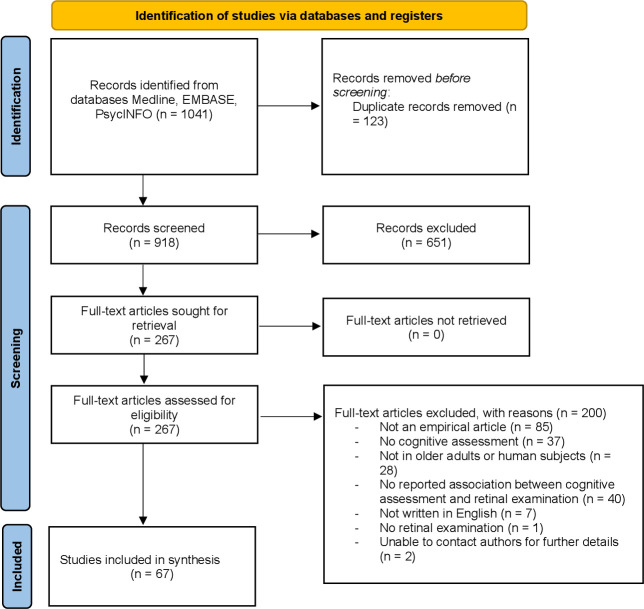
The search identified 821 articles, of which 67 studies were eligible (see figure 1). Most studies included were cross-sectional (60/67; 89.6%), with a few case–controls (2/67; 3.0%) and longitudinal (6/67; 9.0%) studies (table 1). Longitudinal studies had a range of 2–12 years follow-ups. Studies were mostly conducted in USA (13/67; 19.4%), China (9/67; 13.4%), Spain (9/67; 13.4%) and Italy (7/67; 10.4%). The type of cognitive impairment varied between studies with 35 (52.2%) articles looking only at AD and 9 (13.4%) at MCI, and 23 (34.3%) for both conditions. Across all studies, the mean age range was 70.9 years for controls, 72.4 years for AD and 73.0 years for MCI. The ratio of males to females was approximately one-to-one across all studies, with a slight female predominance.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow chart describing the process of study selection.
Table 1.
Characteristics of studies included in the systematic review (n=67)
| Year | Author | Country | Design | Areas of retinal measured | Sample size | Method | |||||||||||
| RNFL | mRNFL | pRNFL | GCC | GC-IPL | MT/MV | CT | FAZ | VD | RVN | Other | |||||||
| 2001 | Parisi45 | Italy | CS | · | 31 | OCT | |||||||||||
| 2006 | Iseri46 | Turkey | CS | · | · | 29 | OCT | ||||||||||
| 2011 | Kesler47 | Israel | CS | · | 78 | OCT | |||||||||||
| 2013 | Kirbas48 | Turkey | CS | · | · | 80 | SD-OCT | ||||||||||
| 2013 | Shen37 | China | L | · | 78 | OCT | |||||||||||
| 2014 | Ascaso49 | Spain | CS | · | · | 90 | OCT | ||||||||||
| 2014 | Gharbiya50 | Italy | CS | · | · | 42 | SD-OCT | ||||||||||
| 2014 | Polo51 | Spain | CS | · | 140 | OCT | |||||||||||
| 2015 | Bambo1 | Spain | CS | · | 112 | OCT | |||||||||||
| 2015 | Bayhan52 | Turkey | CS | · | · | · | 61 | SD-OCT | |||||||||
| 2015 | Feke19 | USA | CS | · | · | 52 | Laser Doppler, OCT | ||||||||||
| 2015 | Gao53 | China | CS | · | · | 72 | OCT | ||||||||||
| 2015 | Gunes54 | Turkey | CC | · | 80 | SD-OCT | |||||||||||
| 2015 | Jentsch21 | Germany | CS | · | · | ·† | 16 | OCT, FLIO | |||||||||
| 2015 | Oktem55 | Turkey | CS | · | 105 | OCT | |||||||||||
| 2015 | Salobrar-Garcia56 | Spain | CS | · | · | 51 | OCT | ||||||||||
| 2015 | Shi57 | China | L | · | 78 | OCT | |||||||||||
| 2016 | Choi42 | Korea | L | · | · | · | 134 | OCT | |||||||||
| 2016 | Cunha26 | Brazil | CS | · | · | · | · | · | 48 | OCT | |||||||
| 2016 | Garcia-Martin58 | Spain | CS | · | · | 225 | OCT | ||||||||||
| 2016 | Knoll59 | USA | CS | · | · | · | 34 | SD-OCT | |||||||||
| 2016 | Pillai60 | USA | CS | · | · | · | 106 | SD-OCT | |||||||||
| 2016 | Trebbastoni27 | Rome | CS | · | 72 | SD-OCT | |||||||||||
| 2017 | Ferrari61 | Italy | CS | · | · | 93 | OCT | ||||||||||
| 2017 | Mendez-Gomez38 | France | L | · | 427 | SD-OCT | |||||||||||
| 2018 | Bulut6 | Turkey | CS | · | · | · | · | 52 | OCTA | ||||||||
| 2018 | Jiang62 | USA | CS | · | · | · | 52 | OCTA, OCT | |||||||||
| 2018 | Lahme63 | Germany | CS | · | · | 74 | OCTA | ||||||||||
| 2018 | Shao64 | USA | CS | · | · | 70 | SD-OCT | ||||||||||
| 2018 | Uchida65 | USA | CS | ·‡ | 124 | OCT | |||||||||||
| 2019 | Almeida13 | Brazil | CS | · | · | · | · | · | 47 | SS-OCT | |||||||
| 2019 | Cipollini66 | Italy | CS | · | · | · | 42 | SD-OCT | |||||||||
| 2019 | Haan22 | Netherlands | CS | · | · | · | ·‡ | 142 | SD-OCT | ||||||||
| 2019 | Haan67 | Netherlands | CS | · | · | · | 86 | FP, SD-OCT, OCTA | |||||||||
| 2019 | Kim68 | South Korea | CS | · | · | · | 47 | OCT | |||||||||
| 2019 | Salobrar-García28 | Spain | CS | · | · | · | ·§ | 90 | OCT | ||||||||
| 2019 | Tao29 | China | CS | · | · | 191 | OCT | ||||||||||
| 2019 | Yoon29 | USA | CS | · | · | · | · | · | 209 | OCTA, SD-OCT | |||||||
| 2019 | Zhang69 | USA | CC | · | · | · | · | · | · | 32 | OCT, OCTA | ||||||
| 2020 | Ashimatey70 | USA | CS | · | 111 | OCTA | |||||||||||
| 2020 | Chua71 | Singapore | CS | · | · | 90 | OCTA | ||||||||||
| 2020 | Criscuolo33 | Italy | CS | · | · | · | · | 83 | SD-OCT, OCTA | ||||||||
| 2020 | Jindahra72 | Thailand | CS | · | · | 58 | OCT | ||||||||||
| 2020 | Jorge73 | Portugal | CS | · | 41 | OCT | |||||||||||
| 2020 | Karakahya40 | Germany | RCT; L | · | · | · | 93 | OCT | |||||||||
| 2020 | Lemmens74 | Belgium | CS | · | 39 | OCT | |||||||||||
| 2020 | Mammadova24 | USA | CS | · | 20 | SD-OCT | |||||||||||
| 2020 | Marquie41 | Spain | L | · | · | 129 | OCT | ||||||||||
| 2020 | Mavilio75 | Italy | CS | · | · | 52 | OCT | ||||||||||
| 2020 | Salobra-Garcia76 | Switzerland | CS | · | · | 32 | OCT, OCTA | ||||||||||
| 2020 | Sanchez31 | Spain | CS | · | · | ·‡ | 930 | OCT | |||||||||
| 2020 | Sen77 | India | CS | · | · | ·‡ | 60 | OCT | |||||||||
| 2020 | Uchida78 | USA | CS | ·‡ | 64 | OCT | |||||||||||
| 2020 | Van De Kreeke32 | Netherlands | CS | · | · | · | · | 298 | OCT, FP | ||||||||
| 2020 | Wu79 | China | CS | · | · | 60 | OCTA | ||||||||||
| 2021 | Biscetti80 | Italy | CS | · | · | · | · | 37 | OCT, OCTA | ||||||||
| 2021 | Janez-Garcia30 | Spain | CS | · | · | · | · | · | 43 | OCT OCTA | |||||||
| 2021 | Li81 | China | CS | · | 71 | OCT | |||||||||||
| 2021 | Mei82 | China | CS | · | · | · | 39 | OCTA | |||||||||
| 2021 | Robbins83 | USA | CS | · | · | · | 122 | OCTA | |||||||||
| 2021 | Robbins84 | USA | CS | · | 278 | OCT | |||||||||||
| 2021 | Wang85 | China | CS | · | · | · | · | 158 | OCTA, FP | ||||||||
| 2021 | Wong86 | Hong Kong | CS | · | 40 | OCTA | |||||||||||
| 2021 | Zabel87 | Poland | CS | · | · | · | · | · | ·‡ | 108 | SD-OCT OCTA | ||||||
| 2021 | Zhao88 | China | CS | · | 59 | OCT | |||||||||||
| 2022 | Montorio89 | Italy | CS | · | · | · | 108 | SD-OCT OCTA | |||||||||
| Total | 29 | 5 | 23 | 22 | 17 | 14 | 9 | 12 | 15 | 6 | 9 | 6415 | |||||
*Focal loss volume and global loss volume.
†Time-resolved autofluorescence of the retina by FLIO.
‡Retinal thickness/volume, mean foveal thichness and juxtafoveal thickness.
§13 IPL, INL, OPL; retinal pigment epithelium thickness.
C, cross-sectional; CC, case–control; CT, Choroidal thickness; FAZ, foveal avascular zone; FLIO, fluorescence lifetime imaging ophthalmoscopy; GCC, macular ganglion cell complex; GC-IPL, ganglion cell-inner plexiform layer; INL, inner nuclear layer; L, longitudinal; mRNFL, macula retinal nerve fibre layer; MT/MV, macular volume/macular thickness; OCT, optical coherence tomography; OCTA, OCT-angiography; OPL, outer plexiform layer; pRNFL, peripapillary retinal nerve fibre layer; RCT, randomised controlled trial; RNFL, retinal nerve fibre layer; RVN, retinal vasculature network; SD-OCT, spectral-domain OCT; VD, vascular/vessel density.
Assessment of retinal abnormalities
Retinal scanning was performed using several techniques (table 1, online supplemental material). The most common method used was OCT (40/67, 59.1%); SD-OCT (17/67); SS-OCT (1/67)), followed by OCTA (18/67; 26.9%) then fundus photography (3/67; 4.5%), Fluorescence lifetime imaging ophthalmoscopy (FLIO) (1/67; 1.5%) and laser Doppler flowmetry (1/67; 1.5%). OCT is a non-invasive method that obtains cross-sectional images of the retina and calculates the thickness of all retinal layers including the nerve fibre layer, GCC; choroid and macula.10 In 12 (17.6%) studies, the Early Treatment of Diabetic Retinopathy Study macular map sectors were used to divide the macula into nine segments to produce a retinal thickness map. The retinal nerve fibre layer (RNFL) thickness was calculated globally, and across either four or six segments.
OCTA acquires images of retinal vasculature to calculate perfusion and vascular density (VD), and foveal avascular zone (FAZ) area6 whereas laser Doppler flowmetry calculates the retinal blood flow rate.19 FLIO measures the autofluorescence intensity emitted by endogenous fluorophores contained within the retina to calculate retinal metabolic activity.20 21 Fundus photography was also employed to obtain detailed images of the fundus within a 50° field of view of the macula, and the optic nerve head to evaluate retinal vasculature.22
As part of the work-up, a full ophthalmological scan was performed in 28 (59.6%) studies prior to retinal imaging, including assessment of best-corrected visual acuity, dilated fundus scan, slit lamp scan of the anterior segment of the eye, intraocular pressure measurement and anatomical ocular measurements with optical biometry. Neuroimaging was performed in 20 (29.4%) studies to exclude alternate diagnoses, and nine (19.1%) studies used standard blood tests to rule out reversible causes of dementia. A comprehensive neuropsychological examination assessing cognitive performance was part of the initial work-up in 11 (23.4%) studies.
