Abstract
Objectives
Invasive meningococcal disease (IMD) is an urgent notifiable disease and its early notification is essential to prevent cases. The objective of the study was to assess the sensitivity of two independent surveillance systems and to estimate the incidence of IMD.
Design
We used capture–recapture model based on two independent surveillance systems, the statutory disease reporting (SDR) system and the microbiological reporting system (MRS) of the Public Health Agency of Catalonia, between 2011 and 2015. The capture–recapture analysis and 95% CIs were calculated using the Chapman formula. Multivariate vector generalised linear model was performed for adjusted estimation.
Measures
The variables collected were age, sex, year of report, size of municipality (<10 000 and ≥10 000), clinical form, death, serogroup, country of birth and type of reporting centre (private and public).
Results
The sensitivity of the two combined surveillance systems was 88.5% (85.0–92.0). SDR had greater sensitivity than the MRS (67.9%; 62.7–73.1 vs 64.7%; 59.4–70.0). In 2014–2015, the sensitivity of both systems was higher (80.6%; 73.2–87.9 vs 73.4%; 65.2–81.6) than in 2011–2013 (59.3%; 52.6–66.0 vs 58.3%; 51.6–65.1). In private centres, the sensitivity was higher for SDR than for MRS (100%; 100–100 vs 4.8%; −4.4–13.9). The adjusted estimate of IMD cases was lower than that obtained using the Chapman formula (279; 266–296 vs 313; 295–330). The estimated adjusted incidence of IMD was 0.7/100 000 persons-year.
Conclusions
The sensitivity of enhanced surveillance through the combination of two complementary sources was higher than for the sources individually. Factors associated with under-reporting in different systems should be analysed to improve IMD surveillance.
Keywords: diagnostic microbiology, epidemiology, molecular diagnostics, infection control
Strengths and limitations of this study.
The use of two surveillance sources as universal statutory disease reporting system based on passive reporting and sentinel microbiological reporting system (MRS) covering 83% of acute hospital beds, offer a wide coverage.
The independence of the two sources was demonstrated by complying with the premise of the capture–recapture method.
Not all centres participate in the MRS, thus not all cases diagnosed had the same probability of being selected from a given source.
The role of the automated electronic reporting of data that might be associated to a greater sensitivity was not analysed.
Background
Invasive meningococcal disease (IMD) continues to be an important cause of morbidity and mortality, mainly in children aged <4 years and adolescents.1
In the European regions, the incidence rate of confirmed IMD cases was 0.62/100 000 persons-year in 2018,2 and in Spain it was 0.86/100 000 persons.3 Six serogroups (A, B, C, W, X, Y) currently cause almost all cases of this life-threatening disease worldwide. Case fatality rate is about 10% in developed countries,4–6 and 40%–65% present with meningitis, but meningococcemia and pneumonia are also frequent,4 being the serogroup involved related both with the case fatality rate7 and the predominant clinical form.8 Serogroup B causes more than a third part of IMD,4 9 but in some countries or population groups the proportion is even higher.10 11 In Spain, from 2009 to 2018, serogroup B accounted for 64% of IMD cases.12 A high proportion, up to 60%13 of IMD cases, are affected by a range of sequelae and health-related impairment in the quality of life of survivors and their families.14
IMD is an urgent notifiable disease and its early notification is essential to provide an adequate public health response in patients and their close contacts to prevent further cases. Epidemiological surveillance allows monitoring of the impact of public health interventions, including vaccination programmes. Therefore, a robust epidemiological and microbiological system with timely and accurate surveillance providing information on the frequency of cases and the distribution of circulating serogroups is crucial.
Evaluations of surveillance systems should be conducted regularly to increase their utility.15–17 There are two reporting systems for the epidemiological surveillance of communicable disease in Catalonia: the statutory disease reporting (SDR) system and the microbiological reporting system (MRS).18
The capture–recapture method is a statistical method for estimating the real incidence of diseases in a population with two or more information sources.19 20 The method is valid if four conditions are met: (1) the population under study has to be closed, that is, there should be no changes during the study period; (2) there must be a method of determining whether an individual identified by one source is the same as an individual identified by the other; (3) each individual must have the same probability of being captured by either system; (4) the systems must be independent.
The aim of this study was to assess the sensitivity of the two surveillance systems in Catalonia (SDR and MRS) using the capture–recapture method and to estimate the incidence of IMD.
Methods
Information sources
Catalonia is a region in the northeast of Spain with a population of 7 508 106 in 2015.21
The SDR is a passive surveillance system through which health professionals report all infectious diseases subject to surveillance. The reporting of cases to the Public Health Agency of Catalonia (PHAC) is mandatory and includes confirmed cases of IMD and is regulated by a decree.18 22
The MRS is a surveillance system that consists of microbiologists notifying laboratory-confirmed microorganisms that cause infectious diseases. The main objectives of the MRS are to confirm suspected cases of infectious diseases through the identification of the microorganisms and serogroups involved and to determine trends and changes in epidemiological patterns and microbiological resistance.23
The MRS was non-compulsory until 2015 and involved 50 healthcare centres representing over 83% of acute hospital beds.24 Confirmed IMD cases were reported by microbiologists including sex, age, clinical presentation (meningitis, bacteraemia of unknown focus and other clinical presentations), serogroup and diagnostic method.
Both systems belong to the PHAC epidemiological surveillance network and, since 2014, transfer information automatically, but the independence of the sources is maintained.
Cases definition, inclusion and exclusion criteria
A confirmed case of IMD was defined as laboratory confirmed if at least one of the following criteria was fulfilled: isolation in cultures or detection of Neisseria meningitidis DNA by PCR in a normally sterile site, detection of gram-negative diplococci or N. meningitidis antigen in cerebrospinal fluid.
Data collection
We made a retrospective study of confirmed IMD cases in Catalonia from January 2011 to December 2015. We extracted all IMD records from the MRS and SDR and linked the databases using the personal identification code (PIC). When the PIC was not available, data on notification, age and sex were used to identify duplicates between the two sources. In cases with inconclusive matching, the hospital was used as a fifth matching criterion.
Estimates were made for the entire 5-year period and by age, sex, year of report, size of municipality (<10 000 and ≥10 000), country of birth, number of hospital beds, clinical form (meningitis, with or without sepsis, sepsis and others), serogroup, death and reporting centre (private or public).
Ethics statement
The study was not submitted for research ethics approval as the activities described were conducted as part of the legislated mandate of the Health Department of Catalonia, the competent authority for surveillance of communicable diseases according to decree 203/2015 of 15 September which created the epidemiological surveillance network of Catalonia.18 All the study activities formed part of public health surveillance and did not require informed consent. Personal data were used only for the matching process and measures to protect the confidentiality of personal data were applied (access to the data restricted to the personnel involved in data analysis, and removal of personal data from the datasets after matching).
Patient and public involvement
No patient involved.
Statistical methods
The total number of IMD cases was estimated using the two-source capture–recapture method, which uses Chapman’s formula,25 developed to reduce bias due to small samples:
where L1 is the number of cases in the SDR dataset, L2 is the number of cases reported to MRS and a is the number of cases captured by both systems. The sensitivity (Se) of case ascertainment by the two sources was also calculated as the proportion of true cases detected by each source, that is, Se (1)=L1/N for source 1 and Se (2)=L2/N for source 2. The sensitivity of both sources combined was calculated as the proportion of cases detected by one of the two sources or both, that is, Se (1, 2) =(L1+L2 -a)/N.
