Abstract
Introduction
Mental health problems frequently interfere with recovery from mild traumatic brain injury (mTBI) but are under-recognised and undertreated. Consistent implementation of clinical practice guidelines for proactive detection and treatment of mental health complications after mTBI will require evidence-based knowledge translation strategies. This study aims to determine if a guideline implementation tool can reduce the risk of mental health complications following mTBI. If effective, our guideline implementation tool could be readily scaled up and/or adapted to other healthcare settings.
Methods and analysis
We will conduct a triple-blind cluster randomised trial to evaluate a clinical practice guideline implementation tool designed to support proactive management of mental health complications after mTBI in primary care. We will recruit 535 adults (aged 18–69 years) with mTBI from six emergency departments and two urgent care centres in the Greater Vancouver Area, Canada. Upon enrolment at 2 weeks post-injury, they will complete mental health symptom screening tools and designate a general practitioner (GP) or primary care clinic where they plan to seek follow-up care. Primary care clinics will be randomised into one of two arms. In the guideline implementation tool arm, GPs will receive actionable mental health screening test results tailored to their patient and their patients will receive written education about mental health problems after mTBI and treatment options. In the usual care control arm, GPs and their patients will receive generic information about mTBI. Patient participants will complete outcome measures remotely at 2, 12 and 26 weeks post-injury. The primary outcome is rate of new or worsened mood, anxiety or trauma-related disorder on the Mini International Neuropsychiatric Interview at 26 weeks.
Ethics and dissemination
Study procedures were approved by the University of British Columbia’s research ethics board (H20-00562). The primary report for the trial results will be published in a peer-reviewed journal. Our knowledge user team members (patients, GPs, policymakers) will co-create a plan for public dissemination.
Trial registration number
ClinicalTrials.gov Registry (NCT04704037).
Keywords: Protocols & guidelines, PRIMARY CARE, Depression & mood disorders, Neurological injury, REHABILITATION MEDICINE, Clinical trials
Strengths and limitations of this study.
We will determine the presence/absence of mental health disorders (primary outcome) through a gold standard assessment structured diagnostic interview.
Triple blinding (patients, providers, outcome assessors) will minimise performance bias.
Prospective recruitment from the most common point of healthcare entry (emergency departments and urgent care centres) will miss cases that present directly to general practitioners (GPs), which will introduce selection bias.
Our case ascertainment method will support generalisability by not solely relying on physician documentation of a diagnosis of mild traumatic brain injury.
This study will rely primarily on patient report to track GP actions and healthcare use, which may be less accurate than administrative data sources.
Introduction
Mild traumatic brain injury (mTBI) is very common, with an annual incidence of 750–1200 per 100 000.1 2 Recovery is frequently complicated by psychiatric comorbidity. At least one in five people with mTBI will experience a major depressive episode, anxiety disorder or post-traumatic stress disorder (PTSD).3–12 This rate is higher than in the general population13 14 and higher than the rate in people with traumatic injuries not involving the head.15 Mental health comorbidity magnifies symptom burden, cognitive impairment15–17 and disability after mTBI.3 18 Cross-lagged analyses suggest that depression and anxiety precede, and presumably cause, chronic disability.19
There are effective treatments for depression, anxiety and PTSD after mTBI.20 Early screening and initiation of treatment for mental health complications (eg, with cognitive–behavioural therapy and/or selective serotonin reuptake inhibitors) have been highlighted in clinical practice guidelines for mTBI as an implementation priority.21 22 However, less than half of patients with a mental health disorder after mTBI receive mental health treatment,3 12 23–25 indicating a major knowledge–practice gap. As with other health conditions, evidence-based knowledge translation strategies may be necessary to accelerate uptake of clinical practice guidelines.26
Guideline implementation tools facilitate clinician behaviour change,27 especially those who engage patients in treatment decision-making.28 We describe here a study protocol for a cluster randomised trial that aims to evaluate the effectiveness of a guideline implementation tool designed to facilitate timely detection and treatment of mental health complications after mTBI. The guideline implementation tool involves deploying automated web-based screening for mental health symptoms and sharing actionable screening test results with patients and their general practitioners (GPs). This approach appeared feasible in a pilot cluster randomised trial.25
The present study targets the transition from acute to community-based primary care because that is often where the continuity of mTBI care fails29 and when mental health complications emerge.4 7 8 30 Patients whose symptoms do not promptly resolve after mTBI typically return to see their GP multiple times during the first 12 weeks after injury,31 32 which is the ideal window to initiate proactive management of mental health complications.33 34 By the time patients with mTBI reach specialty care, they often have intractable symptoms and comorbidities.32 35 36 In our region, GPs are the gateway to both specialised mTBI care and mental health services. Their role and frequent/early contact with patients with mTBI makes GPs an ideal target for the guideline implementation tool.
We hypothesise that the experimental group (guideline implementation tool) will be associated with lower rates of new or worsened mental health disorders compared with usual care control group at 26 weeks post-injury. This study will help determine how to close the gap between knowledge (mental health disorders after mTBI are common, debilitating and treatable) and practice (mental health disorders after mTBI are under-recognised and undertreated).