Assessment of cognitive function and impairment
A summary of the assessment of cognitive function is shown in table 2. Cognitive function was always measured using standard cognitive screening tools, with the most popular one being as MMSE (59/67; 88%), followed by Montreal Cognitive Assessment (MoCA) (9/67; 13.4%), the global clinical dementia rating score (3/67; 4.5%) and the Alzheimer’s Disease Assessment Scale-cognitive subscale (2/67; 3%). These screening tests evaluate various cognitive domains including, orientation, attention, executive functions, memory, language, visuospatial skills, abstract thinking and calculations. Cognitive screening tests were conducted by either neurologists, psychologists, physicians or trained research associates.
Table 2.
Study characteristics of cognitive assessment and score (n=67)
| Year | Author | Mean age of individuals with AD | Mean age of controls | No. of cognitively impaired subjects | Measure | Mean cognitive score | |||
| MCI | AD | Controls | MCI | AD | |||||
| 2001 | Parisi45 | 70.4 | – | – | 17 | MMSE | 23 | – | 16.4 |
| 2006 | Iseri46 | 70.1 | 65.1 | – | 14 | MMSE | 29.4 | – | 18.5 |
| 2011 | Kesler47 | 73.7 | 70.9 | 24 | 30 | MMSE | – | 28.1 | 23.6 |
| 2013 | Kirbas48 | 69.3 | 68.9 | – | 40 | MMSE | 28.7 | – | 21.2 |
| 2013 | Shen37 | – | 74.1 | 18* | – | MMSE | At 25 months:27.7 | At 25 months: 24.6 | – |
| 2014 | Ascaso49 | 72.1 | 72.9 | 21 | 18 | MMSE | 28.8 | – | 19.3 |
| 2014 | Gharbiya50 | 73.1 | 70.3 | – | 21 | MMSE | 28.2 | – | 22.2 |
| 2014 | Polo51 | 74.2 | 74.0 | – | 70 | MMSE | – | – | 16.0 |
| 2015 | Bambo1 | 74.0 | 76.4 | – | 56 | MMSE | – | – | 16.6 |
| 2015 | Bayhan52 | 75.8 | 74.9 | – | 31 | MMSE | 29.3 | – | 17.4 |
| 2015 | Feke19 | 74.3 | 69.1 | 21 | 10 | CDR | 0.0 | 0.5 | 1.0 or 2.0 |
| 2015 | Gao53 | 74.7 | 72.1 | 26 | 25 | MMSE | 28.6 | 25.8 | 19.2 |
| 2015 | Gunes54 | 75.0 | 74.2 | – | 40 | MMSE | – | – | 21.9 |
| 2015 | Jentsch21 | 77.2 | – | – | 16 | MMSE | – | – | 24.0 |
| 2015 | Oktem55 | 75.4 | 70.2 | 35 | 35 | MMSE | 29.0 | 28.0 | 18.0 |
| 2015 | Salobrar-Garcia56 | 79.3 | 72.3 | – | 23 | MMSE | 28.2 | – | 23.3 |
| 2015 | Shi57 | – | 74.1 | 18* | – | MMSE | At baseline: 28.0 | At baseline: 27.0 | – |
| At 25 months: 28.0 | At 25 months: 24.0 | ||||||||
| 2016 | Choi42 | 76.8 | 73.8 | 26 | 42 | MMSE | – | 23.1 | 14.1 |
| 2016 | Cunha26 | 74.8 | 72.3 | – | 24 | MMSE | 29.1 | – | 17.0 |
| 2016 | Garcia-Martin58 | 75.3 | 74.8 | – | 150 | MMSE | 29.8 | – | 18.4 |
| 2016 | Knoll59 | – | 74.0 | 17 | – | MMSE | 29.0 | 27.0 | – |
| 2016 | Pillai60 | 65.8 | 65.1 | 21 | 214,† | MoCA | 26.6 | 21.2 | 16.0 |
| 2016 | Trebbastoni27 | 72.0 | 71.7 | – | 36 | MMSE | At baseline: 28.6 | – | At baseline: 22.7 |
| At 12 months: 28.5 | At 12 months:17.9 | ||||||||
| 2017 | Ferrari61 | 71.3 | 68.3 | 29.0 | 37‡ | MMSE | – | 26.6 | 16.6 |
| 2017 | Mendez-Gomez38 | – | N/A | – | – | MMSE | 27.8 | – | – |
| 2018 | Bulut6 | 74.2 | 72.6 | – | 26 | MMSE | 26.8 | – | 16.9 |
| 2018 | Jiang62 | 73.3 | 67.6 | 19 | 12 | MMSE | 29.5 | 25.7 | 19.9 |
| 2018 | Lahme63 | 68.0 | 66.1 | – | 36 | MMSE | – | – | 22.3 |
| 2018 | Shao64 | 74.0 | 68.0 | 24 | 25 | MMSE | 29.0 | 28.0 | 22.0 |
| 2018 | Uchida65 | 65.3 | 65.1 | 22 | 24† | MoCA | 26.6 | 20.9 | 14.7 |
| 2019 | Almeida | – | 64.6 | 23 | – | MMSE | – | 27.9 | – |
| 2019 | Cipollini66 | 74.0 | 70.0 | – | 25 | MMSE | 29.2 | – | 24.2 |
| 2019 | Haan22 | 65.0 | 67.9 | – | 57 | MMSE | 29.0 | – | 22.0 |
| 2019 | Haan67 | 65.4 | 60.6 | – | 48 | MMSE | 29.0 | – | 23.0 |
| 2019 | Kim68 | 74.2 | 73.6 | 14 | 16 | MMSE | – | 24.2 | 12.1 |
| 2019 | Salobrar-Garcia28 | – | – | – | 50 | MMSE | 28.6 | 19.9 | |
| 2019 | Tao29 | 71.4 | 68.9 | 51 | 73 | MMSE | 28.7 | 28.3 | 19.7 |
| 2019 | Yoon23 | 72.8 | 69.2 | 37 | 39 | MMSE | 29.2 | 22.6 | 20.1 |
| 2019 | Zhang69 | 73.0 | 73.6 | 13 | 3 | MoCA | 27.1 | – | 20.3 |
| 2020 | Ashimatey70 | – | 68.4 | – | 15§ | MoCA | 23.0 | – | 20.0 |
| 2020 | Chua71 | 74.9 | 76.7 | 37 | 24 | MMSE | 24.8 | 23.9 | 20.3 |
| 2020 | Criscuolo33 | – | 73.1 | 54 | – | MMSE | 28.0 | 26.5 | – |
| 2020 | Jindahra72 | 75.6 | 75.8 | 29 | 29 | MoCA | 26.6 | – | 14.5 |
| 2020 | Jorge73 | 65.3 | 66.3 | – | 20 | MoCA | 24.9 | – | 14.4 |
| 2020 | Karakahya40 | 76.8 | 77.2 | – | 13 | MMSE | 28.2 | – | 21.0 |
| 2020 | Lemmens74 | 71.9 | 68.6 | – | 17 | MMSE | 29.3 | – | 17.6 |
| 2020 | Mammadova24 | – | N/A | N/A | N/A | MMSE | 29.2 | – | – |
| 2020 | Marquie41 | – | 65.8 | 15 | – | MMSE | At follow-up: 29.3¶ | At follow-up: 28.3 | – |
| 2020 | Mavilio75 | 71.2 | 69.1 | 16 | 17 | MMSE | 27.1 | 25.1 | 24.8 |
| 2020 | Sanchez31 | 79.0 | 66.0 | 192 | 324 | MMSE | 29.3 | 25.1 | 20.3 |
| 2020 | Santangelo34 | 70.9 | 69.4 | 37 | 43 | MMSE | – | 24.9 | 19.0 |
| 2020 | Salobrar-Garcia76 | – | – | – | 17 | MMSE | 30.0 | – | 26.0 |
| 2020 | Sen77 | 61.5 | 60.9 | – | 40 | MMSE | 28.0 | – | 17.5 |
| 2020 | Uchida78 | 64.7 | 65.1 | – | 14 | MoCA WMS-IV HVLT-R PVF SVF | 27.0 30.5 23.5 40.0 21.0 | - - - - - | 15.5 14.0 12.0 26.0 8.0 |
| 2020 | Van De Kreeke32 | 91.9** | 70.4/92.4†† | – | 23** | MMSE | 29.0†† | – | 24.0 |
| 2020 | Wu79 | 69.9 | 69.0 | 21 | 19 | MMSE | 27.1 | 24.8 | 19.7 |
| 2021 | Biscetti80 | 72.1 | 73.6 | 24‡‡ | – | MMSE | 28.9 | 25.9 | – |
| 2021 | Janez-Carcia30 | 79.2 | 75.7 | – | 19 | MMSE | 28.38 | – | 23.4 |
| 2021 | Li81 | 83.1 | 79.7 | – | 37 | MMSE ADAS-cog CDR | 29.1 3.0 0 | - - - | 7.9 48.4 2.54 |
| 2021 | Mei82 | 73.8 | 74.3 | – | 19 | MMSE | 28.1 | – | 12.8 |
| 2021 | Robbins83 | 62.4 | 68.1 | – | 15 | MMSE | 29.3 | – | 19.36/21.6§§ |
| 2021 | Robbins84 | 72.8 | 69.2 | 74 | 67 | MMSE | 29.0 | 24.5 | 19.8 |
| 2021 | Wang85 | 71.8 | 69.5 | 47 | 62 | MMSE CDR | 28.7 0.03 | 28.0 0.5 | 19.9 1.3 |
| 2021 | Wong86 | 64.9¶¶ | 64.5 | 11 | – | MoCA | 26.9 | 22.8 | – |
| 2021 | Zabel87 | 74.4 | 71.4 | – | 31 | MMSE | 29 | – | 20.5 |
| 2021 | Zhao88 | 70.2 | 66.6 | 23 | 17 | MMSE MoCA ADAS-cog | 28.8 24.9 14.2 | 26.9 20.6 18.0 | 21.2 15.7 31.9 |
| 2022 | Monotorio89 | – | 72.7 | 54 | – | MMSE | 28.4 | 26.5 | – |
*Converted from normal cognition to MCI or MCI to dementia.
†non-AD dementia.
‡Frontotemporal dementia.
§Cognitively abnormal.
¶Subjective cognitive decline, no baseline data available.
**Cognitively impaired nonagenerians.
††Two control groups, one for 65+ and the other for 90+.
‡‡Both MCI and AD were included.
§§MMSE scores for early onset AD and late-onset AD.
¶¶Reported mean for both control groups.
AD, Alzheimer’s disease; AFT, Animal Fluency Test; CDR, clinical dementia rating; CFT, Complex Figure Test; HVLT-R, Hopkins Verbal Learning Test-Revised; MMSE, Mini-Mental State Examination; MoCA, Montreal Cognitive Assessment; PVF, phonemic verbal fluency; SCWT, Stroop Colour Word Test; SVF, Semantic verbal fluency; TMT, Trail Making Test; WMS-IV, Wechsler Memory Scale-Fourth Edition.
AD was diagnosed using the Diagnostic and Statistical Manual of Mental Disorders(DSM-IV) criteria, National Institute of Neurologic and Communicative Disorders and Stroke-Alzheimer’s Disease and Related Disorders Association23 criteria or generally through a combination of both approaches. The most common method to diagnose MCI was through the Peterson’s criteria24 which identifies whether all five criteria are satisfied including, memory complaint corroborated by an informant, objective memory decline, normal general cognitive function, normal functional activities and absent dementia diagnosis.
Association between cognition and retinal measurements
Half of the studies found a significant correlation between RNFL (9/17, 52.9%) and GC-IPL thinning (6/11, 54.5%) with impaired cognition (table 3). Some studies found a significant correlation between macular (14/30, 46.7%), macular retinal nerve fibre layer (mRNFL) (3/5, 60.0%), GCC (8/19, 42.1%), choroidal thickness (CT) (4/9, 44.4%) and pRNFL thinning (5/21, 23.8%) with cognitive performance. These findings did not vary significantly between different OCT devices. Measures of retinal vascular structures using OCTA identified a correlation between VD (7/14, 50.0%) and FAZ area (3/9, 33.3%) with cognitive impairment.
Table 3.
Associations between diagnosed dementia status (eg, AD) and retinal markers
| Year | Author | Method | Areas of retina measured | |||||||||
| RNFL | mRNFL | pRNFL | GCC | GC-IPL | MT | CT | VD | FAZ | Other | |||
| 2001 | Paris45 | OCT |
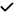
|
– | – | – | – | – | – | – | – | – |
| 2006 | Iseri46 | OCT |
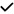
|
– | – | – | – |