The independence of the sources was considered when applying the capture–recapture method.26 27 In the two-by-two table, where a represents cases reported by two sources or combinations of sources, b and c cases reported exclusively by either of the two sources and x the estimated non-reported cases by either of the sources, the OR (OR=ax/bc) should not differ from one.
As a multivariate model, a vector generalised linear model (VGLM) from the generalised additive model framework28 was used to evaluate patient characteristics and the probability of capture by the different sources taking into account the covariates: age (<15 vs ≥15), gender, year of notification (2011–2013 vs 2014–2015), size of the municipality (<10 000 vs ≥10 000), country of birth (Spain vs other), number of hospital beds (<200 vs ≥200) and diagnosis (meningitis vs septicaemia). The outcome for the model is a two column matrix with 0 and 1 indicating if the record is identified by SDR or MRS. We used a backwards stepwise procedure (using likelihood ratio tests, with a p value>0.2 as the criterion for removing variables from the model)29 30 to eliminate covariates, starting with a full model including all described covariates, and we used the parameter estimates from the model to estimate the sizes of population subgroups and calculate incidence rates. The 95% confidence intervals (CI) were calculated, allowing for uncertainty in the total number of cases estimated. For each of the described covariates, VGLM with source notification as outcome was used to test differences in sensitivities. All analyses were made using R software V.3.0.1.
Results
Patient characteristics
Patient characteristics by source are shown in table 1. From 2011 to 2015, 212 IMD cases were reported to the SDR and 202 cases to the MRS, representing an incidence of 0.56 and 0.54/100 000 persons-year, respectively. IMD due to serogroup B was the most frequently reported serogroup (77.4% and 75.7% in the SDR and MRS, respectively). Around 63% of patients were aged<15 years; the mean age was 21.4 for the SDR and 20.5 years for the MRS. Male sex was more frequent in the SDR (52.4%) than in the MRS (49%). The SDR presented the most cases in 2015 (48 cases; 22.6%) and the MRS (61 cases; 30.2%) in 2011. The SDR reported that 84% of patients lived in a municipality of ≥10 000 people compared with 73% in the MRS. In both sources, the number of cases declared in a hospital of ≥200 beds were around 70%. The main clinical form in both sources was meningitis (54.7% and 64.8%, respectively) and sepsis (38.7% and 32.7%, respectively). Reports from private centres represented 10% of cases in the SDR and 0.5% in the MRS. Overall, 22 cases (10.4%) reported by the SDR died compared with 11 cases (5.4%) reported by the MRS.
Table 1.
Sociodemographic, clinical and microbiological characteristics of invasive meningococcal disease cases reported to the SDR and MRS, Catalonia 2011–2015
| SDR (n=212) | MRS (n=202) | |
| Age groups | ||
| Mean (SD) | 21.4 (27.9) | 20.5 (26.7) |
| Median (IQR) | 6 (36) | 6 (32.3) |
| <2 years, n (%) | 62 (29.8%) | 61 (30.7%) |
| 2–4 years, n (%) | 35 (16.8%) | 30 (15.1%) |
| 5–14 years, n (%) | 34 (16.3%) | 35 (17.6%) |
| 15–24 years, n (%) | 12 (5.8%) | 12 (6.0%) |
| 25–34 years, n (%) | 12 (5.8%) | 9 (4.5%) |
| 35–44 years, n (%) | 10 (4.8%) | 12 (6.0%) |
| 45–54 years, n (%) | 9 (4.3%) | 7 (3.5%) |
| ≥55 years, n (%) | 34 (16.3%) | 33 (16.6%) |
| NAs | 1 (0.5%) | 2 (1.0%) |
| Sex, n (%) | ||
| Male | 111 (52.4%) | 99 (49.0%) |
| Female | 101 (47.6%) | 103 (51.0%) |
| Year of report, n (%) | ||
| 2011 | 43 (20.3%) | 61 (30.2%) |
| 2012 | 41 (19.3%) | 29 (14.4%) |
| 2013 | 38 (17.9%) | 30 (14.9%) |
| 2014 | 42 (19.8%) | 34 (16.8%) |
| 2015 | 48 (22.6%) | 48 (23.8%) |
| Size of municipality, n (%) | ||
| <10 000 people | 27 (12.7%) | 28 (13.9%) |
| ≥10 000 people | 177 (83.5%) | 148 (73.3%) |
| NAs | 8 (3.8%) | 26 (12.9%) |
| Country of birth, n (%) | ||
| Spain | 194 (91.5%) | 188 (93.1%) |
| Other countries | 18 (8.5%) | 14 (6.9%) |
| Hospital beds, n (%) | ||
| <200 | 60 (28.3%) | 65 (32.2%) |
| ≥200 | 149 (70.3%) | 137 (67.8%) |
| NAs | 3 (1.4%) | 0 (0.0%) |
| Clinical form, n (%) | ||
| Meningitis | 116 (54.7%) | 131 (64.8%) |
| Septicaemia | 82 (38.7%) | 66 (32.7%) |
| Other forms | 14 (6.6%) | 4 (2.0%) |
| NAs | 0 (0.0%) | 1 (0.5%) |
| Serogroup, n (%) | ||
| A | 0 (0.0%) | 2 (1.0%) |
| B | 164 (77.4%) | 153 (75.7%) |
| C | 26 (12.3%) | 21 (10.4%) |
| W135 | 4 (1.9%) | 6 (3.0%) |
| Y | 5 (2.4%) | 2 (1.0%) |
| Y/ W135 | 1 (0.5%) | 1 (0.5%) |
| Non-groupable | 6 (2.8%) | 4 (2.0%) |
| NAs | 6 (2.8%) | 13 (6.4%) |
| Type of reporting centre | ||
| Private | 21 (10.0%) | 1 (0.5%) |
| Public | 190 (90.0%) | 201 (99.5%) |
MRS, Microbiological reporting system; NAs, Not available; SDR, Statutory disease reporting.
Capture–recapture analysis
The OR was 1.01 (95% CI 0.62 to 1.66), reinforcing the independence of the two sources.
During the period studied, 212 and 202 IMD cases were reported by the SDR and MRS, respectively. Overall, 137 cases (43.8%) coincided in both sources and 36 cases (11.5%) were not reported to either source. The estimated number of cases was 313 (95% CI 295 to 330) (figure 1) and the estimated incidence rate was 0.83/100 000 persons-year.
Figure 1.
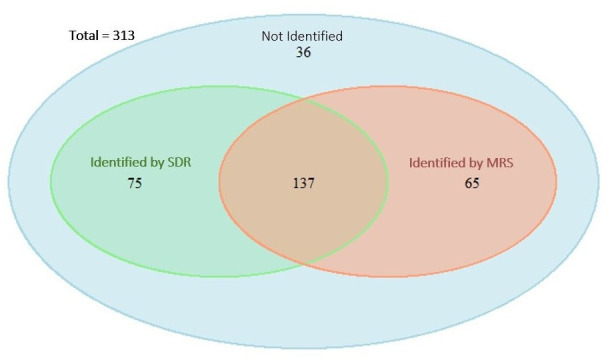
Venn diagram of the capture–recapture analysis of two datasets to estimate the total number of invasive meningococcal disease cases, Catalonia 2011–2015. MRS, microbiological reporting system; SDR, statutory disease reporting.