Methods and analysis
Study design
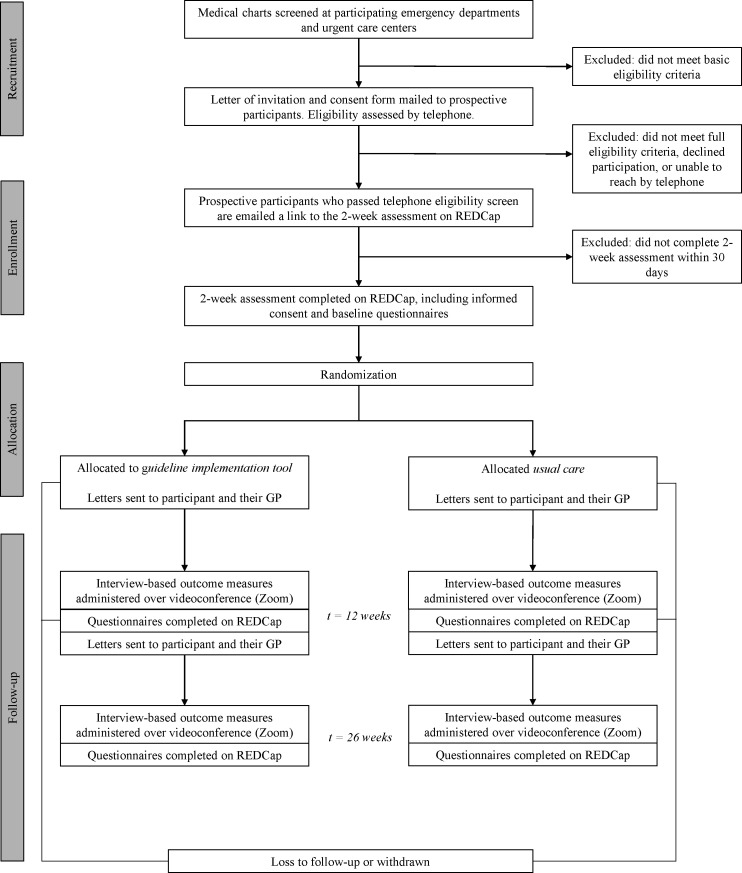
We will conduct a triple-blind (patient, provider, assessor) cluster randomised controlled trial with two arms. Patients and their GPs in the experimental group will receive tailored information about managing mental health complications after mTBI, including actionable screening test results. Patients and their GPs in the usual care control group will receive generic information about mTBI. The informed consent process will not reveal distinct features of each arm to mitigate patient expectancy bias. GPs will be told that their patient is participating in a research study, but they will not be informed about the objectives of the research study or the nature of the intervention. This way, we can observe their behaviour with minimal influence (ie, avoid Hawthorne effects37). Outcome assessors will be blinded to treatment allocations. Figure 1 illustrates the participant timeline. The study is registered on ClinicalTrials.gov (#NCT04704037). Any protocol amendments will be submitted to our institutional review board for approval and posted to ClinicalTrials.gov. The first participant was enrolled on 4 March 2021. We anticipate meeting our recruitment target by March 2023, as planned.
Figure 1.
Overview of study procedures. GP, general practitioner; REDCap, Research Electronic Data Capture.
Setting and participants
Participants will be recruited from six emergency departments (EDs) and two urgent care centres in the Greater Vancouver Area, Canada, which has a catchment area of approximately 1 million patients and 350 000 annual ED visits. Because the diagnosis of mTBI is frequently missed by ED physicians,38 39 we will use the case ascertainment method developed by Pozzato et al,39 40 based on the WHO Neurotrauma Task Force definition of mTBI41 to identify patients presenting to the ED with mTBI. Specifically, research assistants will screen medical charts for patients presenting to these sites with traumatic injury or chief complaints consistent with mTBI. Research assistants will use an algorithm (online supplemental appendix 1) to identify patients with probable or possible mTBI, defined below.
bmjopen-2022-062527supp001.pdf (60.2KB, pdf)
Probable mTBI: plausible mechanism of head trauma by external force with a Glasgow Coma Scale score of 13 or 14 at hospital arrival or other documentation of confusion/disorientation, loss of consciousness less than 30 min, post-traumatic amnesia less than 24 hours or trauma-related intracranial abnormality on head CT. Clinical signs of mTBI must not be accounted for by alcohol or drug intoxication.
Possible mTBI: plausible mechanism of head trauma by external force without clinical indicators for probable mTBI but with emergency physician diagnosis of mTBI or ≥2 post-concussion symptoms and clinical suspicion of mTBI (queried but unclear loss of consciousness, queried but unclear post-traumatic amnesia or head CT ordered). Clinical signs and symptoms of mTBI must not be accounted for by alcohol or drug intoxication.
Patients meeting criteria for probable or possible mTBI on chart review will be mailed a letter of invitation and consent form, and telephoned for further eligibility screening. If chart review reveals intimate partner violence as a possible cause of mTBI, we will use a modified recruitment approach—participants will not be sent the initial letter of invitation and instead telephoned discretely (eg, no voicemail messages will be left). In the eligibility screening phone call, a research assistant will complete a structured interview based on the WHO Neurotrauma Task Force definition of mTBI41 to confirm the patient’s mTBI diagnosis and other eligibility criteria. They will also ask the patient to designate a specific GP or walk-in clinic where they would seek follow-up care.
Inclusion criteria are (1) age 18–69 years old, (2) presentation to ED/urgent care within 72 hours of mTBI, (3) fluent in English, (4) primary residence in British Columbia, (5) able to designate a specific GP or walk-in family medicine clinic where they would seek follow-up care. Exclusion criteria are (1) pre-existing unstable/serious medical condition, (2) pre-existing unstable/severe mental illness (eg, schizophrenia or bipolar disorder requiring hospital admission in the past year or substance use requiring ED visit in the past year). Participants with prior mTBIs (>6 months ago), pre-injury mental health problems or co-occurring orthopaedic injuries will not be excluded.
To help characterise the sample, demographic and injury characteristics will be extracted from ED charts/electronic medical records using a standardised form. Extracted variables will include age, sex, mechanism of injury, Glasgow Coma Scale score at hospital arrival, loss of consciousness, post-traumatic amnesia, other documentation of confusion/disorientation, presence/absence of post-concussion symptoms, toxicology screen results, CT results, discharge diagnosis and discharge disposition.