|
– | – | – |
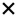 * * |
| 2011 | Kesler47 | OCT |

|
– | – | – | – | – | – | – | – | – |
| 2013 | Kirbas48 | SD-OCT |
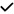
|
– |
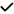
|
– | – | – | – | – | – | – |
| 2013 | Shen37 | OCT |

|
– | – | – | – | – | – | – | – | – |
| 2014 | Ascaso49 | OCT |
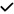
|
– | – | – | – |
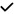
|
– | – | – | – |
| 2014 | Gharbiya50 | SD-OCT | – | – |

|
– | – | – |

|
– | – |
 † † |
| 2014 | Polo51 | OCT |
|
– | – | – | – | – | – | – | – | – |
| 2015 | Bambo | OCT | – | – | ? | – | – | – | – | – | – |
 ‡ ‡ |
| 2015 | Bayhan52 | SD-OCT | – | – | – |

|
– | – |
|
– | – | – |
| 2015 | Feke19 | Laser Doppler/OCT | – | – | – | – | – | – | – | – | – |
 § § |
| 2015 | Gao53 | OCT | – | – |

|
– | – | – | – | – | – | – |
| 2015 | Gunes54 | SD-OCT | – | – |
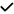
|
– | – | – | – | – | – | – |
| 2015 | Jentsch21 | OCT / FLIO | – | – |

|
– | – | – | – | – | – | ?¶ |
| 2015 | Oktem55 | OCT |
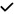
|
– | – | – | – | – | – | – | – | – |
| 2015 | Salobrar-Garcia56 | OCT | – | ? |
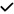
|
– | – | – | – | – | – |
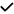 *, ** *, ** |
| 2015 | Shi57 | OCT |
|
– | – | – | – | – | – | – | – | – |
| 2016 | Choi42 | OCT | – | – |

|
– | ? | ? | – | – | – | – |
| 2016 | Cunha26 | OCT | – |

|
|
|

|
|
– | – | – |
 †† †† |
| 2016 | Garcia-Martin58 | OCT |
|
– | – |

|
– | – | – | – | – | – |
| 2016 | Knoll59 | SD-OCT | – | – | ? | – | – | – | – | – | – | – |
| 2016 | Pillai60 | SD-OCT |
|
– | – | – | – | – | – | – | – | – |
| 2016 | Trebbastoni27 | SD-OCT | – | – |
|
– | – | – | – | – | – | – |
| 2017 | Ferrari61 | OCT | – | – |

|
– | AD  MCI MCI
|
– | – | – | – | – |
| 2017 | Mendez-Gomez38 | SD-OCT | – | – | ? | – | – | – | – | – | – | – |
| 2018 | Bulut6 | OCTA | – | – | – | – | – | – |
|
|
|
 ‡‡,§§ ‡‡,§§ |
| 2018 | Jiang62 | OCTA / OCT | – | – | – | – | – | – | – | – | – | ?¶¶ |
| 2018 | Lahme63 | OCTA | – | – | – | – | – | – | – | – | – |
 *** *** |
| 2018 | Shao64 | SD-OCT |
|
– | – | – |
|
– | – | – | – | – |
| 2018 | Uchida65 | OCT | – | – | – | – | – | – | – | – | – |
 ††† ††† |
| 2019 | Almeida13 | SS-OCT | – |