The sensitivity of the SDR was 67.9% (95% CI 62.7% to 73.1%) and that of the MRS was 64.7% (95% CI 59.4% to 70.0%) (p<0.001) (table 2). The sensitivity increased to 88.5% (95% CI 85.0% to 92.0%) when the datasets were combined.
Table 2.
Capture–recapture analysis of all invasive meningococcal disease cases reported to the SDR and MRS stratified by characteristics, Catalonia 2011–2015
| Records in SDR, n | Records in MRS, n | Matched records | Calculated unreported cases | Estimated total number of cases (95% CI) |
Sensitivity SDR (%) (95% CI) |
Sensitivity MRS (%) (95% CI) |
Difference in sensitivities (%) | P value | |
| All cases | 212 | 202 | 137 | 36 | 313 (295 to 330) | 67.9 (62.7 to 73.1) | 64.7 (59.4 to 70.0) | 3.2 | <0.001 |
| Age group | |||||||||
| <15 years | 131 | 126 | 87 | 20 | 190 (177 to 203) | 69.1 (62.6 to 75.7) | 66.5 (59.8 to 73.2) | 2.6 | 0.468 |
| ≥15 years | 80 | 74 | 49 | 16 | 121 (109 to 133) | 66.4 (58.0 to 74.8) | 61.4 (52.7 to 70.1) | 5.0 | |
| Sex | |||||||||
| Male | 111 | 99 | 71 | 16 | 155 (144 to 166) | 71.8 (64.7 to 78.9) | 64.0 (56.5 to 71.6) | 7.8 | 0.588 |
| Female | 101 | 103 | 66 | 20 | 158 (145 to 171) | 64.2 (56.7 to 71.7) | 65.5 (58.1 to 72.9) | −1.3 | |
| Year of report | |||||||||
| 2011–2013 | 122 | 120 | 71 | 35 | 206 (187 to 226) | 59.3 (52.6 to 66.0) | 58.3 (51.6 to 65.1) | 1.0 | <0.001 |
| 2014–2015 | 90 | 82 | 66 | 6 | 112 (106 to 118) | 80.6 (73.2 to 87.9) | 73.4 (65.2 to 81.6) | 7.2 | |
| Size of municipality | |||||||||
| <10 000 people | 27 | 28 | 22 | 2 | 35 (32 to 37) | 78.7 (65.0 to 92.4) | 81.6 (68.7 to 94.6) | −2.9 | 0.100 |
| ≥10 000 people | 177 | 148 | 110 | 23 | 238 (225 to 252) | 74.4 (68.9 to 80.0) | 62.2 (56.1 to 68.4) | 12.2 | |
| Country of birth | |||||||||
| Spain | 194 | 188 | 127 | 32 | 287 (271 to 304) | 67.6 (62.2 to 73.0) | 65.5 (60.0 to 71.0) | 2.1 | 0.696 |
| Other countries | 18 | 14 | 10 | 3 | 25 (20 to 30) | 72.3 (54.7 to 89.9) | 56.2 (36.7 to 75.7) | 16.1 | |
| Hospital beds, n | |||||||||
| <200 | 60 | 65 | 40 | 13 | 97 (87 to 108) | 61.7 (52.1 to 71.4) | 66.9 (57.5 to 76.2) | −5.1 | 0.514 |
| ≥200 | 149 | 137 | 97 | 22 | 210 (197 to 224) | 70.9 (64.7 to 77.0) | 65.2 (58.7 to 71.6) | 5.7 | |
| Clinical form | |||||||||
| Meningitis | 116 | 131 | 84 | 18 | 181 (169 to 193) | 64.2 (57.2 to 71.2) | 72.5 (66.0 to 79.0) | −8.3 | 0.936 |
| Sepsis | 82 | 66 | 50 | 10 | 108 (99 to 117) | 75.9 (67.9 to 84.0) | 61.1 (51.9 to 70.3) | 14.8 | |
| Type of reporting centre | |||||||||
| Private | 21 | 1 | 1 | 0 | 21 (21 to 21) | 100 (100 to 100) | 4.8 (−4.4 to 13.9) | 95.2 | 0.002 |
| Public | 190 | 201 | 136 | 26 | 281 (267 to 295) | 67.7 (62.2 to 73.2) | 71.6 (66.4 to 76.9) | −3.9 | |
| Serogrup | |||||||||
| B | 164 | 153 | 114 | 17 | 220 (209 to 231) | 74.6 (68.8 to 80.3) | 69.6 (63.5 to 75.6) | 5.0 | 0.636 |
| C | 26 | 21 | 16 | 3 | 34 (29 to 39) | 76.7 (62.5 to 90.9) | 61.9 (45.6 to 78.3) | 14.8 | |
MRS, microbiological reporting system; SDR, statutory disease reporting system.
There were no differences in sensitivity between in <15 years and ≥15 years age group (p value=0.468) in either source, although it was higher in the <15 years (69.1%;95% CI 62.6% to 75.7% in the SDR and 66.5%; 95% CI 59.8% to 73.2% in the MRS). The age groups with the highest sensitivity were 2–4 years in the SDR, with 80.3% (95% CI 68.5% to 92.1%), and 35–44 years in the MRS, with 80.5% (95% CI 60.4% to 100.0%) (figure 2).
Figure 2.
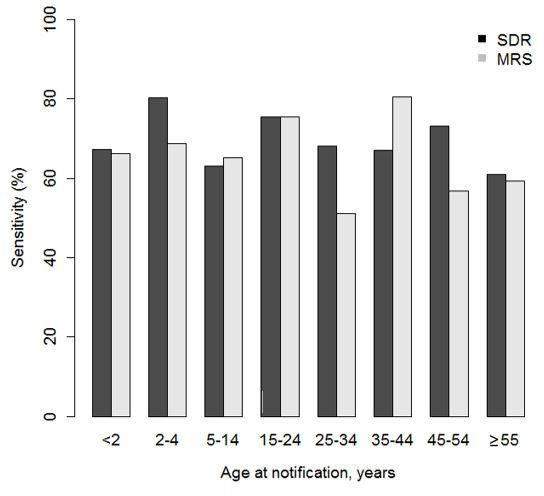
Sensitivities of the SDR and MRS stratified by age groups. Catalonia 2011–2015. MRS, microbiological reporting system; SDR, statutory disease reporting.