Consent and baseline assessment
Patients who are determined to be eligible on telephone screening will be emailed a unique link to a REDCap (Research Electronic Data Capture)42 online survey at approximately 2 weeks post-injury and encouraged to complete it within 24 hours. Patients who open the REDCap survey link and electronically sign the consent form (online supplemental appendix 2) within 30 days will be prompted to fill out a release of health record authorisation form and complete self-report questionnaires, including mental health screening tools recommended in the Ontario Neurotrauma Foundation clinical practice guidelines for mTBI21:
bmjopen-2022-062527supp002.pdf (1.4MB, pdf)
Patient Health Questionnaire (PHQ-9) is a nine-item symptom inventory developed to screen for major depressive disorder in primary care. It has demonstrated strong sensitivity and specificity in people with and without mTBI.43–45 Certain symptoms queried by the PHQ-9 (eg, fatigue) are also commonly associated with mTBI. There is strong evidence that a symptom-inclusive diagnostic approach (ie, counting all possible depressive symptoms towards a diagnosis regardless of aetiological attribution) most accurately identifies depression after mTBI.45 46 Nevertheless, to guard against possible false positives due to symptom overlap between depression and mTBI, we will use a conservative approach developed for TBI,17 47 where a PHQ-9 total score of ≥10 and endorsement (item rating of 2+) of at least one of the cardinal depression symptoms (sadness and/or anhedonia) indicate a positive screen.
Generalized Anxiety Disorder (GAD-7) scale is a seven-item instrument that has been validated as a primary care screening tool not only for generalised anxiety disorder but other anxiety disorders and PTSD.48 49 A cut-off score of ≥10 is optimal for this measure.
Patients will complete the PHQ-9 and GAD-7 again at 12 and 26 weeks post-injury, as a REDCap survey. These two questionnaires take 3–6 min to complete.
Randomisation
Upon completion of the 2-week post-injury REDCap survey, the primary care clinic associated with each enrolled participant will be randomised in a 1:1 allocation ratio using a permuted block randomisation sequence. The sequence will be generated and uploaded to the REDCap project by an individual independent of the research team. Clinics that have already been randomised will retain their assignment when a new patient at that clinic is enrolled.
Interventions
In both treatment arms, patients and their GPs will be sent written information immediately following the 2-week and 12-week assessments. The content of that information will differ between arms.
Usual care control
GPs will be faxed a generic letter drawing their attention to the Ontario Neurotrauma Foundation guidelines for mTBI21 (online supplemental appendix 3A) which we regard as inert given evidence that passive dissemination of clinical practice guidelines is ineffective.50 Patients will be sent instructions about how to access generic education materials about mTBI (from concussion.vch.ca) (online supplemental appendix 3B). Education about mTBI is core usual care practice.21 Mental health screening test results will not be shared with patients or their GPs in the control arm.
bmjopen-2022-062527supp003.pdf (1.6MB, pdf)
bmjopen-2022-062527supp004.pdf (715.5KB, pdf)
Experimental intervention
The guideline implementation tool consists of two components, one for GPs and another for their patients:
GPs will be faxed a tailored letter at 2 and 12 weeks post-injury with their patient’s mental health screening test results, associated mental health treatment recommendations from the Ontario Neurotrauma Foundation guidelines21 and list of local mental health treatment resources (online supplemental appendix 4A). The Ontario Neurotrauma Foundation guidelines recognise selective serotonin reuptake inhibitor medications and cognitive–behavioural therapy as likely effective for treating depression, anxiety disorders and PTSD in people with mTBI,51–53 and suggest that these treatments need not be modified for people with mTBI.33 54 55
Patients will be sent an information package at 2 and 12 weeks post-injury with their mental health screening test results, education about mental health problems after mTBI and a decision-aid that presents treatment options to discuss with their GP (online supplemental appendix 4B). The purpose of this information package is to empower patients to start and/or more actively participate in a discussion about mental health issues with their GP. Similar patient-mediated interventions have been shown to increase the likelihood that physicians prescribe treatment28 as well as strengthen patients’ intention to pursue a particular treatment, enhance their comfort with their treatment decision and improve their adherence to the treatment.56–58
bmjopen-2022-062527supp005.pdf (4.9MB, pdf)
bmjopen-2022-062527supp006.pdf (3.5MB, pdf)
Tailored letters for GPs are automatically generated from the Microsoft Word document template by importing variables from the REDCap database (including the PHQ-9 and GAD-7 scores) with Application Programming Interface software created by Domain7 Solutions for the purpose of this study. Auto-generated letters are stored in REDCap and then sent to GPs by fax through the Health Insurance Portability & Accountability Act-compliant digital fax service (SRFax), integrated with REDCap. Participants received their materials directly from REDCap via a download link with passcode, sent to their email address.
The GP may diagnose and initiate mental health treatment at any time, prompted by the 2-week or 12-week tailored letter, or neither. This timing is based on the Ontario Neurotrauma Foundation guidelines recommendation for early screening,21 and the natural clinical course of depression after mTBI (the natural history of anxiety disorders is less well established), which has a peak incidence at 2–4 months post-injury.4 7 8 30
We designed the guideline implementation tool to target key barriers and facilitators to proactive management of mental health complications after mTBI, identified through qualitative interviews with GPs59 and systematic reviews of clinical practice guideline implementation challenges in other health conditions.60 61 Conceptualised in the Theoretical Domains Framework,62 the targets include: (1) professional role/identity: GPs may be unclear about their role in screening and initiating treatment for mental health complications after mTBI59; (2) memory/decision processes: GPs highlight concise, tailored guideline recommendations as an implementation facilitator60 63 64; (3) environmental context and resources: GPs report having inadequate time65 (eg, to administer standardised questionnaires), infrastructure (eg, treatment algorithms specific to mTBI)59 66 and familiarity with local resources for mental health treatment59; (4) social influences: GPs report that patients with mTBI tend to attribute all of their symptoms to mTBI and might be unaware or resistant to consider that mental health difficulties are contributory.59 The guideline implementation tool provides point-of-care reminders for GPs to consider mental health complications and facilitates the patient encounter by providing actionable screening test results and preparing patients for a discussion about mental health treatment.