|
|
|
|
? | – | – | – | – |
| 2019 | Cipollini66 | SD-OCT | – | – |

|

|
– |
|
– | – | – | – |
| 2019 | Haan22 | SD-OCT | – | – |

|
– | – |

|
– | – | – | – |
| 2019 | Haan67 | SD-OCT / OCTA | – | – | – | – | – | – |

|

|

|
– |
| 2019 | Kim68 | OCT | ? | – | – | – | ? |
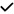
|
– | – | – | – |
| 2019 | Salobrar-Garcia28 | OCT | – | – |
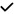
|
– | – |
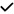
|
– | – | – | – |
| 2019 | Tao29 | OCT | – | – |
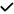
|

|
– | – | – | – | – | – |
| 2019 | Yoon23 | OCTA / SD-OCT |
|
– | – | – |
|
– | – | ? |

|
 ‡‡‡ ?§§§ ‡‡‡ ?§§§ |
| 2019 | Zhang69 | OCT / OCTA | – | – | – | – | – | – | – | ? | – | – |
| 2020 | Ashimatey70 | OCTA | – | – | – | – | – | – | – |
|
– | – |
| 2020 | Chua71 | OCT | – | – | – | – | – | – | – |
|
|
– |
| 2020 | Criscuolo33 | SD-OCT / OCTA |
|
– | – |

|
– | – | – | – | – | – |
| 2020 | Jindahra65 | OCT |
|
– | – | – |
|
– | – | – | – | – |
| 2020 | Jorge73 | OCT | – | – | – | – |

|
– | – | – | – | – |
| 2020 | Karakahya40 | OCT |
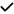
|
– | – | – |
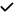
|
– |
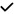
|
– | – | – |
| 2020 | Lemmens74 | OCT |
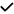
|
– | – | – | – | – | – | – | – | – |
| 2020 | Mammadova24 | SD-OCT |
|
– | – | – | – | – | – | – | – | |
| 2020 | Marquie41 | OCT |
|
– | – |

|
– | – | – | – | – | – |
| 2020 | Mavilio75 | OCT |
|
– | – |

|
– | – | – | – | – | – |
| 2020 | Salobra-Garcia76 | OCT, OCTA | – | – | – | – | – | – |
|
– |

|

|
| 2020 | Sanchez31 | OCT |
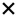
|
– | – |

|
– | – | – | – | – |

|
| 2020 | Santangelo34 | OCT |
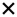
|
– | – | – | – |
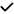
|
– | – | – | – |
| 2020 | Sen77 | OCT |
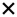
|
– | – |

|
– | – | – | – | – |

|
| 2020 | Uchida78 | OCT | – | – | – | – | – | – | – | – | – |

|
| 2020 | Van De Kreeke32 | OCT |
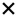
|
– | – |

|

|
– | – |

|
– | – |
| 2020 | Wu79 | OCTA | – | – | – | – | – | – | – | ? | ? | – |
| 2021 | Biscetti80 | OCT | – | – | – |

|

|
– | – |
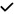
|
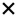
|
– |
| 2021 | Janez-Garcia30 | OCT, OCTA |
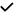
|
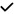
|
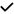
|
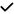
|
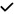
|
– | – | – | – | – |
| 2021 | Li81 | OCT | – | – | – | – | – | – |
|
– | – | – |
| 2021 | Lian | OCT |
|
– | – |
|
– | – | – | – | – | – |
| 2021 | Mei82 | OCTA |
|
– | – |
|
– | – | – |
|
– | – |
| 2021 | Robbins83 | OCTA |
|
– | – | – |

|
– |

|
– | – | – |
| 2021 | Robbins84 | OCT | – | – | – | – | – | – | ? | – | – | – |
| 2021 | Wang85 | OCTA | – | – |

|

|
– | – | – |

|

|
– |
| 2021 | Wong86 | OCTA | – | – | – | – | – | – | – |
|
– | – |
| 2021 | Zabel87 | OCT, OCTA |
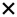
|
– |

|

|
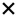 ¶¶ ¶¶ |
– | – |
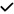
|
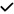 ¶¶,*** ¶¶,*** |
– |
| 2021 | Zhao88 | OCT | – |
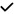
|
– | – | – | – | – | – | – | – |
| 2022 | Montorio89 | OCTA |
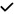
|
– | – |
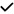
|
– | – | – |