In 2011–2013, sensitivity for the SDR and the MRS were 59.3% (95% CI 52.6% to 66%) and 58.3% (95% CI 51.6% to 65.1%), respectively, lower than that in 2014–2015 (80.6%; 95% CI 73.2% to 87.9%, for the SDR and 73.4%; 95% CI 65.2% to 81.6%, for the MRS (p<0.001)) (table 2). Year 2014 showed the highest sensitivity for both sources: 91.3% (95% CI 83.2% to 99.4%) for the SDR and 73.9% (95% CI 61.2% to 86.6%) for the MRS (figure 3). Year 2011 was the only year in which the MRS had a higher sensitivity than the SDR (56.4%; 95% CI 47.1% to 65.8% and 39.8%; 95% CI 30.6% to 49.0%, respectively). In private centres, the sensitivity of the SDR was 100% (95% CI 100% to 100%) and that of the MRS was 4.8% (95% CI −4.4% to 13.9%). No differences were found in other characteristics analysed.
Figure 3.
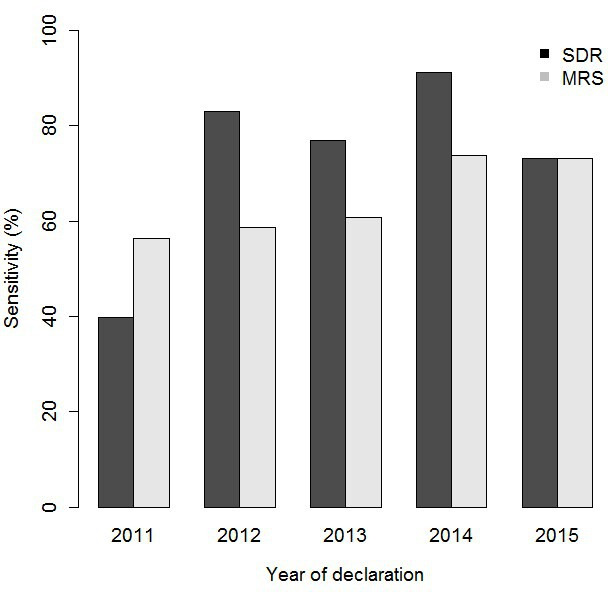
Sensitivities of the SDR and MRS stratified by year of reporting, Catalonia 2011–2015. MRS, microbiological reporting system; SDR, statutory disease reporting.
For meningitis, 116 and 131 cases were reported by the SDR and the MRS, respectively. The estimated number of meningitis cases was 181, and 18 cases were not reported by either source. The highest sensitivity was detected in the MRS (72.5%; 95% CI 66% to 79%) compared with the SDR (64.2%; 95% CI 57.2% to 71.2%) (p<0.001) (table 3). Years 2014–2015 showed a higher sensitivity in both sources compared with 2011–2013: 82.4% (95% CI 72.7% to 92%) in the MRS and 75.6% (95% CI 64.7% to 86.5%) in the SDR. Public centres had a higher sensitivity in the MRS (77.7%; 95% CI 71.4% to 84.0%) and in the SDR (63.9%; 95% CI 56.6% to 71.2%) (p<0.037).
Table 3.
Capture–recapture analysis of meningococcal meningitis reported to the SDR and MRS stratified by characteristics, Catalonia 2011–2015
| Records in SDR, n | Records in MRS, n | Matched records | Calculated unreported cases | Estimated total number of cases (95% CI) |
Sensitivity SDR (%) (95% CI) |
Sensitivity MRS (%) (95% CI) |
Difference in sensitivities (%) | P value | |
| All cases | 116 | 131 | 84 | 18 | 181 (169 to 193) | 64.2 (57.2 to 71.2) | 72.5 (66.0 to 79.0) | −8.3 | <0.001 |
| Age group | |||||||||
| <15 years | 72 | 81 | 51 | 13 | 115 (104 to 125) | 63.1 (54.3 to 72.0) | 71.0 (62.7 to 79.3) | −7.9 | 0.682 |
| ≥15 years | 43 | 49 | 32 | 6 | 66 (60 to 73) | 65.5 (53.9 to 77.0) | 74.6 (64.1 to 85.1) | −9.1 | |
| Sex | |||||||||
| Male | 62 | 69 | 47 | 7 | 91 (84 to 98) | 68.2 (58.6 to 77.8) | 75.9 (67.1 to 84.7) | −7.7 | 0.245 |
| Female | 54 | 62 | 37 | 12 | 91 (81 to 101) | 59.9 (49.8 to 70.0) | 68.7 (59.2 to 78.3) | −8.9 | |
| Year of report | |||||||||
| 2011–2013 | 71 | 82 | 47 | 18 | 124 (111 to 137) | 57.5 (48.8 to 66.2) | 66.4 (58.1 to 74.7) | −8.9 | 0.013 |
| 2014–2015 | 45 | 49 | 37 | 3 | 60 (56 to 64) | 75.6 (64.7 to 86.5) | 82.4 (72.7 to 92.0) | −6.7 | |
| Size of municipality | |||||||||
| <10 000 people | 19 | 20 | 16 | 1 | 24 (22 to 26) | 80.2 (64.1 to 96.2) | 84.4 (69.8 to 99.0) | −4.2 | 0.165 |
| ≥10 000 people | 93 | 93 | 65 | 12 | 133 (124 to 143) | 70.0 (62.2 to 77.8) | 70.0 (62.2 to 77.8) | 0.0 | |
| Country of birth | |||||||||
| Spain | 107 | 120 | 77 | 17 | 167 (155 to 179) | 64.3 (57.0 to 71.5) | 72.1 (65.3 to 78.9) | −7.8 | 0.862 |
| Other countries | 9 | 11 | 7 | 1 | 14 (12 to 17) | 64.3 (39.2 to 89.4) | 78.6 (57.1 to 100.0) | −14.3 | |
| Hospital beds, n | |||||||||
| <200 | 31 | 40 | 23 | 6 | 54 (47 to 61) | 57.7 (44.5 to 70.9) | 74.5 (62.8 to 86.2) | −16.8 | 0.516 |
| ≥200 | 84 | 91 | 61 | 11 | 126 (116 to 135) | 67.1 (58.9 to 75.4) | 72.7 (64.9 to 80.5) | −5.6 | |
| Type of reporting centre | |||||||||
| Private | 9 | 1 | 1 | 0 | 9 (9 to 9) | 100.0 (100.0 to 100.0) | 11.1 (−9.4 to 31.6) | 88.9 | 0.037 |
| Public | 107 | 130 | 83 | 14 | 168 (158 to 178) | 63.9 (56.6 to 71.2) | 77.7 (71.4 to 84.0) | −13.7 | |
| Serogrup | |||||||||
| B | 91 | 100 | 70 | 9 | 130 (122 to 138) | 70.1 (62.2 to 77.9) | 77.0 (69.7 to 84.2) | −6.9 | 0.641 |
| C | 16 | 16 | 11 | 2 | 23 (19 to 27) | 69.3 (50.4 to 88.1) | 69.3 (50.4 to 88.1) | 0 |
MRS, Microbiological reporting system; SDR, Statutory disease reporting.
For septicaemia, 82 cases and 66 cases were reported by the SDR and the MRS, respectively. The sensitivity was higher for the SDR (75.9%; 95% CI 67.9% to 84%) than the MRS (61.1%; 95% CI 51.9% to 70.3%) (table 4). There were 108 estimated cases and 10 cases were not reported by either source. The sensitivity was higher in the <15 years than in the ≥15 years in both sources, but higher in the SDR (81.1%; 95% CI 71.1% to 91.1% vs 71%; 95% CI 59.4% to 82.5% for the MRS; p=0.036), and higher in 2014–2015 than in 2011–2013 (87.6%; 95% CI 78% to 97.3% for the SDR and 71.9%; 95% CI 58.7% to 85.1% for the MRS) (p<0.015).