Outcomes
Primary outcome
The primary outcome is the presence of a new or worsened mental health disorder at 26 weeks (ie, excluding disorders deemed to be pre-existing and stable), as assessed by the Mini International Neuropsychiatric Interview (MINI) V.7.0.2. The MINI is a structured diagnostic interview based on the Diagnostic and Statistical Manual of Mental Health Disorders, Fifth Edition.67 The reliability and validity have been established against comprehensive diagnostic instruments.68 69 The MINI will be administered by trained research assistants, under the supervision of a registered psychologist (NS). Training will consist of completing the ‘Adult Standard MINI Training’ certification from the instrument’s publisher (Harm Research Institute), a study-specific training session with NS, at least two mock MINI administrations and an administration with an actual participant audited by NS.
We will administer the major depressive episode, panic disorder, agoraphobia, social anxiety disorder, specific phobia, generalised anxiety disorder, obsessive-compulsive disorder and PTSD modules of the MINI at 12 and 26 weeks post-injury to determine the presence/absence of these conditions. To ascertain whether a mental health diagnosis is de novo or pre-existing, whenever a participant endorses a MINI module screening question, the research assistant will ask an additional set of standardised questions at the end of the module, as follows: (1) Approximately how long have you been experiencing these symptoms? (in weeks), (2) Were you experiencing these symptoms over the month just before your concussion? (yes/no), (3) Are these symptoms worse now compared with just before your concussion? (yes/no), (4) Has your treatment for these symptoms changed from just before your concussion (for example, you weren’t seeing a counsellor just before your concussion but now you are, or you started a new medication for these symptoms after your concussion, or you are taking a higher dose of a medication that you took before the concussion? (yes/no).
Secondary outcomes
Rivermead Postconcussion Symptom Questionnaire.70 The Rivermead Postconcussion Symptom Questionnaire is one of the most widely used outcome measures in mTBI research. It queries the current severity of 16 common symptoms following mTBI, on a scale from 0 (‘never experienced’) to 4 (‘severe problem’). Items rated 2 or higher are summed to create a total score.
WHO Disability Assessment Schedule 2.0 12-item interview version.71 This disease non-specific structured interview queries health-related difficulties with functional activities across the domains of cognition, self-care, interpersonal relations, mobility, community participation and work/school. The WHO Disability Assessment Schedule 2.0 12-item interview has strong internal consistency, unidimensionality and concurrent validity on people with an mTBI.72 73 Total scores quantify global disability.
Health service use
Using a modified interview version of the Perceived Need for Care Questionnaire,74 we will ask participants (1) whether they received any mental health treatment (information/education, psychotherapy/counselling or medications) since the prior assessment, (2) whether it was their GP who provided, referred for or prescribed the treatment, (3) details about the type and timing of the treatment, and (4) whether the treatment was a continuation of their pre-injury regimen or new/different. We will also access participants’ medication prescriptions from the provincial database (PharmaNet) for the 6 months prior to mTBI and 6 months post-injury observation period. At the 26-week assessment, participants will be asked to sign a PharmaNet consent allowing access to their record.
Self-report questionnaires, including the PHQ-9, GAD-7 and Rivermead Postconcussion Symptom Questionnaire, will be administered as a web-based REDCap survey at the 2-week, 12-week and 26-week time points. The MINI, Perceived Need for Care Questionnaire and WHO Disability Assessment Schedule will be administered by a research assistant through the secure videoconferencing platform Zoom at the 12-week and 26-week time points.
Data management
All data will be collected on REDCap, using the British Columbia Academic Health Science Network for instance. To ensure subject confidentiality, participants will be assigned a unique study number and only this number will be used on any research-related information collected during the course of the study. Data will be downloaded to and accessed from a secure server at the University of British Columbia.
Sample size and statistical power
The target recruitment is 535 patients to yield at least 450 evaluable patients at week 26, allowing for 15% loss to follow-up, consistent with our pilot trial.25 Based on 5000 simulations with the following assumptions: intracluster correlation coefficient=0.2, maximum cluster size=3 and incidence of 20% with a mental health disorder in the control group, we will have >80% power to detect a 10% reduction in the rate of mental health disorder in the guideline implementation tool arm compared with the control arm.
Planned statistical analyses
The treatment policy estimand is of primary interest. The treatment policy estimand evaluates the treatment effect for all randomised patients regardless of adherence to their assigned treatment or use of other treatments (i.e., following the intention-to-treat principle). The outcome variable is the presence of any new or worsened mental health disorder (0/1) at 26 weeks, the primary predictor variable is the treatment arm. To accommodate missing outcome data, a weighted generalised estimating equation approach will be used. This weights each subject’s measurements by the inverse probability that a subject drops out. This procedure restores randomisation balance under the missing at random assumption. Observation-specific weights will be calculated from a logistic regression model that includes predictors of missingness (baseline characteristics and, if available, 12-week PHQ-9 and GAD-7 scores). In planned subgroup analyses, we will examine whether the treatment effect is moderated by baseline mental health symptoms (positive PHQ-9 and/or GAD-7 screen) and patient gender. A detailed statistical analysis plan refining the primary analysis, addressing the secondary outcomes and secondary estimand, and sensitivity analyses will be developed prior to the termination of the trial and unblinding. No interim analyses are planned.
Safety and adverse events
Both the PHQ-9 and the MINI Neuropsychiatric Interview query suicidal ideation. If a participant endorses suicidal ideation on PHQ-9, they will be prompted to complete the Columbia-Suicide Severity Rating Scale-Screener for Primary Care75 as part of the REDCap survey. If a participant endorses suicidal ideation on the MINI, that is, during a Zoom outcome assessment, the assessor will administer the same suicide risk triaging instrument as a standardised interview. In both circumstances, Columbia-Suicide Severity Rating Scale-Screener for Primary Care will be used to triage participants into: (1) no further action required; (2) send a link to download a copy of ‘Coping with Suicidal Thoughts’ (a workbook that provides safety planning support, including crisis line phone numbers); or (3) encouraged to seek an urgent medical care and provide the name and contact that the research team can contact. No other ancillary care is planned.