|
– | – |
| 15/30 | 3/5 | 6/21 | 8/19 | 9/15 | 5/10 | 4/9 | 7/14 | 3/9 | ||||
Key: 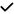 = correlation identified;
= correlation identified;  = no correlation identified; ? = unclear
= no correlation identified; ? = unclear
*Foveal thickness.
†Retinal central subfield thickness.
‡Retinal haemoglobin levels.
§Retinal blood flow.
¶T2, α2 and Q2 in ch2.
**Macular volume.
††GCL++.
‡‡Choroidal flow rate.
§§Outer retinal flow rate.
¶¶Superficial vascular plexus, deep vascular plexus and total retinal vascular network.
***Flow density.
†††Retinal pigment epithelium.
‡‡‡Central subfield thickness.
§§§Perfusion density.
AD, Alzheimer’s disease; CSF, central subfield retinal thickness; FAZ, foveal avascular zone; FLIO, Fluorescence Lifetime Imaging Ophthalmoscopy; GCC, ganglion cell complex; GC-IPL, ganglion cell and inner plexiform layer; mRNFL, macular retinal nerve fibre layer; MT/MV, macular volume/macular thickness; OCT, optical coherence tomography; OCTA, optical coherence tomography-angiography; PRNFL, peripapillary RNFL; RNFL, retinal nerve fibre layer thickness; SD-OCT, spectral-domain OCT; VD, vascular density.
Risk of bias assessment
Risk of bias of the 67 studies are provided in table 4. For over half the studies (39/67, 58.2%), it was unclear whether the index test results were interpreted without the knowledge of the reference standard, and vice versa (37/67, 55.2%). This could contribute to review bias, and thus impact the diagnostic accuracy of the respective clinical tool. The time period between conducting the reference standard and index test was unclear in 17 (25.3%) studies, suggesting that the influence of disease progression bias cannot be excluded. All 67 studies were not representative of the target population as patients with comorbidities that may affect the retina, including diabetes mellitus and hypertension were excluded. This lack of generalisability may interfere with the implementation of retinal scanning in clinical practice. However, the majority of studies (95.5%) provided a clear selection criterion and all studies utilised an accurate reference standard. Partial verification, differential verification, incorporation and clinical review bias were minimal across the included studies. Considering this, the overall risk of bias was moderate, and findings should be interpreted carefully.
Table 4.
Summary of QUADAS score of the 67 included studies
| Year | Author | RS | CSC | ARS | DPB | PVB | DVB | IB | ITE | RSE | ITRB | RSRB | CRB | UTRR | WE | Total |
| 2001 | Parisi45 | N | N | Y | U | U | U | Y | Y | N | U | U | Y | Y | N | 5/14 |
| 2006 | Iseri46 | N | Y | Y | Y | Y | Y | Y | Y | N | U | U | Y | Y | Y | 10/14 |
| 2011 | Kesler47 | N | Y | Y | U | Y | Y | U | U | N | Y | Y | Y | Y | Y | 9/14 |
| 2013 | Kirbas48 | N | Y | Y | U | Y | Y | Y | N | N | U | U | Y | Y | Y | 8/14 |
| 2013 | Shen37 | N | Y | Y | Y | Y | Y | Y | Y | Y | U | U | Y | Y | Y | 11/14 |
| 2014 | Ascaso49 | N | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | Y | Y | N | 11/14 |
| 2014 | Gharbiya50 | N | Y | Y | Y | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | 13/14 |
| 2014 | Polo51 | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 13/14 |
| 2015 | Bambo | N | Y | Y | U | Y | Y | Y | Y | Y | U | U | Y | Y | Y | 10/14 |
| 2015 | Bayhan52 | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 13/14 |
| 2015 | Feke19 | N | Y | Y | U | Y | Y | Y | Y | N | U | U | Y | Y | Y | 10/14 |
| 2015 | Gao53 | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 13/14 |
| 2015 | Gunes54 | N | Y | Y | Y | Y | Y | Y | N | N | U | U | Y | Y | Y | 9/14 |
| 2015 | Jentsch21 | N | Y | Y | U | U | Y | Y | Y | Y | U | U | Y | Y | Y | 9/14 |
| 2015 | Oktem55 | N | N | Y | Y | Y | Y | Y | N | Y | U | U | Y | Y | Y | 9/14 |
| 2015 | Shi57 | N | Y | Y | Y | Y | Y | Y | Y | Y | U | U | Y | Y | Y | 11/14 |
| 2015 | Solabrar-Garcia56 | N | Y | Y | U | Y | Y | Y | Y | N | U | U | Y | Y | Y | 9/14 |
| 2016 | Choi42 | N | Y | Y | Y | Y | Y | Y | Y | Y | U | U | Y | Y | Y | 12/14 |
| 2016 | Cunha26 | N | Y | Y | Y | Y | Y | Y | Y | Y | U | U | Y | Y | Y | 11/14 |
| 2016 | Garcia-Martin58 | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 13/14 |
| 2016 | Knoll59 | N | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | Y | Y | Y | 12/14 |
| 2016 | Pillai60 | N | Y | Y | Y | Y | Y | Y | Y | Y | U | U | Y | Y | Y | 11/14 |
| 2016 | Trebbastoni27 | N | Y | Y | Y | Y | Y | Y | Y | Y | U | U | Y | Y | Y | 11/14 |
| 2017 | Ferrari61 | N | Y | Y | U | Y | Y | Y | Y | N | U | U | Y | Y | Y | 9/14 |
| 2017 | Mendez-Gomez38 | N | N | Y | Y | Y | Y | Y | Y | Y | U | U | Y | Y | Y | 10/14 |
| 2018 | Bulut6 | N | Y | Y | Y | Y | Y | Y | Y | Y | U | U | Y | Y | Y | 11/14 |
| 2018 | Jiang62 | N | Y | Y | U | Y | Y | Y | Y | N | U | U | U | N | N | 6/14 |
| 2018 | Lahme63 | N | Y | Y | U | Y | Y | Y | Y | N | U | U | Y | Y | Y | 9/14 |
| 2018 | Shao64 | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | U | U | Y | Y | 11/14 |
| 2018 | Uchida65 | N | Y | Y | Y | Y | Y | Y | Y | Y | U | U | Y | Y | Y | 11/14 |
| 2019 | Almeida13 | N | Y | Y | Y | Y | Y | Y | Y | Y | U | Y | Y | Y | Y | 12/14 |
| 2019 | Cipollini66 | N | Y | Y | U | Y | Y | Y | Y | Y | U | U | Y | Y | Y | 10/14 |
| 2019 | Haan22 | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 13/14 |
| 2019 | Haan67 | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 13/14 |
| 2019 | Kim68 | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 13/14 |
| 2019 | Salobrar-García28 | N | Y | Y | Y | Y | Y | Y | Y | Y | U | U | Y | Y | Y | 11/14 |
| 2019 | Tao29 | N | Y | Y | N | Y | Y | Y | Y | N | U | U | Y | Y | Y | 9/14 |
| 2019 | Yoon23 | N | Y | Y | Y | Y | Y | Y | Y | Y | U | U | Y | Y | Y | 11/14 |
| 2019 | Zhang69 | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 13/14 |
| 2020 | Ashimatey70 | N | Y | Y | U | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 12/14 |
| 2020 | Chua71 | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 13/14 |
| 2020 | Criscuolo33 | N | Y | Y | U | Y | Y | Y | Y | Y | U | U | Y | Y | Y | 10/14 |
| 2020 | Jindahra72 | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 13/14 |
| 2020 | Jorge73 | N | Y | Y | U | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 12/14 |
| 2020 | Karakahya40 | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 13/14 |
| 2020 | Lemmens74 | N | Y | Y | Y | Y | Y | Y | Y | Y | U | U | Y | Y | Y | 11/14 |
| 2020 | Mammadova24 | N | Y | Y | Y | Y | Y | Y | Y | Y | U | U | Y | Y | Y | 11/14 |
| 2020 | Marguie41 | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 13/14 |
| 2020 | Mavilio75 | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 13/14 |
| 2020 | Sanchez31 | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 13/14 |
| 2020 | Santangelo34 | N | Y | Y | U | Y | Y | Y | Y | N | U | U | Y | Y | Y | 9/14 |
| 2020 | Salobrar-Garcia76 | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 13/14 |
| 2020 | Sen77 | N | Y | Y | Y | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | 12/14 |
| 2020 | Uchida78 | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 13/14 |
| 2020 | Van De Kreeke32 | N | Y | Y | Y | U | Y | Y | Y | N | U | U | Y | Y | Y | 9/14 |
| 2020 | Wu79 | N | Y | Y | Y | Y | Y | Y | Y | Y | U | U | Y | Y | Y | 11/14 |
| 2021 | Biscetti80 | N | Y | Y | Y | Y | Y | Y | Y | Y | U | U | Y | Y | Y | 11/14 |
| 2021 | Janez-Garcia30 | N | U | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 13/14 |
| 2021 | Li81 | N | Y | Y | Y | Y | Y | Y | Y | N | U | U | Y | Y | Y | 10/14 |
| 2021 | Mei82 | N | Y | Y | U | Y | Y | Y | Y | N | U | U | Y | Y | Y | 9/14 |
| 2021 | Robbins83 | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 13/14 |
| 2021 | Robbins84 | N | Y | Y | Y | Y | Y | Y | Y | Y | U | U | Y | Y | Y | 11/14 |
| 2021 | Wang85 | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 13/14 |
| 2021 | Wong86 | N | Y | Y | Y | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | 12/14 |
| 2021 | Zabel87 | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 13/14 |
| 2021 | Zhao88 | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 13/14 |
| 2022 | Montorio89 | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 13/14 |
ARS, Accurate reference standard; CRB, Clinical review bias; DPB, Disease progression bias; DVB, Differential verification bias; IB, Incorporation bias; ITE, Index test execution; ITRB, Index test review bias; N, No; PVB, Partial verification bias; QUADAS, Quality Assessment of Diagnostic Accuracy Studie; RS, Representative spectrum; RSE, Reference standard execution; RSRB, Reference standard review bias; U, Unknown; UTRR, Uninterpretable results reported; WE, Withdrawals explained; Y, Yes.
Discussion
Our review evaluated the relationship between retinal scanning methods and early detection of cognitive impairment in older adults to inform future clinical practice. Over 50% of the studies using OCT identified an association between the thinning of at least one retinal area and cognitive impairment. The future of retinal imaging as a clinically useful tool for measuring cognition in older adults is considered.
Within ophthalmology, retinal imaging devices are primarily used in the diagnosis of retinal disease as well as serial monitoring of retinal conditions such as age-related macular degeneration and response to treatment.10 We identified two main retinal scanning methods, OCT and OCTA in this review, with a more sensitive response from OCT. OCTA was primarily used to measure and evaluate retinal vasculature, but measures of retinal thickness via OCT was considerably more effective in detecting cognitive impairment. Studies using OCTA techniques have resulted in mixed findings.25 This may be due to the varied vessel distribution and morphology, including vessel size and number of anastomoses between participants. The lack of uniformity in vessel size may affect vessel density calculations, as the smaller surface area of capillaries may contribute to a more sensitive measure of perfusion compared with larger vessels.23 Additionally, fewer anastomoses within a vessel network contributes to a higher risk of vascular dysfunction.23 Considering this wide variability in vascular network structure between individuals, OCTA may be suitable for detecting later stages of dementia but may not be reliable in detecting the transition between age-related changes and MCI. Furthermore, not all participants with MCI will convert to dementia, some may revert to normal cognition, thus affecting the accuracy of the results.23 Retinal layer thickness as measured through OCT does not vary as extensively as OCTA and thus, serves as a suitable alternative for the early detection of dementia.