Table 4.
Capture–recapture analysis of meningococcal septicaemia reported to the SDR and MRS stratified by characteristics, Catalonia 2011–2015
| Records in SDR, n | Records in MRS, n | Matched records | Calculated unreported cases | Estimated total number of cases (95% CI) |
Sensitivity SDR (%) (95% CI) |
Sensitivity MRS (%) (95% CI) |
Difference in sensitivities (%) |
P value | |
| All cases | 82 | 66 | 50 | 10 | 108 (99 to 117) | 75.9 (67.9 to 84.0) | 61.1 (51.9 to 70.3) | 14.8 | <0.001 |
| Age at notification, years | |||||||||
| <15 years | 48 | 42 | 34 | 4 | 60 (55 to 64) | 81.1 (71.1 to 91.1) | 71.0 (59.4 to 82.5) | 10.1 | 0.036 |
| ≥15 years | 34 | 23 | 16 | 8 | 49 (40 to 58) | 70.3 (57.4 to 83.1) | 47.5 (33.4 to 61.6) | 22.7 | |
| Sex | |||||||||
| Male | 43 | 27 | 22 | 5 | 53 (47 to 59) | 81.8 (71.3 to 92.2) | 51.3 (37.8 to 64.8) | 30.4 | 0.315 |
| Female | 39 | 39 | 28 | 5 | 55 (49 to 60) | 72.0 (60.0 to 83.9) | 72.0 (60.0 to 83.9) | 0.0 | |
| Year of report | |||||||||
| 2011–2013 | 43 | 34 | 22 | 11 | 66 (56 to 77) | 65.2 (53.6 to 76.7) | 51.5 (39.5 to 63.6) | 13.6 | 0.015 |
| 2014–2015 | 39 | 32 | 28 | 2 | 45 (42 to 48) | 87.6 (78.0 to 97.3) | 71.9 (58.7 to 85.1) | 15.7 | |
| Size of municipality | |||||||||
| <10 000 people | 7 | 7 | 5 | 1 | 10 (8 to 12) | 72.2 (44.0 to 100.0) | 72.2 (44.0 to 100.0) | 0.0 | 0.918 |
| ≥10 000 people | 71 | 52 | 43 | 6 | 86 (80 to 93) | 82.9 (74.9 to 90.8) | 60.7 (50.3 to 71.0) | 22.2 | |
| Country of birth | |||||||||
| Spain | 73 | 63 | 47 | 9 | 98 (90 to 106) | 74.7 (66.1 to 83.3) | 64.5 (55.0 to 74.0) | 10.2 | 0.275 |
| Other countries | 9 | 3 | 3 | 0 | 9 (9 to 9) | 100.0 (100.0 to 100.0) | 33.3 (2.5 to 64.1) | 66.7 | |
| Hospital beds, n | |||||||||
| <200 | 25 | 23 | 16 | 4 | 36 (31 to 42) | 70.0 (55.0 to 85.1) | 64.4 (48.7 to 80.1) | 5.6 | 0.831 |
| ≥200 | 56 | 43 | 34 | 6 | 71 (65 to 78) | 79.2 (69.7 to 88.7) | 60.8 (49.4 to 72.2) | 18.4 | |
| Type of reporting centre | |||||||||
| Private | 9 | 0 | 0 | 0 | 9 (9 to 9) | 100.0 (100.0 to 100.0) | 0 (0.0 to 0.0) | 100 | 0.988 |
| Public | 73 | 66 | 50 | 8 | 97 (89 to 104) | 75.9 (67.3 to 84.4) | 68.6 (59.3 to 77.9) | 7.3 | |
| Serogrup | |||||||||
| B | 66 | 51 | 43 | 4 | 78 (73 to 84) | 84.4 (76.4 to 92.4) | 65.2 (54.7 to 75.8) | 19.2 | 0.661 |
| C | 8 | 4 | 4 | 0 | 8 (8 to 8) | 100 (100 to 100) | 50.0 (15.4 to 84.7) | 50.0 |
MRS, microbiological reporting system; SDR, statutory disease reporting system.
Serogroup B (online supplemental table 1) showed the sensitivity of the SDR was higher than that of the MRS (74.6%; 95% CI 68.8% to 80.3% and 69.6%; 95% CI 63.5% to 75.6%, respectively). There were differences according to the period and the type of centre. In 2014–2015, the sensitivity was 87.1% (95% CI 79.7% to 94.5%) for the SDR and 78.3% (95% CI 69.2% to 87.4%) for the MRS (p<0.002). In private centres, the sensitivity in SDR was 100% compared with 7.1% (95% CI −6.4% to 20.6%) (p=0.004) in MRS. The sensitivity was higher for IMD serogroup C cases in SDR than in MRS (76.7%; 95% CI 62.5% to 90.9% and 62%; 95% CI 45.6% to 78.3%, respectively) (online supplemental table 2).
bmjopen-2021-058003supp001.pdf (113.1KB, pdf)
All 22 deaths were reported in the SDR (Case fatality rate: 10.4%), and the sensitivity of the SDR was higher than that of the MRS (100%; 95 CI% 100% to 100% vs 50%; 95% CI 29.1% to 70.9%, p=0.104). No differences were found in other characteristics analysed (online supplemental table 3).
The results of the multivariate model for all cases are shown in table 5. The variables considered to define the sensitivity of the two sources were year of report (2011–2013 vs 2014–2015) and size of municipality. With these variables in the model, the adjusted estimate of the total number of cases was 279 cases (95% CI 266 to 296) and the estimated incidence rate was 0.7/100 000 persons-year.
Table 5.
Variables defining the sensitivity of the statutory disease reporting system and microbiological reporting system in detecting invasive meningococcal diseases cases. Multivariate model.
| OR (95% CI) | P value | |
| Year of report (2014–2015) | 2.29 (1.35 to 3.89) | 0.002 |
| Size of municipality (≥10 000 people) | 0.51 (0.23 to 1.12) | 0.093 |
n estimate: 279 (266, 296).
Discussion
The sensitivity obtained by combining the two surveillance system for IMD cases was 88.5%, greater than for each source. Globally, the SDR showed higher sensitivity than the MRS, mainly for cases of sepsis, serogroup B and serogroup C, although for meningitis the sensitivity of the MRS was higher than that of the SDR.
Sensitivity of SDR was 67.9%, very close to that of 66.5% found by Andrianou et al in Italy in a study carried out in 2018 using the hospital discharge records system as the external source.31
Other studies found greater sensitivities by combining data systems than we did. Baldovin et al 32 in Italy reported an overall sensitivity of 94.7% by combining four data sources (mandatory notification system, laboratory surveillance, invasive bacterial surveillance and hospital discharge). Jansson et al,33 in Sweden found a global sensitivity of 98.7%, 91.1% for clinical notification and 85.4% for laboratory reporting. In Austria, a good agreement between the National Reference Center for meningococci and the hospital discharge was found, although a clinical review of hospital discharge data was necessary to detect false-positive cases recorded.34
Globally, the sensitivity was similar in children aged<15 years than in persons aged≥15 years in both sources (69.1% for the SDR and 66.5% for the MRS; p=0.468). The differences could be because there is greater sensibilisation to declare paediatric cases than adult cases or because there are differences on IMD incidence according to age.9 Gibson et al 35 in Australia analysed IMD sensitivity in children aged<15 years in three sources: notifiable system, hospitalised patients and mortality data. They found a greater sensitivity (99.5%) than we did, although 15% of hospitalised children were false-positive cases.