No serious adverse events are anticipated. At each outcome assessment, participants will be prompted to respond to the open-ended question ‘Have you been harmed in any way by participating in this research study?’ with a free-text box. Affirmative responses to this question will be forwarded to a Safety Monitoring Committee (physician and psychologist who are not otherwise affiliated with the study) to evaluate the potential adverse event and whether it was caused by participation in the study.
Patient and public involvement
We assembled a Knowledge User Committee to assist with study planning and dissemination. The committee consists of two patient partners with lived experience with mTBI, two GPs and representatives from organisations involved in healthcare insurance/coordination (the provincial worker compensation board, WorkSafeBC, and the provincial motor vehicle insurer, the Insurance Corporation of British Columbia), knowledge translation (Ontario Neurotrauma Foundation and Parachute Canada) and vulnerable populations (Supporting Survivors of Abuse and Brain Injury through Research). The Knowledge User Committee met monthly during the planning phase of the study to review and provide input on key methodological decisions and patient/GP-facing documents. They will be reconvened to co-create a plan for disseminating the trial results.
Ethics and dissemination
All study procedures were approved by the University of British Columbia’s Office of Research Ethics (H20-00562), Vancouver Coastal Health Research Institute and Provincial Health Services Authority. Informed consent will be obtained from all participants.
The primary report for the trial results will be published in a peer-reviewed journal, in accordance with the Consolidated Standards of Reporting Trials. Our Knowledge User Committee will co-create a plan for dissemination to other audiences, including GPs and health service providers and funders. We will also prepare a lay summary of the research findings for our patient participants.
Discussion
Mental health problems frequently interfere with recovery from mTBI but are under-recognised and undertreated. Accordingly, recent clinical practice guidelines recommend early mental health screening and treatment initiation. GPs are optimally positioned to implement this guideline and may be better equipped to do so when their patients arrive with actionable symptom screening test results and knowledge about treatment options. In this study protocol for a cluster randomised trial, we described a plan to evaluate a guideline implementation tool design to support GPs and their patients in this way. Mobilising GPs to provide early proactive intervention may help prevent persistent symptoms and disability after mTBI. The guideline implementation tool was designed to be largely automated and therefore scalable. If found to be effective, it could be widely implemented with minimal resources and readily adapted for different points of entry into the healthcare system.
Random assignment and triple blinding (patients, providers, outcome assessors) will maximise internal validity. Other strengths of this study include prospective recruitment from the most common point of entry into the healthcare system (EDs and urgent care centres) and a case ascertainment method that incorporates but does not rely exclusively on physical documentation of an mTBI diagnosis. These two design features will support generalisability. However, selection bias will remain because some people with mTBI do not seek acute medical care. Another important limitation of this study is the plan to use patient self-report to track GP actions and healthcare use, which will provide only limited insights into the mechanisms underlying an intervention effect. We will access medication prescription data but linking with other administrative data sources, for example, to objectively track psychologist visits, is not feasible in our region.
Supplementary Material
Acknowledgments
The authors wish to the thank Drs Kevin Shi, Hazel Park and Afshin Khazei for their assistance with coordinating recruitment; Drs David Rhine and Frank Besserer for exploring the feasibility of additional recruitment sites; Gwen Farm and Tasha Klotz for their help with assembling materials for this report; Knowledge User Committee members Stephanie Cowle, Teri Thorson, Karen Mason, Linda Calvert, Judy Gargaro, Wenmei Zou, David Hall, Wayne Tang and Raymond Ying.
Footnotes
Twitter: @silverberg_lab, @LLi_1, @patarchambault
Contributors: NS conceived of the study, led its design and implementation, and drafted the manuscript. PB calculated the sample size requirements and created the statistical analysis plan. P-PL co-created the guideline implementation tool and participated in the Knowledge User Committee. All authors (NS, TO, PB, JRB, LCL, WP, FXS and PA) contributed to the study design and funding application, and critically reviewed the manuscript. The Canadian TBI Research Consortium (CTRC) contributed to this study by hosting presentations from the named authors, facilitating discussion about the study, and providing advice that strengthened the design and conduct of the study.
Funding: Canadian Institutes for Health Research (CIHR).
Disclaimer: The funder played no role in the decision to publish the study findings.
Competing interests: NS has a private neuropsychological consulting practice and has received honoraria for providing continuing medical education. The other authors have no potential conflicts to declare.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; peer reviewed for ethical and funding approval prior to submission.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not required.