Although OCT devices have been used for the past two decades, there has been no consistent retinal area that is strongly associated with the cognitive function of older adults. This is consistent across all types of OCT devices. Our findings indicate that thinning of the RNFL and pRNFL may be associated with poorer cognitive function, however, within the last decade, studies have found more varied results for pRNFL, with only 6 (out of 21, 28.6%) studies identifying an association.13 26–30 On the other hand, 45.5% of studies using OCT devices to measure RNFL thickness have identified a positive correlation with cognitive impairment, although studies with larger sample sizes (eg, Sánchez et al,31 930; van de Kreeke et al,32 298) found no significant correlation. Indeed, researchers have failed to consistently identify a correlation between retinal scanning and cognitive impairment, for example, two recent articles identified an association23 24 with RNFL whereas two articles did not.33 34 This lack of consistency is reflected across all retinal areas and the discrepancies may in part be ascribed to differences in sample size, the severity of cognitive impairment, and the OCT technology used in various devices.
Mean RNFL and macular thickness maybe largely dependent on the type of OCT device used.35 The variety of devices identified in this review may thus affect the consistency of results across studies. Moreover, as MCI represents a transition towards dementia, reductions in pRNFL and macular thickness, if any, are likely to be subtle, perhaps even within the normal range, when compared with healthy age-matched control subjects. Furthermore, these cross-sectional studies present data at a single point in time after the participant has been diagnosed with cognitive impairment. The lack of baseline measures from cognitively healthy participants creates difficulty in detecting subtle changes in their cognitive performance. Therefore, our findings need to be interpreted with caution.
The inconsistencies between studies can also be attributed to the lack of sensitivity of cognitive screening tools, such as the MMSE which is largely used to assess cognition, but we know is ineffective in identifying cognitive impairment at its early stages.36 Despite these mixed results, cross-sectional studies present data at a single point in time and therefore, the dynamic change in the relationship between retinal thickness and cognition is unable to be quantified. It seems therefore that with only limited evidence thus far, caution will be needed in interpreting the rate of change of an individual’s RNFL thickness in terms of their cognitive status. Furthermore, given the physiological variations in RNFL thickness, single time point measurements in individual participants are likely to have limited value.
Our review innovates by appraising six well-sized longitudinal studies37–41 (sample size 78–427), to further establish cause-and-effect relationships between retinal scanning and cognitive deterioration. We found that OCT measurements of RNFL thickness including inferior quadrant RNFL thickness37 39 40 and pRNFL thickness38 was able to detect reductions in these areas over time, and was associated with decline in cognitive abilities such as impaired recall,37 immediate and delayed memory37 and episodic memory.38 While cognitive decline was found to be associated with longitudinal reduction in inferior quadrant thickness,38 the association is less clear for other retinal regions around the GCC42 and macular thickness.42 Our results suggest the ability of OCT to potentially detect longitudinal changes in RNFL thickness and declining cognition, although further longitudinal efforts need to be carried out to determine the true nature of cognitive decline with retinal changes.
A systematic review by Ding et al43 evaluated six studies and identified a positive relationship between retinal vascular signs, and information processing speed, verbal memory and executive function. However, the lack of consistency between study findings due to differences in retinal scanning methodology, small sample size and cognitive screening tools were recognised and limited interpretation. An updated review by Heringa et al44 identified a moderately strong association between microvascular and cerebral changes, and dementia diagnosis across 32 studies. They concluded that although retinal vascular assessment can be incorporated into prediction models, only a minority of dementia cases were attributed to retinal vascular changes. These reviews support the potential role of retinal vascular changes in the pathophysiology of cognitive impairment but recommend the need for more prospective data. Our review adds to the existing literature by providing greater insight into the role of OCT in the early detection of cognitive impairment through measures of retinal layer thickness.
Our study has several limitations. First, participants in the included studies were not representative of the sample population and individuals with chronic conditions, such as diabetes mellitus, hypertension and neurological conditions were excluded. These comorbidities are common in the older population and affect the generalisability of our findings. Further studies including patients with these comorbidities are required to identify whether retinal scanning is a viable biomarker in cognitive impairment. Second, some studies were missing data in several domains, including global cognition scores or correlation metrics, which excluded their entry in the review and may compromise publication bias. As noted earlier, most studies have included MMSE and MoCA tests which are not sensitive measures to detect early changes in cognition in dementia, and therefore, diminishes the impact of our findings, as the studies do not provide adequate evidence to endorse retinal imaging as a screening tool. Future retinal imaging studies should include a comprehensive neuropsychological battery to measure specific cognitive domains such as executive function, speed of processing, episodic memory, attention and global cognition as these domains are most impacted in dementia. Third, our search strategy was very specific, and this may have excluded studies that were relevant to our review. Fourth, only 17 (25.4%) studies evaluating OCTA were included in this review resulting in mixed findings. This may explain why other studies specifically assessing OCTA with a larger sample size may have identified a positive correlation.25 Fifth, a major concern is that the studies use different company devices (such as Spectralis, Zeiss, Optovue) to measure retinal neuronal thickness, and comparing across these manufacturers is fruitless, as all the devices use proprietary software and respective postprocessing algorithms for their images.
Our study has some strengths. This is the first systematic review that has evaluated multiple retinal scanning tools across several forms of cognitive impairment. We reviewed extensively more empirical articles than previous systematic reviews,43 44 comprising of a larger, international sample and summarised the recent results of longitudinal studies, adding substantial insight.
Earlier diagnosis of dementia using non-invasive techniques will improve patient care, quality of life, disease management and clinical outcome.4 Cognitive screening tools currently used in routine clinical practice such as MMSE are not sensitive in detecting cognitive impairment in its earlier stages, are time-consuming and can be stressful for the patient.36 OCT is a sensitive alternative that provides a rapid assessment of the retina to detect changes consistent with cognitive impairment, such as RNFL thinning. Advances in OCT technology, especially the advent of Fourier-domain OCT (ED-OCT), and more recently SS-OCT, which improves acquisition speed and resolution of retinal images, will further make accurate quantitative segmented retinal layer analysis possible. Introducing OCT as part of a Government’s health-subsidised care (e.g., Australia’s Medicare Benefits Schedule) could allow optometrists to additionally provide annual cognitive screening to older adults. This would enable earlier detection of cognitive impairment and thus the provision of both pharmacological and non-pharmacological interventions to slow or stabilise disease progression.4
In conclusion, while cross-sectional studies show moderate support between retinal scanning methods and cognitive impairment, recent longitudinal studies provide stronger evidence on the diagnostic utility of OCT in detecting a declining cognitive status. Further longitudinal studies should be conducted to corroborate these findings before retinal scanning can be introduced into clinical practice as a viable tool for detecting cognitive impairment. Studies using more sensitive cognitive screening tools are required to assess the viability of retinal measures as a biomarker in cognitive decline.
Supplementary Material
Footnotes
Twitter: @jsiette
Contributors: JS conceptualised the study and produced with VJ the first draft of the manuscript. BG reviewed the study design and provided feedback. JS and VJ devised the search strategy which was carried out by VJ. JS completed the blinded 5% review of abstracts with BG acting as arbitrator. VJ reviewed the remainder of the abstracts. JS and VJ carried out the full-text review, followed by the data extraction and quality assessment of included articles. VJ developed the initial frameworks with JS providing feedback. JS and RS conducted the full-text review and data extraction of an updated search in March 2022. JC contributed to identification of OCT machines and critical revisions. GL and RS provided essential write-up and feedback on early drafts. All authors contributed to critical revisions of subsequent manuscript drafts and approved the final submission. JS is responsible for the overall content as the guarantor.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request. All data relevant to the study are included in the article or uploaded as online supplemental information. Data are available on reasonable request to the corresponding author.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
We used publicly accessible documents as evidence and did not collect individual personal information from participants. As such it was not necessary to seek an institutional ethics approval before commencing our review.
References
- 1.Bambo MP, Garcia-Martin E, Gutierrez-Ruiz F, et al. Analysis of optic disk color changes in Alzheimer's disease: a potential new biomarker. Clin Neurol Neurosurg 2015;132:68–73. 10.1016/j.clineuro.2015.02.016 [DOI] [PubMed] [Google Scholar]
- 2.Dementia Australia . Dementia statistics: dementia Australia, 2014. Available: https://www.dementia.org.au/statistics2022
- 3.Villemagne VL, Burnham S, Bourgeat P, et al. Amyloid β deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer's disease: a prospective cohort study. Lancet Neurol 2013;12:357–67. 10.1016/S1474-4422(13)70044-9 [DOI] [PubMed] [Google Scholar]
- 4.Dementia Australia . Early diagnosis of dementia: dementia Australia, 2014. Available: https://www.dementia.org.au/information/diagnosing-dementia/early-diagnosis-of-dementia2022