Sensitivity was higher in 2014–2015 than in 2011–2013 for both sources (SDR and MRS). SDR had overall higher sensitivity for IMD cases, septicaemia cases as well as serogroup B and C cases, but not for meningitis cases for which MRS had higher sensitivity. The improvement in notification in the years 2014–2015 may be due to different causes, one could be that there is greater awareness for the notification of infectious diseases to public health surveillance systems, although it should be analysed in subsequent studies. In a different way, Andrianou et al 31 compared the surveillance of the Italian IMD with the registry of hospital discharges, and found a lower sensitivity in 2018 compared with 2015–2017. This yearly evaluation allows the detection of problems in the notification process.
We found a greater sensitivity for meningitis in the MRS than in the SDR (72.5% vs 64.2%) but not for septicaemia (61.1% vs 75.9%). Multiple reasons could explain this fact. A possible explanation is that meningitis has a specific section in MRS for reporting, while septicaemia is reported in bacteraemia of unknown focus section and it could be confused. It is important to determine the reason for this lower sensitivity to septicaemia in order to improve the completeness of MRS reporting. It is difficult to compare our results with those of other studies, since other sources of information were used or the independence of data sources was presumed but not demonstrated,34 which is essential when using the capture–recapture method.
Notification of confirmed cases of IMD by laboratories is essential in epidemiological surveillance.36 Molecular information on circulating serogroups that is required to implement public health measures such as vaccination is essential to control the disease37 and evaluate the impact of available vaccines.
In the absence of automated electronic reporting, monitoring and increasing the speed of laboratory reports may allow the public health department to administer chemoprophylaxis and vaccination to contacts.27 Although a higher sensitivity has been reported for electronic reporting than for paper-based reports by some authors,38 during the study period, automated electronic reporting was used in the SDR but not in the MRS, which may explain, at least in part, why the MRS had a lower sensitivity than the SDR.39
In the multivariate model, the 2014–2015 period and the size of the municipality show a higher sensitivity in the SDR, suggesting that IMD was well recorded in the two surveillance systems, although 36 cases (11.5%) were not captured by either source. This suggests there was under-reporting, despite the clinical severity of the disease. Other authors have also found under-reporting of this disease.40 It is very important to improve reporting by all physicians and microbiologists to the SDR and MRS to assess the impact of interventions such as immunisation.
The estimated IMD incidence rate of 0.7/100 000 persons-year found in the multivariate model is less than that found using capture–recapture (0.83/100 000 persons-year) but higher than that calculated using the SDR (0.56/100 000 persons-year) or MRS data (0.54/100 000 persons-year). Other European studies showed incidence rates of between 0.3932 and 1.18/100 000 persons-year.34
The sensitivity of the two sources were intermediate (67.9% for the SDR and 64.7% for the MRS). The lower sensitivity of the MRS may be due to the fact that the MRS is a sentinel system with a coverage of 83% of acute hospital beds and without private centres. In our series, 21 cases (10%) included in the SDR were reported by private centres, while only 1 case (0.5%) was reported to the MRS; this patient was finally transferred to a public hospital. The inclusion of cases that have an equal probability of selection in one source might lead to an overestimation. Other authors have reported this limitation when the hospital discharge data set includes probable cases, which are not included in the reference centre.34
Death was registered in 22 cases (10.5%), similar to that reported in other European countries,2 but slightly lower than that observed in Italy (14%) using the capture–recapture method.32 All cases were reported to the SDR, but only 50% were reported to the MRS, indicating that clinical data are better in the SDR than in the MRS. Other authors have used mortality data for capture–recapture analysis and concluded that all deaths were reported in notifiable systems.34
The sensitivity of the sources studied for the surveillance of IMD cannot be generalised to other diseases because physicians’ or microbiologists’ perception of the importance of IMD differs from that of other diseases.38
The main strength of this study is that the two sources had wide coverage. The SDR is a universal epidemiological surveillance source and, unlike the MRS, is a sentinel source, with a high coverage of 83%. Cases with PIC accounted for 85.5% of all cases reported to detect whether cases were coincident or not. In addition, the independence of the two sources was demonstrated, complying with the premise of the capture–recapture method.
A limitation of the study was that not all cases had the same probability of being selected from a given source. Cases diagnosed in private centres or public centres that did not participate in the MRS could not be reported by this system and this may explain, at least in part, the lower sensitivity than the SDR. This highlights the importance of including public and private centres to increase the robustness of the MRS. Another limitation was that we did not analyse the role of the electronic surveillance system, although a previous study detected greater sensitivity of the SDR when electronic surveillance was introduced.39
Conclusions
The sensitivity of enhanced surveillance through the combination of two complementary sources (statutory reporting by physicians and microbiological reporting by microbiologists) was higher than that of the individual sources. These systems are complementary and constitute the basic sources of information necessary for adequate epidemiological surveillance of IMD. Specific studies to estimate the factors associated with under-reporting are needed to reinforce epidemiological surveillance of this disease.
Supplementary Material
Footnotes
Collaborators: The Working Group of the Microbiological Reporting System of Catalonia is composed by: M Teresa Bastida (Fundació Hospital Esperit Sant); Frederic Ballester; Isabel Pujol (Hospital Universitari de Sant Joan de Reus); Miguel Ángel Benítez, Alba Cebollero (Consorci de Laboratoris Intercomarcal de l’Alt Penedès); Jordi Vila, Jordi Bosch, (Hospital Clínic); Ana Calderón (Hospital Municipal de Badalona); Margarida Curriu (Hospital Comarcal de Sant Bernabé); M. Ángeles Domínguez, Fe Tubau Quintano (Hospital Universitari de Bellvitge); Jose Manuel Ramírez (Hospital Universitari de Girona Dr. Josep Trueta); Ma José Fusté (Clínica de Terres de l’Ebre); Carme Gallés, Pilar Hernández Pérez, Elisenda Capdevila Gil de Bernabé (Corporació de Salut del Maresme i La Selva); Paula Gassiot (Hospital de Figueres); Frederic Gómez (Hospital Universitari de Tarragona Joan XXIII); Araceli González-Cuevas (Hospital General del Parc Sanitari Sant Joan de Déu); Marius Juanpere (Hospital Móra d’Ebre); Carmen Muñoz-Almagro, Amaresh Pérez-Argüello (Hospital Sant Joan de Déu. Esplugues de Llobregat); Carmina Martí (Hospital General de Granollers); Núria Margall (Hospital de la Santa Creu i Sant Pau); Lurdes Matas, Montserrat Gimenez (Hospital Universitari Germans Trias i Pujol); Montserrat Morta, Glòria Trujillo (Hospital Sant Joan de Déu. Manresa-Fundació Althaia); Sílvia Noguer (Hospital del Vendrell); Montserrat Olsina (Hospital General de Catalunya); Amaia Oteiza (H. Palamós); Pepa Pérez (Catlab-Centre Analítiques Terrassa); Mar Olga Pérez-Moreno (Hospital Verge de la Cinta de Tortosa); Tomás Pumarola, Juanjo González (Hospital Universitari Vall d’Hebron); Xavier Raga (Hospital de Sant Pau i Santa Tecla); Mercè Garcia, Mercè Ribelles (Hospital Universitari Arnau de Vilanova de Lleida); Esther Sanfeliu (Hospital d’Olot Comarcal de la Garrotxa); Goretti Sauca (Hospital de Mataró); Dionisia Fontanals, Isabel Sanfeliu (Corporació Sanitaria Parc Taulí, Sabadell) i Anna Vilamala (Hospital General de Vic). The Working Group of the Epidemiological Surveillance Network of Catalonia is composed by: César Arias, Irene Barrabeig, Neus Camps, Mònica Carol, Núria Follia, Pere Godoy, Ana Martínez, Sofia Minguell, Ignasi Parron, Mª Rosa Sala-Farré, Ariadna Rovira (Agència de Salut Pública de Catalunya), Cristina Rius (Agència de Salut Pública de Barcelona).