References
- 1. Feigin VL, Theadom A, Barker-Collo S, et al. Incidence of traumatic brain injury in New Zealand: a population-based study. Lancet Neurol 2013;12:53–64. 10.1016/S1474-4422(12)70262-4 [DOI] [PubMed] [Google Scholar]
- 2. Langer L, Levy C, Bayley M. Increasing incidence of concussion: true epidemic or better recognition? J Head Trauma Rehabil 2020;35:E60–6. 10.1097/HTR.0000000000000503 [DOI] [PubMed] [Google Scholar]
- 3. Bryant RA, O'Donnell ML, Creamer M, et al. The psychiatric sequelae of traumatic injury. Am J Psychiatry 2010;167:312–20. 10.1176/appi.ajp.2009.09050617 [DOI] [PubMed] [Google Scholar]
- 4. Stein MB, Jain S, Giacino JT, et al. Risk of posttraumatic stress disorder and major depression in civilian patients after mild traumatic brain injury: a TRACK-TBI study. JAMA Psychiatry 2019;76:249–10. 10.1001/jamapsychiatry.2018.4288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Marinkovic I, Isokuortti H, Huovinen A, et al. Prognosis after mild traumatic brain injury: influence of psychiatric disorders. Brain Sci 2020;10. 10.3390/brainsci10120916. [Epub ahead of print: 27 Nov 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rapoport MJ, McCullagh S, Streiner D, et al. The clinical significance of major depression following mild traumatic brain injury. Psychosomatics 2003;44:31–7. 10.1176/appi.psy.44.1.31 [DOI] [PubMed] [Google Scholar]
- 7. Ouellet M-C, Beaulieu-Bonneau S, Sirois M-J, et al. Depression in the first year after traumatic brain injury. J Neurotrauma 2018;35:1620–9. 10.1089/neu.2017.5379 [DOI] [PubMed] [Google Scholar]
- 8. Rao V, Bertrand M, Rosenberg P, et al. Predictors of new-onset depression after mild traumatic brain injury. J Neuropsychiatry Clin Neurosci 2010;22:100–4. 10.1176/jnp.2010.22.1.100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Levin HS, McCauley SR, Josic CP, et al. Predicting depression following mild traumatic brain injury. Arch Gen Psychiatry 2005;62:523–8. 10.1001/archpsyc.62.5.523 [DOI] [PubMed] [Google Scholar]
- 10. Meares S, Shores EA, Taylor AJ, et al. The prospective course of postconcussion syndrome: the role of mild traumatic brain injury. Neuropsychology 2011;25:454–65. 10.1037/a0022580 [DOI] [PubMed] [Google Scholar]
- 11. Hoffman JM, Dikmen S, Temkin N, et al. Development of posttraumatic stress disorder after mild traumatic brain injury. Arch Phys Med Rehabil 2012;93:287–92. 10.1016/j.apmr.2011.08.041 [DOI] [PubMed] [Google Scholar]
- 12. Wang B, Zeldovich M, Rauen K, et al. Longitudinal analyses of the reciprocity of depression and anxiety after traumatic brain injury and its clinical implications. J Clin Med 2021;10:5597. 10.3390/jcm10235597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Delmonico RL, Theodore BR, Sandel ME, et al. Prevalence of depression and anxiety disorders following mild traumatic brain injury. Pm R 2021. 10.1002/pmrj.12657. [Epub ahead of print: 22 Jun 2021]. [DOI] [PubMed] [Google Scholar]
- 14. Hellewell SC, Beaton CS, Welton T, et al. Characterizing the risk of depression following mild traumatic brain injury: a meta-analysis of the literature comparing chronic mTBI to Non-mTBI populations. Front Neurol 2020;11:1–14. 10.3389/fneur.2020.00350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Uiterwijk D, Stargatt R, Humphrey S, et al. The relationship between cognitive functioning and symptoms of depression, anxiety, and post-traumatic stress disorder in adults with a traumatic brain injury: a meta-analysis. Neuropsychol Rev 2021. 10.1007/s11065-021-09524-1. [Epub ahead of print: 25 Oct 2021]. [DOI] [PubMed] [Google Scholar]
- 16. Lange RT, Iverson GL, Rose A. Depression strongly influences postconcussion symptom reporting following mild traumatic brain injury. J Head Trauma Rehabil 2011;26:127–37. 10.1097/HTR.0b013e3181e4622a [DOI] [PubMed] [Google Scholar]
- 17. Terry DP, Brassil M, Iverson GL, et al. Effect of depression on cognition after mild traumatic brain injury in adults. Clin Neuropsychol 2019;33:124–36. 10.1080/13854046.2018.1459853 [DOI] [PubMed] [Google Scholar]
- 18. Hellewell SC, Beaton CS, Welton T, et al. Characterizing the risk of depression following mild traumatic brain injury: a meta-analysis of the literature comparing chronic mTBI to non-mTBI populations. Front Neurol 2020;11:350. 10.3389/fneur.2020.00350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zahniser E, Nelson LD, Dikmen SS, et al. The temporal relationship of mental health problems and functional limitations following mTBI: a TRACK-TBI and TED study. J Neurotrauma 2019;36:1786–93. 10.1089/neu.2018.6172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Silverberg ND, Iaccarino MA, Panenka WJ, et al. Management of concussion and mild traumatic brain injury: a synthesis of practice guidelines. Arch Phys Med Rehabil 2020;101:382–93. 10.1016/j.apmr.2019.10.179 [DOI] [PubMed] [Google Scholar]
- 21. Ontario Neurotrauma Foundation . Guidelines for Concussion/Mild Traumatic Brain Injury & Persistent Symptoms, 2018. Available: https://braininjuryguidelines.org/concussion/
- 22. Department of Veterans Affairs and Department of Defense . VA/DoD clinical practice guideline for the management and rehabilitation of post-acute mild traumatic brain injury version 3.0.; 2021. Available: https://www.healthquality.va.gov/guidelines/Rehab/mtbi/VADoDmTBICPGFinal508.pdf [Accessed 17 Jan 2022].
- 23. Bombardier CH, Fann JR, Temkin NR, et al. Rates of major depressive disorder and clinical outcomes following traumatic brain injury. JAMA 2010;303:1938–45. 10.1001/jama.2010.599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Albrecht JS, Lydecker A, Peters ME, et al. Treatment of depression after traumatic brain injury reduces risk of neuropsychiatric outcomes. J Neurotrauma 2020;37:2542–8. 10.1089/neu.2019.6957 [DOI] [PubMed] [Google Scholar]
- 25. Silverberg ND, Panenka WJ, Lizotte P-P, et al. Promoting early treatment for mild traumatic brain injury in primary care with a guideline implementation tool: a pilot cluster randomised trial. BMJ Open 2020;10:e035527. 10.1136/bmjopen-2019-035527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Prior M, Guerin M, Grimmer-Somers K. The effectiveness of clinical guideline implementation strategies--a synthesis of systematic review findings. J Eval Clin Pract 2008;14:888–97. 10.1111/j.1365-2753.2008.01014.x [DOI] [PubMed] [Google Scholar]
- 27. Bauer MS, Damschroder L, Hagedorn H, et al. An introduction to implementation science for the non-specialist. BMC Psychol 2015;3:32. 10.1186/s40359-015-0089-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fønhus MS, Dalsbø TK, Johansen M, et al. Patient-mediated interventions to improve professional practice. Cochrane Database Syst Rev 2018;9:CD012472. 10.1002/14651858.CD012472.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Seabury SA, Gaudette Étienne, Goldman DP, et al. Assessment of follow-up care after emergency department presentation for mild traumatic brain injury and concussion: results from the TRACK-TBI study. JAMA Netw Open 2018;1:e180210. 10.1001/jamanetworkopen.2018.0210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jorge RE, Robinson RG, Moser D, et al. Major depression following traumatic brain injury. Arch Gen Psychiatry 2004;61:42–50. 10.1001/archpsyc.61.1.42 [DOI] [PubMed] [Google Scholar]
- 31. Galili SF, Bech BH, Vestergaard C, et al. Use of general practice before and after mild traumatic brain injury: a nationwide population-based cohort study in Denmark. BMJ Open 2017;7:e017735. 10.1136/bmjopen-2017-017735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Terry DP, Iverson GL, Panenka W, et al. Workplace and non-workplace mild traumatic brain injuries in an outpatient clinic sample: a case-control study. PLoS One 2018;13:e0198128. 10.1371/journal.pone.0198128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Marshall S, Bayley M, McCullagh S. Ontario Neurotrauma Foundation Guidelines for Concussion/Mild Traumatic Brain Injury & Persistent Symptoms 2018.