- 5.Yoon SP, Thompson AC, Polascik BW, et al. Correlation of OCTA and volumetric MRI in mild cognitive impairment and Alzheimer's disease. Ophthalmic Surg Lasers Imaging Retina 2019;50:709–18. 10.3928/23258160-20191031-06 [DOI] [PubMed] [Google Scholar]
- 6.Bulut M, Kurtuluş F, Gözkaya O, et al. Evaluation of optical coherence tomography angiographic findings in Alzheimer's type dementia. Br J Ophthalmol 2018;102:233–7. 10.1136/bjophthalmol-2017-310476 [DOI] [PubMed] [Google Scholar]
- 7.Dementia Australia . Early warning signs: dementia Australia, 2015. Available: https://www.dementia.org.au/about-dementia/health-professionals/dementia-the-essentials/early-warning-signs2022
- 8.Ong Y-T, Hilal S, Cheung CY, et al. Retinal neurodegeneration on optical coherence tomography and cerebral atrophy. Neurosci Lett 2015;584:12–16. 10.1016/j.neulet.2014.10.010 [DOI] [PubMed] [Google Scholar]
- 9.Nestor SM, Rupsingh R, Borrie M, et al. Ventricular enlargement as a possible measure of Alzheimer's disease progression validated using the Alzheimer's disease neuroimaging initiative database. Brain 2008;131:2443–54. 10.1093/brain/awn146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adhi M, Duker JS. Optical coherence tomography--current and future applications. Curr Opin Ophthalmol 2013;24:213–21. 10.1097/ICU.0b013e32835f8bf8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ito Y, Sasaki M, Takahashi H, et al. Quantitative assessment of the retina using OCT and associations with cognitive function. Ophthalmology 2020;127:107–18. 10.1016/j.ophtha.2019.05.021 [DOI] [PubMed] [Google Scholar]
- 12.Thomson KL, Yeo JM, Waddell B, et al. A systematic review and meta‐analysis of retinal nerve fiber layer change in dementia, using optical coherence tomography. Alzheimers Dement 2015;1:136–43. 10.1016/j.dadm.2015.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Almeida ALM, Pires LA, Figueiredo EA, et al. Correlation between cognitive impairment and retinal neural loss assessed by swept-source optical coherence tomography in patients with mild cognitive impairment. Alzheimers Dement 2019;11:659–69. 10.1016/j.dadm.2019.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang M, Zhu Y, Shi Z, et al. Meta-Analysis of the relationship of peripheral retinal nerve fiber layer thickness to Alzheimer's disease and mild cognitive impairment. Shanghai Arch Psychiatry 2015;27:263–79. 10.11919/j.issn.1002-0829.215100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chan VTT, Sun Z, Tang S, et al. Spectral-Domain OCT measurements in Alzheimer's disease: a systematic review and meta-analysis. Ophthalmology 2019;126:497–510. 10.1016/j.ophtha.2018.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mejia-Vergara AJ, Restrepo-Jimenez P, Pelak VS. Optical coherence tomography in mild cognitive impairment: a systematic review and meta-analysis. Front Neurol 2020;11:578698. 10.3389/fneur.2020.578698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ge Y-J, Xu W, Ou Y-N, et al. Retinal biomarkers in Alzheimer's disease and mild cognitive impairment: a systematic review and meta-analysis. Ageing Res Rev 2021;69:101361. 10.1016/j.arr.2021.101361 [DOI] [PubMed] [Google Scholar]
- 18.Whiting P, Rutjes AWS, Reitsma JB, et al. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol 2003;3:25. 10.1186/1471-2288-3-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feke GT, Hyman BT, Stern RA, et al. Retinal blood flow in mild cognitive impairment and Alzheimer's disease. Alzheimers Dement 2015;1:144–51. 10.1016/j.dadm.2015.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dysli C, Wolf S, Berezin MY, et al. Fluorescence lifetime imaging ophthalmoscopy. Prog Retin Eye Res 2017;60:120–43. 10.1016/j.preteyeres.2017.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jentsch S, Schweitzer D, Schmidtke K-U, et al. Retinal fluorescence lifetime imaging ophthalmoscopy measures depend on the severity of Alzheimer’s disease. Acta Ophthalmol 2015;93:e241–7. 10.1111/aos.12609 [DOI] [PubMed] [Google Scholar]
- 22.den Haan J, van de Kreeke JA, van Berckel BN, et al. Is retinal vasculature a biomarker in amyloid proven Alzheimer's disease? Alzheimers Dement 2019;11:383–91. 10.1016/j.dadm.2019.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoon SP, Grewal DS, Thompson AC, et al. Retinal microvascular and neurodegenerative changes in Alzheimer's disease and mild cognitive impairment compared with control participants. Ophthalmol Retina 2019;3:489–99. 10.1016/j.oret.2019.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mammadova N, Neppl TK, Denburg NL, et al. Reduced retinal thickness predicts age-related changes in cognitive function. Front Aging Neurosci 2020;12:81. 10.3389/fnagi.2020.00081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Song A, Johnson N, Ayala A, et al. Optical coherence tomography in patients with Alzheimer's disease: what can it tell us? Eye Brain 2021;13:1–20. 10.2147/EB.S235238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cunha LP, Lopes LC, Costa-Cunha LVF, et al. Macular thickness measurements with frequency Domain-OCT for quantification of retinal neural loss and its correlation with cognitive impairment in Alzheimer's disease. PLoS One 2016;11:e0153830. 10.1371/journal.pone.0153830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trebbastoni A, D'Antonio F, Bruscolini A, et al. Retinal nerve fibre layer thickness changes in Alzheimer's disease: results from a 12-month prospective case series. Neurosci Lett 2016;629:165–70. 10.1016/j.neulet.2016.07.006 [DOI] [PubMed] [Google Scholar]
- 28.Salobrar-García E, de Hoz R, Ramírez AI, et al. Changes in visual function and retinal structure in the progression of Alzheimer's disease. PLoS One 2019;14:e0220535. 10.1371/journal.pone.0220535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tao R, Lu Z, Ding D, et al. Perifovea retinal thickness as an ophthalmic biomarker for mild cognitive impairment and early Alzheimer's disease. Alzheimers Dement 2019;11:405–14. 10.1016/j.dadm.2019.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jáñez-García L, Bachtoula O, Salobrar-García E, et al. Roughness of retinal layers in Alzheimer's disease. Sci Rep 2021;11:11804. 10.1038/s41598-021-91097-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sánchez D, Castilla-Marti M, Marquié M, et al. Evaluation of macular thickness and volume tested by optical coherence tomography as biomarkers for Alzheimer's disease in a memory clinic. Sci Rep 2020;10:1580. 10.1038/s41598-020-58399-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van de Kreeke JA, Legdeur N, Badissi M, et al. Ocular biomarkers for cognitive impairment in nonagenarians; a prospective cross-sectional study. BMC Geriatr 2020;20:155. 10.1186/s12877-020-01556-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Criscuolo C, Cennamo G, Montorio D, et al. Assessment of retinal vascular network in amnestic mild cognitive impairment by optical coherence tomography angiography. PLoS One 2020;15:e0233975. 10.1371/journal.pone.0233975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Santangelo R, Huang S-C, Bernasconi MP, et al. Neuro-Retina might reflect Alzheimer's disease stage. J Alzheimers Dis 2020;77:1455–68. 10.3233/JAD-200043 [DOI] [PubMed] [Google Scholar]
- 35.Costa-Cunha LVF, Cunha LP, Malta RFS, et al. Comparison of Fourier-domain and time-domain optical coherence tomography in the detection of band atrophy of the optic nerve. Am J Ophthalmol 2009;147:56–63. 10.1016/j.ajo.2008.07.020 [DOI] [PubMed] [Google Scholar]
- 36.Mitchell AJ. A meta-analysis of the accuracy of the mini-mental state examination in the detection of dementia and mild cognitive impairment. J Psychiatr Res 2009;43:411–31. 10.1016/j.jpsychires.2008.04.014 [DOI] [PubMed] [Google Scholar]
- 37.Shen Y, Shi Z, Jia R, et al. The attenuation of retinal nerve fiber layer thickness and cognitive deterioration. Front Cell Neurosci 2013;7:142. 10.3389/fncel.2013.00142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Méndez-Gómez JL, Rougier M-B, Tellouck L, et al. Peripapillary retinal nerve fiber layer thickness and the evolution of cognitive performance in an elderly population. Front Neurol 2017;8:93. 10.3389/fneur.2017.00093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shi Z, Zhu Y, Wang M, et al. The utilization of retinal nerve fiber layer thickness to predict cognitive deterioration. J Alzheimers Dis 2016;49:399–405. 10.3233/JAD-150438 [DOI] [PubMed] [Google Scholar]
- 40.Karakahya RH, Özcan Tuba Şaziye, Özcan T. Salvage of the retinal ganglion cells in transition phase in Alzheimer's disease with topical coenzyme Q10: is it possible? Graefes Arch Clin Exp Ophthalmol 2020;258:411–8. 10.1007/s00417-019-04544-3 [DOI] [PubMed] [Google Scholar]
- 41.Marquié M, Valero S, Castilla-Marti M, et al. Association between retinal thickness and β-amyloid brain accumulation in individuals with subjective cognitive decline: Fundació ACE healthy brain initiative. Alzheimers Res Ther 2020;12:37. 10.1186/s13195-020-00602-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Choi SH, Park SJ, Kim NR. Macular ganglion cell -Inner plexiform layer thickness is associated with clinical progression in mild cognitive impairment and Alzheimers disease. PLoS One 2016;11:e0162202. 10.1371/journal.pone.0162202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ding J, Patton N, Deary IJ, et al. Retinal microvascular abnormalities and cognitive dysfunction: a systematic review. Br J Ophthalmol 2008;92:1017–25. 10.1136/bjo.2008.141994 [DOI] [PubMed] [Google Scholar]
- 44.Heringa SM, Bouvy WH, van den Berg E, et al. Associations between retinal microvascular changes and dementia, cognitive functioning, and brain imaging abnormalities: a systematic review. J Cereb Blood Flow Metab 2013;33:983–95. 10.1038/jcbfm.2013.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parisi V, Restuccia R, Fattapposta F, et al. Morphological and functional retinal impairment in Alzheimer's disease patients. Clin Neurophysiol 2001;112:1860–7. 10.1016/S1388-2457(01)00620-4 [DOI] [PubMed] [Google Scholar]
- 46.Iseri PK, Altinaş O, Tokay T, et al. Relationship between cognitive impairment and retinal morphological and visual functional abnormalities in Alzheimer disease. J Neuroophthalmol 2006;26:18–24. 10.1097/01.wno.0000204645.56873.26 [DOI] [PubMed] [Google Scholar]
- 47.Kesler A, Vakhapova V, Korczyn AD, et al. Retinal thickness in patients with mild cognitive impairment and Alzheimer's disease. Clin Neurol Neurosurg 2011;113:523–6. 10.1016/j.clineuro.2011.02.014 [DOI] [PubMed] [Google Scholar]
- 48.Kirbas S, Turkyilmaz K, Anlar O, et al. Retinal nerve fiber layer thickness in patients with Alzheimer disease. J Neuroophthalmol 2013;33:58–61. 10.1097/WNO.0b013e318267fd5f [DOI] [PubMed] [Google Scholar]