Contributors: PC is the guarantor and analysed and interpreted data, studied conception and design of the study and writes the manuscript. MV and NS did statistical analysis. GC revised and collected data. TG, SH and LR collected data. MJ revised the study and AD did critical revision and got funding.
Funding: This work was supported partially by CIBER of Epidemiology and Public Health (CIBERESP), Carlos III Health Institute and the Catalan Agency for the Management of Grants for University Research (AGAUR grant number 2017/SGR 1342). The funding sources played no part in the design of the study and collection, analysis and interpretation of data and in writing the manuscript.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Contributor Information
Working Group of the Microbiological Reporting System of Catalonia and Working Group of the Epidemiological Surveillance Network of Catalonia:
Maria Teresa Bastida, Frederic Ballester, Isabel Pujol, Miguel Angel Benítez, Alba Cebollero, Jordi Vila, Jordi Bosch, Ana Calderon, Margarida Curriu, M Angeles Dominguez, Fe Tubau Quintano, Jose Manuel Ramirez, Ma Jose Fusté, Carme Gallés, Pilar Hernandez Pérez, ElisendaCapdevila GildeBernabe, Paula Gassiot, Frederic Gómez-Bertomeu, Araceli González-Cuevas, Marius Juanpere, Carmen Muñoz-Almagro, Amaresch Perez Arguello, Carmina Martí, Nuria Margall, Lurdes Matas, Montserrat Giménez, Montserrat Morta, Gloria Trujillo, Silvia Noguer, Montserrat Olsina, Amaia Oteiza Ubanell, Pepa Perez, Mar Olga Pérez-Moreno, Tomas Pumarola, Juanjo Gonzales, Xavier Raga, Mercè Garcia, Mercè Ribelles, Esther Sanfeliu, Goretti Sauca, Dionisia Fontanals, Isabel Sanfeliu, Anna Vilamala, César Arias, Irene Barrabeig, Neus Camps, Mònica Carol, Núria Follia, Pere Godoy, Ana Martínez, Sofia Minguell, Ignasi Parron, Ma Rosa Sala-Farré, Ariadna Rovira, and Cristina Rius
Data availability statement
Data are available upon reasonable request. The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1. World Health Organization . Laboratory methods for the diagnosis of meningitis caused by Neisseria meningitidis, Streptococcus pneumoniae, and Haemophilus influenzae: WHO manual. 2nd ed. Geneve: WHO, 2011. [Google Scholar]
- 2. European Center for Disease Prevention and Control . Surveillance atlas on infectious diseases, 2018. Available: https://www.ecdc.europa.eu/en/meningococcal-disease/surveillance-and-disease-data/atlas
- 3. Centro Nacional de Epidemiología . Enfermedades de declaración obligatoria. España; 2018. https://www.isciii.es/QueHacemos/Servicios/VigilanciaSaludPublicaRENAVE/EnfermedadesTransmisibles/Documents/INFORMES/INFORMESRENAVE/RENAVE_cierre_EDO_2018.pdf
- 4. Stephens DS. Neisseria meningitidis. In: Bennet JE, Dolin R, Blaser MJ, eds. Principles and practice of infectious diseases. 9th edn. Philadelphia: Elsevier, 2019: 2585–607. [Google Scholar]
- 5. Nuttens C, Findlow J, Balmer P, et al. Evolution of invasive meningococcal disease epidemiology in Europe, 2008 to 2017. Euro Surveill 2022;27:2002075. 10.2807/1560-7917.ES.2022.27.3.2002075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang B, Santoreneos R, Giles L, et al. Case fatality rates of invasive meningococcal disease by serogroup and age: a systematic review and meta-analysis. Vaccine 2019;37:2768–82. 10.1016/j.vaccine.2019.04.020 [DOI] [PubMed] [Google Scholar]
- 7. Beebeejaun K, Parikh SR, Campbell H, et al. Invasive meningococcal disease: timing and cause of death in England, 2008-2015. J Infect 2020;80:286–90. 10.1016/j.jinf.2019.12.008 [DOI] [PubMed] [Google Scholar]
- 8. Loenenbach AD, van der Ende A, de Melker HE, et al. The clinical picture and severity of invasive meningococcal disease serogroup W compared with other serogroups in the Netherlands, 2015-2018. Clin Infect Dis 2020;70:2036–44. 10.1093/cid/ciz578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Acevedo R, Bai X, Borrow R, et al. The global meningococcal initiative meeting on prevention of meningococcal disease worldwide: epidemiology, surveillance, hypervirulent strains, antibiotic resistance and high-risk populations. Expert Rev Vaccines 2019;18:15–30. 10.1080/14760584.2019.1557520 [DOI] [PubMed] [Google Scholar]
- 10. Salama M, Kopel E, Jaffe J, et al. Surveillance of invasive meningococcal disease in the Tel Aviv district, Israel, 2007-2017. Vaccine 2019;37:6186–91. 10.1016/j.vaccine.2019.08.055 [DOI] [PubMed] [Google Scholar]
- 11. Van CP, Nguyen TT, Bui ST, et al. Invasive meningococcal disease remains a health threat in Vietnam people's army. Infect Drug Resist 2021;14:5261–9. 10.2147/IDR.S339110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Red Nacional de Vigilancia Epidemiológica . Enfermedad meningocócica. Vigilancia de la temporada 2017-2018. Available: https://www.isciii.es/QueHacemos/Servicios/VigilanciaSaludPublicaRENAVE/EnfermedadesTransmisibles/Documents/archivos%20A-Z/Enfer_Meningoc%C3%B3cica/RENAVE_EMI-2017-18.pdf
- 13. Igidbashian S, Bertizzolo L, Tognetto A, et al. Invasive meningococcal disease in Italy: from analysis of national data to an evidence-based vaccination strategy. J Prev Med Hyg 2020;61:e152–61. 10.15167/2421-4248/jpmh2020.61.2.1589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Olbrich KJ, Müller D, Schumacher S, et al. Systematic review of invasive meningococcal disease: sequelae and quality of life impact on patients and their caregivers. Infect Dis Ther 2018;7:421–38. 10.1007/s40121-018-0213-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. CDC Guidelines Working Group . Updated guidelines for evaluating public health surveillance systems. Morb Mortal Wkly Rep 2001;50:1–35. [PubMed] [Google Scholar]
- 16. World Health Organization . Protocol for the evaluation of epidemiological surveillance systems. Available: https://apps.who.int/iris/handle/10665/63639 [Accessed 06 Sep 2020].