- 34. Giummarra MJ, Lennox A, Dali G, et al. Early psychological interventions for posttraumatic stress, depression and anxiety after traumatic injury: a systematic review and meta-analysis. Clin Psychol Rev 2018;62:11–36. 10.1016/j.cpr.2018.05.001 [DOI] [PubMed] [Google Scholar]
- 35. Mooney G, Speed J. The association between mild traumatic brain injury and psychiatric conditions. Brain Inj 2001;15:865–77. 10.1080/02699050110065286 [DOI] [PubMed] [Google Scholar]
- 36. King NS, Kirwilliam S. Permanent post-concussion symptoms after mild head injury. Brain Inj 2011;25:462–70. 10.3109/02699052.2011.558042 [DOI] [PubMed] [Google Scholar]
- 37. Borg J, Holm L, Cassidy JD, et al. Diagnostic procedures in mild traumatic brain injury: results of the who collaborating centre Task force on mild traumatic brain injury. J Rehabil Med 2004:61–75. 10.1080/16501960410023822 [DOI] [PubMed] [Google Scholar]
- 38. Ryu WHA, Feinstein A, Colantonio A, et al. Early identification and incidence of mild TBI in Ontario. Can J Neurol Sci 2009;36:429–35. 10.1017/s0317167100007745 [DOI] [PubMed] [Google Scholar]
- 39. Pozzato I, Cameron ID, Meares S, et al. A surveillance study to determine the accuracy of mild traumatic brain injury diagnosis in an emergency department: protocol for a retrospective cohort study. BMJ Open 2017;7:e016222. 10.1136/bmjopen-2017-016222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pozzato I, Meares S, Kifley A, et al. Challenges in the acute identification of mild traumatic brain injuries: results from an emergency department surveillance study. BMJ Open 2020;10:e034494. 10.1136/bmjopen-2019-034494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Holm L, Cassidy JD, Carroll LJ, et al. Summary of the who collaborating centre for neurotrauma Task force on mild traumatic brain injury. J Rehabil Med 2005;37:137–41. 10.1080/16501970510027321 [DOI] [PubMed] [Google Scholar]
- 42. Harris PA, Taylor R, Minor BL, et al. The REDCap Consortium: building an international community of software platform partners. J Biomed Inform 2019;95:103208. 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gilbody S, Richards D, Brealey S, et al. Screening for depression in medical settings with the patient health questionnaire (PHQ): a diagnostic meta-analysis. J Gen Intern Med 2007;22:1596–602. 10.1007/s11606-007-0333-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wittkampf KA, Naeije L, Schene AH, et al. Diagnostic accuracy of the mood module of the patient health questionnaire: a systematic review. Gen Hosp Psychiatry 2007;29:388–95. 10.1016/j.genhosppsych.2007.06.004 [DOI] [PubMed] [Google Scholar]
- 45. Dyer JR, Williams R, Bombardier CH, et al. Evaluating the psychometric properties of 3 depression measures in a sample of persons with traumatic brain injury and major depressive disorder. J Head Trauma Rehabil 2016;31:225–32. 10.1097/HTR.0000000000000177 [DOI] [PubMed] [Google Scholar]
- 46. Cook KF, Bombardier CH, Bamer AM, et al. Do somatic and cognitive symptoms of traumatic brain injury confound depression screening? Arch Phys Med Rehabil 2011;92:818–23. 10.1016/j.apmr.2010.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fann JR, Bombardier CH, Dikmen S, et al. Validity of the patient health Questionnaire-9 in assessing depression following traumatic brain injury. J Head Trauma Rehabil 2005;20:501–11. 10.1097/00001199-200511000-00003 [DOI] [PubMed] [Google Scholar]
- 48. Spitzer RL, Kroenke K, Williams JBW, et al. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med 2006;166:1092–7. 10.1001/archinte.166.10.1092 [DOI] [PubMed] [Google Scholar]
- 49. Löwe B, Decker O, Müller S, et al. Validation and standardization of the generalized anxiety disorder screener (GAD-7) in the general population. Med Care 2008;46:266–74. 10.1097/MLR.0b013e318160d093 [DOI] [PubMed] [Google Scholar]
- 50. Grimshaw JM, Thomas RE, MacLennan G, et al. Effectiveness and efficiency of guideline dissemination and implementation strategies. Health Technol Assess 2004;8:144. 10.3310/hta8060 [DOI] [PubMed] [Google Scholar]
- 51. Sander AM, Clark AN, Arciniegas DB, et al. A randomized controlled trial of acceptance and commitment therapy for psychological distress among persons with traumatic brain injury. Neuropsychol Rehabil 2021;31:1105–29. 10.1080/09602011.2020.1762670 [DOI] [PubMed] [Google Scholar]
- 52. Carlson KF, Kehle SM, Meis LA, et al. Prevalence, assessment, and treatment of mild traumatic brain injury and posttraumatic stress disorder: a systematic review of the evidence. J Head Trauma Rehabil 2011;26:103–15. 10.1097/HTR.0b013e3181e50ef1 [DOI] [PubMed] [Google Scholar]
- 53. Fann JR, Hart T, Schomer KG. Treatment for depression after traumatic brain injury: a systematic review. J Neurotrauma 2009;26:2383–402. 10.1089/neu.2009.1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Veterans Affairs/Department of Defense . Management of Concussion-Mild traumatic brain injury (MTBI) clinical practice guidelines, 2016. Available: https://www.healthquality.va.gov/guidelines/rehab/mtbi/