- 49.Ascaso FJ, Cruz N, Modrego PJ, et al. Retinal alterations in mild cognitive impairment and Alzheimer's disease: an optical coherence tomography study. J Neurol 2014;261:1522–30. 10.1007/s00415-014-7374-z [DOI] [PubMed] [Google Scholar]
- 50.Gharbiya M, Trebbastoni A, Parisi F, et al. Choroidal thinning as a new finding in Alzheimer's disease: evidence from enhanced depth imaging spectral domain optical coherence tomography. J Alzheimers Dis 2014;40:907–17. 10.3233/JAD-132039 [DOI] [PubMed] [Google Scholar]
- 51.Polo V, Garcia-Martin E, Bambo MP, et al. Reliability and validity of Cirrus and spectralis optical coherence tomography for detecting retinal atrophy in Alzheimer's disease. Eye 2014;28:680–90. 10.1038/eye.2014.51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bayhan HA, Aslan Bayhan S, Celikbilek A, et al. Evaluation of the chorioretinal thickness changes in Alzheimer's disease using spectral-domain optical coherence tomography. Clin Exp Ophthalmol 2015;43:145–51. 10.1111/ceo.12386 [DOI] [PubMed] [Google Scholar]
- 53.Gao L, Liu Y, Li X, et al. Abnormal retinal nerve fiber layer thickness and macula lutea in patients with mild cognitive impairment and Alzheimer's disease. Arch Gerontol Geriatr 2015;60:162–7. 10.1016/j.archger.2014.10.011 [DOI] [PubMed] [Google Scholar]
- 54.Güneş A, Demirci S, Tök L, et al. Evaluation of retinal nerve fiber layer thickness in Alzheimer disease using spectral-domain optical coherence tomography. Turk J Med Sci 2015;45:1094–7. [PubMed] [Google Scholar]
- 55.Oktem EO, Derle E, Kibaroglu S, et al. The relationship between the degree of cognitive impairment and retinal nerve fiber layer thickness. Neurol Sci 2015;36:1141–6. 10.1007/s10072-014-2055-3 [DOI] [PubMed] [Google Scholar]
- 56.Salobrar-Garcia E, Hoyas I, Leal M. Analysis of retinal peripapillary segmentation in early Alzheimer's disease patients. Biomed Res Int 2015;2015:636548. 10.1155/2015/636548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shi Z, Wu Y, Wang M, et al. Greater attenuation of retinal nerve fiber layer thickness in Alzheimer's disease patients. J Alzheimers Dis 2014;40:277–83. 10.3233/JAD-131898 [DOI] [PubMed] [Google Scholar]
- 58.Garcia-Martin E, Bambo MP, Marques ML, et al. Ganglion cell layer measurements correlate with disease severity in patients with Alzheimer's disease. Acta Ophthalmol 2016;94:e454–9. 10.1111/aos.12977 [DOI] [PubMed] [Google Scholar]
- 59.Knoll B, Simonett J, Volpe NJ, et al. Retinal nerve fiber layer thickness in amnestic mild cognitive impairment: case-control study and meta-analysis. Alzheimers Dement 2016;4:85–93. 10.1016/j.dadm.2016.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pillai JA, Bermel R, Bonner-Jackson A, et al. Retinal nerve fiber layer thinning in Alzheimer's disease: a case-control study in comparison to normal aging, Parkinson's disease, and non-Alzheimer's dementia. Am J Alzheimers Dis Other Demen 2016;31:430–6. 10.1177/1533317515628053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ferrari L, Huang S-C, Magnani G, et al. Optical coherence tomography reveals retinal neuroaxonal thinning in frontotemporal dementia as in Alzheimer's disease. J Alzheimers Dis 2017;56:1101–7. 10.3233/JAD-160886 [DOI] [PubMed] [Google Scholar]
- 62.Jiang H, Wei Y, Shi Y, et al. Altered macular microvasculature in mild cognitive impairment and Alzheimer disease. J Neuroophthalmol 2018;38:292–8. 10.1097/WNO.0000000000000580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lahme L, Esser EL, Mihailovic N, et al. Evaluation of ocular perfusion in Alzheimer's disease using optical coherence tomography angiography. J Alzheimers Dis 2018;66:1745–52. 10.3233/JAD-180738 [DOI] [PubMed] [Google Scholar]
- 64.Shao Y, Jiang H, Wei Y, et al. Visualization of focal thinning of the ganglion Cell-Inner plexiform layer in patients with mild cognitive impairment and Alzheimer's disease. J Alzheimers Dis 2018;64:1261–73. 10.3233/JAD-180070 [DOI] [PubMed] [Google Scholar]
- 65.Uchida A, Pillai JA, Bermel R, et al. Outer retinal assessment using spectral-domain optical coherence tomography in patients with Alzheimer's and Parkinson's disease. Invest Ophthalmol Vis Sci 2018;59:2768–77. 10.1167/iovs.17-23240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cipollini V, Abdolrahimzadeh S, Troili F, et al. Neurocognitive assessment and retinal thickness alterations in Alzheimer disease: is there a correlation? J Neuroophthalmol 2020;40:370–7. 10.1097/WNO.0000000000000831 [DOI] [PubMed] [Google Scholar]
- 67.den Haan J, van de Kreeke JA, Konijnenberg E, et al. Retinal thickness as a potential biomarker in patients with amyloid-proven early- and late-onset Alzheimer's disease. Alzheimers Dement 2019;11:463–71. 10.1016/j.dadm.2019.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim J-I, Kang B-H. Decreased retinal thickness in patients with Alzheimer’s disease is correlated with disease severity. PLoS One 2019;14:e0224180. 10.1371/journal.pone.0224180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang YS, Zhou N, Knoll BM, et al. Parafoveal vessel loss and correlation between peripapillary vessel density and cognitive performance in amnestic mild cognitive impairment and early Alzheimer's disease on optical coherence tomography angiography. PLoS One 2019;14:e0214685. 10.1371/journal.pone.0214685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ashimatey BS, D'Orazio LM, Ma SJ, et al. Lower retinal capillary density in minimal cognitive impairment among older Latinx adults. Alzheimers Dement 2020;12:e12071. 10.1002/dad2.12071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chua J, Hu Q, Ke M, et al. Retinal microvasculature dysfunction is associated with Alzheimer's disease and mild cognitive impairment. Alzheimers Res Ther 2020;12:161. 10.1186/s13195-020-00724-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jindahra P, Hengsiri N, Witoonpanich P, et al. Evaluation of retinal nerve fiber layer and ganglion cell layer thickness in Alzheimer's disease using optical coherence tomography. Clin Ophthalmol 2020;14:2995–3000. 10.2147/OPTH.S276625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jorge L, Canário N, Martins R, et al. The Retinal Inner Plexiform Synaptic Layer Mirrors Grey Matter Thickness of Primary Visual Cortex with Increased Amyloid β Load in Early Alzheimer's Disease. Neural Plast 2020;2020:8826087 10.1155/2020/8826087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lemmens S, Van Craenendonck T, Van Eijgen J, et al. Combination of snapshot hyperspectral retinal imaging and optical coherence tomography to identify Alzheimer's disease patients. Alzheimers Res Ther 2020;12:144. 10.1186/s13195-020-00715-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mavilio A, Sisto D, Prete F, et al. RE-PERG in early-onset Alzheimer's disease: a double-blind, electrophysiological pilot study. PLoS One 2020;15:e0236568. 10.1371/journal.pone.0236568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Salobrar-Garcia E, Méndez-Hernández C, Hoz Rde, et al. Ocular vascular changes in mild Alzheimer's disease patients: foveal avascular zone, choroidal thickness, and ONH hemoglobin analysis. J Pers Med 2020;10. 10.3390/jpm10040231. [Epub ahead of print: 15 11 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sen S, Saxena R, Vibha D, et al. Detection of structural and electrical disturbances in macula and optic nerve in Alzheimer's patients and their correlation with disease severity. Semin Ophthalmol 2020;35:116–25. 10.1080/08820538.2020.1748203 [DOI] [PubMed] [Google Scholar]
- 78.Uchida A, Pillai JA, Bermel R, et al. Correlation between brain volume and retinal photoreceptor outer segment volume in normal aging and neurodegenerative diseases. PLoS One 2020;15:e0237078. 10.1371/journal.pone.0237078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wu J, Zhang X, Azhati G, et al. Retinal microvascular attenuation in mental cognitive impairment and Alzheimer's disease by optical coherence tomography angiography. Acta Ophthalmol 2020;98:e781–7. 10.1111/aos.14381 [DOI] [PubMed] [Google Scholar]
- 80.Biscetti L, Lupidi M, Luchetti E, et al. Novel noninvasive biomarkers of prodromal Alzheimer disease: the role of optical coherence tomography and optical coherence tomography-angiography. Eur J Neurol 2021;28:2185–91. 10.1111/ene.14871 [DOI] [PubMed] [Google Scholar]
- 81.Li M, Li R, Lyu J-H, et al. Relationship between Alzheimer's disease and retinal choroidal thickness: a cross-sectional study. J Alzheimers Dis 2021;80:407–19. 10.3233/JAD-201142 [DOI] [PubMed] [Google Scholar]
- 82.Mei X, Qiu C, Zhou Q, et al. Changes in retinal multilayer thickness and vascular network of patients with Alzheimer's disease. Biomed Eng Online 2021;20:97. 10.1186/s12938-021-00931-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Robbins CB, Grewal DS, Stinnett SS, et al. Assessing the retinal microvasculature in individuals with early and late-onset Alzheimer's disease. Ophthalmic Surg Lasers Imaging Retina 2021;52:336–44. 10.3928/23258160-20210528-06 [DOI] [PubMed] [Google Scholar]
- 84.Robbins CB, Grewal DS, Thompson AC, et al. Choroidal structural analysis in Alzheimer disease, mild cognitive impairment, and cognitively healthy controls. Am J Ophthalmol 2021;223:359–67. 10.1016/j.ajo.2020.09.049 [DOI] [PubMed] [Google Scholar]
- 85.Wang X, Zhao Q, Tao R, et al. Decreased retinal vascular density in Alzheimer's disease (AD) and mild cognitive impairment (MCI): an optical coherence tomography angiography (OCTA) study. Front Aging Neurosci 2020;12:572484. 10.3389/fnagi.2020.572484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wong MNK, Lai DWL, Chan HH-L, et al. Neural and retinal characteristics in relation to working memory in older adults with mild cognitive impairment. Curr Alzheimer Res 2021;18:185–95. 10.2174/1567205018666210608114044 [DOI] [PubMed] [Google Scholar]
- 87.Zabel P, Kaluzny JJ, Zabel K, et al. Quantitative assessment of retinal thickness and vessel density using optical coherence tomography angiography in patients with Alzheimer's disease and glaucoma. PLoS One 2021;16:e0248284. 10.1371/journal.pone.0248284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhao A, Fang F, Li B, et al. Visual abnormalities associate with hippocampus in mild cognitive impairment and early Alzheimer's disease. Front Aging Neurosci 2020;12:597491. 10.3389/fnagi.2020.597491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Montorio D, Criscuolo C, Breve MA, et al. Radial peripapillary vessel density as early biomarker in preperimetric glaucoma and amnestic mild cognitive impairment. Graefes Arch Clin Exp Ophthalmol 2022. 10.1007/s00417-022-05561-5. [Epub ahead of print: 22 Jan 2022]. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-054657supp001.pdf (395.2KB, pdf)
bmjopen-2021-054657supp002.pdf (218.3KB, pdf)
Data Availability Statement
Data are available on reasonable request. All data relevant to the study are included in the article or uploaded as online supplemental information. Data are available on reasonable request to the corresponding author.