- 17. Romaguera RA, German RR, Klaucke DN. Evaluating public health surveillance. In: Principles and practice of public health surveillance. New York: Oxford University Press, 2000: 176–93. [Google Scholar]
- 18. Generalitat de Catalunya . Decret 2013/2015, de 15 de setembre, pel qual ES creA La Xarxa de Vigilancia Epidemiològica I ES regulen ELS sistemes de notificació de malalties de declaració obligatòria I ELS brots epidèmics. DOGC 2015;6958:1–19. [Google Scholar]
- 19. Laska EM. The use of capture-recapture methods in public health. Bull World Health Organ 2002;80:845. [PMC free article] [PubMed] [Google Scholar]
- 20. Freixa Blanxart M, Gurdia Olmos J, Honrubia Serrano ML. Validation of the capture –recapture method. Psichotema 2000;12:231–5. [Google Scholar]
- 21. Statistical Institute of Catalonia , 2015. Population at 1 January, 2015. Available: https://www.idescat.cat/pub/?id=pmh&n=446&lang=en [Accessed 18 Apr 2022].
- 22. Generalitat de Catalunya . Manual de notificació per ALS declarants al sistema de notificació de malalties de declaració obligatòria (MDO), 2016. Available: https://canalsalut.gencat.cat/web/.content/_Professionals/Vigilancia_epidemiologica/documents/arxius/MANUAL_MDO_2016.pdf [Accessed 27 Jan 2021].
- 23. Generalitat de Catalunya . Manual de procediment de notificació microbiològica obligatòria (SNMC), 2016. Available: https://canalsalut.gencat.cat/web/.content/_Professionals/Vigilancia_epidemiologica/documents/arxius/manual_procediment.pdf [Accessed 27 Jan 2021].
- 24. Ciruela P, Izquierdo C, Broner S. Epidemiology of invasive pneumococcal disease in Catalonia report 2012-2016 Generalitat de Catalunya; 2018. https://canalsalut.gencat.cat/web/.content/_Professionals/Vigilancia_epidemiologica/documents/arxius/invasive_pneumococcal_2012_2016_ang.pdf [Accessed 27 Jan 2021]. [Google Scholar]
- 25. Chapman DG. Some properties of the hypergeometric distribution with applications to zoological sample censuses. Berkeley: University of California Press, 1951. [Google Scholar]
- 26. Ballivet S, Salmi LR, Dubourdieu D. Capture-Recapture method to determine the best design of a surveillance system. Application to a thyroid cancer registry. Eur J Epidemiol 2000;16:147–53. 10.1023/A:1007605122984 [DOI] [PubMed] [Google Scholar]
- 27. Tilling K, Sterne JA, Wolfe CD. Estimation of the incidence of stroke using a capture-recapture model including covariates. Int J Epidemiol 2001;30:1351–9. 10.1093/ije/30.6.1351 [DOI] [PubMed] [Google Scholar]
- 28. Yee TW, Stoklosa J, Huggins RM. The VGAM Package for Capture-Recapture Data Using the Conditional Likelihood. J Stat Softw 2015;65:1–33 http://www.jstatsoft.org/ 10.18637/jss.v065.i05 [DOI] [Google Scholar]
- 29. Maldonado G, Greenland S. Simulation study of confounder-selection strategies. Am J Epidemiol 1993;138:923–36. 10.1093/oxfordjournals.aje.a116813 [DOI] [PubMed] [Google Scholar]
- 30. LaPorte RE, Dearwater SR, Chang YF, et al. Efficiency and accuracy of disease monitoring systems: application of capture-recapture methods to injury monitoring. Am J Epidemiol 1995;142:1069–77. 10.1093/oxfordjournals.aje.a117560 [DOI] [PubMed] [Google Scholar]
- 31. Andrianou XD, Riccardo F, Caporali MG, et al. Evaluation of the National surveillance system for invasive meningococcal disease, Italy, 2015-2018. PLoS One 2021;16:e0244889. 10.1371/journal.pone.0244889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Baldovin T, Lazzari R, Cocchio S, et al. Invasive meningococcal disease in the Veneto region of Italy: a capture-recapture analysis for assessing the effectiveness of an integrated surveillance system. BMJ Open 2017;7:e012478. 10.1136/bmjopen-2016-012478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jansson A, Arneborn M, Ekdahl K. Sensitivity of the Swedish statutory surveillance system for communicable diseases 1998-2002, assessed by the capture-recapture method. Epidemiol Infect 2005;133:401–7. 10.1017/S0950268804003632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Berghold C, Berghold A, Fülöp G, et al. Invasive meningococcal disease in Austria 2002: assessment of completeness of notification by comparison of two independent data sources. Wien Klin Wochenschr 2006;118:31–5. 10.1007/s00508-005-0502-0 [DOI] [PubMed] [Google Scholar]
- 35. Gibson A, Jorm L, McIntyre P. Using linked birth, notification, hospital and mortality data to examine false-positive meningococcal disease reporting and adjust disease incidence estimates for children in New South Wales, Australia. Epidemiol Infect 2015;143:2570–9. 10.1017/S0950268814003355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vázquez JA, Taha MK, Findlow J, et al. Global meningococcal initiative: guidelines for diagnosis and confirmation of invasive meningococcal disease. Epidemiol Infect 2016;144:3052–7. 10.1017/S0950268816001308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ratnayake R, Allard R. Challenges to the surveillance of meningococcal disease in an era of declining incidence in Montréal, Québec. Can J Public Health 2013;104:e335–9. 10.17269/cjph.104.3755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. O'Lorcain P, Bennett DE, Morgan SL, et al. A retrospective assessment of the completeness and timeliness of meningococcal disease notifications in the Republic of Ireland over a 16-year period, 1999-2015. Public Health 2018;156:44–51. 10.1016/j.puhe.2017.11.027 [DOI] [PubMed] [Google Scholar]
- 39. Carmona G, Vilaró M, Ciruela P, et al. Hepatitis A surveillance: sensitivity of two information sources. BMC Infect Dis 2018;18:633. 10.1186/s12879-018-3552-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gómez JA, Wetzler Malbrán P, Vidal G, et al. Estimation of the real burden of invasive meningococcal disease in Argentina. Epidemiol Infect 2019;147:e311:1–10. 10.1017/S0950268819002024 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-058003supp001.pdf (113.1KB, pdf)
Data Availability Statement
Data are available upon reasonable request. The datasets used and analysed during the current study are available from the corresponding author on reasonable request.