- 55. Silverberg ND, Panenka WJ. Antidepressants for depression after concussion and traumatic brain injury are still best practice. BMC Psychiatry 2019;19:9–11. 10.1186/s12888-019-2076-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gagliardi AR, Légaré F, Brouwers MC, et al. Patient-mediated knowledge translation (PKT) interventions for clinical encounters: a systematic review. Implement Sci 2016;11:26. 10.1186/s13012-016-0389-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Perestelo-Perez L, Rivero-Santana A, Sanchez-Afonso JA, et al. Effectiveness of a decision aid for patients with depression: A randomized controlled trial. Health Expect 2017;20:1096–105. 10.1111/hex.12553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. LeBlanc A, Herrin J, Williams MD, et al. Shared decision making for antidepressants in primary care: a cluster randomized trial. JAMA Intern Med 2015;175:1761–70. 10.1001/jamainternmed.2015.5214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Silverberg ND, Otamendi T, Dulai A, et al. Barriers and facilitators to the management of mental health complications after mild traumatic brain injury. Concussion 2021;6:CNC92. 10.2217/cnc-2020-0022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Cabana MD, Rand CS, Powe NR, et al. Why Don't Physicians Follow Clinical Practice Guidelines? JAMA 1999;282:1458–65. 10.1001/jama.282.15.1458 [DOI] [PubMed] [Google Scholar]
- 61. Grimshaw JM, Thomas RE, MacLennan G, et al. Effectiveness and efficiency of guideline dissemination and implementation strategies. Health Technol Assess 2004;8:1–72. 10.3310/hta8060 [DOI] [PubMed] [Google Scholar]
- 62. Atkins L, Francis J, Islam R, et al. A guide to using the theoretical domains framework of behaviour change to investigate implementation problems. Implementation Science 2017;12:1–18. 10.1186/s13012-017-0605-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Gagliardi AR, Brouwers MC, Palda VA, et al. How can we improve guideline use? A conceptual framework of implementability. Implement Sci 2011;6:26. 10.1186/1748-5908-6-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Rashidian A, Eccles MP, Russell I. Falling on stony ground? A qualitative study of implementation of clinical guidelines' prescribing recommendations in primary care. Health Policy 2008;85:148–61. 10.1016/j.healthpol.2007.07.011 [DOI] [PubMed] [Google Scholar]
- 65. Lawton R, Heyhoe J, Louch G, et al. Using the theoretical domains framework (TDF) to understand adherence to multiple evidence-based indicators in primary care: a qualitative study. Implement Sci 2016;11:113. 10.1186/s13012-016-0479-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zonfrillo MR, Master CL, Grady MF, et al. Pediatric providers' self-reported knowledge, practices, and attitudes about concussion. Pediatrics 2012;130:1120–5. 10.1542/peds.2012-1431 [DOI] [PubMed] [Google Scholar]
- 67. American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 5th Ed. Arlington, VA: American Psychiatric Publishing, 2013. [Google Scholar]
- 68. v SD, Harnett-Sheehan K, Raj BA. The measurement of disability. Int Clin Psychopharmacol 1996;11:89–95. 10.1097/00004850-199606003-00015 [DOI] [PubMed] [Google Scholar]
- 69. Sheehan DV, Lecrubier Y, Sheehan KH. The Mini-International neuropsychiatric interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 1998;59 Suppl (20:22-33;quiz 34-57. [PubMed] [Google Scholar]
- 70. Eyres S, Carey A, Gilworth G, et al. Construct validity and reliability of the Rivermead Post-Concussion symptoms questionnaire. Clin Rehabil 2005;19:878–87. 10.1191/0269215505cr905oa [DOI] [PubMed] [Google Scholar]
- 71. Federici S, Bracalenti M, Meloni F. World Health organization disability assessment schedule 2.0: an international systematic review. Disabil Rehabil 2016;8288:1–34. [DOI] [PubMed] [Google Scholar]
- 72. Snell DL, Iverson GL, Panenka WJ, et al. Preliminary validation of the world Health organization disability assessment schedule 2.0 for mild traumatic brain injury. J Neurotrauma 2017;34:3256–61. 10.1089/neu.2017.5234 [DOI] [PubMed] [Google Scholar]
- 73. Snell DL, Siegert RJ, Silverberg ND. Rasch analysis of the world Health organization disability assessment schedule 2.0 in a mild traumatic brain injury sample. Brain Inj 2020;34:610–8. 10.1080/02699052.2020.1729417 [DOI] [PubMed] [Google Scholar]
- 74. Meadows G, Harvey C, Fossey E, et al. Assessing perceived need for mental health care in a community survey: development of the perceived need for care questionnaire (PNCQ). Soc Psychiatry Psychiatr Epidemiol 2000;35:427–35. 10.1007/s001270050260 [DOI] [PubMed] [Google Scholar]
- 75. Zero Suicide . Columbia-Suicide severity rating Scale-Screener for primary care. Available: https://cssrs.columbia.edu/the-columbia-scale-c-ssrs/cssrs-for-communities-and-healthcare [Accessed 24 Jun 2021].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-062527supp001.pdf (60.2KB, pdf)
bmjopen-2022-062527supp002.pdf (1.4MB, pdf)
bmjopen-2022-062527supp003.pdf (1.6MB, pdf)
bmjopen-2022-062527supp004.pdf (715.5KB, pdf)
bmjopen-2022-062527supp005.pdf (4.9MB, pdf)
bmjopen-2022-062527supp006.pdf (3.5MB, pdf)